Abstract
BAM1 is a plastid-targeted β-amylase of Arabidopsis thaliana specifically activated by reducing conditions. Among eight different chloroplast thioredoxin isoforms, thioredoxin f1 was the most efficient redox mediator, followed by thioredoxins m1, m2, y1, y2, and m4. Plastid-localized NADPH-thioredoxin reductase (NTRC) was also able partially to restore the activity of oxidized BAM1. Promoter activity of BAM1 was studied by reporter gene expression (GUS and YFP) in Arabidopsis transgenic plants. In young (non-flowering) plants, BAM1 was expressed both in leaves and roots, but expression in leaves was mainly restricted to guard cells. Compared with wild-type plants, bam1 knockout mutants were characterized by having more starch in illuminated guard cells and reduced stomata opening, suggesting that thioredoxin-regulated BAM1 plays a role in diurnal starch degradation which sustains stomata opening. Besides guard cells, BAM1 appears in mesophyll cells of young plants as a result of a strongly induced gene expression under osmotic stress, which is paralleled by an increase in total β-amylase activity together with its redox-sensitive fraction. Osmotic stress impairs the rate of diurnal starch accumulation in leaves of wild-type plants, but has no effect on starch accumulation in bam1 mutants. It is proposed that thioredoxin-regulated BAM1 activates a starch degradation pathway in illuminated mesophyll cells upon osmotic stress, similar to the diurnal pathway of starch degradation in guard cells that is also dependent on thioredoxin-regulated BAM1.
Keywords: Disulphide, guard cell, osmoregulation, redox, starch
Introduction
Starch is the major storage biopolymer synthesized by plants. Thanks to its physicochemical properties, starch represents a convenient way for plants to store large amounts of chemical energy and organic matter without altering the osmotic balance of the cell. Up to half of the photoassimilated carbon in a day can enter the pathway of starch biosynthesis in leaves (Stitt and Quick, 1989; Rao and Terry, 1995; Teusink et al., 1998; Zeeman et al., 2004). Leaf starch is transiently accumulated in a daily cycle. Soluble carbohydrates resulting from starch degradation are mainly exported to sink tissues or used in the same cell where they are produced. Alternatively, starch-derived soluble sugars can also participate to osmotic adjustment under water stress (Basu et al., 2007; Lee et al., 2008) or take part in the osmotic regulation of specialized cells, notably guard cells (Talbott and Zeiger, 1993; Lascève et al., 1997; Vavasseur and Raghavendra, 2005; Lawson, 2009). Many environmental and genetic factors influence starch biosynthesis and degradation (e.g. photoperiod, light quality, temperature, sugars, senescence, etc). Genetically impaired leaf starch breakdown gives rise to a common phenotype in Arabidopsis and other species, characterized by stunted plant growth and exaggerated accumulation of starch in leaves (starch excess, sex) (Caspar et al., 1991; Huber and Hanson, 1992; Eimert et al., 1995).
Beta-maltose, exported from the chloroplast during the night, is the major product of leaf starch breakdown (Weise et al., 2005) and β-amylases are the only enzymes able to produce β-maltose in plants. The Arabidopsis genome codes for nine β-amylase-like proteins and four of them are targeted to chloroplasts (Lao et al., 1999; Sparla et al., 2006; Fulton et al., 2008). Different nomenclatures have been reported for β-amylases, here the one recently proposed by Fulton et al. (2008) is adopted and chloroplast β-amylases are thus named BAM1 (At3g23920, formerly TR-BAMY, Sparla et al., 2006; and BMY7, Kaplan and Guy, 2005), BAM2 (At4g00490), BAM3 (At4g17090, formerly CT-BMY, Lao et al., 1999; and BMY8, Kaplan and Guy, 2005) and BAM4 (At5g55700). These four β-amylases play different roles in starch degradation in chloroplasts (Fulton et al., 2008). BAM3 is the main β-amylase of Arabidopsis leaves and bam3 mutants display a strong sex phenotype both in Arabidopsis (Kaplan and Guy, 2005) and potato (Scheidig et al., 2002). BAM3 is probably the major player in the normal degradation of leaf starch during the night (for a review, see Zeeman et al., 2010). BAM1 is redox-regulated and only active in vitro under reducing conditions (Sparla et al., 2006). Loss of function bam1 mutants show no sex phenotype (Kaplan and Guy, 2005), but analysis of double and multiple mutants indicate that mutation of BAM1 exacerbates the sex phenotype of the single bam3 mutant (Fulton et al., 2008). The role of BAM2 is marginal, while BAM4, which is preferentially expressed in vascular tissues (Francisco et al., 2010) and may be catalytically inactive as a β-amylase, has been proposed to have an important regulatory function, possibly as a maltose sensor modulating the rate of starch breakdown. Accordingly, bam4 knockout mutants also feature the typical sex phenotype (Fulton et al., 2008).
In comparison with mesophyll cells, our current knowledge of starch metabolism in guard cells is still incomplete. Guard cells have a remarkable metabolic plasticity and their mechanisms of osmoregulation, which often involve starch metabolism, vary with species and environmental conditions (Lawson, 2009). While massive uptake of potassium ions from the apoplast builds up guard cell turgor leading to stomatal opening during the day, the synthesis of organic anions (such as malate2–) or sometimes the uptake of inorganic anions, represents a flexible response to sustain membrane potential (Amodeo et al., 1996; Talbott and Zeiger, 1996). Synthesis of malate occurs in the cytosol of guard cells using carbon skeletons derived from starch degradation in the light. Besides potassium, sucrose may also contribute to lower the osmotic potential of guard cells when stomata are open. Sucrose accumulation could derive from CO2 fixation in guard cells but, since the photosynthetic activity of these cells is limited, more often derives from either degradation of starch accumulated in guard cell chloroplasts or direct uptake from the apoplast (Vavasseur and Raghavendra, 2005).
Stomatal closure at night requires potassium to exit from the guard cells and, possibly, sucrose to be removed for nocturnal starch biosynthesis. Storage and mobilization of starch in guard cells thus generally follow an opposite rhythm with respect to mesophyll cells, where starch is synthesized during the day and degraded throughout the following night. The maltose transporter MEX1 is the major transporter responsible for the export of carbon skeletons resulting from starch degradation in the stroma of mesophyll chloroplasts (Niittylä et al., 2004). Although there is no evidence yet for such a transporter in the chloroplast envelope of guard cells, glucose and maltose are the predominant metabolites released by guard cell chloroplasts in the light (Ritte and Raschke, 2003), implying that β-amylases could play a role in daily starch degradation in guard cell chloroplasts.
The present work indicates that one of the four Arabidopsis β-amylase isoforms targeted to chloroplasts, thioredoxin-regulated BAM1 (formerly TR-BAMY, Sparla et al., 2006) in young plants (up to 26 d) is preferably expressed in guard cells, where starch may be preferably degraded during the day rather than in the night (Vavasseur and Raghavendra, 2005; Lawson, 2009). This localization is consistent with the activation of BAM1 by reduced thioredoxins which need light to be reduced (Buchanan and Balmer, 2005). Indeed, knockout mutants lacking BAM1 accumulate starch in guard cells and are impaired in stomatal opening, suggesting that BAM1 plays a role in starch degradation in guard cells to sustain stomata opening during the day. BAM1 is also expressed in the mesophyll cells of adult plants at the flowering stage (more than 31 d), and it is strongly expressed in the mesophyll of osmotically stressed immature plants (younger than 26 d), suggesting that a diurnal, redox-regulated pathway of starch degradation may be activated even in cells which concomitantly synthesize starch in the light. Finally, BAM1 is expressed in roots where it could be activated by NADPH-thioredoxin reductase (NTRC), recently proposed to be a major regulator of starch biosynthesis in amyloplasts (Michalska et al., 2009).
Materials and methods
Thioredoxin specificity and activation of recombinant BAM1 by NTRC
Recombinant BAM1 was expressed and purified as described in Sparla et al. (2006). Recombinant isoforms of Arabidopsis chloroplast thioredoxins were expressed and purified as in Collin et al. (2003, 2004). Thioredoxin specificity was evaluated by testing the activation rate of different thioredoxins on pre-oxidized BAM1. Oxidized BAM1 was obtained by incubating pure recombinant enzyme for 3 h at room temperature in the presence of 20 mM oxidized DTT, followed by desalting in 100 mM Tricine, pH 7.9, using a PD10 column (GE Healthcare). Oxidized BAM1 (0.1 mg) was then incubated in the presence of 0.5 mM reduced DTT and thioredoxin f1, x, y1, y2, m1, m2, m3, m4, at 1 μM each. At different incubation times, β-amylase activity was measured with p-nitrophenyl maltopentaoside (PNPG5) as described in Sparla et al. (2006).
Chloroplast-localized NADPH-dependent thioredoxin reductase (NTRC, At2g41680) from Arabidopsis was expressed and purified in recombinant form and tested for its capability to restore the activity of pre-oxidized BAM1. Oxidized BAM1 was obtained as previously described, and desalted in 50 mM TRIS-HCl, pH 7.6.
Two different molar ratios between BAM1 and NTRC (1:10 and 1:100, respectively) were tested. Before measuring the activity with PNPG5, oxidized BAM1 was incubated for 3 h at room temperature in the presence of NTRC, 0.1 mg ml−1 BSA and 0.5 mM NADPH. Blanks without NTRC or without NADPH were null.
Fully reduced BAM1 was obtained incubating BAM1 in the presence of 20 mM reduced DTT.
Plant material and growth conditions
Arabidopsis thaliana plants (ecotype Columbia) were grown on soil or in hydroponic solution in a growth chamber at constant temperature of 22 °C, under 12/12 h light/dark cycle and photosynthetic photon flux density of 100 μmol m−2 s−1.
For hydroponic cultures, seeds were stratified at 4 °C for 4 d in seedholders filled with 0.65% (w/v) agarose and containing one seed each. Seedholders, obtained from 0.5 ml test tubes, were lodged in the perforated lid of the black polyethylene box filled with hydroponic solution (full-strength composition: 1.25 mM KNO3; 1.5 mM Ca(NO3)2; 0.75 mM MgSO4; 0.5 mM KH2PO4; 50 μM Fe(II)-EDTA; 50 μM H3BO3; 1 μM ZnSO4; 0.7 μM CuSO4; 12 μM MnSO4; 0.24 μM Na2MoO4; 0.1 mM Na2SiO3). Starting from the third week of growth, the nutrient solution was changed every week.
For experiments of osmotic stress, 24-d-old hydroponically grown plants were transferred to a hydroponic medium supplemented with 450 mM mannitol.
T-DNA line
The bam1 T-DNA insertion mutant line from the SALK Institute (SALK_039895) was obtained from the European Arabidopsis Stock Centre (NASC, Nottingham, UK). Insertion site of the T-DNA was confirmed by PCR on genomic DNA and cDNA obtained by total RNA, using the T-DNA specific primer 5′-TGGTTCACGTAGTGGGCCATCG-3′ in combination with BAM1 specific primers 5′-AGAACGTATAGAGAAGGAGGGATTG-3′ and 5′-CCGTCTCTGAACCTTGTGTTGTAGTA-3′ as in Fulton et al. (2008).
YFP and GUS lines
The YFP (Yellow Fluorescent Protein)-coding sequence and the GUS (β-glucuronidase)-coding sequence were both fused to the BAM1 promoter (base positions –2195 to –1). The 2195 bp promoter fragment was amplified by PCR using genomic DNA extracted from Arabidopsis leaves as template. The pair of primers, both carrying an EcoRI restriction site, were as follows: forward primer 5′-CATGGAATTCTATTTGAATCAATTTGACCCAGA-3′ and reverse primer 5′-CATGGAATTCTTTTCTCTCTATACGCGAGAAAACG-3′.
After digestion, the promoter was cloned upstream of the GUS or YFP coding regions into a modified pGreen0029 binary vector (Hellens et al., 2000), where the GUS or YFP coding sequence, fused with the nos terminator, was previously inserted in the polylinker between KpnI-SacI restriction sites.
The pGreenBAM1promoter::GUS or pGreenBAM1promoter::YFP construct was transferred into GV3101-pSoup Agrobacterium strain (Hellens et al., 2000) and Arabidopsis plants were transformed by the floral dip method (Clough and Bent, 1998) and screened on half-strength MS agar medium containing 50 mg l−1 kanamycin. The presence of the insertions was confirmed by PCR on genomic DNA with the following specific primers: forward primer inside BAM1 promoter sequence 5′-CACCGTCCATTCTGACTCTTTT-3′; reverse primer inside the GUS coding sequence 5′-CGGCTAACGTATCCACGCCGTAT-3′; reverse primer inside the YFP coding sequence 5′-CGGTGGTGCAGATGAACTT-3′.
Eleven and four independent BAM1promoter::GUS and BAM1promoter::YFP T1 plants, respectively, were screened and selected for comparable localization of reporter genes.
Subsequent work was conducted on T2 plants. A single GUS line was subjected to GUS staining and a single YFP line was analysed for its fluorescence.
Histochemical GUS assay and detection of YFP fluorescence
For histochemical GUS assays, 2–5 plants at various developmental stages and under stress conditions, were treated as described in Jefferson et al. (1987). Stained plants were examined by bright-field microscopy using a Nikon Eclipse 90-I microscope. The images were processed using the software NIS-Element A-R 3.0. Two independently grown batches of plants of the same GUS line were analysed.
The transgenic plants (mature hydroponically grown and seedlings) transformed with YFP were analysed by confocal microscopy, using a Nikon PCM2000 (Bio-Rad, Germany) laser scanning confocal imaging system. For the YFP detection, excitation was at 488 nm and emission between 530/560 nm. For chlorophyll detection, excitation was at 488 nm and detection over 600 nm.
Each experiment was repeated twice (independently grown batches of plants of the same YFP line) and for each time and condition at least three plants were analysed. The images show representative leaves.
Analysis of starch content
Starch content was visualized on individual hydroponically grown plants after decolorization with hot 80% (v/v) ethanol and stained with iodine (Scheidig et al., 2002). Subcellular localization of starch content was observed using a Nikon Eclipse 90-I microscope and the images were processed using the software NIS-Element A-R 3.0. Thirty-six stomata for both the wild type and bam1 mutant were randomly selected and their guard cells were analysed for starch content using the software ImageJ 1.38 for Windows. The amount of starch per guard cell was calculated as the total pixel area of the dark brown circles isolated in each cell. The experiment was replicated three times. Plants were 26-d-old and 2/3 leaves per plant were analysed after 6 h of light exposure in the growth chamber. Values of starch accumulation in the guard cells were analysed by one-way completely randomized ANOVA and means comparison was performed by Duncan's test at significance level of 0.01 (CoStat, CoHort Software, USA).
Quantification of starch content in plant leaves was obtained using the entire rosette of usually three plants (0.3 g fresh weight, on average) for each data point. Plants collected were immediately frozen in liquid N2. Starch was extracted and determined by measuring the amount of free glucose released from insoluble starch after treatment with α-amylase and amyloglucosidase, as described in Smith and Zeeman (2006). The analysis was performed on three independent batches of plants.
Detection of total β-amylase activity in plants
Soluble proteins were extracted from leaves of 26-d-old, hydroponically grown plants after 4 h of treatment with 450 mM mannitol or control solution. In a typical experiment, all leaves of each plant were homogenized on ice in 0.5 ml of 100 mM Tricine, pH 7.9, 10 mM 2-mercaptoethanol, and 1 mM phenylmethanesulphonylfluoride (PMSF) using a TissueRuptor (Qiagen). The homogenate was spun down in a microfuge at 16 000 g for 25 min at room temperature. The resulting supernatant was transferred to a clean tube on ice for the following measurements. Total protein concentration was determined according to the Bradford method and β-amylase activity was assayed at 37 °C with PNPG5 as described in Sparla et al. (2006). Total β-amylase activity is referred to as total PNPG5-activity measured after 1 h incubation at room temperature in 20 mM reduced DTT. The redox-sensitive fraction of β-amylase activity corresponds to the difference between total PNPG5-activity (20 mM reduced DTT) and the PNPG5-activity measured after 1 h of incubation at room temperature in 20 mM oxidized DTT. Each measure was replicated on a minimum of 30 plants.
Statistical analysis
Before analysis, homogeneity of variances at P=0.05 was confirmed by Bartlett's test. Data on β-amylase activity were analysed by one-way completely randomized ANOVA and means comparison was performed by Duncan's test at significance level of 0.05 (CoStat, CoHort Software, USA).
Stomatal aperture measurement and statistical analysis
Analyses were performed on 26-d-old plants after 6 h of light exposure. Abaxial epidermal peels were fixed with medical spray adhesive Hansaplast (Hansaplast®, Italy) on a microscope slide, immediately observed with an image analyser-Axioplan microscope (Zeiss, Jena, Germany) connected to a Sony CCD-IRIS camera (SSC-M37CE, Sony, Japan). The captured images were then processed using ImageJ 1.38 for Windows. Stomata were randomly selected from the digital image collection, obtained from a set of leaves of six independently grown plants for both the wild type and the bam1 mutant. Stomatal apertures were calculated as the ratio between the width and length of the pore (an average of 70 stomatal apertures were analysed for each genotype).
Data on stomatal aperture were analysed by one-way completely randomized ANOVA and means comparison was performed by Duncan's test at significance level of 0.01 (CoStat, CoHort Software, USA).
Results and discussion
Among all major plastidial thioredoxin isoforms of Arabidopsis, BAM1 is preferably activated by thioredoxin f
BAM1 is a thioredoxin-regulated β-amylase, being totally inactive in the oxidized state and fully activated by reduction (Sparla et al., 2006). Higher plants contain a large number of thioredoxin isoforms (Lemaire et al., 2007) and eight plastidial thioredoxins isoforms of Arabidopsis, representing all major types f, m, x, and y, for their capability to regulate BAM1 activity were compared here.
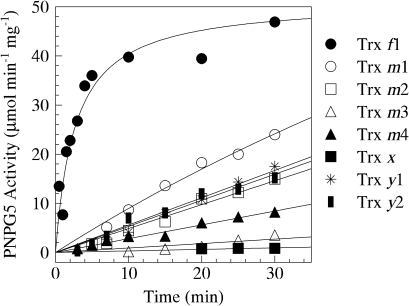
The catalytic activity of BAM1 under oxidizing conditions (3 h incubation in 20 mM oxidized DTT) was close to zero. The incubation of pre-oxidized enzyme, after the complete removal of oxidized DTT, with 0.5 mM reduced DTT for 30 min did not lead to any significant reactivation (not shown), but BAM1 activity was rapidly (t1/2 ≈1 min) and fully restored if 1 μM thioredoxin f1 was also present in the incubation medium (Fig. 1). All other thioredoxins tested, at the same concentration of 1 μM, were much less efficient than thioredoxin f1. However, thioredoxins m1, m2, y1, y2, and m4, in this order, were all able to reduce BAM1 to some extent, although the initial activation rates were at least 20-fold lower, compared with thioredoxin f1. The effect of thioredoxins m3 and x was negligible (Fig. 1).
Fig. 1.
Specificity of BAM1 for chloroplast thioredoxin isoforms. Activation kinetics of oxidized BAM1 were analysed by measuring PNPG5-depedent activity after incubation of the pre-oxidized enzyme in the presence of 0.5 mM reduced DTT alone or plus 1 μM thioredoxins. Activities obtained under same conditions but in the absence of BAM1 were subtracted. Symbols: full circles, thioredoxin f1; open circles, thioredoxin m1; open squares, thioredoxin m2; open triangles, thioredoxin m3; full triangles, thioredoxin m4; full squares, thioredoxin x; stars, thioredoxin y1 and half full squares, thioredoxin y2.
Alternative activation of BAM1 by chloroplast NADPH-thioredoxin reductase (NTRC)
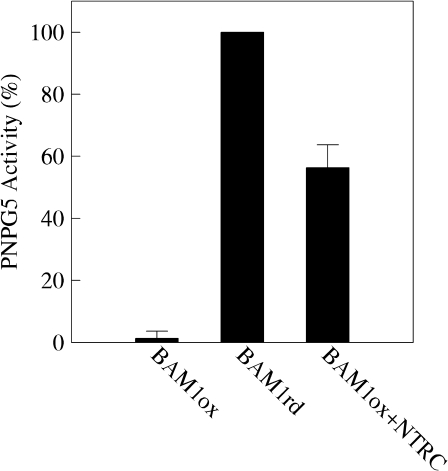
To investigate the possibility that BAM1 could be regulated by an alternative way to the classical ferredoxin/thioredoxin system (Schürmann and Buchanan, 2008), recombinant Arabidopsis NADPH-thioredoxin reductase (NTRC) was tested for its capability to reductively activate oxidized BAM1. NTRC is a plastidial flavo-reductase containing a thioredoxin domain (Michalska et al., 2009). Incubation of pre-oxidized BAM1 (0.5 μM) for 3 h with 5 μM NTRC and 0.5 mM NADPH allowed recovery of about half of the BAM1 maximal activity reached by incubation with 20 mM DTT (Fig. 2). A 10-fold higher NTRC to BAM1 ratio did not increase the activation level of BAM1 (data not shown).
Fig. 2.
Activation of BAM1 by NTRC. Activation of oxidized BAM1 (0.5 μM) was analysed by measuring PNPG5-dependent activity after 3 h of incubation at room temperature in the presence of NTRC (5 μM) and 0.5 mM NADPH. The release of p-nitrophenol from PNPG5 in the absence of BAM1 was null. Data represent mean values ±SD of three independent experiments made on different BAM1 preparations.
NTRC can thus activate BAM1 at a much slower rate than reduced thioredoxin f1. In illuminated chloroplasts at least, it is likely that the capability of NTRC to regulate BAM1 is fully overcome by the ferredoxin/thioredoxin system connected to Photosystem I. The response of the Calvin cycle enzymes to thioredoxin f is indeed in the same time frame as BAM1 (Marri et al., 2009).
BAM1 promoter drives the expression of reporter genes in roots, in guard cells of non-flowering plants, and in the mesophyll of adult plants
To analyse the promoter activity of BAM1, a 2195 bp fragment located upstream of the start codon was fused to the GUS or to the YFP cassette within a binary vector for Agrobacterium-mediated stable transformation of Arabidopsis plants. Second generation (T2) transgenic lines were analysed weekly during the whole growing season by means of YFP fluorescence and histochemical GUS staining.
Both YFP and GUS plants showed clear expression of BAM1 in roots starting from the first week of growth until full development (Figs 3, 4). Expression was restricted to tips of developed roots and stele (Figs 3, 4). BAM1 is thus not restricted to chloroplasts but is also present in heterotrophic tissues. These results are qualitatively confirmed by publicly available microarray data which show, in addition, that BAM1 may be the major β-amylase among plastid-targeted isoforms in roots (see Supplementary Fig. S1 at JXB online). BAM3, which predominates in leaves and controls starch degradation in the night (Fulton et al., 2008), is almost undetectable in roots.
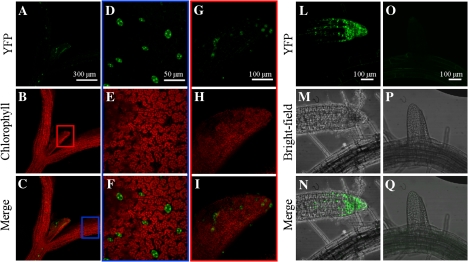
Fig. 3.
Localization of YFP fluorescence under the control of the BAM1 promoter, in a representative 7-d-old seedling. (A–C) Shoot; (D–F) cotyledon (magnification of the portion delimited by the blue rectangle); (G–I) first leaf (magnification of the portion delimited by the red rectangle); (L–N) primary root tip; (O–Q) developing secondary root.
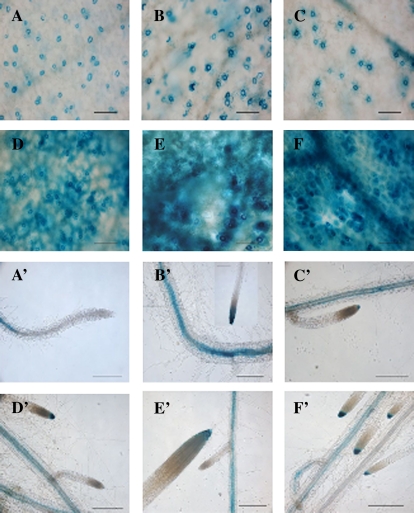
Fig. 4.
Localization of BAM1promoter::GUS activity during different stages of Arabidopsis development. The figure shows a typical expression pattern obtained from two independent experiments (scale bar=100 μm). (A, A') 10-d-old plants; (B, B’) 17-d-old plants; (C, C’) 24-d-old plants; (D, D') 31-d-old plants; (E, E') 38-d-old plants; (G, G') 45-d-old plants.
How BAM1 could be regulated in roots is unclear. Both thioredoxin f and m (de Dios Barajas-Lopez et al., 2007) and y1 (Collin et al., 2003), all active with BAM1, were found to be expressed in roots, and amyloplasts are known to contain all the necessary components (ferredoxin:NADP reductase, ferredoxin, ferredoxin:thioredoxin reductase) for a NADPH-dependent, thioredoxin-mediated regulatory system (Balmer et al., 2006). However, NTRC is also present in heterotrophic plastids (Michalska et al., 2009) and is proposed to play a major role in regulating starch biosynthesis in amyloplasts by activating ADP-glucose pyrophosphorylase (Michalska et al., 2009). This picture might be more complicated if NTRC might concomitantly activate a starch-degrading enzyme like BAM1 in the same plastids, but more data are necessary to address this point.
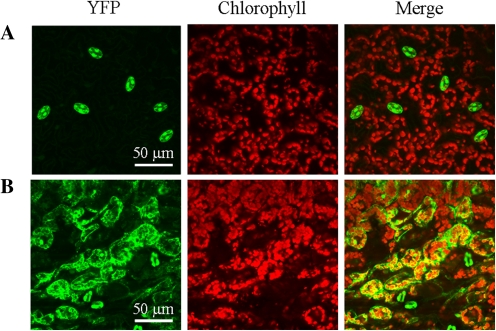
Besides roots, the expression of BAM1 in photosynthetic tissues was very peculiar. In the aerial portion of 7-d-old YFP seedlings, BAM1 was exclusively expressed in guard cells of cotyledons and first leaf (Fig. 3). Specific expression in guard cells was confirmed in GUS plants analysed from 10–24 d of growth, although it was also observed in vascular tissues on occasion (Fig. 4). In one-week-older plants (31 d), coincident with the emergence of the inflorescence, strong GUS activity made its appearance in mesophyll cells and leaf veins (Fig. 4).
The unexpected finding that the BAM1 promoter drove reporter genes expression in guard cells fits with the in vitro observation that BAM1 is reductively activated by thioredoxins. Similar to roots, BAM1 transcripts in guard cells are also the most abundant among plastid-targeted β-amylases (BAM1 to 4), as shown by microarray data (see Supplementary Fig. S2 at JXB online). Little is known about thioredoxins in guard cells, but the promoter of pea thioredoxin f was shown to be active in guard cells of transgenic Arabidopsis (de Dios Barajas-Lopez et al., 2007) and microarray data suggest that the whole set of thioredoxins, including f-type isoforms may be expressed in guard cells.
Guard cell metabolism is highly specialized for the accumulation of solutes (including malate and sucrose), which are required to support stomatal opening during the day. Since direct sugar production via carbon assimilation in guard cells is low (Outlaw and De Vlieghere-He, 2001), the source of sucrose and carbon skeletons in the light might rather depend on starch breakdown, with an additional supply from surrounding cells (Outlaw, 2003). In fact, the rate of starch breakdown in guard cells was found to be correlated with the degree of stomatal aperture (Outlaw and Manchester, 1979), and thioredoxin-dependent BAM1 may well be involved in this process since chloroplast thioredoxins are reduced in the light by Photosystem I. On the other hand, when stomata are closed during darkness, osmotically active carbohydrates (including sucrose) might be stored as insoluble starch in chloroplasts and it makes sense that under these conditions the starch-degrading enzyme BAM1 would be inactivated by oxidized thioredoxins. In order to test these hypotheses, an Arabidopsis mutant impaired in BAM1 expression was analysed.
BAM1 knockout mutants have no sex phenotype but increased starch content in guard cells in the light and reduced stomata opening
A T-DNA insertion line in which BAM1 expression is virtually nil, as confirmed by RT-PCR experiments on homozygous lines (T3) using gene-specific primers (see Supplementary Fig. S3 at JXB online), was used in these experiments. The presence of the T-DNA insertion within BAM1 coding sequence was also supported by PCR experiments on genomic DNA (see Supplementary Fig. S3 at JXB online).
Total leaf starch content was qualitatively detected by iodine staining in 24-d-old plants grown on hydroponics. As expected, starch increased during the day and decreased during the night and no significant differences were observed between the wild type and the mutants (see Supplementary Fig. S4 at JXB online), confirming that BAM1 did not contribute significantly to primary starch degradation in mesophyll cells of young Arabidopsis plants (see also Kaplan and Guy, 2005; Fulton et al., 2008).
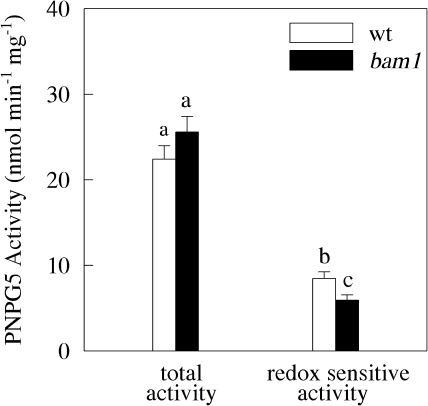
Total β-amylase activity measured in the leaves of these plants was also similar, although the redox-sensitive portion of this activity was slightly higher in wild-type plants (38%) than in the mutants (24%) (Fig. 5). Clearly, BAM1 does not control the starch degradation pathway of young Arabidopsis leaves. This result is not surprising, however, since BAM1 is mostly expressed in guard cells in these 24-d-old plants.
Fig. 5.
Beta-amylase activity in wild-type and bam1 plants, hydroponically grown under normal conditions. Total PNPG5-activity was measured on soluble proteins extracted from leaves after 1 h of incubation in the presence of 20 mM reduced DTT. Redox-sensitive activity was obtained by subtracting from total PNPG5-activity the same activity measured after 1 h of incubation with oxidized DTT. Values are the means ±SE (n=30 independent protein extractions). Statistical analysis by ANOVA underlined significant differences (P <0.001) between wild-type and bam1 plants. A Duncan's Test was conducted to compare means and statistically significantly different values (P <0.05) are indicated by different letters in the graphs.
Studies on chloroplast β-amylases have shown that only plants mutated in bam3 or bam4, but not bam1 or bam2, displayed the characteristic sex phenotype, together with an elevated starch content during the night (indicative of diminished starch depolymerization in mesophyll cells) (Kaplan and Guy, 2005; Fulton et al., 2008). However, the fact that BAM1 can partially take over the BAM3 role in starch degradation under particular conditions (e.g. the down-regulation of BAM3) is indicated by the fact that bam1/bam3 double mutants showed a more severe sex phenotype than the bam3 mutants (Fulton et al., 2008). In light of our current results, it is tempting to explain the different phenotypes (bam1, bam3, bam1/bam3 mutants) by supposing that a lack of BAM3 might drive the expression of BAM1 in the mesophyll of young plants, where it would normally be restricted to guard cells.
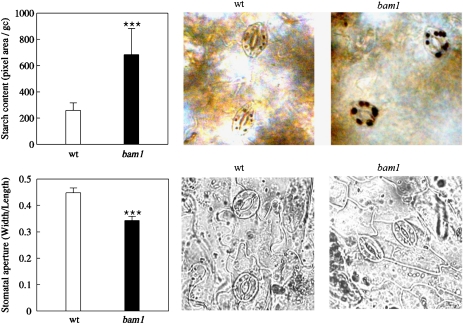
Interesting phenotypic differences between wild-type and bam1 plants were discovered by focusing our attention on guard cells. As depicted in Fig. 6, in normal growth conditions and after 6 h of light, guard cells of bam1 knockout plants clearly contained more starch than wild-type leaves. Moreover, stomata were wide open in wild-type plants, much more than in bam1 mutants. In spite of these effects, hydroponically grown bam1 plants did not show any macroscopic phenotype suggesting that, at least in our experimental conditions, the residual functionality of stomata guaranteed normal growth.
Fig. 6.
Starch content in guard cells and stomatal aperture analysis on wild-type and bam1 mutant plants. Leaves were collected from plants hydroponically grown for 26 d, after 6 h of light exposure. Both measurements were performed throughout digital image processing. After iodine staining, starch content (upper graph) was quantified as the total pixel area of the starch accumulations visible in chloroplasts of single guard cells. Values are means ±SE (n=72 guard cells). In the pictures on the right the typical starch accumulation observed in wild-type and bam1 guard cells. In the lower graph, stomatal aperture measurement was determined as the ratio between width and length of the stomatal pore. Values are means ±SE (n=70 stomata). Statistical analysis by ANOVA and a Duncan's Test (P <0.01) were conducted. Statistically significant differences are indicated by three stars in the graphs. (This figure is available in colour at JXB online.)
Altogether, our data strongly suggest that BAM1 plays an important role in the process of starch degradation occurring in guard cell chloroplasts in the light. Lack of BAM1 impairs diurnal starch degradation in these specialized cells and, as a consequence, stomatal opening. The in vitro properties of BAM1 are consistent with starch degradation in guard cells being triggered in the light through the activation of BAM1 by thioredoxins (preferably of the f-type), reduced in turn by Photosystem I through ferredoxin:thioredoxin reductase.
BAM1 is induced in mesophyll cells of young plants under osmotic stress
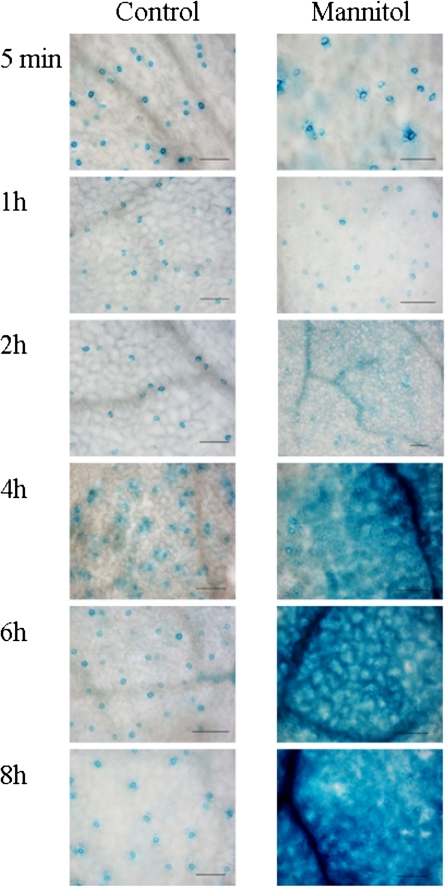
BAM1 promoter activity was analysed further under osmotic stress conditions. To do this, 24-d-old BAM1promoter::GUS and BAM1promoter::YFP plants, grown hydroponically, were exposed to 450 mM mannitol treatments (Figs 7, 8). GUS activity showed an initial response after 2 h and strong induction starting from 4 h of treatment (Fig. 7). Induction of GUS activity was first localized in veins, followed by a secondary GUS induction in mesophyll cells. A similar induction pattern by osmotic stress of BAM1 promoter activity in mesophyll cells besides guard cells was observed in YFP plants (Fig. 8). Interestingly, microarray data (see Supplementary Fig. S5 at JXB online) indicate that the same osmotic stress conditions which induce BAM1 expression are symmetrically responsible for the repression of BAM3 (see also Sparla et al., 2006). In osmotically stressed plants (as well as in bam3 mutants), BAM1 may thus become the major chloroplast β-amylase, suggesting that under these particular conditions starch degradation could be redox-regulated (i.e. light-activated) like in guard cells.
Fig. 7.
Induction of BAM1promoter::GUS activity under control conditions and in the presence of 450 mM mannitol. Treatment was applied 3 h after beginning of the light period. GUS activity was measured after 5 min, 1 h, 2 h, 4 h, 6 h, and 8 h of treatment (scale bar=100 μm).
Fig. 8.
Localization of YFP fluorescence in leaves of plants transformed with BAM1promoter::YFP. (A) Representative control leaf; (B) representative leaf after 10 h of 450 mM mannitol treatment. Each image represents a 3D reconstruction from a Z stack confocal acquisition.
Osmotic stress activates diurnal starch degradation in the wild type but not in bam1 mutants
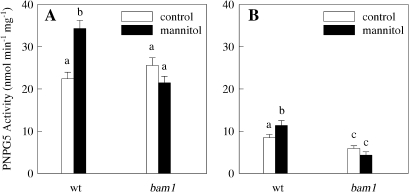
In parallel experiments the total β-amylase activity was measured in wild-type and bam1 plants, both treated with 450 mM mannitol for 4 h during the light period or kept in nutrient solution. Mannitol treatment caused an increase of about 50% of total β-amylase activity in wild-type plants, but no significant changes in bam1 plants were detected (Fig. 9). The redox-sensitive fraction of total β-amylase activity under osmotic stress was also slightly stimulated in wild-type plants while it remained unchanged in bam1 plants. However, the increase in total β-amylase activity of osmotically stressed wild-type plants (Fig. 9A) could not be entirely ascribed to redox-sensitive β-amylases (Fig. 9B) and apparently also involved other (redox-insensitive) β-amylases. Although osmotic stress might lead to unpredictable changes in the activities of the several β-amylases (including the expected repression of BAM3 activity), it could safely be concluded that BAM1 is induced under osmotic stress both at the gene (Figs 7, 8) and at the activity level (Fig. 9). As a result, it is possible that BAM1 exerts a control on leaf starch degradation under osmotic stress, and since this enzyme is strictly redox-regulated, this would imply that leaf starch breakdown may occur in the light under these specific conditions. To clarify this point, the effect of osmotic stress on the capacity of accumulating starch in leaves during the light period was measured in wild-type and bam1 plants.
Fig. 9.
Beta-amylase activity in wild-type and bam1 mutant plants, hydroponically grown under normal conditions (control) or in the presence of 450 mM mannitol for 4 h (mannitol). (A) Soluble proteins extracted from leaves were incubated for 1 h in the presence of 20 mM reduced DTT before measuring PNPG5-activity; (B) redox-sensitive fraction obtained by subtracting from total PNPG5-activity (A) the same activity measured after 1 h of incubation with oxidized DTT. Values are the means ±SE (n=30 independent protein extractions). Statistical analysis by ANOVA underlined significant differences (P <0.001) between wild-type and bam1 plants. A Duncan's Test was conducted to compare means and statistically significantly different values (P <0.05) are indicated by different letters in the graphs.
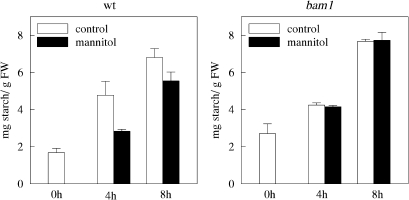
As depicted in Fig. 10, under normal growth conditions, total starch content in wild-type and bam1 plants increased 3-fold during 8 h of illumination (starch was measured from the 3rd to the 11th hour of the light cycle). Leaf starch content was instead lower in wild type plants treated with 450 mM mannitol, the rate of starch accumulation being 24% less than in control conditions (Fig. 10). The observed decrease of leaf starch accumulation could, in principle, derive from two contrasting effects: either inhibition of starch biosynthesis (possibly caused by a decrease in net photosynthetic rate) or activation of starch degradation. However, starch accumulation was not affected by osmotic stress in bam1 plants which maintained exactly the same rate observed in control conditions. Therefore, it seems more likely that the observed decrease of starch accumulation caused by osmotic stress in wild-type plants is a consequence of the activation of starch degradation in the light, rather than an inhibition of starch biosynthesis, which was apparently not affected. In this framework, BAM1 activity seems to be at the onset of the starch degradation pathway in the light. Since bam1 plants appeared to be less tolerant to osmotic stress (see Supplementary Fig. S6 at JXB online), leaf starch degradation catalysed by BAM1 may be part of the normal adaptation response to stress in Arabidopsis. Maltose, the product of β-amylase activity, has been reported to play a role as an osmoprotectant under various stress conditions (Kaplan and Guy, 2004; Rizhsky et al., 2004).
Fig. 10.
Starch content in wild-type and bam1 plants under osmotic stress conditions. Twenty-six-day-old plants were osmotically stressed 3 h after switching on the light, by adding 450 mM mannitol to the hydroponic solution. Starch was quantified as described by Smith and Zeeman (2006). Five independent experiments were performed. Data are means values ±SE (n=15).
Conclusion
The present results are consistent with a scenario in which BAM1, in photosynthetic cells, is a light-activated enzyme involved in diurnal starch degradation under particular conditions. In leaves of young, non-flowering plants BAM1 is mainly expressed in guard cells. Although the activity of BAM1 in guard cells could not be directly assessed, efficient activation of BAM1 by reduced thioredoxins in vitro (e.g. thioredoxin f) strongly suggests that BAM1 in guard cells is activated in the light, when thioredoxins are kept reduced by Photosystem I, and inactivated at night. Consistently, mutants lacking BAM1 contain large amounts of starch in guard cells in the middle of the day, when starch degradation could support stomatal opening. Stomatal opening is indeed reduced when guard cells of bam1 mutants are filled with starch. The regulation of BAM1 thus perfectly matches the need of guard cells to produce osmolytes from starch during the day, and insoluble starch overnight when turgor release in guard cells induces the stomata to close.
Although BAM1 has a marginal role in starch degradation in mesophyll cells (Fulton et al., 2008), this situation clearly changes under osmotic stress, when BAM1 abruptly appears in mesophyll cells even in young plants, apparently as a substitute for BAM3 which is symmetrically repressed (Hruz et al., 2008; Sparla et al., 2006). In this particular condition, primary starch accumulation during photosynthesis is apparently counteracted by starch hydrolysis catalysed by redox-regulated BAM1. Day-time leaf starch breakdown triggered by BAM1 would produce osmolytes (maltose) as an active response against the osmotic stress.
In conclusion, starch degradation also clearly occurs in the light, although in particular cell types and/or conditions, and it is shown here that in both guard cells and osmotically stressed mesophyll cells, diurnal starch degradation is triggered by thioredoxin-regulated BAM1.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Microarray data on the expression of BAM1, BAM2, BAM3, and BAM4 genes in root.
Supplementary Fig. S2. Microarray data on the expression of BAM1, BAM2, BAM3, and BAM4 genes in guard and mesophyll cells.
Supplementary Fig. S3. Analysis of Arabidopsis line (SALK_039895) carrying a T-DNA insertion within the BAM1 coding sequence.
Supplementary Fig. S4. Starch content in wild-type and bam1 plants.
Supplementary Fig. S5. Microarray data on the expression of BAM1, BAM2, BAM3, and BAM4 genes in plants exposed to osmotic and salt-stress conditions.
Supplementary Fig. S6. Mannitol effect on wild-type and bam1 plants.
Supplementary Material
Acknowledgments
This project is funded by the Ministero della Pubblica Istruzione (PRIN 2008) and by the University of Bologna (Progetto Strategico 2006).
References
- Amodeo G, Talbott LD, Zeiger E. Use of potassium and sucrose by onion guard cells during a daily cycle of osmoregulation. Plant and Cell Physiology. 1996;37:575–579. [Google Scholar]
- Balmer Y, Vensel WH, Cai N, Manieri W, Schürmann P, Hurkman WJ, Buchanan BB. A complete ferredoxin/thioredoxin system regulates fundamental processes in amyloplasts. Proceedings of the National Academy of Sciences, USA. 2006;103:2988–2993. doi: 10.1073/pnas.0511040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu PS, Ali M, Chaturvedi SK. Osmotic adjustment increases water uptake, remobilization of assimilates and maintains photosynthesis in chickpea under drought. Indian Journal of Experimental Biology. 2007;45:261–267. [PubMed] [Google Scholar]
- Buchanan BB, Balmer Y. Redox regulation: a broadening horizon. Annual Review of Plant Biology. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- Caspar T, Lin T-P, Kakefuda G, Benbow L, Preiss J, Somerville C. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiology. 1991;95:1181–1188. doi: 10.1104/pp.95.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: amplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Collin V, Issakidis-Bourguet E, Marchand C, Hirasawa M, Lancelin J-M, Knaff DB, Miginiac-Maslow M. The Arabidopsis plastidial thioredoxins. Journal of Biological Chemistry. 2003;278:23747–23752. doi: 10.1074/jbc.M302077200. [DOI] [PubMed] [Google Scholar]
- Collin V, Lamkemeyer P, Miginiac-Maslow M, Hirasawa M, Knaff DB, Dietz KJ, Issakidis-Bourguet E. Characterization of plastidial thioredoxins from Arabidopsis belonging to the new y-type. Plant Physiology. 2004;136:4088–4095. doi: 10.1104/pp.104.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios Barajas-Lopez J, Serrato AJ, Olmedilla A, Chueca A, Sahrawy M. Localization in roots and flowers of pea chloroplastic thioredoxin f and thioredoxin m proteins reveals new roles in nonphotosynthetic organs. Plant Physiology. 2007;145:946–960. doi: 10.1104/pp.107.105593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimert K, Wang SM, Lue WI, Chen J. Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. The Plant Cell. 1995;7:1703–1712. doi: 10.1105/tpc.7.10.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco P, Li J, Smith SM. The gene encoding the catalytically inactive beta-amylase BAM4 involved in starch breakdown in Arabidopsis leaves is expressed preferentially in vascular tissues in source and sink organs. Journal of Plant Physiology. 2010;167:890–895. doi: 10.1016/j.jplph.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, et al. Beta-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. The Plant Cell. 2008;20:1040–1058. doi: 10.1105/tpc.107.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Molecular Biology. 2000;42:819–832. doi: 10.1023/a:1006496308160. [DOI] [PubMed] [Google Scholar]
- Huber SC, Hanson KR. Carbon partitioning and growth of a starchless mutant of Nicotiana sylvestris. Plant Physiology. 1992;99:1449–1454. doi: 10.1104/pp.99.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Advances in Bioinformatics. 2008;2008:420747. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Guy CL. β-amylase induction and the protective role of maltose during temperature shock. Plant Physiology. 2004;135:1674–1684. doi: 10.1104/pp.104.040808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Guy CL. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. The Plant Journal. 2005;44:730–743. doi: 10.1111/j.1365-313X.2005.02565.x. [DOI] [PubMed] [Google Scholar]
- Lascève G, Leymarie J, Vavasseur A. Alterations in light-induced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant, Cell and Environment. 1997;20:350–358. [Google Scholar]
- Lao NT, Schoneveld O, Mould RM, Hibberd JM, Gray JC, Kavanagh TA. An Arabidopsis gene encoding a chloroplast targeted β-amylase. The Plant Journal. 1999;20:519–527. doi: 10.1046/j.1365-313x.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Lawson T. Guard cell photosynthesis and stomatal function. New Phytologist. 2009;181:13–34. doi: 10.1111/j.1469-8137.2008.02685.x. [DOI] [PubMed] [Google Scholar]
- Lee BR, Jin YL, Jung WJ, Avice JC, Morvan-Bertrand A, Ourry A, Park CW, Kim TH. Water-deficit accumulates sugars by starch degradation—not by de novo synthesis—in white clover leaves (Trifolium repens) Physiologia Plantarum. 2008;134:403–411. doi: 10.1111/j.1399-3054.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- Lemaire SD, Michelet L, Zaffagnini M, Massot V, Issakidis-Bourguet E. Thioredoxins in chloroplasts. Current Genetics. 2007;51:343–365. doi: 10.1007/s00294-007-0128-z. [DOI] [PubMed] [Google Scholar]
- Marri L, Zaffagnini M, Collin V, Issakidis-Bourguet E, Lemaire SD, Pupillo P, Sparla F, Miginiac-Maslow M, Trost P. Prompt and easy activation by specific thioredoxins of Calvin cycle enzymes of Arabidopsis thaliana associated in the GAPDH/CP12/PRK supramolecular complex. Molecular Plant. 2009;2:259–269. doi: 10.1093/mp/ssn061. [DOI] [PubMed] [Google Scholar]
- Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proceedings of the National Academy of Sciences, USA. 2009;106:9908–9913. doi: 10.1073/pnas.0903559106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. A previously unknown maltose transporter essential for starch degradation in leaves. Science. 2004;303:87–89. doi: 10.1126/science.1091811. [DOI] [PubMed] [Google Scholar]
- Outlaw WH., Jr. Integration of cellular and physiological functions of guard cells. Critical Reviews in Plant Sciences. 2003;22:503–529. [Google Scholar]
- Outlaw WH, Jr., De Vlieghere-He X. Transpiration rate. An important factor controlling the sucrose content of the guard cell apoplast of broad bean. Plant Physiology. 2001;126:1716–1724. doi: 10.1104/pp.126.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw WH, Jr., Manchester J. Guard cell starch concentration quantitatively related to the stomatal aperture. Plant Physiology. 1979;64:79–82. doi: 10.1104/pp.64.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M, Terry N. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet. Plant Physiology. 1995;107:1313–1321. doi: 10.1104/pp.107.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritte G, Raschke K. Metabolite export of isolated guard cell chloroplasts of Vicia faba. New Phytologist. 2003;159:195–202. doi: 10.1046/j.1469-8137.2003.00789.x. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology. 2004;134:1683–1696. doi: 10.1104/pp.103.033431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidig A, Fröhlich A, Schulze S, Lloyd JR, Kossmann J. Downregulation of a chloroplast-targeted beta-amylase leads to a starch-excess phenotype in leaves. The Plant Journal. 2002;30:581–591. doi: 10.1046/j.1365-313x.2002.01317.x. [DOI] [PubMed] [Google Scholar]
- Schürmann P, Buchanan BB. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxidants and Redox Signaling. 2008;10:1235–1274. doi: 10.1089/ars.2007.1931. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nature Protocols. 2006;1:1342–1345. doi: 10.1038/nprot.2006.232. [DOI] [PubMed] [Google Scholar]
- Sparla F, Costa A, Lo Schiavo F, Pupillo P, Trost P. Redox regulation of a novel plastid-targeted β-amylase of Arabidopsis thaliana. Plant Physiology. 2006;141:840–850. doi: 10.1104/pp.106.079186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Quick WP. Photosynthetic carbon partitioning: its regulation and possibilities for manipulation. Physiologia Plantarum. 1989;77:633–641. [Google Scholar]
- Talbott LD, Zeiger E. Sugar and organic acid Accumulation in guard cells of Vicia faba in response to red and blue light. Plant Physiology. 1993;102:1163–1169. doi: 10.1104/pp.102.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Central roles for potassium and sucrose in guard cell osmoregulation. Plant Physiology. 1996;111:1051–1057. doi: 10.1104/pp.111.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusink B, Walsh MC, van Dam K, Westerhoff HV. The danger of metabolic pathways with turbo design. Trends in Biochemical Sciences. 1998;23:162–169. doi: 10.1016/s0968-0004(98)01205-5. [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS. Guard cell metabolism and CO2 sensing. New Phytologist. 2005;165:665–682. doi: 10.1111/j.1469-8137.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- Weise SE, Kim KS, Stewart RP, Sharkey TD. β-Maltose is the metabolically active anomer of maltose during transitory starch degradation. Plant Physiology. 2005;137:756–761. doi: 10.1104/pp.104.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeman SC, Smith SM, Smith AM. The breakdown of starch in leaves. New Phytologist. 2004;163:247–261. doi: 10.1111/j.1469-8137.2004.01101.x. [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Kossmann J, Smith AM. Starch: its metabolism, evolution and biotechnological modification in plants. Annual Review of Plant Biology. 2010;61:209–234. doi: 10.1146/annurev-arplant-042809-112301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.