Abstract
Methylating drugs such as temozolomide (TMZ) are widely used in the treatment of brain tumors including malignant glioblastoma. The mechanism of TMZ-induced glioblastoma cell death and apoptosis, however, is not fully understood. Here, we tested the potential involvement of AMP-activated protein kinase (AMPK) in this process. We found that methylating agents TMZ and N-methyl-N′-nitro-N-nitrosoguanidine induce AMPK activation in primary cultured human glioblastoma and glioblastoma cell lines. TMZ-induced O6-methylguanine production is involved in AMPK activation. O6-benzylguanine, an O6-methylguanine-DNA methyltransferase inhibitor, enhances TMZ-induced O6-methylguanine production, leading to enhanced reactive oxygen species production, which serves as an upstream signal for AMPK activation. Activation of AMPK is involved in TMZ-induced glioblastoma cell death and apoptosis. AMPK inhibitor (Compound C) or AMPKα siRNA knockdown inhibits TMZ-induced glioblastoma cell death and apoptosis, whereas AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside enhances it. In further studies, we found that activation of AMPK is involved in TMZ-induced p53 activation and subsequent p21, Noxa, and Bax up-regulation. Activation of AMPK by TMZ also inhibits mTOR complex 1 (mTORC1) signaling and promotes anti-apoptosis protein Bcl-2 down-regulation, which together mediate TMZ-induced pro-cell apoptosis effects. Our study suggests that activation of AMPK by TMZ contributes to glioblastoma cell apoptosis, probably by promoting p53 activation and inhibiting mTORC1 signaling.
Keywords: AMP Kinase, Anticancer Drug, Cancer Therapy, Cell Death, DNA Methylation
Introduction
Astrocytic tumors are the most common primary brain tumors. Of the astrocytic tumors, malignant glioblastoma is the most malignant form and has the worst prognosis. Complete surgical resection of glioblastoma is difficult, and the tumor generally recurs within a year after radiation and chemotherapy regardless of the initial response to these treatment modalities (1). Of the chemotherapeutic agents used to treat glioblastoma, alkylating agents including O6-methylating agent temozolomide (TMZ)5 are the most widely used.
Despite the use of O6-methylating agents (TMZ for example) in glioblastoma therapy, the median survival times of patients suffering from the most severe form glioblastoma are still remarkably low (12–14 months). There is an urgent need for improving glioblastoma therapy. One goal that needs to be reached in achieving this would be to improve our knowledge of the mechanism of alkylating agent-induced death in glioblastoma cells (2). Here, we focus on AMPK, a recently discovered kinase that is involved in anti-tumor growth/survival effects (3–9).
AMP-activated protein kinase (AMPK) is a metabolic-sensing protein kinase, which plays an essential role as an energy-sensor mainly in ATP-deprived conditions (10). In the activated states, AMPK down-regulates several anabolic enzymes and thus shuts down the ATP-consuming metabolic pathways. Interestingly, several recent reports have observed the strong pro-apoptotic potential of AMPK in activated conditions such as AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR)-treated cells or constitutively active AMPK mutant (3, 5–9, 11). For example, activation of AMPK by AICAR inhibits the growth of EGF receptor vIII-expressing glioblastomas (5). Interestingly, certain chemotherapy drugs can also activates AMPK, mediating pro-cell death and apoptosis effects (12). For example, a recent paper suggests that AMPK activation is a key player for doxorubicin-induced cancer cell apoptosis (12). However, the potential effects of AMPK activation on TMZ-induced glioblastoma cell death and apoptosis have not been studied.
Here, we found for the first time that methylating agents TMZ and N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) induce AMPK activation in both primary cultured human glioblastoma cells and glioblastoma cell lines, which mediates cell death and apoptosis. We found that activation of AMPK is involved in TMZ-induced p53 activation and mTORC1 inhibition.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
AKT1, T-S6K, AMPKα1, AMPKα2, TSC1 (Hamartin), TSC2 (Tuberin), Bcl-2, Bcl-XL, p21, p53, AKT, ERK1/2, LKB1, goat anti-rabbit IgG-HRP, and goat anti-mouse IgG-HRP antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Compound C, rapamycin, and AICAR were purchased from Calbiochem (San Diego, CA). O6-benzylguanine (O6-BG), TMZ, MNNG, monoclonal mouse anti-β-actin, H2O2, and N-acetylcysteine (NAc) were obtained from Sigma (St. Louis, MO). T-AMPKα and all phosphorylation antibodies were purchased from Cell Signaling Technology (Bevery, MA).
Cell Culture
Glioblastoma U87MG and U373MG cells were maintained in DMEM (Sigma), supplemented with a 10% FBS (Sigma), penicillin/streptomycin (1:100; Sigma) and 4 mm l-glutamine (Sigma), in a CO2 incubator at 37 °C.
Primary Human Glioblastoma Cell Cultures
Following informed consent, tumor samples classified as glioblastoma based on World Health Organization (WHO) criteria were obtained from patients undergoing surgical treatment at Brain Hospital affiliated with Nanjing Medical University. Within 1–3 h after surgical removal, tumors were washed and enzymatically dissociated into single cells. Red blood cells were removed by differential centrifugation. Tumor cells were then cultured serum medium consisting of DMEM (Invitrogen) with 10% FBS (Invitrogen) with basic FGF and EGF (50 ng/ml each; Calbiochem). The culture dish and plates were precoated with poly-l-lysine/laminin mixture (Invitrogen).
Cell Viability Assay (MTT Dye Assay)
Cell viability was measured by the 3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method. Briefly, cells were collected and seeded in 96-well plates at a density of 5 × 105 cells/cm2. Different seeding densities were optimized at the beginning of the experiments. 20 μl of MTT tetrazolium salt (Sigma) dissolved in Hanks' balanced solution at a concentration of 5 mg/ml was added to each well with the indicated treatment and incubated in CO2 incubator for 4 h. Finally, the medium was aspirated from each well, and 150 μl of dimethyl sulfoxide (Sigma) was added to dissolve formazan crystals, and the absorbance of each well was obtained using a Dynatech MR5000 plate reader at a test wavelength of 490 nm with a reference wavelength of 630 nm.
Western Blotting
Aliquots of 40 μg of protein from each sample were separated by 10–12% SDS-PAGE and transferred onto a PVDF membrane (Millipore, Bedford, MA). After blocking with 10% instant nonfat dry milk for 1 h, membranes were incubated with specific antibodies overnight at 4 °C followed by incubation with secondary antibodies for 1 h. Antibody binding was detected with the enhanced chemiluminescence (ECL) detection system (Amersham Biosciences).
Immunoprecipitation
U87MG cells treated with the appropriate stimuli were treated with lysis buffer, 200 mm NaCl (pH 7.4), 1% Triton X-100, 10% glycerol, 0.3 mm EDTA, 0.2 mm Na3VO4, and protease inhibitor mixtures (Roche Applied Sciences). Aliquots of 800 μg of proteins from each sample were precleared by incubation with 20 μl of protein A/G-Sepharose (beads) (Amersham Biosciences) for 1 h at 4 °C. Precleared samples were incubated with primary antibodies in lysis buffer overnight at 4 °C. To this was added 35 μl of protein A/G beads, and the samples were incubated for 2 h at 4 °C. The beads were washed five times with phosphate-buffered saline (PBS) and once with lysis buffer, boiled, separated by 10% SDS-PAGE, and transferred onto a PVDF membrane followed by Western blotting analysis.
Quantification of Apoptotic Cells
To detect apoptotic cells, cells were stained with DNA dye Hoechst 33342 (Sigma). Cells with the indicated treatment were fixed with 4% formaldehyde in PBS for 10 min at 4 °C. Cells were then incubated with 5 μg/ml Hoechst 33342 to stain the nuclei for 5 min. After washing with PBS, the apoptotic cells were observed under a confocal fluorescence microscope (Leica TCS SMD FCS, Leica, Germany). Cells exhibiting condensed chromatin nuclei (Hoechst 33342 stain, Blue) were scored as apoptotic cells. For each Hoechst experiment, at least 100 cells in five random scope fields were counted for apoptotic rate.
The Cell Apoptosis ELISA Detection Kit (Roche Applied Science) was used to detect apoptosis in glioblastoma cell lines with different treatments according to the manufacturer's protocol. Briefly, after the indicated treatments, the cytoplasmic histone/DNA fragments from cells were extracted and bound to immobilized anti-histone antibody. Subsequently, the peroxidase-conjugated anti-DNA antibody was used for the detection of immobilized histone/DNA fragments. After the addition of substrate for peroxidase, the spectrophotometric absorbance of the samples was determined by using Dynatech MR5000 plate reader at 405 nM.
RNA Interference (RNAi)
siRNA for AMPKα1/α2 (sc-45312), LKB1 (sc-35816), ATM (sc-29761), and Bcl-2 (sc-29214) were purchased from Santa Cruz Biotechnology. SignalSilence® mTOR siRNA was purchased from Cell Signaling Technology. U87MG or primary cultured glioblastoma cells were cultured and seeded in a 6-well plate 1 day prior to transfection and cultured to 70% confluence the following day. For RNAi experiments, 3.2 μl of PLUS™ Reagent (Invitrogen) was diluted in 90 μl of RNA dilution water (Invitrogen) for 5 min in room temperature. Then, 10 μl of siRNA (10 μm) or control (scramble) siRNA (10 μm) was added to the PLUS™ Reagent and incubated for 5 min at room temperature. 3.6 μl of Lipofectamine (Invitrogen) was then added to the complex. After a 30-min incubation, the transfection complex was formed. Finally, the complex was added to the wells containing 1 ml of medium with the final siRNA concentration of 100 nm. Target protein expression was determined by Western blotting 48 h after transfection, successfully knocked down cells were used for further experiments.
p53 and TSC1/2 shRNA
Human TSC1 (sc-37437-SH)/TSC2 (sc-36762-SH) and p53 shRNA (sc-29435-sh) plasmids were purchased from Santa Cruz Biotechnology. U87MG cells were cultured and seeded in a 6-well plate 1 day prior to transfection and cultured to 70% confluence the following day. For shRNA experiments, 3.2 μl of PLUS™ Reagent was diluted in 90 μl of RNA dilution water for 5 min in room temperature. Then, 2 μg of plasmid or 2 μg of control plasmid was added to the PLUS™ Reagent and incubated for 5 min at room temperature. 3.6 μl of Lipofectamine was then added to the complex. After a 30-min incubation, the transfection complex was formed and added to U87MG cells. Cells were then cultured for additional 48 h, followed by puromycin (0.5 μm) selection. TSC2/TSC1 or p53 expression was detected by Western blotting 48 h later. Successfully transfected stable cells were cultured and stored for further experiments.
Reactive Oxygen Species (ROS) Production Detection
Cultured U87MG cells were loaded with 1 μm fluorescent dye dihydrorhodamine 2 h before treatment, which reacts with ROS in cells and results in a change of fluorescence. After the indicated treatments, U87MG cells were trypsinized, suspended in ice-cold PBS, and fixed in 70% ethyl alcohol at −20 °C. The changes in fluorescence in drug-treated cells were quantified by fluorescence-activated cell sorter analysis. Induction of ROS generation was expressed in arbitrary units (versus control).
Statistical Analysis
The values in the figures are expressed as the means ± S.D. The figures in this study are representative of at least three independent experiments. Values of p < 0.05 were considered as statistically significant (ANOVA) with a Newman-Keuls post test.
RESULTS
TMZ Induces AMPK Activation in Glioblastoma Cells
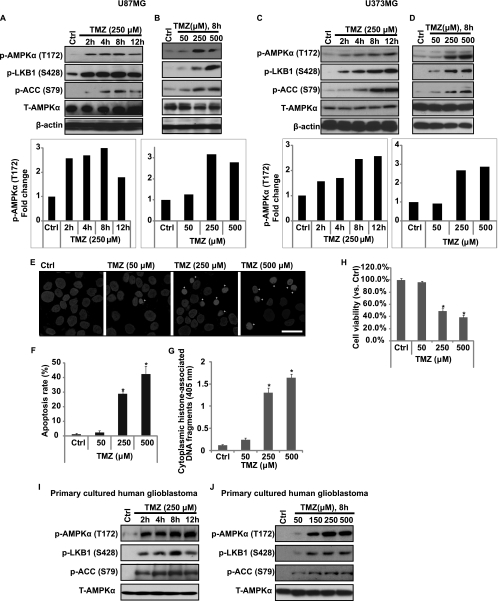
First, we tested the possible effect of TMZ on AMPK activation in cultured glioblastoma cells. Essential to activation of AMPK is its phosphorylation at Thr-172 by an upstream kinase LKB1 (13). LKB1 is activated allosterically by binding to the pseudokinase STRAD and the adaptor protein MO25. The LKB1-STRAD-MO25 heterotrimeric complex represents the biologically active unit that is capable of phosphorylates and activates AMPK. Phosphorylation of LKB at Ser-428 influences the ability of LKB1 to bind and phosphorylate AMPK at Thr-172 (14). After AMPK activation, AMPK phosphorylates and inactivates acetyl-CoA carboxylase (ACC) (Ser-79) (15), which is well established as a key indicator for AMPK activity. As shown in Fig. 1, A–D, in two different glioblastoma cell lines (U87MG and U373MG), TMZ clearly induced AMPK and its upstream molecular LKB1 and downstream molecular ACC phosphorylation in a both time- and dose-dependent manner. As expected, TMZ induced U87MG cell death (Fig. 1H, reduced cell viability, MTT assay) and cell apoptosis (Fig. 1, E–G) in a dose-dependent manner, the cell apoptosis was confirmed by two different methods including Hoechst 33342 assay (Fig. 1, E and F) and histone/DNA ELISA (Fig. 1G). Importantly, TMZ also induced strong AMPK activation in primary cultured human glioblastoma cells in a both dose- and time-dependent manner (Fig. 1, I and J).
FIGURE 1.
TMZ induces AMPK activation in glioblastoma cells. Glioblastoma cell line U87MG (A and B) or U373MG (C and D) was treated with TMZ (250 μm) and cultured for different time points (0, 2, 4, 8, and 12 h) or treated with different doses of TMZ (0, 50, 250, and 500 μm) and cultured for 8 h. p-AMPKα (Thr-172), T-AMPKα, p-ACC (Ser-79), p-LKB1 (Ser-428), and β-actin were detected by Western blotting. AMPKα phosphorylation was quantified. U87MG cells were treated with different doses of TMZ (0, 50, 250, and 500 μm), and cell apoptosis was detected by Hoechst 33342 assay (E and quantified in F) and histone/DNA ELISA (G) 36 h later. Cell viability was detected by MTT assay (H) 48 h after TMZ treatment. Primary cultured human glioblastoma cells (I and J) were treated with TMZ (250 μm) and cultured for different time points (0, 2, 4, 8, and 12 h) or treated with different doses of TMZ (0, 50, 150, 250, and 500 μm) and cultured for 8 h. p-AMPKα (Thr-172), T-AMPKα, p-ACC (Ser-79), and p-LKB1 (Ser-428) were detected by Western blotting. Experiments in this figure were repeated at least three times, and similar results were obtained. *, p < 0.05 versus untreated group (Ctrl lane) (ANOVA). Scale bar, 25 μm.
O6MeG, ROS, and LKB1 Are Involved in TMZ-induced AMPK Activation
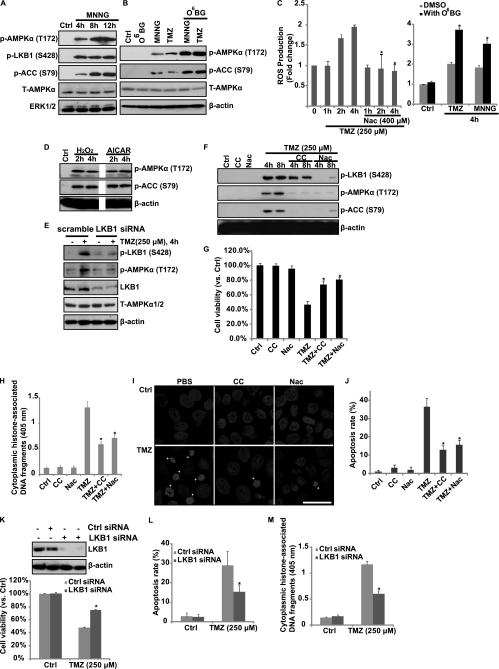
As shown in Fig. 1, TMZ induced strong AMPK activation in cultured glioblastoma cells. Further, MNNG, another DNA-methylating agent, was also able to induce AMPK activation (Fig. 2A). We then tested the possible upstream signals for AMPK activation by TMZ. Previous studies have identified ROS as strong AMPK activators (16–18), so we next tested the possible involvement of ROS production in TMZ-induced AMPK activation. As shown in Fig, 2C, TMZ induced ROS production in U87MG cells, NAc, an antioxidant, largely inhibited TMZ-induced ROS production and AMPK activation (Fig. 2, C and F). As expected, direct H2O2 treatment induced strong AMPK activation; so did the AMPK activator AICAR (Fig. 2D). Here, we suggest that ROS production that leads to AMPK activation by TMZ is associated with induction of DNA damage. To support this, we found that O6-BG, an O6-methylguanine-DNA methyltransferase (MGMT) inhibitor, which is reportedly to enhance TMZ-induced O6-MeG production (the lethal DNA damage by TMZ) (2, 19, 20), caused enhanced ROS generation and downstream AMPK activation (Fig. 2, B and C). Further, the other DNA-methylating agent MNNG was also able to induce ROS production and AMPK activation, which was also enhanced by O6-BG (Fig. 2, A–C). So, we indicate that TMZ causes DNA damage which leads to ROS production, the latter serving as an upstream signal for AMPK activation. Knockdown of LKB1 by specific siRNA reduced TMZ-induced AMPK activation, indicating that LKB1 might be the upstream signal for AMPK activation in response to TMZ (Fig. 2E). Importantly, NAc and Compound C, which inhibited TMZ-induced AMPK activation (Fig. 2F), also inhibited TMZ-induced cell death (reduced cell viability) and apoptosis (detected by Hoechst staining and histone/DNA ELISA) (Fig. 2, G–J). Further, knockdown of the upstream signal LKB1 also diminished TMZ-induced cell death and apoptosis (Fig. 2, K–M). These results indicated that AMPK activation might be involved in TMZ-induced cell death/apoptosis in cultured glioblastoma cells.
FIGURE 2.
O6-MeG, ROS, and LKB1 are involved in TMZ-induced AMPK activation. A, glioblastoma U87MG cells were treated with 10 μm MNNG and cultured for 4, 8, and 12 h. p-AMPKα (Thr-172), p-ACC (Ser-79), p-LKB1 (Ser-428), T-AMPKα, and ERK1/2 were detected by Western blotting. B, U87MG cells were either left untreated or pretreated with O6-BG (10 μm) for 2 h, followed by TMZ (250 μm) or MNNG (10 μm) treatment for 8 h. AMPK activation was detected by Western blotting using the antibodies mentioned above. C, U87MG cells were pretreated with anti-oxidant NAc (400 μm) or O6-BG (10 μm) for the indicated time periods, followed by TMZ (250 μm) or MNNG (10 μm) treatment and cultured for the indicated times. ROS production was detected. D, U87MG cells were treated with 500 μm H2O2 or 1 mm AICAR and cultured for 2 and 4 h. p-AMPKα, p-ACC, and β-actin were detected by Western blotting. E, U87MG cells transfected with scramble (control) or LKB1 siRNAs were treated with 250 μm TMZ. LKB1/AMPK activation was detected by Western blotting. U87MG cells were pretreated with 10 μm Compound C (CC) or NAc (400 μm) for 2 h, followed by TMZ (250 μm) treatment. AMPK activation was detected by Western blotting 4 and 8 h later (F), cell viability was detected by MTT assay after 48 h (G), cell apoptosis was detected by histone/DNA ELISA (H), and Hoechst staining (I and quantified in J) after 36 h. U87MG cells transfected with scramble (Ctrl) or LKB1 siRNA were treated with 250 μm TMZ, and cell death and apoptosis were detected using the same methods described above (K–M). Experiments in this figure were repeated at least three times, and similar results were obtained. *, p < 0.05 versus TMZ-treated group in Ctrl cells (ANOVA). Scale bar, 25 μm.
AMPK Activation Is Involved in TMZ-induced Cell Death/Apoptosis in Vitro
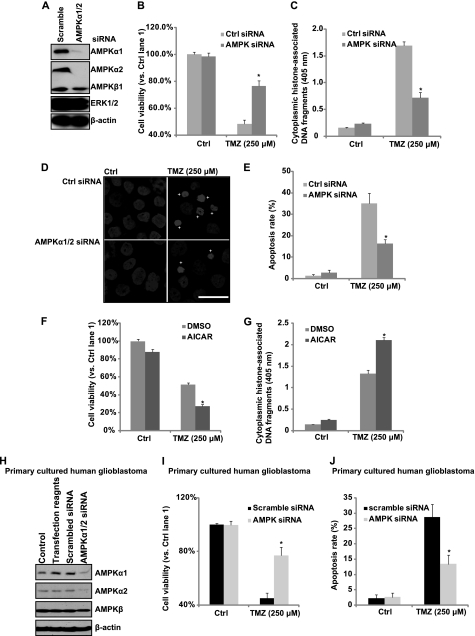
To confirm further that AMPK activation is involved in TMZ-induced glioblastoma cell death/apoptosis, AMPKα siRNA was used. As shown in Fig. 3A, AMPKα1/2 siRNAs efficiently knocked down both AMPKα1 and AMPKα2 in cultured U87MG cells, whereas control (scramble) siRNA had almost no effect on AMPKα1/2 expression. TMZ-induced cell death and apoptosis were largely inhibited in AMPKα1/2 knockdown cells (Fig. 3, B–E). However, enforced AMPK activation by AICAR enhanced TMZ-induced cell death and apoptosis in cultured U87MG cells (Fig. 3, F and G). Importantly, knockdown of AMPKα1/2 by specific siRNAs in primary cultured glioblastoma cells (Fig. 3H) also inhibited TMZ-induced cell death and apoptosis (Fig. 3, I and J). Taking these results into account, we suggest that AMPK activation is involved in TMZ-induced cell apoptosis in cultured glioblastoma cells.
FIGURE 3.
AMPK activation is involved in TMZ-induced cell death in vitro. A, glioblastoma U87MG cells were transfected with control (scrambled) or AMPKα1/2 siRNA for 48 h. AMPKα1/2, AMPKβ, ERK1/2, and β-actin expression levels were detected by Western blotting. Successfully AMPKα knocked down cells were used for further experiments. U87MG cells transfected with control or AMPKα siRNA were treated with 250 μm TMZ. B, cell viability was detected by MTT assay after 48 h. C–E, cell apoptosis was detected by histone/DNA ELISA (C) and Hoechst 33342 staining (D and quantified in E) after 36 h. F and G, U87MG cells were pretreated with AICAR (1 mm) followed by 250 μm TMZ treatment. Cell viability was detected by MTT assay after 48 h (F). Cell apoptosis was detected by histone/DNA ELISA after 36 h (G). H, primary cultured human glioblastoma cells were either left untreated or transfected with control (scramble) or AMPKα1/2 siRNA for 48 h. The expression level of AMPKα1/2, AMPKβ, and β-actin were detected by Western blotting. Successfully AMPKα knocked down cells were used for further experiments. DMSO, dimethyl sulfoxide. I, primary cultured human glioblastoma cells transfected with control or AMPKα siRNA were treated with 250 μm TMZ. Cell viability was detected by MTT assay after 48 h. J, cell apoptosis was detected by Hoechst 33342 staining after 36 h. Experiments in this figure were repeated at least three times, and similar results were obtained. *, p < 0.05 versus TMZ-treated group in control group (ANOVA). Scale bar, 25 μm.
AMPK Activation Is Involved in TMZ-induced p53 Activation in Vitro
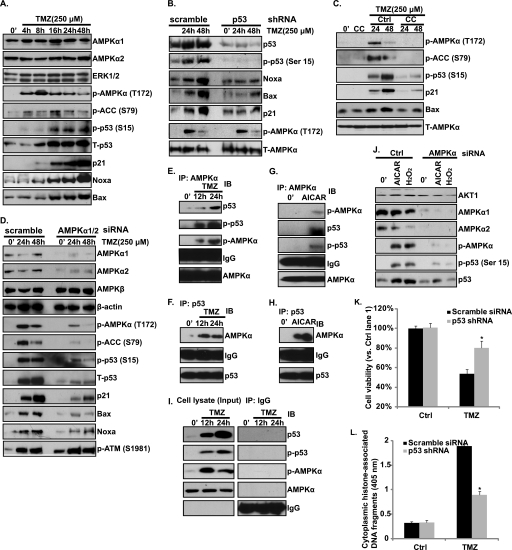
Because we have showed that TMZ induces AMPK activation, which contributes to glioblastoma cell death and apoptosis (Figs. 1–3), we next tried to identity the downstream targets of AMPK activation. We first focused on p53 because p53 is a key mediator for TMZ-induced glioblastoma cell death (2, 21–25), and previous studies have shown that AMPK phosphorylates and activates p53 directly, leading to apoptotic cell death (6, 7). As shown in Fig. 4A, TMZ-induced p53 phosphorylation (at serine 15) and up-regulation/stabilization in U87MG cells. p21 and pro-apoptotic proteins Noxa and Bax were also induced after TMZ treatment (Fig. 4A). To confirm further the requirement of p53 to TMZ-induced glioblastoma cell apoptosis, we generated a p53 knockdown stable U87MG cell by introducing a p53 shRNA plasmid to wild-type U87MG cells through the methods mentioned above (Fig. 4B). TMZ-induced cell death and apoptosis were largely inhibited in the shRNA-transfected low p53 expression stable U87MG cells (Fig. 4, K and L), again supporting that requirement of p53 for TMZ-induced cell apoptosis. Further, p53 knockdown by shRNA largely inhibited TMZ-induced p21 and Noxa/Bax expression, suggesting that p21/Noxa/Bax was induced in a p53-dependent manner after TMZ treatment (Fig. 4B). Importantly, inhibition of the activation of AMPK by Compound C (Fig. 4C) and AMPKα1/2 siRNAs (Fig. 4D) efficiently reduced TMZ-induced p53 activation (phosphorylation at Ser-15 and accumulation) as well as downstream p21/Noxa/Bax induction (Fig. 4, C and D), indicating that activation of AMPK is involved in TMZ-induced p53 activation. We then tested whether there was a direct link between AMPK and p53 in TMZ-treated glioblastoma cells by using a co-immunoprecipitation assay, and we found that AMPKα formed a complex with p53, where AMPK directly phosphorylates p53 (Ser-15) after TMZ or AICAR treatment (Fig. 4, E–I). Notably, AICAR and H2O2, two other AMPK activation agents, were also able to induce p53 activation, which was also inhibited by AMPK siRNA (Fig. 4J). Importantly, the induction of p53/p21 by TMZ was also inhibited by AMPKα1/2 siRNA knockdown in primary cultured human glioblastoma cells (Fig. 5J). Taking all of these results into account, we suggest that TMZ induces AMPK activation, activated AMPK directly binds to and phosphorylates p53, and activated p53 then mediates glioma cell apoptosis, probably by regulation the expression of its downstream target proteins including p21, Noxa, and Bax.
FIGURE 4.
AMPK activation is involved in TMZ-induced p53 activation. A, glioblastoma U87MG cells were treated with 250 μm TMZ and cultured for the indicated times (0, 4, 8, 16, 24 and 48 h). AMPKα1, AMPKα2, ERK1/2, p-AMPKα (Thr-172), p-ACC (Ser-79), phosphorylation (Ser-15) and expression of p53 as well as p21, Noxa, and Bax were detected. B, p53 successfully knocked down U87MG cells (by tranfecting cell with a p53 shRNA plasmid and puromycin selection as described under “Experimental Procedures”) were treated with TMZ (250 μm) and cultured for 24 and 48 h. Phosphorylation (Ser-15) and expression of p53, p21, Noxa, Bax, p-AMPKα (Thr-172) and T-AMPK were detected. C, U87MG cells were pretreated with the AMPK inhibitor Compound C (CC, 10 μm) for 2 h, followed by 250 μm TMZ treatment for 24 h and 48 h. p-AMPKα (Thr-172), p-ACC (Ser-79), T-AMPKα, p-p53 (Ser-15), p21, and Bax were detected. D, U87MG cells transfected with control or AMPKα1/2 siRNA were treated with TMZ (250 μm) for 24 and 48 h. AMPKα1/2, AMPKβ, β-actin, p-AMPKα (Thr-172), p-ACC (Ser-79) as well as phosphorylation (Ser-15) and the expression level of p53, p21, Noxa, Bax, and p-ATM (Ser 1981) were detected. E–H, the association between AMPK and p53 after TMZ (250 μm, 12 and 24 h) (E and F) or AICAR (1 mm, 24 h) (G and H) was detected by co-immunoprecipitation (IP). I, IgG was used here as an internal control, whereas 30 μg of total cell lysate was used as input control. J, U87MG cells transfected with control or AMPKα1/2 siRNA were treated with AICAR (1 mm) or H2O2 (500 μm) for 24 h. The expression levels of AMPKα1/2, p-AMPKα (Thr-172), and AKT1, phosphorylation (Ser-15), and expression level of p53 were detected. IB, immunoblotting. K and L, p53 shRNA-transfected stable U87MG cells were treated with TMZ (250 μm). Cell viability was detected by MTT assay after 48 h (K). Cell apoptosis was detected by histone/DNA ELISA (L) after 36 h. Experiments in this figure were repeated at least three times and similar results were obtained. *, p < 0.05 versus same treatment in scramble siRNA-transfected group (ANOVA).
FIGURE 5.
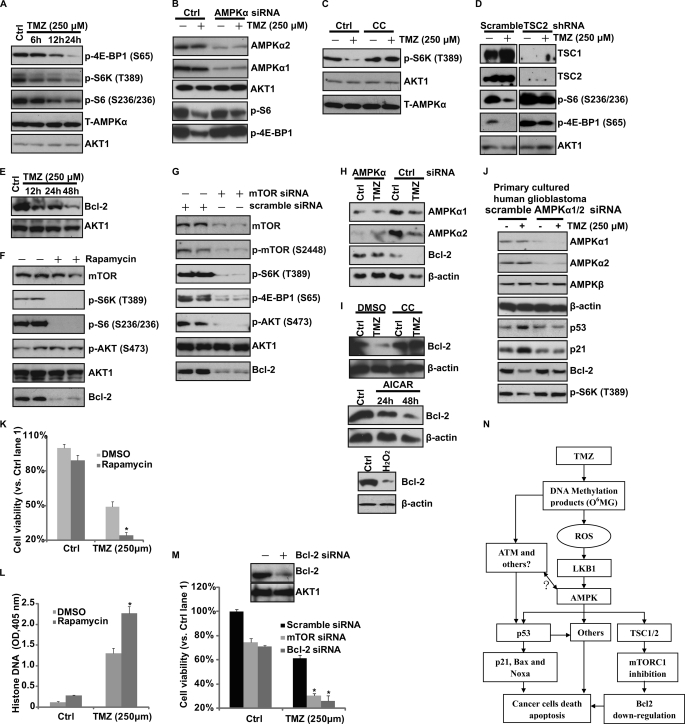
AMPK activation mediates TMZ-induced mTORC1 inhibition in vitro. A, glioblastoma U87MG cells were treated with TMZ (250 μm) for the indicated times. p-S6K (Thr-389), p-4E-BP1 (Ser-65), p-S6 (Ser-235/236), T-AMPKα, and AKT1 were detected. B, U87MG cells transfected with scramble (Ctrl) or AMPKα1/2 siRNA were treated with TMZ (250 μm) for 24 h. p-4E-BP1 (Ser-65), p-S6 (Ser-235/236), and relative loading controls were detected. C, U87MG cells were pretreated with Compound C (CC, 10 μm) for 2 h followed by 250 μm TMZ treatment for 24 h. p-S6K (Thr-389), AKT1, and T-AMPKα were detected. D, U87MG cells were transfected with TSC2/TSC1 shRNA for 48 h. Successfully transfected cells were selected by puromycin. After selection, cells were treated with TMZ (250 μm) for 24 h. p-4E-BP1 (Ser-65), p-S6 (Ser-235/236), TSC1, TSC2, and AKT1 were detected. E, glioblastoma U87MG cells were treated with TMZ (250 μm) for the indicated times. Bcl-2 expression was detected by Western blotting. F, U87MG cells were treated with rapamycin (100 nm) for 12 h. mTOR, p-S6K (Thr-389), p-S6 (Ser-235/236), p-AKT (Ser-473), AKT1, and Bcl-2 were detected. G, U87MG cells were transfected with scramble (Ctrl) or mTOR siRNA for 48 h. mTOR, p-mTOR (Ser-2448), p-S6K (Thr-389), p-AKT (Ser-473), AKT1, and Bcl-2 were detected. H, U87MG cells transfected with control (scramble) or AMPKα1/2 siRNA were treated with TMZ (250 μm) for 48 h. The expression levels of AMPKα1/2, Bcl-2, and β-actin were detected. I, U87MG cells were pretreated with Compound C (CC, 10 μm) for 2 h, followed by 250 μm TMZ treatment for 48 h. Bcl-2 and β-actin were detected. The effects of AICAR (1 mm) and H2O2 (250 μm, 24 h) on Bcl-2 expression were also detected. J, primary cultured human glioblastoma cells transfected with control (scramble) or AMPKα1/2 siRNA were treated with TMZ (250 μm) for 48 h. The expression levels of AMPKα1/2, AMPKβ, p53, p21, Bcl-2, and p-S6K as well as loading control β-actin were detected. K and L, U87MG cells were pretreated with rapamycin (100 nm) for 2 h, followed by 250 μm TMZ treatment. Cell viability (48 h later) was detected by MTT assay. Cell apoptosis (36 h later) was detected by histone/DNA ELISA. M, U87MG cells were transfected with scramble (Ctrl), mTOR, or Bcl-2 siRNA for 48 h followed by TMZ (250 μm) treatment for another 48 h. Cell viability was detected by MTT assay. N, proposed signal pathway involved in this study is shown. TMZ induces AMPK activation in glioblastoma cells, DNA damage caused by TMZ plays an important role in ROS production and following LKB1/AMPK activation. Activation of AMPK contributes TMZ-induced glioblastoma cell apoptosis, probably by regulating p53 (positively) and mTORC1 (negatively) pathways as well as altering the expression of apoptosis-associated proteins, including p21, Bax, Noxa, and Bcl-2. In addition to AMPK, ATM and possible other DNA repair proteins may also be involved in p53 activation by TMZ. Experiments in this figure were repeated at least three times, and similar results were obtained. *, p < 0.05 versus the TMZ-treated group (ANOVA).
AMPK Activation Mediates TMZ-induced mTORC1 (mTOR Complex 1) Inhibition in Vitro
One of the most studied downstream targets of AMPK activation is mTORC1 inhibition. AMPK activation has been postulated to mediate its pro-death/anti-growth effects primarily through mTORC1 inhibition (11, 26, 27). We next tested the effects of TMZ on mTORC1 inhibition. As shown in Fig. 5A, TMZ inhibited the phosphorylation of S6K, S6, and 4ebp1, the indicators of mTORC1 activation. Here, we found that AMPK is the key mediator for TMZ-induced mTORC1 inhibition because inhibition of AMPK by AMPK inhibitor Compound C and AMPKα1/2 siRNA reduced the inhibitory effects on mTORC1 by TMZ (Fig. 5, B and C). Previous studies have shown that activation of AMPK inhibits mTORC1 primarily, although it phosphorylates and activates TSC2, the upstream inhibitor protein of mTOR. shRNA knockdown TSC2/TSC1 almost reversed TMZ-induced mTORC1 inhibition (Fig. 5D), indicating that AMPK inhibits mTORC1 in a TSC1/2-dependent manner. mTORC1 is well known to regulate ribosomal gene expression by increasing translation initiation. Among the genes that is regulated by mTOC1, anti-apoptotic protein Bcl-2 has been implicated in the resistance of tumor cells to the pro-apoptotic effects (17, 28). A recent paper suggested that Bcl-2 is regulated by mTORC1(12). Inhibition of mTORC1 by rapamycin or doxorubicin down-regulates Bcl-2, which mediates cancer cell apoptosis (12). Here, we found that TMZ treatment (Fig. 5E) as well as direct mTORC1 inhibition by rapamycin (Fig. 5F) or mTOR siRNA (Fig. 5G) down-regulates the expression level of Bcl-2 in U87MG cells. Importantly, inhibition of AMPK by Compound C or by AMPK siRNA reversed TMZ-induced Bcl-2 down-regulation, whereas enforced activation of AMPK by H2O2 and AICAR caused Bcl-2 down-regulation (Fig. 5, H and I). Notably, TMZ-induced mTORC1 (p-S6K) inhibition as well as Bcl-2 down-regulation were reversed by AMPKα1/2 siRNA knockdown in primary cultured human glioblastoma cells (Fig. 5J). As expected, rapamycin or mTOR siRNA largely enhanced TMZ-induced cell death and apoptosis (Fig. 5, K–M), and Bcl-2 knockdown by specific siRNA sensitized U87MG cell to TMZ (Fig. 5M). Taking all of these results into account, we suggest that TMZ treatment induces AMPK activation, which inhibits mTORC1signaling in a TSC1/TSC2-dependent manner. mTORC1 inhibition causes Bcl-2 down-regulation which is important to cell apoptosis in glioblastoma cells.
DISCUSSION
Here, we found for the first time that DNA-methylating agents TMZ and MNNG induce AMPK activation in cultured glioblastoma cells in vitro (Fig. 1 and Fig. 2, A and B). We suggest that ROS production that leads to LKB1/AMPK activation by TMZ is associated with induction of DNA-damaging O6-MG. (Fig. 2). Activation of AMPK mediates TMZ-induced glioblastoma cell death and apoptosis in vitro. Although AMPK inhibitor (Compound C) or AMPKα siRNA knockdown inhibits TMZ-induced glioblastoma cell death and apoptosis, AMPK activator AICAR enhanced it (Figs. 2 and 3). We found that AMPK is involved in TMZ-induced p53 activation and induction of pro-apoptosis proteins Noxa and Bax (Fig. 4). Further, activation of AMPK by TMZ also inhibits mTORC1 signaling in a TSC2-dependent manner (Fig. 5), which causes anti-apoptosis protein Bcl-2 down-regulation (Fig. 5) and mediates TMZ-induced pro-apoptosis effects.
During the last years, anticancer drugs with methylating properties, including TMZ, have received much attention, notably in the therapy of malignant glioblastomas. These drugs target DNA, inducing about a dozen DNA methylation products. Studies with MGMT-deficient cells (19) and MGMT-transfected isogenic cell lines (20) revealed that one of the lesions, the minor alkylation product O6-MeG, is a most potent killing lesion (29, 30). It acts as a powerful trigger of apoptosis, probably by inducing p53 (2). Here, we suggest that O6-MeG-DNA may also be related to TMZ-induced ROS production, which then serves as an upstream signal for LKB1 phosphorylation and downstream AMPK activation (Fig. 2). O6-BG, an MGMT inhibitor, enhances TMZ-induced O6-MeG production, leading to enhanced ROS generation and downstream AMPK activation (Fig. 2).
By using multiple different cellular methods, it is well established by several groups that p53 is required for TMZ-induced cell apoptosis and cytotoxicity (2, 21–25). However, published studies also suggest that p53 and p21 seem only required for G2-M arrest duration (31, 32). However, the above conclusion is mainly based on works done in p53-“deficient” U87MG cells where a vector expressing human papilloma virus E6 (U87MG-E6) was transfected (31, 32). To address this issue, here we generated p53-knockdown stable cells by transfecting a p53 shRNA plasmid directly to p53-wild-type U87MG cells, followed by a puromycin selection of stable cells (Fig. 4B). We found that TMZ-induced cell apoptosis is largely inhibited in these p53-knockdown stable glioblastoma cells (Fig. 4, K and L), and we conclude that p53 is required for TMZ-induced cell death and apoptosis in glioblastoma cells. Importantly, we found that p53 is required for TMZ-induced Noxa and Bax expression, two pro-apoptotic proteins. Noxa belongs to the BH3-only branch of Bcl-2 proteins, able to activate pro-apoptotic Bax to trigger mitochondrial membrane permeabilization, cytochrome c release, and downstream apoptosis (33). Noxa/Bax was identified as a target of p53 and mediates apoptosis in response to agents that induce p53-mediated cell death both in vitro and in vivo (33). Our conclusion that p53 is required for TMZ-induced cytotoxicity, probably by inducing pro-apoptotic proteins Noxa/Bax expression, is consistent with previous studies. For example, it is shown that cell apoptosis and Noxa transcription in response to TMZ were inhibited by abrogation of p53 expression using siRNA knockdown (25). However, p53 mimetic agents designed to stabilize the wild-type conformation of p53 sensitize glioma cells for TMZ cytotoxicity (21). Also, previous studies have shown that the p53/p21-related cell cycle arrest was intimately linked to TMZ toxicity. Tumors with a dysfunctional p53 cycle and a weak cell cycle response to DNA damage were extremely unresponsive to TMZ even with the aid of MGMT inactivators (34). Further, tumors with wild-type p53 but lacking a robust increase in p21 protein level were also resistant to TMZ (34).
Our study demonstrates that AMPK activation is involved in TMZ-induced p53 activation (phosphorylation at Ser-15/up-regulation) and p21 up-regulation (Fig. 4), which is consistent with previous studies. For example, activation of AMPK by AICAR suppresses the growth of hepatoma HepG2 cells and induces expression of wild-type p53 and, secondarily, p21 (35). It also enhances the phosphorylation of Ser-15 in the transactivation domain of p53, which activates p53 and induces apoptosis (6, 7, 36). Intriguingly, this site (Ser-15) is phosphorylated by purified AMPK in vitro (6, 7). In addition, ectopic expression of wild-type AMPK kinase LKB1 inhibits the growth and increases the concentration of p21 in LKB1-deficient A549 lung adenocarcinoma cells (8, 37). By using an immunoprecipitation assay, here we demonstrated that AMPK binds to and phosphorylates p53 (Ser-15) directly in response to TMZ treatment (Fig. 4) and then activates p53 and could be the key mechanism mediates glioblastoma cell death/apoptosis in vitro. Because activation of p53 is so important in regulating the TMZ cytotoxic effect, it is not surprising to see that kinases other than AMPK are also able to regulate p53 phosphorylation (Ser-15) after TMZ treatment. It is noted that knockdown of ATM by siRNA also reduced p53 phosphorylation and accumulation (see supplemental Fig. S1). At this point, we do not know whether there is a direct link between AMPK activation and ATM, and we suggest that AMPK is among several kinases that regulate p53 activation in response to TMZ.
Besides positively regulating p53, AMPK activation might also induce tumor cell death or inhibit tumor cell growth by several other mechanisms, of which, the most studied is by activating TSC2, which then inhibits mTORC1 signaling and protein synthesis (38, 39). The regulation of TSC2 and mTOR by AMPK has special implications because the PI3K-Akt signaling pathway is constitutively active in many cancers, including glioblastomas, most notably those that have either inactivating mutations of the PTEN gene (e.g. gliomas, melanoma, advanced prostate cancer, and endometrial carcinomas) (40) or overexpression of an activated member of the epidermal growth factor (EGF) receptor family (e.g. Her2/NEW in breast cancer) (9, 41). One important consequence of the constitutive activation of AKT is phosphorylation of TSC2 at a site different from that phosphorylated by AMPK. This leads to inhibition of TSC2, activation of mTORC1 signaling, and an increase in protein synthesis, which have been involved in tumor growth and survival. Here, we show that activation of AMPK inhibits mTORC1 activation after TMZ treatment in glioblastoma cell line (both cell lines and primary culture) in vitro (Fig. 5). Knockdown TSC2 or AMPK reverses the inhibitory effects of TMZ on mTORC1 activation in glioblastoma cell (Fig. 5). Based on these findings, we suggest that activation of AMPK by TMZ inhibits the activation of mTORC1 in a TSC2-dependent manner; inhibition of mTORC1 facilitates TMZ-induced glioblastoma cell death/apoptosis probably by down-regulating pro-apoptosis protein Bcl-2 (Fig. 5).
In summary, here we found for the first time that TMZ induces AMPK activation in cultured glioblastoma cells. ROS production, as a result of DNA damage after TMZ treatment, serves as an upstream signal for LKB1/AMPK activation. Activation of AMPK contributes to TMZ-induced glioblastoma cell apoptosis, probably by promoting p53 activation and inhibiting mTORC1 signaling as well as altering the expression level of apoptosis-related proteins, including p21, Noxa, Bax, and Bcl-2.
Supplementary Material
This work was supported by National Natural Science Foundation of China Grants 30701032 and 81072661, the New Century Excellent Talents Program supported by Ministry of Education of China (2009) (Y. Y.), Fundamental Research Funds for the Central Universities Grant JKZ2009006, Natural Science Foundation of Jiangsu Province Grant BK2009298, and “Major Drug Discovery” science and technology major projects of China Grant 2009ZX09303-001.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- TMZ
- temozolomide
- ACC
- acetyl-CoA carboxylase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-riboside
- AMPK
- AMP-activated protein kinase
- ATM
- ataxia telangiectasia mutated
- MGMT
- O6-methylguanine-DNA methyltransferase
- MNNG
- N-methyl-N′-nitro-N-nitrosoguanidine
- MTT
- 3-(4,5-dimethylthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- mTOR
- mammalian target of rapamycin
- mTORC1
- mTOR complex 1
- O6-BG
- O6-benzylguanine
- O6-MeG
- O6-methylguanine
- NAc
- N-acetylcysteine
- ROS
- reactive oxygen species.
REFERENCES
- 1.Conrad C. A., Milosavljevic V. P., Yung W. K. (1995) Neurol. Clin. 13, 795–812 [PubMed] [Google Scholar]
- 2.Roos W. P., Batista L. F., Naumann S. C., Wick W., Weller M., Menck C. F., Kaina B. (2007) Oncogene 26, 186–197 [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Gil M., Pesi R., Perna S., Allegrini S., Giannecchini M., Camici M., Tozzi M. G. (2003) Neuroscience 117, 811–820 [DOI] [PubMed] [Google Scholar]
- 4.Pan W., Yang H., Cao C., Song X., Wallin B., Kivlin R., Lu S., Hu G., Di W., Wan Y. (2008) Oncol. Rep. 20, 1553–1559 [PubMed] [Google Scholar]
- 5.Guo D., Hildebrandt I. J., Prins R. M., Soto H., Mazzotta M. M., Dang J., Czernin J., Shyy J. Y., Watson A. D., Phelps M., Radu C. G., Cloughesy T. F., Mischel P. S. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 12932–12937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones R. G., Plas D. R., Kubek S., Buzzai M., Mu J., Xu Y., Birnbaum M. J., Thompson C. B. (2005) Mol. Cell 18, 283–293 [DOI] [PubMed] [Google Scholar]
- 7.Okoshi R., Ozaki T., Yamamoto H., Ando K., Koida N., Ono S., Koda T., Kamijo T., Nakagawara A., Kizaki H. (2008) J. Biol. Chem. 283, 3979–3987 [DOI] [PubMed] [Google Scholar]
- 8.Xiang X., Saha A. K., Wen R., Ruderman N. B., Luo Z. (2004) Biochem. Biophys. Res. Commun. 321, 161–167 [DOI] [PubMed] [Google Scholar]
- 9.Luo Z., Saha A. K., Xiang X., Ruderman N. B. (2005) Trends Pharmacol. Sci. 26, 69–76 [DOI] [PubMed] [Google Scholar]
- 10.Hardie D. G., Carling D., Carlson M. (1998) Annu. Rev. Biochem. 67, 821–855 [DOI] [PubMed] [Google Scholar]
- 11.Cao C., Lu S., Kivlin R., Wallin B., Card E., Bagdasarian A., Tamakloe T., Chu W. M., Guan K. L., Wan Y. (2008) J. Biol. Chem. 283, 28897–28908 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Ji C., Yang B., Yang Y. L., He S. H., Miao D. S., He L., Bi Z. G. (2010) Oncogene, in press [DOI] [PubMed] [Google Scholar]
- 13.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Z., Dong Y., Zhang M., Cui M. Z., Cohen R. A., Riek U., Neumann D., Schlattner U., Zou M. H. (2006) J. Biol. Chem. 281, 6366–6375 [DOI] [PubMed] [Google Scholar]
- 15.Winder W. W., Hardie D. G. (1996) Am. J. Physiol. Endocrinol. Metab. 270, E299–E304 [DOI] [PubMed] [Google Scholar]
- 16.Kim D. S., Woo E. R., Chae S. W., Ha K. C., Lee G. H., Hong S. T., Kwon D. Y., Kim M. S., Jung Y. K., Kim H. M., Kim H. K., Kim H. R., Chae H. J. (2007) Life Sci. 80, 314–323 [DOI] [PubMed] [Google Scholar]
- 17.Zhang J. F., Liu J. J., Lu M. Q., Cai C. J., Yang Y., Li H., Xu C., Chen G. H. (2007) Transpl. Immunol. 17, 162–168 [DOI] [PubMed] [Google Scholar]
- 18.Kim W. H., Lee J. W., Suh Y. H., Lee H. J., Lee S. H., Oh Y. K., Gao B., Jung M. H. (2007) Cell. Signal. 19, 791–805 [DOI] [PubMed] [Google Scholar]
- 19.Preuss I., Thust R., Kaina B. (1996) Int. J. Cancer 65, 506–512 [DOI] [PubMed] [Google Scholar]
- 20.Kaina B., Fritz G., Mitra S., Coquerelle T. (1991) Carcinogenesis 12, 1857–1867 [DOI] [PubMed] [Google Scholar]
- 21.Hermisson M., Klumpp A., Wick W., Wischhusen J., Nagel G., Roos W., Kaina B., Weller M. (2006) J. Neurochem. 96, 766–776 [DOI] [PubMed] [Google Scholar]
- 22.Tentori L., Lacal P. M., Benincasa E., Franco D., Faraoni I., Bonmassar E., Graziani G. (1998) J. Pharmacol. Exp. Ther. 285, 884–893 [PubMed] [Google Scholar]
- 23.D'Atri S., Tentori L., Lacal P. M., Graziani G., Pagani E., Benincasa E., Zambruno G., Bonmassar E., Jiricny J. (1998) Mol. Pharmacol. 54, 334–341 [DOI] [PubMed] [Google Scholar]
- 24.Naumann S. C., Roos W. P., Jöst E., Belohlavek C., Lennerz V., Schmidt C. W., Christmann M., Kaina B. (2009) Br. J. Cancer 100, 322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong J. L., Veal G. J., Redfern C. P., Lovat P. E. (2007) Apoptosis 12, 613–622 [DOI] [PubMed] [Google Scholar]
- 26.Gwinn D. M., Shackelford D. B., Egan D. F., Mihaylova M. M., Mery A., Vasquez D. S., Turk B. E., Shaw R. J. (2008) Mol. Cell 30, 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleason C. E., Lu D., Witters L. A., Newgard C. B., Birnbaum M. J. (2007) J. Biol. Chem. 282, 10341–10351 [DOI] [PubMed] [Google Scholar]
- 28.Romano M. F., Avellino R., Petrella A., Bisogni R., Romano S., Venuta S. (2004) Eur. J. Cancer 40, 2829–2836 [DOI] [PubMed] [Google Scholar]
- 29.Meikrantz W., Bergom M. A., Memisoglu A., Samson L. (1998) Carcinogenesis 19, 369–372 [DOI] [PubMed] [Google Scholar]
- 30.Kaina B., Ziouta A., Ochs K., Coquerelle T. (1997) Mutat. Res. 381, 227–241 [DOI] [PubMed] [Google Scholar]
- 31.Hirose M., Kuroda Y. (1998) Cancer Lett. 129, 165–171 [DOI] [PubMed] [Google Scholar]
- 32.Hirose Y., Berger M. S., Pieper R. O. (2001) Cancer Res. 61, 5843–5849 [PubMed] [Google Scholar]
- 33.Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. (2000) Science 288, 1053–1058 [DOI] [PubMed] [Google Scholar]
- 34.Bocangel D. B., Finkelstein S., Schold S. C., Bhakat K. K., Mitra S., Kokkinakis D. M. (2002) Clin. Cancer Res. 8, 2725–2734 [PubMed] [Google Scholar]
- 35.Imamura K., Ogura T., Kishimoto A., Kaminishi M., Esumi H. (2001) Biochem. Biophys. Res. Commun. 287, 562–567 [DOI] [PubMed] [Google Scholar]
- 36.Bode A. M., Dong Z. (2004) Nat. Rev. Cancer 4, 793–805 [DOI] [PubMed] [Google Scholar]
- 37.Jimenez A. I., Fernandez P., Dominguez O., Dopazo A., Sanchez-Cespedes M. (2003) Cancer Res. 63, 1382–1388 [PubMed] [Google Scholar]
- 38.Inoki K., Zhu T., Guan K. L. (2003) Cell 115, 577–590 [DOI] [PubMed] [Google Scholar]
- 39.Cheng S. W., Fryer L. G., Carling D., Shepherd P. R. (2004) J. Biol. Chem. 279, 15719–15722 [DOI] [PubMed] [Google Scholar]
- 40.Cantley L. C., Neel B. G. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 4240–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou B. P., Hung M. C. (2003) Semin. Oncol. 30, 38–48 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.