Abstract
Neutrophils are the primary effector cells during inflammation, but can also control excessive inflammatory responses by secreting anti-inflammatory cytokines. However, the mechanisms modulating their plasticity remain unclear. We now show that systemic serum amyloid A-1 (SAA-1) controls the plasticity of neutrophil differentiation. SAA-1 not only induced anti-inflammatory IL-10-secreting neutrophils but also promoted invariant NKT (iNKT) cell interaction with these neutrophils, a process that limits their suppressive activity by reducing IL-10 and enhancing IL-12 production. Because SAA-1-producing melanomas promote differentiation of IL-10-secreting neutrophils, harnessing iNKT cells could be useful therapeutically by reducing the frequency of immunosuppressive neutrophils and restoring tumor specific immune responses.
Keywords: invariant NKT cell, neutrophil, tumor evasion, Interleukin-10, serum amyloid A-1
Several types of myeloid cells can promote tumor progression either directly, by inhibiting tumor specific immune responses, or indirectly by promoting angiogenesis, tumor growth and tissue remodeling1. The best characterized are tumor infiltrating macrophages that have properties of alternatively activated macrophages2, and myeloid derived suppressor cells (MDSC), a heterogenous population of immunosuppressive myeloid cells3. MDSC are capable of differentiating into immunosuppressive macrophages and neutrophils4, and are characterized by high arginase and iNOS production, the metabolic products of which include diffusible and highly reactive peroxynitrites5. Recent reports have highlighted the ability of neutrophils not only to promote inflammation, but also to exhibit anti-inflammatory properties, depending on tumor microenvironment cues such as secretion of tumor growth factor-β6. Interleukin (IL)-10-secreting neutrophils proliferate during bacterial infections due to the co-triggering of TLR-MyD88 and C-type lectin receptor (CLR)-Syk-dependent pathways7.
These results support a previously unanticipated plasticity for neutrophils, similar to that seen in macrophages2, raising the possibility that alternative polarization of neutrophils can be harnessed by tumors to dampen tumor immune responses and facilitate tumor growth. However, it remains unclear whether neutrophils acquire their anti-inflammatory properties at the site of inflammation, or whether systemic signals, released during acute and chronic inflammatory processes, are capable of modulating the phenotype of circulating neutrophils, hence affecting a significantly larger pool of cells. Furthermore, although neutrophils have the capacity to directly promote inflammatory reactions by influencing the activity of other immune cells and tissues8, it remains unclear whether the interaction of neutrophils with other immune cells may also modulate their pro- and anti-inflammatory properties.
In the present study, we find that the major acute-phase reactant serum amyloid A-1 (SAA-1), one of the first and most abundant proteins secreted during the physiological response to infections and injuries, controls the plasticity of neutrophil differentiation. We show that SAA-1 induces IL-10 secretion from neutrophils and also promotes their ability to interact with invariant NKT (iNKT) cells, a process that limits their suppressive activity by reducing IL-10 and enhancing IL-12 production. These findings underscore the plasticity of neutrophils, as cells capable of having pro- and anti-inflammatory properties and highlight the role of iNKT cells as important modulators of inflammatory responses.
RESULTS
Immunosuppressive IL-10 secreting neutrophils in melanoma patients
To address whether melanomas induce the expansion of leukocytes with suppressive properties we compared the frequency of tumor specific CD8+ T cells expanded in vitro from whole blood samples of melanoma patients and from Ficoll purified peripheral blood mononuclear cells (PBMC). The results of these experiments demonstrated that the expansion of melan-A26-35 specific CD8+ T cells within total leukocyte cultures was greatly reduced in comparison with their frequency within Ficoll purified PBMC (Fig. 1a and Supplementary Fig. 1a). In contrast, blood samples from healthy donors showed a similar expansion of melan-A26-35 specific CD8+ T cells in total leukocytes and PBMC, albeit at a lower frequency as expected (Fig. 1a).
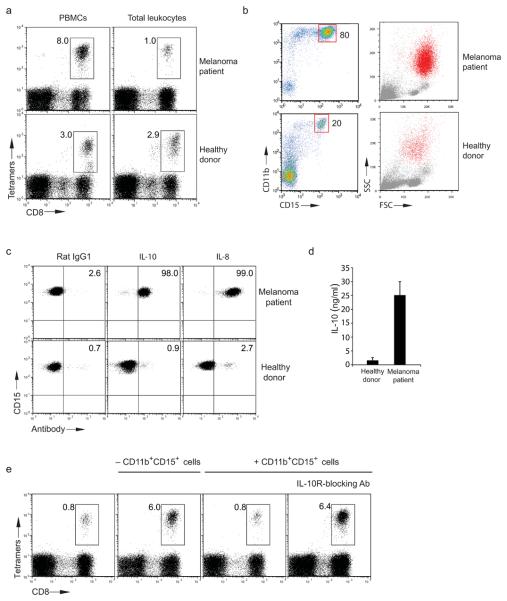
Figure 1. Proliferation of immunosuppressive CD11b+CD15+ cells in melanoma patients.
(a) Expansion of melan-A26-35 tetramer specific CD8+ T cells from PBMC or total leukocytes of one HLA-A2+ melanoma patient and one healthy donor. (b) Percentage and forward/side scatter of CD11b+CD15+cells purified from one melanoma patient (top) and one healthy donor (bottom). Red dots in forward/side scatter plot indicate back gating of CD11b+CD15+cells. (a,b) The melanoma patient tested is represented as green symbol in Supplementary Fig. 1b. (c) Intracellular staining with anti-IL-10 and anti-IL-8 of CD11b+CD15+cells purified from the melanoma patient Δ (Supplementary Fig. 2a) and one healthy donor. (d) Cumulative IL-10 secretion from CD11b+CD15+ cells from seven healthy donors and 10 melanoma patients ( Supplementary Fig. 2a) (error bars, SD). (e) Expansion of melan-A26-35 tetramer specific CD8+ T cells from total leukocytes of one HLA-A2+ melanoma patient (red, Supplementary Fig. 1b) depleted (middle-left) or not depleted (left) of CD11b+CD15+cells. Percentages of melan-A26-35 tetramer specific CD8+ T cells after adding 10% purified CD11b+CD15+ cells to CD11b+CD15+ depleted total leukocytes with (right) or without blocking anti-IL10R antibody (middle-right). Data are representative of seven (a), five (c) and three (e) experiments (summarized in Supplementary Fig. 1a, 2a and 1c).
Supplementary Fig. 2a) (error bars, SD). (e) Expansion of melan-A26-35 tetramer specific CD8+ T cells from total leukocytes of one HLA-A2+ melanoma patient (red, Supplementary Fig. 1b) depleted (middle-left) or not depleted (left) of CD11b+CD15+cells. Percentages of melan-A26-35 tetramer specific CD8+ T cells after adding 10% purified CD11b+CD15+ cells to CD11b+CD15+ depleted total leukocytes with (right) or without blocking anti-IL10R antibody (middle-right). Data are representative of seven (a), five (c) and three (e) experiments (summarized in Supplementary Fig. 1a, 2a and 1c).
When we compared the cellular profile of total leukocytes from melanoma patients and healthy volunteers we observed that neutrophils (as defined by staining with CD11b and CD15 antibodies) were expanded in the peripheral blood of a large proportion of melanoma patients (Fig. 1b and Supplementary Fig. 1b and 2a). Consistent with the phenotypic features of neutrophils, CD11b+CD15+ cells were removed after Ficoll purification and had a poly-segmented nuclear morphology (data not shown). Further phenotypic profiling revealed that neutrophils from melanoma patients, unlike CD11b+CD15+ cells from healthy donors, constitutively synthesized IL-10 and IL-8 (Fig. 1c) and had enhanced expression of arginase-1 (Supplementary Fig. 2b). Spontaneous production of IL-10 by neutrophils purified from melanoma patients was further confirmed by ELISA (Fig. 1d).
The frequency of neutrophils detected in the blood of melanoma patients correlated with the staging of disease (Supplementary Fig. 2a), and neutrophils isolated from patients with late stage melanoma also had an increased capacity to suppress lymphocyte proliferation in mixed lymphocyte reactions (MLR) (Supplementary Fig. 2a). We then asked whether the presence of high numbers of IL-10-secreting neutrophils in the blood of melanoma patients could account for the observed suppression of melan-A26-35 specific CD8+ T cell proliferation. Consistent with this hypothesis, we observed that depletion of CD11b+CD15+ cells from the leukocytes of melanoma patients rescued the expansion of melan-A26-35 specific CD8+ T cells (Fig. 1e, middle-left). Conversely, proliferation of melan-A26-35 specific CD8+ T cell was again abolished after adding back to the same cultures autologous purified CD11b+CD15+ cells (Fig. 1e, middle-right). The immunosuppressive property of CD11b+CD15+ cells purified from melanoma patients was mediated by IL-10 secretion, as addition of IL-10 receptor blocking antibody rescued melan-A26-35 specific CD8+ T cell proliferation (Fig. 1e, right). Control CD11b+CD15+ cells purified from healthy donors (which did not produce IL-10) failed to inhibit melan-A26-35 specific CD8+ T cell expansion (Supplementary Fig. 2d). Cumulative results with all the melan-A26-35 specific CD8+ T cell expansion experiments using PBMC in the presence or absence of purified CD11b+CD15+ cells and with or without IL-10 receptor blocking antibody are shown (Supplementary Fig. 1a and 1c).
SAA-1 controls differentiation of immunosuppressive IL-10 secreting neutrophils
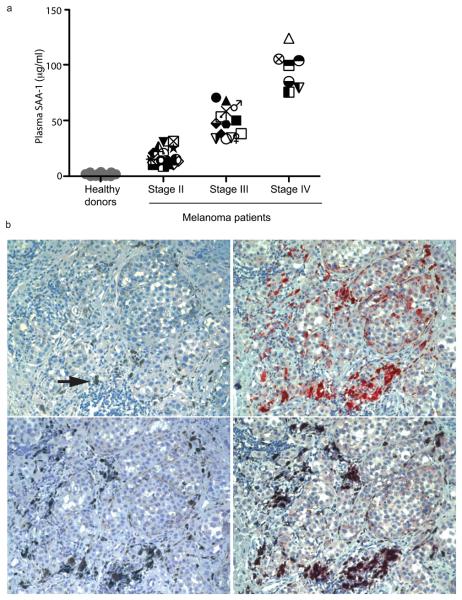
The observation that circulating IL-10-secreting neutrophils in a large proportion of melanoma patients undergo a unimodal shift in phenotype, strongly suggests that their expansion and phenotypic modification are the result of systemic signal(s), which may be harnessed by the tumor, as an evasion mechanism to hamper melanoma specific immune responses. To identify which factors are responsible for the in vivo expansion of IL-10-secreting neutrophils in melanoma patients we compared the cytokine profiles in the plasma of 40 melanoma patients with those of 30 normal control subjects. Although several cytokines were elevated in most patients, such as granulocyte-macrophage colony stimulating factor (GM-CSF), IL-13, IL-10 and IL-8 (Supplementary Fig. 3), we found that in all patients studied the concentration of the acute phase response protein SAA-1 was several orders of magnitude higher than that of the other cytokines measured (Fig. 2a). These results were further confirmed by measuring SAA-1 plasma concentrations in an additional 10 melanoma patients, in which we observed a correlation between SAA-1 concentrations and the frequency of CD11b+CD15+ cells (Supplementary Fig. 1b). In addition, amounts of SAA-1 were higher than those of the C reactive protein (CRP), another acute phase response protein. Interestingly, we observed a positive correlation between the concentrations of SAA-1 and disease staging (Fig. 2a). Although the major source of SAA-1 during inflammation is the liver, peripheral production of this protein has been reported9. Indeed, staining of primary melanoma sections from five patients with SAA-1 specific antibodies revealed the presence of SAA-1 positive tumor cells and tumor infiltrating macrophages (Fig. 2b).
Figure 2. SAA-1 is found in plasma and primary tumors of melanoma patients.
(a) SAA-1 levels in plasma from 40 melanoma patients and 30 healthy donors were determined by ELISA. Patients are identified by unique symbols (Supplementary Fig. 2a and Supplementary Fig. 3). (b) Immunohistochemistry of primary melanoma serial sections. Top-left, melanin location (arrow; × 20); top-right, staining of tumor cells with SAA-1 antibody (× 20); bottom-left, staining with anti-human CD68 antibody (× 20); bottom-right, double staining with anti-SAA-1 and anti-CD68 antibodies (× 20), indicating that cells positive for SAA-1 expression are also tumor associated macrophages.
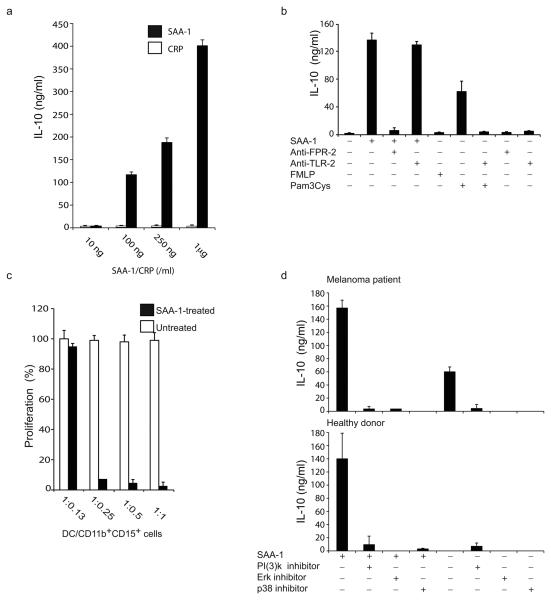
We reasoned that the high systemic plasma amounts of SAA-1 together with its production within primary melanomas could account for the global shift in the phenotype of circulating neutrophils. To address this possibility, we sorted CD11b+CD15+ cells from healthy donors and observed that exposure to increasing concentrations of SAA-1, but not to CRP (Fig. 3a) or the potent neutrophil activator formyl-peptide (FMLP) (Fig. 3b), induced IL-10 secretion in a dose-dependent manner. This effect was not caused by the presence of endotoxin in the SAA-1 preparation as the endotoxin removing compound polymyxin failed to reduce IL-10 production10 (Supplementary Fig. 4). Consistent with the ability of SAA-1 to induce production of IL-10, we observed that SAA-1-treated CD11b+CD15+ cells isolated from healthy donors acquired the capacity to suppress T cell proliferation (Fig. 3c).
Figure 3. SAA -1 induces IL-10 production from human neutrophils.
(a). IL-10 secretion by SAA-1 treated CD11b+CD15+ cells purified from healthy donors in response to increasing concentrations of SAA-1 . (b) IL-10 secretion by CD11b+CD15+ cells from healthy donors, treated with SAA-1, Pam3Cys or FMLP in the presence or absence of blocking FPR-2 and TLR-2 antibodies. (c) T cell proliferation of alloreactive T cells stimulated by allogeneic DC, with graded numbers of CD11b+CD15+ cells from third party healthy donors pretreated or not with SAA-1 (100 % proliferation corresponds to 7 × 104 cpm). (d) IL-10 release from CD11b+CD15+ cells purified from the melanoma patient △ (as shown in Supplementary Fig. 2a) (top panel), and one healthy donor (bottom panel) pre-incubated or not with the PI(3) kinase inhibitor LY294002, the Erk inhibitor U1026 and the p38 inhibitor SB203580 and then treated or not with SAA-1. Data are representative of five independent experiments (error bars, SD).
These results demonstrate the presence of SAA-1 in the plasma and primary tumors of melanoma patients and define SAA-1 as an acute phase response protein capable of inducing IL-10 production in neutrophils. These findings are consistent with the observation that both SAA-1 concentrations in blood and the frequency of IL-10-producing neutrophils positively correlate with the staging of the disease.
Mechanisms controlling IL-10 secretion from SAA-1 treated neutrophils
The best characterized receptors for SAA-1 are the G-protein coupled Formyl Peptide Like-111,12, recently renamed FPR213 and the Toll-like receptor (TLR)-211,14, both of which are expressed by neutrophils (Supplementary Fig. 5a). To identify the receptor involved in SAA-1-dependent IL-10 production, we treated neutrophils purified from healthy donors with SAA-1 in the presence or absence of FPR2 and TLR2 blocking antibodies. The addition of FPR2 blocking antibody prevented SAA-1-dependent IL-10 release (Fig. 3b). In contrast, the TLR2 blocking antibody blocked the effect of the TLR-2 agonist Pam3Cys, but had no effect on SAA-1-dependent neutrophil activation (Fig. 3b).
As it has been shown previously that FPR2 triggers the activation of the mitogen-activated protein kinases Erk 1/2, PI(3)K and p3815,16, which are also important for IL-10 production by neutrophils7, we pre-treated purified neutrophils with p38, Erk and PI(3)K kinase inhibitors for 1 h, and then stimulated them with SAA-1 for 24 h. Inhibitors of all three kinases, at doses that did not affect neutrophil viability (data not shown), strongly inhibited IL-10 production by SAA-1 treated neutrophils from melanoma patients and healthy donors (Fig. 3d). Importantly, the above inhibitors also prevented spontaneous secretion of IL-10 by neutrophils purified from melanoma patients (Fig. 3d, top), indicating that MAPK and PI(3)K pathways are constitutively activated in these cells. Indeed, ex vivo intracellular staining with antibodies directed against Akt (a PI(3)K target), Erk and p38 (Supplementary Fig. 5b) or with antibodies specific for the phosphorylated forms of these three kinases confirmed that they are constitutively phosphorylated in neutrophils purified from melanoma patients (Fig. 4e).
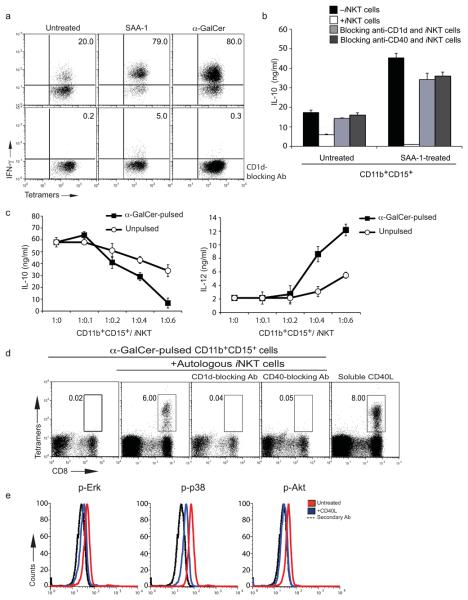
Figure 4. SAA-1 treatment of human neutrophils promotes crosstalk with iNKT cells.
(a) Intracellular anti-IFN-γ staining of CD1d/α-GalCer tetramer positive human iNKT cells co-cultured with untreated (left), SAA-1 (middle) or α-GalCer (right) treated CD11b+CD15+ cells from the melanoma patient ⊗ without (top) or with (bottom) CD1d blocking antibody. (b) IL-10 release from CD11b+CD15+ cells from the melanoma patient ▼ untreated or treated with SAA-1 and incubated with iNKT cells with or without CD1d and CD40 blocking antibodies (error bars, SD). (c) IL-10 and IL-12 release from α-GalCer pulsed or unpulsed CD11b+CD15+ cells from the melanoma patient ● incubated with iNKT cells at the indicated ratios (error bars, SD). (a,b,c) Symbols denote patients in Supplementary Fig. 2b. (d) Frequency of melan-A26-35 tetramer specific CD8+ T cells from total leukocytes of one melanoma patient (brown, Supplementary Fig. 1b) incubated with autologous melan-A26-35 peptide pulsed DC and α-GalCer pulsed autologous CD11b+CD15+ cells with or without autologous iNKT cells (plots 1 to 4). Effect of blocking anti-CD1d (plot 3) and anti-CD40 (plot 4) antibodies is shown. Addition of soluble CD40L to CD11b+CD15+ cells restores melan-A26-35-specific CD8+ T cell expansion in the absence of α-GalCer and autologous iNKT cells (plot 5). (e) Intracellular staining of melanoma patients' CD11b+CD15+ cells with anti-kinase specific antibodies after incubation with soluble CD40L.
Thus, SAA-1 modulation of IL-10 production by neutrophils is mediated by the G-protein coupled receptor FPR2 via activation of multiple nonredundant kinase pathways.
SAA-1 promotes crosstalk between neutrophils and iNKT cells
The finding that SAA-1 induces the differentiation of immunosuppressive neutrophils has important general implications, as SAA-1 is amongst the first acute response proteins to be secreted during microbial infections and the differentiation of IL-10 producing immunosuppressive neutrophils is likely to be a common feature of inflammatory responses. Indeed, hepatic acute phase response proteins can induce the differentiation of MDSC in a model of sepsis17. In addition, we have observed that the expansion of MDSC in mice (CD11b+/GR-1+ cells) and humans (CD11b+CD15+ cells) infected with influenza virus18 correlates with increased SAA-1 concentrations in the serum (data not shown).
iNKT cells are activated by TLR-dependent dendritic cell (DC) maturation events by the combined secretion of soluble factors19 and up-regulation of endogenous lipid agonists presented by CD1d molecules20,21. Activated iNKT cells exert a variety of immunomodulatory functions, mediated largely by their constitutive expression of CD40L and the release of cytokines such as IFN-γ and IL-422. Since MDSC differentiation in influenza virus infected mice is controlled by iNKT cells via a CD1d- and CD40-dependent mechanism 18 and because neutrophils express both CD40 and CD1d, which can be further enhanced by their incubation with IFN-γ (Supplementary Fig. 5c), we asked whether iNKT cells can modulate the activation and differentiation of IL-10-producing neutrophils. Neutrophils directly purified from melanoma patients (Fig. 4a, top left), but not from healthy donors (Supplementary Fig. 6a), were capable of promoting the activation of iNKT cells in the absence of any further stimulation. In turn, co-incubation of neutrophils with iNKT cells led to a reduction in the amount of IL-10 secreted by neutrophils from melanoma patients (Fig. 4b). This effect was proportional to the numbers of iNKT cells added and it was associated with the simultaneous increase in IL-12 production as shown by ELISA (Fig. 4c) and by intracellular staining of CD11b+CD15+ cells (Supplementary Fig. 6c). The crosstalk between neutrophils and iNKT cells was shown to be CD1d dependent, as the addition of anti-CD1d blocking antibodies prevented IFN-γ production by iNKT cells (Fig. 4a and Supplementary Fig. 6a) and restored IL-10 release by neutrophils (Fig. 4b).
As neutrophils from healthy donors failed to trigger iNKT cell activation, we asked whether SAA-1 could modulate their capacity to interact with iNKT cells. Consistent with this hypothesis, SAA-1 treatment of neutrophils from healthy donors triggered a CD1d-dependent activation of iNKT cells to a degree comparable to that obtained after pulsing neutrophils with the strong iNKT cell agonist alpha-GalactosylCeramide (α-GalCer) (Fig. 4a and Supplementary Fig. 6a). Moreover SAA-1 could further increase the capacity of neutrophils from melanoma patients to promote activation of iNKT cells, and this activation led to a reduction in IL-10 release by SAA-1-treated neutrophils from both melanoma patients and healthy donors (Fig. 4b and data not shown). These results indicate that neutrophils purified from patients with an elevated SAA-1 concentration, such as melanoma patients, are capable of spontaneously activating iNKT cells, and that activation of iNKT cells can modulate the release of IL-10 by immunosuppressive neutrophils.
Next, we set out to identify the molecular mechanisms responsible for the crosstalk between iNKT cells and neutrophils and showed that the addition of a CD40 blocking antibody abolished the ability of iNKT cells to reduce the amounts of IL-10 released by immunosuppressive neutrophils (Fig. 4b). Three sets of experiments further confirmed that the ability of iNKT cells to down-modulate the SAA-1-dependent IL-10 production by neutrophils was CD40L dependent. Firstly, incubation of IL-10-secreting neutrophils from melanoma patients with increasing concentrations of soluble CD40L reduced production of IL-10 and increased synthesis of IL-12 (Supplementary Fig. 6b). Secondly, addition of soluble CD40L to neutrophils of melanoma patients rescued melan-A26-35-CD8+ T cell proliferation (Fig. 4d, last plot). Finally, we observed that the CD40L-dependent reduction in IL-10 secretion by neutrophils from melanoma patients was associated with reduced phosphorylation of Erk, Akt and p38 MAP kinase (Fig. 4e). Consistent with the above results, we observed that pulsing neutrophils isolated from melanoma patients with the iNKT cell agonist α-GalCer further enhanced the ability of iNKT cells to abolish IL-10 production by neutrophils (Fig. 4c) and completely abolished their immunosuppressive activity in a CD40- and CD1d-dependent manner (Fig. 4d, middle-left, middle, and middle-right). Incubation of α-GalCer-pulsed CD11b+CD15+ cells with iNKT cells did not result in lysis of neutrophils by iNKT cells, ruling out that the loss of suppressive activity was simply due to cell death (Supplementary Fig. 7a). In addition to reduced IL-10 and increased IL-12 secretion, iNKT-treated neutrophils had reduced reactive oxygen species (ROS) production, suggesting an overall change from an immunosuppressive to a more inflammatory phenotype (Supplementary Fig. 7a).
These results demonstrate that in addition to driving the differentiation of IL-10-producing neutrophils, SAA-1 promotes their crosstalk with iNKT cells. This crosstalk is CD1d dependent and by activating the CD40 pathway in neutrophils it provides a negative feedback loop capable of reducing IL-10 production and limiting their immunosuppressive activity.
iNKT cells suppress expansion of IL-10 secreting neutrophils in vivo
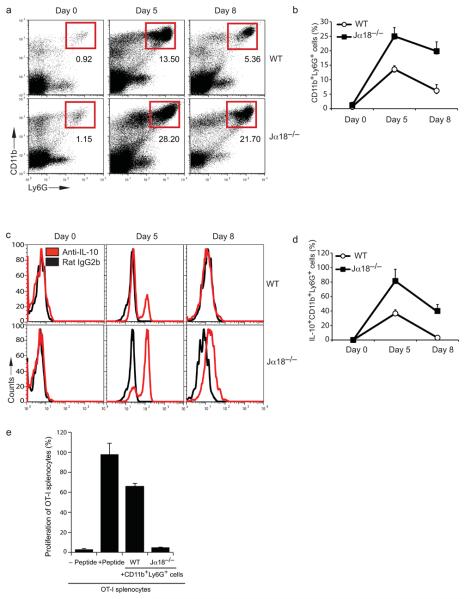
To further investigate the ability of iNKT cells to modulate the SAA-1-dependent differentiation of IL-10-producing neutrophils, recombinant SAA-1 was injected subcutaneously into wild-type (WT) and iNKT-deficient Tcra-Jtm1Tgi mice (hereafter referred to as Jα18−/−) once a day for 5 days and the frequency of neutrophils (CD11b+Ly6G+ cells) was measured in blood samples at different time points during treatment. Consistent with previous findings demonstrating the ability of SAA-1 to induce neutrophilia11, we observed an expansion of CD11b+Ly6G+ cells in SAA-1 injected WT mice, which declined 3 days after the last SAA-1 injection (Fig. 5a and b). However, injection of SAA-1 into Jα18−/− mice induced a higher percentage of CD11b+Ly6G+ cells compared to WT mice (28.2% vs 13.5%), which declined more slowly than in SAA-1-injected WT mice (Fig. 5a and 5b). Critically, in contrast with WT neutrophils, a high proportion of CD11b+Ly6G+ cells from SAA-1 injected Jα18−/− mice produced IL-10 (Fig. 5c and d), indicating that the lack of iNKT cells allows the expansion of IL-10 secreting neutrophils. Finally, CD11b+Ly6G+ cells isolated from Jα18−/− mice were capable of suppressing proliferation of OT-I cytotoxic T lymphocytes, much more efficiently than CD11b+Ly6G+ cells purified from SAA-1 injected WT mice (Fig. 5e). As a control, we demonstrated that injection of SAA-1 into TLR-2 deficient mice induces an expansion of IL-10 secreting CD11b+Ly6G+ cells comparable to that obtained in SAA-1 injected WT mice (data not shown).
Figure 5. Expansion of immunosuppressive IL-10 secreting neutrophils in SAA-1 injected Jα18−/− mice.
(a) Percentage of CD11b+Ly6G+cells in the blood of WT and Jα18−/− mice (n = 6) injected daily subcutaneously with SAA-1 for 5 days. Blood samples were collected before injection (day 0) and at day 5 and day 8 (i.e. 3 days after the last SAA-1 injection). (b) Cumulative data of the frequency of CD11b+Ly6G+ cells from six WT and Jα18−/− mice injected with SAA-1, as described in (a) (error bars, SD). (c) Ex vivo intracellular IL-10 staining of circulating CD11b+Ly6G+ from either WT or Jα18−/− mice injected daily subcutaneously with SAA-1 for 5 days. (Red, staining with anti-IL-10 antibodies; black, isotype control staining). (d) Cumulative data of the frequency of IL-10 positive CD11b+Ly6G+ cells from six WT and six Jα18−/− mice injected with SAA-1, as described in (c) (error bars, SD). (e) Proliferation of CFSE labeled OT-I splenocytes pulsed with the SIINFEKL peptide in the presence of 10% CD11b+Ly6G+ cells sorted from the blood of SAA-1 injected WT or Jα18−/− mice at day 5. OTI proliferation was analyzed at day 4 (error bars, SD).
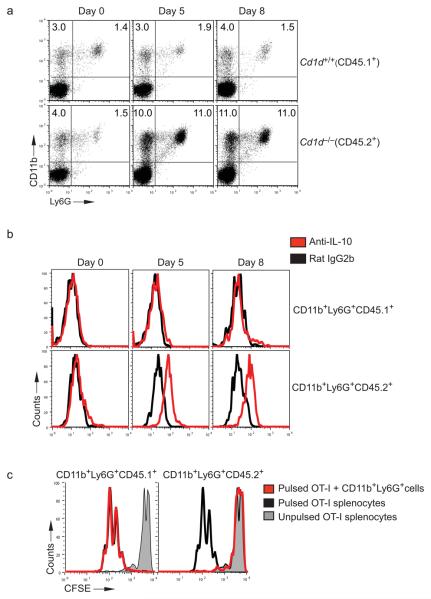
These results could depend either on direct crosstalk between neutrophils and iNKT cells or on the trans-modulation of neutrophils by soluble factors released by iNKT cells. To distinguish between these two possibilities, we reconstituted lethally irradiated B6.SJL mice (CD45.1+) with a 50:50 mixture of CD1d+CD45.1+ and CD1d−CD45.2+ bone marrow (Supplementary Fig. 7b). We reasoned that if the iNKT cell-dependent effect on IL-10-secreting neutrophils was also CD1d-dependent, SAA-1 injection into chimeric mice should preferentially induce the expansion of IL-10-secreting CD1d negative CD45.2+/+ neutrophils. Before injecting SAA-1, we confirmed equal engrafting of both CD45.1 and CD45.2 hematopoietic cells (data not shown) and positive selection of iNKT cells (Supplementary Fig. 7c). After injecting SAA-1 daily for 5 consecutive days, we observed a greater expansion of circulating CD11b+Ly6G+ cells derived from the Cd1d−/− donor bone marrow (i.e. CD45.2+), as compared with CD11b+Ly6G+ originating from the WT bone marrow (i.e. CD45.1+) (Fig. 6a). Importantly, purified blood CD11b+Ly6G+ cells originated from the Cd1d−/−CD45.2+ bone marrow produced IL-10 and were capable of suppressing OT-I proliferation (Fig. 6b and c), while purified blood CD11b+Ly6G+ cells derived from the Cd1d+/+CD45.1+ bone marrow remained IL-10 negative and did not have any suppressive effect on OT-I cytotoxic T lymphocyte proliferation (Fig. 6b and c).
Figure 6. Expansion of immunosuppressive IL-10 secreting Cd1d−/− neutrophils in SAA-1 injected Cd1d+/+/Cd1d−/− mixed bone marrow chimeras.
(a) Frequency of CD11b+Ly6G+ cells in Cd1d+/+/Cd1d−/− chimeric mice injected daily subcutaneously with SAA-1 (120 μg/kg) for 5 days. Blood samples were collected at day 0, day 5 and day 8 after the initial injection and stained with CD11b and Ly6G antibodies. Gating on CD45.1+ (Cd1d+/+) cells (top), and on CD45.2+ (Cd1d−/−) cells (bottom). (b) Intracellular staining with anti-IL-10 antibody on CD11b+Ly6G+ cells derived from either CD45.1+Cd1d−/− bone marrow (top) or CD45.2+Cd1d−/− bone marrow (bottom). Data are representative of two experiments with 3 mice each. (c) Proliferation at day 4 of CFSE labeled OT-I splenocytes pulsed with the SIINFEKL peptide with (red) or without (black) CD11b+Ly6G+ cells from either CD45.1+Cd1d+/+ cells (left) or CD45.2+Cd1d−/− cells (right) sorted from the blood of SAA-1 injected chimeric mice at day 5. Gray line, peptide unpulsed control proliferation.
These findings provide conclusive evidence that the in vivo crosstalk between iNKT cells and SAA-1-treated neutrophils is CD1d dependent, and results in a large reduction of IL-10 secretion.
Discussion
In this study we have shown that, in both humans and mice, the acute phase response protein SAA-1 induces the expansion and differentiation of IL-10-secreting neutrophils, which can suppress antigen specific T cell responses. In addition, we have observed that SAA-1 also promotes the interaction between neutrophils and iNKT cells in a CD1d- and CD40-dependent manner, which reverses their suppressive phenotype by reducing IL-10 production. The effect of SAA-1 is mediated by the binding of SAA-1 to the G-protein coupled receptor FPR2 and is dependent on the activation of MAPK and PI(3)K. Conversely, the CD40-dependent signaling pathway activated by the crosstalk with iNKT cells de-phosporylates MAPK and PI(3)K.
The observation that SAA-1 also promotes the interaction between neutrophils and iNKT cells opens the avenue to new therapeutic strategies harnessing this immunomodulatory T cell subset. Multiple mechanisms influencing iNKT-cell activation have emerged, indicating an important link between inflammation and iNKT cells23, which results in enhanced CD40-dependent DC24,25 and B cell activation26. For example, during microbial infections, inflammatory cytokines, such as IL-12 and IL-18, enhance basal iNKT cell autoreactivity and promote secretion of interferon-γ19-21,27. In addition, during inflammatory responses, iNKT-cell activation can be influenced by increased expression of surface CD1d molecules by activated antigen presenting cells28,29, secretion of G-CSF30, and/or by increased expression of enzymes leading to the biosynthesis of endogenous self lipids20,21,31.
Our in vivo data underscore the importance of iNKT cell numbers in modulating the overall frequency of neutrophils and their differentiation in IL-10-secreting cells via CD1d-dependent interactions. Indeed, injection of SAA-1 into WT mice reduced numbers of IL-10-secreting neutrophils as compared with the numbers observed in SAA-1-injected iNKT cell-deficient mice. In addition, injection of SAA-1 in mixed bone marrow chimeric mice, reconstituted with haematopoietic cells half of which lacked CD1d molecules, resulted in proliferation of IL-10-secreting immunosuppressive neutrophils only within the Cd1d−/− cell population. Further experiments are warranted to understand in which location iNKT cells modulate the proliferation and differentiation of neutrophils. However, given the ability of SAA-1 in inducing neutrophilia11 and the presence of iNKT cells in the bone marrow32,33, it is tempting to speculate that iNKT cells may modulate neutrophils' differentiation and expansion in the bone marrow, as well as in the periphery, possibly by interacting with granulocyte-monocyte precursors, which we have shown to be CD1d positive (data not shown).
iNKT cells are comprised of a functionally heterogeneous population of distinct subsets, differing by CD434 and NK1.1 expression35,36, their anatomical location37, cytokine profiles38 and effector functions37. As such, it remains unclear whether specific iNKT cell subsets mediate downregulation of IL-10 secretion from SAA-1-differentiated neutrophils in vivo. The functional diversity of iNKT cells has recently been extended with the discovery of a subset of IL-17-producing iNKT cells (NKT17 cells) that lack the expression of both CD4 and NK1.138, are able to promote airway neutrophilia39 and exert effector function within epithelia, such as the skin40. Given the link between IL-17 secretion and neutrophil recruitment41, further experiments are warranted to address the role of NKT17 cells38,40,42,43 in the modulation of IL-10 production by SAA-1 differentiated neutrophils. The release of IL-17 by iNKT cells may represent a host defense mechanism to attract anti-inflammatory neutrophils in inflamed tissues and convert them into pro-inflammatory neutrophils.
The reasons for the large expansion of IL-10-secreting neutrophils in melanoma patients, in the face of circulating iNKT cells, remain unclear. However, it is possible that the abundance of IL-10-producing neutrophils in melanoma patients may be accounted for by a defect in the numbers and activity of iNKT cells, as it has previously been reported that cancer patients often have lower frequencies of circulating iNKT cells than healthy volunteers44-46, and defects in activation47. Since we showed that the ability of human iNKT cells from melanoma patients to abolish the production of IL-10 by neutrophils is enhanced upon their incubation with strong iNKT cell agonists, such as α-GalCer, pharmacologically harnessing the interaction between iNKT cells and neutrophils may provide a new ‘conditioning’ regimen aimed at increasing the therapeutic efficacy of subsequent vaccination strategies.
Expansion of suppressive IL-10-secreting neutrophils in melanoma patients correlated with elevated amounts of SAA-1 and with stage of disease. These findings confirm and extend previously published results demonstrating a high frequency of circulating immunosuppressive neutrophils in a number of tumor types48-50, and suggest that SAA-1 is a negative prognostic marker in cancer patients51. As the proliferation of suppressive neutrophils during microbial infections is a mechanism to control the unwanted bystander effects of neutrophils7, 17, our results highlight a novel mechanism used by tumors to impair tumor specific immune responses - by harnessing the anti-inflammatory properties of this acute phase protein. By exploiting the plasticity of neutrophils, the tumor microenvironment induces the proliferation of large numbers of immunosuppressive IL-10-secreting cells enhancing local and systemic levels of SAA-1. Consistent with our findings, it has been shown that a proportion of renal cell carcinomas secrete SAA-152 and that SAA-3, another member of the SAA family, is capable of conditioning the pre-metastatic niche of tumor cell infiltration by facilitating the accumulation of myeloid cells in pre-metastatic lungs53, although secretion of IL-10 in these tumors was not investigated. These results indicate that SAA-1 secreted within the tumor microenvironment (i.e. by tumor cells and tumor infiltrating macrophages) contributes to total SAA plasma concentrations. However, since it known that soluble factors secreted within the tumor microenvironment (such as TNF, IL-6 and IL-1) can enhance SAA-1 transcription54,55, the relative contribution of liver- and tumor-derived SAA-1 remains unknown at present.
SAA-1 is secreted rapidly during viral and bacterial infections and we anticipate that the results described in this manuscript can be extended to inflammatory processes dependent on microbial infections. Indeed, influenza virus infection of mice lacking iNKT cells results in the expansion of immunosuppressive CD11b+Ly6G+ cells, which infiltrate inflamed lungs and greatly reduce the ability of these infected mice to mount antigen specific immune responses18. As SAA-1 concentrations in plasma are significantly increased during influenza infection56 and to much higher amounts than in melanoma patients (data not shown), it is very likely that the proliferation of suppressive CD11b+Ly6G+ cells, observed in influenza virus-infected mice, and of CD11b+CD15+ cells observed in influenza virus-infected individuals is also correlated with the enhanced SAA-1 plasma concentrations.
In conclusion, our results describe a novel mechanism for the control of inflammatory processes via local and systemic secretion of SAA-1 that leads to the proliferation and differentiation of anti-inflammatory neutrophils, but also promotes the crosstalk of neutrophils with iNKT cells, which in turn modulates their suppressive properties. These findings underscore the plasticity of neutrophils, as cells capable of having pro- and anti-inflammatory properties and highlight the role of iNKT cells as important modulators of inflammatory responses. This powerful physiological mechanism designed to control excessive inflammation can be exploited by tumors to impair tumor specific immune responses. Harnessing iNKT cells may reduce the frequency of immunosuppressive neutrophils, which may have important implications in vaccination strategies.
Materials and Methods
Reagents
MHC class I and CD1d tetramers were prepared as described previously57,58. SAA-1 protein was from Peprotech. Soluble CD40L was from Alexis. Anti FPR-2 antibody was from GENOVAC, while phospho-p44/42 Erk, phospho-p38 and phospho-Akt antibodies were from Cell Signalling. Other antibodies used for FACS staining were purchased from eBioscience. Samples were acquired either on a FACS Calibur™ using CellQuest™ software or on a Cyan Cytometer and analyzed using Flowjo software. Antibodies used in western blot analyses were arginase 1 (N-20) (Santa-Cruz Biotechnology) and anti-glyceraldehyde-3-phoshate dehydrogenase (GAPDH, AbD Serotec). Kinase inhibitors were SB203580 (p38), U1026 (Erk) and LY294002 (PI(3)K) (from Calbiochem Merck). To detect ROS production we used 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (DCFDA-H2) (Invitrogen) as described previously59. Pam3Cys was used at 10 μg/ml (Invivogen). α-GalCer was solubilized at 200 μg/ml in vehicle (0.5% Tween-20/PBS). Anti-human CD1d blocking antibody was from BD Pharmingen.
Mice
Female C57BL/6 mice, OT-I TCR transgenic and B6.SJL-PtprcaPep3b/BoyJ (B6Ly5.1, CD45 1+/+) were maintained in the Biological Services Unit, John Radcliffe Hospital, University of Oxford and used according to established University of Oxford institutional guidelines under the authority of a UK Home Office project license. Other mice used lacked the Jα18 TCR gene segment60 (Tcra-Jtm1Tgi mice, hereafter referred to as Jα18−/−) lacking Vα14 iNKT cells but with other lymphoid cell lineages) or Cd1d gene (Cd1d−/−, CD45.2+/+, kindly provided by Luc Van Kaer, Vanderbilt University School of Medicine, Nashville, TN)61.
Isolation and differentiation of human neutrophils
Neutrophils were purified from the peripheral blood of melanoma patients and healthy donors using a Ficoll gradient: leukocytes pelleted as a layer over red blood cells were collected and then purified using anti-CD11b-coated magnetic beads (Miltenyi Biotec). A proportion higher than 95% was stained with anti-CD11b and anti-CD15 specific antibodies (Supplementary Fig. 1c). Neutrophils from healthy donors and melanoma patients were pre-incubated with Erk, p38 and PI(3) kinase inhibitors (1 μM), with anti-CD40 antibody (5 μg/ml, R&D Systems) for 1 h at 37 °C, and then cultured with 250 ng/ml of SAA-1 protein for 24 h. SAA-1 or α-GalCer (100 ng/ml) were added to human neutrophils for 24 h in the presence of iNKT cells. Soluble CD40L was added to untreated neutrophils from melanoma patients for 24 h.
Human iNKT cells and DC
iNKT cells and DC were generated from either healthy blood donors or from melanoma patients as previously described20.
OT-I proliferation assays
Splenocytes from OT-I TCR transgenic mice were pulsed with 2 μg/ml of SIINFEKL peptide for 1 h at 37 °C, washed and labeled with 5 μM carboxyluorescein succinimidyl ester (CFSE). Sorted CD11b+Ly6G+ cells (2 × 104) were cultured in 96-well flat bottom plates with 2 × 105 CFSE labeled-OT-I splenocytes. Cells were analyzed using a FACS Calibur™ with CellQuest™ software 4 days later. Data are expressed as percentage proliferation of SIINFEKL peptide pulsed and CFSE labeled-OT-I splenocytes, in the presence of CD11b+Ly6G+ cells, as compared with proliferation of peptide pulsed and CFSE labeled-OT-I splenocytes in the absence of CD11b+Ly6G+ cells (100%).
MLR
PBMC (2 × 105) were mixed with allogeneic irradiated (5000 rad) DC (5 × 104) in 200 μl RPMI 5% human AB serum in 96-well flat bottom plates. Cells were incubated at 37 °C, 5% CO2 for 5 days and then 1 μCi/well 3H-thymidine (Perkin Elmer life Sciences) was added for 15–18 h. 3H-thymidine incorporation was measured using a Wallac Microbeta Jet 1450 reader (Perkin Elmer). CD11b+CD15+ cell-mediated inhibition of lymphocytes proliferation was carried out by co-culturing irradiated SAA-1 treated CD11b+CD15+ cells (5 × 104) from healthy donors, together with PBMC and irradiated DC. Data are expressed as percentage of PBMC proliferation driven by allogeneic irradiated DC in the presence of irradiated CD11b+CD15+ cells, as compared with alloreactive PBMC proliferation in the absence of CD11b+CD15+ cells (100%).
ELISA
Plasma of melanoma patients was collected after 2 h of sedimentation. Blood samples were obtained under an ethically approved protocol, after gaining written informed consent from patients. Cytokines were quantified by using a FlowCytomix kit (Bender MedSystems) according to the manufacturer's instructions. SAA-1 protein and CRP were measured using ELISA kits (Invitrogen and Elica, respectively) performed according to the manufacturers' instructions. IL-10 and IL-12 were measured using ELISA kits (BD Biosciences).
Intracellular staining
Human or mouse neutrophils were stained with surface markers for 20 min at 4°C. Cells were washed twice in cold PBS, fixed and permeabilized with a Foxp3 Fixation/Permeabilization Concentrate and Diluent kit (eBioscience) according to the manufacturer's instructions. Cells were washed twice with Permabilization buffer (eBioscience) and stained with specific antibodies for 20 min at 4 °C.
Immunohistochemistry
Tissue sections of primary tumors (4-μm sections) were deparaffinized and rehydrated. Antigen demasking was performed in 50 mM Tris/2 mM EDTA pH 9.0 using a Philips Whirlpool Sixth Sense microwave on a steaming program. Staining with PG-M1 (anti-human CD68, Dako) was carried out using the Novolink Polymer Detection System (RE7280-K, Leica) and substrate developed with silver-grey peroxidase substrate kit (SK-4700, Vector Labs). Staining with anti-SAA-1 antibody (Abcam) was carried out using Dako REAL Detection System, Alkaline Phosphatase/RED. Primary antibody incubation was performed overnight in the cold room. Reaction was visualized by RED chromagen, Dako Fuchsin + Substrate-Chromagen System (Dako). Tissue sections were counterstained with Gill Nr 3 haematoxylin (Sigma Aldrich) and mounted in Aquatex (Merck).
Mixed bone marrow chimera mice
Recipient mice were γ-irradiated twice with 450 rad, and then reconstituted with a mixture of bone marrow derived from Cd1d−/− and B6.SJL (totally 5 × 106 cells) to achieve a 50:50 chimera. Eight weeks after reconstitution, mice were tested for chimerism and at 10 weeks chimeras were injected subcutaneously with SAA-1 protein (120 μg/kg) for 5 days.
MelanA26-35 specific CD8+ T cell expansion
Human DCs were pulsed for 3 h with 100 ng/ml MelanA26-35 (ELAGIGILTV) peptide in serum-free medium. Cells were washed thoroughly and incubated with autologous 1 × 106 PBMC, pre-incubated with IL-10 receptor blocking antibody for 1 h (10 μg/ml R&D Systems), at a 1:10 ratio in RPMI-10% fetal calf serum. Autologous CD11b+CD15+ cells were purified as described and added to the culture at a 1:1 ratio with DC. Specific MelanA26-35 CD8+ cells were expanded in IL-2-containing medium and analyzed at day 10–15 by tetramer staining, as described previously62.
Supplementary Material
Acknowledgments
This work was supported by the Cancer Research UK (grant C399/A2291) and the UK Medical Research Council (VC). MM is supported by the Oxford NIHR Biomedical Research Centre. Clinical sample processing was supported by the Oxford Experimental Cancer Medicine Centre.
We wish to than Richard Lisle and Kevin Hollowood (Oxford NIHR Biomedical Research Centre) for their help in preparing melanoma sample sections and analyzing the stained samples. We wish to thank Giorgio Napolitani, Angus Stock and Moira Johnson for helpful discussions and critical reading of the manuscript and Paolo Polzella for technical support.
Footnotes
Contributions
C.D.S. designed and performed the experiments, prepared the figures and contributed to write the manuscript; R.A. performed specific experiments; I.K. and M.M. obtained consent from melanoma patients and collected blood samples; S.B., M.J., and R.A. performed tissue staining of melanoma sections; M.S. provided reagents and contributed to the writing of the manuscript; V.C. designed the experiments and wrote the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Santo C, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Cross A, Edwards SW, Bucknall RC, Moots RJ. Secretion of oncostatin M by neutrophils in rheumatoid arthritis. Arthritis Rheum. 2004;50:1430–1436. doi: 10.1002/art.20166. [DOI] [PubMed] [Google Scholar]
- 9.Urieli-Shoval S, Linke RP, Matzner Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol. 2000;7:64–69. doi: 10.1097/00062752-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs DM, Morrison DC. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977;118:21–27. [PubMed] [Google Scholar]
- 11.He RL, et al. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood. 2009;113:429–437. doi: 10.1182/blood-2008-03-139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dufton N, et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye RD, et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 16.He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–4079. doi: 10.4049/jimmunol.177.6.4072. [DOI] [PubMed] [Google Scholar]
- 17.Sander LE, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Santo C, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 20.Salio M, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paget C, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salio M, Cerundolo V. Linking inflammation to natural killer T cell activation. PLoS Biol. 2009;7:e1000226. doi: 10.1371/journal.pbio.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermans IF, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 25.Silk JD, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli G, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 28.Raghuraman G, Geng Y, Wang CR. IFN-beta-mediated up-regulation of CD1d in bacteria-infected APCs. J Immunol. 2006;177:7841–7848. doi: 10.4049/jimmunol.177.11.7841. [DOI] [PubMed] [Google Scholar]
- 29.Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol. 2005;175:3584–3593. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 30.Morris ES, et al. Induction of natural killer T cell-dependent alloreactivity by administration of granulocyte colony-stimulating factor after bone marrow transplantation. Nat Med. 2009;15:436–441. doi: 10.1038/nm.1948. [DOI] [PubMed] [Google Scholar]
- 31.Fox LM, et al. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda JL, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 35.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 36.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowe NY, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coquet JM, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doisne JM, et al. Skin and peripheral lymph node invariant NKT cells are mainly retinoic acid receptor-related orphan receptor (gamma)t+ and respond preferentially under inflammatory conditions. J Immunol. 2009;183:2142–2149. doi: 10.4049/jimmunol.0901059. [DOI] [PubMed] [Google Scholar]
- 41.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michel ML, et al. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rachitskaya AV, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tahir SM, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–4050. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 45.Dhodapkar MV, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molling JW, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 47.van der Vliet HJ, et al. Circulating myeloid dendritic cells of advanced cancer patients result in reduced activation and a biased cytokine profile in invariant NKT cells. J Immunol. 2008;180:7287–7293. doi: 10.4049/jimmunol.180.11.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 49.Wislez M, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63:1405–1412. [PubMed] [Google Scholar]
- 50.Rodriguez PC, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Findeisen P, et al. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol. 2009;27:2199–2208. doi: 10.1200/JCO.2008.18.0554. [DOI] [PubMed] [Google Scholar]
- 52.Paret C, Schon Z, Szponar A, Kovacs G. Inflammatory Protein Serum Amyloid A1 Marks a Subset of Conventional Renal Cell Carcinomas with Fatal Outcome. Eur Urol. 2009 doi: 10.1016/j.eururo.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 54.Uhlar CM, Whitehead AS. The kinetics and magnitude of the synergistic activation of the serum amyloid A promoter by IL-1 beta and IL-6 is determined by the order of cytokine addition. Scand J Immunol. 1999;49:399–404. doi: 10.1046/j.1365-3083.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- 55.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 56.Whicher JT, Chambers RE, Higginson J, Nashef L, Higgins PG. Acute phase response of serum amyloid A protein and C reactive protein to the common cold and influenza. J Clin Pathol. 1985;38:312–316. doi: 10.1136/jcp.38.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunbar PR, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J Immunol. 2000;165:6644–6652. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 58.Karadimitris A, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98:3294–3298. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corzo CA, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 61.Mendiratta SK, et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 62.Salio M, et al. Mature dendritic cells prime functionally superior melan-A-specific CD8+ lymphocytes as compared with nonprofessional APC. J Immunol. 2001;167:1188–1197. doi: 10.4049/jimmunol.167.3.1188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.