Abstract
An astonishing number of extracellular matrix glycoproteins are expressed in dynamic patterns in the developing and adult nervous system. Neural stem cells, neurons, and glia express receptors that mediate interactions with specific extracellular matrix molecules. Functional studies in vitro and genetic studies in mice have provided evidence that the extracellular matrix affects virtually all aspects of nervous system development and function. Here we will summarize recent findings that have shed light on the specific functions of defined extracellular matrix molecules on such diverse processes as neural stem cell differentiation, neuronal migration, the formation of axonal tracts, and the maturation and function of synapses in the peripheral and central nervous system.
Neurons and glia express receptors that interact with numerous extracellular glycoproteins. These regulate processes such as stem cell differentiation, neuronal migration, and the formation of axonal tracts and synapses.
Extracellular matrix (ECM) glycoproteins are widely expressed in the developing and adult nervous system. Tremendous progress has been made in defining the roles of specific ECM components in controlling the behavior of neurons and glia (Sanes 1989; Reichardt and Tomaselli 1991; Venstrom and Reichardt 1993; Milner and Campbell 2002; Nakamoto et al. 2004). Here, we will provide an overview of ECM functions in the nervous system, emphasizing recent findings that have shed light on the mechanisms by which ECM glycoproteins regulate such diverse processes as neural stem cell (NSC) behavior, neuronal migration, formation of axonal processes and their myelin sheets, and synapse formation and function.
NEURAL STEM CELL BEHAVIOR AND NEURONAL MIGRATION
NSCs give rise to neurons and glia, and the ECM provides a microenvironment that modulates NSC behavior (Perris and Perissinotto 2000; Sobeih and Corfas 2002; Zimmermann and Dours-Zimmermann 2008). Radial glial cells (RGCs) of the developing central nervous system (CNS) are a well-studied class of NSCs (Fig. 1) (Temple 2001; Fishell and Kriegstein 2003; Kriegstein and Noctor 2004; Noctor et al. 2007; Malatesta et al. 2008; Miller and Gauthier-Fisher 2009). RGCs are also precursors of neural progenitors maintained in the adult brain (Kokovay et al. 2008; Miller and Gauthier-Fisher 2009). RGCs have a radial morphology, with apical processes contacting the ventricle and basal processes extending across the respective CNS structures (Fig. 1). Many neurons use basal RGC processes as a scaffold for migration. The ECM shapes the niche where NSCs reside, modulates their maintenance and differentiation, and influences migration of their progeny (Sobeih and Corfas 2002; Porcionatto 2006; von Holst 2008).
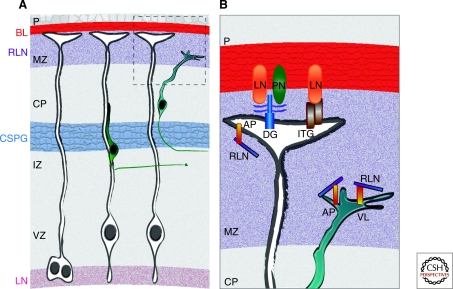
Figure 1.
ECM molecules in the developing neocortex. (A) Overview of some ECM molecules found in the embryonic neocortex. Laminin (LN) is a major component of the basal lamina (BL) under the pia mater (P) and is also found in the ventricular zone (VZ). Reelin (RLN) is secreted in the marginal zone (MZ) by Cajal-Retzius cells. Chondroitin sulfate proteoglycans (CSPGs) are concentrated in the subplate region above the intermediate zone (IZ). (B) Higher magnification schematic of the boxed region in (A). RGC endfeet interact with ECM molecules in the BL, such as LN and perlecan (PN), through the integrin (ITG) and dystroglycan (DG) receptors. Radial glia and neurons engage in reelin signaling via the ApoER2 (AP) and VLDLR (VL) receptors.
Laminins
The ECM forms a basal lamina (BL) surrounding the brain and blood vessels throughout the CNS (Timpl and Brown 1996; Erickson and Couchman 2000). In the neocortex, the BL at the pial surface is contacted by RGCs’ endfeet (Fig. 1). A number of studies have shown that the pial BL is crucial for neocortical development. Removal of the BL leads to detachment of RGC fibers, affecting RGC survival and cortical lamination (Sievers et al. 1986; von Knebel Doeberitz et al. 1986; Sievers et al. 1994; Radakovits et al. 2009). Laminins are major components of the BL (Timpl et al. 1979) and are also present in the VZ of the developing neocortex (Campos et al. 2004; Lathia et al. 2007). Laminins promote the expansion, migration, and differentiation of NSCs in vitro (Drago et al. 1991; Liesi 1992; Liesi et al. 1992; Kearns et al. 2003; Campos et al. 2004; Flanagan et al. 2006; Hall et al. 2008; Ma et al. 2008; Silva et al. 2009; Pierret et al. 2010). Expression of several laminin subunits in cultured NSCs is dependent on the transcription factor RE1 Silencing Factor (REST) (Otto et al. 2007; Sun et al. 2008). REST regulates neurogenesis by repressing neurogenic genes in nonneuronal tissues (Schoenherr et al. 1996; Chen et al. 1998). REST-null embryonic stem cells have defects in cell adhesion, NSC generation and neuronal differentiation, phenotypes that can be rescued by exogenously added laminin (Sun et al. 2008). However, the effects of laminins on NSCs and the importance of the REST/Laminin interaction still await examination in vivo. Mice lacking laminin γ1 die during embryogenesis (Smyth et al. 1999); those bearing a mutation affecting solely the laminin γ1 nidogen-binding domain survive until birth and display disruptions of the pial BL and neuronal ectopias (Halfter et al. 2002). Inactivation of laminin γ1 in a subset of cortical neurons causes cortical lamination defects (Chen et al. 2009). However, defects of NSC maintenance or differentiation have not been reported in these mutants.
In vivo evidence for a role of laminins in controlling NSC behavior comes from studies of their dystroglycan and integrin receptors. Human patients with mutations in enzymes that glycosylate dystroglycan show cortical neuronal ectopias (Yoshida et al. 2001; Beltran-Valero de Bernabe et al. 2002). Mice without dystroglycan in the CNS or bearing mutations in dystroglycan glycosyltransfserases display BL disruptions and neuronal migration defects (Grewal et al. 2001; Michele et al. 2002; Moore et al. 2002). Inactivation of β1 integrins in RGCs results in abnormal neocortical lamination and fusion of cerebellar folia (Graus-Porta et al. 2001; Blaess et al. 2004). These abnormalities are caused by detachment of RGCs from the pia and disorganization of the pial BL and cortical marginal zone (MZ) (Fig. 1) (Graus-Porta et al. 2001; Blaess et al. 2004; Radakovits et al. 2009). In the neocortex of β1-deficient animals, neurons associate with intact RGCs and migrate, but form ectopias in the MZ (Graus-Porta et al. 2001). Similar phenotypes are observed in mice lacking the α6 integrin subunit or both α6 and α3, which heterodimerize with β1 to form laminin receptors (Georges-Labouesse et al. 1998; De Arcangelis et al. 1999; Colognato et al. 2005). Deletion of β1 integrin solely in migrating neurons results in normal neocortical lamination, indicating that abnormalities in neuronal migration are secondary to defects in RGCs (Graus-Porta et al. 2001; Belvindrah et al. 2007a).
Disruption of β1 integrin function in the VZ by antibody injections leads to detachment of RGC apical processes (Loulier et al. 2009). Apical detachment of RGCs is also observed in mice lacking laminin α2 (Loulier et al. 2009). Thus, β1 integrins and laminins appear to maintain both apical and basal RGC processes. In addition, loss of laminin γ1 prevents neurons from migrating towards the MZ (Chen et al. 2009), a phenotype that differs from those resulting from loss of β1 integrins, indicating that other surface receptors are involved. For example, β4, β8, α3, α4, and α5 integrins have been implicated in NSC development, neocortical lamination, and/or neuronal migration (Murgia et al. 1998; Mobley et al. 2009; Stanco et al. 2009; Marchetti et al. 2010). Furthermore, ECM molecules and integrins likely play context-dependent roles. For example, in the adult brain, β1 integrins and laminins α2/α4 are required for the formation of cell chains in the rostral migratory stream (RMS) (Belvindrah et al. 2007b).
Proteoglycans
Proteoglycans are prominently expressed in the nervous system (Gu et al. 2007; Gu et al. 2009; Abaskharoun et al. 2010), and enzymatic digestion of chondroitin sulfate proteoglycans disrupts the development of NCSs in culture (von Holst et al. 2006; Gu et al. 2009). Yet, no major abnormalities have been described in the CNS of mice lacking proteoglycans, likely because of either functional redundancy or early embryonic lethality (Hartmann and Maurer 2001; Zimmermann and Dours-Zimmermann 2008). One exception is the BL component perlecan. Genetic ablation of perlecan results in exencephaly following massive BL disruptions, or in neuronal ectopias in mutant brains with less severe BL defects (Haubst et al. 2006; Giros et al. 2007). In the latter cases, cell cycle progression in NSCs is affected, likely because of decreased levels of sonic hedgehog (Giros et al. 2007). Interestingly, proliferation of granule cell precursors in the cerebellum is also affected in mice lacking β1 integrins, a phenotype that is caused at least in part by defective sonic hedgehog signaling (Blaess et al. 2004).
Tenascins
Tenascin-C (TN-C) is expressed in the CNS in regions of active neurogenesis (Bartsch et al. 1992; Jankovski and Sotelo 1996). Tenascin-R (TN-R) expression is prominent in myelinating glia, in subsets of interneurons and in the deepest layers of the olfactory bulbs (Saghatelyan et al. 2004; Huang et al. 2009). In NSCs in culture, TN-C facilitates the switch from production of neuronal to glial progenitors (Lillien and Raphael 2000; Garcion et al. 2004; Liao et al. 2008), whereas TN-R inhibits migration of NSC-derived neurons (Huang et al. 2009). In vivo, TN-C regulates myelinating glial lineage development and glomerulogenesis in the olfactory bulbs (OBs) (Garcion et al. 2001; Treloar et al. 2009). TN-R promotes detachment of chain-migrating neuroblasts in the RMS and their migration within the OBs. Interestingly, OB expression of TN-R is activity-dependent and reduced on odor deprivation (Saghatelyan et al. 2004).
Reelin
Reelin is one of the best-studied ECM glycoproteins in the CNS. During development, reelin is secreted by specific cell types in laminated brain structures, including the neocortex (Fig. 1). Reelin binds to the lipoprotein receptors ApoER2 and VLDLR (D'Arcangelo et al. 1999), which are expressed by migrating neurons and RGCs (Luque et al. 2003). ApoER2 and VLDLR bind to the adaptor protein Dab1, which is phosphorylated by Src-family kinases on reelin binding to its receptors (Howell et al. 1999; Arnaud et al. 2003). Phosphorylated Dab1 recruits signaling molecules including PI3K (Bock et al. 2003), Crk/CrkL (Ballif et al. 2004; Chen et al. 2004; Huang et al. 2004), and Lis1 (Assadi et al. 2003). Mutations in reelin signaling in humans cause lissencephaly and cerebellar hypoplasia (Hong et al. 2000), and in mice severe CNS abnormalities characterized most notably by severe lamination defects in the cerebellum, hippocampus and neocortex (Mariani et al. 1977; Caviness and Korde 1981; Caviness 1982; Goffinet 1983; Goffinet et al. 1984; Hoffarth et al. 1995). Defective neocortical lamination is caused by failure of newborn neurons to move past their predecessors, creating a disorganized cytoarchitecture lacking the typical inside-out layering pattern of the normal neocortex. Because the number and types of neocortical neurons generated appears unaffected in reeler mutants (Caviness 1973), reelin is thought to primarily control migration. However, the cellular mechanism by which reelin regulates cell positioning is not known. Reelin has variably been proposed to be a chemoattractant (Gilmore and Herrup 2000), repellent (Ogawa et al. 1995; Schiffmann et al. 1997), stop (Sheppard and Pearlman 1997), or detachment (Sheppard and Pearlman 1997; Dulabon et al. 2000; Sanada et al. 2004) signal for migrating neurons.
A role for reelin as a detachment signal is supported by the observation that postmigratory neurons remain associated with RGC fibers in reeler mice, creating a “traffic jam” (Pinto-Lord et al. 1982), and by studies suggesting that reelin down-regulates integrin-mediated adhesion, allowing migrating neurons to detach from the RGC scaffold (Dulabon et al. 2000; Sanada et al. 2004). However, when reelin-responsive and nonresponsive neurons coexist, wild-type cells migrate normally (Sanada et al. 2004; Olson et al. 2006; Hammond et al. 2010). Genetic studies also do not support a role for integrins on migrating neurons in reelin signaling (Belvindrah et al. 2007a). In addition, the detachment hypothesis does not account for migration defects in early-born neurons, which do not migrate along RGCs (Nadarajah et al. 2001). Finally, cell-autonomous perturbations of reelin signaling in radially migrating neurons block their movement and addition of recombinant reelin to slice cultures from reeler mice restores migration (Jossin et al. 2004; Olson et al. 2006; Young-Pearse et al. 2007; Hashimoto-Torii et al. 2008), indicating that reelin promotes motility. Because recent observations show that neocortical neurons migrate by glia-dependent and glia-independent modes (Nadarajah et al. 2001), it has been proposed that reelin stimulates detachment of neurons from RGCs as well as glia-independent migration (Luque et al. 2003; Cooper 2008).
Studies demonstrating cross talk between reelin and other signaling pathways, such as integrins (Dulabon et al. 2000; Calderwood et al. 2003; Sanada et al. 2004), Notch (Hashimoto-Torii et al. 2008), amyloid precursor protein (Young-Pearse et al. 2007) and thrombospondins (Blake et al. 2008) indicate that reelin is part of a complex developmental paradigm with distinct mechanisms of action in different brain regions (Trommsdorff et al. 1999; Benhayon et al. 2003; Beffert et al. 2006; Hack et al. 2007; Forster et al. 2010).
AXONAL GROWTH AND MYELINATION
Wiring of the nervous system depends on a coordinated sequence of events, including axon growth to precise targets and their subsequent myelination. CNS myelination is performed by oligodendrocytes, whereas Schwann cells myelinate peripheral nerves (Sherman and Brophy 2005; Simons and Trajkovic 2006). The ECM is crucial for axon formation and myelination (Colognato et al. 2005; Chernousov et al. 2008) (Fig. 2), and is a component of glial scar tissue at sites of CNS injury (Rolls et al. 2009).
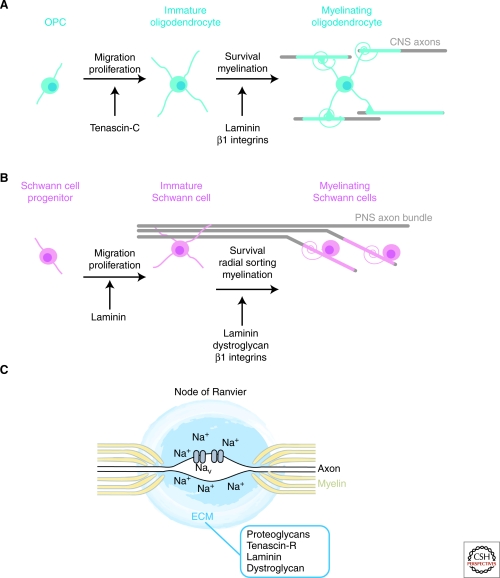
Figure 2.
ECM and myelination. (A) Oligodendroglia differentiate in sequential stages to generate mature oligodendrocytes. Each oligodendrocyte myelinates several CNS axons. Tenascin-C, laminin, and their β1 integrin receptors play roles at different developmental stages, as indicated. (B) Schwann cells myelinate peripheral nerves. Immature Schwann cells sort out axonal bundles to individually myelinate each axon. Laminin regulates all stages of Schwann cell development, whereas dystroglycan and β1 integrin receptors control axonal sorting and myelination. (C) The ECM surrounding nodes of Ranvier may regulate the local concentration of cations and clusters voltage-gated sodium channels, which allow for saltatory electrical conductivity. Several proteoglycans, tenascin-R, laminin and dystroglycan contribute to the formation of nodal matrices. Nav, voltage-gated channel; Na+, sodium cations.
Laminins
Some of the first results implicating ECM molecules in nervous system development showed that laminins promote neurite outgrowth in an integrin-dependent manner (Calof and Reichardt 1985; Lander et al. 1985a; Lander et al. 1985b; Hall et al. 1987; Tomaselli and Reichardt 1988). These findings have been extended by others (e.g., Gomez and Letourneau 1994; Luckenbill-Edds 1997; Esch et al. 1999; Menager et al. 2004). Laminins have also been implicated in axonal guidance in vivo. For example, ablation of Drosophila laminin A results in pathfinding defects in sensory nerves (Garcia-Alonso et al. 1996). In Xenopus, laminin-1 modulates growth cone behavior of retinal neurons, converting the attraction cue provided by netrin-1 into repulsion (Hopker et al. 1999). In mice, laminin γ1 deficiency results in abnormal branching of myelinated axons from the corpus callosum (Chen et al. 2009). The mutants also show abnormal neuronal migration (see below), impaired activation of integrin downstream effectors like focal adhesion kinase and paxillin, and disrupted AKT/GSK-3β signaling, which has been implicated in neurite growth (Yoshimura et al. 2005). The exact mechanisms underlying these abnormalities await investigation.
In the peripheral nervous system (PNS), Schwann cells are surrounded by a basal lamina (BL) that contains laminin 2 (α2β1γ1), laminin 8 (α4β1γ1), and laminin 10 (α5β1γ1) (Feltri and Wrabetz 2005). Schwann cells migrate into peripheral nerve and use a mechanism known as radial sorting to establish a 1:1 association with each larger diameter axon (Shy 2009) (Fig. 2). Human patients suffering from congenital muscle dystrophy (MDC) lack laminin α2 and develop demyelinating peripheral neuropathy (Shorer et al. 1995; Di Muzio et al. 2003). In laminin α2-deficient mice, sorting and axon myelination are impaired following reduced Schwann cell proliferation and inability to extend myelin sheets (Bray et al. 1977; Helbling-Leclerc et al. 1995; Feltri et al. 2002). These defects are most prominent in nerve roots. Conversely, loss of laminin α4 leads to axonal sorting defects that are pronounced in distal nerve; when both laminin α2 and α4 are missing, all axon segments are affected (Wallquist et al. 2005; Yang et al. 2005). Similarly, mice lacking laminin γ1 in Schwann cells show decreased Schwann cell proliferation, differentiation, and survival, radial sorting impairment, hypomyelination and reduced nerve conduction velocity (Yu et al. 2005; Yu et al. 2007). Mice lacking β1 integrin in Schwann cells show radial axonal sorting defects and hypomyelination, but normal Schwann cell proliferation and survival (Feltri et al. 2002), suggesting that β1 integrins only mediate some laminin functions. Dystroglycan is mainly expressed postnatally in Schwann cells but is another crucial laminin receptor. Dystroglycan-null mice have abnormally folded myelin and reduced clustering of sodium channels at the nodes of Ranvier (Saito et al. 2003).
In the CNS, oligodendrocytes derive mainly from precursors residing in the ventral VZ and ganglionic eminences. They proliferate and migrate before becoming mature myelinating cells (Bradl and Lassmann 2010). Oligodendrocytes are not associated with a BL and each cell extends multiple sheets able to myelinate several axons (Colognato et al. 2005; Simons and Trotter 2007) (Fig. 2). Expression of laminins correlates with the onset of CNS myelination (Colognato et al. 2002; Colognato et al. 2005), and varied degrees of defects have been found in white matter tracts of patients suffering from MDC (Caro et al. 1999; Leite et al. 2005). Mice lacking laminin α2 have a developmental delay in oligodendrocyte maturation, resulting in hypomyelination (Chun et al. 2003; Relucio et al. 2009). The degree of developmental delay is region-specific, which may reflect different laminin α2 requirements (Relucio et al. 2009). Abnormalities in Fyn signaling, which is modulated by laminins in cultured oligodendrocytes, were observed in the mutant brains, suggesting one explanation for the temporary stall in oligodendrocyte differentiation (Colognato et al. 2004; Relucio et al. 2009). Interestingly, β1 integrins not only affect PNS but also CNS myelination (Relvas et al. 2001; Barros et al. 2009). Deletion of β1 integrins in the CNS results in thinner myelin sheaths in several regions, and cultured oligodendrocytes require β1integrin signaling via Akt to extend myelin sheets (Barros et al. 2009).
Proteoglycans
A major obstacle for regeneration after CNS injury is the axon growth-inhibitory activity of the glial scar (Rolls et al. 2009). Chondroitin sulfate proteoglycans (CSPGs) are main scar components and found up-regulated in injured rat brains and spinal cords (Silver and Miller 2004; Galtrey and Fawcett 2007). In vitro, phosphacan and all soluble hyaluronan-binding CSPGs (aggrecan, versican, neurocan, and brevican) inhibit axonal growth (Bandtlow and Zimmermann 2000; Yamaguchi 2000). Enzymatic digestion of CSPGs reduces their inhibitory activity (McKeon et al. 1995; Smith-Thomas et al. 1995) and promotes axon regrowth and functional recovery after spinal cord injury (Moon et al. 2001; Bradbury et al. 2002; Yick et al. 2003; Caggiano et al. 2005; Bai et al. 2010).
Proteoglycans have been proposed to participate in the assembly of the extracellular meshwork surrounding nodes of Ranvier (Fig. 2). Differential proteoglycan expression is observed in central versus peripheral nodes of Ranvier and between large and small diameter CNS axons (Peles and Salzer 2000; Melendez-Vasquez et al. 2005). In brevican-deficient mice, the CNS nodal matrix composition is reorganized; components typically associated with large diameter nodes, such as phosphacan and TN-R, no longer show a diameter-dependent association (Bekku et al. 2009). Molecular alterations of the nodal ECM are also observed in a mouse model lacking the versican splice variant V2 (Dours-Zimmermann et al. 2009). However, no conduction velocity defects were obvious in either of these mutants (Bekku et al. 2009; Dours-Zimmermann et al. 2009). In contrast, loss of brain-specific hyaluronan-binding link protein 1 (Bral1), which also localizes over nodes of Ranvier and forms complexes with brevican and versican V2, inhibits the stabilization of nodal matrices and is thought to impair accumulation of cations at nodes, resulting in slow conduction velocities (Bekku et al. 2010).
Tenascins
TN-R and TN-C have been implicated in neurite growth. In vitro, both TNs promote or retard neuritogenesis, depending on the neuronal cell types (Faissner and Kruse 1990; Pesheva et al. 1993; Taylor et al. 1993; Lochter et al. 1994; Rigato et al. 2002; Mercado et al. 2004). Although no axonal pathfinding defects have been reported in TN-R mutant mice, TN-R acts as a repellent for optic axons in zebrafish (Becker et al. 2003; Becker et al. 2004). In the OB, TN-C is an inhibitory boundary molecule, preventing axonal growth of sensory neurons before glomerulogenesis (Treloar et al. 2009).
TN-R and TN-C also regulate myelinating glia and axonal function (Fig. 2). TN-R is expressed in immature and mature oligodendrocytes, and TN-C in oligodendrocyte precursor cells (OPCs) (Fuss et al. 1993; Czopka et al. 2009). TN-R facilitates OPC differentiation in vitro, whereas oligodendrocyte maturation is reduced on TN-C substrates. Conversely, loss of TN-C accelerates oligodendrocyte differentiation (Pesheva et al. 1997; Garwood et al. 2004; Czopka et al. 2009). Despite these opposing effects, TN-C and TN-R inhibit extension of myelin sheets by oligodendrocytes in vitro (Garcion et al. 2004; Czopka et al. 2009). However, neither TN-R nor TN-C knockout mice show myelination abnormalities (Kiernan et al. 1999; Weber et al. 1999). TN-C mutants have increased migration and reduced rate of OPC proliferation, but decreased cell death in myelination areas likely corrects for any reduction in oligodendrocyte density (Garcion et al. 2001). Interestingly, in TN-R knockout mice, expression of phosphacan along white matter tracts is perturbed and axonal conduction velocity is decreased (Weber et al. 1999), suggesting that TN-R may have an essential function in ECM assembly at nodes of Ranvier.
Thrombospondin Type-1 Repeat Proteins
ECM proteins sharing thrombospondin type-1 repeats regulate axon outgrowth and guidance. These include members of the thrombospondin (TSP) family, F-spondin, SCO-spondin, and others (Adams and Tucker 2000; Tucker 2004; Meiniel et al. 2008). TSP isoform-1 is the best-characterized member of the TSP family and is secreted by astroglia. TSP1 promotes neurite outgrowth in many types of cultured neurons (Neugebauer et al. 1991; O'Shea et al. 1991; Osterhout et al. 1992). This effect is mediated by β1 integrins in retinal and sympathetic neurons (Tomaselli et al. 1990; DeFreitas et al. 1995). TSP1 is also detected along white matter tracts and promotes migration of OPCs (Scott-Drew and ffrench-Constant 1997). Additionally, TSP1 levels are up-regulated at sites of injury, and correlate with the capacity of axons to regenerate (Moller et al. 1996; Hoffman and O'Shea 1999a; Hoffman and O'Shea 1999b).
F-spondin is expressed in the floor plate and in developing peripheral nerves. It inhibits adhesion and influences migration of neural crest cells, promotes commissural axon outgrowth, and acts as a contact-repellent molecule for embryonic motor neurons (Klar et al. 1992; Burstyn-Cohen et al. 1998; Burstyn-Cohen et al. 1999; Debby-Brafman et al. 1999; Tzarfati-Majar et al. 2001). F-spondin is also thought to influence repair in injured peripheral sensory neurons (Burstyn-Cohen et al. 1998). SCO-spondin is secreted by ependymal cells of the subcommissural organ (SCO) in the developing vertebrate brain (Gobron et al. 1996; Goncalves-Mendes et al. 2003; Meiniel et al. 2008). TSR motifs of SCO-spondin induce neurite extension in neuronal cell lines in a β1-integrin-dependent fashion; immunohistochemical evidence suggests it may control axonal development in vivo (Bamdad et al. 2004; Caprile et al. 2009; Hoyo-Becerra et al. 2010).
Netrins and Slits
The secreted molecules netrins and slits are part of two of the major protein families with crucial roles in axonal outgrowth and guidance. They provide instructive cues repelling or attracting axons depending on the repertoire of receptors presented at the surface of the neuronal growth cones and the activated intracellular signaling pathways. Netrins and slits also function in a variety of other processes within and outside the CNS, controlling cell adhesion, migration and polarity (Killeen and Sybingco 2008; Bradford et al. 2009; Ypsilanti et al. 2010).
Netrins are evolutionary related to the ECM molecule laminin and contain binding sites for heparan sulfate proteoglycans (HSPG), glycolipids and the integrins α3β1 and a6β4 (Bradford et al. 2009). The first identified netrin ortholog, Unc6, was found in Caenorhabditis elegans. Unc6 mutants showed axon guidance defects and an uncoordinated (Unc) crawling phenotype (Hedgecock et al. 1990). Netrins were then found in many other organisms including Drosophila, zebrafish and mammals. In vertebrates, the netrin family comprises the secreted netrin-1, netrin-3, and netrin-4 proteins and the glycosylphosphatidylinostol (GPI)-membrane anchored netrins G1 and G2 (reviewed in Cirulli and Yebra 2007; Bradford et al. 2009). Netrins are dynamically expressed in the developing CNS and in all species described so far netrin-1 is secreted by midline cells. The chemoattractant effects of netrin-1 are mediated through axonal receptors of the deleted in colorectal cancer (Dcc) family, which include the vertebrate Dcc and neogenin, the C. elegans UNC40 and the Drosophila Frazzled (Fra) proteins (Chan et al. 1996; Keino-Masu et al. 1996; Kolodziej et al. 1996). More recently, the Down syndrome cell adhesion molecule (Dscam) has also been shown to act as a netrin receptor promoting axonal attraction (Ly et al. 2008). Repulsive netrin-1 effects are mediated solely through Unc5 receptors or in combination with Dcc (Hong et al. 1999; Keleman and Dickson 2001). Netrin-1 acts both as a short-range and a long-range guidance cue and is particularly significant for the steering of comissural axons. For example, mouse mutants for netrin-1 or Dcc completely lack the corpus callosum and hippocampal comissure, among defects in numerous other axonal tracts (reviewed in Barallobre et al. 2005).
The first member of the slit family was identified in Drosophila as a midline glia secreted protein (Kidd et al. 1999), but slits have since been discovered in several species (Ypsilanti et al. 2010). In mammals there are three slit genes (Slit1-3), all of which are expressed in the CNS (Itoh et al. 1998). Slits are glycoproteins that function as ligands for Roundabout (Robo) receptors. They act as major axonal repulsion cue and also inhibit axonal attraction (Stein and Tessier-Lavigne 2001; Killeen and Sybingco 2008; Ypsilanti et al. 2010). There are three Robo proteins in the CNS of Drosophila and of most vertebrates (Robo/Robo1, Leak/Robo2, and Robo3). Yet, Robos are not the only receptors for slits and vice-versa. For example, the EVA-1 transmembrane protein functions as a SLT-1/slit co-receptor in C. elegans and the interaction of HSPGs with slit proteins is required or potentiates their activity in some axonal tracts (Hu 2001; Piper et al. 2006; Fujisawa et al. 2007; Seiradake et al. 2009). As for netrin/Dcc, slit/Robo signaling is also essential for the establishment of many axonal tracts. For instance, mouse mutants for both Slit1 and Slit2 show axon guidance errors in a variety of pathways, including the corticofugal, callosal, and thalamocortical tracts (Bagri et al. 2002).
During development, the netrin and slit pathways are best known for their function in dorsal-ventral axonal guidance but they also play a role in anterior-posterior and longitudinal guidance (Killeen and Sybingco 2008). The two guidance cues are often tightly coordinated as exemplified in many studies of midline crossing by commissural axons in vertebrates and invertebrates. In brief, commissural axons are at first attracted towards the midline by netrin and are insensitive to the slit repulsive cue because its reception by the growth cone is transiently repressed. In flies, this negative regulation of the slit-Robo pathway is performed by commissureless, which is only transiently expressed in precrossing commissural neurons, ensuring that at that stage newly synthesized robo proteins are not trafficked to the growth cones but instead are targeted for degradation (Keleman et al. 2002; Keleman et al. 2005). In mice, this function is provided by the Robo3.1 isoform, which is also transiently expressed in precrossing neurons, although the precise mechanism involved is not yet clear (Chen et al. 2008). Once across the midline, axons increase their robo expression (but specifically down-regulate Robo3.1 in mice) and thus acquire slit sensitivity. In this way, the slit/Robo chemorepellent activity forces axons away from the midline and prevents their re-entrance. In addition, Robo seems also able to inhibit the attraction mediated by the netrin attractant receptor Dcc, possibly explaining how postcrossing axons lose their sensitivity to netrin (Stein and Tessier-Lavigne 2001; reviewed in Dickson and Gilestro 2006; Evans and Bashaw 2010; Ypsilanti et al. 2010).
Although considerable progress has been made in determining the function of netrins and slits during axonal guidance, many questions await further investigation, such as how the different ligands and receptor subtypes precisely mediate varying effect in different contexts and in a temporal manner. In this respect, it will be crucial to further investigate the interactions of netrins and slits with additional coreceptors including other ECM molecules.
SYNAPTOGENESIS AND NEURAL CIRCUIT FORMATION
Synapses are surrounded by a protein meshwork secreted by neurons and astrocytes (Dityatev and Schachner 2006). The vertebrate neuromuscular junction (NMJ), where motoneurons contact muscle fibers (Fig. 3), has served as a model to study ECM functions at peripheral synapses. In the CNS, the ECM forms perineuronal nets (PNNs) enwrapping neuronal cell bodies and processes (Fig. 4), which affect their development and function (Celio et al. 1998).
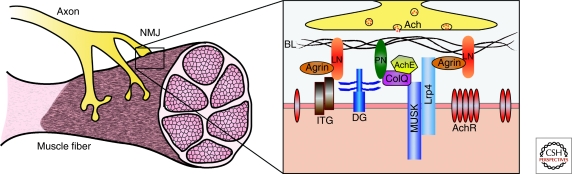
Figure 3.
ECM molecules at the neuromuscular junction. ECM molecules (BL) are required for NMJ development and function. The heparan sulfate proteoglycan agrin binds to its receptor, Lrp4, and regulates postsynaptic NMJ development through the receptor tyrosine kinase, MuSK. Laminins (LN) are required at the NMJ to promote presynaptic differentiation, as well as postsynaptic maturation via integrin (ITG) and α-dystroglycan (DG) receptors. ITG and DG receptors also bind perlecan (PN) in the BL, which recruits collagen Q (ColQ). ColQ can also bind MuSK and is important for AchR clustering and regulation of Ach levels via recruitment of acetylcholinesterase (AchE) to the NMJ.
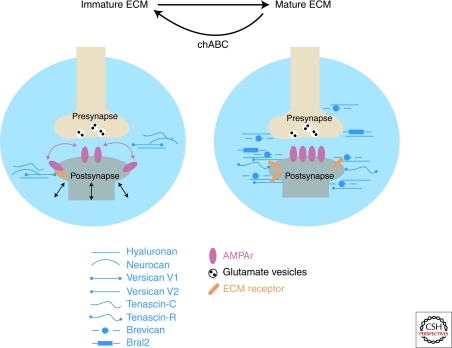
Figure 4.
ECM changes at CNS synapses. Synapses are embedded into an ECM meshwork (blue) composed of hyaluronan, chondroitin sulfate proteglycans (CSPGs), tenascins, and others. The composition of the ECM changes during development. For example, neurocan, versican V1, and tenascin-C are abundant in the immature CNS, whereas tenascin-R, versican V2, and Bral1 are prominent in the mature CNS. The mature ECM is thought to restrict dendritic spine motility and lateral diffusion of AMPA receptors (AMPAr). Chondroitinase ABC (chABC) digestion of CSPGs can restore juvenile spine dynamics.
The Neuromuscular Junction
At the NMJ, motoneuron terminals release acetylcholine (ACh), which binds ACh receptors (AChr) at postsynaptic membranes, leading to muscle contraction (Wu et al. 2010). NMJs are embedded in a specialized BL containing collagen IV, laminins, heparan sulfate proteoglycans (HSPs) and various other glycoproteins (Fig. 3) (Patton 2003).
Agrin and Laminins
Agrin is a HSP released by motoneurons into the BL. In addition, muscle fibers and Schwann cells produce distinct agrin isoforms (Werle 2008). Agrin-deficient mice lack NMJs (Gautam et al. 1996), and agrin can induce postsynaptic-like membranes in denervated muscles (Gesemann et al. 1995; Jones et al. 1997). Agrin binds to low-density lipoprotein receptor-related protein 4 (Lrp4), which interacts with MuSK, a receptor tyrosine kinase that acts as a signalosome for postsynaptic NMJ development (Fig. 3) (Glass et al. 1996; Strochlic et al. 2005; Kim et al. 2008; Zhang et al. 2008; Wu et al. 2010). A short agrin form consisting of its MuSK-activating and laminin-binding domains is sufficient to restore NMJs in agrin mutant mice when expressed by muscle, suggesting that agrin function does not depend on its local deposition at synapses (Lin et al. 2008).
Agrin, other HSPs, and nidogens stabilize networks of laminin, the most prominent noncollagenous glycoprotein of the BL at the NMJ (Fig. 3) (Massoulie and Millard 2009). β2-containing laminins bind calcium channels, inducing their clustering and consequent assembly of presynaptic proteins. Deletion of laminin β2 results in loss of NMJ presynaptic active zones where neurotransmitters are released (Noakes et al. 1995; Knight et al. 2003; Nishimune et al. 2004; Miner et al. 2006; Fox et al. 2007). Laminin α4 is also required for presynaptic differentiation (Ichikawa et al. 2005) and for the correct apposition between active zones and postsynaptic sites (Patton et al. 2001). Laminins play additional roles in postsynaptic maturation. Clustering of AChr is delayed in muscles of laminin α5 mutant mice and arrested in α4/α5 double mutants (Nishimune et al. 2008). Furthermore, agrin-induced aggregation of AChr in myotubes correlates with laminin recruitment (Montanaro et al. 1998). This laminin-mediated effect is MuSK-independent, occurring instead via the dual agrin/laminin receptor α-dystroglycan (Montanaro et al. 1998; Nishimune et al. 2008), which plays vital roles in maturation of the NMJ and central synapses (Grady et al. 2000; Jacobson et al. 2001; Pilgram et al. 2010). Integrins are additional laminin receptors at the NMJ (Barros and Muller 2005). β1-integrins modulate AChr clustering in cultured myotubes (Martin and Sanes 1997). In vivo, ablation of β1-integrins in motoneurons has little effect on NMJ formation, but its loss in muscle leads to defective motoneuron-muscle interactions, resulting in excessive nerve branching and preventing normal NMJ presynaptic differentiation (Schwander et al. 2004). These defects resemble the phenotype of agrin-null mice, indicating β1-integrins may be required for the presentation of agrin and/or laminin to motor nerve terminals (Schwander et al. 2004).
Collagens
The most abundant BL protein at NMJs is collagen IV (Sanes 2003). Collagen IV chains α1 and 2 are implicated in NMJ nerve terminal maturation, while α3/6 chains are required for their maintenance (Fox et al. 2007). CollagenQ (ColQ), another collagen at the NMJ, anchors acetylcholinesterase (AChE), a serine hydrolase controlling ACh levels, to the ECM (Bon et al. 1997; Sigoillot et al. 2010b), and it is required for AChr clustering and synaptic gene expression via its interaction with MuSK (Sigoillot et al. 2010a; Sigoillot et al. 2010b). ColQ binds perlecan, which associates with dystroglycan, laminin and β1-integrins (Talts et al. 1999; Bix et al. 2004). Perlecan also stabilizes AChE to NMJs (Peng et al. 1999; Arikawa-Hirasawa et al. 2002), but it is unclear if it cooperates with ColQ in this function.
Central Synapses
In the CNS, the carbohydrate hyaluronan (HA) forms the backbone of PNNs. During nervous system maturation, many ECM molecules at PNNs are replaced by others of the same family, allowing for maintenance of overall ECM structure (Rauch 2004). For example, neurocan, versican V1, and tenascin-C are abundant in the juvenile rodent CNS, whereas brevican, versican V2, tenascin-R, Bral2, and HA synthases are prominent in the mature CNS (Bruckner et al. 2000; Bekku et al. 2003; Carulli et al. 2006; Carulli et al. 2007; Galtrey et al. 2008) (Fig. 4). Because some ECM components are inhibitory for cell adhesion and fiber outgrowth (Pesheva et al. 1989; Morganti et al. 1990; Angelov et al. 1998), the ECM has originally been thought of as inhibiting synaptogenesis, a view that has recently changed.
Chondroitin Sulfate Proteoglycans
In the model of ocular dominance plasticity, monocular deprivation leads to an ocular dominance shift in young animals that is not observed in adults. Reactivation of ocular dominance plasticity in adults can be achieved following enzymatic degradation of CSPGs (Pizzorusso et al. 2002). Brief monocular deprivation increases dendritic spine motility and occludes subsequent effects of ECM degradation, indicating that this mechanism may act to permit synapse remodeling during ocular dominance plasticity (Oray et al. 2004). Degradation of CSPGs at PNNs also renders subsequently acquired fear memories susceptible to erasure, implicating PNNs in the formation of stable memories (Gogolla et al. 2009). Finally, ECM removal restores juvenile AMPA-type neurotransmitter receptor (AMPAr) mobility in mature neurons, suggesting that PNNs compartmentalize neuronal surfaces and participate in short-term synaptic plasticity (Frischknecht et al. 2009). In sum, PNNs contribute to the formation of neural circuitry by restricting structural changes at synapses (Fig. 4), and by modulating experience-dependent synaptic plasticity. Key players are likely molecules regulating perisynaptic ECM proteolysis in an activity-dependent manner (Nakamura et al. 2000; Berardi et al. 2004; Lochner et al. 2006; Frischknecht et al. 2008; Lee et al. 2008).
Reelin
Reelin regulates not only neuronal migration but also synapse development and function (Dityatev and Schachner 2006; Rogers and Weeber 2008). In the adult neocortex, reelin is secreted by GABAergic interneurons (Alcantara et al. 1998; Sinagra et al. 2005). Reduction or loss of reelin signaling hampers arborization of hippocampal and frontal cortex neuronal dendrites, and reduces dendritic spine density (Liu et al. 2001; Niu et al. 2004; Matsuki et al. 2008; Niu et al. 2008). Conversely, transgenic mice overexpressing reelin show increased synaptic contacts and hypertrophy of hippocampal dendritic spines (Pujadas et al. 2010). Reelin signaling is also involved in synaptic plasticity; mice heterozygous for reelin or ApoER2 show impaired hippocampal long-term potentiation (LTP) (Weeber et al. 2002; Beffert et al. 2005; Chen et al. 2005; Qiu et al. 2006; Rogers and Weeber 2008). Reelin signals through ApoER2 to enhance LTP via a mechanism involving the activity-dependent splicing of an ApoER2 exon that encodes a domain required for reelin-induced tyrosine phosphorylation of NMDA-type receptors (NMDAr) (Beffert et al. 2005; Beffert et al. 2006). Additionally, reelin participates in the recruitment, trafficking, and composition of NMDAr, contributing to the developmental switch of NMDAr subunits from NR2B to NR2A (Sinagra et al. 2005; Groc et al. 2007; Campo et al. 2009).
Thrombospondins
Astrocytes play an integral role in the development of synapses (Stevens 2008), and TSPs are key astrocyte-derived molecules regulating synaptogenesis. TSP1 and 2 are secreted by immature astrocytes, correlating with the onset of synaptogenesis (Ullian et al. 2001; Christopherson et al. 2005). Applying TSP1 and 2 to cultured retinal ganglion cells increases the number of excitatory synapses. Conversely, TSP1/2 double KO mice show reduced cortical synapse density (Christopherson et al. 2005). TSP1/2 interact with the gabapentin receptor α2γ-1, which can mediate their synaptogenic activity (Eroglu et al. 2009). TSP1/2 induced synapses are presynaptically active but postsynaptically silent (Christopherson et al. 2005), suggesting that other signals are required to convert these immature synapses into functional ones. TSP1 also accelerates formation of immature synapses in cultured hippocampal neurons (Xu et al. 2010). This effect depends on neuroligin1 (NL1) (Xu et al. 2010), which together with its neurexin ligands induces formation of synapses lacking AMPAr (Graf et al. 2004). TSP1 also binds to the reelin receptors ApoER2 and VLDLR (Blake et al. 2008). In addition, production of TSP-1 by astrocytes is enhanced by type IV collagen, an effect that depends on α1β1 integrins (Yonezawa et al. 2010). Given that type IV collagen plays important roles in presynaptic specialization at NMJs (Fox et al. 2007) and that β1 integrins are required for hippocampal LTP (Chan et al. 2006; Huang et al. 2006), it will be interesting to examine if these molecules have coordinated functions with TSPs at central synapses.
Other ECM Proteins
Other ECM molecules have been implicated in the formation and plasticity of central synapses (Dityatev and Schachner 2006; Galtrey and Fawcett 2007; Lee et al. 2008; Faissner et al. 2010), such as neuronal pentraxins (NPs) and tenascins. The neuronal activity-regulated pentraxin (Narp) and the neuronal pentraxin NP1 are axonal-derived lectins enriched at excitatory synapses. The neuronal pentraxin receptor (NPR) associates with Narp and NP1, and its extracellular domain is released into the ECM. NP proteins contribute to synaptogenesis by clustering AMPAr (Xu et al. 2003; Bjartmar et al. 2006; Sia et al. 2007). In addition, NPR ectodomain cleavage by TACE is essential for metabotropic glutamate receptor-dependent long-term depression (LTD) (Cho et al. 2008). TN-R and TN-C have also been implicated in forms of synaptic plasticity. Although TN-C affects LTP and LTD in the CA1 hippocampal area via L-type calcium channels (Evers et al. 2002; Strekalova et al. 2002), loss of TN-R leads to elevated basal excitatory synaptic transmission and reduced perisomatic GABAergic inhibition (Bukalo et al. 2001; Bukalo et al. 2007). Therefore, ECM components affect synapse development and function in complex ways, in which different ECM molecules have specific effects that are likely mediated by distinct receptors.
CONCLUDING REMARKS
The genome of mammals encodes a vast range of different ECM glycoproteins that affect nearly all aspects of nervous system development and function. Although substantial progress has been made to define the functions of specific ECM molecules in the nervous system, many challenges remain. For example, which mechanisms control the composition and structure of ECM assemblies in different parts of the nervous system? How do these assemblies affect the activity of secreted growth factors and morphogens, and how do cells integrate information provided by complex ECM mixtures? Finally, how does the three-dimensional ECM architecture and its mechanical properties affect cell behavior? Advances in genomics, proteomics, genetics, and systems level approaches will undoubtedly help provide answers to these questions.
ACKNOWLEDGMENTS
This work was supported by funding from the National Institutes of Health (S.J.F., NS060355; U.M., NS046456, MH078833), the Skaggs Institute for Chemical Biology (U.M.), and the Dorris Neuroscience Center (U.M).
Footnotes
Editors: Richard Hynes and Kenneth Yamada
Additional Perspectives on Extracellular Matrix Biology available at www.cshperspectives.org
REFERENCES
- Abaskharoun M, Bellemare M, Lau E, Margolis RU 2010. Expression of hyaluronan and the hyaluronan-binding proteoglycans neurocan, aggrecan, and versican by neural stem cells and neural cells derived from embryonic stem cells. Brain Res 1327: 6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Tucker RP 2000. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn 218: 280–299 [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, D'Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E 1998. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci 18: 7779–7799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelov DN, Walther M, Streppel M, Guntinas-Lichius O, Neiss WF, Probstmeier R, Pesheva P 1998. Tenascin-R is antiadhesive for activated microglia that induce downregulation of the protein after peripheral nerve injury: A new role in neuronal protection. J Neurosci 18: 6218–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y 2002. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci 5: 119–123 [DOI] [PubMed] [Google Scholar]
- Arnaud L, Ballif BA, Forster E, Cooper JA 2003. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol 13: 9–17 [DOI] [PubMed] [Google Scholar]
- Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, et al. 2003. Interaction of reelin signaling and Lis1 in brain development. Nat Genet 35: 270–276 [DOI] [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M 2002. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33: 233–248 [DOI] [PubMed] [Google Scholar]
- Bai F, Peng H, Etlinger JD, Zeman RJ 2010. Partial functional recovery after complete spinal cord transection by combined chondroitinase and clenbuterol treatment. Pflugers Arch 460: 657–666 [DOI] [PubMed] [Google Scholar]
- Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA 2004. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol 14: 606–610 [DOI] [PubMed] [Google Scholar]
- Bamdad M, Volle D, Dastugue B, Meiniel A 2004. αa1β1-integrin is an essential signal for neurite outgrowth induced by thrombospondin type 1 repeats of SCO-spondin. Cell Tissue Res 315: 15–25 [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR 2000. Proteoglycans in the developing brain: New conceptual insights for old proteins. Physiol Rev 80: 1267–1290 [DOI] [PubMed] [Google Scholar]
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E 2005. The Netrin family of guidance factors: Emphasis on Netrin-1 signalling. Brain Res Brain Res Rev 49: 22–47 [DOI] [PubMed] [Google Scholar]
- Barros CS, Muller U 2005. Cell adhesion in nervous system development: Integrin fuctions in glia cells. In Integrins in Development (ed. Danen E). Landes Bioscience, Georgetown, TX [Google Scholar]
- Barros CS, Nguyen T, Spencer KS, Nishiyama A, Colognato H, Muller U 2009. Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 136: 2717–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch S, Bartsch U, Dorries U, Faissner A, Weller A, Ekblom P, Schachner M 1992. Expression of tenascin in the developing and adult cerebellar cortex. J Neurosci 12: 736–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Schweitzer J, Feldner J, Becker T, Schachner M 2003. Tenascin-R as a repellent guidance molecule for developing optic axons in zebrafish. J Neurosci 23: 6232–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Schweitzer J, Feldner J, Schachner M, Becker T 2004. Tenascin-R as a repellent guidance molecule for newly growing and regenerating optic axons in adult zebrafish. Mol Cell Neurosci 26: 376–389 [DOI] [PubMed] [Google Scholar]
- Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J 2006. Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci 26: 2041–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, et al. 2005. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47: 567–579 [DOI] [PubMed] [Google Scholar]
- Bekku Y, Rauch U, Ninomiya Y, Oohashi T 2009. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem 108: 1266–1276 [DOI] [PubMed] [Google Scholar]
- Bekku Y, Su WD, Hirakawa S, Fassler R, Ohtsuka A, Kang JS, Sanders J, Murakami T, Ninomiya Y, Oohashi T 2003. Molecular cloning of Bral2, a novel brain-specific link protein, and immunohistochemical colocalization with brevican in perineuronal nets. Mol Cell Neurosci 24: 148–159 [DOI] [PubMed] [Google Scholar]
- Bekku Y, Vargova L, Goto Y, Vorisek I, Dmytrenko L, Narasaki M, Ohtsuka A, Fassler R, Ninomiya Y, Sykova E, et al. 2010. Bral1: Its role in diffusion barrier formation and conduction velocity in the CNS. J Neurosci 30: 3113–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, et al. 2002. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet 71: 1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Muller U 2007a. β1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci 27: 13854–13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvindrah R, Hankel S, Walker J, Patton BL, Muller U 2007b. β1 integrins control the formation of cell chains in the adult rostral migratory stream. J Neurosci 27: 2704–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhayon D, Magdaleno S, Curran T 2003. Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Brain Res Mol Brain Res 112: 33–45 [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Maffei L 2004. Extracellular matrix and visual cortical plasticity: Freeing the synapse. Neuron 44: 905–908 [DOI] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Hook M, et al. 2004. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J Cell Biol 166: 97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. 2006. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci 26: 6269–6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess S, Graus-Porta D, Belvindrah R, Radakovits R, Pons S, Littlewood-Evans A, Senften M, Guo H, Li Y, Miner JH, et al. 2004. β1-integrins are critical for cerebellar granule cell precursor proliferation. J Neurosci 24: 3402–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake SM, Strasser V, Andrade N, Duit S, Hofbauer R, Schneider WJ, Nimpf J 2008. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J 27: 3069–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, Herz J 2003. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem 278: 38772–38779 [DOI] [PubMed] [Google Scholar]
- Bon S, Coussen F, Massoulie J 1997. Quaternary associations of acetylcholinesterase. II. The polyproline attachment domain of the collagen tail. J Biol Chem 272: 3016–3021 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636–640 [DOI] [PubMed] [Google Scholar]
- Bradford D, Cole SJ, Cooper HM 2009. Netrin-1: Diversity in development. Int J Biochem Cell Biol 41: 487–493 [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H 2010. Oligodendrocytes: Biology and pathology. Acta Neuropathol 119: 37–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GM, Perkins S, Peterson AC, Aguayo AJ 1977. Schwann cell multiplication deficit in nerve roots of newborn dystrophic mice. A radioautographic and ultrastructural study. J Neurol Sci 32: 203–212 [DOI] [PubMed] [Google Scholar]
- Bruckner G, Grosche J, Schmidt S, Hartig W, Margolis RU, Delpech B, Seidenbecher CI, Czaniera R, Schachner M 2000. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J Comp Neurol 428: 616–629 [DOI] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A 2001. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neurosci 104: 359–369 [DOI] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A 2007. Hippocampal metaplasticity induced by deficiency in the extracellular matrix glycoprotein tenascin-R. J Neurosci 27: 6019–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Frumkin A, Xu YT, Scherer SS, Klar A 1998. Accumulation of F-spondin in injured peripheral nerve promotes the outgrowth of sensory axons. J Neurosci 18: 8875–8885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Tzarfaty V, Frumkin A, Feinstein Y, Stoeckli E, Klar A 1999. F-Spondin is required for accurate pathfinding of commissural axons at the floor plate. Neuron 23: 233–246 [DOI] [PubMed] [Google Scholar]
- Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA 2005. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma 22: 226–239 [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Fujioka Y, de Pereda JM, Garcia-Alvarez B, Nakamoto T, Margolis B, McGlade CJ, Liddington RC, Ginsberg MH 2003. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc Natl Acad Sci 100: 2272–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calof AL, Reichardt LF 1985. Response of purified chick motoneurons to myotube conditioned medium: Laminin is essential for the substratum-binding, neurite outgrowth-promoting activity. Neurosci Lett 59: 183–189 [DOI] [PubMed] [Google Scholar]
- Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P 2009. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One 4: e5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C 2004. β1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131: 3433–3444 [DOI] [PubMed] [Google Scholar]
- Caprile T, Osorio G, Henriquez JP, Montecinos H 2009. Polarized expression of integrin β1 in diencephalic roof plate during chick development, a possible receptor for SCO-spondin. Dev Dyn 238: 2494–2504 [DOI] [PubMed] [Google Scholar]
- Caro PA, Scavina M, Hoffman E, Pegoraro E, Marks HG 1999. MR imaging findings in children with merosin-deficient congenital muscular dystrophy. AJNR Am J Neuroradiol 20: 324–326 [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Fawcett JW 2007. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol 501: 83–94 [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW 2006. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol 494: 559–577 [DOI] [PubMed] [Google Scholar]
- Caviness VS Jr, 1973. Time of neuron origin in the hippocampus and dentate gyrus of normal and reeler mutant mice: an autoradiographic analysis. J Comp Neurol 151: 113–120 [DOI] [PubMed] [Google Scholar]
- Caviness VS Jr, 1982. Neocortical histogenesis in normal and reeler mice: A developmental study based upon [3H]thymidine autoradiography. Brain Res 256: 293–302 [DOI] [PubMed] [Google Scholar]
- Caviness VS Jr, Korde MG 1981. Monoaminergic afferents to the neocortex: a developmental histofluorescence study in normal and Reeler mouse embryos. Brain Res 209: 1–9 [DOI] [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L 1998. Perineuronal nets: Past and present. Trends Neurosci 21: 510–515 [DOI] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL 2006. β β1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci 26: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG 1996. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87: 187–195 [DOI] [PubMed] [Google Scholar]
- Chen ZF, Paquette AJ, Anderson DJ 1998. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet 20: 136–142 [DOI] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J 2005. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci 25: 8209–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M 2008. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 58: 325–332 [DOI] [PubMed] [Google Scholar]
- Chen ZL, Haegeli V, Yu H, Strickland S 2009. Cortical deficiency of laminin gamma1 impairs the AKT/GSK-3β signaling pathway and leads to defects in neurite outgrowth and neuronal migration. Dev Biol 327: 158–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Ochalski PG, Tran TS, Sahir N, Schubert M, Pramatarova A, Howell BW 2004. Interaction between Dab1 and CrkII is promoted by Reelin signaling. J Cell Sci 117: 4527–4536 [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S 2008. Regulation of Schwann cell function by the extracellular matrix. Glia 56: 1498–1507 [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB, Xu D, Hopf C, Kim JA, Reddy RC, Petralia RS, Perin MS, Linden DJ, et al. 2008. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron 57: 858–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA 2005. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120: 421–433 [DOI] [PubMed] [Google Scholar]
- Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T 2003. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol 163: 397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Yebra M 2007. Netrins: Beyond the brain. Nat Rev Mol Cell Biol 8: 296–306 [DOI] [PubMed] [Google Scholar]
- Colognato H, ffrench-Constant C, Feltri ML 2005. Human diseases reveal novel roles for neural laminins. Trends Neurosci 28: 480–486 [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C 2002. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol 4: 833–841 [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C 2004. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol 167: 365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA 2008. A mechanism for inside-out lamination in the neocortex. Trends Neurosci 31: 113–119 [DOI] [PubMed] [Google Scholar]
- Czopka T, Von Holst A, Schmidt G, Ffrench-Constant C, Faissner A 2009. Tenascin C and tenascin R similarly prevent the formation of myelin membranes in a RhoA-dependent manner, but antagonistically regulate the expression of myelin basic protein via a separate pathway. Glia 57: 1790–1801 [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T 1999. Reelin is a ligand for lipoprotein receptors. Neuron 24: 471–479 [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E 1999. Synergistic activities of α3 and α6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development 126: 3957–3968 [DOI] [PubMed] [Google Scholar]
- Debby-Brafman A, Burstyn-Cohen T, Klar A, Kalcheim C 1999. F-Spondin, expressed in somite regions avoided by neural crest cells, mediates inhibition of distinct somite domains to neural crest migration. Neuron 22: 475–488 [DOI] [PubMed] [Google Scholar]
- DeFreitas MF, Yoshida CK, Frazier WA, Mendrick DL, Kypta RM, Reichardt LF 1995. Identification of integrin α 3 β1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron 15: 333–343 [DOI] [PubMed] [Google Scholar]
- Di Muzio A, De Angelis MV, Di Fulvio P, Ratti A, Pizzuti A, Stuppia L, Gambi D, Uncini A 2003. Dysmyelinating sensory-motor neuropathy in merosin-deficient congenital muscular dystrophy. Muscle Nerve 27: 500–506 [DOI] [PubMed] [Google Scholar]
- Dickson BJ, Gilestro GF 2006. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol 22: 651–675 [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M 2006. The extracellular matrix and synapses. Cell Tissue Res 326: 647–654 [DOI] [PubMed] [Google Scholar]
- Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR 2009. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci 29: 7731–7742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Nurcombe V, Bartlett PF 1991. Laminin through its long arm E8 fragment promotes the proliferation and differentiation of murine neuroepithelial cells in vitro. Exp Cell Res 192: 256–265 [DOI] [PubMed] [Google Scholar]
- Dulabon L, Olson EC, Taglienti MG, Eisenhuth S, McGrath B, Walsh CA, Kreidberg JA, Anton ES 2000. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron 27: 33–44 [DOI] [PubMed] [Google Scholar]
- Erickson AC, Couchman JR 2000. Still more complexity in mammalian basement membranes. J Histochem Cytochem 48: 1291–1306 [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. 2009. Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139: 380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G 1999. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci 19: 6417–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ 2010. Axon guidance at the midline: Of mice and flies. Curr Opin Neurobiol 20: 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers MR, Salmen B, Bukalo O, Rollenhagen A, Bosl MR, Morellini F, Bartsch U, Dityatev A, Schachner M 2002. Impairment of L-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J Neurosci 22: 7177–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Kruse J 1990. J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron 5: 627–637 [DOI] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C 2010. Contributions of astrocytes to synapse formation and maturation—Potential functions of the perisynaptic extracellular matrix. Brain Res Rev 63: 26–38 [DOI] [PubMed] [Google Scholar]
- Feltri ML, Wrabetz L 2005. Laminins and their receptors in Schwann cells and hereditary neuropathies. J Peripher Nerv Syst 10: 128–143 [DOI] [PubMed] [Google Scholar]
- Feltri ML, Graus-Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, et al. 2002. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J Cell Biol 156: 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Kriegstein AR 2003. Neurons from radial glia: The consequences of asymmetric inheritance. Curr Opin Neurobiol 13: 34–41 [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES 2006. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res 83: 845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S 2010. Emerging topics in Reelin function. Eur J Neurosci 31: 1511–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, et al. 2007. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 129: 179–193 [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Fejtova A, Viesti M, Stephan A, Sonderegger P 2008. Activity-induced synaptic capture and exocytosis of the neuronal serine protease neurotrypsin. J Neurosci 28: 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED 2009. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12: 897–904 [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Wrana JL, Culotti JG 2007. The slit receptor EVA-1 coactivates a SAX-3/Robo mediated guidance signal in C. elegans. Science 317: 1934–1938 [DOI] [PubMed] [Google Scholar]
- Fuss B, Wintergerst ES, Bartsch U, Schachner M 1993. Molecular characterization and in situ mRNA localization of the neural recognition molecule J1–160/180: A modular structure similar to tenascin. J Cell Biol 120: 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW 2007. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54: 1–18 [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW 2008. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci 27: 1373–1390 [DOI] [PubMed] [Google Scholar]
- Garcia-Alonso L, Fetter RD, Goodman CS 1996. Genetic analysis of Laminin A in Drosophila: Extracellular matrix containing laminin A is required for ocellar axon pathfinding. Development 122: 2611–2621 [DOI] [PubMed] [Google Scholar]
- Garcion E, Faissner A, ffrench-Constant C 2001. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development 128: 2485–2496 [DOI] [PubMed] [Google Scholar]
- Garcion E, Halilagic A, Faissner A, ffrench-Constant C 2004. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 131: 3423–3432 [DOI] [PubMed] [Google Scholar]
- Garwood J, Garcion E, Dobbertin A, Heck N, Calco V, ffrench-Constant C, Faissner A 2004. The extracellular matrix glycoprotein Tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. Eur J Neurosci 20: 2524–2540 [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR 1996. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85: 525–535 [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Mark M, Messaddeq N, Gansmuller A 1998. Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol 8: 983–986 [DOI] [PubMed] [Google Scholar]
- Gesemann M, Denzer AJ, Ruegg MA 1995. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol 128: 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore EC, Herrup K 2000. Cortical development: Receiving reelin. Curr Biol 10: R162–166 [DOI] [PubMed] [Google Scholar]
- Giros A, Morante J, Gil-Sanz C, Fairen A, Costell M 2007. Perlecan controls neurogenesis in the developing telencephalon. BMC Dev Biol 7: 29–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, et al. 1996. Agrin acts via a MuSK receptor complex. Cell 85: 513–523 [DOI] [PubMed] [Google Scholar]
- Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, Dastugue B, Meiniel A 1996. SCO-spondin: A new member of the thrombospondin family secreted by the subcommissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci 109: 1053–1061 [DOI] [PubMed] [Google Scholar]
- Goffinet AM 1983. The embryonic development of the cerebellum in normal and reeler mutant mice. Anat Embryol (Berl) 168: 73–86 [DOI] [PubMed] [Google Scholar]
- Goffinet AM, So KF, Yamamoto M, Edwards M, Caviness VS Jr, 1984. Architectonic and hodological organization of the cerebellum in reeler mutant mice. Brain Res 318: 263–276 [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C 2009. Perineuronal nets protect fear memories from erasure. Science 325: 1258–1261 [DOI] [PubMed] [Google Scholar]
- Gomez TM, Letourneau PC 1994. Filopodia initiate choices made by sensory neuron growth cones at laminin/fibronectin borders in vitro. J Neurosci 14: 5959–5972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves-Mendes N, Simon-Chazottes D, Creveaux I, Meiniel A, Guenet JL, Meiniel R 2003. Mouse SCO-spondin, a gene of the thrombospondin type 1 repeat (TSR) superfamily expressed in the brain. Gene 312: 263–270 [DOI] [PubMed] [Google Scholar]
- Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR 2000. Maturation and maintenance of the neuromuscular synapse: Genetic evidence for roles of the dystrophin–glycoprotein complex. Neuron 25: 279–293 [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM 2004. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 119: 1013–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U 2001. β1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31: 367–379 [DOI] [PubMed] [Google Scholar]
- Grewal PK, Holzfeind PJ, Bittner RE, Hewitt JE 2001. Mutant glycosyltransferase and altered glycosylation of alpha-dystroglycan in the myodystrophy mouse. Nat Genet 28: 151–154 [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Stephenson FA, Verrier D, Manzoni OJ, Chavis P 2007. NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. J Neurosci 27: 10165–10175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WL, Fu SL, Wang YX, Li Y, Lu HZ, Xu XM, Lu PH 2009. Chondroitin sulfate proteoglycans regulate the growth, differentiation and migration of multipotent neural precursor cells through the integrin signaling pathway. BMC Neurosci 10: 128–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu WL, Fu SL, Wang YX, Li Y, Wang XF, Xu XM, Lu PH 2007. Expression and regulation of versican in neural precursor cells and their lineages. Acta Pharmacol Sin 28: 1519–1530 [DOI] [PubMed] [Google Scholar]
- Hack I, Hellwig S, Junghans D, Brunne B, Bock HH, Zhao S, Frotscher M 2007. Divergent roles of ApoER2 and Vldlr in the migration of cortical neurons. Development 134: 3883–3891 [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U 2002. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci 22: 6029–6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PE, Lathia JD, Caldwell MA, Ffrench-Constant C 2008. Laminin enhances the growth of human neural stem cells in defined culture media. BMC Neurosci 9: 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DE, Neugebauer KM, Reichardt LF 1987. Embryonic neural retinal cell response to extracellular matrix proteins: developmental changes and effects of the cell substratum attachment antibody (CSAT). J Cell Biol 104: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond VE, So E, Cate HS, Britto JM, Gunnersen JM, Tan SS 2010. Cortical layer development and orientation is modulated by relative contributions of Reelin-negative and -positive neurons in mouse chimeras. Cereb Cortex 20: 2017–2026 [DOI] [PubMed] [Google Scholar]
- Hartmann U, Maurer P 2001. Proteoglycans in the nervous system–the quest for functional roles in vivo. Matrix Biol 20: 23–35 [DOI] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Torii M, Sarkisian MR, Bartley CM, Shen J, Radtke F, Gridley T, Sestan N, Rakic P 2008. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubst N, Georges-Labouesse E, De Arcangelis A, Mayer U, Gotz M 2006. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development 133: 3245–3254 [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH 1990. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4: 61–85 [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, et al. 1995. Mutations in the laminin α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet 11: 216–218 [DOI] [PubMed] [Google Scholar]
- Hoffarth RM, Johnston JG, Krushel LA, van der Kooy D 1995. The mouse mutation reeler causes increased adhesion within a subpopulation of early postmitotic cortical neurons. J Neurosci 15: 4838–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JR, O'Shea KS 1999a. Thrombospondin expression in nerve regeneration I. Comparison of sciatic nerve crush, transection, and long-term denervation. Brain Res Bull 48: 413–420 [DOI] [PubMed] [Google Scholar]
- Hoffman JR, O'Shea KS 1999b. Thrombospondin expression in nerve regeneration II. Comparison of optic nerve crush in the mouse and goldfish. Brain Res Bull 48: 421–427 [DOI] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E 1999. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion [see comments]. Cell 97: 927–941 [DOI] [PubMed] [Google Scholar]
- Hong SE, Shugart YY, Huang DT, Shahwan SA, Grant PE, Hourihane JO, Martin ND, Walsh CA 2000. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet 26: 93–96 [DOI] [PubMed] [Google Scholar]
- Hopker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C 1999. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature 401: 69–73 [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA 1999. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev 13: 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyo-Becerra C, Lopez-Avalos MD, Cifuentes M, Visser R, Fernandez-Llebrez P, Grondona JM 2010. The subcommissural organ and the development of the posterior commissure in chick embryos. Cell Tissue Res 339: 383–395 [DOI] [PubMed] [Google Scholar]
- Hu H 2001. Cell-surface heparan sulfate is involved in the repulsive guidance activities of Slit2 protein. Nat Neurosci 4: 695–701 [DOI] [PubMed] [Google Scholar]
- Huang Y, Magdaleno S, Hopkins R, Slaughter C, Curran T, Keshvara L 2004. Tyrosine phosphorylated Disabled 1 recruits Crk family adapter proteins. Biochem Biophys Res Commun 318: 204–212 [DOI] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF 2006. Distinct roles of the β1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci 26: 11208–11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang L, Niu R, Liao H 2009. Tenascin-R distinct domains modulate migration of neural stem/progenitor cells in vitro. In Vitro Cell Dev Biol Anim 45: 10–14 [DOI] [PubMed] [Google Scholar]
- Ichikawa N, Kasai S, Suzuki N, Nishi N, Oishi S, Fujii N, Kadoya Y, Hatori K, Mizuno Y, Nomizu M, et al. 2005. Identification of neurite outgrowth active sites on the laminin α4 chain G domain. Biochemistry 44: 5755–5762 [DOI] [PubMed] [Google Scholar]
- Itoh A, Miyabayashi T, Ohno M, Sakano S 1998. Cloning and expressions of three mammalian homologues of Drosophila slit suggest possible roles for Slit in the formation and maintenance of the nervous system. Brain Res Mol Brain Res 62: 175–186 [DOI] [PubMed] [Google Scholar]
- Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S 2001. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J Cell Biol 152: 435–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C 1996. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: Cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol 371: 376–396 [DOI] [PubMed] [Google Scholar]
- Jones G, Meier T, Lichtsteiner M, Witzemann V, Sakmann B, Brenner HR 1997. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc Natl Acad Sci 94: 2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Ignatova N, Hiesberger T, Herz J, Lambert de Rouvroit C, Goffinet AM 2004. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J Neurosci 24: 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns SM, Laywell ED, Kukekov VK, Steindler DA 2003. Extracellular matrix effects on neurosphere cell motility. Exp Neurol 182: 240–244 [DOI] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M 1996. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87: 175–185 [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ 2001. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron 32: 605–617 [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribeiro C, Dickson BJ 2005. Comm function in commissural axon guidance: cell-autonomous sorting of Robo in vivo. Nat Neurosci 8: 156–163 [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ 2002. Comm sorts robo to control axon guidance at the Drosophila midline. Cell 110: 415–427 [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS 1999. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96: 785–794 [DOI] [PubMed] [Google Scholar]
- Kiernan BW, Garcion E, Ferguson J, Frost EE, Torres EM, Dunnett SB, Saga Y, Aizawa S, Faissner A, Kaur R, et al. 1999. Myelination and behaviour of tenascin-C null transgenic mice. Eur J Neurosci 11: 3082–3092 [DOI] [PubMed] [Google Scholar]
- Killeen MT, Sybingco SS 2008. Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev Biol 323: 143–151 [DOI] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ 2008. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 135: 334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A, Baldassare M, Jessell TM 1992. F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell 69: 95–110 [DOI] [PubMed] [Google Scholar]
- Knight D, Tolley LK, Kim DK, Lavidis NA, Noakes PG 2003. Functional analysis of neurotransmission at β2-laminin deficient terminals. J Physiol 546: 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E, Shen Q, Temple S 2008. The incredible elastic brain: How neural stem cells expand our minds. Neuron 60: 420–429 [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN 1996. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 87: 197–204 [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC 2004. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 27: 392–399 [DOI] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF 1985a. Laminin is associated with the “neurite outgrowth-promoting factors” found in conditioned media. Proc Natl Acad Sci 82: 2183–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Reichardt LF 1985b. Purification of a factor that promotes neurite outgrowth: Isolation of laminin and associated molecules. J Cell Biol 101: 898–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP, ffrench-Constant C 2007. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol 505: 630–643 [DOI] [PubMed] [Google Scholar]
- Lee TW, Tsang VW, Birch NP 2008. Synaptic plasticity-associated proteases and protease inhibitors in the brain linked to the processing of extracellular matrix and cell adhesion molecules. Neuron Glia Biol 4: 223–234 [DOI] [PubMed] [Google Scholar]
- Leite CC, Lucato LT, Martin MG, Ferreira LG, Resende MB, Carvalho MS, Marie SK, Jinkins JR, Reed UC 2005. Merosin-deficient congenital muscular dystrophy (CMD): A study of 25 Brazilian patients using MRI. Pediatr Radiol 35: 572–579 [DOI] [PubMed] [Google Scholar]
- Liao H, Huang W, Schachner M, Guan Y, Guo J, Yan J, Qin J, Bai X, Zhang L 2008. β1 integrin-mediated effects of tenascin-R domains EGFL and FN6–8 on neural stem/progenitor cell proliferation and differentiation in vitro. J Biol Chem 283: 27927–27936 [DOI] [PubMed] [Google Scholar]
- Liesi P 1992. Neuronal migration on laminin involves neuronal contact formation followed by nuclear movement inside a preformed process. Exp Neurol 117: 103–113 [DOI] [PubMed] [Google Scholar]
- Liesi P, Seppala I, Trenkner E 1992. Neuronal migration in cerebellar microcultures is inhibited by antibodies against a neurite outgrowth domain of laminin. J Neurosci Res 33: 170–176 [DOI] [PubMed] [Google Scholar]
- Lillien L, Raphael H 2000. BMP and FGF regulate the development of EGF-responsive neural progenitor cells. Development 127: 4993–5005 [DOI] [PubMed] [Google Scholar]
- Lin S, Maj M, Bezakova G, Magyar JP, Brenner HR, Ruegg MA 2008. Muscle-wide secretion of a miniaturized form of neural agrin rescues focal neuromuscular innervation in agrin mutant mice. Proc Natl Acad Sci 105: 11406–11411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WS, Pesold C, Rodriguez MA, Carboni G, Auta J, Lacor P, Larson J, Condie BG, Guidotti A, Costa E 2001. Down-regulation of dendritic spine and glutamic acid decarboxylase 67 expressions in the reelin haploinsufficient heterozygous reeler mouse. Proc Natl Acad Sci 98: 3477–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA 2006. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol 66: 564–577 [DOI] [PubMed] [Google Scholar]
- Lochter A, Taylor J, Fuss B, Schachner M 1994. The extracellular matrix molecule janusin regulates neuronal morphology in a substrate- and culture time-dependent manner. Eur J Neurosci 6: 597–606 [DOI] [PubMed] [Google Scholar]
- Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton B, et al. 2009. β1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol 7: e1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckenbill-Edds L 1997. Laminin and the mechanism of neuronal outgrowth. Brain Res Brain Res Rev 23: 1–27 [DOI] [PubMed] [Google Scholar]
- Luque JM, Morante-Oria J, Fairen A 2003. Localization of ApoER2, VLDLR and Dab1 in radial glia: Groundwork for a new model of reelin action during cortical development. Brain Res Dev Brain Res 140: 195–203 [DOI] [PubMed] [Google Scholar]
- Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E 2008. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell 133: 1241–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP 2008. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol 8: 90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Appolloni I, Calzolari F 2008. Radial glia and neural stem cells. Cell Tissue Res 331: 165–178 [DOI] [PubMed] [Google Scholar]
- Marchetti G, Escuin S, van der Flier A, De Arcangelis A, Hynes RO, Georges-Labouesse E 2010. Integrin α5β1 is necessary for regulation of radial migration of cortical neurons during mouse brain development. Eur J Neurosci 31: 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Crepel F, Mikoshiba K, Changeux JP, Sotelo C 1977. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc Lond B Biol Sci 281: 1–28 [DOI] [PubMed] [Google Scholar]
- Martin PT, Sanes JR 1997. Integrins mediate adhesion to agrin and modulate agrin signaling. Development 124: 3909–3917 [DOI] [PubMed] [Google Scholar]
- Massoulie J, Millard CB 2009. Cholinesterases and the basal lamina at vertebrate neuromuscular junctions. Curr Opin Pharmacol 9: 316–325 [DOI] [PubMed] [Google Scholar]
- Matsuki T, Pramatarova A, Howell BW 2008. Reduction of Crk and CrkL expression blocks reelin-induced dendritogenesis. J Cell Sci 121: 1869–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Hoke A, Silver J 1995. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol 136: 32–43 [DOI] [PubMed] [Google Scholar]
- Meiniel O, Meiniel R, Lalloue F, Didier R, Jauberteau MO, Meiniel A, Petit D 2008. The lengthening of a giant protein: When, how, and why? J Mol Evol 66: 1–10 [DOI] [PubMed] [Google Scholar]
- Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL 2005. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia 52: 301–308 [DOI] [PubMed] [Google Scholar]
- Menager C, Arimura N, Fukata Y, Kaibuchi K 2004. PIP3 is involved in neuronal polarization and axon formation. J Neurochem 89: 109–118 [DOI] [PubMed] [Google Scholar]
- Mercado ML, Nur-e-Kamal A, Liu HY, Gross SR, Movahed R, Meiners S 2004. Neurite outgrowth by the alternatively spliced region of human tenascin-C is mediated by neuronal α7β1 integrin. J Neurosci 24: 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, et al. 2002. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418: 417–422 [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier-Fisher A 2009. Home at last: neural stem cell niches defined. Cell Stem Cell 4: 507–510 [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL 2002. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res 69: 286–291 [DOI] [PubMed] [Google Scholar]
- Miner JH, Go G, Cunningham J, Patton BL, Jarad G 2006. Transgenic isolation of skeletal muscle and kidney defects in laminin β2 mutant mice: Implications for Pierson syndrome. Development 133: 967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley AK, Tchaicha JH, Shin J, Hossain MG, McCarty JH 2009. β8 integrin regulates neurogenesis and neurovascular homeostasis in the adult brain. J Cell Sci 122: 1842–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller JC, Klein MA, Haas S, Jones LL, Kreutzberg GW, Raivich G 1996. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia 17: 121–132 [DOI] [PubMed] [Google Scholar]
- Montanaro F, Gee SH, Jacobson C, Lindenbaum MH, Froehner SC, Carbonetto S 1998. Laminin and α-dystroglycan mediate acetylcholine receptor aggregation via a MuSK-independent pathway. J Neurosci 18: 1250–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW 2001. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 4: 465–466 [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, et al. 2002. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418: 422–425 [DOI] [PubMed] [Google Scholar]
- Morganti MC, Taylor J, Pesheva P, Schachner M 1990. Oligodendrocyte-derived J1–160/180 extracellular matrix glycoproteins are adhesive or repulsive depending on the partner cell type and time of interaction. Exp Neurol 109: 98–110 [DOI] [PubMed] [Google Scholar]
- Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, Giancotti FG 1998. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J 17: 3940–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL 2001. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci 4: 143–150 [DOI] [PubMed] [Google Scholar]
- Nakamoto T, Kain KH, Ginsberg MH 2004. Neurobiology: New connections between integrins and axon guidance. Curr Biol 14: R121–123 [PubMed] [Google Scholar]
- Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, Miura R, Yamaguchi Y, Okada Y 2000. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem 275: 38885–38890 [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF 1991. Vitronectin and thrombospondin promote retinal neurite outgrowth: Developmental regulation and role of integrins. Neuron 6: 345–358 [DOI] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS 2004. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 432: 580–587 [DOI] [PubMed] [Google Scholar]
- Nishimune H, Valdez G, Jarad G, Moulson CL, Muller U, Miner JH, Sanes JR 2008. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol 182: 1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G 2004. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron 41: 71–84 [DOI] [PubMed] [Google Scholar]
- Niu S, Yabut O, D'Arcangelo G 2008. The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci 28: 10339–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP 1995. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature 374: 258–262 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR 2007. Neural stem and progenitor cells in cortical development. Novartis Found Symp 288: 96–58 [PubMed] [Google Scholar]
- O'Shea KS, Liu LH, Dixit VM 1991. Thrombospondin and a 140 kd fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron 7: 231–237 [DOI] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K 1995. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14: 899–912 [DOI] [PubMed] [Google Scholar]
- Olson EC, Kim S, Walsh CA 2006. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci 26: 1767–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oray S, Majewska A, Sur M 2004. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44: 1021–1030 [DOI] [PubMed] [Google Scholar]
- Osterhout DJ, Frazier WA, Higgins D 1992. Thrombospondin promotes process outgrowth in neurons from the peripheral and central nervous systems. Dev Biol 150: 256–265 [DOI] [PubMed] [Google Scholar]
- Otto SJ, McCorkle SR, Hover J, Conaco C, Han JJ, Impey S, Yochum GS, Dunn JJ, Goodman RH, Mandel G 2007. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci 27: 6729–6739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL 2003. Basal lamina and the organization of neuromuscular synapses. J Neurocytol 32: 883–903 [DOI] [PubMed] [Google Scholar]
- Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR 2001. Properly formed but improperly localized synaptic specializations in the absence of laminin α4. Nat Neurosci 4: 597–604 [DOI] [PubMed] [Google Scholar]
- Peles E, Salzer JL 2000. Molecular domains of myelinated axons. Curr Opin Neurobiol 10: 558–565 [DOI] [PubMed] [Google Scholar]
- Peng HB, Xie H, Rossi SG, Rotundo RL 1999. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol 145: 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perris R, Perissinotto D 2000. Role of the extracellular matrix during neural crest cell migration. Mech Dev 95: 3–21 [DOI] [PubMed] [Google Scholar]
- Pesheva P, Gennarini G, Goridis C, Schachner M 1993. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1–160/180. Neuron 10: 69–82 [DOI] [PubMed] [Google Scholar]
- Pesheva P, Gloor S, Schachner M, Probstmeier R 1997. Tenascin-R is an intrinsic autocrine factor for oligodendrocyte differentiation and promotes cell adhesion by a sulfatide-mediated mechanism. J Neurosci 17: 4642–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesheva P, Spiess E, Schachner M 1989. J1–160 and J1–180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol 109: 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierret C, Morrison JA, Rath P, Zigler RE, Engel LA, Fairchild CL, Shi H, Maruniak JA, Kirk MD 2010. Developmental cues and persistent neurogenic potential within an in vitro neural niche. BMC Dev Biol 10: 5–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgram GS, Potikanond S, Baines RA, Fradkin LG, Noordermeer JN 2010. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol Neurobiol 41: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Lord MC, Evrard P, Caviness VS Jr, 1982. Obstructed neuronal migration along radial glial fibers in the neocortex of the reeler mouse: A Golgi-EM analysis. Brain Res 256: 379–393 [DOI] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C 2006. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron 49: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L 2002. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248–1251 [DOI] [PubMed] [Google Scholar]
- Porcionatto MA 2006. The extracellular matrix provides directional cues for neuronal migration during cerebellar development. Braz J Med Biol Res 39: 313–320 [DOI] [PubMed] [Google Scholar]
- Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martinez A, Delgado-Garcia JM, Soriano E 2010. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci 30: 4636–4649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ 2006. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem 85: 228–242 [DOI] [PubMed] [Google Scholar]
- Radakovits R, Barros CS, Belvindrah R, Patton B, Muller U 2009. Regulation of radial glial survival by signals from the meninges. J Neurosci 29: 7694–7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U 2004. Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci 61: 2031–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF, Tomaselli KJ 1991. Extracellular matrix molecules and their receptors: functions in neural development. Annu Rev Neurosci 14: 531–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H 2009. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci 29: 11794–11806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relvas JB, Setzu A, Baron W, Buttery PC, LaFlamme SE, Franklin RJ, ffrench-Constant C 2001. Expression of dominant-negative and chimeric subunits reveals an essential role for β1 integrin during myelination. Curr Biol 11: 1039–1043 [DOI] [PubMed] [Google Scholar]
- Rigato F, Garwood J, Calco V, Heck N, Faivre-Sarrailh C, Faissner A 2002. Tenascin-C promotes neurite outgrowth of embryonic hippocampal neurons through the alternatively spliced fibronectin type III BD domains via activation of the cell adhesion molecule F3/contactin. J Neurosci 22: 6596–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Weeber EJ 2008. Reelin and apoE actions on signal transduction, synaptic function and memory formation. Neuron Glia Biol 4: 259–270 [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M 2009. The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10: 235–241 [DOI] [PubMed] [Google Scholar]
- Saghatelyan A, de Chevigny A, Schachner M, Lledo PM 2004. Tenascin-R mediates activity-dependent recruitment of neuroblasts in the adult mouse forebrain. Nat Neurosci 7: 347–356 [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, et al. 2003. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron 38: 747–758 [DOI] [PubMed] [Google Scholar]
- Sanada K, Gupta A, Tsai LH 2004. Disabled-1-regulated adhesion of migrating neurons to radial glial fiber contributes to neuronal positioning during early corticogenesis. Neuron 42: 197–211 [DOI] [PubMed] [Google Scholar]
- Sanes JR 1989. Extracellular matrix molecules that influence neural development. Annu Rev Neurosci 12: 491–516 [DOI] [PubMed] [Google Scholar]
- Sanes JR 2003. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 278: 12601–12604 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Bernier B, Goffinet AM 1997. Reelin mRNA expression during mouse brain development. Eur J Neurosci 9: 1055–1071 [DOI] [PubMed] [Google Scholar]
- Schoenherr CJ, Paquette AJ, Anderson DJ 1996. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci 93: 9881–9886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Shirasaki R, Pfaff SL, Muller U 2004. β1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J Neurosci 24: 8181–8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Drew S, ffrench-Constant C 1997. Expression and function of thrombospondin-1 in myelinating glial cells of the central nervous system. J Neurosci Res 50: 202–214 [DOI] [PubMed] [Google Scholar]
- Seiradake E, von Philipsborn AC, Henry M, Fritz M, Lortat-Jacob H, Jamin M, Hemrika W, Bastmeyer M, Cusack S, McCarthy AA 2009. Structure and functional relevance of the Slit2 homodimerization domain. EMBO Rep 10: 736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Pearlman AL 1997. Abnormal reorganization of preplate neurons and their associated extracellular matrix: An early manifestation of altered neocortical development in the reeler mutant mouse. J Comp Neurol 378: 173–179 [DOI] [PubMed] [Google Scholar]
- Sherman DL, Brophy PJ 2005. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6: 683–690 [DOI] [PubMed] [Google Scholar]
- Shorer Z, Philpot J, Muntoni F, Sewry C, Dubowitz V 1995. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. J Child Neurol 10: 472–475 [DOI] [PubMed] [Google Scholar]
- Shy ME 2009. Biology of peripheral inherited neuropathies: Schwann cell axonal interactions. Adv Exp Med Biol 652: 171–181 [DOI] [PubMed] [Google Scholar]
- Sia GM, Beique JC, Rumbaugh G, Cho R, Worley PF, Huganir RL 2007. Interaction of the N-terminal domain of the AMPA receptor GluR4 subunit with the neuronal pentraxin NP1 mediates GluR4 synaptic recruitment. Neuron 55: 87–102 [DOI] [PubMed] [Google Scholar]
- Sievers J, Pehlemann FW, Gude S, Berry M 1994. A time course study of the alterations in the development of the hamster cerebellar cortex after destruction of the overlying meningeal cells with 6-hydroxydopamine on the day of birth. J Neurocytol 23: 117–134 [DOI] [PubMed] [Google Scholar]
- Sievers J, von Knebel Doeberitz C, Pehlemann FW, Berry M 1986. Meningeal cells influence cerebellar development over a critical period. Anat Embryol (Berl) 175: 91–100 [DOI] [PubMed] [Google Scholar]
- Sigoillot SM, Bourgeois F, Lambergeon M, Strochlic L, Legay C 2010a. ColQ controls postsynaptic differentiation at the neuromuscular junction. J Neurosci 30: 13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigoillot SM, Bourgeois F, Legay C 2010b. Cholinesterases regulation in the absence of ColQ. Chem Biol Interact 187: 84–89 [DOI] [PubMed] [Google Scholar]
- Silva A, Pereira J, Oliveira CR, Relvas JB, Rego AC 2009. BDNF and extracellular matrix regulate differentiation of mice neurosphere-derived cells into a GABAergic neuronal phenotype. J Neurosci Res 87: 1986–1996 [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH 2004. Regeneration beyond the glial scar. Nat Rev Neurosci 5: 146–156 [DOI] [PubMed] [Google Scholar]
- Simons M, Trajkovic K 2006. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci 119: 4381–4389 [DOI] [PubMed] [Google Scholar]
- Simons M, Trotter J 2007. Wrapping it up: The cell biology of myelination. Curr Opin Neurobiol 17: 533–540 [DOI] [PubMed] [Google Scholar]
- Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P 2005. Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci 25: 6127–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW 1995. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci 108: 1307–1315 [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D 1999. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 144: 151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobeih MM, Corfas G 2002. Extracellular factors that regulate neuronal migration in the central nervous system. Int J Dev Neurosci 20: 349–357 [DOI] [PubMed] [Google Scholar]
- Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES 2009. Netrin-1-α3β1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci 106: 7595–7600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M 2001. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science 291: 1928–1938 [DOI] [PubMed] [Google Scholar]
- Stevens B 2008. Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals 16: 278–288 [DOI] [PubMed] [Google Scholar]
- Strekalova T, Sun M, Sibbe M, Evers M, Dityatev A, Gass P, Schachner M 2002. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol Cell Neurosci 21: 173–187 [DOI] [PubMed] [Google Scholar]
- Strochlic L, Cartaud A, Cartaud J 2005. The synaptic muscle-specific kinase (MuSK) complex: New partners, new functions. Bioessays 27: 1129–1135 [DOI] [PubMed] [Google Scholar]
- Sun YM, Cooper M, Finch S, Lin HH, Chen ZF, Williams BP, Buckley NJ 2008. Rest-mediated regulation of extracellular matrix is crucial for neural development. PLoS One 3: e3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R 1999. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J 18: 863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Pesheva P, Schachner M 1993. Influence of janusin and tenascin on growth cone behavior in vitro. J Neurosci Res 35: 347–362 [DOI] [PubMed] [Google Scholar]
- Temple S 2001. The development of neural stem cells. Nature 414: 112–117 [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC 1996. Supramolecular assembly of basement membranes. Bioessays 18: 123–132 [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR 1979. Laminin–a glycoprotein from basement membranes. J Biol Chem 254: 9933–9937 [PubMed] [Google Scholar]
- Tomaselli KJ, Reichardt LF 1988. Peripheral motoneuron interactions with laminin and Schwann cell-derived neurite-promoting molecules: Developmental regulation of laminin receptor function. J Neurosci Res 21: 275–285 [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Hall DE, Flier LA, Gehlsen KR, Turner DC, Carbonetto S, Reichardt LF 1990. A neuronal cell line (PC12) expresses two β1-class integrins-α1 β1 and α3 β1-that recognize different neurite outgrowth-promoting domains in laminin. Neuron 5: 651–662 [DOI] [PubMed] [Google Scholar]
- Treloar HB, Ray A, Dinglasan LA, Schachner M, Greer CA 2009. Tenascin-C is an inhibitory boundary molecule in the developing olfactory bulb. J Neurosci 29: 9405–9416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J 1999. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97: 689–701 [DOI] [PubMed] [Google Scholar]
- Tucker RP 2004. The thrombospondin type 1 repeat superfamily. Int J Biochem Cell Biol 36: 969–974 [DOI] [PubMed] [Google Scholar]
- Tzarfati-Majar V, Burstyn-Cohen T, Klar A 2001. F-spondin is a contact-repellent molecule for embryonic motor neurons. Proc Natl Acad Sci 98: 4722–4727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA 2001. Control of synapse number by glia. Science 291: 657–661 [DOI] [PubMed] [Google Scholar]
- Venstrom KA, Reichardt LF 1993. Extracellular matrix. 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 7: 996–1003 [DOI] [PubMed] [Google Scholar]
- von Holst A 2008. Tenascin C in stem cell niches: redundant, permissive or instructive? Cells Tissues Organs 188: 170–177 [DOI] [PubMed] [Google Scholar]
- von Holst A, Sirko S, Faissner A 2006. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci 26: 4082–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Knebel Doeberitz C, Sievers J, Sadler M, Pehlemann FW, Berry M, Halliwell P 1986. Destruction of meningeal cells over the newborn hamster cerebellum with 6-hydroxydopamine prevents foliation and lamination in the rostral cerebellum. Neurosci 17: 409–426 [DOI] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lannergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, et al. 2005. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. J Neurosci 25: 3692–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P, Bartsch U, Rasband MN, Czaniera R, Lang Y, Bluethmann H, Margolis RU, Levinson SR, Shrager P, Montag D, et al. 1999. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J Neurosci 19: 4245–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J 2002. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 277: 39944–39952 [DOI] [PubMed] [Google Scholar]
- Werle MJ 2008. Cell-to-cell signaling at the neuromuscular junction: the dynamic role of the extracellular matrix. Ann N Y Acad Sci 1132: 13–18 [DOI] [PubMed] [Google Scholar]
- Wu H, Xiong WC, Mei L 2010. To build a synapse: Signaling pathways in neuromuscular junction assembly. Development 137: 1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Xiao N, Xia J 2010. Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat Neurosci 13: 22–24 [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P 2003. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron 39: 513–528 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y 2000. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci 57: 276–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, et al. 2005. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. J Cell Biol 168: 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yick LW, Cheung PT, So KF, Wu W 2003. Axonal regeneration of Clarke's neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol 182: 160–168 [DOI] [PubMed] [Google Scholar]
- Yonezawa T, Hattori S, Inagaki J, Kurosaki M, Takigawa T, Hirohata S, Miyoshi T, Ninomiya Y 2010. Type IV collagen induces expression of thrombospondin-1 that is mediated by integrin alpha1beta1 in astrocytes. Glia 58: 755–767 [DOI] [PubMed] [Google Scholar]
- Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, et al. 2001. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell 1: 717–724 [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K 2005. GSK-3β regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 120: 137–149 [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ 2007. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 27: 14459–14469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y, Chedotal A 2010. Moving away from the midline: new developments for Slit and Robo. Development 137: 1939–1952 [DOI] [PubMed] [Google Scholar]
- Yu WM, Yu H, Chen ZL 2007. Laminins in peripheral nerve development and muscular dystrophy. Mol Neurobiol 35: 288–297 [DOI] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL 2005. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci 25: 4463–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L 2008. LRP4 serves as a coreceptor of agrin. Neuron 60: 285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT 2008. Extracellular matrix of the central nervous system: From neglect to challenge. Histochem Cell Biol 130: 635–653 [DOI] [PubMed] [Google Scholar]