Abstract
Objectives
To investigate the biological activities of Justicia pectoralis Jacq. (Acanthaceae), an herbal medicine used in Costa Rica (CR) for the management of menopausal symptoms and dysmenorrhea.
Study design
The aerial parts of Justicia pectoralis were collected, dried and extracted in methanol. To establish possible mechanisms of action of JP for the treatment of menopausal symptoms, the estrogenic and progesterone agonist, and antiinflammatory activities were investigated.
Main outcome measures
The methanol extract (JP-M) was tested in ER and PR binding assays, a COX-2 enzyme inhibition assay, the ERβ-CALUX assay in U2-OS cells, as well as reporter and endogenous gene assays in MCF-7 K1 cells.
Results
The JP-M extract inhibited COX-2 catalytic activity (IC50 4.8µg/ml); bound to both ERα and ERβ (IC50 50 µg/ml and 23.1µg/ml, respectively); induced estrogen-dependent transcription in the ERβ-CALUX; and bound to the progesterone receptor (IC50 22.8 µg/ml). The extract also modulated the expression of endogenous estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells at a concentration of 20 µg/ml. Activation of a 2 ERE-construct in transiently transfected MCF-7 cells by the extract was inhibited by the estrogen receptor antagonist ICI 182,780, indicating that the effects were mediated through the estrogen receptor. Finally, the extract weakly enhanced the proliferation of MCF-7 cells, however this was not statistically significant as compared with DMSO controls.
Conclusions
Extracts of J. pectoralis have estrogenic, progestagenic and anti-inflammatory effects, and thus have a plausible mechanism of action, explaining its traditional use for menopause and PMS.
Keywords: Justicia pectoralis, Costa Rica, ERβ-CALUX, estrogenic, PTGES, pS2, herbal medicine, menopause
1.0 Introduction
The menopausal transition represents a continuum of change that begins with diminished ovarian reserve, followed by the menstrual irregularity of the peri-menopause, and culminates with the cessation of menses and the loss of reproductive capacity in the postmenopausal woman [1,2]. Prior to the outcomes of the Women’s Health Initiative, hormone therapy (HT; estrogen and/or progesterone) was the gold standard for the symptomatic treatment of menopause [3,4]. The WHI study, found that treating women for greater than 5 years with estrogen plus progestin lead to a significant increase in the risk for breast cancer [5]. Increased risk of endometrial cancer, ovarian cancer, blood clots, strokes, and the increased risk for gallbladder disease are also side effects of the continued use of hormone therapy [6]. Today, several types of HT are available and are commonly prescribed for the treatment for menopausal symptoms, despite the adverse events associated with the treatment regimen. Understandably, some women find the side effects associated with HT unacceptable and forgo treatment for their menopausal symptoms. Additionally, for women with a high risk of developing estrogen-responsive cancers or heart disease, HT is not recommended, and thus these women have limited therapeutic options. For this reason, many women find alternative and complementary therapies for menopause and PMS a very attractive option [3,4].
In non-industrialized nations, plant-based medicines are used to treat all aspects of women’s health, including menstrual problems, conception, pregnancy, birth, lactation, and menopause, as health care facilities are scarce [7, 8, 9]. In Central America, medicinal plants continue to be the most economically and culturally suitable treatment for a wide variety of health conditions, including those related to women’s health. Many of these plants have neither been thoroughly documented, nor scientifically investigated. Reliable information on the safety and efficacy of these plants is critical not only for women living in Central America, many of whom depend heavily on herbal medicine, but also for most women worldwide who are searching for alternative natural therapies for menstrual and menopausal problems.
In Costa Rica, the average age at onset of menopause is 50.6 years, and Costa Rican women experience menopausal symptoms similar to their counterparts in the U.S [7]. Many Costa Rican women do not use HT to treat menopausal symptoms, but instead use specific herbal medicines [7]. As part of an ongoing international collaboration between the University of Illinois and the University of Costa Rica, we have previously reported on several plant species used by Costa Rican women for the treatment of menopause and dysmenorrhea [7, 8]. More recently, we have identified Justicia pectoralis Jacq. (Acanthaceae), commonly referred to as “tilo or carpenter’s bush”, is a medicinal plant with a long history of traditional use in Central America. In Costa Rica, dried ethanol extracts of the aerial parts of J. pectoralis are commonly sold as an over-the-counter sleep aid under the name of Estilo©. While in the markets, dried plant material is widely advertized as a treatment for menopause and other menstrual ailments [10].
Extracts of J. pectoralis are used alone or in combination with other plants for the management of menopausal symptoms and dysmenorrhea, however the estrogenic, progestagenic and anti-inflammatory activities have not been investigated. In this work, we report the estrogen and progestagen agonist effects of this plant in competitive ER and PR binding assays, the ERβ CALUX (Chemically Activated Luciferase Expression assay; the 2ERE-luceriferase assay in MCF-7 breast cancer cells transiently transfected ER with a 2ERE-luciferase construct. The extract was also tested in cell proliferation assays using breast cancer line MCF-7. Finally, inhibition of cyclooxygenase II (COX-2) activity was determined to assess anti-inflammatory activity, as the COX-2 enzyme has been linked to dysmenorrhea.
2.0 Methods
2.1 International Agreements
This study was performed under a collaborative agreement between the University of Illinois at Chicago (UIC) and the University of Costa Rica (UCR). The Memorandum of Agreement was signed by authorities from both UIC and UCR in September of 2003 and renewed for 2008–2012.
2.2 Plant Collections, Extraction and Compound Isolation
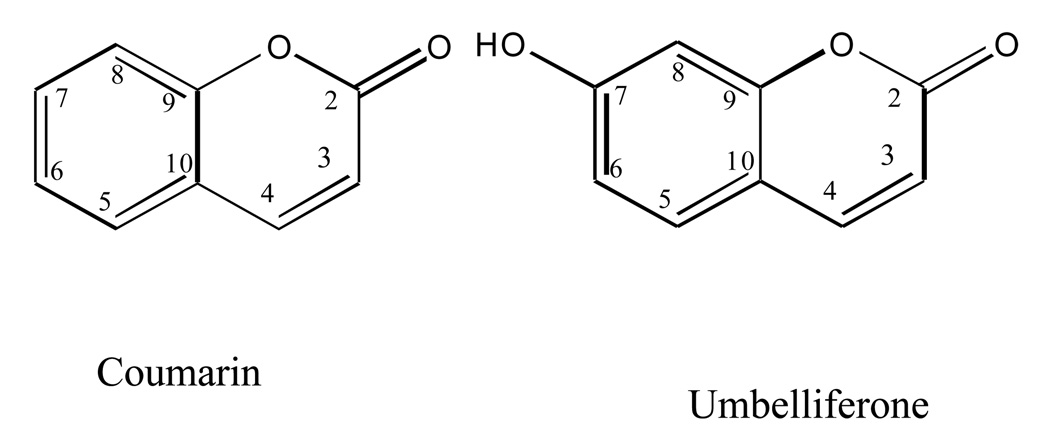
One kilogram (dry weight) of cultivated J. pectoralis aerial parts were collected in “El Arca de las Hierbas”, Santa Barbara, Heredia, Costa Rica and authenticated by botanist Dr. Jorge Laurito. Herbarium specimens have been deposited in the Universidad de Costa Rica Herbarium. The aerial parts of the plant were dried, finely ground and extracted by maceration in 5L of MeOH, at room temperature for 24 hours over three days, giving a crude methanol extract (1:5). The solvent was removed from crude extract under reduced pressure. The dried methanol extract was re-suspended in 700 ml of deionized H2O partitioned with (1.5 L) petroleum ether (PE), and (1.5 L) ethyl acetate (EtOAc), to afford dried PE (12.58g), EtOAc (16.9g), and H2O (52.84g) fractions, after solvent evaporation under reduced pressure. The EtOAc partition was separated on a reversed phase silica gel 60 reverse-phase C-18 packing material in a glass column (45.7 × 3.8 cm, 270 g, 230–400 mesh) (Fisher, Hanover Park, IL) and eluted with a methanol-water gradient (50 % up to 100 % MeOH) yielding C-17 fractions. Fractions C2–C4 were combined and separated on preparative normal phase TLC using a solvent system of 9:2 PE: EtOAc, yielding compound 1 (200 mg), coumarin. Fractions C-8 and C-9 were combined and re-crystallized in chloroform, yielding compound 2 (20 mg), umbelliferone. Fractions C11–C13 were combined and subjected to CC on silica gel, Sephadex LH-20 gel, followed by preparative reverse-phase C18 HPLC using an acetonitrile–water system (30: 70 to 40: 60) to yield compound 3 (3 mg), justicidin B. Isolated compounds were identified using LC/MS, NMR and by comparison of data to known compounds.
2.3 Cell culture and RNA extraction
MCF-7 K1 human breast cancer cells were maintained in MEM (Sigma-Aldrich Corp., St. Louis, MO) supplemented with 5% calf serum (Hyclone, Logan, UT) as previously described [9] Four days prior to treatment the cells were sub-cultured in phenol red-free MEM containing 5% charcoal dextran-treated calf-serum. The media was changed on day 2 and day 4 of culture. Cells were treated with 10 nM E2 alone or in combination with 20 µg/ml of the plant extract. Cell proliferation assays were performed as previously described using optical density measurements [9].
Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. RNA was further purified using RN-easy columns (Qiagen, Valencia, CA) and treatment with ribonuclease-free deoxyribonuclease 1 (Qiagen, Valencia, CA). The human osteoblastic osteosarcoma cell line U2-OS (ATCC) was cultured and maintained in a 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium (DF, Gibco) supplemented with 7.5% fetal calf serum [9].
2.4 ERE Reporter gene assay
MCF-7 cells were co-transfected with a plasmid containing a 2ERE-luciferase reporter construct and the control plasmid, Renilla luciferase (Promega Corp., Madison, WI), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) diluted in serum-free, antibiotic-free OptiMEM (Invitrogen, Carlsbad, CA). Six hours after transfection, media were changed to phenol red-free media containing charcoal-dextran stripped serum and the cells were treated with one of the following: vehicle solvent (DMSO); vehicle solvent plus ICI 182,780 (10 µM); E2 (10 nM) dissolved in ethanol; estrogen (E2) plus ICI; plant extract (20 µg/ml) plus vehicle solvent; or plant extract (20 µg/ml) plus ICI 182,790 for 16 h. Reporter activity was measured using a dual-luciferase assay according to the manufacturer’s directions (Promega Corp., Madison, WI). Relative luciferase activity is shown are the mean ± sem from at least three independent determinations, variation was less than 10% between runs.
2.5 Progesterone Receptor Binding Assay
Affinity of the crude extracts to the progesterone receptor was determined by competitive radio-ligand binding assays using 3H-progesterone as previously described [8]. Briefly, the J. pectoralis methanol extract and subsequent fractions (50 µg/ml) were diluted in 3.21 nM [1,2,6,7-3H(N)]-progesterone and 50 µL of incubation buffer (10 mM Tris, pH 7.4, containing 1.5 mM EDTA and 10% (v/v) glycerol). Thirty µl of an appropriate dilution of the progesterone receptor was added and incubated for 16–18 h. As a carrier protein, 1 mg/ml of casein was added to the incubation buffer. The unbound radio-labeled ligand was removed by incubation with 300µL dextran-coated charcoal for 15 min at 4°C. Samples were centrifuged at 6000 × g for 10 min at 4°C. Binding affinity was determined by adding 100 µL of the supernatant to 3 ml of scintillation cocktail and counted for 1 min. All determinations were carried out in triplicate. The median inhibitory concentration (IC50) was determined by testing the binding affinity of the extracts to the progesterone receptor in concentrations of 1 to 50 µg/ml. All experiments were performed in triplicate.
2.6 ER Binding Assay
The relative binding affinity of the herbal extracts to full-length ERα and ERβ was determined in a competitive radio-ligand-binding assay. The plant extracts and sub-fractions were dissolved in DMSO and initially tested at concentrations of 50 µg/ml exactly as described [8, 9]. Incubations were carried out at room temperature for 2h. The percent inhibition of 3[H] estradiol binding to each ER was determined as follows: [(dpm sample-dpm blank)/dpm DMSO-dpm blank) × 100%]. The binding capability of the sample was calculated in comparison to that of estradiol (50 mM, 100%) and the data was recorded as the average ± SD of three determinations (Doyle et al., 2009). The median inhibitory concentration (IC50) was determined by testing the binding affinity of the extracts to the estrogen receptors in concentrations of 1 to 50 µg/ml (performed in triplicate).
2.7 ERβ-CALUX reporter gene assay
The Chemically Activated Luciferase Expression (ER-CALUX) assay was performed as previously described [9, 11]. For transient transfections, the U2-OS cells were plated in 24-well tissue culture plates [9, 11, 12]. After culturing for one day, cells were transfected with 1 mg reporter plasmid (33 ERE-TATA-Luc), 200 ng SV2-lacZ and 200 ng expression plasmid (pSG5-neo-hERβ) using the calcium phosphate co-precipitation method. Luciferase activity was corrected for transfection efficiency by measuring LacZ expression as a result of SV2-lacZ co-transfection [9, 11, 12].
Transfected human osteosarcoma cell line (U2-OS) ERβ CALUX cells were plated in 96-well plates (6000 cells/well) with phenol red-free DF medium supplemented with 5% dextran-coated charcoal-stripped fetal calf serum at a volume of 200 µl per well. Two days later, the medium was refreshed and the cells were incubated with compounds to be tested (dissolved in DMSO). After 24h, the medium was removed and the cells lysed in 30 µL Triton-lysis buffer and luciferase activity was measured using a luminometer for 0.1 min/well. Luciferase activity per well was measured as relative light units (RLUs). Fold induction was calculated by dividing the mean value of light units from exposed and non-exposed (solvent control) wells. Luciferase induction, as a percentage of maximal 17β-estradiol (E2) activity, was calculated by setting the highest fold induction of E2 at 100%. Estradiol equivalents (EEQ) were calculated by interpolating test sample data in an E2 standard curve.
2.8 pS2, PR, PTGES protein expression
The effect of the J. pectoralis on the expression of the estrogen responsive genes pS2, PR, and PTGES in MCF-7 cells was determined according to the previously published methods [9, 13, 14]. Four days prior to E2 treatment, MCF-7 cells were sub-cultured in phenol red-free MEM media containing 5% charcoal-dextran-treated calf serum. On day two of the assay, the media was changed. Cells were treated with 10 nM E2 (Sigma-Aldrich Corp., St. Louis, MO) for 4h. Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed in a total volume of 20 µl using 200 U reverse transcriptase, 50 pmol random hexamer, and 1 mM deoxy-NTP (New England Biolabs, Beverly, MA). The resulting cDNA was then diluted to a total volume of 100 µl with sterile H2O.
2.9 Real-time PCR analysis
Each real-time PCR reaction consisted of 1 µl diluted RT product, 1x SYBR Green PCR Master Mix (PE Applied Biosystems, Foster City, CA), and 50 nM forward and reverse primers: 5′-CTTCCTTTTCCTGGGCTTCG-3′ (PTGES forward), 5′-GAAGACCAGGAAGTGCATCCA-3′ (PTGES reverse), 5′-ATGGCCACCATGGAGAACAAGG-3′ (pS2 forward) and 5′-CATAAATTCACACTCCTCTTCTGG-3′ (pS2 reverse), 5′ -GAGCTCATCAAGGCAATT-3′ (PR forward) and 5′ -CCATCCCTGCCAATATCT-3′ (PR reverse) [9]. Reactions were carried out on an ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems) for 40 cycles (95°C for 15 sec, 60°C for 1 min) after an initial 10-min incubation at 95°C. The fold change in expression of each gene was calculated using the comparative Ct method, with the ribosomal protein 36B4 mRNA as an internal control.
2.10 Spectrophotometric Assay of Human COX 2 Enzyme
Enzymatic activity was determined based on the oxidation of N’,N’,N’,N’-tetramethyl-p-phenylenediamine (TMPD) during the reduction of PGG2 to PGH2. [15]. Briefly, in a 96-well plate, 5 µL of sample (200 µg/ml) dissolved in DMSO, 128 µl of 0.1M Tris-HCl buffer (pH 8.0), 2 µL of heme (0.75PM), 2 µl of TMPD and 10 µL of 0.612 mg/ml of COX-2. The plate was incubated for 5 minutes at 25°C to allow the sample to bind to the enzyme. The reaction was initiated by adding 50 µL of 1 mM arachidonic acid to each (Total volume = 197 µl). The maximum velocity at 590nm was immediately recorded in Synergy HT multi-detection microplate reader (Biotek, Winooski VT). Remaining enzymatic activity was calculated using the following equation: [(sample-blank)/(control-blank)] X100. Nimesulide, a selective COX-2 inhibitor was used as the positive control in this assay.
2.11 Cyclooxygenase Enzyme Immunoassay (COX-2 EIA)
Extracts active in the preliminary screening TMPD assays were further tested in the cyclooxygenase-2 enzyme immunoasssay. This assay assesses human recombinant cyclooxygenase (COX-2) catalytic activity by measuring prostaglandin E2 (PGE2) production exactly as previously described [8]. Generally, reaction mixtures were prepared in 100 mM Tris-HCl buffer, pH 8.0, containing 1 µM heme, 500 µM phenol, 300 µM epinephrine, sufficient amounts of COX-2 to generate 150 ng of PGE2/mL, and 1–20 µg/ml of test samples. The reaction was initiated by the addition of arachidonic acid (final concentration, 10 µM) and incubated for 10 min at room temperature (final volume, 200 µl). The reaction was terminated by adding 20 µl of the reaction mixture to 180 µl of indomethacin, and PGE2 concentration quantified by an ELISA method. Following transfer to a 96-well plate coated with a goat anti-mouse IgG (Jackson Immuno Research Laboratories), the tracer (PGE2-acetylcholinesterase; Cayman Chemical, Ann Arbor, MI) and primary antibody (mouse antiPGE2; Monsanto, St. Louis, MO) were added. Plates were then incubated at room temperature overnight, reaction mixtures were removed, and wells were washed with a solution of 10 mM potassium phosphate buffer (pH 7.4) containing 0.01% sodium azide and 0.05% Tween 20. Ellman’s reagent (200 µL) was added to each well, and the plate was incubated at 37°C for 3–5 h, until the control wells yielded OD = 0.5–1.0 at 412 nm. A standard curve with PGE2 (Cayman Chemical, Ann Arbor, MI) was generated from the same plate, which was used to quantify the PGE2 levels produced in the presence of test samples. Results were expressed as a percentage relative to a control (solvent-treated samples). All determinations were performed in duplicate, and values generally agreed within 10%. Dose-response curves were generated for the calculation of IC50s.
2.12 Statistics
Statistical analyses were performed using the SAS® statistical package (SAS Institute, Cary, NC). One-way ANOVA was used, followed by Dunnett test for pair-wise comparison between the DMSO control and each of the other extracts. A general linear model with two main effects and their interaction was used to analyze the real time RT-PCR data, followed by post hoc comparison between the control and each of the other extracts. Statistical significance was ascribed to the data when p < 0.05.
3.0 Results
3.1 Effects on ER and PR binding
A methanol extract of J. pectoralis (JP-M) significantly displaced [3H]-17β-estradiol from both ERα and ERβ (59% and 71%, respectively at 50 µg/ml) demonstrating a slight preference for ERβ, but this was not statistically significant. In the progesterone receptor (PR) competitive binding assay, the methanol extract of JP displaced [3H]-progesterone from the PR by 75% at 50µg/mL (IC50 of 22.8 µg/ml, p < 0.05). An EtOAc partition of the methanol extract was found to be active in the ER binding assay. The EtOAc partition was fractionated using C18 reversed phase column chromatography, which led to the identification of fractions, C-9 and C11-13 (combined), with ER binding activity of 99% and 92%, respectively. Umbelliferone was isolated from fraction C9 and justicidin-B was isolated from C11–C13. However, these fractions also contain other as yet unidentified compounds.
3.2 Effects of JP-M on reporter gene assays
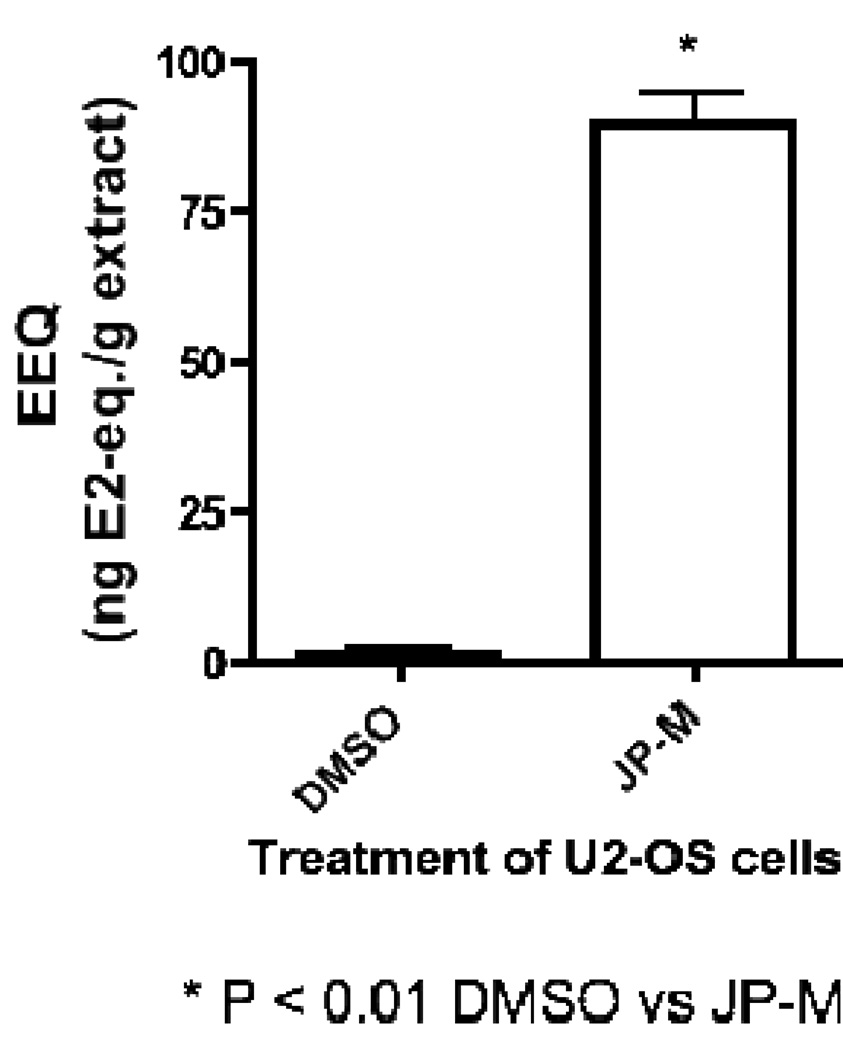
The JP-M extract exhibited estrogenic activity in the ER CALUX® reporter gene assay, increasing luciferase activity with an estrogen equivalence (EEQ) of 90 ng/g of sample, indicating the ability of the extract to induce transcription of an estrogen responsive reporter gene through ERβ in stably transfected U2-OS cells (Figure 1). Data shown in Figure 1 are expressed in estradiol equivalents (EEQ), the concentration in nanograms of E2 with equivalent activity to 1 g of sample.
Figure 1.
Results of the ER-CALUX® reporter gene assay demonstrating the ability of the JP-M extract to induce transcription of an estrogen responsive luciferase reporter gene through activation of ERβ in stably transfected U2-OS cells. Data represent the mean ± SD of three separate experiments performed in triplicate.
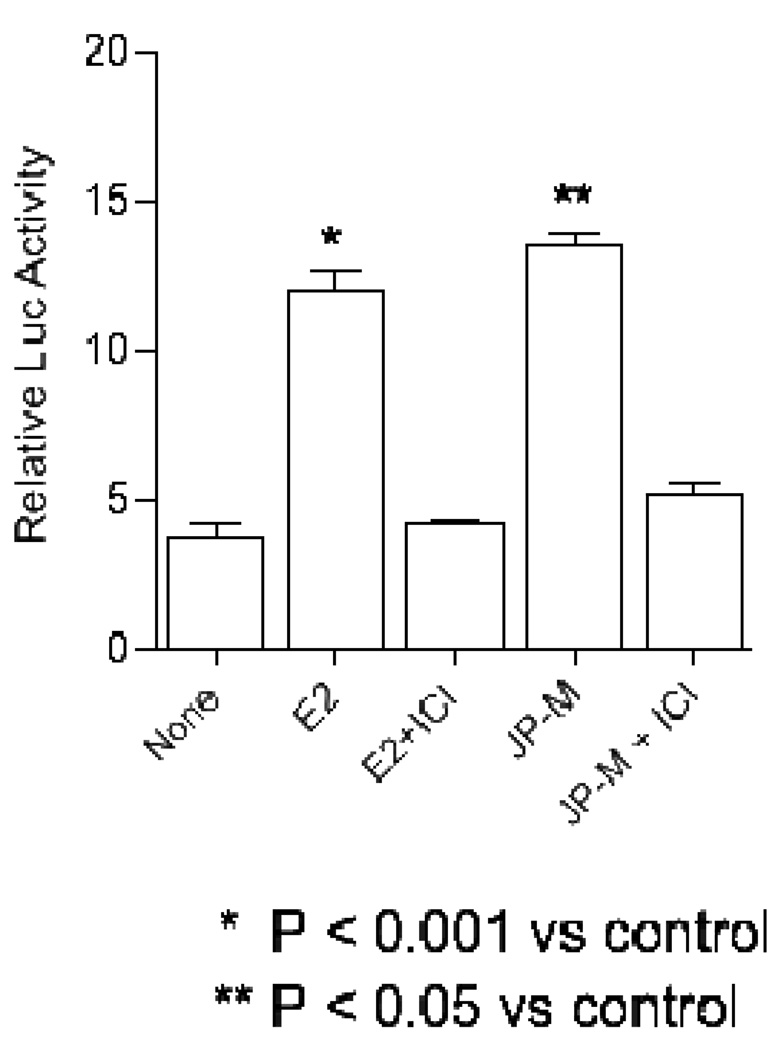
The estrogenic agonist activities of the JP-M extract were further investigated in the presence and absence of E2, in MCF-7 cells transiently transfected with a 2ERE-luciferase reporter construct. Both the JP-M extract (20 µg/ml) and the E2 positive control (10 nM) increased ERE-transcriptional activation, as measured by the fold increase in luciferase activity in MCF-7 cells (Figure 2). E2 significantly increased luciferase activity by approximately 11.5 fold (p < 0.001) over DMSO control, and JP-M treatment significantly increased luciferase activity by ~ 13 fold (p < 0.05) above the DMSO control. Both the JP-M- and E2-induction of ERE-dependent luciferase activity was inhibited by the co-treatment of the cells with the estrogen receptor antagonist ICI 182,780 (Figure 2), indicating that the activities of the extract, like estradiol, is mediated via the activation of the estrogen receptor.
Figure 2.
The ER antagonist ICI 182780 specifically blocks the induction of luciferase activity by JP-M through ERα in MCF-7 cells transiently transfected with a pERE-luciferase plasmid. Cells were treated with 10 nM E2 or 10 nM E2 + 1 µM ICI 182780 or 20 µg/ml JP-M or 20 µg/ml JP-M + 1 µM ICI 182780 to assess induction. Relative luciferase units were measured by luminometer. Results are expressed as fold induction above control (vehicle solvent). Abbreviations: E2 = 17β-estradiol; JP-M = Justicia pectoralis; ICI = ICI 182,780. Data represent the mean ± SD of three separate experiments performed in triplicate.
3.3 Effect of JP-M on endogenous gene expression in MCF-7 cells
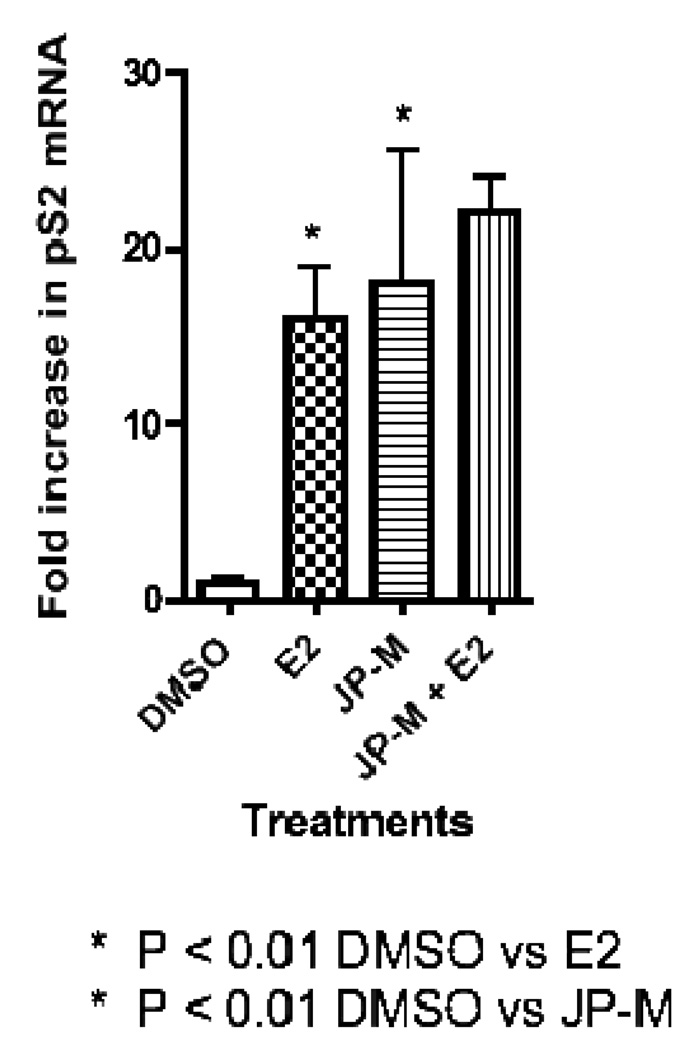
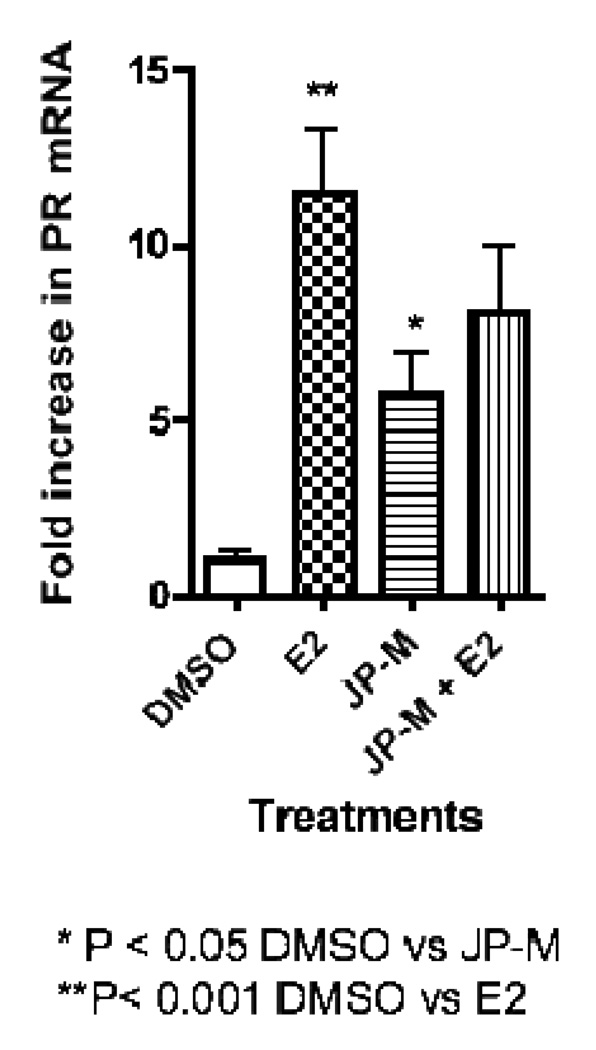
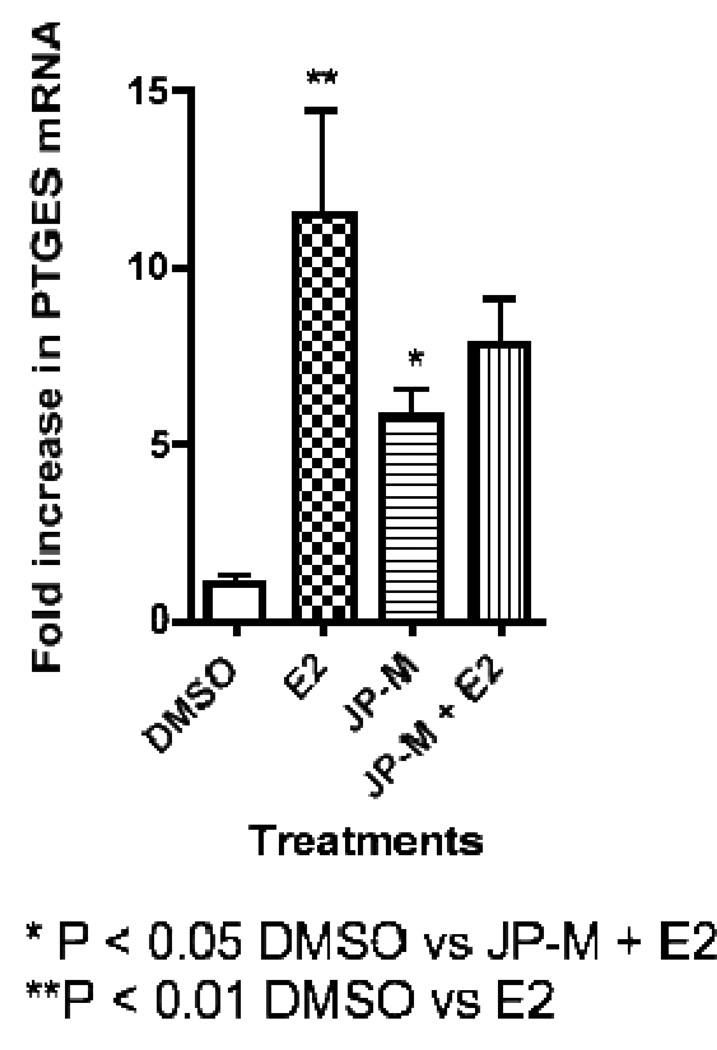
Real-time-PCR analysis of JP-M treated MCF-7 cells revealed that the extract induced the transcription of the estrogen-responsive endogenous genes pS2, PR, and PTGES (Figure 3, 4, 5). The JP-M extract alone significantly enhanced the expression of PR mRNA by 6.5 fold (p < 0.05; Figure 3), pS2 mRNA by 18.0 fold (p < 0.01; Figure 4) and PTGES mRNA by 6.0 fold (p < 0.05; Figure 5) above DMSO control. The potential for synergistic or antagonistic effects of the extracts in the presence of E2 in the MCF-7 cells was also investigated. The JP-M extract (20 µg/ml) in combination with E2 (10 nM) enhanced pS2 expression by 18-fold but this was not statistically significant as compared with E2 alone (Figure 3). In the presence of 10 nM E2, JP-M did not significantly increase mRNA levels of PTGES or PR above E2 alone (Figures 4–5). In fact, expression of PR and PTGES mRNA in the presence of the JP-M extract and E2 was slightly reduced, but this was not statistically significant.
Figure 3.
Results of RT-PCR analysis of endogenous estrogen responsive genes in MCF-7 cells after treatment with plant extracts (20 µg/ml). Data is represented as fold increase in mRNA above control for (3) pS2, (4) PR, (5) PTGES. Extracts are abbreviated: JP-M = Justicia pectoralis. MCF-7 cells were treated with the JP-M extract (20 µg/ml) + E2 (10 nM) to assess synergistic or antagonistic effects. Data represent the mean ± SD of three separate experiments performed in triplicate.
Figure 4.
Results of RT-PCR analysis of endogenous estrogen responsive genes in MCF-7 cells after treatment with plant extracts (20 µg/ml). Data is represented as fold increase in mRNA above control for (3) pS2, (4) PR, (5) PTGES. Extracts are abbreviated: JP-M = Justicia pectoralis. MCF-7 cells were treated with the JP-M extract (20 µg/ml) + E2 (10 nM) to assess synergistic or antagonistic effects. Data represent the mean ± SD of three separate experiments performed in triplicate.
Figure 5.
Results of RT-PCR analysis of endogenous estrogen responsive genes in MCF-7 cells after treatment with plant extracts (20 µg/ml). Data is represented as fold increase in mRNA above control for (3) pS2, (4) PR, (5) PTGES. Extracts are abbreviated: JP-M = Justicia pectoralis. MCF-7 cells were treated with the JP-M extract (20 µg/ml) + E2 (10 nM) to assess synergistic or antagonistic effects. Data represent the mean ± SD of three separate experiments performed in triplicate.
3.4 Effects on MCF-7 cell proliferation
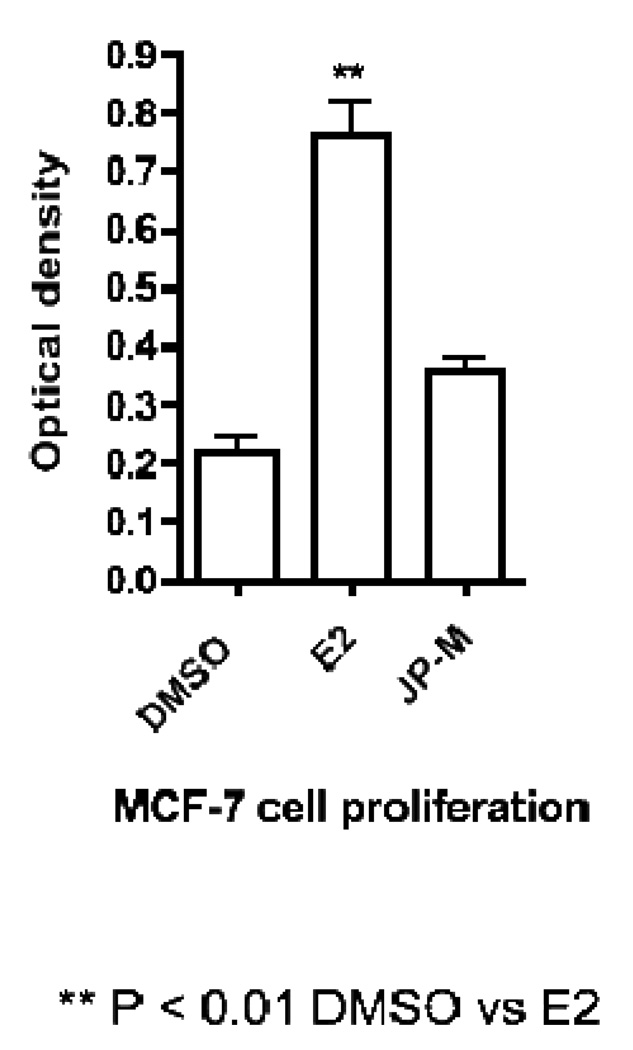
Treatment of the MCF-7 cell line with 20 µg/ml of JP-M extract was not cytotoxic, but actually weakly enhanced MCF-7 cell proliferation, although this was not statistically different from DMSO controls.
3.5 Effects on COX-2 activity
The JP-M extract, aqueous, and EtOAc partitions were tested for COX-2 inhibitory activity. At 10 µg/ml, the methanol extract displayed significant inhibition in the COX-2 EIA assay, suppressing prostaglandin production by 89% at 50 µg/ml with an IC50 of 4.8 µg/ml and the EtOAc partition inhibited COX-2 activity by 94% at 50 µg/ml, (IC50 of 4.3 µg/ml) (data not shown). Coumarin and umbelliferone (Compounds 1 and 2; Figure 7) were isolated from the by activity-guided fractionation on a reversed phase C18 silica gel column. It was determined that the primary compound responsible for the inhibition of COX-2 activity was coumarin. In the COX-2 colorimetric assay, coumarin inhibited COX-2 activity significantly with an IC50 of 0.54 µM, while umbelliferone was not active in this assay.
Figure 7.
Stuctures of the two coumarins, coumarin and umbelliferone isolated from Justicia pectoralis.
4.0 Discussion
Over the past 10 years, Costa Rica has implemented health care programs to improve women's health, particularly in the field of reproduction (e.g., high coverage of institutional deliveries and prenatal care) [7, 9]. Nevertheless, like most other countries, it faces key challenges associated with rapid changes in age demographics and epidemiologic transition, such as cardiovascular disease, control and treatment of cervical cancer, breast cancer, poor nutrition, mental health, and the problems associated with menopause [7]. Women in Costa Rica enter menopause around the same age as women in the U.S. and have similar symptoms [7]. However, unlike the their American counterparts, many women in Costa Rica consider menopause as a natural phenomenon and treat the symptoms with herbs, teas, and other alternative therapies. Interestingly, Latina women in the U.S. also tend not to use HT and opt for more natural remedies for menopause such as diet, exercise and herbal remedies [16].
One of the plants widely found in the local markets and sold to treat PMS and menopause in Costa Rica is J. pectoralis [10]. The aerial parts of the part are sold alone, or in combination with other herbs to treat the symptoms of “Menopausia”, and these herbs are reportedly used for “cooling”. We had previously reported on the estrogenicity of some of the other herbals used by Costa Rican women [Doyle], however J. pectoralis due to its unusual biological activities. Interestingly, other J. pectoralis and other Justicia species are similarly employed in the management of reproductive disorders in the developing world, including Cameroon and Ecuador [17, 18], and have been found to have estrogenic effects in rodent models [17]. In Cuba, J. pectoralis was one of the medicinal plants commonly used by women during pregnancy, although no indication was given [18]. Furthermore, in Trinidad and Tobago, Justicia species are used to treat inflammatory conditions, such as Justicia secunda for the treatment of dysmenorrhea, and Justicia pectoralis is used to treat prostatitis [20]. The high presence of anti-inflammatory coumarins in these plants may explain these uses [17]. However, the use of extracts of J. pectoralis for the management of menopausal and PMS symptoms in Costa Rica appears novel, and this species has not previously been scientifically investigated for potential hormone-like effects.
Since menopausal symptoms are directly associated with a reduction in circulating estrogen and progesterone levels, it is logical to hypothesize that traditional therapies used to treat menopausal symptoms may have estrogenic or progestagenic effects. The data presented in this work suggest that extracts of J. pectoralis act as estrogen and progesterone agonists in vitro, along with its anti-inflammatory activities. Estrogens and progestins have been used for many years as a hormonal therapy for the management of menopausal and PMS symptoms [21]. Estrogens, either alone or in combination with progestins, have been shown to be the most effective clinical treatment for the relief of vasomotor symptoms (hot flashes) and have also been shown to relieve vaginal atrophy, prevent the loss of BMD, and reduce fracture risk, including clinical fractures of the vertebrae and hip [22]. Women with an intact uterus are often given estrogens in combination with progesterone, which is intended to avoid the development of endometrial hyperplasia and endometrial cancer [23]. Doses usually recommended are the lowest possible dose, for the shortest duration needed to relieve symptoms.
In this work we have shown that extracts of J. pectoralis from Costa Rica enhanced the expression of both ER and PR-regulated genes, indicating a plausible mechanism of action for this plant in the treatment of menopause or PMS. Up-regulation of the expression of ERE-reporter genes and endogenous genes pS2, PR and PTGES in MCF-7 cells are indicative of estrogenic agonist effects via ERα [13, 14]. It is well known that pS2 expression is regulated in MCF-7 cells by estrogens, and that pS2 is predominantly, but not exclusively, expressed in ER-dependent cancers. In addition to pS2, mRNA expression of the PR and PTGES genes in MCF-7 cells are also regulated in a characteristic manner by estrogenic compounds [13, 14]. The extract also up-regulated an estrogen-responsive 2ERE-reporter gene in transiently transfected MCF-7 cells, which was inhibited by the estrogen antagonist ICI 182,780, indicating that ER α is involved in the mechanism. These data support the estrogen agonist effects of J. pectoralis. Additionally, the extract bound to the progesterone receptor and up-regulated the expression of PR mRNA, suggesting a potential progesterone agonist effect as well.
Along with estrogen and progesterone agonist effects of J. pectoralis, the extract significantly inhibited the activity of the COX-2 enzyme in vitro, demonstrating the potential for anti-inflammatory activities. Justicia pectoralis is a plant well known in traditional medicine as an anti-inflammatory herb [17, 24]. Previous investigators have isolated coumarin and umbelliferone from the leaves of J. pectoralis [17]. Interestingly, J. pectoralis has also been used traditionally in Brazil as an anti-inflammatory, with coumarin and umbeliferone reported in the active extracts [24]. However, neither the extract nor coumarin had previously been reported to inhibit COX-2 activity. Thus, results from our work support the previous reports, and further suggests that coumarin exerts its anti-inflammatory effects through the inhibition of the activity of the COX-2 enzyme. Since dysmenorrhea is associated with the imbalance of prostaglandins that is controlled both by the COX and estrogen pathways, our results suggest at least two mechanisms of action by which J. pectoralis maybe useful for the treatment for PMS and dysmenorrhea.
Interestingly, although the J. pectoralis extract showed significant estrogen/progesterone agonist activities in vitro, it did not significantly alter the proliferation of MCF-7 breast cancer cells at concentrations up to 20 µg/ml. This was perplexing for two reasons, first, there are reports of J. pectoralis and justicidin B having cytotoxic effects [25, 26]. Justicidin B reportedly showed strong cytotoxicity against chronic myeloid leukemia LAMA-8 and K-562 cell lines and on the chronic lymphoid leukemia SKW-3 cell line [26]. However, in MCF=7 cells no cytotoxicity was observed. It is possible that higher concentrations of the extract were needed to inhibit MCF-7 cell proliferation. But more likely the lack of cytotoxicity was due to the low concentrations of justicidin B in our extract (less than 0.0002%). Secondly, it may also be plausible that since the extract contains high concentrations of coumarins that inhibit COX-2, that these compounds may neutralize the proliferative effects of estrogen/progesterone agonists in MCF-7 cells. It is well known that COX-2 inhibitors have chemopreventative activities in breast cancer cells [27], including MCF-7 cells [28]. Thus, the idea that an herbal extract can have estrogenic and progestagenic effects without enhancing estrogen-responsive cancers it a very interesting concept and one worthy of further research.
Conclusion
Justicia pectoralis is a medicinal plant used by women in Costa Rica to treat symptoms associated with PMS and menopause. Our data demonstrates that extracts of Justicia pectoralis act as an estrogen and progesterone agonists, and inhibits the activity of the COX-2 enzyme in vitro, thereby supporting the traditional use of this plant. In vivo studies, to investigate the safety and efficacy should be undertaken as this plant appears to be widely used by women in developing countries.
Figure 6.
Results of MCF-7 cell proliferation data after treatment with DMSO control, E2 (10 nM) or JP-M (20 µg/ml).
Acknowledgements
This study was made possible by Grant Numbers R21-AT002381-02 (GM) and R01-CA130932-02 (JF). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or NIH. We would like to gratefully acknowledge Mr. Juan-Carlos Brenes, University of Costa Rica for his assistance in the plant collections and technical help, as well as Dr. Harry Besselink, BioDetection Systems B.V., Amsterdam, The Netherlands for his assistance with the ERβ CALUX® assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Houmard BS, Seifer DB. Predicting the Onset of Menopause. In: Seifer DB, Kennard EA, editors. Menopause: Endocrinology and Management. Totwa, New Jersey: Humana Press; 1999. pp. 109–118. [Google Scholar]
- 2.Wijma K, Melin A, Nedstrand E, Hammar M. Treatment of menopausal symptoms with applied relaxation: A pilot study. J Behav Ther Exp Psychiatry. 1997;28:251–261. doi: 10.1016/s0005-7916(97)00030-x. [DOI] [PubMed] [Google Scholar]
- 3.Cass I, Runowicz CD. Non-hormonal alternatives to treating menopausal symptoms. Am J Manag Care. 1998;4:732–735. [PubMed] [Google Scholar]
- 4.Taylor M. Alternatives to conventional hormone replacement therapy. Compr Ther. 1997;23:514–532. [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Guay MP, Dragomir A, Pilon D, Moride Y, Perreault S. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiol Drug Saf. 2007;16:17–27. doi: 10.1002/pds.1273. [DOI] [PubMed] [Google Scholar]
- 7.Locklear TD, Doyle BJ, Caceres A, Perez A, Mahady GB. Menopause, a Universal Female Experience, Lessons from Central America. Cur Rev Women’s Health. 2007;4:1–10. [Google Scholar]
- 8.Michel J, Duarte RE, Caceres A, Yao P, Huang Y, Bolton J, Soejarto DD, Mahady GB. Q’eqchi Maya medicine for women’s health: In vitro evaluation of Guatemalan plants in estrogen and serotonin bioassay. J. Ethnopharmacol. 2007;114:92–101. doi: 10.1016/j.jep.2007.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle BJ, Frasor J, Bellows LE, Locklear TD, Perez A, Gomez-Laurito J, Mahady GB. Estrogenic effects of herbal medicines from Costa Rica used for the management of menopausal symptoms. Menopause. 2009;16:748–755. doi: 10.1097/gme.0b013e3181a4c76a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locklear TD, Doyle BJ, Perez A, Laurito-Gomez J, Brenes JC, Huang Y, Mahady GB. Alternative therapies for PMS and dysmenorrhea from Central America: Justicia pectoralis Jacq.: Mechanisms of action. FASEB Journal. 2007;21:A436. [Google Scholar]
- 11.Sonneveld E, Jansen HJ, Riteco JA, Brouwer A, van der Burg B. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol Sci. 2005;83:136–148. doi: 10.1093/toxsci/kfi005. [DOI] [PubMed] [Google Scholar]
- 12.Legler J, van den Brink CE, Brouwer A, Murk AJ, van der Saag PT, Vethaak AD, van der Burg B. Development of a stably transfected estrogen receptor-mediated luciferase reporter gene assay in the human T47D breast cancer cell line. Toxicol Sci. 1999;48:55–66. doi: 10.1093/toxsci/48.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Frasor J, Danes JM, Komm B, Chang KC, Lyttle R, Katzenellenbogen B. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 14.Frasor J, Weaver AE, Pradhan M, Metha K. Synergistic up-regulation of prostaglandin E synthase expression in breast cancer cells by 17β-estradiol and pro-inflammatory cytokines. Endocrinology. 2008;149:6272–6279. doi: 10.1210/en.2008-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riendeau D, Charleson S, Cromlish W, Mancini JA, Wong E, Guay J. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can J Physiol Pharmacol. 1997;75:1088–1095. [PubMed] [Google Scholar]
- 16.Lino CS, Taveira ML, Viana GSB, Matos FJA. Analgesic and anti-inflammatory activities of Justicia pectoralis Jacq. and its main constituents: coumarin and umbelliferone.". Phytotherapy Research. 1997;11:211–215. [Google Scholar]
- 17.Oliveria AFM. Chromatographic screening of medicinal Acanthaceae: Justicia pectoralis Jacq. and J. genarussa Burm. Revista Brasileria de Plantas Medicinais. 2000;3:37–41. [Google Scholar]
- 18.Macías-Peacok B, Pérez-Jackson L, Suárez-Crespo MF, Fong-Domínguez CO, Pupo-Perera E. [Use of medicinal plants during pregnancy.] [Article in Spanish] Rev Med Inst Mex Seguro Soc. 2009;47:331–334. [PubMed] [Google Scholar]
- 19.Tene V, Malagón O, Finzi PV, Vidari G, Armijos C, Zaragoza T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J Ethnopharmacol. 2007;111:63–81. doi: 10.1016/j.jep.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Lans C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. J Ethnobiol Ethnomed. 2007;3:13–25. doi: 10.1186/1746-4269-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 22.Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007;16:600–631. doi: 10.1089/jwh.2006.0311. [DOI] [PubMed] [Google Scholar]
- 23.Gold EB, Block G, Crawford S. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;159:1189–1199. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- 24.Leal LK, Ferreira AA, Bezerra GA, Matos FJ, Viana GS. Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: a comparative study. J Ethnopharmacol. 2000;70:151–159. doi: 10.1016/s0378-8741(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 25.Vasilev N, Elfahmi, Bos R, Kayser O, Momekov G, Konstantinov S, Ionkova I. Production of justicidin B, a cytotoxic arylnaphthalene lignan from genetically transformed root cultures of Linum leonii. J Nat Prod. 2006;69:1014–1017. doi: 10.1021/np060022k. [DOI] [PubMed] [Google Scholar]
- 26.Joseph H, Gleye J, Moulis C, Mensah LJ, Roussakis C, Gratas C, Justicidin B. a cytotoxic principle from Justicia pectoralis. J Nat Prod. 1988;51:599–600. doi: 10.1021/np50057a030. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Su B, Chen S. A COX-2 inhibitor nimesulide analog selectively induces apoptosis in Her2 over-expressing breast cancer cells via cytochrome c dependent mechanisms. Biochem Pharmacol. 2009;77:1787–1794. doi: 10.1016/j.bcp.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, He C, Zhou K, Wang J, Kang JX. Coptis extracts enhance the anticancer effect of estrogen receptor antagonists on human breast cancer cells. Biochem Biophys Res Commun. 2009;378:174–178. doi: 10.1016/j.bbrc.2008.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]