Abstract
Aims
We have previously shown that 17-β-estradiol (E2) protects cardiomyocytes exposed to simulated ischaemia–reperfusion (I/R) by differentially regulating pro-apoptotic p38α mitogen-activated protein kinase (p38α MAPK) and pro-survival p38β. However, little is known about how E2 modulation of these kinases alters apoptotic signalling. An attractive downstream target is p53, a well-known mediator of apoptosis and a substrate of p38α MAPK. The aim of this study was to determine whether the cytoprotective actions of oestrogen involve regulation of p53 via cardiac p38 MAPKs.
Methods and results
Cultured rat cardiomyocytes underwent hypoxia followed by reoxygenation (H/R) to simulate I/R. We found that inhibiting p53 significantly reduced apoptosis. Phosphorylation of p53 at serine 15 [p-p53(S15)] increased after H/R in a p38α MAPK- and reactive oxygen species (ROS)-dependent manner. E2 at 10 nM effectively inhibited p-p53(S15) and mitochondrial translocation of p53. Blocking p53 led to augmented p38β activity and attenuated ROS, suggesting suppression of this antioxidant kinase by p53. The use of a specific agonist for each oestrogen receptor (ER) isoform, ERα and ERβ, demonstrated that both isoforms participate in preventing cell death by inhibiting p53 in the mitochondria-centred apoptotic processes.
Conclusion
Our results demonstrate that during H/R stress, cardiomyocytes undergo p53-dependent apoptosis following phosphorylation of p53 by p38α MAPK, leading to p38β suppression. E2 protects cardiomyocytes by inhibiting p38α-p53 signalling in apoptosis.
Keywords: Oestrogen, Cardiomyocyte, p38, p53, Apoptosis
1. Introduction
The incidence of ischaemic heart disease (IHD) and sequelae such as myocardial infarction (MI) is lower in pre-menopausal women than in age-matched men. Gender difference in IHD narrows, however, once women become post-menopausal. In addition, women who are on hormone replacement therapy fare better after MI than those who are not,1 adding to a long-held belief that the female hormone, oestrogen, may be responsible for the observed cardioprotection. Although population-based trials so far have been inadequate to dispel controversy regarding the complex actions of oestrogen or synthesized supplements in cardiovascular disease, controlled experiments have shown collectively that oestrogen promotes cardiomyocyte survival under ischaemia-related stress.2
The ability of oestrogen to prevent apoptosis may be part of a molecular mechanism responsible for its cytoprotective effects on the heart, although details of the mechanism are yet to be fully elucidated. Patten et al.3 demonstrated that 17-β-estradiol (E2) curbed apoptosis by activating the phospho-inositide-3 kinase (PI3K)/Akt pathway in an animal model of MI. We have previously shown that E2 protects cardiomyocytes against simulated ischaemia–reperfusion (I/R) injury by differentially regulating p38α mitogen-activated protein kinase (MAPK) and its pro-survival counterpart, p38β. We also described an important link between PI3K/Akt and p38β central to the E2-dependent cell survival.4
Of the four known isoforms of p38 MAPKs, p38α and p38β are abundant in cardiomyocytes, whereas p38γ and p38δ are not expressed in the heart.5 p38α MAPK is best studied and stimulated by hypoxia or ischaemia among other stressors. On the other hand, p38β induced by E2 suppresses reactive oxygen species (ROS) formation and prevents apoptosis.4 How the two cardiac p38 MAPKs with opposing effects on cell death modulate the fate of a cardiomyocyte is not clear. An attractive downstream target in the kinase interplay is p53, a well-known mediator of apoptosis and a substrate of p38α MAPK.
p53 is phosphorylated by p38α MAPK in a cell- and stress-specific manner. For example, p53 phosphorylation by p38 MAPK at serine 15 regulates p53-dependent neuronal death.6 The post-translational modification by this kinase is thought to enhance p53 function in apoptosis.7,8 Previous studies indicate that both proteins play a large part in hypoxia-induced injury. However, little is known about the p38–p53 interaction within a cardiomyocyte. Moreover, how oestrogen influences these important regulatory molecules to effect myocyte survival is a clinically relevant question. Thus, in order to investigate whether oestrogen rescues cardiomyocytes by regulating p53 via cardiac p38 MAPKs, we used a model of rat cardiomyocytes exposed to simulated I/R injury in the form of hypoxia–reoxygenation (H/R). Here, we report new findings that summate how E2 attenuates mitochondria-centred cell death by modulating an apoptotic signalling between p38α MAPK and p53 via p38β.
2. Methods
2.1. Harvest and culture of neonatal rat cardiomyocytes
All studies were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine, and the Research and Development and Animal Use Committees of the Long Beach Veterans Affairs Medical Center. The investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Myocytes were isolated from the hearts of 1- to 3-day-old rats (Sprague–Dawley) using a neonatal cardiomyocyte isolation system kit (Worthington) as reported previously.4 See Supplementary material online for further details.
2.2. Simulated I/R
Cells were synchronized in serum-free L-15 Leibovitz medium overnight and treated with 10 nM E2 (Sigma-Aldrich) 30 min prior to start of 18 h hypoxia, at the time of starting hypoxia, or at the start of 1 h reoxygenation following hypoxia. The E2 effect on cell survival was greatest when E2 was present prior to starting hypoxia. The subsequent experiments were performed with reagents of interest or control vehicle added 30 min prior to start of hypoxia. Further details are in the Supplementary material online.
2.3. Apoptosis assays
Early stages of apoptosis were assessed by annexin V-fluorescein isothiocyanate (FITC) microscopy kit (Pharmingen) according to the manufacturer's protocol. A minimum of 200 cells were counted per visual field (see Supplementary material online, Tables S1 and S2).
For the assessment of the intrinsic apoptotic pathway, the release of cytochrome c (Cyt c) from mitochondria was examined by immunoblotting (see Supplementary material online).
2.4. Transfection of small interfering RNA
After cells were grown 70% confluent, small interfering RNA (siRNA) directed against p53 (Ambion) was transfected using Oligofectamine™ (Invitrogen) according to the manufacturer's instruction. The final siRNA concentration was 200 nM. The cells were subjected to H/R after 48 h post-transfection.
2.5. ROS detection
Intracellular ROS was imaged by Image-iT™ LIVE green ROS detection kit (Molecular Probes) with nuclei counterstained with Hoechst 33342. A minimum of 300 were counted per visual field.
2.6. p38 immunoprecipitation and kinase studies
Cells were lysed and p38β was immunoprecipitated using anti-p38β antibody (#sc-6187, Santa Cruz Biotechnology) conjugated to Sepharose beads. Immunoprecipitated p38β was subjected to kinase assays by incubating with activating transcription factor 2 (ATF2).4 The phosphorylated ATF2 was separated on 10% SDS–PAGE gel and detected by autoradiography. Western blotting of immunoprecipitated p38β was performed to demonstrate equal amounts of immunoprecipitated kinase used.
2.7. Adenoviral infection
Adenoviruses expressing dominant negative p38α MAPK (p38αDN) were generously provided by Dr Jiahuai Han (Scripps Research Institute, La Jolla, CA, USA).4 Viruses were amplified and titrated in 293 cells. The suppression of the p38 MAPK activity was confirmed by immunoprecipitation of the kinase from the infected cardiomyocytes and kinase assay as described above (data not shown). Viral stocks at a multiplicity of infection of 25–50 particles/cell were used to infect cardiomyocytes overnight. The cells were fed fresh medium the following day and further incubated for 24 h until ready for experiments.
2.8. Statistical analysis
All experiments were repeated at least three times. Data underwent one-way analysis of variance using GB-Stat software for statistical significance at P-value <0.05.
3. Results
3.1. Oestrogen inhibits p53-dependent apoptosis following H/R
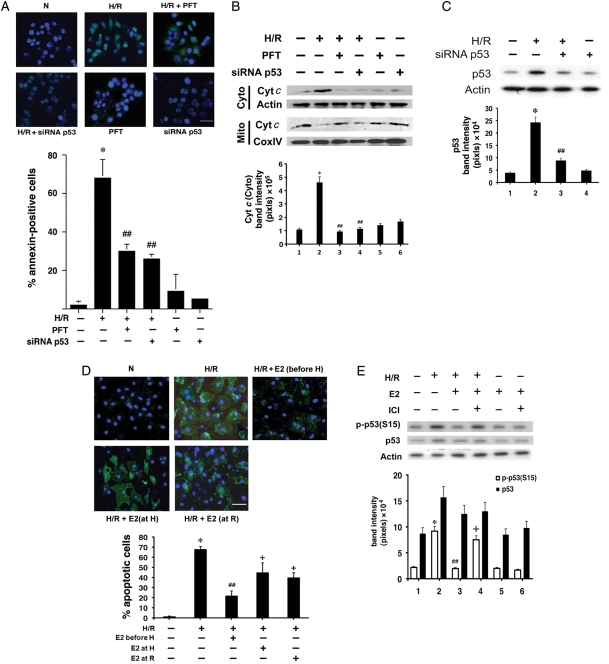
To test the relevance of p53 in our in vitro apoptosis model, neonatal rat cardiomyocytes were cultured and subjected to H/R after treatment with either a p53-specific chemical inhibitor, pifithrin-alpha (PFT), or siRNA directed against p53 (siRNA p53) (Figure 1A and B; see Supplementary material online, Table S1). Transfection with siRNA p53 achieved inhibition of ∼80% of endogenous p53 expression under H/R (Figure 1C). When p53 was inhibited, H/R-related death was significantly reduced, resulting in nearly 60% reduction in the number of apoptotic cells (Figure 1A). The intrinsic pathway of apoptosis, marked by the release of Cyt c from mitochondria to cytosol, was also examined after p53 inhibition (Figure 1B). Blocking p53 with PFT or siRNA p53 resulted in a significant reduction in Cyt c translocation. The effect of PFT or siRNA p53 at normoxia was not statistically significant from the untreated condition. The results confirmed that H/R-induced cardiomyocyte apoptosis was dependent on p53 in our model. Of note, Figure 1A and B suggests apoptosis as a major mode of cardiomyocyte death induced by H/R-related stress in our model, consistent with our previous report that few cells die from necrosis (<5% of the total cell population) following H/R.4
Figure 1.
(A) Apoptosis assay. After H/R with or without PFT (1 µM) or siRNA p53, apoptotic cardiomyocytes were identified by annexin V-FITC. The apoptotic cells appeared green, and the nuclei co-stained with Hoechst 33342 blue. N, normoxia. The white scale bar represents 25 µm. Mean% apoptotic cells ± standard error (n = 3) are shown in a bar graph. *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. (B) Cyt c release into cytosol. After indicated treatments, immunoblotting was performed on the mitochondrial (Mito) and cytosolic (Cyto) fraction for Cyt c and a marker protein (CoxIV for mitochondria and actin for cytosol). Representative immunoblots are shown with quantitative analysis (n = 3). *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. (C) p53 inhibition with siRNA p53. To confirm inhibition of p53 expression by siRNA p53, immunoblotting for p53 was done 2 days following siRNA transfection, with actin as a loading control. Representative blots are shown with quantitative analysis (n = 3). *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. (D) Time course of E2 exposure on cell survival. Cardiomyocytes were treated with 10 nM E2 for 30 min prior to hypoxia (before H), at the time of starting hypoxia (at H), or at the start of reoxygenation (at R). Apoptotic cells were identified in a manner similar to (A). The white scale bar represents 25 µm. Mean% apoptotic cells ± standard error (n = 3) are shown in a bar graph. *P < 0.05 vs. normoxia, ##P < 0.01 vs. H/R, and +P < 0.05 vs. H/R. (E) The effect of E2 on p-p53(S15). Immunoblotting was performed for p-p53(S15), p53, and actin. The concentration of E2 and ICI was 10 and 100 nM, respectively. Representative immunoblots are shown, with quantitative analysis (n = 3). *P < 0.05 vs. normoxia, ##P < 0.05 vs. H/R, and +P < 0.05 vs. H/R + E2.
After establishing that p53 inhibition leads to better cardiomyocyte survival, we treated the cells with a physiological concentration of E2 (10 nM) at different time points to detect the most optimal E2 effect on cell survival. Cells were treated with E2 for 30 min prior to starting 18 h hypoxia, at the beginning of hypoxia, or at the beginning of reoxygenation following hypoxia (Figure 1D). The 18 h hypoxic time had been determined previously to achieve the maximal degree of apoptosis before cells become necrotic, as neonatal rat cells are relatively resistant to hypoxia if exposed for a shorter duration.4 Longer exposure and incubation risk a higher likelihood of contamination by non-myocytes. In our model, the anti-apoptotic effect was greatest when the cells were pre-incubated with E2 for 30 min prior to hypoxia, compared with when E2 was added at the start of hypoxia or reoxygenation. It is worth noting that adding E2 in the latter two time points still yielded statistically significant protection from hypoxic injury, suggesting that E2 is protective against relatively acute as well as latent insult from H/R. Subsequent experiments requiring E2 were performed with the 30 min pre-incubation of the cells with the hormone before starting hypoxia.
After establishing the relevance of p53 and E2 separately in our H/R model, we then examined the effect of E2 on p53. In the presence of E2, there was a moderate decrease in total p53 protein (Figure 1E). Previously, we have reported that E2 enhanced cardiomyocyte survival by inhibiting pro-apoptotic p38α MAPK activation following H/R.4 p38 MAPK is known to activate p53 by phosphorylating the latter at serine 15.8 We hypothesized that E2-mediated down-regulation of p38α MAPK might antagonize this form of post-translational modification/activation of p53. Consistent with this, p53 phosphorylation at serine 15 [p-p53(S15)] increased with H/R, whereas adding E2 significantly reduced p-p53(S15) (Figure 1E). Attenuation of p-p53(S15) was dependent on the oestrogen receptors (ERs), as ICI182780 (ICI), a specific ER inhibitor, reversed the E2 effect.
3.2. H/R-induced p53 depends on p38α MAPK
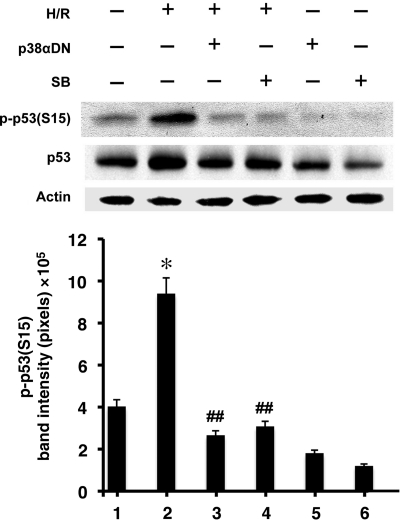
After establishing the role of p53 in cardiomyocyte apoptosis, we examined whether or not p53 activation was dependent on p38α MAPK, inhibition of which was essential in cytoprotection by E2.4 In order to block endogenous p38α MAPK, we expressed dominant negative p38αDN, via adenovirus infection, carrying a TGY180–182 → AGF mutation in the phosphorylation site of the kinase.9 This mutant prevents hypoxia-induced activation of endogenous p38α MAPK.4 We predicted that inactivating p38α MAPK would diminish the subsequent phosphorylation and activation of p53. Accordingly, little p-p53(S15) was observed in the presence of p38αDN during H/R, corrected for modest change in total p53 protein concentration (Figure 2). Similarly, the use of a chemical inhibitor of p38α MAPK, SB203580 (SB), also attenuated p-p53(S15). Although SB inhibits both p38α and p38β MAPKs, the latter is not activated by H/R stress alone, whereas the former is significantly up-regulated by the same stimulus.4 Therefore, SB is likely to inhibit p38α MAPK to a much greater degree than p38β in H/R. Overall, these results indicate that H/R-induced p53 depends on p38α MAPK.
Figure 2.
The effect of p38α MAPK on p-p53(S15). Western blotting of p-p53(S15), p53, and actin was done after treatment with p38αDN or SB (1 µM). Representative immunoblots are shown with quantitative analysis (n = 3) of p-p53(S15). *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R.
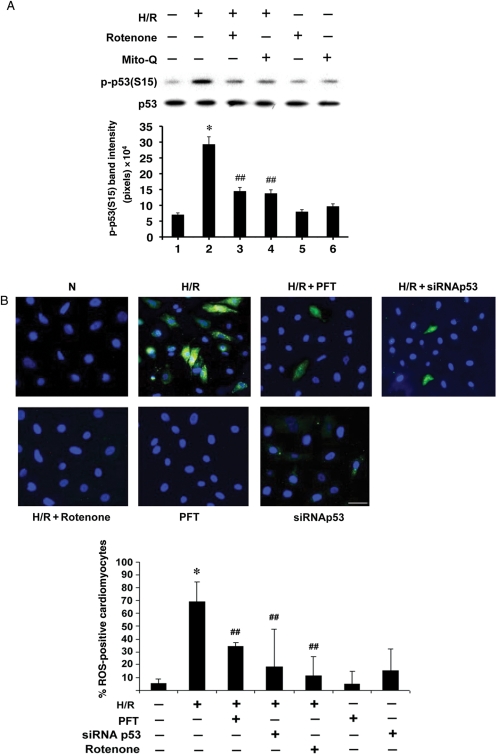
p38α MAPK activation follows a burst of ROS in H/R stress, whereas the antioxidant effect of E2 effectively quenches the radical oxygen formation.4 We posited that if the upstream trigger of the kinase, i.e. ROS, was removed, p38α MAPK-dependent p53 activation would be diminished. Consistent with this postulation, p-p53(S15) was reduced when known ROS inhibitors, rotenone and Mito-Q®, were present during H/R (Figure 3A). Both inhibitors specifically act on mitochondria. Thus, we concluded that the mitochondrial source of ROS upstream of p38α MAPK contributed to p53 activation.
Figure 3.
(A) The effect of ROS on p-p53(S15). Western blotting of p-p53(S15) and p53 after treatment with rotenone (2.5 µM) or Mito-Q® (10 µM) was done, with representative immunoblots and quantitative analysis (n = 3) shown. *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. (B) The effect of p53 on ROS. Intracellular ROS was detected by ROS-dependent deacetylation and oxidation of 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (D2CFDA) to green fluorescein. Representative images of cells are shown with quantitative analysis (n = 3). The bars represent mean ± standard error. *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. N, normoxia. The white scale bar represents 25 µm. The ROS-positive cells appeared green fluorescent, whereas nuclei stained blue with Hoechst 33342. The concentration of PFT and rotenone was 1 and 2.5 µM, respectively.
To further detail the relationship between p53 and ROS, we tested if the reverse were true, i.e. if suppression of p53 would affect ROS production. When we inhibited p53 with PFT or siRNA p53, the intracellular ROS level was markedly reduced, despite applying H/R (Figure 3B). This suggested p53 exerting a positive feedback on ROS production. Combined with the evidence that ROS stimulates p38α MAPK-dependent p53, this finding argues for an apoptotic loop sustained by the ROS–p38α MAPK–p53 interaction in H/R. Our data are also consistent with published reports demonstrating ROS-dependent p53 up-regulation10 as well as p53-mediated augmentation of ROS generation in non-cardiac models,11,12 attesting to the interdependency between ROS and p53 in cellular redox regulation.13 We have previously shown that directly inhibiting p38α MAPK with SB does not significantly alter ROS production, demonstrating that ROS act upstream to the kinase in the apoptotic pathway.4 Nevertheless, it is conceivable that p53 activated by factors other than p38α MAPK during H/R may fuel the ROS production. This is consistent with the results shown in Figure 3B.
3.3. p53 inhibits p38β
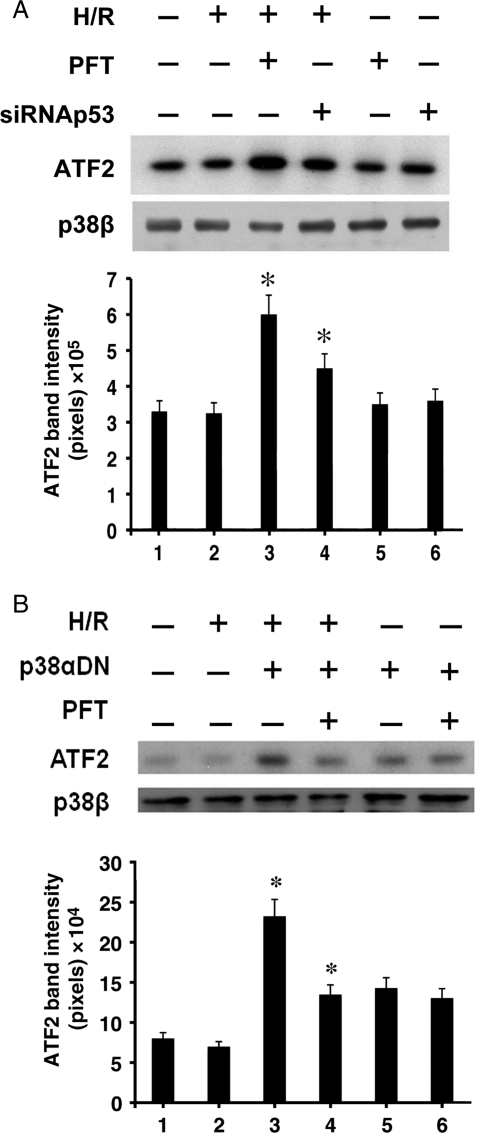
Part of E2-mediated cardiomyocyte survival depends on the hormone's signalling to p38β.4 During H/R, the p38β activation by E2 leads to suppression of ROS and ROS-dependent p38α MAPK, whereas the absence of E2 renders p38β inactive. We wondered if the dormant state of p38β during H/R was due to a negative control by p53. In the presence of E2, however, the kinase would be active, as E2 is known to stimulate the pro-survival kinase via PI3K/Akt signalling.4 In order to see whether p53 inhibits p38β, we examined the p38β activity while silencing p53 (Figure 4A). Cells treated with siRNA p53 or PFT underwent H/R, after which p38β was immunoprecipitated for an in vitro kinase assay with a purified substrate, ATF2. Inhibiting p53 significantly augmented the p38β activity in H/R, determined by the level of radiolabelled ATF2. H/R stress alone did not affect the p38β function. This finding supports our hypothesis that p38β is negatively controlled by p53 during H/R. As a control, western blotting was performed on immunoprecipitated p38β to ensure equal amounts of p38β used in the kinase reactions.
Figure 4.
(A) The effect of p53 on p38β activity. Following treatment with PFT (1 µM) or siRNAp53, the p38β kinase activity was assessed in an in vitro kinase assay, with purified ATF2 as a substrate and p38β immunoprecipitated from the cell lysate. A representative image of radiolabelled ATF2 is shown with quantitative analysis (n = 3). *P < 0.05 vs. H/R. Western blotting of immunoprecipitated p38β used in the kinase assay is shown. (B) The effect of p38α MAPK on p38β activity. The p38β kinase activity was assessed in a similar manner as in (A) after blocking p38α MAPK with p38αDN. A representative radiograph of phosphorylated ATF2 is shown with quantitative analysis (n = 3). *P < 0.05 vs. H/R. Western blotting of immunoprecipitated p38β protein used in the kinase assay is shown.
Considering that p38α MAPK promotes p53 activation and p53 inhibits p38β, it follows that p38α MAPK regulates p38β via p53. To confirm this relationship, we selectively inhibited p38α MAPK by expressing p38αDN and examined the p38β kinase activity (Figure 4B). The p38β kinase activity was low as expected post-H/R, a condition that activates native p38α MAPK. However, with suppression of p38α MAPK, the p38β kinase became active in hypoxic conditions. When p53 was blocked in the presence of p38αDN, the active state of p38β was maintained, subscribing to a notion that the influence of p38α MAPK on p53 has a significant role in the control of p38β. This finding is consistent with the idea that H/R-activated p38α MAPK upstream dictates p38β function via p53. To our knowledge, this is the first evidence that the survival kinase, p38β, is under the negative regulation by p53 in cardiomyocyte apoptosis.
3.4. Both ERα and ERβ contribute to p53 inhibition and cardiomyocyte survival
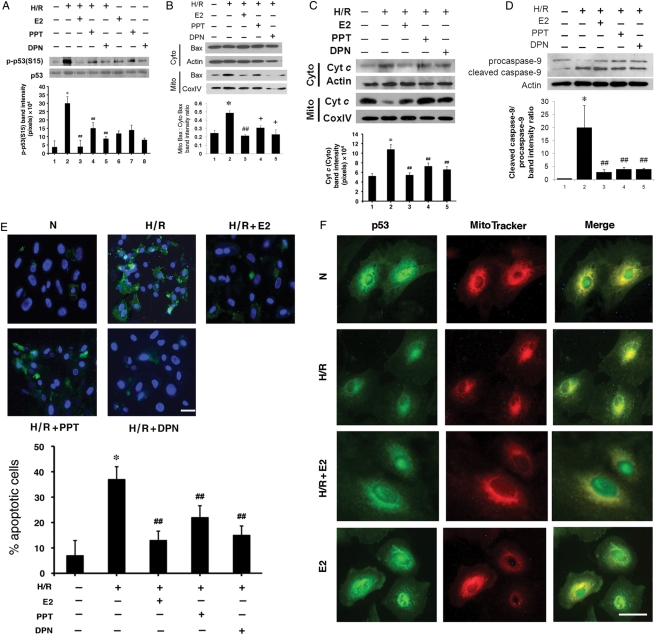
Both isoforms of ERs, ERα and ERβ, are present in a cardiomyocyte,14 but it is not clear which is responsible for the cytoprotection from H/R stress. Data from in vivo models utilizing knockout mice lacking each isoform provide no consensus in this regard.15,16 We sought to address this issue by delineating which ER prevented the p38–p53 interaction and myocyte death. To this end, we used a specific agonist for each ER isoform. PPT is a well-known ERα agonist,17 and DPN is a specific ERβ agonist.18 In the presence of PPT, there was a significant reduction of p-p53(S15) induced during H/R (Figure 5A). A similar result was obtained with DPN, indicating that both isoforms were involved. Either agonist alone was not as effective as E2. This observation implicates a role of ERα/β heterodimers in E2-mediated p38–p53 inhibition. Supporting this notion are reports of ER heterodimers taking part in genomic and non-genomic actions of E2.19,20 We then asked if the effect of ER isoforms on p53 modulation extends to the p53 function in apoptosis. ROS serves as a major stimulus for cell death in H/R and there is a dynamic relationship between p53 and ROS.4,10,13 As most of ROS following H/R stems from mitochondria, we focused on the aspects of mitochondria-driven apoptosis affected by p53. One of the earliest key events controlled by p53 is translocation of pro-apoptotic Bax from cytosol to mitochondria.21 As seen in Figure 5B, the ratio of mitochondrial to cytosolic Bax increased with H/R, whereas E2 reversed this ratio. Both PPT and DPN mimicked the E2 effect, consistent with the data on p-p53(S15). Bax, once redistributed to mitochondria, is believed to insert into the outer membrane of the organelle, thereby releasing Cyt c into cytosol.22 E2 as well as PPT and DPN were able to attenuate Cyt c translocation, a hallmark of mitochondria-driven apoptosis (Figure 5C). Then, we tested to see whether treatment with E2, PPT, or DPN prevented caspase-9 activation, an important step closely associated with Cyt c release in the mitochondria-centred apoptosis.23 Caspase-9 activation involves cleavage of the procaspase protein into smaller activated units, which in turn process other caspases to propagate the death signal.24 We examined whether E2 and the agonists influenced this crucial step (Figure 5D). As expected, caspase-9 activation increased, as reflected by the augmented ratio of cleaved caspase-9 to procaspase-9 following H/R. In contrast, E2 effectively curtailed this process, thereby blocking the apoptotic cascade. PPT and DPN also had a similar inhibitory effect on the caspase-9 activation. Finally, we thought that the mitigation of the pro-apoptotic events by PPT and DPN as shown above should lead to better cardiomyocyte survival. Indeed, cells treated with PPT or DPN were protected from undergoing significant apoptosis, indicated by less annexin V staining (Figure 5E; see Supplementary material online, Table S2). Again, the degree of protection was better with E2 than either agonist alone, suggesting involvement of both receptor isoforms. In sum, both PPT and DPN consistently attenuated the key steps of early apoptosis, as did E2, and we conclude that E2 renders cytoprotection from mitochondria-driven cell death by invoking both subtypes of ER.
Figure 5.
(A) The effect of ER isoforms on p-p53(S15). Immunoblotting was performed for p-p53(S15) and total p53 after treatment with E2, PPT or DPN, each at 10 nM. Representative blots are shown, with quantitative analysis (n = 3) of p-p53(S15). *P < 0.01 vs. normoxia and ##P < 0.05 vs. H/R. (B) The effect of ER isoforms on Bax translocation. After treating with E2, PPT, or DPN (each at 10 nM), the mitochondrial (Mito) and cytosolic (Cyto) fractions were separated, and immunoblotted for Bax and a marker protein (CoxIV for mitochondria and actin for cytosol). Representative blots are shown, with quantitative analysis (n = 3) of the mitochondrial Bax-to-cytosolic Bax ratio. *P < 0.01 vs. normoxia, ##P < 0.01 vs. H/R, and +P < 0.05 vs. H/R. (C) The effect of ER isoforms on Cyt c release. The mitochondrial (Mito) and cytosolic (Cyto) fractions were separated and immunoblotted for Cyt c and a marker protein (CoxIV for mitochondria and actin for cytosol) in a manner similar to (B). Representative immunoblots are shown with quantitative analysis (n = 3) of Cyt c released into cytosol. *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. (D) The effect of ER isoforms on caspase-9 activation. Immunoblotting was performed for procaspase-9 or cleaved caspase-9 after treatment with 10 nM E2, PPT, or DPN. Representative blots are shown, with quantitative analysis (n = 3) of the cleaved caspase-9 to procaspase-9 ratio. *P < 0.05 vs. normoxia and ##P < 0.05 vs. H/R. (E) The effect of ER isoforms on cardiomyocyte survival. Apoptotic cells were detected in a similar manner as in Figure 1A. The final concentration of E2, PPT, and DPN was 10 nM. Representative images of apoptotic cells are shown, with the white scale bar representing 25 µm. Mean% (±standard error) of apoptotic cells is presented with quantitative analysis (n = 3). *P < 0.01 vs. normoxia and ##P < 0.05 vs. H/R. (F) E2 alters subcellular translocation of p53. After H/R with or without E2 (10 nM), cardiomyocytes were labelled with MitoTracker, fixed, permeabilized and stained with anti-p53 primary antibody, followed by FITC-conjugated secondary antibody, and visualized under a fluorescence microscope. The photographs of the same cells labelled with anti-p53 antibody (green) or MitoTracker (red) were taken and merged to demonstrate co-localization (yellow) of p53 and mitochondria. A white scale bar represents 25 µm.
We have demonstrated so far that E2 antagonizes the apoptotic cascade associated with p53. To further characterize the hormone's influence on the p53 function in mitochondria-centred apoptosis, we performed immunofluorescence studies to see whether E2 would alter p53 subcellular localization (Figure 5F). In normoxia, p53 was present in both the nucleus and perinuclear areas. Co-staining with a mitochondrial probe (MitoTracker) identified the p53-rich perinuclear regions to be mitochondria. Subjecting the cells to H/R led to strong staining of p53 in mitochondria, with nuclei now essentially devoid of p53. This is consistent with previous reports, demonstrating mitochondrial accumulation of p53 during hypoxia-induced apoptosis in established cell lines.25,26 In contrast, treating cardiomyocytes with E2 during H/R altered p53 redistribution, restoring the dual subcellular pattern similar to that seen in normoxia. This suggests that not only E2 blocks p38α-dependent activation of p53, but it also prevents p53 from physically associating with mitochondria. Does E2 also change the expression of anti-apoptotic proteins known to function at mitochondria? Bcl-2 is a major mitochondrial anti-apoptotic protein whose gene expression falls under the negative regulation by p53 in non-cardiac cells.27,28 However, in our model of cultured cardiomyocytes, the level of Bcl-2 protein did not change significantly following H/R, consistent with previous reports.10,22,29 Treatment with E2, PPT, or DPN made little difference in this regard (see Supplementary material online, Figure S1). Thus, we concluded that a majority of E2-mediated cytoprotection in H/R is not due to up-regulation of Bcl-2 protein expression. Of note, an increased Bcl-2 level has been linked to ERβ in one report utilizing ERβ knockout mice.30 The negative finding of our data contrasting that of the aforementioned study may be due to the differences between the experimental models, but it should also be noted that Bcl-2 detected from the knockout mice were from whole heart homogenate, making it impossible to distinguish whether the protein up-regulation was from cardiomyocytes or other cell types present in the organ.
4. Discussion
The anti-apoptotic actions of E2 are an important part of the cardioprotective mechanisms of the female sex hormone.3,4,31 Recently, we have shown that H/R-triggered apoptosis of cardiomyocytes is mitigated by E2 via differential modulation of p38α and p38β MAPKs.4 Here, we have presented a line of evidence that supports a regulatory relationship between the cardiac p38 MAPKs and p53 and that it is a target of the hormone's salutary actions.
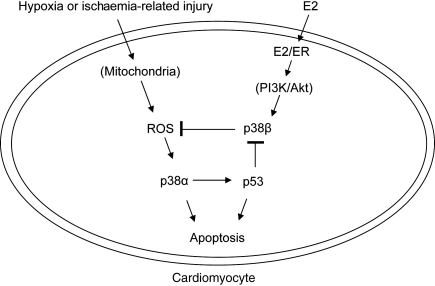
Specifically, we report several novel findings as follows. First, E2 protects cardiomyocytes from p53-dependent apoptosis. This occurs by inhibiting p38α MAPK-mediated p53 activation and phosphorylation. Secondly, p53-dependent apoptosis involves inhibition of p38β. The ability of E2/ER to activate p38β was shown to occur by signalling to the PI3K/Akt pathway.4 Akt is known to inhibit p53 in various cell types by promoting nuclear translocation of Mdm2 and Mdm2-mediated degradation of p53.32 A similar mechanism may be at work in E2-mediated down-regulation of p53 during H/R in myocytes. In addition, E2 blocks mitochondrial translocation of p53 and multiple steps in the mitochondria-centred death signalling. Thirdly, both ER isoforms are involved in this cytoprotection. A schematic diagram summarizing the major findings is pictured in Figure 6. Taken together, the findings shed new light on the mechanism by which oestrogen confers cytoprotection during hypoxic stress.
Figure 6.
A working model of E2-dependent protection of a cardiomyocyte under H/R stress. Following H/R or simulated I/R, there is a burst of mitochondrial ROS. The oxygen radicals stimulate p38α MAPK, which phosphorylates and activates p53. p53 in turn represses p38β. E2 binds and activates ERs (possibly in heterodimers), and the E2/ER complex activates p38β via PI3K/Akt.
We have previously noted that p38β is central to E2-mediated survival of cardiomyocytes and that this kinase is essential to quenching ROS.4 Our current work demonstrates, for the first time to our knowledge, that cardiac p38β is under the negative control by p53 during apoptosis. This potentially explains why the kinase remains inactive during hypoxia or ischaemia,4,33 conditions that promote p53 activity. We surmise that when the cell is exposed to oestrogen, p38β induction by the hormone serves as a key step to overcome the p38α MAPK–p53 apoptotic signalling. Antagonizing p38β also provides another way for p53 to increase ROS production against the antioxidant properties of the kinase. The mechanism by which p38β quenches mitochondrial ROS is unclear and is a subject of ongoing investigation by our laboratory. It is possible that p38β may interact with the mitochondrial fraction of p53 in apoptosis. The p38 MAPK (most likely p38α MAPK, given the commercial antibody used in the referenced study) is found in mitochondria.34 Whether or not the p38β isoform translocates to mitochondria, and if so, what part of the mitochondrial redox apparatus is subjected to the kinase actions and the role of the ER isoforms in this context remain to be answered.
Our data indicate that both ERα and ERβ contribute to p53 inhibition and subsequent cell survival (Figure 5). This observation opens a possibility that ER heterodimers may be at work in the interception of myocyte apoptosis. A classically accepted model is that E2-activated ERs form homodimers to bind DNA and act as transactivator. Nevertheless, functional ERα/β heterodimers have been suggested in transcription19,35 and found in the plasma membrane pool.20 Although the ratio of the two ER homologues varies in different tissues,36 both are present in cardiomyocytes.14 Animal models are yet to yield clear consensus on the extent of contribution by each ER receptor in cardioprotection, likely because experimental models, endpoints, and applied doses of ER agonists vary widely from one report to another (for review, see Murphy and Steenbergen37). A comparison of cardiomyocytes negative for an ER subtype in combination with the use of subtype-specific agonists may better define the role of each receptor in I/R. Another consideration is participation of GPR30, now known as G-protein-coupled ER (GPER).38 E2 stimulation of GPER activates PI3K,39 and the receptor is reported to have beneficial haemodynamic effects on the heart.40,41 Although the role of GPER in cardiomyocyte apoptosis remains largely unknown, E2-mediated cytoprotection may incur this novel receptor as well as ERα and ERβ.
Our work is based on simulated I/R in vitro. A potential source of pitfall is the use of neonatal myocytes, rendering a possibility that the observations made from this model may not reflect the underlying pathology behind the ischaemic injury more prevalent in adults. However, despite the obvious differences between neonatal and adult cardiomyocytes, salient features are similar between them, such as intracellular localization of ER isoforms42 and p38 MAPK expression.43 With these relevant protein characteristics uniform between neonatal and adult cells, we believe that the findings from this study are likely applicable to the ischaemic events in adult myocytes.
Finally, the conclusions drawn from our study add to much needed clarification of gender dichotomy and provide a mechanistic basis for potential application of ER ligands in IHD.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work is supported by a grant from the National Heart Lung Blood Institute at the National Institutes of Health (K08HL089066 to J.K.K).
Supplementary Material
References
- 1.Shlipak MG, Angeja BG, Go AS, Frederick PD, Canto JG, Grady D. Hormone therapy and in-hospital survival after myocardial infarction in postmenopausal women. Circulation. 2001;104:2300–2304. doi: 10.1161/hc4401.98414. doi:10.1161/hc4401.98414. [DOI] [PubMed] [Google Scholar]
- 2.Patten RD, Karas RH. Estrogen replacement and cardiomyocyte protection. Trends Cardiovasc Med. 2006;16:69–75. doi: 10.1016/j.tcm.2006.01.002. doi:10.1016/j.tcm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. doi:10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 4.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281:6760–6767. doi: 10.1074/jbc.M511024200. doi:10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, et al. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. doi:10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Mao XO, Sun Y, Xia Z, Greenberg DA. p38 mitogen-activated protein kinase mediates hypoxic regulation of Mdm2 and p53 in neurons. J Biol Chem. 2002;277:22909–22914. doi: 10.1074/jbc.M200042200. doi:10.1074/jbc.M200042200. [DOI] [PubMed] [Google Scholar]
- 7.Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999;18:6845–6854. doi: 10.1093/emboj/18.23.6845. doi:10.1093/emboj/18.23.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chouinard N, Valerie K, Rouabhia M, Huot J. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem J. 2002;365:133–145. doi: 10.1042/BJ20020072. doi:10.1042/BJ20020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, et al. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. doi:10.1016/S1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 10.von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation. 1999;99:2934–2941. doi: 10.1161/01.cir.99.22.2934. [DOI] [PubMed] [Google Scholar]
- 11.Drane P, Bravard A, Bouvard V, May E. Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene. 2001;20:430–439. doi: 10.1038/sj.onc.1204101. doi:10.1038/sj.onc.1204101. [DOI] [PubMed] [Google Scholar]
- 12.Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA. 1996;93:11848–11852. doi: 10.1073/pnas.93.21.11848. doi:10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. doi:10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grohe C, Kahlert S, Lobbert K, Stimpel M, Karas RH, Vetter H, et al. Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Lett. 1997;416:107–112. doi: 10.1016/s0014-5793(97)01179-4. doi:10.1016/S0014-5793(97)01179-4. [DOI] [PubMed] [Google Scholar]
- 15.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. doi: 10.1016/j.yjmcc.2004.11.013. doi:10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Jeanes HL, Tabor C, Black D, Ederveen A, Gray GA. Oestrogen-mediated cardioprotection following ischaemia and reperfusion is mimicked by an oestrogen receptor (ER)alpha agonist and unaffected by an ER beta antagonist. J Endocrinol. 2008;197:493–501. doi: 10.1677/JOE-08-0071. doi:10.1677/JOE-08-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. doi:10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Baudry J, Katzenellenbogen JA, Katzenellenbogen BS. Molecular basis for the subtype discrimination of the estrogen receptor-beta-selective ligand, diarylpropionitrile. Mol Endocrinol. 2003;17:247–258. doi: 10.1210/me.2002-0341. doi:10.1210/me.2002-0341. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24:7681–7694. doi: 10.1128/MCB.24.17.7681-7694.2004. doi:10.1128/MCB.24.17.7681-7694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. doi:10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 21.Schuler M, Maurer U, Goldstein JC, Breitenbucher F, Hoffarth S, Waterhouse NJ, et al. p53 triggers apoptosis in oncogene-expressing fibroblasts by the induction of Noxa and mitochondrial Bax translocation. Cell Death Differ. 2003;10:451–460. doi: 10.1038/sj.cdd.4401180. doi:10.1038/sj.cdd.4401180. [DOI] [PubMed] [Google Scholar]
- 22.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. doi:10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. doi:10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 24.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. doi:10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. doi:10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 26.Sansome C, Zaika A, Marchenko ND, Moll UM. Hypoxia death stimulus induces translocation of p53 protein to mitochondria. Detection by immunofluorescence on whole cells. FEBS Lett. 2001;488:110–115. doi: 10.1016/s0014-5793(00)02368-1. doi:10.1016/S0014-5793(00)02368-1. [DOI] [PubMed] [Google Scholar]
- 27.Haldar S, Negrini M, Monne M, Sabbioni S, Croce CM. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994;54:2095–2097. [PubMed] [Google Scholar]
- 28.Wu Y, Mehew JW, Heckman CA, Arcinas M, Boxer LM. Negative regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene. 2001;20:240–251. doi: 10.1038/sj.onc.1204067. doi:10.1038/sj.onc.1204067. [DOI] [PubMed] [Google Scholar]
- 29.Strosznajder R, Gajkowska B. Effect of 3-aminobenzamide on Bcl-2, Bax and AIF localization in hippocampal neurons altered by ischemia–reperfusion injury. The immunocytochemical study. Acta Neurobiol Exp (Wars) 2006;66:15–22. doi: 10.55782/ane-2006-1583. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, et al. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R972–R978. doi: 10.1152/ajpregu.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelzer T, Schumann M, Neumann M, deJager T, Stimpel M, Serfling E, et al. 17beta-estradiol prevents programmed cell death in cardiac myocytes. Biochem Biophys Res Commun. 2000;268:192–200. doi: 10.1006/bbrc.2000.2073. doi:10.1006/bbrc.2000.2073. [DOI] [PubMed] [Google Scholar]
- 32.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–467. doi: 10.1016/s0968-0004(02)02166-7. doi:10.1016/S0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 33.Saurin AT, Martin JL, Heads RJ, Foley C, Mockridge JW, Wright MJ, et al. The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 2000;14:2237–2246. doi: 10.1096/fj.99-0671com. doi:10.1096/fj.99-0671com. [DOI] [PubMed] [Google Scholar]
- 34.Kong JY, Klassen SS, Rabkin SW. Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Mol Cell Biochem. 2005;278:39–51. doi: 10.1007/s11010-005-1979-6. doi:10.1007/s11010-005-1979-6. [DOI] [PubMed] [Google Scholar]
- 35.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. doi:10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 36.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. doi:10.1210/en.138.3.863. [DOI] [PubMed] [Google Scholar]
- 37.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia–reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. doi:10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. doi:10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. doi:10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 40.Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- 41.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. doi:10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedram A, Razandi M, Aitkenhead M, Levin ER. Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem. 2005;280:26339–26348. doi: 10.1074/jbc.M414409200. doi:10.1074/jbc.M414409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, et al. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia–reperfusion in vivo. J Biol Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. doi:10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.