Abstract
Phytochelatins mediate tolerance to heavy metals in plants and some fungi by sequestering phytochelatin-metal complexes into vacuoles. To date, only Schizosaccharomyces pombe Hmt1 has been described as a phytochelatin transporter and attempts to identify orthologous phytochelatin transporters in plants and other organisms have failed. Furthermore, recent data indicate that the hmt1 mutant accumulates significant phytochelatin levels in vacuoles, suggesting that unidentified phytochelatin transporters exist in fungi. Here, we show that deletion of all vacuolar ABC transporters abolishes phytochelatin accumulation in S. pombe vacuoles and abrogates 35S-PC2 uptake into S. pombe microsomal vesicles. Systematic analysis of the entire S. pombe ABC transporter family identified Abc2 as a full-size ABC transporter (ABCC-type) that mediates phytochelatin transport into vacuoles. The S. pombe abc1 abc2 abc3 abc4 hmt1 quintuple and abc2 hmt1 double mutant show no detectable phytochelatins in vacuoles. Abc2 expression restores phytochelatin accumulation into vacuoles and suppresses the cadmium sensitivity of the abc quintuple mutant. A novel, unexpected, function of Hmt1 in GS-conjugate transport is also shown. In contrast to Hmt1, Abc2 orthologs are widely distributed among kingdoms and are proposed as the long-sought vacuolar phytochelatin transporters in plants and other organisms.
Keywords: ABC Transporter, HPLC, Sulfur, Xenobiotics, Yeast, Cadmium, Fission Yeast, GS-bimane Conjugates, High Molecular Weight Complexes.
Introduction
Heavy metal contamination is a serious worldwide environmental problem caused by almost two centuries of intense industrial and mining activities, combined with an inappropriate disposal of residual waste (1, 2). Detrimental effects of heavy metals on human health may arise from occupational exposure, food intake and long-term exposure to metals in the environment, and have been linked to diabetes, hypertension, myocardial infarction, diminished lung function and certain types of cancer (3, 4). Non-essential heavy metals such as cadmium (Cd), lead (Pb), and mercury (Hg) interfere with the function of essential metals, including copper, zinc and manganese, by displacing them from their functional binding sites in proteins, thus interfering with biochemical and physiological functions (for reviews see Refs. 5–7). Because of the intrinsically high reactivity of heavy metals, exposure to elevated concentrations of both essential and non-essential metals impairs metabolism. Therefore, organisms have developed mechanisms to sense, transport, and mobilize essential metals, and other mechanisms to detoxify non-essential heavy metals (5–8). One of the most studied mechanisms mediating heavy metal detoxification involves phytochelatins, which are present in plants, algae, Schizosaccharomyces pombe and, surprisingly, Caenorhabditis elegans (5, 6, 9–13).
Phytochelatins (PCs)4 are glutathione-derived peptides synthesized in the cytosol by the enzyme phytochelatin synthase (14–16). In plants and S. pombe, cytosolic phytochelatin-metal complexes are transported into vacuoles (17–20). In plants, PCs can also undergo long distance transport between shoots and roots (21–23) and this transport affects the shoot-to-root distribution of Cd. In the case of plants and S. pombe, PC-Cd complexes inside the vacuole bind sulfide and free Cd2+, forming high molecular weight complexes (HMWCs) that surround cadmium-sulfur clusters, providing enhanced stability and vacuolar Cd accumulation capacity (24–27). Cd2+, as a free ion, can also be transported into vacuoles by vacuolar Ca2+/H+ antiporters (19, 28, 29). As for phytochelatin transporters, early studies in isolated vacuoles indicated that PC transport was mediated by ABC-type transporters (18, 19). Furthermore, a vacuolar half-size ABC transporter required for Cd tolerance, Hmt1 (heavy metal tolerance 1), was proposed to function as the S. pombe vacuolar PC transporter (19, 26).
For more than 10 years the role of Hmt1 in Cd tolerance was undisputed, but attempts to identify Hmt1 orthologs in plants were unsuccessful (30, 31). This was particularly intriguing after the completion of the Arabidopsis genome project, where it was clear that this reference plant lacks an Hmt1 ortholog, but retains the capacity to sequester PCs and Cd in vacuoles to form HMWCs (30, 32). Hmt1-like proteins are not abundant in nature, being restricted to a limited number of organisms, and their function is not completely understood (33). The closest Hmt1 homologues in Arabidopsis are the ATM transporters localized in the mitochondrion (30, 31, 33, 34). ATM3 mediates the maturation of iron-sulfur clusters (35) and functions in the synthesis of molybdenum cofactor (36). Interestingly, overexpression of ATM3 in Arabidopsis enhances cadmium resistance (37).
The first evidence indicating that Hmt1 may have a role in Cd tolerance other than PC transport came from work in C. elegans, where PC synthase and Hmt1 homologues were identified (12, 13, 38). RNAi studies, and more recently deletion analyses, revealed that the C. elegans Hmt1 and PC synthase have an additive effect on Cd tolerance, suggesting that Hmt1 and PC synthase do not function in a simple linear pathway (38, 39). In addition, an HMT1 protein from the non-PC producing Drosophila melanogaster was able to rescue the Cd sensitivity of the S. pombe hmt1 mutant (33). Conversely, S. pombe Hmt1 conferred tolerance to Cd in Escherichia coli and Saccharomyces cerevisiae, organisms devoid of phytochelatins (40). Furthermore, recent data showed that the S. pombe hmt1 mutant accumulates significant levels of PCs in vacuoles (33), suggesting that other, yet unidentified proteins mediate PC transport into vacuoles. Altogether these results might explain why the search for a Hmt1-related PC transporters in other organisms has been futile and re-opens the original question regarding the identity of vacuolar PC uptake transporters.
Here we report that Abc2, a full-size ABC transporter of the MRP/ABCC subfamily, mediates accumulation of PCs in S. pombe vacuoles. Systematic analyses of ABC transporter deletion mutants, Cd sensitivity assays, complementation experiments, PC content, and HMWCs in purified vacuoles demonstrate that Abc2 mediates PC accumulation into vacuoles. We further show a new role for Hmt1 in GS-conjugate transport. The identification of Abc2 as a PC transporter expands the current model of Cd tolerance mediated by PCs and opens the possibility to identify the long-sought PC transporters in plants and other organisms. These transporters, in combination with other genes, hold the potential to enhance the heavy metal tolerance and accumulation capacity of organisms for their use in the bioremediation of soils and waters contaminated with heavy metals, or to circumvent the accumulation of non-essential metals in the edible parts of crop plants.
EXPERIMENTAL PROCEDURES
Yeast Strains and Growth Conditions
The S. pombe strains used are described in supplemental Table S1. Deletion mutants were constructed using standard techniques (41). For Cd sensitivity assays, S. pombe wild type (h- ura4-D18 leu1–32) and mutants were grown in liquid YES (yeast extract with supplements) medium and 1:10 serial dilutions were spotted on YES plates containing 5–100 μm CdCl2 as described elsewhere (42). The S. cerevisiae strain Sm14 (MATa, ura3, leu2, his3, trp3, lys2, suc2, ycf::hisG, yhl035c::Leu3, yll015w::Kan-MX6, yll048c:: TRP1-MX6) was used to generate a PC-synthase integration strain (Sm15) by inserting the TaPCS1 coding sequence under the control of the CUP1–1 promoter (TaPCS1::cup1–1).
Cloning of Hmt1, Abc2, and Abc4 for Complementation Experiments
The Abc2 coding sequence was amplified from genomic DNA using the following primers (Fw 5′-agggaatattaagcttatgaattctgcttacaaaggattcacattt-3′, Rv 5′- ttactagtggatccgagctctcaaataagtccactctcttttgcgaggga-3′). pRep41, but not the Abc2 PCR fragment, was digested with SalI and BamHI to prevent self-ligation and used to directly recombine Abc2 into pRep41 using the In-FusionTMcloning system (Clontech). All cloned genes were confirmed by sequencing. SpAbc4 was amplified from S. pombe genomic DNA using the following primers (Fw 5′-caccatggaaaactttcaccatcgtccgtttaaa-3′, Rv 5′-tcatgctgatttcgaacctcgagtttcagg-3′) and cloned into pENTR/D-TOPOTM(Invitrogen). SpAbc4 was recombined into a pRep41 GatewayTM-compatible vector (pRep41-GW) using LR clonase II (Invitrogen). pRep41 and pRep41-YFP were modified to be GatewayTM-compatible (pRep41-GW and pRep41-GW-YFP) by inserting a GatewayTM cassette into the SmaI site, followed by ligation with T4 DNA ligase as recommended by the manufacturer (Invitrogen). For Abc2 localization studies, S. pombe Abc2 coding sequence was amplified from genomic DNA using the following primers (Fw 5′-caccatgaattctgcttacaaaggattcacattt-3′, Rv 5′-aataagtccactctcttttgcgagggaata-3′) and cloned into pENTR/D-TOPOTM following the manufacturer's recommendations (Invitrogen). The resulting plasmid, pENTR-Abc2 was recombined into pRep41-GW-YFP using the LR clonase II reaction (Invitrogen) to generate pRep41-Abc2-YFP. The Hmt1 coding sequence was amplified by PCR from cDNA using the following primers (Fw 5′-ggggacaagtttgtacaa aaaagcaggcttcaccatggttctacgttacaacagcccac-3′,Rv 5′-ggggaccactttgtacaagaaagctgggtcttaatgagtttcagcagaagttttt-3′, att sites are marked in bold) and recombined into pDONR221 through a BP clonase II reaction (Invitrogen). pDONR221-Hmt1 was then used to transfer Hmt1 into pRep41-GW using LR clonase II (Invitrogen). For Hmt1 expression in S. cerevisiae, pDONR221-Hmt1 was recombined into pYES-DEST52 (Invitrogen) using LR Clonase II (Invitrogen)(43).
For complementation experiments, S. pombe wild type, hmt1 and the quintuple mutant abc1 abc2 abc3 abc4 hmt1 (abc1–4hmt1) were transformed with pRep41, pRep41-Hmt1, pRep41-Abc2 or pRep41-Abc4 using the lithium-acetate method (44) and transformants were selected on Edinburgh minimal medium plates, without leucine, plus supplements (adenine, uracil, histidine, 225 mg/liter each) (EMMS without Leu). Independent transformants were grown on liquid EMMS without Leu medium and 1:10 serial dilutions were spotted on selective plates containing 5–100 μm CdCl2. For thiamine repression experiments, 50 μm of thiamine was added to the liquid culture to repress the nmt1-driven gene expression. For expression of Hmt1 in S. cerevisiae, Sm14 and Sm15 strains were transformed with pYES-DEST52 (Invitrogen) or pYES-DEST52-Hmt1 using the lithium-acetate method, and transformants were selected on dextrose-containing YNB-Ura medium. For Cd exposure experiments, yeast were grown on liquid galactose/raffinose YNB-Ura medium, supplemented with 50 μm of CuCl2 to induce TaPCS1 expression, and serial dilutions (1:10) were spotted on galactose/raffinose selective plates containing 5–1000 μm CdCl2. Expression of Hmt1, Abc1, Abc2, Abc3, and Abc4 during Cd exposure was examined using publicly available microarrays (43).
GS-bimane and Abc2-YFP Confocal Imaging
S. pombe transformed with pRep41, pRep41-Hmt1, pRep41-Abc2 or pRep41-Abc4 were grown on EMMS without Leu medium to an A600 nm of 0.5–1.0 and 50 μm of monochlorobimane (Sigma-Aldrich), dissolved in dimethylformamide, was added to the medium, and cultures were incubated overnight. The following morning, cells were washed twice with Milli-Q water (Millipore), mixed 1:1 with 0.1% low melting agarose (Sigma) and placed on microscope slides. GS-bimane fluorescence imaging was done with a Nikon TE-200U microscope equipped with a Yokogawa Nipkow spinning disk confocal head, Chroma HQ480/40 band-pass emission filter and a Roper CascadeII 512b EM CCD camera, using the violet-blue excitation line. For YFP imaging, S. pombe cells were transformed with pRep41-Abc2-YFP and grown on EMMS medium without leucine. Cells in log phase were mixed 1:1 with 0.1% low melting agarose, placed on microscope slides and excited with an argon laser (488 nm). The YFP signal was detected using a Chroma HQ525/50 band-pass emission filter. Images were processed using Image J. (http://rsbweb.nih.gov/ij).
Gel Filtration Chromatography for HMWC Detection in Cell Extracts
HMWCs in cell extracts were separated by size exclusion chromatography using a Superdex 75 10/300 GL column equilibrated with 20 mm Tris pH 7.7 (GE Healthcare) and a BioLogic DuoFlow FPLC system (Bio-Rad) at flow rate of 1 ml/min. S. pombe cells were grown on YES liquid medium to a A600 nm 1.0 and then exposed to 200 μm CdCl2 for 20 h. Cells were harvested by centrifugation, washed twice with 20 mm Tris, pH 7.7, and broken with glass beads. Cell debris was eliminated by centrifugation (20 000 × g, 10 min) and the supernatant was cleared using 0.45 μm filters (Millipore). 250 μl of sample (0.25–0.5 mg of protein/ml) were applied to the column and the absorbance at 254 nm was recorded. Molecular weights were estimated using the Gel Filtration LMW Calibration Kit (GE Healthcare), and chromatograms were normalized using the protein concentration of the extracts determined with the Bradford reagent (Sigma-Aldrich).
Vacuole Isolation and Vacuolar PC and HMWC Measurements
For the isolation of intact vacuoles for subsequent PC and HMWC analyses, S. pombe cells were cultured in EMMS selective medium supplemented with or without Cd as described (33). Briefly, 200-ml volumes of stationary phase cultures were diluted into 1.5 liters of EMMS and grown for 4–6 h at 30 °C after which time CdCl2 was added to a final concentration of 500 μm to activate PC production. Cells were cultured in the presence of CdCl2 for an additional 18 h, collected by centrifugation, converted to spheroplasts, disrupted and subjected to fractionation by differential centrifugation as described (33). PC content was analyzed in intact vacuoles by HPLC, followed by thiol quantification with Ellman's reagent as described (45). For PC analyses in cell extracts, S. pombe cells were cultured in EMM medium to stationary phase and diluted 5-fold with fresh EMM medium before adding CdCl2 (200 μm) to activate PC synthesis. After 18–24h of culturing at 30 °C with shaking, cells were collected by centrifugation at 4,000 × g for 5 min and subjected to two rounds of re-suspension/centrifugation in water and then in lysis buffer containing 50 mm Tris-HCl, pH 7.8, 10 mm 2-mercaptoethanol and a mixture of protease inhibitors (pepstatin, aprotinin, and leupeptin, 1 μg/ml each and PMSF 1 mm). After the final centrifugation, cells were broken by sonication at 4 °C in the same lysis buffer and cell debris was cleared by low-speed centrifugation at 3,500 × g for 10 min. PCs were analyzed in the supernatants by reverse-phase (RP)-HPLC on a Microsorb-MV 100–5 C18, 150 × 4.6-mm HPLC column (Varian, Inc) followed by thiol quantification with Ellman's reagent as previously described (45). HMWC's in intact vacuoles were analyzed by gel-filtration FPLC using an AKTA purifier FPLC system (GE Healthcare). Aliquots of vacuoles (100 μg protein) were injected onto a Superdex peptide 10/300GL column (GE Healthcare) equilibrated with 50 mm Tris-HCl, pH7.8. The column was developed with the same buffer at a flow rate of 0.5 ml/min. Fractions (1 ml) were collected, reacted with Ellman's reagent and analyzed spectrophotometrically at 412 nm as described (45). The elution profiles of protein markers including cytochrome c (Mr 12,500), aprotinin (Mr 6,512), and vitamin B12 (Mr 1,355) (GE Healthcare) were analyzed using the same conditions, and detected in collected fractions using the UV detector of the FPLC system.
A LMWC standard (a mixture of different chain length PCs) was synthesized in vitro using purified AtPCS1-FLAG (45). HMWC standards were produced from LMWC by addition of CdCl2 (500 μm final) and Na2S (10-fold molar access to PCs in LMWC). In vitro-synthesized LMWC and HMWC were separated by FPLC as described above.
35S-PC2 Synthesis and Uptake Assays
35S-PC2 was synthesized using [35S]GSH (Perkin-Elmer) and recombinant AtPCS1. 35S-PC2 synthesis was carried out in 100 mm Tris (pH 8.0) containing 100 μm CdCl2, 3 mm GSH, [35S]GSH (30 μl, 60 μCi, 2.22 MBq) and 5 μg of AtPCS1-His6 for 1 h at 37 °C. The reaction (0.1 ml) was stopped by adding 10 μl of perchloric acid 30% (v/v), mixed vigorously for 1 min and centrifuged 20,000 × g x10 min. The supernatant was filtered using a 0.22 μm Ultrafree-MC filtering device (Millipore), and the flow-through was used for phytochelatin purification, as previously described (23). Purified 35S-PC2 was resuspended in 100 mm Tris (pH 8.0), 500 μm DTT, and 500 μm PC2 (non-radoactive, Anaspec). Unless otherwise noted, 0.025 μCi of 35S-PC2 were used for transport assays using S. pombe microsomal vesicles prepared as described previously for S. cerevisae (46).
RESULTS
Hmt1 and PC Synthase Have Additive Effects on Cd Tolerance
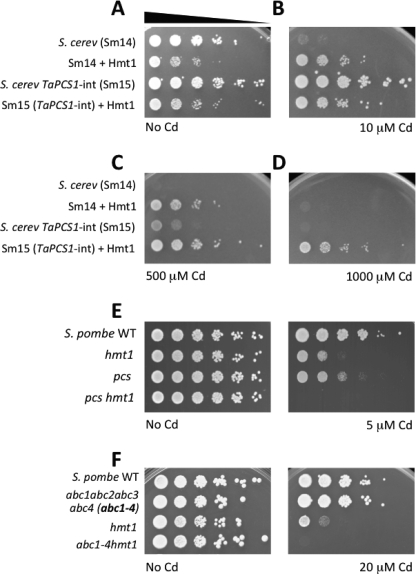
Expression of S. pombe hmt1+ or pcs+ cDNAs in S. cerevisiae dramatically increases Cd tolerance (15, 16, 40). To test whether Hmt1 and PC synthase have an additive effect on Cd tolerance in yeast, we expressed S. pombe hmt1+ in a S. cerevisiae strain engineered to synthesize phytochelatins (strain Sm15). S. cerevisiae strain Sm14, the parental strain of Sm15, lacks 4 ATP-binding cassette transporters (YDR135C, YHL035C, YLL015W, YLL048C) that mediate metal tolerance and is Cd hypersensitive (Fig. 1, A and B), thus providing a suitable background to study proteins that mediate metal tolerance. Expression of hmt1+ in the absence of Cd reduced the fitness of both S. cerevisiae strains (Fig. 1A). In the presence of Cd, however, hmt1+ increased the Cd tolerance of the Sm14 yeast strain (Fig. 1, B and C). Integration of the Triticum aestivum PCS1 gene (TaPCS1) into the yeast genome increased the Cd tolerance of the Sm15 strain compared with Sm14 (Fig. 1, B and C). The reduction in fitness induced by Hmt1 was also observed at low Cd concentration (10 μm) in the Sm15, TaPCS1-expressing strain (Fig. 1B). However, heterologous expression of hmt1+ increased the Cd tolerance of the PCS integration strain even further compared with Sm14 (Fig. 1, C and D). Co-expression of hmt1+ and TaPCS1 (Sm15 + Hmt1) allowed the Cd-sensitive strain to tolerate remarkably high concentrations of Cd (up to 1 mm, Fig. 1D). These results suggest that Hmt1 and PC synthase have additive effects on Cd detoxification. To test whether Hmt1 and PC synthase also have additive effects on Cd tolerance in S. pombe, we generated an hmt1 pcs double mutant and compared its Cd sensitivity to hmt1 and pcs single mutants. Growth of the hmt1 pcs double mutant strain was more sensitive to Cd compared with the single hmt1 or pcs deletion mutants (Fig. 1E), suggesting that in S. pombe Hmt1 and PC synthase do not act in a single linear pathway but have additive effects.
FIGURE 1.
Cadmium tolerance mediated by Hmt1, PC synthase and ABC transporters. A–D, Hmt1 and PC synthase have additive effects on cadmium tolerance in S. cerevisiae and S. pombe. A, S. cerevisiae strain Sm14 lacks 4 ABC transporters (ydr135C/ycf1, yhl035c, yll015w, yll048c) and (B) is Cd hypersensitive. Integration of TaPCS1 in Sm14 (strain Sm15) rescues the Cd sensitivity (B and C). Heterologous expression of S. pombe Hmt1 enhances Cd tolerance in both Sm14 and Sm15 strains (panels B and C). D, co-expression of Hmt1 and PC synthase (Sm15 + Hmt1) dramatically increases the Cd tolerance compared with yeast expressing either PC synthase or Hmt1 (1000 μm panel). E, deletion of hmt1+ or pcs+ in S. pombe results in Cd sensitivity and deletion of both genes (pcs hmt1) exhibits an additive negative effect on Cd tolerance. F, vacuolar full-size ABC transporters function in cadmium tolerance in S. pombe. Deletion of all vacuolar ABC transporters (Abc2, Abc3, Abc4, and Hmt1) induces Cd hypersensitivity compared with hmt1. Abc1 is part of the Cluster II.1 of S. pombe ABC transporters, together with Abc2, Abc3, and Abc4, but Abc1-GFP fusions show a pattern typical of endoplasmic reticulum (47).
ABC-type Transporters Contribute to Cd Tolerance in S. pombe
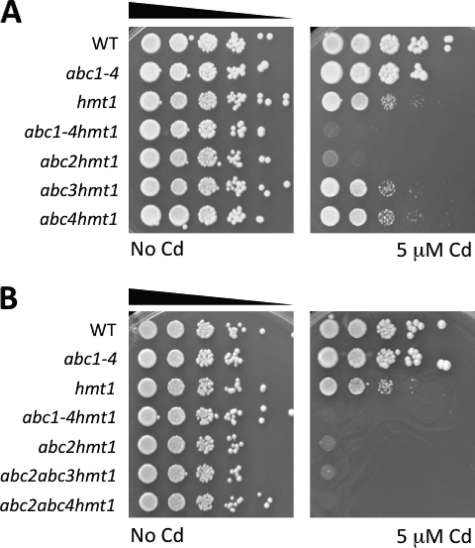
PC transport into vacuoles was thought to be mediated solely by Hmt1 (19). However, recent data demonstrate that PCs can be transported into vacuoles in an Hmt1-independent manner by a yet unidentified transporter (33). PC transport into vacuoles displays biochemical properties typical of ABC-type transporters (ATP-dependent, inhibited by vanadate but insensitive to uncouplers) (18, 19). Of the 11 ABC transporters encoded in the S. pombe genome, only Hmt1, Abc2, Abc3, and Abc4 localize to the vacuolar membrane, as determined by GFP-protein fusions (47). Abc1 is part of the Cluster II.1 of S. pombe ABC transporters (Abc1, Abc2, Abc3, and Abc4), but Abc1 localizes to the cell perimeter (47). To determine whether ABC transporters, other than Hmt1, contribute to vacuolar PC accumulation, and hence to Cd tolerance, we generated a S. pombe mutant strain lacking all vacuolar ABC transporters and Abc1, and assessed its Cd sensitivity. The abc1 abc2 abc3 abc4 hmt1 quintuple mutant (abc1–4 hmt1) was found to be extremely Cd sensitive, compared with hmt1 (Fig. 1F). The abc1 abc2 abc3 abc4 quadruple mutant did not exhibit substantial Cd sensitivity, likely due to the presence of a functional Hmt1 (Fig. 1F; and data presented further below). Nevertheless, the dramatic sensitivity of the abc1–4 hmt1 quintuple mutant compared with hmt1 (Fig. 1F) suggested that full-size ABC transporters also mediate Cd tolerance. In contrast, pdr1 bfr1, a double mutant of a different clade of S. pombe ABC transporters (Cluster I), did not show any Cd sensitivity (supplemental Fig. S1A). Analyses of publicly available microarray data show that none of the vacuolar ABC transporters are significantly (>2-fold) induced by Cd exposure (supplemental Fig. S1B). To analyze the possibility that vacuolar morphology could be altered by the lack of vacuolar ABC transporters, thus causing Cd sensitivity, we analyzed the integrity of the vacuolar membrane using the fluorescent dye FM4–64. All mutants analyzed showed wild type-like vacuoles suggesting that Cd sensitivity of the abc1–4 hmt1 quintuple mutant was not due to changes in vacuolar morphology (supplemental Fig. S1C).
To establish which of the full-length ABC transporters mediate Cd tolerance, we systematically analyzed different combinations of abc mutants using the hmt1 background. The abc2 hmt1 double mutant strain was found to exhibit the most dramatic Cd sensitivity, while the Cd tolerance of abc3 hmt1 and abc4 hmt1 was no different from the hmt1 single mutant (Fig. 2A). However, the abc2 hmt1 double mutant strain was slightly less Cd sensitive than the abc1–4 hmt1 quintuple mutant, suggesting that other members of the Cluster II.1 of S. pombe ABC transporters could play a minor role in conferring Cd tolerance (Fig. 2A). To identify which ABC transporter mediated this residual Cd tolerance, we generated triple mutants and evaluated their Cd tolerance. The triple mutant abc2 abc4 hmt1 was found to exhibit identical Cd sensitivity compared with the abc1–4 hmt1 quintuple mutant suggesting that, among the full-length vacuolar ABC transporters, Abc2 is the major contributor to Cd tolerance followed by Abc4 (Fig. 2B).
FIGURE 2.
Systematic deletion of vacuolar ABC transporters reveals the function of the vacuolar transporter Abc2 in cadmium tolerance. A, Cd sensitivity of ABC transporter mutants in the hmt1 background was compared with the single hmt1 mutant. The abc2 hmt1 deletion mutant showed the strongest effect on Cd sensitivity compared with abc3 hmt1 and abc4 hmt1 mutants. B, cadmium sensitivity of triple abc hmt1 mutants showed that Cd sensitivity of abc2 abc4 hmt1 is identical to the quintuple abc1–4 hmt1 mutant. Yeast strains were grown in rich medium (YES + supplements), and 1:10 serial dilutions were spotted onto plates containing 5 μm CdCl2.
Abc2 Mediates PC Accumulation in Vacuoles
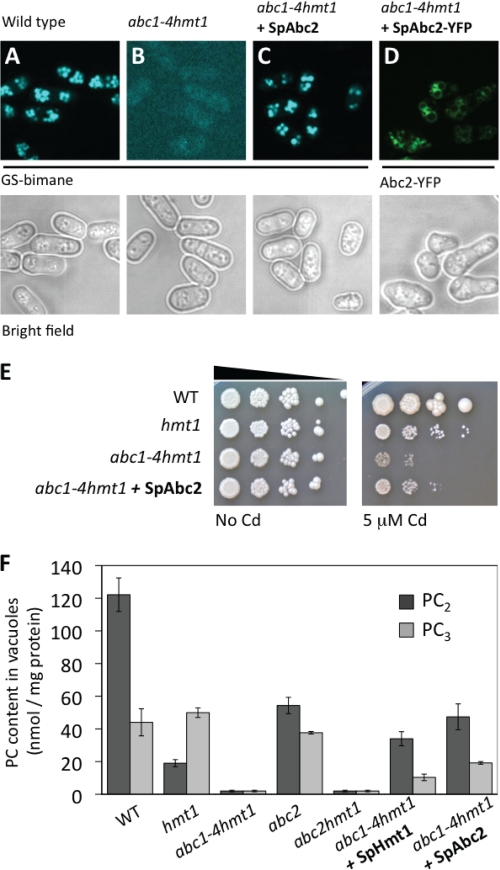
To directly determine the function of Abc2 in Cd tolerance, the coding sequence of Abc2 (abc2+) was cloned into pREP41 (pREP41 and pREP41-GW-YFP) to evaluate intracellular localization, Cd tolerance and PC accumulation in vacuoles. To confirm that Abc2 was functionally active when expressed in the abc1–4 hmt1 quintuple mutant, we evaluated the accumulation of glutathione-bimane conjugates (GS-bimane) into vacuoles. Monochlorobimane is a permeable dye that forms a fluorescent impermeable compound (GS-bimane) when conjugated with glutathione in the cytosol by glutathione S-transferases. GS-bimane is actively transported into vacuoles by ABC-type transporters and it is visualized as intense fluorescent bodies under UV excitation (Fig. 3A) (33, 47, 48). In S. pombe, Abc2 and Abc4 are required for vacuolar accumulation of GS-X conjugates (47), thus providing a good test for evaluating functional activity of the recombinant protein. The abc1–4 hmt1 quintuple mutant was unable to compartmentalize GS-bimane conjugates into vacuoles and displayed only residual fluorescence from cytosolic GS-bimane conjugates (Fig. 3B). GS-bimane conjugates, although fluorescent, are not accumulated to high levels in the cytosol, possibly because of product inhibition of cytosolic glutathione S-transferases by GS-bimane. Expression of Abc2 in the abc1–4 hmt1 quintuple mutant was sufficient to restore GS-bimane fluorescence in vacuoles (Fig. 3C), and cells expressing Abc2-YFP fusions displayed fluorescence at the vacuolar membrane (Fig. 3D), demonstrating that Abc2, expressed from pREP41, was functional and properly localized. Moreover, expression of Abc2 enhanced the Cd tolerance of the abc1–4 hmt1 quintuple mutant (Fig. 3E).
FIGURE 3.
The vacuolar transporter Abc2 rescues the cadmium sensitivity of the S.pombe abc1–4 hmt1 quintuple mutant and restores phytochelatin accumulation in vacuoles. A and B, GS-bimane accumulation was used to assess the function of Abc2. GS-bimane conjugates are accumulated in wild-type vacuoles but not in the abc1–4 hmt1 quintuple mutant. C, expression of Abc2 in the abc1–4 hmt1 quintuple mutant restored GS-bimane accumulation in vacuoles. D, S. pombe cells expressing Abc2-YFP displayed fluorescence characteristic of the vacuolar membrane. Accumulation of GS-bimane in vacuoles and the Abc2-YFP fluorescent pattern demonstrates that Abc2, expressed from pRep41, was functional and properly localized. E, expression of Abc2 in the abc1–4 hmt1 quintuple mutant reduced the Cd sensitivity of the abc1–4 hmt1 quintuple mutant. F, PC accumulation in vacuoles was decreased in the hmt1 mutant and abolished in the abc1–4 hmt1 quintuple and the abc2 hmt1 double mutant. Expression of Abc2 in the abc1–4 hmt1 quintuple mutant restored the accumulation of PCs in vacuoles. Hmt1 also restored vacuolar accumulation of PCs.
To determine whether full-size ABC transporters mediate PC accumulation in vivo, we analyzed the PC content in highly purified vacuoles. Vacuoles isolated from wild-type cells exposed to Cd showed high levels of PCs (Fig. 3F). The hmt1 mutant strain showed a reduction in PC2 content but the PC3 content remained unaffected. Likewise, the abc2 mutant showed a significant reduction in PC2 content. In contrast, vacuoles isolated from either the abc1–4 hmt1 quintuple or abc2 hmt1 double mutant showed undetectable levels of either PC2 or PC3 (Fig. 3F). Expression of Abc2 restored accumulation of both PC2 and PC3 in abc1–4 hmt1 vacuoles, demonstrating that Abc2 is sufficient to mediate PC accumulation in S. pombe vacuoles in vivo (Fig. 3F). Expression of Hmt1 also restored the accumulation of PCs in vacuoles (Fig. 3F) and, as previously reported, Cd tolerance (26) (supplemental Fig. S2). These results suggest that both Hmt1 and Abc2 mediate vacuolar accumulation of PCs. One central implication of this finding is that, in contrast to Hmt1, plants have Abc2 homologues (MRP/ABCC subfamily of ABC-type transporters), providing a new avenue to address the long-standing problem of how PCs are sequestered into plant vacuoles.
To further analyze whether ABC transporters mediate PC2 uptake, radiotracer experiments using 35S-PC2 were performed using microsomal vesicles obtained from wild type, hmt1 and the abc1–4 hmt1 quintuple mutant strains. PC transport in wild type vesicles was ATP-driven (34.2 ± 0.1 pmol PC2/(mg prot × min)−1, n = 3 ± S.E.), 70% inhibited by 1 mm vanadate (10.9 ± 3.4 pmol PC2/(mg prot × min)−1, n = 3 ± S.E.) and not abrogated by the uncoupler NH4Cl (5 mm, 24.36 ± 5.33 pmol PC2/(mg prot × min)−1, n = 3 ± S.E.). All these properties reflect an ABC transporter-mediated PC uptake activity. Vesicles obtained from the hmt1 single mutant showed a reduced but substantial uptake of both PC2 and PC2-Cd, which was dramatically reduced in the abc1–4 hmt1 quintuple mutant (supplemental Fig. S3), suggesting that full-size ABC transporters, in parallel to the half-size ABC transporter Hmt1, mediate phytochelatin transport into S. pombe vacuoles.
Expression of Abc4 in the abc1–4 hmt1 quintuple mutant also restored GS-bimane accumulation in vacuoles (supplemental Fig. S4A), but did not noticeably enhance the Cd tolerance of the abc1–4 hmt1 quintuple mutant (supplemental Fig. S4B). In addition and in contrast to the abc1–4 hmt1 quintuple mutant, hmt1 and abc2 hmt1 mutants showed GS-bimane accumulation in vacuoles, suggesting overlapping functions of this clade of ABC transporters in vacuolar GS-bimane uptake (supplemental Fig. S4C).
PC Content and HMWCs in S. pombe abc Mutants
To analyze the possibility that the lack of vacuolar PCs in the abc1–4 hmt1 quintuple mutant was caused by a lack of PC synthesis, we quantified the total PC content in whole cell extracts obtained from the different abc and hmt1 mutants and complemented strains. All strains show detectable levels of PCs in cell extracts, suggesting that PC synthesis remained functional (supplemental Fig. S5A). However, the hmt1, abc2 hmt1, and abc1–4 hmt1 mutant strains showed a decrease in PC content, suggesting that the absence of Hmt1 and ABC transporters partially impairs PC synthesis or degradation to different extents (supplemental Fig. S5A). Expression of either Hmt1 or Abc2 in the abc1–4 hmt1 quintuple mutant partially restored the PC content in cell extracts compared with wild type (supplemental Fig. S5A).
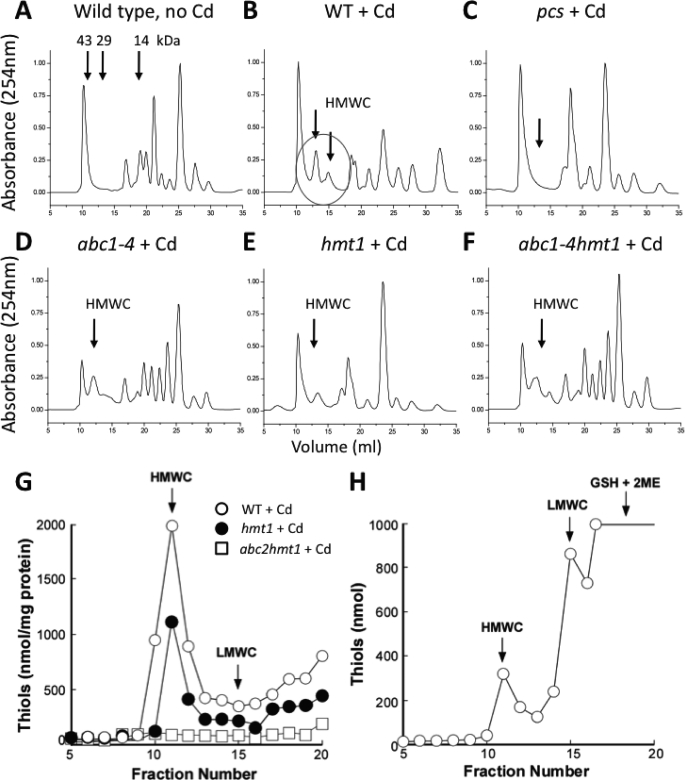
Cadmium, PCs, and sulfide form high molecular weight complexes (HMWCs) surrounding cadmium-sulfur cores (CdS), and these complexes are considered the final step in Cd sequestration inside vacuoles (25, 27). The inability to form HMWCs results in Cd sensitivity (49). To determine whether formation of HMWCs was impaired in the abc1–4 hmt1 mutant, HMWCs were analyzed by size-exclusion chromatography in cell extracts obtained from Cd-exposed cells (Fig. 4, A and B). As described elsewhere, HMWCs were mainly composed of PCs, as detected by HPLC-MS (supplemental Fig S5B) and, as expected, the S. pombe PC-deficient mutant (pcs) was unable to synthesize HMWCs (Fig. 4C). Unexpectedly, all S. pombe abc mutants, including hmt1, show peaks corresponding to HMWCs (Fig. 4, D–F). To determine whether these HMWC were inside the vacuole or located in the cytosol, HMWCs were further analyzed in vacuole preparations obtained from the abc mutants. S. pombe wild-type vacuoles showed HMWCs (Fig. 4G, open circles) in the same fractions compared with in vitro synthesized HMWCs (Fig. 4H). Vacuoles from the hmt1 mutant showed a decreased content of HMWCs compared with wild type (Fig. 4G) while vacuoles isolated from the abc2 hmt1 double mutant did not show any traces of HMWCs (Fig. 4G). Accumulation of HMWCs in the cytosol is highly toxic and impairs PC synthesis (49), suggesting that PC synthesis inhibition, rather than an increased PC degradation rate, is in part responsible for the extreme Cd sensitivity of the abc1–4 hmt1 and abc2 hmt1 mutants (Figs. 1F and 2).
FIGURE 4.
Phytochelatin-containing High Molecular Weight Complexes (HMWCs) are absent in vacuoles of the S. pombe abc2 hmt1 double mutant but not in the hmt1 single mutant. A–C, HMWCs, detected in whole cell extracts by size-exclusion chromatography, were formed after Cd exposure, and their synthesis was PC-dependent. D, quadruple abc1–4, (E) the single hmt1, and (F) the quintuple abc1–4 hmt1 mutant showed HMWC-like compounds (marked with arrows). The presence of phytochelatins in these high-molecular weight fractions was confirmed by HPLC-MS (supplemental Fig. S5). G, HMWCs in purified vacuoles from S. pombe wild type (open circles) have an identical elution pattern compared with a HMWC standard synthesized in vitro (H). Vacuoles isolated from the hmt1 mutant showed a reduced content of HMWCs (G, filled circles) whereas no HMWCs were detected in purified vacuoles isolated from the abc2 hmt1 double mutant (G, open squares).
S. pombe Hmt1 Is a Half-size ABC Transporter with Broad Substrate Specificity
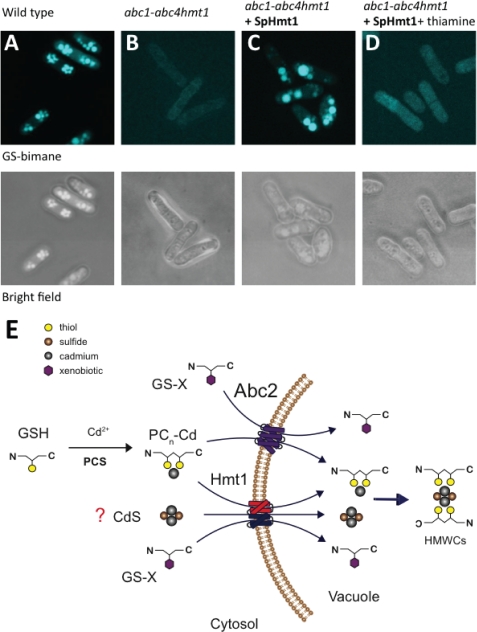
During Hmt1 complementation experiments of the abc1–4 hmt1 quintuple mutant we noticed that expression of Hmt1 also restored GS-bimane fluorescence in vacuoles (Fig. 5, A–C). Vacuolar accumulation of GS-bimane conjugates was found to be Hmt1-dependent because the addition of thiamine to the growth medium, which represses nmt1-driven expression of hmt1+, dramatically reduced the accumulation of GS-bimane in vacuoles (Fig. 5D). This was unexpected, as transport of GS-conjugates has been reported to be mediated by full-length ABC transporters (i.e. AtMRP1, ScYCF1), and not half-size transporters such as Hmt1 (47, 48, 50). The physiological substrate that allows Hmt1 to confer a high degree of Cd tolerance is not known (see “Discussion”). However, these results suggest that the half-size transporter Hmt1, similarly to full-size ABC transporters of the ABCC subfamily, can transport glutathione-S-conjugates.
FIGURE 5.
Hmt1 is a half-size ABC transporter with broad substrate specificity. A and B, GS-bimane conjugates were sequestered in wild-type vacuoles but not in the abc1–4hmt1 quintuple mutant. C, expression of Hmt1 in the abc1–4 hmt1 quintuple mutant restored the accumulation of GS-bimane into vacuoles. D, addition of thiamine to the culture media to repress nmt1-driven expression of hmt1+ abolishes GS-bimane accumulation in vacuoles. E, proposed model for cadmium tolerance, phytochelatin transport, and GS-conjugate sequestration in S. pombe vacuoles. Abc2 and Hmt1 mediate accumulation of PCs and GS-bimane conjugates into vacuoles and both contribute to Cd tolerance in S. pombe. Hmt1 also contributes to Cd tolerance via a PC-independent mechanism. Based on similar features between Hmt1 and the ABC transporters from the mitochondrion (ATMs), one additional function for Hmt1 could be the detoxification of CdS clusters from the cytosol (see “Discussion”).
DISCUSSION
Since its discovery 15 years ago, S. pombe Hmt1 has stood alone as the only known PC transporter (19), despite numerous genetic screens to identify PC transporters in plants and other organisms. Here, we demonstrate through systematic analyses of deletion mutants, Cd tolerance assays, PC content in purified vacuoles and complementation experiments, that S. pombe Abc2, a full-size ABC transporter, also mediates PC accumulation into vacuoles.
PC Synthase and Hmt1 Mediate Cd Tolerance through Independent Mechanisms
The capacity of Hmt1 to confer Cd tolerance in a PC-independent manner suggests that Hmt1 may have other functions in addition to PC transport (38–40) (Fig. 1). This is also evident in the S. pombe hmt1 mutant, which is Cd sensitive despite the fact that it retains the ability to synthesize PCs and transport them into vacuoles to form HMWCs (Figs. 1, 2, 3E, and 4E). These results have encouraged several groups to re-assess the function of Hmt1 (33, 40) (Fig. 1). The first suggestion that Hmt1 may have a function other than PC transport came from work in C. elegans, where it was shown that hmt1–1 and pcs-1 have an additive effect on Cd tolerance compared with single mutants (38). An additive effect of Hmt1 is not expected in a linear model when PC synthase is deleted. We have found that Hmt1 and PC synthase also have additive effects on Cd tolerance in both S. cerevisiae and S. pombe (Fig. 1), suggesting that they do not function in a strictly linear pathway (19). Moreover, in C. elegans, Hmt1 and PC synthase localize to different tissues, supporting the hypothesis that they cannot operate in a simple linear metal detoxification pathway (39).
PC Transport Mediated by Full-size ABC Transporters
The finding that vacuoles isolated from the S. pombe hmt1 mutant exposed to Cd contain high levels of PCs, suggests the existence of an Hmt1-independent mechanism for accumulation of PCs into vacuoles (Fig. 3F) (33). PC uptake into vacuole preparations shows biochemical properties characteristic of ABC-type transporters (18, 19), as confirmed in the present study (see “Results” section). The S. pombe genome encodes a relatively small number of ABC transporters (11 transporters), compared with 29 in S. cerevisiae and >100 in A. thaliana (31, 47, 51, 52). Of the 11 S. pombe ABC transporters, only Hmt1, Abc2, Abc3, and Abc4 are targeted to the vacuolar membrane (Fig. 3D) (47) and deletion of all vacuolar ABC transporters caused Cd hypersensitivity (Figs. 1F and 2) and abolished transport and accumulation of PCs into vacuoles (Fig. 3F and supplemental Fig. S3). Systematic characterization of different abc deletion mutants led us to identify Abc2 as a major contributor, among the full-size ABC transporters, to Cd tolerance (Fig. 2). Expression of Abc2 in the abc1–4 hmt1 mutant partially restores Cd tolerance and PC accumulation into vacuoles (Fig. 3, E and F). Plants do not have orthologs of the half-size ABC transporter Hmt1, which has hampered the discovery of plant PC transporters. However, and in contrast to Hmt1, plants and other organisms that produce PCs have Abc2 homologues (MRP/ABCC subfamily of ATP binding cassette transporters, supplemental Fig. S6).
Substrate Specificity of S. pombe ABC Transporters
In a recent analysis of the 11 S. pombe ABC transporters, Abc2 was found to be required for accumulation of red pigments inside vacuoles (47). In a broad analysis, single and multiple ABC mutants were exposed to more than 20 different compounds, including antibiotics, metal ions and oxidizing agents. The Cd sensitivity of a abc2 abc4 hmt1 mutant was noted but not investigated further (47). Here we show that Abc2, as other members of the MRP/ABCC family, mediates accumulation of GS-bimane conjugates (Fig. 3C) and, more interestingly, PC accumulation in vacuoles (Fig. 3F). Abc2 is a homologue of S. cerevisiae YCF1, a glutathione-S-conjugate pump with broad substrate specificity (47, 48). YCF1 does not mediate PC uptake (48), but based on the broad substrate specificity of Abc2 (Fig. 3), it will be interesting to determine whether, in addition to PCs, Abc2 also mediates transport of GS2-Cd complexes. The great plasticity of ABC transporters might not be surprising considering the variety of molecules that need to be mobilized and detoxified by the limited number of ABC transporters present in S. pombe. In addition, we cannot rule out at this point a possible interaction between ABC transporters, which has been documented to modify the kinetic properties of ABC transporters (53–55) and could explain why Hmt1 and Abc2 alone do not restore the vacuolar PC content to wild type levels (Fig. 3F). It also remains unclear whether post-transcriptional regulation plays a role in cadmium tolerance by regulating the trafficking and turnover of Hmt1 and Abc2.
Mechanism Mediating Heavy Metal Tolerance by Hmt1
A key remaining question is the chemical nature of the Hmt1 substrate in vivo. Hmt1 requires GSH to confer Cd tolerance (40), but analyses of the peptide content of vacuolar HMWCs in S. pombe have failed to detect significant amounts of GSH inside vacuoles (24, 25, 56). Moreover, vacuolar vesicles isolated from S. pombe overexpressing Hmt1 did not show significant uptake rates of GSH or GS2-Cd (19), suggesting that the GSH requirement of Hmt1 may not be related to GS2-Cd transport. It is possible that Hmt1 may have an additional yet unidentified substrate, which is highly toxic when it accumulates in the cytosol of hmt1 mutants.
The closest homologue to Hmt1 in S. pombe is Atm1 (supplemental Fig. S6), a mitochondrial half-size ABC transporter that mediates export of FeS clusters from the mitochondrial matrix to the cytosol (47, 57). The function of Atm1 in S. cerevisiae also requires GSH (58). Therefore, it is tempting to speculate that Hmt1 may transport CdS clusters from the cytosol into vacuoles (Fig. 5E) and that either GSH is required to synthesize these clusters or, as recently determined for FeS clusters in plants and bacteria, GSH and/or PC2 help to stabilize these clusters (59, 60). Transport of CdS-PC clusters by Hmt1 could explain why the abc2 single mutant still accumulates PCs in the vacuole (Fig. 3F). In addition, active detoxification of CdS clusters by Hmt1 in the abc2 single mutant could also explain why the PC content in this mutant remained unaffected (Fig. 5E and supplemental Fig. S5A) and why abc mutants are not Cd hypersensitive (Figs. 1F and 2). On the other hand, accumulation of CdS clusters in the cytosol is expected to be highly toxic, impairing the activity FeS-containing enzymes and PC synthesis (49). Impairment of PC synthesis explains the decreased PC content in cell extracts obtained from hmt1 and the abc1–4hmt1 quintuple mutant (supplemental Fig. S5A). Moreover, formation of HMWC around CdS cores is a spontaneous process that takes place in the vacuole where all the components are readily available (25). In hmt1 and abc mutants, traces of HMWC were detected in cell extracts, but not in purified vacuoles (Fig. 4), suggesting that HMWCs are also formed in the cytosol, impairing PC synthesis, and explaining the extreme Cd sensitivity of the hmt1, abc2 hmt1, and abc1–4hmt1mutants, even though they retain the ability to synthesize PCs (supplemental Fig. S5A).
In conclusion, we have determined that Abc2, a full-size ABC transporter of the MRP/ABCC family, mediates accumulation of PCs in vacuoles, and we have demonstrated that Hmt1 and Abc2 have distinct and also overlapping functions in Cd detoxification. These findings modify the original model of Cd tolerance mediated by Hmt1 (Fig. 5E) and present the possibility to identify the long-sought PC transporters in other organisms. Tissue-specific expression of PC transporters may be useful for bioremediation of soils and waters contaminated with heavy metals and to exclude heavy metal accumulation in edible tissues of plants used for livestock and human nutrition.
Supplementary Material
Acknowledgments
We thank Kaoru Takegawa for providing the S. pombe abc1abc2abc3abc4 mutant strain, Angus Murphy for providing an abc2 abc3 hmt1 mutant, and Maja Schellenberg for technical assistance in transport experiments.
Note Added in Proof
A report describing the independent identification of the related AtABCC1 and AtABCC2 as vacuolar phytochelatin uptake transporters in Arabidopsis thaliana is in press (Song, W.-Y., Park, J., Mendoza-Cózatl, D., Suter-Grotemeyer, M., Shim, D., Hörtensteiner, S., Geisler, M., Weder, B., Rea, P., Rentsch, D., Schroeder, J. I., Lee, Y., and Martinoia, E. (2010) Proc. Natl. Acad. Sci. U.S.A., in press).
This work was supported, in whole or in part, by grants from the NIEHS, National Institutes of Health (P42 ES010337, to J. I. S. and P. R.), the Energy Biosciences Office of the Department of Energy (DE-FG02-03ER15449, to J. I. S.), the EU Project PHIME (Contract no. FOOD-CT-2006-0016253, to E. M.), National Science Foundation (MCB-0923731, to O. K. V.), and Cornell University Agricultural Experiment Station CUAES Hatch (NYC-125433, to O. K. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6 and Table S1.
- PC
- phytochelatin
- abc1–4
- S. pombe abc1 abc2 abc3 abc4 quadruple mutant
- abc1–4hmt1
- S. pombe abc1 abc2 abc3 abc4 hmt1 quintuple mutant
- ABC transporters
- ATP-binding cassette transporters
- ATM
- ABC transporters of the mitochondrion
- Cd
- cadmium
- CdS
- cadmium-sulfur clusters
- FeS
- iron-sulfur clusters
- GS-bimane
- bimane-S-glutathione
- Hmt1
- heavy metal tolerance factor 1
- HMWC
- high molecular weight complexes
- HPLC-MS
- reversed phase high performance liquid chromatography coupled to mass spectrometry
- MCB
- monochlorobimane
- MRP
- multidrug resistance protein
- YFP
- yellow fluorescent protein.
REFERENCES
- 1.Ogunseitan O. A., Schoenung J. M., Saphores J. D., Shapiro A. A. (2009) Science 326, 670–671 [DOI] [PubMed] [Google Scholar]
- 2.Satarug S., Garrett S. H., Sens M. A., Sens D. A. (2010) Environ. Health Perspect. 118, 182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan J. A., Scheckel K. G., Berti W. R., Brown S. L., Casteel S. W., Chaney R. L., Hallfrisch J., Doolan M., Grevatt P., Maddaloni M., Mosby D. (2004) Environ. Sci. Technol. 38, 18A-24A [DOI] [PubMed] [Google Scholar]
- 4.Guo Y., Huo X., Li Y., Wu K., Liu J., Huang J., Zheng G., Xiao Q., Yang H., Wang Y., Chen A., Xu X. (2010) Sci. Total. Environ. 408, 3113–3117 [DOI] [PubMed] [Google Scholar]
- 5.Mendoza-Cózatl D., Loza-Tavera H., Hernández-Navarro A., Moreno-Sánchez R. (2005) FEMS Microbiol. Rev. 29, 653–671 [DOI] [PubMed] [Google Scholar]
- 6.Clemens S. (2006) Biochimie 88, 1707–1719 [DOI] [PubMed] [Google Scholar]
- 7.Verbruggen N., Hermans C., Schat H. (2009) Curr. Opin. Plant Biol. 12, 364–372 [DOI] [PubMed] [Google Scholar]
- 8.Zenk M. H. (1996) Gene 179, 21–30 [DOI] [PubMed] [Google Scholar]
- 9.Grill E., Winnacker E. L., Zenk M. H. (1985) Science 230, 674–676 [DOI] [PubMed] [Google Scholar]
- 10.Mutoh N., Hayashi Y. (1988) Biochem. Biophys. Res. Commun. 151, 32–39 [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y., Isobe M., Mutoh N., Nakagawa C. W., Kawabata M. (1991) Methods Enzymol. 205, 348–358 [DOI] [PubMed] [Google Scholar]
- 12.Clemens S., Schroeder J., Degenkolb T. (2001) Eur. J. Biochem. 268, 3640–3643 [DOI] [PubMed] [Google Scholar]
- 13.Vatamaniuk O. K., Bucher E. A., Ward J. T., Rea P. A. (2001) J. Biol. Chem. 276, 20817–20820 [DOI] [PubMed] [Google Scholar]
- 14.Ha S. B., Smith A. P., Howden R., Dietrich W. M., Bugg S., O'Connell M. J., Goldsbrough P. B., Cobbett C. (1999) Plant Cell 11, 1153–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vatamaniuk O. K., Mari S., Lu Y. P., Rea P. A. (1999) Proc. Natl. Acad. Sci., U.S.A. 96, 7110–7115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens S., Kim E. J., Neumann D., Schroeder J. I. (1999) EMBO J. 18, 3326–3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vögeli-Lange R., Wagner G. J. (1990) Plant Physiol. 92, 1086–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salt D. E., Rauser W. E. (1995) Plant Physiol. 107, 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz D. F., Ruscitti T., McCue K. F., Ow D. W. (1995) J. Biol. Chem. 270, 4721–4728 [DOI] [PubMed] [Google Scholar]
- 20.Van Belleghem F., Cuypers A., Semane B., Smeets K., Vangronsveld J., d'Haen J., Valcke R. (2007) New Phytol. 173, 495–508 [DOI] [PubMed] [Google Scholar]
- 21.Gong J. M., Lee D., Schroeder J. I. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10118–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen A., Komives E. A., Schroeder J. I. (2006) Plant Physiol. 141, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendoza-Cózatl D. G., Butko E., Springer F., Torpey J. W., Komives E. A., Kehr J., Schroeder J. I. (2008) Plant J. 54, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reese R. N., Winge D. R. (1988) J. Biol. Chem. 263, 12832–12835 [PubMed] [Google Scholar]
- 25.Wu J. S., Sung H. Y., Juang R. H. (1995) Biochem. Mol. Biol. Int. 36, 1169–1175 [PubMed] [Google Scholar]
- 26.Ortiz D. F., Kreppel L., Speiser D. M., Scheel G., McDonald G., Ow D. W. (1992) EMBO J. 11, 3491–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speiser D. M., Ortiz D. F., Kreppel L., Scheel G., McDonald G., Ow D. W. (1992) Mol. Cell. Biol. 12, 5301–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salt D. E., Wagner G. J. (1993) J. Biol. Chem. 268, 12297–12302 [PubMed] [Google Scholar]
- 29.Shigaki T., Barkla B. J., Miranda-Vergara M. C., Zhao J., Pantoja O., Hirschi K. D. (2005) J. Biol. Chem. 280, 30136–30142 [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Fernández R., Davies T. G., Coleman J. O., Rea P. A. (2001) J. Biol. Chem. 276, 30231–30244 [DOI] [PubMed] [Google Scholar]
- 31.Rea P. A. (2007) Annu. Rev. Plant. Biol. 58, 347–375 [DOI] [PubMed] [Google Scholar]
- 32.Howden R., Goldsbrough P. B., Andersen C. R., Cobbett C. S. (1995) Plant Physiol. 107, 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sooksa-Nguan T., Yakubov B., Kozlovskyy V. I., Barkume C. M., Howe K. J., Thannhauser T. W., Rutzke M. A., Hart J. J., Kochian L. V., Rea P. A., Vatamaniuk O. K. (2009) J. Biol. Chem. 284, 354–362 [DOI] [PubMed] [Google Scholar]
- 34.Chen S., Sánchez-Fernández R., Lyver E. R., Dancis A., Rea P. A. (2007) J. Biol. Chem. 282, 21561–21571 [DOI] [PubMed] [Google Scholar]
- 35.Kushnir S., Babiychuk E., Storozhenko S., Davey M. W., Papenbrock J., De Rycke R., Engler G., Stephan U. W., Lange H., Kispal G., Lill R., Van Montagu M. (2001) Plant Cell. 13, 89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teschner J., Lachmann N., Schulze J., Geisler M., Selbach K., Santamaria-Araujo J., Balk J., Mendel R. R., Bittner F. (2010) Plant Cell. 22, 468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D. Y., Bovet L., Kushnir S., Noh E. W., Martinoia E., Lee Y. (2006) Plant Physiol. 140, 922–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vatamaniuk O. K., Bucher E. A., Sundaram M. V., Rea P. A. (2005) J. Biol. Chem. 280, 23684–23690 [DOI] [PubMed] [Google Scholar]
- 39.Schwartz M. S., Benci J. L., Selote D. S., Sharma A. K., Chen A. G., Dang H., Fares H., Vatamaniuk O. K. (2010) PLoS One 5, e9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prévéral S., Gayet L., Moldes C., Hoffmann J., Mounicou S., Gruet A., Reynaud F., Lobinski R., Verbavatz J. M., Vavasseur A., Forestier C. (2009) J. Biol. Chem. 284, 4936–4943 [DOI] [PubMed] [Google Scholar]
- 41.Forsburg S. L., Rhind N. (2006) Yeast 23, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy P. J., Vashisht A. A., Hoe K. L., Kim D. U., Park H. O., Hayles J., Russell P. (2008) Toxicol. Sci. 106, 124–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen D., Toone W. M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bähler J. (2003) Mol. Biol. Cell. 14, 214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okazaki K., Okazaki N., Kume K., Jinno S., Tanaka K., Okayama H. (1990) Nucleic Acids Res. 18, 6485–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vatamaniuk O. K., Mari S., Lu Y. P., Rea P. A. (2000) J. Biol. Chem. 275, 31451–31459 [DOI] [PubMed] [Google Scholar]
- 46.Nagy R., Grob H., Weder B., Green P., Klein M., Frelet-Barrand A., Schjoerring J. K., Brearley C., Martinoia E. (2009) J. Biol. Chem. 284, 33614–33622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwaki T., Giga-Hama Y., Takegawa K. (2006) Microbiology 152, 2309–2321 [DOI] [PubMed] [Google Scholar]
- 48.Li Z. S., Lu Y. P., Zhen R. G., Szczupka M., Thiele D. J., Rea P. A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vande Weghe J. G., Ow D. W. (2001) Mol. Microbiol. 42, 29–36 [DOI] [PubMed] [Google Scholar]
- 50.Lu Y. P., Li Z. S., Rea P. A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 8243–8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Higgins C. F. (1992) Annu. Rev. Cell. Biol. 8, 67–113 [DOI] [PubMed] [Google Scholar]
- 52.Decottignies A., Goffeau A. (1997) Nat. Genet. 15, 137–145 [DOI] [PubMed] [Google Scholar]
- 53.Mo W., Zhang J. T. (2009) Expert. Opin. Drug. Metab. Toxicol. 5, 1049–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trompier D., Alibert M., Davanture S., Hamon Y., Pierres M., Chimini G. (2006) J. Biol. Chem. 281, 20283–20290 [DOI] [PubMed] [Google Scholar]
- 55.Yang Y., Liu Y., Dong Z., Xu J., Peng H., Liu Z., Zhang J. T. (2007) J. Biol. Chem. 282, 8821–8830 [DOI] [PubMed] [Google Scholar]
- 56.Plocke D. J., Kägi J. H. (1992) Eur. J. Biochem. 207, 201–205 [DOI] [PubMed] [Google Scholar]
- 57.Broderick J. B. (2007) Nat. Chem. Biol. 3, 243–244 [DOI] [PubMed] [Google Scholar]
- 58.Sipos K., Lange H., Fekete Z., Ullmann P., Lill R., Kispal G. (2002) J. Biol. Chem. 277, 26944–26949 [DOI] [PubMed] [Google Scholar]
- 59.Iwema T., Picciocchi A., Traore D. A., Ferrer J. L., Chauvat F., Jacquamet L. (2009) Biochemistry 48, 6041–6043 [DOI] [PubMed] [Google Scholar]
- 60.Rouhier N., Unno H., Bandyopadhyay S., Masip L., Kim S. K., Hirasawa M., Gualberto J. M., Lattard V., Kusunoki M., Knaff D. B., Georgiou G., Hase T., Johnson M. K., Jacquot J. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7379–7384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.