Abstract
Permanent scars form upon healing of tissue injuries such as those caused by ischemia (myocardial infarction, stroke), trauma, surgery, and inflammation. Current options in reducing scar formation are limited to local intervention. We have designed a systemically administered, target-seeking biotherapeutic for scar prevention. It consists of a vascular targeting peptide that specifically recognizes angiogenic blood vessels and extravasates into sites of injury, fused with a therapeutic molecule, decorin. Decorin prevents tissue fibrosis and promotes tissue regeneration by inhibiting TGF-β activity and by other regulatory activities. The decorin-targeting peptide fusion protein had substantially increased neutralizing activity against TGF-β1 in vitro compared with untargeted decorin. In vivo, the fusion protein selectively accumulated in wounds, and promoted wound healing and suppressed scar formation at doses where nontargeted decorin was inactive. These results show that selective targeting yields a tissue-healing and scar-reducing compound with enhanced specificity and potency. This approach may help make reducing scar formation by systemic drug delivery a feasible option for surgery and for the treatment of pathological processes in which scar formation is a problem.
Keywords: CAR peptide, angiogenesis, proteoglycan, cell-penetrating peptide
Numerous growth factors and other agents that could potentially enhance tissue regeneration have been identified, but their therapeutic application has been limited in clinical medicine for several reasons: It is difficult to maintain bioactivity of locally applied therapeutic agents in regenerating tissue because of lack of retention of the agent, poor tissue penetration, and instability of protein therapeutics in the protease rich environment of an injured tissue. Moreover, injuries to organs beneath the skin or to multiple sites of injury further limit the effectiveness of local treatments. Clearly, systemic approaches to tissue repair would be valuable.
The response to tissue injury in adult mammals is focused on quick sealing of an injury (restoration of the tissue continuity). Rapid proliferation of fibroblasts and enhanced extracellular matrix production by them replaces the damaged tissue with fibrotic tissue, resulting in loss of function and scar formation (1–3). An important driver of this process is transforming growth factor-β1 (TGF-β1), and suppressing the activity of this growth factor has become a focus of efforts to develop compounds that prevent scar formation and fibrosis (1–5).
Decorin, a small chondroitin/dermatan sulfate proteoglycan, has been proposed to be a physiological TGF-β inhibitor, which limits the duration of TGF-β responses in inflammation and tissue repair (4). The ability of decorin to prevent tissue fibrosis and promote tissue regeneration has been observed in numerous injury models (4, 6–18). The TGF-β blockade is likely the main factor in the antiscarring activity of decorin, but effects on some other growth factors and on collagen fibrillogenesis may also contribute (13–18).
Despite the positive results in animal models (8–12, 15–18), decorin has not reached the clinic. One reason is that as a protein therapeutic, it is relatively difficult to manufacture. Enhancing the activity of decorin is a potential solution to this problem, as it would make it possible to achieve the same result with a lower dosage. Here we have investigated whether adding a wound-targeting function to decorin would enhance its activity. We recently used in vivo phage display to identify peptides that home to angiogenic blood vessels in regenerating tissues (19). Phage expressing the most potent wound-homing peptide obtained, CARSKNKDC (CAR) homed up to 200-fold more than control phage to blood vessels in regenerating skin, skeletal muscle, and tendons. The homing was selective; there was no accumulation in normal tissues. In this study, we have used the CAR peptide to generate a CAR–decorin fusion protein for targeted delivery of decorin into injured tissues. The three different isoforms of TGF-β can have opposing effects on fibrosis (5); TGF-β1 is the main scar-inducing form and TGF-β2 augments the profibrotic actions of TGF-β1, whereas TGF-β3 counteracts scar development (5, 20, 21). As decorin is thought to inhibit all TGF-β isoforms (22), we also studied the TGF-β inhibition profile of the CAR-targeted decorin.
Results
Construction of Recombinant Decorins.
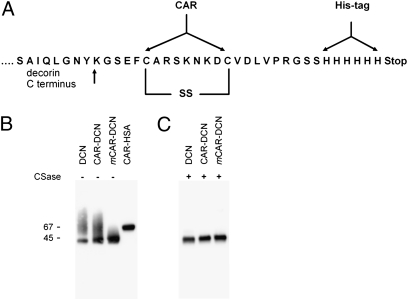
We produced a wound-directed recombinant decorin fusion protein, CAR–decorin, by adding a wound homing peptide, CAR (sequence CARSKNKDC), which recognizes angiogenic blood vessels in skin wounds and in other regenerating tissues, to an extended C terminus of human decorin (Fig. 1) (19). We also generated native decorin, human serum albumin with the CAR peptide (CAR–HSA), and decorin with an inactive CAR peptide variant (mCAR–DCN). We have previously shown that replacing two basic amino acids in the CAR sequence with neutral ones (CARSKNKDC mutated to CAQSNNKDC in mCAR) eliminates the wound homing activity (19). His-tagged fusion proteins were produced in a mammalian cell expression system. The yields of purified fusion proteins were 5–15 mg/L of cell culture medium, providing ample material for characterization and in vivo treatment studies.
Fig. 1.
Cloning and production of recombinant fusion proteins. (A) Schematic representation of the CAR–decorin fusion protein showing the insertion of the CAR peptide sequence and a polyhistidine-tag C-terminal of full-length decorin. (B) Gel electrophoretic analysis of recombinant decorins and CAR–HSA. The recombinant proteins were expressed in mammalian cells, purified on a Ni-column, separated on gradient SDS/PAGE gels, and detected with a monoclonal anti–6-histidine tag antibody. The decorins migrate as sharp bands at 45 kDa with a smear above the sharp band. (C) Digestion of the recombinant decorins with chondroitinase ABC before electrophoresis removed the smear. Thus, the sharp bands correspond to the core proteins and the smear is caused by the chondroitin sulfate chain attached to most of the decorin molecules (7, 8, 22, 23).
In SDS gel electrophoresis, decorin and the decorin fusion proteins migrated as sharp bands at 45 kDa with a smear above the band (Fig. 1). The sharp bands correspond to the core proteins, and the smear is caused by heterogeneity in the chondroitin sulfate chain attached to most of the decorin molecules (23). Mass spectrometry confirmed protein identities as decorin (or HSA), and differential scanning calorimetry produced a sharp peak with a melting temperature of Tm = 49.3 °C, indicating native protein folding.
Activities of Decorin and CAR Peptide Are Retained in the Fusion Protein.
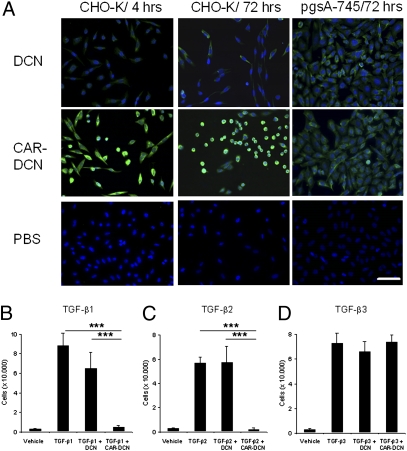
We tested the activity of the CAR homing motif in the decorin fusion protein in cultured Chinese hamster ovary cells (CHO-K). In line with the strong binding of the synthetic CAR peptide to CHO-K cells (ref. 19; Fig. S1), CAR–decorin bound to these cells more avidly than decorin (Fig. 2A, 4 h). Fig. 2A shows that the increased binding of CAR–decorin to the CHO cells was not seen when we used mutant CHO cells (pgsA-745) that lack the receptor for the CAR (19). Decorin inhibits CHO-K cell proliferation, which is dependent on autologous TGF-β production (7, 23), but CAR–decorin was active at 10-fold lower concentrations than decorin (Fig. S1B). Decorin also inhibits cell spreading (24), and CAR–decorin was more potent in this regard than decorin (Fig. 2A, 72 h). Thus, the CAR peptide imparts its binding specificity and other activities to the CAR–decorin fusion protein that make it more potent than decorin itself.
Fig. 2.
Cell binding and inhibition of cell spreading and TGF-β–dependent cell proliferation by decorins. (A) The culture media of CHO-K and pgsA-745 cells were supplemented with 0.3 μg/mL of decorins daily for 4 h or 3 d. After washing, the decorins were detected with anti–his-tag antibody and FITC-conjugated secondary antibody (green). The CAR-targeted decorin bound to CHO-K cells more (4 h) and inhibited cell spreading more strongly (3 d) than unmodified decorin, whereas the two decorins show identical low binding to mutant pgsA-745 cells that lack the receptor for the CAR peptide (19). Cell nuclei were stained by DAPI (blue). (Scale bar, 50 μm.) (B) CHO-K cells were grown in the presence of TGF-β1, (C) TGF-β2, or (D) TGF-β3 (all at 7.5 ng/mL) and 0.3 μg/mL of decorins. Half of the medium was changed daily for 4 d (7). Error bars represent mean ± SD. Two to four separate experiments were performed in triplicate. CAR-targeted decorin was particularly potent in inhibiting TGF-β1– and -β2–driven cell proliferation (B and C, ***P ≤ 0.001 both compared with control and decorin; ANOVA).
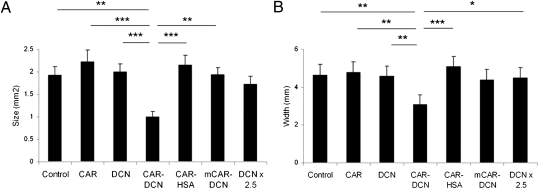
Fig. 4.
Reduced scar formation during wound healing in mice treated with CAR–decorin. Mice with full thickness skin wounds received daily i.v. injections as indicated from day 3 after the wounding until day 14. Scars were harvested on day 21, and the cross-sectional area (A) and the width (diameter) (B) of the scars were quantified by examining two microscopic sections from each wound. The results are expressed as the average of the two values. There were seven animals, each with four wounds, in every treatment group. *P < 0.05, **P < 0.01, ***P < 0.001; ANOVA. The results are expressed as mean ± SEM, n = 28.
Enhanced TGF-β1 Neutralizing Activity of CAR-Modified Decorin.
To determine whether CAR–decorin can inhibit proliferation induced by exogenously added TGF-β, we tested CHO-K cells cultured in the presence of the three isoforms of TGF-β. Decorin partially inhibited cell proliferation induced by the major scar-inducing isoform, TGF-β1, whereas CAR–decorin completely suppressed this TGF-β1 activity (Fig. 2B; P < 0.001). CAR–decorin was significantly more active than decorin used at 100-fold higher concentrations (Fig. S1C; P < 0.001). Neither protein affected TGF-β1–stimulated pgsA-745 cells (Fig. S1D). CAR–decorin also almost completely inhibited TGF-β2–induced CHO cell proliferation at a concentration at which decorin was inactive (Fig. 2C; P < 0.001). Remarkably, CAR–decorin had no effect on cell proliferation induced by TGF-β3 (Fig. 2D).
Systemic Delivery of Decorin to Wounds.
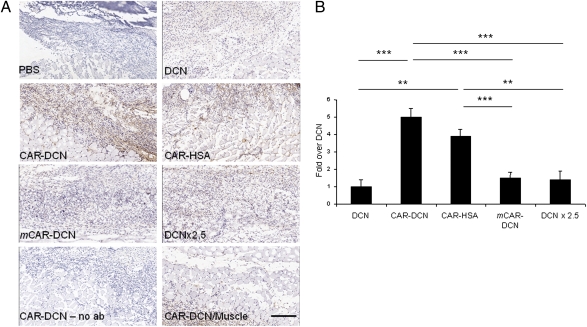
Decorin has been reported to home to angiogenic vasculature through a core protein-dependent interaction (25, 26), an effect possibly further enhanced by the binding of its glycosaminoglycan side chain to integrins (27). We found that adding the CAR peptide to decorin greatly enhanced the accumulation of i.v.-injected decorin in skin wounds in mice as detected by an anti–His-tag antibody (the recombinant proteins contained a His tag). We observed an almost fivefold stronger signal in the wound granulation tissue in mice that received CAR–decorin compared with nonmodified decorin (Fig. 3 and Fig. S2). The difference persisted even when a 2.5-fold higher dose of decorin was used (Fig. 3; P < 0.001), and after multiple daily injections (days 3–5) of the recombinant decorins had been given (Fig. S2). CAR–decorin also penetrated into granulation tissue more than nonmodified decorin (Fig. S2). The mCAR–decorin control protein accumulated in wounds to approximately the same extent as nonmodified decorin (Fig. 3). The normal tissues (skin and underlying muscle) around the skin wound were almost completely negative for decorin immunoreactivity regardless of which decorin protein was used. The accumulation of the CAR–decorin in wound tissue parallels the accumulation we have obtained in using the CAR peptide to deliver bacteriophage and fluorescein into wounds (19). The results show that homing peptide-enhanced delivery can markedly increase the concentration of decorin in wounds.
Fig. 3.
Homing of CAR–decorin in wound tissue. Mice with full thickness skin wounds received an i.v. injection of His-tagged fusion proteins on day 5 after wounding. After 4 h, the location of decorin was determined with an anti–His-tag antibody (brown). (A) The wounds of mice injected with nontargeted decorin (DCN) or mCAR–DCN were weakly positive, whereas strong staining was observed in CAR–DCN wounds. The accumulation of CAR–HSA in the wounds confirmed the ability of the CAR peptide to enhance targeting to the wounds. No staining was observed in normal skeletal muscle underlying the skin wounds of mice treated with any of the decorins (shown for CAR–DCN; CAR–DCN/muscle). No staining was seen in wound tissue when class-matched mouse IgG was substituted for the anti–6-histidine tag antibody (no ab). (Scale bar, 200 μm.) (B) Quantitative analysis of homing in skin wounds. The statistical significance was examined with ANOVA; *P < 0.05, **P ≤ 0.01, ***P < 0.001, n = 16 per group. The results are expressed as mean ± SD.
Wound Treatment with CAR-Modified Decorin.
Next, we examined the effect of the peptide-modified decorins on wound healing. I.v. administration of decorins was started on day 3 after wounding, when granulation tissue first starts to form in wounds (5). The treatment was continued for 7 or 11 d in three independent treatment experiments with 20 mice in each treatment group (n = 140). The daily dose of 40 μg/d (by tail vein injection) was chosen on the basis of previous decorin studies (8, 25, 26). The dose was doubled on days 4–6, coincident with the peak in TGF-β expression in wounds (5).
The 3-d delay in starting the treatment was designed to rule out the possibility that decorin would accumulate into the wounds by extravasation during the inflammatory phase and to preserve a window for the recruitment of inflammatory cells to the wounds (5). The influx of inflammatory cells into a wound contributes to subsequent scar formation (1–3, 28, 29), but inflammatory cells are also important in combating infection, particularly in humans (28). Furthermore, macrophages, especially the early influx, are crucial for all aspects of skin wound healing in mice (29–31), not just for the removal of necrotic material (28). Indeed, we found that the accumulation of macrophages and neutrophils in wounds treated with CAR–decorin did not differ from controls (Fig. S3). By limiting the duration of the therapy to the period when granulation tissue is formed, we left the early, TGF-β–induced accumulation of inflammatory cells untouched and preserved the beneficial effects of macrophages on subsequent tissue regeneration (29–31).
Daily inspection of the wounds (n = 560) showed no wound rupture during the treatment trials. Histological analysis of wounds at day 10 showed that the amount of granulation tissue was strikingly smaller in the groups that received CAR–decorin; the reduction was ≈50% relative to control groups (Figs. S4 and S5) at the 10-d timepoint. Nonmodified decorin, synthetic CAR peptide alone, CAR–HSA, or mCAR–decorin produced no statistically significant change in the amount of the granulation tissue compared with the vehicle control (Figs. S4 and S5). We also tested nonmodified decorin at a 2.5-fold higher dose (100/200 μg/d; DCN × 2.5) and saw no statistically significant change in wound size compared with the control wounds.
After the granulation tissue has formed, myofibroblasts cause contraction of the wound into scar tissue. A treatment trial ending at day 14 showed that the difference seen in the size of the granulation tissue by CAR–decorin treatment persisted through the wound contraction process (Figs. S4 and S5). Wound length (the longitudinal diameter of the wound) was also significantly decreased in groups receiving CAR–decorin (Fig. S4). Wound closure measurements indicated acceleration of closure in the animals that received CAR–decorin (Fig S6). The small differences to the controls became highly significant for CAR–decorin when combined results from three experiments were analyzed, and there may have been a slight effect by the nontargeted decorins as well in this assay (Table S1). A significantly shorter distance between the tips of the epithelial tongues was measured for the CAR–DCN treated wounds compared with control wounds in the histological analysis (Fig. S6B).
Reduction of Scar Formation by Wound-Targeted Decorin.
To determine whether the reduction seen in the granulation tissue and scar formation by CAR–decorin treatment persisted in fully healed wounds, we treated mice between days 3 and 14 after wounding and collected the scar tissue on day 21. The scars of CAR–decorin-treated mice were nearly 50% smaller than the scars in the other groups (P < 0.001, Fig. 4). The length of the scars (hyperproliferative epidermis) was also significantly decreased in the group that received CAR–decorin (Fig. 4). These results show that homing peptide-targeted decorin administered during wound healing can significantly reduce the size of the resultant scar.
Pharmacokinetics of Recombinant Decorins in Vivo.
The enhanced effect of CAR–decorin appears to be based on active targeting rather than other mechanisms, such as pharmacokinetic factors. The results described above show that, whereas CAR–HSA homes to granulation tissue (Fig. 3), it has no effect on the wounds (Fig. 4 and Fig. S4). This result excludes the possibility that prolonging the half-life of the CAR peptide in the circulation by its coupling to decorin would have rendered the peptide itself active in wound healing. Moreover, the various recombinant decorins used in this study had identical half-lives in the circulation of normal mice without wounds (Fig. S7), excluding the possibility that the activity of CAR–decorin would be due to unusual pharmacokinetics (other than the homing to wounds).
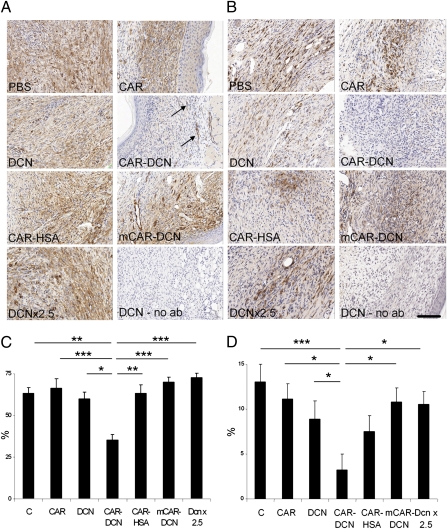
CAR–Decorin Inhibits the Expression of TGF-β–Induced Genes in Wounds.
TGF-β1 is likely to be an important target of decorin in wound healing, because TGF-β1 plays a key role in scar formation (1–5), and decorin inhibits TGF-β–dependent responses (4, 6–18). CAR–decorin inhibited gene expression of several TGF-β–induced genes that play a role in scar formation (32–35) by about 50% at day 5 after wounding when TGF-β activity peaks in wounds (5) (Fig. S8). The transformation of fibroblasts to α-SMA–positive myofibroblasts, which is a TGF-β–dependent process and responsible for converting granulation tissue to permanent scar tissue (36), was significantly reduced (Fig. 5A). This result is in agreement with previous data showing that decorin can block the TGF-β–induced transdifferentiation of myogenic cells to myofibroblasts (9). Connective tissue growth factor (CTGF/CCN2) is required for TGF-β–induced transdifferentiation of fibroblasts to myofibroblasts (32–34). CAR–decorin also reduced the expression of CCN2 mRNA and protein at various timepoints during granulation tissue formation (Fig. 5 and Fig. S8). As myofibroblasts are responsible for converting the granulation tissue into permanent scar (36), these data provide an explanation for the reduced scar formation we observe in the mice treated with the targeted decorin.
Fig. 5.
α-SMA–positive fibroblasts (myofibroblasts) and connective tissue growth factor (CCN2/CTGF) in wounds treated with targeted decorin. Representative sections from wounds collected on day 10 after wounding are shown for α-SMA–positive myofibroblasts (A) and for CCN2/CTGF (B). A wound from a decorin-treated mouse was stained with class-matched mouse IgG as a specificity control (no ab). The wounds of mice treated with CAR–DCN showed diminished myofibroblast reaction and CCN2/CTGF protein expression in their skin wounds. The arrows indicate α-SMA–positive smooth muscle cells in the walls of blood vessels in CAR–DCN treated wound. Quantitative analysis of α-SMA–positive myofibroblasts (C) and CCN2/CTGF protein expression (D) in skin wounds at day 10. The statistical significance was examined with ANOVA, *P < 0.05, **P ≤ 0.01, ***P ≤ 0.001. The results are expressed as mean ± SEM, n = 16. (Scale bars, 150 μm.) (C) PBS/BSA control treatment; CAR refers to the CAR peptide.
Discussion
We show here that a wound-targeted, systemic treatment with decorin can significantly reduce scar formation in skin wounds. Our targeting scheme makes use of the uniqueness of regenerating angiogenic blood vessels (19, 37–41) and provides an opportunity for therapeutic manipulation of tissue injury and regeneration.
We show that targeted decorin accumulates in the granulation tissue in the wounded dermis, especially in the bottom of the dermis where angiogenic blood vessels form. The fibroblasts in the bottom layers of a skin wound are the main cell type responsible for the permanent, hypertrophic scar (42), and these cells produce more of the major scar-inducing β1 isoform of TGF-β than cells derived from the other layers of the wound (42). Normally, the production of endogenous decorin is suppressed in these regions that are responsible for the production of permanent scar (42). Thus, our targeting scheme delivers decorin to the regions of the wound that are primarily responsible for scar formation.
A surprising result in our study was that, although decorin binds to all three isoforms of TGF-β (22), CAR–decorin neutralized TGF-β1 and -β2, but was inactive against TGF-β3. The explanation for this selectivity may lie in the heparan sulfate-binding properties of CAR and the TGF-β isoforms. CAR is a heparin-binding peptide (19) and both TGF-β1 and TGF-β2 also bind to heparin, whereas TGF-β3 does not (43, 44). Moreover, heparan sulfate proteoglycan binding potentiates the biological activity of TGF-β1 and TGF-β2 (43, 45). Thus, we suggest that the proximity created by the binding of CAR, TGF-β1, and TGF-β2 to heparan sulfate proteoglycans at the cell surface may enhance the interaction of CAR–decorin with TGF-β1 and TGF-β2, resulting in more effective blocking of TGF-β1 and -β2 than would be the case without the heparan sulfate binding. In this context it is worth noting that latent TGF-β binding proteins 1 and 4, which keep TGF-β in a latent form, are anchored to the cell surface through heparan sulfate binding (46, 47).
The differential neutralizing activity of CAR–decorin against the TGF-β isoforms has important implications for scar formation; TGF-β1 is the isoform responsible for scar formation (5) and TGF-β2 augments the activity of TGF-β1 in inducing scar formation (5). TGF-β3, in contrast, has antiscarring activity (5), demonstrated recently in randomized clinical trials (20). Thus, CAR–decorin could inhibit TGF-β1– and -β2–induced scar formation, while leaving the beneficial TGF-β3 activity untouched (20, 21).
The stimulation of fibroblast growth and extracellular matrix production by TGF-β1 in scar formation is primarily mediated by CCN2 (32–34). To enhance wound fibroblast proliferation and collagen synthesis, as well as myofibroblast transdifferentiation, CCN2 requires the presence of epidermal growth factor (EGF) (32–34). Interestingly, decorin also antagonizes EGF by binding to EGF receptors (25, 26, 48). Thus, wound-targeted decorin, by virtue of being able to block both TGF-β and EGF signaling, may be superior to therapeutic approaches that only inhibit TGF-β. This view is further supported by reports that decorin also inhibits myostatin, an important contributor to scarring in some organs (17, 18). The myostatin inhibition may be, at least in part, due to up-regulation of follistatin expression by decorin, as follistatin is an inhibitor of both myostatin and another scar-inducing TGF-β family member, activin (17, 18). Finally, decorin is likely to be a physiological regulator of scar formation (4, 8), as decorin expression is induced in inflamed tissues (4), a truncated, inactive form of decorin accumulates in hypertrophic scarring and during aging in skin (49, 50), decorin expression is suppressed during scarring (41, 51), and decorin null mice exhibit accentuated scarring (14, 52) and impaired regenerative potential in response to injury (52).
Systemic treatment of injured tissues has obvious advantages: a systemically administered therapeutic agent will reach the site of injury regardless of the location, and will not be exposed to the same extent to the protease-rich, destructive environment prevalent after the injury as a locally applied agent. The increased efficacy of homing peptide-enhanced delivery of a therapeutic agent we have demonstrated here may make systemic treatment of tissue injuries a feasible option. This opens up unique possibilities in traumatology and surgery, as well as in the treatment of tissue injuries that result in permanent scar formation (myocardial infarction, stroke) in general medicine. The CAR peptide also targets tumor vasculature (53) and decorin has antitumor activities (54, 55.). The CAR peptide also targets inflammation in the lungs (53). These findings suggest that CAR-targeted decorin could also have applications in the treatment of cancer and inflammatory conditions that involve TGF-β activity.
Materials and Methods
Fusion Proteins.
The cloning strategy of recombinant decorins is described in detail in SI Materials and Methods. A map of the extended C terminus of the decorin fusion proteins is shown in Fig. 1. The decorin and HSA constructs were expressed in 293-F cells using the FreeStyle 293 expression system from Invitrogen, according to the manufacturer's instructions. The cells were cultured for 48 h and the decorins were isolated from the media on Ni-NTA agarose beads (Qiagen) using 5 mL of beads per 500 mL of media. After an overnight incubation at +4 °C, the beads were washed with PBS, and decorin was eluted with PBS containing 300 mM imidazole, dialyzed against PBS, and stored at −80 °C.
Wound-Healing Model and Treatment Schedule.
All animal experiments were reviewed and approved by the institutional animal care and use committees of Sanford-Burnham Medical Research Institute, La Jolla and University of California at Santa Barbara. Eight-week-old male BALB/c mice (weighing 23–25 g) were anesthetized with 4% isoflurane and 1.5 L/min of oxygen, and the anesthesia was maintained at ≈1.5% isoflurane at 1 L/min of oxygen. Skin was shaved, cleaned, and disinfected with betadine and 70% alcohol. Treatment trials were conducted on mice that had circular, 8-mm diameter, full thickness (including panniculus carnosus muscle) excision wounds in the dorsal skin. The wounds were first marked by a biopsy punch and then cut with scissors. All skin wounds were left uncovered without a dressing.
The treatments were started 3 d after wounding and consisted of daily tail vein injections. The dose for decorins was 40 μg per injection in 150 μL PBS was selected on the basis of previous decorin-treatment studies (8, 24, 25), except that the dose was doubled on days 4–6 to coincide with the expected peak of TGF-β expression in the wounds (5). The dose for CAR–HSA was 65 μg per injection. BSA (40 μg and 80 μg on days 4–6) was used as a control protein. A synthetic CAR peptide (19) was administered at 1 μg (2 μg on days 4–6) per injection. The wounds were inspected and photographed daily, and scored for complete reepithelialization. At the end of the treatment period, the animals were killed, and the wounded tissue collected and processed for analyses.
Cloning and characterization of the recombinant proteins, cell culture, binding assays, TGF-β assays, processing of tissue samples, image analysis, immunohistochemistry, quantification of immuno- and histochemistry, sandwich ELISA, and real-time PCR were performed using standard methods (SI Materials and Methods).
Statistical Analysis.
For comparisons of multiple groups, statistical analysis was carried out by two-way analysis of variance (ANOVA) complemented by the Bonferroni post hoc test for pair wise comparisons between the test groups. Comparison of mRNA expression was analyzed using the Student's paired t test. In the frequency outcome variable (percentage of complete reepithelialization), the groups were compared with the χ2 test. P values <0.05 were considered statistically significant. The significance level shown refers to two-tailed test.
Supplementary Material
Acknowledgments
We thank Drs. Eva Engvall, Kazuki Sugahara, and Hannele Uusitalo-Järvinen for comments on the manuscript; Dr. Venkata Ramana Kotamraju for skillful peptide synthesis; Dr. William P. Sheffield (McMaster University, Hamilton, ON, Canada) for human albumin cDNA; Dr. Gary Grotendorst for help with CCN2/CTGF immunohistochemistry; Dr. Dave Carrino for help with chondroitinase treatment; and Paul Kirsch for help with the manuscript. This work was supported by Grants PO1 CA 82713 and Cancer Center Support Grant CA 30199 from the National Cancer Institute, and DAMD17-02-1-0315 and X81XWH-08-2-0032 from the Department of Defense. T.A.H.J. received support from the Academy of Finland, Sigrid Juselius Foundation, Instrumentarium Research Foundation (both Helsinki, Finland), and Competitive Research Funding of the Pirkanmaa Hospital District, Tampere University Hospital.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016233107/-/DCSupplemental.
References
- 1.Aarabi S, Longaker MT, Gurtner GC. Hypertrophic scar formation following burns and trauma: New approaches to treatment. PLoS Med. 2007;4:e234. doi: 10.1371/journal.pmed.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 4.Border WA, Ruoslahti E. Transforming growth factor-β in disease: The dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunner G, Blakytny R. Extracellular regulation of TGF-β activity in wound repair: Growth factor latency as a sensor mechanism for injury. Thromb Haemost. 2004;92:253–261. doi: 10.1160/TH04-05-0324. [DOI] [PubMed] [Google Scholar]
- 6.Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 8.Border WA, et al. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, et al. Transforming growth factor-β1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weis SM, et al. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005;24:313–324. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Faust SM, Lu G, Wood SC, Bishop DK. TGFβ neutralization within cardiac allografts by decorin gene transfer attenuates chronic rejection. J Immunol. 2009;183:7307–7313. doi: 10.4049/jimmunol.0902736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan W, et al. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-β/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther. 2009;20:1190–1200. doi: 10.1089/hum.2008.204. [DOI] [PubMed] [Google Scholar]
- 13.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj J. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 14.Järveläinen H, et al. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 15.Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ. Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci. 2004;19:1226–1242. doi: 10.1111/j.1460-9568.2004.03184.x. [DOI] [PubMed] [Google Scholar]
- 16.Grisanti S, et al. Decorin modulates wound healing in experimental glaucoma filtration surgery: A pilot study. Invest Ophthalmol Vis Sci. 2005;46:191–196. doi: 10.1167/iovs.04-0902. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, et al. Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther. 2007;15:1616–1622. doi: 10.1038/sj.mt.6300250. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, et al. Relationships between transforming growth factor-β1, myostatin, and decorin: Implications for skeletal muscle fibrosis. J Biol Chem. 2007;282:25852–25863. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- 19.Järvinen TAH, Ruoslahti E. Molecular changes in the vasculature of injured tissues. Am J Pathol. 2007;171:702–711. doi: 10.2353/ajpath.2007.061251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson MW, et al. Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet. 2009;373:1264–1274. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- 21.Bandyopadhyay B, et al. A “traffic control” role for TGFβ3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol. 2006;172:1093–1105. doi: 10.1083/jcb.200507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hildebrand A, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988;336:244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan MM, et al. Matricellular hevin regulates decorin production and collagen assembly. J Biol Chem. 2006;281:27621–27632. doi: 10.1074/jbc.M510507200. [DOI] [PubMed] [Google Scholar]
- 25.Seidler DG, et al. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 26.Goldoni S, et al. An antimetastatic role for decorin in breast cancer. Am J Pathol. 2008;173:844–855. doi: 10.2353/ajpath.2008.080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiedler LR, et al. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of α2β1 integrin activity. J Biol Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 28.Ashcroft GS, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 29.Lucas T, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 30.Goren I, et al. A transgenic mouse model of inducible macrophage depletion: Effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–147. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. FASEB J. 2004;18:469–479. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 33.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 34.Leask A, Abraham DJ. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 35.Midwood K, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 36.Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 37.Friedlander M, et al. Involvement of integrins α v β 3 and α v β 5 in ocular neovascular diseases. Proc Natl Acad Sci USA. 1996;93:9764–9769. doi: 10.1073/pnas.93.18.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St Croix B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 39.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 40.Christian S, et al. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Dodd C, Shankowsky HA, Scott PG, Tredget EE Wound Healing Research Group. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest. 2008;88:1278–1290. doi: 10.1038/labinvest.2008.101. [DOI] [PubMed] [Google Scholar]
- 43.Lyon M, Rushton G, Gallagher JT. The interaction of the transforming growth factor-βs with heparin/heparan sulfate is isoform-specific. J Biol Chem. 1997;272:18000–18006. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- 44.Rider CC. Heparin/heparan sulphate binding in the TGF-β cytokine superfamily. Biochem Soc Trans. 2006;34:458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 45.Iwao K, et al. Heparan sulfate deficiency leads to Peters anomaly in mice by disturbing neural crest TGF-β2 signaling. J Clin Invest. 2009;119:1997–2008. doi: 10.1172/JCI38519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q, et al. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-β (TGF-β) by modulating assembly of latent TGF-β-binding protein-1. J Biol Chem. 2007;282:26418–26430. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]
- 47.Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-β binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Iozzo RV, Moscatello DK, McQuillan DJ, Eichstetter I. Decorin is a biological ligand for the epidermal growth factor receptor. J Biol Chem. 1999;274:4489–4492. doi: 10.1074/jbc.274.8.4489. [DOI] [PubMed] [Google Scholar]
- 49.Carrino DA, et al. Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J Biol Chem. 2003;278:17566–17572. doi: 10.1074/jbc.M300124200. [DOI] [PubMed] [Google Scholar]
- 50.Carrino DA, et al. Age-related differences in human skin proteoglycans. Glygobiol. 2010 doi: 10.1093/glycob/cwq162. 10.1093/glycob/cwq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayani K, et al. Delayed appearance of decorin in healing burn scars. Histopathology. 2000;36:262–272. doi: 10.1046/j.1365-2559.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 52.Baghy K, et al. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 2010 doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urakami T, et al. Peptide-directed highly selective targeting of pulmonary arterial hypertension. Am J Pathol. 2010 doi: 10.1016/j.ajpath.2011.02.032. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldoni S, Iozzo RV. Tumor microenvironment: Modulation by decorin and related molecules harboring leucine-rich tandem motifs. Int J Cancer. 2008;123:2473–2479. doi: 10.1002/ijc.23930. [DOI] [PubMed] [Google Scholar]
- 55.Iozzo RV, Schaefer L. Proteoglycans in health and disease: Novel regulatory signaling mechanisms evoked by the small leucine-rich proteoglycans. FEBS J. 2010;277:3864–3875. doi: 10.1111/j.1742-4658.2010.07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.