Abstract
The moss Rhynchostegium pallidifolium (Mitt.) A. Jaeger, which often forms large pure colonies on soils and rocks, inhibited the hypocotyls and root growth of cress (Lepidium sativum L.) seedlings when R. pallidifolium and cress were incubated together on agar medium. The inhibition of cress was greater at the close position from the moss than at the far position from the moss. 3-Hydroxy-β-ionone was found in the medium and concentration of 3-hydroxy-β-ionone in the medium was greater at the close position than at the far position from R. pallidifolium, suggesting that R. pallidifolium may secrete 3-hydroxy-β-ionone into the medium. Exogenously applied 3-hydroxy-β-ionone inhibited the growth of hypocotyls and roots of cress at concentrations greater than 1 and 3 µM, respectively. Considering the growth inhibitory activity and concentrations found in the medium, 3-hydroxy-β-ionone was estimated to be able to cause 46–64% of the observed growth inhibition of cress hypocotyls and roots by R. pallidifolium. Therefore, 3-hydroxy-β-ionone may play an important role in the allelopathic activity of R. pallidifolium and may help competition with neighboring plants resulting in the formation of pure colonies.
Key words: allelopathy, growth inhibitor, 3-hydroxy-β-ionone, phytotoxicity, Rhynchostegium pallidifolium
Bryophytes are almost free from attack by micro-organism and insects, and their herbarium specimens usually do not need special treatment against insects and micro-organism. In addition, many bryophyte species have their own particular odors and tastes.1 These bryophyte characteristics are probably attributed to chemical constituents inherent in their structures. In fact, many biologically active substances, such as phenolics and terpenoides, have been isolated from bryophytes.2–5
Several higher plants can not grow well in places where some bryophytes occurred. Some bryophytes dominate plant communities and form large pure colonies on soils and rocks on sunny places of lowland to upland areas including marshy places.1,6,7 Therefore, allelopathic chemical interactions may play an important role in the domination of bryophytes in these plant communities. In contrast to higher plants, however, there only was a preliminary study on allelopathy of bryophytes. The moss Rhynchostegium pallidifolium (Mitt.) A. Jaeger, which belongs to Brachytheciaceae family of Bryopsida (moss) class, Bryophyta division, also forms large pure colonies and possesses strong allelopathic activity. An allelopathic substance of the moss was recently isolated and identified as 3-hydroxy-β-ionone.8
Allelopathic Activity of R. pallidifolium
When cress seeds were grown on agar MS medium with R. pallidifolium, the growth of cress hypocotyls and roots was inhibited (Fig. 1). The inhibition was the greatest at the position of 10 mm from R. pallidifolium and the smallest at the position of 40 mm. Cress seedlings may grow with R. pallidifolium without competition for nutrients, because nutrients are considered to be unnecessary during the germination stage of seeds where most nutrients are withdrawn from seed reserves.9 In addition, no significant pH changes occurred in the medium during the period of the incubation. These results suggest that the inhibitory effect of R. pallidifolium on cress may not be due to competitive interference for nutrients and pH changes in the medium, but rather due to an alleloapthic effect.
Figure 1.
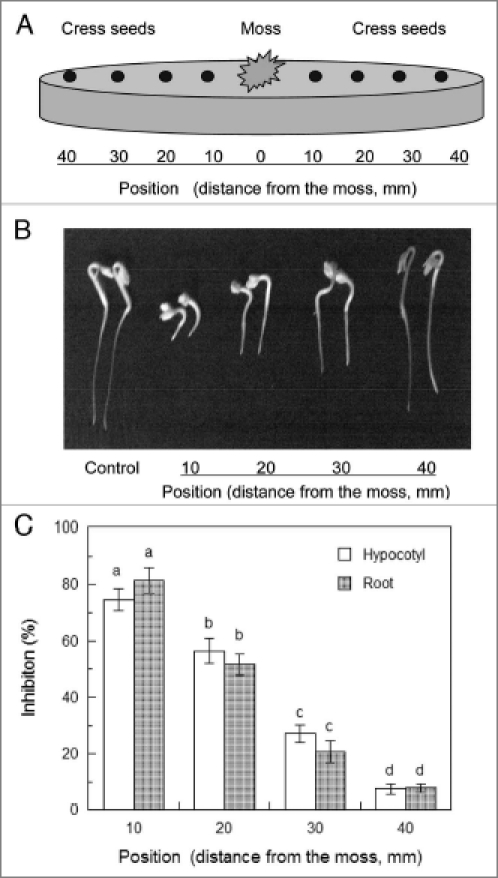
Effect of R. pallidifolium on the growth of cress. R. pallidifolium was transplanted onto agar mS medium and grown at 25°C with a 12-h photoperiod for 5 days as described previously.9 Cress seeds was then placed on the medium (A) and incubated at 25°C with a 12-h photoperiod for 2 days (B). The length of cress hypocootyls and roots was mesured and inhibition % was deterined by the formula: [(control plant length - plant length incubated with R. pallidifolium)/control plant length] × 100. Control cress was incubated on the medium in the absence of R. pallidifolium (C). Different letters indicate significant differences (p < 0.05) according to Tukey's test.
3-Hydroxy-β-Ionone Concentration
3-Hydroxy-β-ionone was found in the medium, but the concentration in the medium differed at the position from R. pallidifolium (Table 1). The highest concentration of 3-hydroxy-β-ionone was at the position of 10 mm from R. pallidifolium and the lowest concentration was at the position of 40 mm from the moss. 3-Hydroxy-β-ionone was also found in soils under R. pallidifolium.9 These results suggest that 3-hydroxy-β-ionone was probably secreted by R. pallidifolium into the medium.
Table 1.
Concentration of 3-hydroxy-β-ionone in growth medium
| Position (mm) | Concentration (µM) |
| 10 | 11.3 ± 1.4 |
| 20 | 5.6 ± 1.1 |
| 30 | 2.1 ± 0.4 |
| 40 | 0.1 ± 0.1 |
Inhibitory Activity of 3-Hydroxy-β-Ionone
3-Hydroxy-β-ionone inhibited the growth of hypocotyls and roots of cress at concentrations greater than 1 and 3 µM, respectively (Fig. 2). When inhibition of cress hypocotyls and roots was plotted against the logarithm of the concentrations of 3-hydroxy-β-ionone as described by Streibig,10 good logistic functions were obtained. The equations of the functions of 3-hydroxy-β-ionone were Y = [(0.552 − 75.290)/{1 + (X/72.372)0.958}] + 75.290; (r2 = 0.998) and Y = [(0.313 − 84.479)/{1 + (X/68.973)1.298}] + 84.479; (r2 = 0.999) for hypocotyls and roots, respectively. Y in the equations indicates the growth inhibition (%) and X indicates the concentration (µM) of 3-hydroxy-β-ionone as shown in Figure 2.
Figure 2.
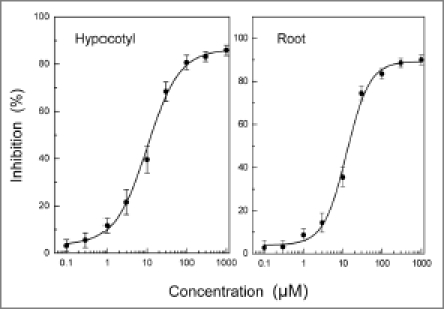
Effects of 3-hydroxy-β-ionone on hypocotyl and root growth of cress seedlings. Cress seeds were incubated in aqueous solution of 3-hydroxy-β-ionone for 2 days and the length of hypocotyls and root of cress seedlings were measured as described previously.9 Inhibition % was then determined as described in Figure 1.
Contribution of 3-Hydroxy-β-Ionone to Allelopathy of R. pallidifolium
To quantify the contribution of 3-hydroxy-β-ionone to overall allelopathy of R. pallidifolium, the potential growth inhibition on cress caused by secreted 3-hydroxy-β-ionone in the medium was calculated using the derived equations of logistic functions and substituting X values with the concentrations found in the growth medium (Table 1). These values in Table 2 indicate that 3-hydroxy-β-ionone in the medium has the potential to inhibit 43.7 and 43.5% for cress hypocotyl and root growth, respectively, at the potion 10 mm from the moss and 4.2 and 4.1% for cress hypocotyl and root growth, respectively, at the potion 40 mm from the moss.
Table 2.
Potential growth inhibition on cress by 3-hydroxy-β-ionone found in growth medium
| Potential growth inhibition (%) | ||
| Position (mm) | Hypocotyl | Root |
| 10 | 43.7 | 43.5 |
| 20 | 32.3 | 23.9 |
| 30 | 16.6 | 9.7 |
| 40 | 4.2 | 4.1 |
The potential growth inhibition on cress caused by 3-hydroxy-β-ionone was calculated using the equations of logistic functions with substituting X values by 3-hydroxy-β-ionone concentrations found in the growth medium (Table 1) as described in the text.
The contribution of 3-hydroxy-β-ionone to the growth inhibition on cress by R. pallidifolium was calculated by the formula: [potential growth inhibition values (Table 2)]/[measured growth inhibition (Fig. 1C)] × 100. The values in Table 3 show that 3-hydroxy-β-ionone accounts for 57.3–63.5% and 46.3–53.3% of the observed growth inhibition of cress hypocotyls and roots, respectively, by R. pallidifolium (Fig. 1C). Therefore, the contribution of 3-hydroxy-β-ionone to the growth inhibition may explain about 46–64% of the allelopathic activity of R. pallidifolium against cress.
Table 3.
Contribution of 3-hydroxy-β-ionone to the growth inhibition by R. pallidifolium
| Contribution (%) | ||
| Position (mm) | Hypocotyl | Root |
| 10 | 63.5 | 53.3 |
| 20 | 57.3 | 46.3 |
| 30 | 61.3 | 46.8 |
| 40 | 57.3 | 52.3 |
It is suggested that, although mechanisms of the exudation are not well understood, plants are able to secrete a wide variety of compounds from root cells by plasmalemma-derived exudation, endoplasmic-derived exudation, and proton-pumping mechanisms.12,13 Through the exudation of compounds, plants are able to regulate the soil microbial community in their immediate vicinity and inhibit the growth of competing plant species.11–13 Considering the inhibitory activity of 3-hydroxy-β-ionone and the secretion level of 3-hydroxy-β-ionone, 3-hydroxy-β-ionone may play a very important role in R. pallidifolium defense mechanism in the rhizosphere as an allelopathic substance and may help competition with neighboring plants resulting in the formation of pure colonies.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/11642
References
- 1.Ando H, Matsuo A. Applied bryology. In: Cramer J, editor. Advance Bryology. Vol. 2. Berlin: International Association of Bryologists; 1984. pp. 133–229. [Google Scholar]
- 2.Asakawa Y. Biologically active substances from bryophytes. In: Chopra RN, Bhatla SC, editors. Bryophyte development: Physiology and Biochemistry. Boca Raton, Fla: CRC Press; 1990. pp. 259–287. [Google Scholar]
- 3.Asakawa Y. Chemosystematics of the Hepaticae. Phytochemistry. 2004;65:623–669. doi: 10.1016/j.phytochem.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Basile A, Sorbo S, López-Sáez JA, Cobianchi RC. Effects of seven pure flavonoids from mosses on germination and growth of Tortula muralis HEDW. (Bryophyta) and Raphanus sativus l. (Magnoliophyta) Phytochemistry. 2003;62:1145–1151. doi: 10.1016/s0031-9422(02)00659-3. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Wurtele ES, LaMotte CE. Abscisic acid is present in liverworts. Phytochemistry. 1994;37:625–627. [Google Scholar]
- 6.Blime JM. The role of bryophytes in temperate forest ecosystems. Hikobia. 2001;13:267–289. [Google Scholar]
- 7.Tsubota H, Kuroda A, Masuzaki H, Nakahara M, Deguchi H. Preliminary study on allelopathic activity of bryophytes under laboratory conditions using the sandwich method. J Hattori Bot Lab. 2006;100:517–525. [Google Scholar]
- 8.Kato-Noguchi H, Seki T, Shigemori H. Allelopathy and allelopathic substance in the moss Rhynchostegium pallidifolium. J Plant Physiol. 2010;167:468–471. doi: 10.1016/j.jplph.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Fuerst EP, Putnam AR. Separating the competitive and allelopathic components of interference: Theoretical principles. J Chem Ecol. 1983;8:937–944. doi: 10.1007/BF00982203. [DOI] [PubMed] [Google Scholar]
- 10.Streibig JC. Herbicide bioassay. Weed Res. 1988;28:479–484. [Google Scholar]
- 11.McCully E. Roots in soil: unearthing the complexities of roots and their rhizospheres. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:695–718. doi: 10.1146/annurev.arplant.50.1.695. [DOI] [PubMed] [Google Scholar]
- 12.Hawes MC, Gunawardena U, Miyasaka S, Zhao X. The role of root border cells in plant defense. Trends Plant Sci. 2000;5:128–133. doi: 10.1016/s1360-1385(00)01556-9. [DOI] [PubMed] [Google Scholar]
- 13.Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004;9:26–32. doi: 10.1016/j.tplants.2003.11.008. [DOI] [PubMed] [Google Scholar]


