Abstract
Neurotransmitter release is triggered by Ca2+ binding to a low affinity Ca2+ sensor, mostly synaptotagmin-1, which catalyzes SNARE-mediated synaptic vesicle fusion. Tomosyn negatively regulates Ca2+-dependent neurotransmitter release by sequestering target SNAREs through the C-terminal VAMP-like domain. In addition to the C terminus, the N-terminal WD40 repeats of tomosyn also have potent inhibitory activity toward Ca2+-dependent neurotransmitter release, although the molecular mechanism underlying this effect remains elusive. Here, we show that through its N-terminal WD40 repeats tomosyn directly binds to synaptotagmin-1 in a Ca2+-dependent manner. The N-terminal WD40 repeats impaired the activities of synaptotagmin-1 to promote SNARE complex-mediated membrane fusion and to bend the lipid bilayers. Decreased acetylcholine release from N-terminal WD40 repeat-microinjected superior cervical ganglion neurons was relieved by microinjection of the cytoplasmic domain of synaptotagmin-1. These results indicate that, upon direct binding, the N-terminal WD40 repeats negatively regulate the synaptotagmin-1-mediated step of Ca2+-dependent neurotransmitter release. Furthermore, we show that synaptotagmin-1 binding enhances the target SNARE-sequestering activity of tomosyn. These results suggest that the interplay between tomosyn and synaptotagmin-1 underlies inhibitory control of Ca2+-dependent neurotransmitter release.
Keywords: Exocytosis, Membrane Fusion, Membrane Trafficking, Protein-Protein Interactions, Signal Transduction
Introduction
Neurotransmitter release arises from Ca2+-dependent exocytosis of synaptic vesicles (1). At the presynaptic active zone, synaptic vesicles are docked beside Ca2+ channels and primed to become fusion-competent. Upon reaching presynaptic nerve terminals, action potentials open Ca2+ channels. The resulting Ca2+ influx triggers fusion of primed synaptic vesicles to the presynaptic plasma membrane. Exocytotic efficacy of synaptic vesicles is adequately controlled (1–3). The number of synaptic vesicles in the readily releasable pool (RRP)2 is extremely small (1–2% of the total number of vesicles in a presynaptic terminal) (4–6), and the vast majority of synaptic vesicles reside in the presynaptic nerve terminal despite regular Ca2+ influx (2, 3). A subset of the residing vesicles (10–20% of the total vesicles) constitutes the recycling pool (7, 8), which undergoes exo- and endocytosis under stimulation at physiological frequency (2, 3). The synaptic vesicles in the recycling pool are accordingly mobilized to prevent depletion of the RRP (2, 3, 9), thereby enabling neurons to respond to an appropriate frequency of action potentials.
The vesicle fusion process is catalyzed by three soluble N-ethylmaleimide-sensitive fusion protein attachment protein (SNAP) receptors (SNAREs), syntaxin-1, SNAP-25, and VAMP2 (10, 11). Syntaxin-1 and SNAP-25 are localized within the presynaptic plasma membrane as target SNAREs (t-SNAREs), and VAMP2 resides in synaptic vesicles as a vesicular SNARE (v-SNARE). The three SNARE proteins are thought to assemble in a zipper-like fashion and form a stable SNARE complex. As a consequence, the synaptic vesicles and the presynaptic plasma membrane are pulled into close apposition, leading to mixing of the two lipid bilayers (10–13). In this process, synaptotagmin-1, a synaptic vesicle protein with two C2 domains that both bind to Ca2+, serves as a Ca2+ sensor (14–20). Upon Ca2+ binding, synaptotagmin-1 binds to the SNARE complex (21) and induces positive curvature of the target membrane by inserting the hydrophobic loops in the C2 domains into the membrane (22–25). This synaptotagmin-1-mediated membrane bending leads to the fast synaptic vesicle fusion that occurs in response to Ca2+ influx (22–30). On the other hand, it is largely unknown how synaptotagmin-1 is adequately inactivated to control the exocytotic efficacy of synaptic vesicles.
Tomosyn, a syntaxin-1-binding protein that we originally identified (31), sequesters t-SNAREs on the presynaptic plasma membrane through the C-terminal VAMP-like domain and thereby inhibits SNARE complex formation (31–36). Consistent with an inhibitory activity toward SNARE complex formation, the genetic ablation of tomosyn in mice and Caenorhabditis elegans leads to enhancement of neurotransmitter release (34, 37, 38), and its overexpression in superior cervical ganglion (SCG) neurons inhibits neurotransmitter release induced by an action potential (39). Accumulating evidence suggests that tomosyn controls the exocytotic efficacy of synaptic vesicles. Paired pulse facilitation at mossy fiber synapses of hippocampi is decreased in the tomosyn-deficient mice (34). In response to repetitive presynaptic action potentials, tomosyn-overexpressing neurons show severe synaptic depression in contrast to the remarkable synaptic facilitation seen in control neurons (39). However, it is unclear how tomosyn controls Ca2+-dependent exocytosis because the C-terminal VAMP-like domain sequesters t-SNAREs in a Ca2+-independent manner.
The N-terminal WD40 repeats of tomosyn are also responsible for potent inhibition of neurotransmitter release. We have demonstrated that acetylcholine release from SCG neurons is potently inhibited by microinjecting a tomosyn fragment encompassing the N-terminal WD40 repeats (34). It has been demonstrated that catecholamine secretion is potently inhibited in chromaffin cells by overexpressing the N-terminal WD40 repeats of tomosyn (40). Intriguingly, the inhibitory activity of the N-terminal WD40 repeats in chromaffin cells was dependent on Ca2+ concentration (40, 41). Similarly, we reported that the inhibitory activity of tomosyn in SCG neurons is influenced by Ca2+ concentration (39). Although we have shown that tomosyn oligomerizes the SNARE complex through its N-terminal WD40 repeats (34), this does not account for the Ca2+-dependent inhibitory activity of the N-terminal WD40 repeats because the oligomerization takes place in a Ca2+-independent manner. Therefore, the N-terminal WD40 repeats must functionally interact with a Ca2+-responsive protein(s) involved in the regulation of synaptic vesicle fusion. In C. elegans, tomosyn associates with synaptic vesicles through the N-terminal WD40 repeats (38), raising the possibility that the N-terminal WD40 repeats may negatively regulate the function of synaptic vesicles. However, the binding partner(s) on synaptic vesicles for the N-terminal WD40 repeats remains unknown. To understand the molecular mechanism by which tomosyn controls neurotransmitter release, it is crucial to elucidate how the N-terminal WD40 repeats inhibit neurotransmitter release.
In this study, we demonstrate that tomosyn directly binds to synaptotagmin-1 through the N-terminal WD40 repeats, in a Ca2+-dependent manner, and impairs synaptotagmin-1 catalysis. These results indicate that tomosyn negatively regulates the synaptotagmin-1-mediated step of Ca2+-dependent neurotransmitter release through the N-terminal WD40 repeats. We also demonstrate that this binding positively regulates the activity of the C-terminal VAMP-like domain of tomosyn to form the tomosyn-SNARE complex. These results raise the possibility that the interplay between tomosyn and synaptotagmin-1 underlies the inhibitory control of Ca2+-dependent neurotransmitter release.
EXPERIMENTAL PROCEDURES
Recombinant Proteins
The MBP-fused full-length tomosyn-1 (amino acids 1–1116) (MBP-tomosyn) and the MBP-fused large N-terminal fragment of tomosyn-1 (amino acids 1–949) (MBP-tomosyn-N) were expressed in Sf21 cells and purified as described previously (34). The MBP-fused fragment encompassing both the tail domain and the VAMP-like domain (amino acids 933–1116) (MBP-tomosyn-C) was expressed in Escherichia coli and purified as described previously (35). Full-length syntaxin-1 (amino acids 1–289), the cytoplasmic domain of syntaxin-1 (syntaxin-1-ΔTM) (amino acids 1–265), full-length SNAP-25 (amino acids 1–206), full-length VAMP2 (amino acids 1–116), and the cytoplasmic domain of synaptotagmin-1 (ΔTM-synaptotagmin-1) (amino acids 80–421) were expressed in E. coli as GST fusion proteins and purified as described previously (34, 35). To remove the GST tags, the GST fusion proteins were digested with PreScission protease (GE Healthcare) or thrombin and then further purified as described previously (34, 35).
Immunoprecipitation of Tomosyn from Synaptosomes
Synaptosomes were isolated from rat brains as described previously (42). One milligram of the synaptosomes was solubilized with Buffer A containing 10 mm HEPES/NaOH, pH 7.4, 140 mm NaCl, 5 mm NaHCO3, 1.2 mm NaH2PO4, 1 mm MgCl2, 10 mm glucose, 5 mm KCl, 1 mm EGTA, 1.3 mm CaCl2, and 1% (w/v) Triton X-100 at 4 °C for 60 min followed by ultracentrifugation at 100,000 × g at 4 °C for 20 min. The supernatant was incubated with an anti-tomosyn pAb immobilized on protein A-Sepharose at 4 °C for 60 min. The immobilized antibodies were washed with Buffer A three times, and the bound proteins were solubilized in SDS sample buffer with boiling. The samples were subjected to SDS-PAGE followed by immunoblotting with an anti-tomosyn pAb, an anti-synaptotagmin-1 mAb, and an anti-VAMP2 mAb.
Direct Binding of Synaptotagmin-1 to Tomosyn
ΔTM-synaptotagmin-1 (3400 pmol) was incubated with 80 pmol of MBP-tomosyn or MBP immobilized on 20 μl of amylose beads in Buffer B containing 20 mm Tris/HCl, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm dithiothreitol (DTT), and 0.5% (w/v) Nonidet P-40 at 4 °C for 5 h. After the beads had been extensively washed with Buffer B three times, the bound proteins were eluted in the SDS sample buffer with boiling. The samples were subjected to SDS-PAGE followed by CBB staining.
Direct Binding of Synaptotagmin-1 to N-terminal WD40 Repeats of Tomosyn in Response to Ca2+
ΔTM-synaptotagmin-1 (2000 pmol) was incubated with 100 pmol of MBP-tomosyn-N, MBP-tomosyn-C, or MBP immobilized on 20 μl of amylose beads in 1 ml of Buffer B or 1 ml of Buffer C containing 20 mm Tris/HCl, pH 7.5, 150 mm NaCl, 1 mm CaCl2, 1 mm DTT, and 0.5% (w/v) Nonidet P-40 at 4 °C overnight. After the beads had been extensively washed with Buffer B or Buffer C three times, the bound proteins were eluted in SDS sample buffer with boiling. The samples were subjected to SDS-PAGE followed by CBB staining. To obtain the Kd value for the binding of synaptotagmin-1 to N-terminal WD40 repeats, ΔTM-synaptotagmin-1 was titrated in Buffer C, and the amounts of bound and free ΔTM-synaptotagmin-1 were quantified and plotted. The Kd value was calculated by Scatchard analysis.
Direct Bindings of t-SNAREs to N-terminal WD40 Repeats of Tomosyn and Full-length Tomosyn
For analysis of the binding of syntaxin-1 or SNAP-25 to the N-terminal WD40 repeats of tomosyn, various concentrations of syntaxin-1-ΔTM or SNAP-25 were incubated with 100 pmol of MBP-tomosyn-N immobilized on 20 μl of amylose beads in 1 ml of Buffer C at 4 °C overnight. After the beads had been extensively washed with Buffer C three times, the bound proteins were eluted in the SDS sample buffer with boiling. The samples were subjected to SDS-PAGE followed by CBB staining or immunoblotting with an anti-syntaxin-1 mAb or an anti-SNAP-25 mAb. The amounts of bound and free syntaxin-1-ΔTM or SNAP-25 were quantified and plotted. The Kd values were calculated by Scatchard analysis. For analysis of the binding of syntaxin-1 and SNAP-25 to full-length tomosyn, equal concentrations of syntaxin-1-ΔTM and SNAP-25 were incubated with 100 pmol of MBP-tomosyn immobilized on 20 μl of amylose beads at various concentrations in 1 ml of Buffer C at 4 °C overnight. The bound proteins were eluted in the same manner as described above, and the Kd value was calculated. To compare the binding of t-SNAREs to tomosyn and the binding of synaptotagmin-1 to tomosyn, equal concentrations of syntaxin-1-ΔTM, SNAP-25, and ΔTM-synaptotagmin-1 were incubated with 100 pmol of MBP-tomosyn immobilized on 20 μl of amylose beads at various concentrations in 1 ml of Buffer C at 4 °C overnight, and the bound proteins were eluted in the same manner as described above.
Liposome Preparation
All lipids were purchased from Avanti Polar Lipids, and the SNARE-bearing liposomes were prepared as reported previously with a slight modification (13). Briefly, for preparation of t-SNARE vesicles, 150 μl of a 10 mm lipid mixture composed of 85 mol % 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 15 mol % 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS) dissolved in chloroform was dried down in 10 × 75-mm test tubes by a gentle stream of N2 gas, and any remaining traces of chloroform were then removed under vacuum for more than 30 min. The lipid films were dissolved in 500 μl of liposome buffer A (50 mm Tris/HCl, pH 8.0, 100 mm KCl, 10% (w/v) glycerol, 1 mm DTT, and 0.8% (w/v) n-octyl β-d-glucopyranoside) containing 0.8 mg/ml syntaxin-1 and 0.8 mg/ml SNAP-25 for the t-SNARE vesicles by gentle agitation for 15 min at room temperature (RT). For preparation of fluorescently labeled v-SNARE vesicles, 100 μl of a 3 mm lipid mixture composed of 82 mol % POPC, 15 mol % DOPS, 1.5 mol % N-(7-nitro-2,1,3-benzoxadiaziole-4-yl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (NBD-DPPE), and 1.5 mol % N-(lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl-sn-glycero-3-phophoethanolamine (rhodamine-DPPE) dissolved in chloroform was dried in 10 × 75-mm test tubes in the same manner as described above. The lipid film was dissolved in 100 μl of liposome buffer B (50 mm Tris/HCl, pH 8.0, 100 mm KCl, 10% (w/v) glycerol, 1 mm DTT, and 1% (w/v) n-octyl β-d-glucopyranoside) containing 0.4 mg/ml VAMP2 by gentle agitation for 15 min at RT. The samples were diluted 3-fold with reconstitution buffer (25 mm HEPES/KOH, pH 7.4, 100 mm KCl, 10% (w/v) glycerol, and 1 mm DTT) so that the level of n-octyl β-d-glucopyranoside was below its critical micellar concentration. Then the samples were dialyzed against 4 liters of reconstitution buffer supplemented with 4 g of Biobeads SM-2 (Bio-Rad) using 7-kDa cutoff membranes at RT for 1 h. The dialysis was continued at 4 °C overnight with no buffer change. The dialysates were mixed with equal volumes of 80% (w/v) Nycodenz (Sigma) dissolved in reconstitution buffer. For the t-SNARE vesicles, 1.3 ml of the sample was overlaid with 1 ml of 30% (w/v) Nycodenz dissolved in reconstitution buffer followed by 1 ml of reconstitution buffer lacking glycerol. For the v-SNARE vesicles, 700 μl of the sample was overlaid with 1 ml of 30% (w/v) Nycodenz dissolved in reconstitution buffer followed by 1.5 ml of reconstitution buffer lacking glycerol. The samples were centrifuged at 200,000 × g at 4 °C for 4 h. The vesicles were harvested from the 0/30% Nycodenz interface.
Liposome Fusion Assay
ΔTM-synaptotagmin-1 (120 pmol), MBP-tomosyn-N (120 pmol), MBP-tomosyn-C (120 pmol), the mixture of ΔTM-synaptotagmin-1 (120 pmol) and MBP-tomosyn-N (120 pmol), and the mixture of ΔTM-synaptotagmin-1 (120 pmol) and MBP-tomosyn-C (120 pmol) were incubated in 30 μl of reconstitution buffer supplemented with 1 mm CaCl2 at 4 °C for 1 h, respectively. Then the t-SNARE vesicles, within which 60 pmol of each of t-SNAREs was embedded, were added to each sample and incubated in 50 μl of reconstitution buffer supplemented with 1 mm CaCl2 at 4 °C for 1 h. As positive and negative controls, the t-SNARE vesicles and liposomes with no t-SNARE proteins (control vesicles) were simply incubated in 50 μl of reconstitution buffer supplemented with 1 mm CaCl2 at 4 °C for 1 h, respectively. The samples were mixed with 8 μl of fluorescently labeled v-SNARE vesicles on ice, and immediately 50 μl of each of the samples was placed in a 96-well plate (Nunc) on an ice-cold metal block followed by overlaying with 50 μl of mineral oil (Sigma). The increase in NBD fluorescence upon lipid mixing was monitored at 1-min intervals for 60 min at 37 °C using a fluorometer (Fluoroskan Ascent FL, Thermo Scientific) with the filters set at 460 (excitation) and 538 nm (emission). Then 10 μl of 2.5% (w/v) Triton X-100 was added to terminate the fusion reactions and dequench the NBD fluorescence. To normalize the fusion-dependent fluorescence, the lowest NBD fluorescent signals and the maximum signals after Triton X-100 addition were set to 0 and 100% fluorescence, respectively.
Liposome Tubulation Assay
Brain lipid extract (Folch fraction I) was dried and resuspended at 1 mg/ml in a buffer containing 50 mm HEPES/KOH, pH 7.4, and 100 mm KCl. Folch liposomes were produced by sonication. ΔTM-synaptotagmin-1 (9 μm), MBP-tomosyn-N (9 μm), and a mixture of ΔTM-synaptotagmin-1 (9 μm) and MBP-tomosyn-N (9 μm) were incubated in a buffer containing 25 mm HEPES/KOH, pH 7.4, 100 mm KCl, 1 mm CaCl2, and 1 mm DTT at 4 °C for 3 h, respectively. Folch liposomes (0.2 mg/ml) were added to each sample and incubated at RT for 30 min. For the control reaction, 0.2 mg/ml Folch liposomes were simply suspended in buffer. The samples were loaded on a carbon-coated grid (glow-discharged) and stained with 2% (w/v) uranyl acetate. Negatively stained samples were viewed on an H-7650 transmission electron microscope (Hitachi, Tokyo, Japan) operated at 80 kV, and images were recorded at a nominal magnification of 20,000 using a 1024 × 1024 charge-coupled device camera (Tietz Video and Image Processing Systems, Gauting, Germany).
Synaptic Transmission between SCG Neurons
Postnatal day 7 Wistar ST rats were decapitated under diethyl ether anesthesia according to the guidelines of the Physiological Society of Japan. Isolated SCG neurons were maintained in culture for 5–6 weeks as described (43, 44). In brief, SCGs were dissected, desheathed, and incubated with collagenase (0.5 mg/ml; Worthington) in L-15 (Invitrogen) at 37 °C for 10 min. Following enzyme digestion, semidissociated ganglia were triturated gently through small pore glass pipettes until a cloudy suspension was observed. After washing by low speed centrifugation at 1300 rpm for 3 min, the collected cells were plated onto coverslips in plastic dishes (Corning; 35-mm diameter; approximately one ganglion per dish) containing a growth medium of 84% Eagle's minimal essential medium (Invitrogen), 10% fetal calf serum (Invitrogen), 5% horse serum (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 25 ng/ml nerve growth factor (2.5 S, grade II; Alomone Laboratories). The cells were maintained at 37 °C in a 95% air, 5% CO2-humidified incubator, and the medium was changed twice per week. Excitatory postsynaptic potential (EPSP) recordings and injection of the recombinant proteins were performed as described previously (45–47). Electrophysiological data collected using software written by the late L. Tauc (Centre National de la Recherche Scientifique, Gif-sur-Yvette, France) were analyzed with Origin (Microcal Software Inc.). Statistical significance was determined by Bonferroni post hoc test after one-way analysis of variance.
Assay for Synaptotagmin-1-enhanced Tomosyn-SNARE Complex Formation
Various concentrations of ΔTM-synaptotagmin-1 were incubated with 220 pmol of MBP-tomosyn or MBP-tomosyn-N immobilized on 50 μl of amylose beads in Buffer B or Buffer C at 4 °C for 5 h to allow synaptotagmin-1-tomosyn complex formation. After the beads had been extensively washed, the beads were incubated with 220 pmol of syntaxin-1-ΔTM and 220 pmol of SNAP-25 in Buffer B or Buffer C at 4 °C for 3 h to allow tomosyn-SNARE complex formation. After the beads had been extensively washed with Buffer B or Buffer C three times, the bound proteins were eluted in SDS sample buffer with boiling. Then the samples were subjected to SDS-PAGE followed by CBB staining or immunoblotting with the anti-syntaxin-1 mAb or the anti-SNAP-25 mAb.
RESULTS
Direct Binding of Tomosyn to Synaptotagmin-1
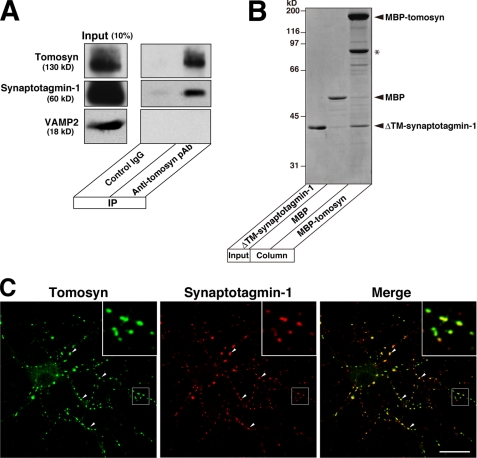
In this study, we sought to elucidate the molecular mechanism by which the N-terminal WD40 repeats of tomosyn inhibit neurotransmitter release in response to Ca2+. In C. elegans, TOM-1, a tomosyn orthologue, has been shown to be associated with synaptic vesicles through its N-terminal WD40 repeats, although the binding partner(s) on synaptic vesicles remains unknown (38). In addition, a proteomics analysis of synaptic vesicles isolated from rat brain identified tomosyn (48). Therefore, we first examined whether mammalian tomosyn associated with a synaptic vesicle protein(s). Synaptosomes isolated from rat brains were extracted with Triton X-100 and immunoprecipitated with the anti-tomosyn pAb or a control IgG followed by immunoblotting with the anti-tomosyn pAb and various antibodies against synaptic vesicle proteins. Of the synaptic vesicle proteins we tested, synaptotagmin-1 was efficiently co-immunoprecipitated with tomosyn (Fig. 1A and data not shown). We next assessed the binding of tomosyn to synaptotagmin-1 using purified recombinant proteins. MBP-fused full-length tomosyn (MBP-tomosyn) and MBP alone were immobilized on amylose resin and incubated with the cytoplasmic domain of synaptotagmin-1 (ΔTM-synaptotagmin-1). ΔTM-synaptotagmin-1 specifically bound to the immobilized MBP-tomosyn but not to MBP (Fig. 1B). These results indicate that tomosyn directly binds to synaptotamin-1. Cultured hippocampal primary neurons at 15 days in vitro were doubly immunostained with the anti-tomosyn pAb and the anti-synaptotagmin-1 mAb. In agreement with the association of tomosyn with synaptic vesicles in C. elegans, a subset of tomosyn co-localized with synaptotagmin-1 (Fig. 1C). In addition, we observed that tomosyn co-localized with a subset of synaptotagmin-1 in the CA3 area of mouse hippocampus (supplemental Fig. 1).
FIGURE 1.
Direct binding of tomosyn to synaptotagmin-1. A, co-immunoprecipitation (IP) of synaptotagmin-1 with tomosyn from synaptosomes. The synaptosomes isolated from rat brains were solubilized with Triton X-100 and immunoprecipitated with anti-tomosyn pAb. Control IgG was used as a negative control. The immunoprecipitates were subjected to SDS-PAGE followed by immunoblotting with the respective antibodies. The results shown are representative of three independent experiments. B, direct binding of tomosyn to synaptotagmin-1. MBP-tomosyn or MBP alone was immobilized on amylose beads and incubated with ΔTM-synaptotagmin-1. The bound proteins were eluted with SDS sample buffer and subjected to SDS-PAGE followed by CBB staining. The asterisk indicates degraded MBP-tomosyn. The result shown is representative of three independent experiments. C, co-localization of tomosyn and synaptotagmin-1 in cultured hippocampal primary neurons. The cultured hippocampal primary neurons were double immunostained with anti-tomosyn pAb and anti-synaptotagmin-1 mAb at 15 days in vitro. Insets are enlarged images of the boxed areas. The arrowheads indicate the representative areas where tomosyn co-localizes with synaptotagmin-1. Bar, 20 μm.
Binding of Tomosyn to Synaptotagmin-1 through N-terminal WD40 Repeats
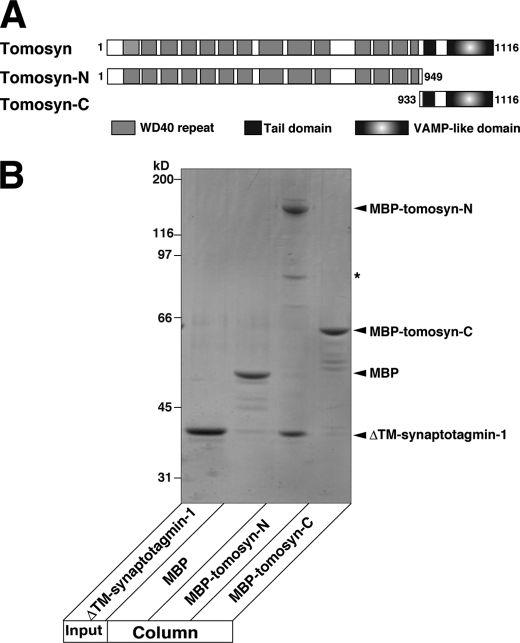
We sought to determine which region of tomosyn was responsible for binding to synaptotagmin-1. We made two kinds of MBP-fused fragments of tomosyn, a fragment encompassing the N-terminal WD40 repeats (MBP-tomosyn-N) and a fragment encompassing the tail domain and the C-terminal VAMP-like domain (MBP-tomosyn-C) (Fig. 2A). MBP-tomosyn-N, MBP-tomosyn-C, and MBP alone were immobilized on amylose beads and incubated with ΔTM-synaptotagmin-1. ΔTM-synaptotagmin-1 specifically bound to MBP-tomosyn-N but not to MBP-tomosyn-C or MBP (Fig. 2B). These results indicate that, through its N-terminal WD40 repeats, tomosyn binds to synaptotagmin-1.
FIGURE 2.
Direct binding of N-terminal WD40 repeats of tomosyn to synaptotagmin-1. A, schematic representation of tomosyn and its fragments used in this study. B, direct binding of the N-terminal WD40 repeats of tomosyn to synaptotagmin-1. MBP-tomosyn-N, MBP-tomosyn-C, or MBP alone was immobilized on amylose beads and incubated with ΔTM-synaptotagmin-1 in the absence of Ca2+. The bound proteins were eluted with SDS sample buffer and subjected to SDS-PAGE followed by CBB staining. The asterisk indicates degraded MBP-tomosyn-N. The result shown is representative of three independent experiments.
Ca2+-dependent Binding of N-terminal WD40 Repeats of Tomosyn to Synaptotagmin-1
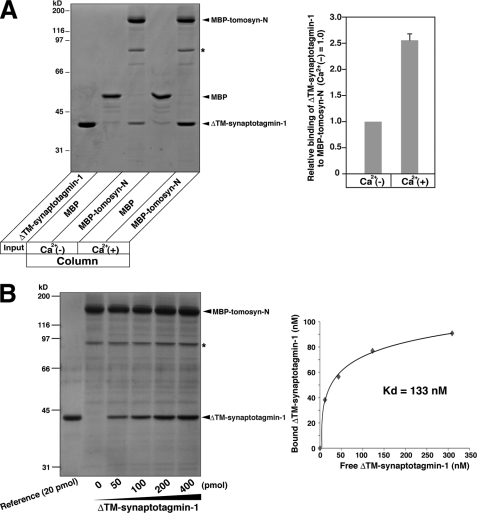
We next examined the effect of Ca2+ on synaptotagmin-1 binding. MBP-tomosyn-N immobilized on amylose beads was incubated with ΔTM-synaptotagmin-1 in the presence and absence of Ca2+, and the amounts of bound synaptotagmin-1 were compared. In the presence of Ca2+, ΔTM-synaptotagmin-1 bound to MBP-tomosyn-N 2.5-fold more than in the absence of Ca2+ (Fig. 3A). The apparent Kd value for the binding in the presence of Ca2+ was estimated to be 133 nm (Fig. 3B). These results indicate that, upon Ca2+ binding, synaptotagmin-1 potently binds to tomosyn.
FIGURE 3.
Ca2+-dependent binding of N-terminal WD40 repeats of tomosyn to synaptotagmin-1. A, the effect of Ca2+ on the binding of the N-terminal WD40 repeats of tomosyn to synaptotagmin-1. MBP-tomosyn-N or MBP was immobilized on amylose beads and incubated with DTM-synaptotagmin-1 in the absence or presence of Ca2+. The bound proteins were eluted with SDS sample buffer and subjected to SDS-PAGE followed by CBB staining. The result shown is representative of three independent experiments. The right panel shows quantification of the binding of ΔTM-synaptotagmin-1 to MBP-tomosyn-N. The error bar represents S.D. B, estimation of the binding affinity of the N-terminal WD40 repeats of tomosyn to synaptotagmin-1 in the presence of Ca2+. MBP-tomosyn-N (100 pmol) was immobilized on amylose beads and incubated with the indicated amounts of ΔTM-synaptotagmin-1 in the presence of Ca2+. The bound proteins were eluted with SDS sample buffer and subjected to SDS-PAGE followed by CBB staining. The amounts of bound and free ΔTM-synaptotagmin-1 were quantified and plotted in the right panel. The results shown are representative of three independent experiments. The apparent Kd value was calculated by Scatchard analysis. The asterisks indicate degraded MBP-tomosyn-N.
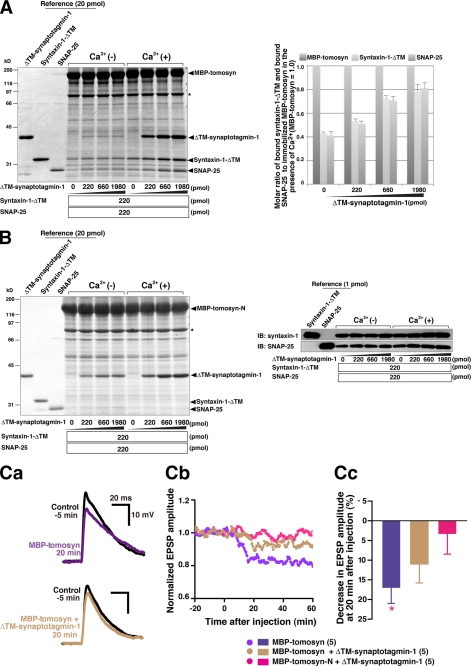
We previously reported that the N-terminal WD40 repeats directly bind to syntaxin-1 and SNAP-25, thereby catalyzing oligomerization of the SNARE complex (34). Therefore, we assessed the binding between tomosyn-N and syntaxin-1 or SNAP-25 in the same manner as for ΔTM-synaptotagmin-1 except that the cytoplasmic domain of syntaxin-1 (syntaxin-1-ΔTM) or SNAP-25 was used instead of ΔTM-synaptotagmin-1. Although binding of syntaxin-1-ΔTM and SNAP-25 to MBP-tomosyn-N was easily detected by immunoblotting, it was hard to detect the bound proteins by CBB staining (supplemental Fig. 2, A and B). These results indicate that syntaxin-1 and SNAP-25 bind to the N-terminal WD40 repeats much less strongly than synaptotagmin-1. It has been shown that the C-terminal VAMP-like domain of tomosyn, syntaxin-1, and SNAP-25 assemble into a SNARE complex-like structure (33). Therefore, we also assessed binding between full-length tomosyn and t-SNAREs. MBP-tomosyn immobilized on amylose beads was incubated with various concentrations of syntaxin-1-ΔTM and SNAP-25, and the bound proteins were subjected to SDS-PAGE followed by CBB staining. The apparent Kd value for the binding of MBP-tomosyn to t-SNAREs was estimated to be 285 nm (supplemental Fig. 2C). Furthermore, we incubated MBP-tomosyn immobilized on amylose beads with equal amounts of t-SNAREs and ΔTM-synaptotagmin-1 at various concentrations. t-SNAREs bound to MBP-tomosyn as much as ΔTM-synaptotagmin-1 did (supplemental Fig. 3). These results indicate that assembly of t-SNAREs and the C-terminal VAMP-like domain is comparable in affinity to the binding of synaptotagmin-1 to the N-terminal WD40 repeats.
Inhibition of Synaptotagmin-1 Functions by N-terminal WD40 Repeats of Tomosyn
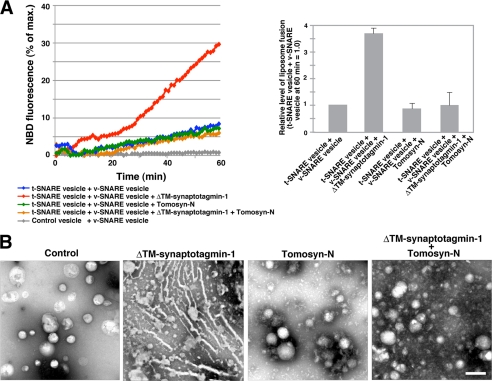
Upon Ca2+ binding, synaptotagmin-1 promotes SNARE-mediated membrane fusion (22–25). Therefore, we examined the effects of tomosyn on the function of synaptotagmin-1 in Ca2+-dependent membrane fusion. Syntaxin-1 and SNAP-25 were incorporated into liposomes (referred to as t-SNARE vesicles), and VAMP2 was incorporated into liposomes containing a quenched mixture of NBD-labeled phosphatidylethanolamine and rhodamine-labeled phosphatidylethanolamine (referred to as v-SNARE vesicles) as reported previously (13). The t-SNARE vesicles and the v-SNARE vesicles were incubated in a buffer containing Ca2+, and SNARE-mediated liposome fusion was monitored by measuring the increase in NBD fluorescence upon dilution of the fluorescent lipids. Consistent with the findings of previous reports (22–25), performing the reaction in the presence of ΔTM-synaptotagmin-1 increased the NBD fluorescence much more than was seen with reactions in which the t-SNARE vesicles were simply incubated with the v-SNARE vesicles (Fig. 4A), indicating that synaptotagmin-1 promotes SNARE-mediated liposome fusion. In the presence of MBP-tomosyn-N, the reaction showed a profile of NBD fluorescence increase similar to that of the reaction of t-SNARE vesicles with v-SNARE vesicles, indicating that the N-terminal WD40 repeats of tomosyn have no effect on SNARE-mediated liposome fusion in the absence of synaptotagmin-1. Importantly, in the presence of both ΔTM-synaptotagmin-1 and MBP-tomosyn-N, the reaction showed a much lower increase in NBD fluorescence than the reaction in the presence of ΔTM-synaptotagmin-1 alone. These results indicate that the N-terminal WD40 repeats of tomosyn decrease the activity of synaptotagmin-1 to promote SNARE-mediated membrane fusion. We also examined the effect of tomosyn-C on SNARE-mediated membrane fusion. Consistent with the findings of our recent report (49), an excess amount of MBP-tomosyn-C inhibited liposome fusion due to t-SNARE sequestering of the VAMP-like domain (supplemental Fig. 4A). In the presence of both MBP-tomosyn-C and ΔTM-synaptotagmin-1, the reaction also showed potent inhibition of liposome fusion, indicating that ΔTM-synaptotagmin-1 has no effect on the inhibitory activity of tomosyn-C. These results support no binding of tomosyn-C to synaptotagmin-1 as shown in Fig. 2B.
FIGURE 4.
Inhibition of synaptotagmin-1 functions by N-terminal WD40 repeats of tomosyn. A, inhibition of the activity of synaptotagmin-1 to promote SNARE-mediated membrane fusion by the N-terminal WD40 repeats of tomosyn. The t-SNARE vesicles were incubated with or without ΔTM-synaptotagmin-1 alone, MBP-tomosyn-N alone, or both ΔTM-synaptotagmin-1 and MBP-tomosyn-N in the presence of Ca2+ and then incubated with the v-SNARE vesicles at 37 °C. As a negative control, liposomes with no t-SNARE proteins (control vesicles) were incubated with the v-SNARE vesicles. The increase in NBD fluorescence upon liposome fusion was monitored for 60 min. The result shown is representative of three independent experiments. The right panel shows the relative level of liposome fusion at 60 min. Error bars represent S.D. B, inhibition of the membrane bending activity of synaptotagmin-1 by the N-terminal WD40 repeats of tomosyn. Folch liposomes were incubated with ΔTM-synaptotagmin-1 alone, MBP-tomosyn-N alone, or both ΔTM-synaptotagmin-1 and MBP-tomosyn-N in the presence of Ca2+. The samples were stained with uranyl acetate followed by electron microscopy analysis. As a negative control, Folch liposomes were simply stained with uranyl acetate. Bar, 200 nm. The results shown are representative of three independent experiments.
Upon Ca2+ binding, synaptotagmin-1 induces positive membrane curvature by inserting the hydrophobic loops of the C2 domains into the target membrane (22, 25). This membrane bending activity underlies the promotion of SNARE-mediated membrane fusion by synaptotagmin-1. Therefore, we next examined the effect of tomosyn on the membrane bending activity of synaptotagmin-1. Various combinations of ΔTM-synaptotagmin-1 and MBP-tomosyn-N were incubated with Folch liposomes in the presence of Ca2+ followed by negative staining with uranyl acetate. Consistent with the findings of previous reports (22, 25), ΔTM-synaptotagmin-1 deformed the liposomes into tubules (Fig. 4B). MBP-tomosyn-N did not affect the morphology of the liposomes. Importantly, ΔTM-synaptotagmin-1 did not induce tubulation of liposomes in the presence of MBP-tomosyn-N. These results indicate that the N-terminal WD40 repeats of tomosyn inhibit the membrane bending activity of synaptotagmin-1 and are in good agreement with the results of the liposome fusion assays as shown in Fig. 4A. In addition, consistent with no binding of tomosyn-C to synaptotagmin-1, MBP-tomosyn-C could not inhibit the liposome tubulation induced by ΔTM-synaptotagmin-1 (supplemental Fig. 4B). Collectively, these results indicate that tomosyn inhibits the synaptotagmin-1-mediated step of synaptic vesicle fusion through the direct binding between the N-terminal WD40 repeats and synaptotagmin-1.
Role of Binding of N-terminal WD40 Repeats of Tomosyn to Synaptotagmin-1 in Neurotransmitter Release
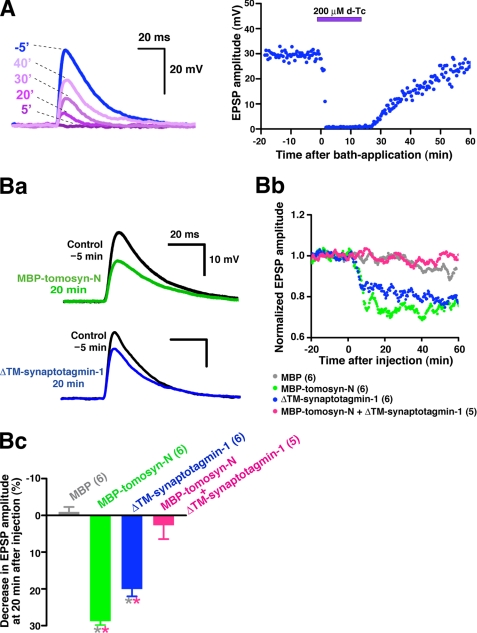
We validated the inhibitory activity of the WD40 repeats of tomosyn toward synaptotagmin-1 function at the cell level. In agreement with the partial co-localization observed in hippocampal neurons (Fig. 1C and supplemental Fig. 1), a subset of tomosyn co-localized with synaptotagmin-1 in SCG neurons in culture as shown by double immunostaining with the anti-tomosyn pAb and the anti-synaptotagmin-1 mAb (supplemental Fig. 5). Therefore, we used the ideal synapse of cultured SCG neurons for a functional study with microinjection of recombinant proteins. Numerous previous studies have reported that SCG neurons in culture form cholinergic synapses (44, 47, 50–57). Indeed, in this study, neurotransmission was reversibly abolished by an antagonist for nicotinic acetylcholine receptors, d-tubocurarine, confirming that the synapses formed in culture represent a model for fast synapses (Fig. 5A). Fragments of tomosyn or/and synaptotagmin-1 were microinjected into a presynaptic SCG neuron, and evoked EPSPs were monitored as acetylcholine release (45–47). Consistent with our previous reports (34, 35), MBP-tomosyn-N reduced acetylcholine release, whereas MBP alone did not (Fig. 5B). ΔTM-synaptotagmin-1 reduced acetylcholine release, suggesting that the soluble fragment of synaptotagmin-1 perturbs the function of endogenous synaptotagmin-1. To examine whether the MBP-tomosyn-N-induced inhibition was a result of direct binding to synaptotagmin-1, MBP-tomosyn-N was co-injected along with ΔTM-synaptotagmin-1. Although both MBP-tomosyn-N and ΔTM-synaptotagmin-1 slowed the rate of EPSP decay in addition to reducing the peak amplitude (Fig. 5Ba), MBP or a mixture of MBP-tomosyn-N and ΔTM-synaptotagmin-1 did not affect the EPSP waveform (data not shown). The mixture reduced the inhibition induced by each alone (Fig. 5, Bb and Bc), suggesting that ΔTM-synaptotagmin-1 blocks MBP-tomosyn-N binding to endogenous synaptoagmin-1, thereby abolishing the inhibitory activity of the N-terminal WD40 repeats. Concomitantly, these results indicate that, upon MBP-tomosyn-N binding, ΔTM-synaptotagmin-1 has no perturbing activity on the function of endogenous synaptotagmin-1. In contrast, MBP-tomosyn-C slowed the rate of EPSP rise and reduced the peak amplitude (supplemental Fig. 4Ca), suggesting reduced acetylcholine release due to the t-SNARE-sequestering activity of the VAMP-like domain. Co-injection of MBP-tomosyn-C and ΔTM-synaptotagmin-1 did not affect the rate of EPSP rise (supplemental Fig. 4Ca) and did not reduce the inhibition induced by each alone (supplemental Fig. 4, Cb and Cc), confirming no binding of tomosyn-C to synaptotagmin-1. Taken together, our results indicate that tomosyn targets synaptic vesicles through binding of its N-terminal WD40 repeats to synaptotagmin-1 and thereby inhibits the synaptotagmin-1-mediated step of Ca2+-dependent neurotransmitter release.
FIGURE 5.
Role of binding of N-terminal WD40 repeats of tomosyn to synaptotagmin-1 in neurotransmitter release. A, using cholinergic synapses formed between cultured SCG neurons for the functional study, evoked EPSPs were recorded every 10 s. To confirm the cholinergic synaptic transmission, d-tubocurarine (d-Tc) was drop-applied at time 0 to a concentration of 200 μm in the bath. The left panel shows EPSP traces before and after bath application of d-tubocurarine from one representative experiment. The right panel shows the decrease and recovery in EPSP amplitudes induced by d-tubocurarine and the washout. B, perturbing effects of tomosyn and synaptotagmin-1 fragments on neurotransmitter release. MBP-tomosyn-N, ΔTM-synaptotagmin-1, MBP, or the mixture of MBP-tomosyn-N and ΔTM-synaptotagmin-1 was microinjected into presynaptic SCG neurons at time 0 (50 μm each in the pipette). Ba, change in EPSP traces with MBP-tomosyn-N or ΔTM-synaptotagmin-1 from one representative experiment. Bb, normalized, averaged, and smoothed EPSP amplitudes (n = 5 or 6). Bc, decrease in EPSP amplitude at 20 min after injection. * Grey, p < 0.05, versus MBP. * Pink, p < 0.05, versus MBP-tomosyn-N+ΔTM-synaptotagmin-1. Error bars represent S.E. Parenthetical numbers indicate the number of trials.
Enhanced Tomosyn-SNARE Complex Formation by Synaptotagmin-1
Tomosyn sequesters t-SNAREs through the C-terminal VAMP-like domain and thereby inhibits neurotransmitter release (31–35). We finally examined whether the binding of synaptotagmin-1 to tomosyn affected formation of the tomosyn-SNARE complex. MBP-tomosyn immobilized on amylose beads was incubated with various concentrations of ΔTM-synaptotagmin-1 in the absence or presence of Ca2+ and incubated with fixed amounts of syntaxin-1-ΔTM and full-length SNAP-25 to allow the tomosyn-SNARE complex to form. Then the bound proteins were subjected to SDS-PAGE followed by CBB staining. In the presence of Ca2+, ΔTM-synaptotagmin-1 dose-dependently bound to MBP-tomosyn (Fig. 6A). Under this condition, binding of syntaxin-1-ΔTM and SNAP-25 to MBP-tomosyn was increased in a synaptotagmin-1 concentration-dependent manner. We also carried out a similar binding assay using MBP-tomosyn-N. In the presence of Ca2+, ΔTM-synaptotagmin-1 dose-dependently bound to MBP-tomosyn-N (Fig. 6B). Consistent with the results shown in supplemental Fig. 2 and our previous report (34), syntaxin-1-ΔTM and SNAP-25 weakly bound to MBP-tomosyn-N as detected by immunoblotting but not CBB staining. However, these binding events were not affected by ΔTM-synaptotagmin-1 binding to MBP-tomosyn-N. These results indicate that the binding of synaptotagmin-1 to tomosyn promotes the activity of the C-terminal VAMP-like domain to form the tomosyn-SNARE complex. In addition, the reduction in EPSP amplitude and the slowed decay with MBP-tomosyn were partially rescued by co-injection of ΔTM-synaptotagmin-1, but the recovery was less than that with MBP-tomosyn-N (Fig. 6C). This remaining inhibition of MBP-tomosyn in the presence of ΔTM-synaptotagmin-1 can be explained by enhanced tomosyn-SNARE complex formation upon synaptotagmin-1 binding.
FIGURE 6.
Enhanced tomosyn-SNARE complex formation by synaptotagmin-1. A, effect of synatopatmin-1 binding on tomosyn-SNARE complex formation. MBP-tomosyn immobilized on amylose beads was incubated with various concentrations of ΔTM-synaptotagmin-1 in the absence or presence of Ca2+. After being washed, the beads were incubated with syntaxin-1-ΔTM and full-length SNAP-25 in the absence or presence of Ca2+. The bound proteins were eluted with SDS sample buffer and subjected to SDS-PAGE followed by CBB staining. The asterisk indicates degraded MBP-tomosyn. The result shown is representative of three independent experiments. The right panel shows the molar ratio of immobilized MBP-tomosyn, bound syntaxin-1, and bound SNAP-25 in the presence of Ca2+. Error bars represent S.D. B, no involvement of synaptotagmin-1 in the binding of N-terminal WD40 repeats to t-SNAREs. MBP-tomosyn-N immobilized on amylose beads was incubated with various concentrations of ΔTM-synaptotagmin-1 in the absence or presence of Ca2+ followed by reaction with syntaxin-1-ΔTM and full-length SNAP-25 in the same manner as described in A. The asterisk indicates degraded MBP-tomosyn-N. The right panels show immunoblotting (IB) with anti-syntaxin-1 mAb and anti-SNAP-25 mAb. The result shown is representative of three independent experiments. C, perturbing effects of synatotagmin-1 on inhibition of neurotransmitter release by full-length tomosyn. Evoked EPSPs were recorded every 10 s. MBP-tomosyn or a mixture of MBP-tomosyn and ΔTM-synaptotagmin-1 was microinjected into presynaptic SCG neurons at time 0 in the same manner as described in the legend for Fig. 5B. Ca, changes in EPSP traces with MBP-tomosyn or the mixture of MBP-tomosyn and ΔTM-synaptotagmin-1 from one representative experiment. Cb, normalized, averaged, and smoothed EPSP amplitudes (n = 5). Cc, decrease in EPSP amplitude at 20 min after injection. *, p < 0.05, versus MBP-tomosyn-N+ΔTM-synaptotagmin-1. Error bars represent S.E. The graphs of the mixture of MBP-tomosyn-N and ΔTM-synaptotagmin-1 are repetitions of Fig. 5, Bb and Bc. Parenthetical numbers indicate the number of trials.
Overall, our results indicate that the Ca2+-dependent binding between tomosyn and synaptotagmin-1 regulates the inhibitory activity of tomosyn as well as the catalytic activity of synaptotagmin-1. These results suggest that the interplay between tomosyn and synaptotagmin-1 underlies negative control of Ca2+-dependent neurotransmitter release.
DISCUSSION
In this study, we demonstrated that tomosyn directly binds to synaptotagmin-1 through the N-terminal WD40 repeats in a Ca2+-dependent manner and thereby inhibits the synaptotagmin-1-mediated step of Ca2+-dependent neurotransmitter release. Tomosyn sequesters t-SNAREs through the C-terminal VAMP-like domain, leading to inhibition of SNARE complex formation. Therefore, our results indicate that tomosyn potently inhibits neurotransmitter release by targeting synaptic vesicles through the N-terminal WD40 repeats and the presynaptic plasma membrane through the C-terminal VAMP-like domain.
Tomosyn controls exocytotic efficacy of the synaptic vesicles (34, 39). However, it remains unclear how tomosyn controls Ca2+-dependent exocytosis because the C-terminal VAMP-like domain sequesters t-SNAREs in a Ca2+-independent manner. Some studies in chromaffin cells suggest that the Ca2+ responsiveness of tomosyn may be confined to the N-terminal WD40 repeats because its inhibitory activity is relieved in the presence of a high Ca2+ concentration (40, 41). We recently reported that the N-terminal WD40 repeats oligomerize the SNARE complex, leading to inhibition of neurotransmitter release (34). However, this does not account for the Ca2+ responsiveness of tomosyn because the oligomerization occurs in a Ca2+-independent manner. By contrast, the binding between the N-terminal WD40 repeats and synaptotagmin-1 in this study was Ca2+-dependent. Synaptotagmin-1 is a major Ca2+ sensor protein on synaptic vesicles (48) and plays pivotal roles in the regulation of Ca2+-dependent exocytosis of synaptic vesicles. Therefore, impairment of synaptotagmin-1 function by Ca2+-dependent binding of the N-terminal WD40 repeats of tomosyn likely underlies the control of Ca2+-dependent exocytosis exerted by tomosyn.
The direct binding between tomosyn and synaptotagmin-1 enhanced the activity of tomosyn to sequester t-SNAREs (Fig. 6) and impaired the catalytic activity of synaptotagmin-1. Ca2+ strengthened this binding (Fig. 3), indicating that the synaptotagmin-1-tomosyn-SNARE complex is formed after Ca2+ influx. These findings raise the possibility that synaptotagmin-1, cooperating with tomosyn on synaptic vesicles, acts as an alternative Ca2+ sensor to negatively control the exocytotic efficacy of synaptic vesicles, sensing Ca2+ concentration changes near Ca2+ channel clusters. In response to a rise in Ca2+ concentration, synaptotagmin-1 on synaptic vesicles catches tomosyn and inactivates its own catalysis for membrane fusion. Simultaneously, the synaptotagmin-1-tomosyn complex enhances the sequestering of t-SNAREs on the plasma membrane and blocks SNARE assembly. Eventually, formation of the Ca2+-dependent synaptotagmin-1-tomosyn-SNARE complex ensures inactivation of the fusion machineries on both the donor and target membranes and thereby inhibits priming of synaptic vesicles. Evidence is accumulating that tomosyn regulates RRP size. Tomosyn-deficient mice show reduced paired pulse facilitation in hippocampi (34), suggesting that, without tomosyn, the first action potential depletes synaptic vesicles in the RRP. Tomosyn-overexpressing presynaptic SCG neurons show small EPSPs but cannot respond to subsequent repetitive action potentials, thus inducing severe synaptic depression (39). The above mentioned interplay between synaptotagmin-1 and tomosyn seems to be ideal for controlling the number of primed synaptic vesicles in the RRP. Further studies will be required to address these concerns.
The N-terminal WD40 repeats directly bind to syntaxin-1 and SNAP-25, thereby catalyzing oligomerization of the SNARE complex (34). In our binding assays, the N-terminal WD40 repeats bound to synaptotagmin-1 much more strongly than to syntaxin-1 and SNAP-25 (supplemental Fig. 2). These results suggest that the inhibitory activity of the N-terminal WD40 repeats upon neurotransmitter release might mostly depend on impairment of synaptotagmin-1 function rather than oligomerization of the SNARE complex. However, given that the N-terminal WD40 repeats act as a catalyst for oligomerization of the SNARE complex, the weak binding to t-SNAREs may be sufficient to potently inhibit neurotransmitter release. Therefore, it remains unclear which binding event is the most important determining factor for the inhibitory activity of the N-terminal WD40 repeats. It also remains unclear whether the inhibitory activity of the N-terminal WD40 repeats toward synaptotagmin-1 function and their oligomerization activity toward the SNARE complex are mutually exclusive or compatible. Although we demonstrated that tomosyn potently bound to synaptotagmin-1 through the N-terminal WD40 repeats and thereby inhibited the synaptotagmin-1-mediated step of synaptic vesicle exocytosis, most of the results were obtained by assays using purified recombinant proteins. Indeed, experiments such as the binding assay, the liposome fusion assay, the liposome tubulation assay, and the electrophysiological analysis were carried out under non-physiological conditions in terms of the endogenous expression levels of tomosyn and synaptotagmin-1. The Ca2+ concentration (1 μm) used in the binding assay, the liposome fusion assay, and the liposome tubulation assay was also not relevant to physiological Ca2+ influx (10–50 μm). Therefore, we cannot rule out the possibility that tomosyn may require an additional cofactor(s) that promotes binding for robust exertion of its inhibitory activity toward synaptotagmin-1 in vivo.
Recently, the crystal structure of the N-terminal WD40 repeats of Sro7, a yeast orthologue of tomosyn, was solved (58). Based on the solved structure, Sro7 is suggested to bind to Sec9, a yeast counterpart of SNAP-25, through the N-terminal WD40 repeats and thereby inhibit SNARE complex formation. Although Sec9 binds to Sro7 through both the N-terminal region and the SNARE motifs, the N-terminal region of Sec9 is not conserved in mammalian SNAP-25 (58). As far as we can tell, the N-terminal WD40 repeats of tomosyn have no inhibitory activity toward SNARE complex formation (data not shown). Therefore, the inhibitory activity of the N-terminal WD40 repeats of Sro7 toward SNARE complex formation may not be evolutionarily conserved. On the other hand, the binding of tomosyn to synaptotagmin-1 is in agreement with the association of tomosyn with synaptic vesicles in C. elegans (38), raising the possibility that the activity of the N-terminal WD40 repeats to inhibit synaptotagmin-1 function might be evolutionarily conserved between nematodes and mammals. The binding of synaptotagmin-1 to the N-terminal WD40 repeats enhanced formation of the tomosyn-SNARE complex through the C-terminal VAMP-like domain (Fig. 6). We recently reported that tomosyn adopts two conformational states upon reciprocal intramolecular binding of the tail domain (35). In one conformational state, in which the tail domain binds to the N-terminal WD40 repeats, tomosyn potently inhibits SNARE complex formation through the C-terminal VAMP-like domain. In the other conformational state, in which the tail domain binds to the C-terminal VAMP-like domain, the inhibitory activity of the C-terminal VAMP-like domain is decreased. Therefore, the binding of synaptotagmin-1 to the N-terminal WD40 repeats may stabilize the former conformational state of tomosyn, leading to an enhancement of tomosyn-SNARE complex formation. Protein kinase A (PKA) phosphorylation of the serine residues in the N-terminal WD40 repeats reduces tomosyn-SNARE complex formation (39). Therefore, PKA phosphorylation may interfere with synaptotagmin-1 binding and stabilize the latter conformational state of tomosyn, leading to inactivation of tomosyn. Structural studies of full-length tomosyn, the synaptotagmin-1-tomosyn complex, and the synaptotagmin-1-tomosyn-SNARE complex will be required to confirm this.
In summary, we show here that Ca2+-dependent binding between tomosyn and synaptotagmin-1 regulates the catalytic activity of synaptotagmin-1 and the inhibitory activity of tomosyn. These results suggest that the interplay between tomosyn and synaptotagmin-1 underlies inhibitory control of Ca2+-dependent neurotransmitter release. Of note, this is the first report to show the mechanism of inactivation of synaptotagmin-1.
Supplementary Material
Acknowledgments
We thank Drs. Mitsunori Fukuda (Tohoku University, Sendai, Japan) and Masami Takahashi (Kitasato University, Kanagawa, Japan) for kindly providing us with the plasmids to express synaptotagmin-1.
This work was supported by grants-in-aid for the national project on Targeted Proteins Research Program (to T. S.), for scientific research C (to T. S.), for scientific research on priority areas (to T. S. and S. M.), for scientific research on innovative areas (to Y. Y.), and for the Global Center of Excellence Program F11 (to T. S.) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, by a grant provided by The Ichiro Kanehara Foundation (to T. S.), and by a grant provided by The Uehara Memorial Foundation (to T. S.). Part of this work was also supported by Core Research for Evolutional Science and Technology, the Japan Science and Technology Agency (to N. M. and K. I.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- RRP
- readily releasable pool
- SNAP
- soluble N-ethylmaleimide-sensitive fusion protein attachment protein
- v-SNARE
- vesicular SNARE
- t-SNARE
- target SNARE
- SCG
- superior cervical ganglion
- EPSP
- excitatory postsynaptic potential
- VAMP
- vesicle-associated membrane protein
- MBP
- maltose binding protein
- pAb
- polyclonal antibody
- CBB
- Coomassie Brilliant Blue
- NBD
- N-(7-nitro-2,1,3-benzoxadiaziole-4-yl).
REFERENCES
- 1.Sudhof T. C. (2004) Annu. Rev. Neurosci. 27, 509–547 [DOI] [PubMed] [Google Scholar]
- 2.Südhof T. C. (2000) Neuron 28, 317–320 [DOI] [PubMed] [Google Scholar]
- 3.Rizzoli S. O., Betz W. J. (2005) Nat. Rev. Neurosci. 6, 57–69 [DOI] [PubMed] [Google Scholar]
- 4.Schikorski T., Stevens C. F. (2001) Nat. Neurosci. 4, 391–395 [DOI] [PubMed] [Google Scholar]
- 5.Sakaba T., Schneggenburger R., Neher E. (2002) Neurosci. Res. 44, 343–356 [DOI] [PubMed] [Google Scholar]
- 6.Rizzoli S. O., Betz W. J. (2004) Science 303, 2037–2039 [DOI] [PubMed] [Google Scholar]
- 7.Harata N., Ryan T. A., Smith S. J., Buchanan J., Tsien R. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12748–12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lange R. P., de Roos A. D., Borst J. G. (2003) J. Neurosci. 23, 10164–10173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harata N., Pyle J. L., Aravanis A. M., Mozhayeva M., Kavalali E. T., Tsien R. W. (2001) Trends Neurosci. 24, 637–643 [DOI] [PubMed] [Google Scholar]
- 10.Jahn R., Scheller R. H. (2006) Nat. Rev. Mol. Cell Biol. 7, 631–643 [DOI] [PubMed] [Google Scholar]
- 11.Rizo J., Rosenmund C. (2008) Nat. Struct. Mol. Biol. 15, 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. (1998) Nature 395, 347–353 [DOI] [PubMed] [Google Scholar]
- 13.Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 14.Sutton R. B., Davletov B. A., Berghuis A. M., Südhof T. C., Sprang S. R. (1995) Cell 80, 929–938 [DOI] [PubMed] [Google Scholar]
- 15.Shao X., Fernandez I., Südhof T. C., Rizo J. (1998) Biochemistry 37, 16106–16115 [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M., Kanno E., Mikoshiba K. (1999) J. Biol. Chem. 274, 31421–31427 [DOI] [PubMed] [Google Scholar]
- 17.Augustine G. J. (2001) Curr. Opin. Neurobiol. 11, 320–326 [DOI] [PubMed] [Google Scholar]
- 18.Fernandez I., Araç D., Ubach J., Gerber S. H., Shin O., Gao Y., Anderson R. G., Südhof T. C., Rizo J. (2001) Neuron 32, 1057–1069 [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Chacón R., Königstorfer A., Gerber S. H., García J., Matos M. F., Stevens C. F., Brose N., Rizo J., Rosenmund C., Südhof T. C. (2001) Nature 410, 41–49 [DOI] [PubMed] [Google Scholar]
- 20.Chapman E. R. (2008) Annu. Rev. Biochem. 77, 615–641 [DOI] [PubMed] [Google Scholar]
- 21.Tang J., Maximov A., Shin O. H., Dai H., Rizo J., Südhof T. C. (2006) Cell 126, 1175–1187 [DOI] [PubMed] [Google Scholar]
- 22.Martens S., Kozlov M. M., McMahon H. T. (2007) Science 316, 1205–1208 [DOI] [PubMed] [Google Scholar]
- 23.Stein A., Radhakrishnan A., Riedel D., Fasshauer D., Jahn R. (2007) Nat. Struct. Mol. Biol. 14, 904–911 [DOI] [PubMed] [Google Scholar]
- 24.Xue M., Ma C., Craig T. K., Rosenmund C., Rizo J. (2008) Nat. Struct. Mol. Biol. 15, 1160–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui E., Johnson C. P., Yao J., Dunning F. M., Chapman E. R. (2009) Cell 138, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. (1994) Cell 79, 717–727 [DOI] [PubMed] [Google Scholar]
- 27.Yoshihara M., Littleton J. T. (2002) Neuron 36, 897–908 [DOI] [PubMed] [Google Scholar]
- 28.Shin O. H., Rhee J. S., Tang J., Sugita S., Rosenmund C., Südhof T. C. (2003) Neuron 37, 99–108 [DOI] [PubMed] [Google Scholar]
- 29.Nishiki T., Augustine G. J. (2004) J. Neurosci. 24, 8542–8550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young S. M., Jr., Neher E. (2009) Neuron 63, 482–496 [DOI] [PubMed] [Google Scholar]
- 31.Fujita Y., Shirataki H., Sakisaka T., Asakura T., Ohya T., Kotani H., Yokoyama S., Nishioka H., Matsuura Y., Mizoguchi A., Scheller R. H., Takai Y. (1998) Neuron 20, 905–915 [DOI] [PubMed] [Google Scholar]
- 32.Hatsuzawa K., Lang T., Fasshauer D., Bruns D., Jahn R. (2003) J. Biol. Chem. 278, 31159–31166 [DOI] [PubMed] [Google Scholar]
- 33.Pobbati A. V., Razeto A., Böddener M., Becker S., Fasshauer D. (2004) J. Biol. Chem. 279, 47192–47200 [DOI] [PubMed] [Google Scholar]
- 34.Sakisaka T., Yamamoto Y., Mochida S., Nakamura M., Nishikawa K., Ishizaki H., Okamoto-Tanaka M., Miyoshi J., Fujiyoshi Y., Manabe T., Takai Y. (2008) J. Cell Biol. 183, 323–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto Y., Mochida S., Kurooka T., Sakisaka T. (2009) J. Biol. Chem. 284, 12480–12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashery U., Bielopolski N., Barak B., Yizhar O. (2009) Trends Neurosci. 32, 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gracheva E. O., Burdina A. O., Holgado A. M., Berthelot-Grosjean M., Ackley B. D., Hadwiger G., Nonet M. L., Weimer R. M., Richmond J. E. (2006) PLoS Biol. 4, e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen J. M., Madison J. M., Dybbs M., Kaplan J. M. (2006) Neuron 51, 303–315 [DOI] [PubMed] [Google Scholar]
- 39.Baba T., Sakisaka T., Mochida S., Takai Y. (2005) J. Cell Biol. 170, 1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yizhar O., Lipstein N., Gladycheva S. E., Matti U., Ernst S. A., Rettig J., Stuenkel E. L., Ashery U. (2007) J. Neurochem. 103, 604–616 [DOI] [PubMed] [Google Scholar]
- 41.Yizhar O., Matti U., Melamed R., Hagalili Y., Bruns D., Rettig J., Ashery U. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2578–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizoguchi A., Ueda T., Ikeda K., Shiku H., Mizoguti H., Takai Y. (1989) Brain. Res. Mol. Brain Res. 5, 31–44 [DOI] [PubMed] [Google Scholar]
- 43.Mochida S., Nonomura Y., Kobayashi H. (1994) Microsc. Res. Tech. 29, 94–102 [DOI] [PubMed] [Google Scholar]
- 44.Mochida S. (1995) J. Physiol. Paris 89, 83–94 [DOI] [PubMed] [Google Scholar]
- 45.Mochida S., Sheng Z. H., Baker C., Kobayashi H., Catterall W. A. (1996) Neuron 17, 781–788 [DOI] [PubMed] [Google Scholar]
- 46.Mochida S., Westenbroek R. E., Yokoyama C. T., Itoh K., Catterall W. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2813–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma H., Mochida S. (2007) Neurosci. Res. 57, 491–498 [DOI] [PubMed] [Google Scholar]
- 48.Takamori S., Holt M., Stenius K., Lemke E. A., Grønborg M., Riedel D., Urlaub H., Schenck S., Brügger B., Ringler P., Müller S. A., Rammner B., Gräter F., Hub J. S., De Groot B. L., Mieskes G., Moriyama Y., Klingauf J., Grubmüller H., Heuser J., Wieland F., Jahn R. (2006) Cell 127, 831–846 [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto Y., Fujikura K., Sakaue M., Okimura K., Kobayashi Y., Nakamura T., Sakisaka T. (2010) Biochem. Biophys. Res. Commun. 399, 24–30 [DOI] [PubMed] [Google Scholar]
- 50.O'Lague P. H., Obata K., Claude P., Furshpan E. J., Potter D. D. (1974) Proc. Natl. Acad. Sci. U.S.A. 71, 3602–3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rees R., Bunge R. P. (1974) J. Comp. Neurol. 157, 1–11 [DOI] [PubMed] [Google Scholar]
- 52.Ko C. P., Burton H., Bunge R. P. (1976) Brain Res. 117, 437–460 [DOI] [PubMed] [Google Scholar]
- 53.Ko C. P., Burton H., Johnson M. I., Bunge R. P. (1976) Brain Res. 117, 461–485 [DOI] [PubMed] [Google Scholar]
- 54.Johnson M., Ross D., Meyers M., Rees R., Bunge R., Wakshull E., Burton H. (1976) Nature 262, 308–310 [DOI] [PubMed] [Google Scholar]
- 55.O'Lague P. H., Potter D. D., Furshpan E. J. (1978) Dev. Biol. 67, 424–443 [DOI] [PubMed] [Google Scholar]
- 56.Wakshull E., Johnson M. I., Burton H. (1979) J. Neurophysiol. 42, 1426–1436 [DOI] [PubMed] [Google Scholar]
- 57.Krapivinsky G., Mochida S., Krapivinsky L., Cibulsky S. M., Clapham D. E. (2006) Neuron 52, 485–496 [DOI] [PubMed] [Google Scholar]
- 58.Hattendorf D. A., Andreeva A., Gangar A., Brennwald P. J., Weis W. I. (2007) Nature 446, 567–571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.