Abstract
Despite the enormous number of studies demonstrating changes in the chaperone-like activity of α-crystallins in vitro, little is known about how these changes influence life-long lens transparency in vivo. Using the γB-crystallin I4F mutant protein as a target for αA-crystallins, we examined how cataract phenotypes are modulated by interactions between α-crystallins with altered chaperone-like activities and γB-I4F proteins in vivo. Double heterozygous α-crystallin knock-out αA(+/−) αB(+/−) mice with a decreased amount of α-crystallins were used to simulate reduced total α-crystallin chaperone-like activity in vivo. We found that triple heterozygous αA(+/−) αB(+/−) γB(I4F/+) mice developed more severe whole cataracts than heterozygous γB(I4F/+) mice. Thus, total chaperone-like activity of α-crystallins is important for maintaining lens transparency. We further tested whether mutant αA-crystallin Y118D proteins with increased chaperone-like activity influenced the whole cataract caused by the γB-I4F mutation. Unexpectedly, compound αA(Y118D/+) γB(I4F/+) mutant lenses displayed severe nuclear cataracts, whereas the lens cortex remained unaffected. Thus, the synergistic effect of αA-Y118D and γB-I4F mutant proteins is detrimental to the transparency only in the lens core. α-Crystallins with different chaperone-like activities are likely required in the lens cortex and nucleus for maintaining transparency.
Keywords: Eye, Genetics, Heat Shock Protein, Mouse, Protein-Protein Interactions, α-Crystallins, Cataract, Chaperone-like Activity, Lens, Light Scattering
Introduction
The eye lens is a transparent organ composed of bulk elongated fiber cells and a monolayer of epithelial cells covering the anterior surface (1–3). Ninety percent of all lens proteins are crystallins, which are subdivided into three classes, α, β, and γ. Lens transparency is believed to depend on the short-range order of crystallin proteins and the appropriate interactions among crystallin and non-crystallin proteins (4, 5). During aging and cataractogenesis, changes in lens protein interactions may result in high molecular weight aggregates that scatter light (6). Protein aggregation can also disrupt normal protein functions and/or create new pathological functions in the lens. Mechanisms for regulating the interactions among crystallin and non-crystallin proteins are not well understood.
α-Crystallins, consisting of αA- and αB-crystallin subunits, exist as polydispersed heteromeric aggregates in the lens. Life-long lens transparency is hypothesized to rely on the chaperone-like function of α-crystallins that acts as a “sink” for binding to denatured proteins to prevent abnormal protein aggregation induced by various risk factors (7). The chaperone-like activity of α-crystallins can be measured in vitro based on their ability to prevent heat or chemically induced, nonspecific protein aggregation of target proteins such as insulin and α-lactalbumin (8–13). Recent genetic, structural, and biochemical studies have revealed many new crystallin gene mutations, significantly improved the structural information of α- and γ-crystallin proteins, and elucidated various altered biochemical properties of α- and γ-crystallin mutant proteins that cause cataracts in humans and in mice (14–17).
Studies of α-crystallin mutations that cause cataracts have revealed altered chaperone-like activity of recombinant mutant α-crystallins in vitro. Because most α-crystallin mutant proteins display a decrease in chaperone-like activity in vitro, it is generally accepted that decreased chaperone-like activity of α-crystallin mutants is one of the mechanisms for cataract formation (11, 13, 18–21). However, several recent studies have indicated that mutant α-crystallins with increased chaperone-like activity also cause cataract formation (9, 22, 23). Thus, proper chaperone-like activity of α-crystallin proteins is probably required for maintaining lens transparency. The mechanistic difference between cataracts caused by reduced or increased chaperone-like activity of α-crystallin remains unknown. It is often difficult to interpret the results of the chaperone-like activity assays in vitro because these tests utilize non-lens proteins rather than native lens target proteins as binding substrates. The regulation of chaperone-like activity of α-crystallin in vivo is unclear due to the lack of a method that can be used to directly evaluate chaperone-like function during lens development or aging.
We reported previously that the mutant γB-I4F crystallin protein is a target protein for wild-type α-crystallins in vitro and in vivo (24). The γB-I4F mutation causes a dominant cataract, and homozygous lenses develop a much denser opacity than heterozygous lenses (24). Gel filtration analysis of lens homogenates shows that γB-I4F mutant proteins form high molecular weight aggregates with α-crystallins (24). Increased water-insoluble proteins are associated with cataract formation in γB-I4F lenses. These results indicate that endogenous α-crystallin proteins are unable to prevent the abnormal protein aggregation triggered by a large amount of γB-I4F mutant proteins in the lens. The more severe cataract of homozygous mutant mice is caused by the dosage effect of γB-I4F mutant proteins. Therefore, the γB-I4F mutant protein is probably an appropriate target protein in vivo to test the effect of altered chaperone-like activity of α-crystallins in cataract formation. Decreased chaperone-like activity in the lens can presumably be recapitulated in vivo by using α-crystallin heterozygous knock-out mice because these mice develop clear lenses despite a decrease in the amount of α-crystallins (25, 26). Increased chaperone-like activity in the lens can be reproduced by the αA-Y118D mutant protein with increased chaperone-like activity in vitro, which we reported recently (9).
In this study, we used the γB-I4F mutant protein as an in vivo target of α-crystallins to determine how altered α-crystallins affect lens transparency. Cataract severity has been quantitatively evaluated using a new method that we developed for measuring the light scattered from cataractous mouse lenses. The results suggest that lens transparency relies on different levels of αA-crystallin chaperone-like activity in the lens cortex and nucleus.
EXPERIMENTAL PROCEDURES
Mice
This study followed the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and an Animal Care and Use Committee-approved animal protocol (University of California, Berkeley). The generation and genotyping of αA(Y118D/Y118D) and γB(I4F/I4F) mice were described previously (21, 27). Double heterozygous αA(Y118D/+) γB(I4F/+) mice were generated by intercrossing αA(Y118D/Y118D) mice with γB(I4F/I4F) mice, and triple heterozygous αA(+/−) αB(+/−) γB(I4F/+) mice were generated by intercrossing αA(−/−) αB(−/−) mice with γB(I4F/I4F) mice. The αA(−/−) αB(−/−) mice were a generous gift from Dr. Eric Wawrousek at NEI (28). The γB(I4F/+) heterozygous mice were generated by crossing homozygous γB-I4F mice with C57BL wild-type mice.
Expression, Purification, and in Vitro Binding of Recombinant Wild-type and Mutant αA and γB Proteins
Mouse wild-type αA, mutant αA-Y118D, wild-type γB, and mutant γB-I4F cDNAs were subcloned into a bacterial expression plasmid and prepared as described previously (8, 24, 29). Recombinant proteins that were exclusively in the inclusion bodies were solubilized in 8 m urea followed by stepwise buffer dialysis as described previously (30). For the in vitro binding analysis, 0.2 mg of each recombinant protein was mixed and heated to 45 °C for 30 min before being loaded into the Pharmacia Superose HR-6 column connected to the ÄKTA FPLC system (GE Healthcare). These peaks were collected in different tubes with different elution volumes. Equal volumes of eluted solutions (20 μl) were mixed with loading buffer and loaded onto a 12.5% SDS-polyacrylamide gel for separation. Proteins were detected by Coomassie Blue staining for qualitative (not quantitative) examination of the protein compositions in the peaks.
Cloning and Expression Plasmids
Lens total RNA was isolated from wild-type and homozygous αA(Y118D/Y118D) or γB(I4F/I4F) mutant lenses with TRIzol reagent (Invitrogen). Total RNA was used to generate cDNA using the Superscript first-strand synthesis system for the RT-PCR kit (Invitrogen). Coding region inserts were amplified using the Platinum® Pfx DNA polymerase kit (Invitrogen). Primers used to amplify the αA inserts were 5′-GCGAATTCAGATGGACGTCACCATTCA and 3′-AGACGTGGGAGCAGGTCCACCTCCACCCCTAGGCG, and primers used to amplify the γB inserts were 5′-CGGAATTCAGATGGGAAAG and 3′-CGGGATCCCCACCTCCACCG. PCR was run using the following steps: 1) 94 °C for 2 min; 2) 94 °C for 15 s, 55 °C for 30 s, and 68 °C for 1 min for 35 cycles; and 3) 68 °C for 3 min. PCR products were purified from a 1% agarose gel. The αA and γB PCR products and the pEGFP-N1 and pDsRed-N1 RFP2 vectors (Clontech) were cut with the restriction enzymes EcoRI and BamHI (New England Biolabs, Ipswich, MA) for 2 h at 37 °C. Purified vectors and PCR products were ligated using the T4 DNA ligase kit (Promega, Madison, WI). Plasmids were purified after transformation into TOP10 bacteria (Invitrogen) from individual bacterial clones (plasmid purification kit, Qiagen, Valencia, CA), and clones were sequenced with an Applied Biosystems 48-capillary model 3730 genetic analyzer system to confirm the presence or absence of point mutations in the αA- and γB-crystallin inserts.
Lens Epithelial Cell Transfection
Mouse immortalized lens epithelial cells were generated as described previously, and plasmid transfection using SuperFect reagent (Qiagen) was also performed as described previously (31). Transfected cells on 35-mm plastic tissue culture dishes were observed for GFP and RFP signals after 24 and 48 h. After 48 h, cells were fixed with 4% paraformaldehyde for 5 min. Fixed cells were washed two times with 1× PBS for 2 min per wash. Cells were stained with rhodamine phalloidin (Molecular Probes, Eugene, OR) for 2 h at room temperature, washed three times with 1× PBS for 5 min per wash, and mounted with DAPI VectorShield mounting medium (Vector Laboratories, Inc., Burlingame, CA). Images were collected with a Zeiss Axiovert 200 fluorescence microscope.
Surface Plots and Plot Profiles of Lens Imaging
Twenty-one-day-old fresh lenses were carefully dissected under a dissecting microscope (MZ16, Leica) and imaged with a digital camera. Surface plots and plot profiles of white light intensity were generated using ImageJ from pictures of fresh lenses. The outer edge of the lens in the pictures, which was bright, was cropped out to remove background noise from surface plots and plot profiles. Plot profile data were exported and plotted using Excel.
Light Scattering Quantification
Light scattering from lenses was measured using the HR 2000CG_UV_NIR high-resolution spectrometer and a QP400–2-UV-VIS fiber-optic cable (both from Ocean Optics, Dunedin, FL). Lenses were carefully transferred to the tip of the fiber-optic cable, and excess PBS was removed with a Kimwipe. Lenses on the fiber-optic cable were illuminated with a white light source perpendicular to the equator of the lens. The light source intensity was held constant between samples. Scattered light was captured by the fiber with a whole acceptance of angle of 24.8°. Spectra were recorded and saved for later comparison. Each lens was measured twice in succession to show repeatability. Each measurement took less than 1 min, including transferring the lenses to the fiber-optic cable tip and taking a snapshot of the light intensity profile.
The measurements were represented as graphs with the wavelength on the x axis and the light intensity on the y axis. Denser cataracts scattered more light, and thus, the light scattering intensity measured by the detector increased. Measurements were stored as ASCII files, and MATLAB was used to calculate the area under the curve. At least six lenses from three different mice for each genotype were used to determine the average light scattering intensity. Normalized intensity was calculated by subtracting the area under the curve measured with wild-type lenses, which do not scatter light. The average normalized intensity and standard error were plotted in Excel. Statistical significance was determined using the Student's t test.
RESULTS
Recombinant αA-Y118D Proteins Bind to Mutant γB-I4F but Not Wild-type γB-Crystallins in Vitro
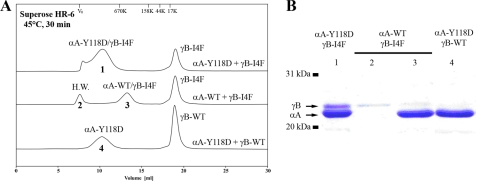
To evaluate the interactions between wild-type or mutant αA-crystallins and wild-type or mutant γB-crystallins, we performed in vitro binding assays using recombinant proteins produced from a bacterial expression system. Purified recombinant proteins were mixed and heated to induce protein aggregation before separation by gel filtration analysis. We tested three groups of mixed recombinant proteins: 1) mutant αA-Y118D with mutant γB-I4F, 2) αA-WT with mutant γB-I4F, and 3) mutant αA-Y118D with γB-WT. Eluted proteins were separated on SDS-polyacrylamide gels. We found that mutant αA-Y118D proteins bound to a large amount of mutant γB-I4F proteins (Fig. 1 A, peak 1, and B, lane 1) and did not bind to wild-type γB-crystallins (Fig. 1 A, peak 4, and B, lane 4). In comparison, wild-type αA-crystallins bound to a small amount of γB-I4F (Fig. 1 A, peak 3, and B, lane 3), and γB-I4F mutant proteins alone also formed a small amount of high molecular weight aggregates (Fig. 1 A, peak 2, and B, lane 2). The small shoulder in peak 1 may be similar to that of peak 2. Compared with wild-type αA-crystallin, mutant αA-Y118D proteins probably have increased binding affinity for γB-I4F mutant proteins. The lag in the elution of peak 3 from the column, relative to peaks 1 and 4, was probably due to the fact that the average molecular weight of αA-Y118D mutant protein aggregates was larger than that of the wild-type αA-crystallin protein aggregates (9). We further examined the interaction between mutant αA-Y118D and γB-I4F crystallins in transfected cells.
FIGURE 1.
In vitro binding assay showing more αA-Y118D/γB-I4F aggregates than wild-type αA/γB-I4F aggregates. Equal amounts of recombinant αA-crystallin and γB-crystallin proteins were mixed and heated at 45 °C for 30 min before chromatographic analysis. A, gel filtration chromatograms revealed that the α-crystallin peak became bigger with high molecular weight components when mixing the recombinant αA-Y118D proteins with recombinant γB-I4F mutant proteins (top curve), but not when the recombinant αA-Y118D proteins were mixed with recombinant wild-type γB proteins (bottom curve). When recombinant wild-type αA and recombinant γB-I4F mutant proteins were mixed together, there was a small mixed peak (middle curve, peak 3) and a high molecular weight (H.W.) peak (middle curve, peak 2). B, the protein components in the peaks (numbers 1–4) in A were examined by a Coomassie Blue-stained SDS-polyacrylamide gel. Lanes 1–4 correspond to peaks 1–4. The upper band was the recombinant γB-I4F mutant protein, and the lower band was either recombinant αA-Y118D or recombinant wild-type αA protein. Lane 1 showed that peak 1 contains both αA-Y118D and γB-I4F; lane 2 revealed that the high molecular weight peak 2 contains only γB-I4F; lane 3 had mainly αA-WT with a small amount of γB-I4F; lane 4 contained only αA-Y118D without γB-WT protein.
Mutant αA-Y118D and γB-I4F Proteins Form Aggregates in Co-transfected Cells
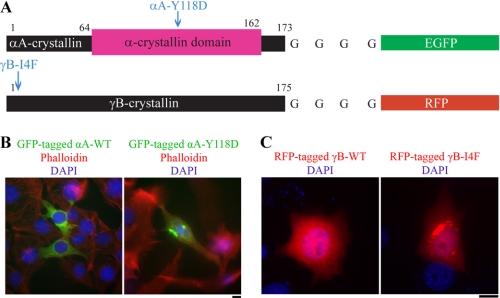
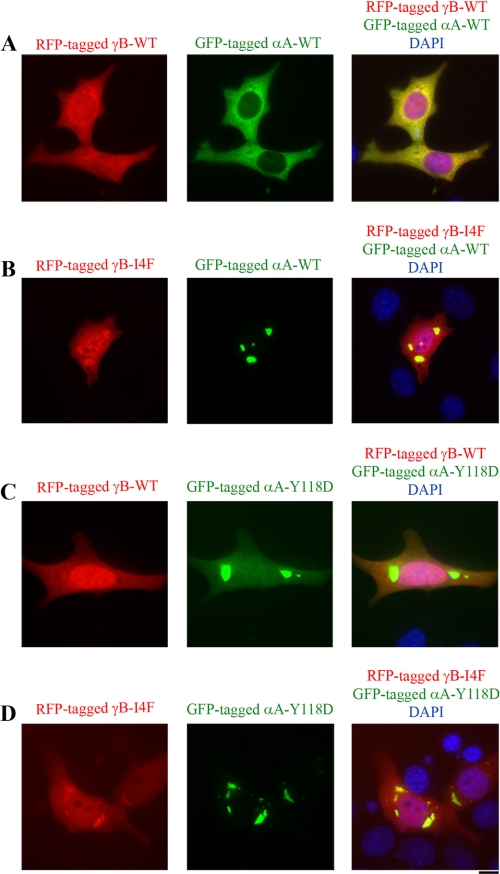
Expression plasmids for wild-type αA-crystallin, mutant αA-Y118D crystallin tagged with GFP, wild-type γB-crystallin, or mutant γB-I4F crystallin tagged with RFP were transfected individually into a mouse immortalized lens epithelial cell line (Fig. 2A). GFP-tagged wild-type αA-crystallins alone were uniformly distributed in the cytosol, whereas mutant αA-Y118D proteins alone formed cytoplasmic protein aggregates (Fig. 2B). Similarly, RFP-tagged wild-type γB-crystallins alone were uniformly distributed in cells without aggregates, whereas mutant γB-I4F proteins alone formed cytoplasmic aggregates (Fig. 2C). Co-transfection of wild-type and mutant crystallins revealed that αA-WT and γB-WT proteins maintained uniform protein distribution in the cytosol, whereas co-expression of αA-WT and γB-I4F proteins caused large aggregates (Fig. 3, A and B). In addition, co-expression of αA-Y118D and γB-WT showed that only a very small amount of γB-WT proteins was co-localized with αA-Y118D aggregates (Fig. 3C). In contrast, a large amount of γB-I4F mutant proteins was present in the αA-Y118D aggregates (Fig. 3D). These results indicate that mutant γB-I4F is truly a target protein for both αA-WT and mutant αA-Y118D in cells, as suggested by the binding data in vitro. Thus, the γB-I4F mutant protein might serve as a target substrate in vivo to determine how the interactions between α-crystallins with altered chaperone-like activity and γB-I4F mutant proteins influence lens transparency.
FIGURE 2.
Expression of GFP-tagged αA-crystallins or RFP-tagged γB-crystallins in immortalized mouse lens epithelial cells. A, illustration of wild-type or mutant αA-crystallins tagged with GFP and wild-type or mutant γB-crystallins tagged with RFP (not drawn to scale). Point mutations are marked. B, fluorescent images of GFP-tagged αA-crystallins (green) with phalloidin-stained F-actin (red) and DAPI-stained nuclei (blue) in immortalized mouse lens epithelial cells. Only αA-Y118D proteins formed aggregates in transfected cells. C, fluorescent images of RFP-tagged γB-crystallins (red) with DAPI-stained nuclei (blue) in immortalized mouse lens epithelial cells. Only γB-I4F mutant proteins formed aggregates. Scale bars, 20 μm.
FIGURE 3.
Co-expression of wild-type or mutant αA- and γB-crystallins in immortalized mouse lens epithelial cells. Fluorescent images are of GFP-tagged αA-crystallins (green), RFP-tagged γB-crystallins (red), and the merged images with DAPI-stained nuclei (blue) in transfected mouse lens epithelial cells. A, co-expression of αA-WT and γB-WT. B, co-expression of wild-type αA and γB-I4F. Wild-type αA-crystallin proteins were present in the aggregates of mutant γB-I4F proteins. C, co-expression of mutant αA-crystallin (αA-Y118D) and wild-type γB. The αA-Y118D aggregates contained a small amount of γB-WT. D, co-expression of mutant αA-Y118D and mutant γB-I4F. Mutant αA-Y118D proteins were present in the aggregates of γB-I4F proteins. Scale bar, 20 μm.
Altered Chaperone-like Activity of α-Crystallins Increases the Severity of Cataracts Caused by the γB-I4F Mutation
The αA(+/−) αB(+/−) double heterozygous knock-out mice, which display clear lenses like wild-type mice (data not shown), were used to reduce lens total chaperone-like activity of α-crystallins in vivo. The αA-Y118D heterozygous mutant mice, which develop mild nuclear cataracts (27), were used to evaluate the affect of αA-crystallin with increased chaperone-like activity. Thus, we generated αA(+/−) αB(+/−) γB(I4F/+) triple heterozygous mutant mice and αA(Y118D/+) γB(I4F/+) double heterozygous mutant mice and compared their lens phenotypes.
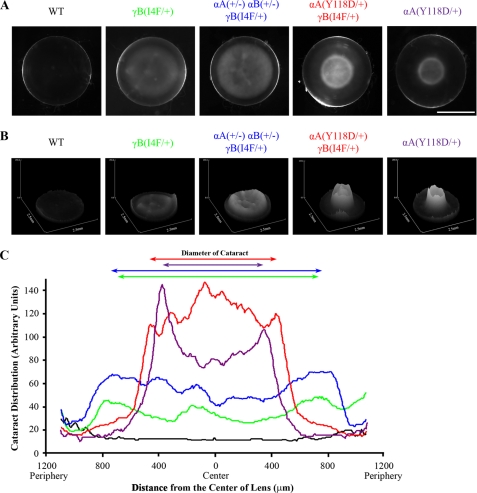
In contrast to the mild cataracts seen in γB(I4F/+) lenses, the αA(+/−) αB(+/−) γB(I4F/+) lenses had more severe cataracts, but increased opacity was less obvious in the lens core, as shown by lens pictures, densitometric surface plots, and linear plot profiles of these cataracts (Fig. 4). Quantitative measurements of light scattered from these cataracts further showed that the αA(+/−) αB(+/−) γB(I4F/+) lenses had a statistically significant increase in light scattering compared with γB(I4F/+) lenses (Fig. 5). We tried to evaluate cataract formation in younger mice. However, due to cold cataract formation in neonatal lenses (31) and the very mild cataract phenotype in triple heterozygous lenses, we were unable to obtain reliable evidence to determine whether the cataracts in triple heterozygous mice developed earlier or were more severe at neonatal stages.
FIGURE 4.
Comparison of cataracts in different mutant mouse lines. A, images of wild-type, γB(I4F/+), αA(+/−) αB(+/−) γB(I4F/+), αA(Y118D/+) γB(I4F/+), and αA(Y118D/+) lenses at postnatal day 21. Scale bar, 1 mm. B, surface plots of opacity distribution in different lenses using ImageJ software. These plots revealed that only the αA(Y118D/+) γB(I4F/+) lens has dense nuclear opacity in the lens core. C, plot profiles generated by ImageJ showed cataract distribution (y axis) relative to the distance from the center of the lens (x axis). The diameter of the cataract, from peak to peak, is represented by arrows above the plot. The cataract in the αA(Y118D/+) γB(I4F/+) lens is concentrated in the lens core.
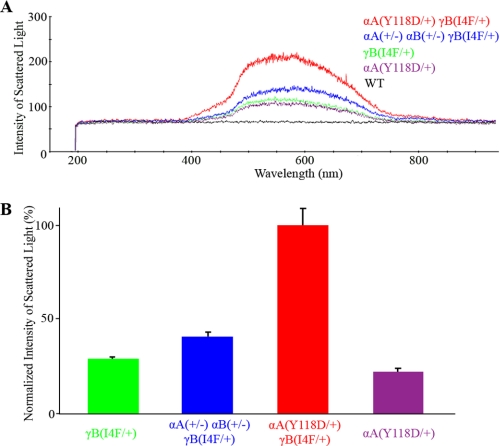
FIGURE 5.
Quantitative measurements of light scattering from compound mutant lenses reveals a statistically significant increase in light scattering caused by altered α-crystallin chaperone-like activity. A, the αA(+/−) αB(+/−) γB(I4F/+) and αA(Y118D/+) γB(I4F/+) lenses scattered more light, as measured by intensity over the visible wavelength, than γB(I4F/+) and αA(Y118D/+) lenses. The wild-type lens represents the base line of the light scattering intensity. B, bar graph of the mean ± S.D. of normalized intensity for each genotype (at least six lenses). The intensity of scattered light was arbitrarily normalized based on 0% for wild-type lenses and 100% for αA(Y118D/+) γB(I4F/+) lenses. The difference between γB(I4F/+) and αA(Y118D/+) γB(I4F/+) lenses and the difference between γB(I4F/+) and αA(+/−) αB(+/−) γB(I4F/+) lenses were statistically significant (p < 0.001).
Unexpectedly, the αA(Y118D/+) γB(I4F/+) double mutant lens had a dense cataract that was predominantly in the lens nucleus (Fig. 4). Light scattering measurements confirmed that compound mutant lenses scattered more light than γB(I4F/+) lenses (Fig. 5). The combination of the amount of light scattered by αA(Y118D/+) lenses and γB(I4F/+) lenses was about 50% of the amount of light scattered by αA(Y118D/+) γB(I4F/+) double heterozygous lenses. Therefore, the dense nuclear cataracts in double heterozygous mutant lenses likely resulted from enhanced interaction between αA-Y118D and γB-I4F mutant proteins.
DISCUSSION
This study provides direct in vivo evidence demonstrating that lens transparency is influenced by the interactions between α-crystallins and a native target substrate, γB-I4F crystallin. Although αA(+/−) αB(+/−) mice develop normal lenses, increased light scattering from cataractous αA(+/−) αB(+/−) γB(I4F/+) lenses indicates that the function of α-crystallin is impaired in αA(+/−) αB(+/−) mice. A normal amount of α-crystallins is important for lens transparency. It is well known that αA-crystallins undergo posttranslational modifications to alter the chaperone-like activity during lens development or during aging of a normal lens (32). The precise regulation of the chaperone-like activity of α-crystallin and the proper binding affinity between α-crystallin and other lens proteins are likely required for establishing and maintaining lens transparency.
Increased binding affinity of αA-Y118D mutant proteins to γB-I4F mutant proteins probably generates more protein aggregates, leading to light scattering in the lens. These results reveal that the synergistic effect of αA-Y118D and γB-I4F mutant protein interactions is detrimental to the transparency only in the lens core. Thus, α-crystallins with different chaperone-like activity are likely needed in the lens cortex and nucleus for maintaining transparency. This work supports the notion that lens transparency may require high chaperone-like activity of α-crystallins in the lens cortex, but low chaperone-like activity of α-crystallins in the lens nucleus.
Aberrant Aggregates of αA-Y118D Mutant Proteins in Cultured Cells
We have confirmed that the αA-Y118D crystallin is a gain-of-function mutant that binds strongly to γB-I4F mutant proteins in transfected cells and in recombinant protein binding assays. Endogenous αA-crystallin proteins were not detectable in the immortalized mouse lens epithelial cells, whereas αB-crystallins could be detected by Western blotting (data not shown). Increased chaperone-like activity (or increased binding affinity) of αA-Y118D mutant proteins may cause abnormal αA-Y118D protein aggregation or binding to other endogenous proteins. It is possible that the αA-Y118D mutant protein by itself is unstable or causes precipitation of normal proteins within lens cells. Currently, it is unclear why αA-Y118D mutant proteins form aggregates in transfected cells and what the other proteins are in these aggregates. We confirmed that these αA-Y118D aggregates are not co-localized with the lysosome marker Lamp-1, the Golgi marker giantin, or the endoplasmic reticulum marker calreticulin (data not shown). For future studies, it will be very interesting to investigate the molecular basis for these aggregates and to determine whether ubiquitination is associated with the mutant protein aggregates.
In Vivo Chaperone-like Activity of α-Crystallins Is Essential for Lens Transparency
Previous studies were unable to prove the necessity for utilizing both alleles of αA and αB genes because αA(+/−) and αB(+/−) mice develop clear lenses despite a decrease in the amount of αA and αB proteins, respectively (25, 26). More severe cataracts in αA(+/−) αB(+/−) γB(I4F/+) lenses indicate that α-crystallins expressed from both gene alleles are essential for maintaining lens transparency. Decades of in vitro studies have suggested that α-crystallins with chaperone-like activity may serve a protective function in the lens (7, 8). Recessive cataracts caused by αA-crystallin null mutations in humans and in mice indicate that αA-crystallin is essential for establishing and/or maintaining transparency in wild-type lenses (25, 33). However, α-crystallins have not been directly shown to prevent cataract formation caused by other mutations in vivo. Opacity differences between γB(I4F/+) and αA(+/−) αB(+/−) γB(I4F/+) lenses reveal the first in vivo evidence that α-crystallins suppress cataract formation induced by the unstable γB-I4F mutant protein. Thus, γB-I4F mutant proteins are a useful native “denatured” target protein for wild-type and mutant α-crystallins for in vitro and in vivo chaperone-like activity assays.
Previous studies of the γB-I4F mutation suggested that mutant γB-I4F proteins were less heat-stable (24). It is likely that an insufficient amount of α-crystallin proteins results in the aggregation of unstable γB-I4F mutant proteins shown in Fig. 1A (H.W. peak 2). The molar ratio of α-crystallin multimers was much lower than that of γB-I4F protein monomers in the in vitro binding assay. Presumably, an insufficient amount of α-crystallins also cause increased aggregation and insolubility of γB-I4F mutant proteins in αA(+/−) αB(+/−) γB(I4F/+) mutant lenses in vivo. Further experiments will be needed to elucidate the mechanisms for cataractogenesis in αA(+/−) αB(+/−) γB(I4F/+) and αA(Y118D/+) γB(I4F/+) mutant lenses. However, it is difficult to separate the effects of mutant proteins versus increased chaperone-like activity in vivo. It may be interesting to determine whether an extra amount of wild-type αA-crystallin, expressed from a transgenic mouse line, can inhibit cataracts caused by the γB-I4F mutation.
Quantitative Light Scattering Measurements Accurately Determine Cataract Severity in Mouse Lenses
We have implemented and tested a quantitative method to determine light scattering in cataractous lenses among different mouse strains using a fiber-optic cable and spectrometer. Previous studies have used qualitative scoring methods that rely on the judgment of researchers or imaging the lens on a grid background (34). These methods cannot be used to quantify cataract severity. Without the light scattering intensity quantification, it would be difficult to demonstrate a significant difference between γB(I4F/+) and αA(+/−) αB(+/−) γB(I4F/+) lenses. Moreover, this new quantification method can be easily established in any laboratory and is an excellent tool for vision researchers to use to quantitatively study cataractogenesis in mice.
This work was supported, in whole or in part, by National Institutes of Health Grant EY013849 (to X. G.) from NEI.
- RFP
- red fluorescent protein.
REFERENCES
- 1.Lovicu F. J., McAvoy J. W. (2005) Dev. Biol. 280, 1–14 [DOI] [PubMed] [Google Scholar]
- 2.Lovicu F. J., Robinson M. L. (2004) Development of the ocular lens, Cambridge University Press, Cambridge, UK [Google Scholar]
- 3.Piatigorsky J. (1981) Differentiation 19, 134–153 [DOI] [PubMed] [Google Scholar]
- 4.Bloemendal H., De Jong W., Jaenicke R., Lubsen N. H., Slingsby C., Tardieu A. (2004) Prog. Biophys. Mol. Biol. 86, 407–485 [DOI] [PubMed] [Google Scholar]
- 5.Delaye M., Tardieu A. (1983) Nature 302, 415–417 [DOI] [PubMed] [Google Scholar]
- 6.Benedek G. B. (1997) Invest. Ophthalmol. Vis. Sci. 38, 1911–1921 [PubMed] [Google Scholar]
- 7.Derham B. K., Harding J. J. (1999) Prog. Retin. Eye Res. 18, 463–509 [DOI] [PubMed] [Google Scholar]
- 8.Horwitz J. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 10449–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Q., Ding L., Phan K. B., Cheng C., Xia C. H., Gong X., Horwitz J. (2009) Invest. Ophthalmol. Vis. Sci. 50, 2919–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murugesan R., Santhoshkumar P., Sharma K. K. (2007) Mol. Vis. 13, 2301–2309 [PubMed] [Google Scholar]
- 11.Singh D., Raman B., Ramakrishna T., Rao Ch. M. (2006) Mol. Vis. 12, 1372–1379 [PubMed] [Google Scholar]
- 12.Srinivas V., Raman B., Rao K. S., Ramakrishna T., Rao Ch. M. (2003) Protein Sci. 12, 1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Li C., Lu Q., Su T., Ke T., Li D. W., Yuan M., Liu J., Ren X., Zhang Z., Zeng S., Wang Q. K., Liu M. (2008) Biochim. Biophys. Acta 1782, 303–309 [DOI] [PubMed] [Google Scholar]
- 14.Ghosh J. G., Houck S. A., Clark J. I. (2007) PLoS ONE 2, e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graw J. (2009) Exp. Eye Res. 88, 173–189 [DOI] [PubMed] [Google Scholar]
- 16.Hejtmancik J. F. (2008) Semin. Cell Dev. Biol. 19, 134–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pande A., Zhang J., Banerjee P. R., Puttamadappa S. S., Shekhtman A., Pande J. (2009) Biochem. Biphys. Res. Commun. 382, 196–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perng M. D., Muchowski P. J., van den Ijssel P., Wu G. J., Hutcheson A. M., Clark J. I., Quinlan R. A. (1999) J. Biol. Chem. 274, 33235–33243 [DOI] [PubMed] [Google Scholar]
- 19.Shroff N. P., Cherian-Shaw M., Bera S., Abraham E. C. (2000) Biochemistry 39, 1420–1426 [DOI] [PubMed] [Google Scholar]
- 20.Gu F., Luo W., Li X., Wang Z., Lu S., Zhang M., Zhao B., Zhu S., Feng S., Yan Y. B., Huang S., Ma X. (2008) Hum. Mutat. 29, 769. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Zhang X., Luo L., Wu M., Zeng R., Cheng G., Hu B., Liu B., Liang J. J., Shang F. (2006) Invest. Ophthalmol. Vis. Sci. 47, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xi J. H., Bai F., Gross J., Townsend R. R., Menko A. S., Andley U. P. (2008) J. Biol. Chem. 283, 5801–5814 [DOI] [PubMed] [Google Scholar]
- 23.Andley U. P., Hamilton P. D., Ravi N. (2008) Biochemistry 47, 9697–9706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H., Du X., Wang M., Huang Q., Ding L., McDonald H. W., Yates J. R., 3rd, Beutler B., Horwitz J., Gong X. (2005) J. Biol. Chem. 280, 25071–25078 [DOI] [PubMed] [Google Scholar]
- 25.Brady J. P., Garland D., Duglas-Tabor Y., Robison W. G., Jr., Groome A., Wawrousek E. F. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brady J. P., Garland D. L., Green D. E., Tamm E. R., Giblin F. J., Wawrousek E. F. (2001) Invest. Ophthalmol. Vis. Sci. 42, 2924–2934 [PubMed] [Google Scholar]
- 27.Xia C. H., Liu H., Chang B., Cheng C., Cheung D., Wang M., Huang Q., Horwitz J., Gong X. (2006) Invest. Ophthalmol. Vis. Sci. 47, 3004–3010 [DOI] [PubMed] [Google Scholar]
- 28.Morozov V., Wawrousek E. F. (2006) Development 133, 813–821 [DOI] [PubMed] [Google Scholar]
- 29.Horwitz J., Huang Q. L., Ding L., Bova M. P. (1998) Methods Enzymol. 290, 365–383 [DOI] [PubMed] [Google Scholar]
- 30.Mayer M., Buchner J. (2004) Methods Mol. Med. 94, 239–254 [DOI] [PubMed] [Google Scholar]
- 31.Wang K., Cheng C., Li L., Liu H., Huang Q., Xia C. H., Yao K., Sun P., Horwitz J., Gong X. (2007) Invest. Ophthalmol. Vis. Sci. 48, 3719–3728 [DOI] [PubMed] [Google Scholar]
- 32.Sharma K. K., Santhoshkumar P. (2009) Biochim. Biophys. Acta 1790, 1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pras E., Frydman M., Levy-Nissenbaum E., Bakhan T., Raz J., Assia E. I., Goldman B., Pras E. (2000) Invest. Ophthalmol. Vis. Sci. 41, 3511–3515 [PubMed] [Google Scholar]
- 34.Sanderson J., Marcantonio J. M., Duncan G. (2000) Invest. Ophthalmol. Vis. Sci. 41, 2255–2261 [PubMed] [Google Scholar]