Abstract
Bisphenol A (BPA) is used as the backbone for plastics and epoxy resins, including various food and beverage containers. BPA has also been detected in 95% of random urine samples and ovarian follicular fluid of adult women. Few studies have investigated the effects of BPA on antral follicles, the main producers of sex steroid hormones and the only follicles capable of ovulation. Thus, this study tested the hypothesis that postnatal BPA exposure inhibits antral follicle growth and steroidogenesis. To test this hypothesis, antral follicles isolated from 32-day-old FVB mice were cultured with vehicle control (dimethyl sulfoxide [DMSO]), BPA (4.4–440μM), pregnenolone (10 μg/ml), pregnenolone + BPA 44μM, and pregnenolone + BPA 440μM. During the culture, follicles were measured for growth daily. After the culture, media was subjected to ELISA for hormones in the estradiol biosynthesis pathway, and follicles were processed for quantitative real-time PCR of steroidogenic enzymes. The results indicate that BPA (440μM) inhibits follicle growth and that pregnenolone cotreatment was unable to restore/maintain growth. Furthermore, BPA 44 and 440μM inhibit progesterone, dehydroepiandrosterone, androstenedione, estrone, testosterone, and estradiol production. Pregnenolone cotreatment was able to increase production of pregnenolone, progesterone, and dehydroepiandrosterone and maintain androstenedione and estrone levels in BPA-treated follicles compared with DMSO controls but was unable to protect testosterone or estradiol levels. Furthermore, pregnenolone was unable to protect follicles from BPA-(44–440 μM) induced inhibition of steroidogenic enzymes compared with the DMSO control. Collectively, these data show that BPA targets the estradiol biosynthesis pathway in the ovary.
Keywords: bisphenol A, steroidogenesis, ovary, antral follicle growth, StAR, pregnenolone
Bisphenol A (BPA), 2,2-bis-4-hydroxyphenyl propane, is an estrogenic compound originally synthesized in 1963 to prevent miscarriage. BPA eventually became widely used as the backbone for epoxy resins, durable clear polycarbonate plastics such as reusable plastic food containers, food and beverage can liners, infant formula cans, baby bottles, and dental sealants, among many other plastic-based products. Unfortunately, aging, heating, and contact with acids and bases, including those commonly found in cleaning supplies and detergents, cause the BPA polymers to break apart. In a process commonly referred to as “leaching,” BPA seeps into the contents of various food packages, into saliva, and/or into dust particles, providing ample entry ways for BPA into animal physiological systems, including humans (Can et al., 2005; Kang et al., 2006; Lenie et al., 2008; Vandenberg et al., 2007; vom Saal and Hughes, 2005; Welshons et al., 2006).
Although BPA exposure can occur via food and drink (ingested), BPA can also be inhaled. Once in the lungs, BPA can travel through the bloodstream and into enterohepatic flow, where it can act as if ingested orally and travel through the digestive system. Various studies have detected BPA in blood, saliva, and human tissues as well as in 95% of human urine samples taken in a random sampling (Calafat et al., 2005, 2008; Ouchi and Watanabe, 2002). Additionally, though BPA is thought to be conjugated to glucuronide in the digestive system and to exit the body via the urinary system, the chemical is not solely confined to the digestive system. BPA has also been detected in mammary tissue, breast milk, amniotic fluid, and ovarian follicular fluid, indicating BPA travels not only through the digestive system but also through the venous network and thus into the many other organ and tissue systems of the body (Ikezuki et al., 2002; Lenie et al., 2008; Vandenberg et al., 2007, 2009).
Recent research has suggested that BPA persists in the human system longer than originally thought. In a study comparing BPA levels with fasting time, researchers found BPA persisting in the body well past the given 4–6 h half-life of BPA. Specifically, following 17-h exposure, BPA levels only decreased 56% instead of the expected 100% elimination. This study suggests that BPA has a longer half-life than previously estimated; there are significant non-food source exposures of BPA or, more likely, a combination of the two explanations (Stahlhut et al., 2009; Tillet, 2009). Collectively, these findings suggest that humans are constantly exposed to BPA.
Studies focusing on the effects of BPA exposure on the rodent female reproductive system have shown that maternal exposure to BPA during gestation can cause adverse developmental effects in the offspring. Specifically, BPA has been shown to induce early puberty onset, changes in weight gain, early vaginal opening (Honma et al., 2002), and ovarian morphological abnormalities in the offspring. The ovarian abnormalities include cystic ovaries (Hartshorne, 1997) and cystadenomas, hemorrhagic follicles, and large antral-like follicles seemingly unable to ovulate (Hartshorne, 1997). BPA also can cause progressive proliferative lesions of the oviduct (Savabieasfahani et al., 2006) and leiomyomas in the uterus (Newbold et al., 2007). Furthermore, BPA increases estrogen receptor alpha (Esr1) and estrogen receptor beta (Esr2) receptor expression levels in the uterus (Richter et al., 2007).
Few studies have investigated the direct effects of BPA on the ovarian follicle itself, the functional unit of the ovary and the main steroid hormone producer of the female reproductive system. Previous studies have focused mainly on meiotic abnormalities, such as cell cycle arrest, meiotic aneuploidy, and congression failure during the cell cycle (Eichenlaub-Ritter et al., 2008; Hunt et al., 2003) or on morphological indicators of ovulation, such as observing a lack of corpora lutea in the ovary (Hartshorne, 1997). Although BPA has been reported to be present in follicular fluid of adult human ovaries, previously published studies have not focused on antral follicles of adult humans, and as a result, little information is available regarding the effect of postnatal BPA exposure on the proper functioning of adult human ovarian follicles. Furthermore, little information is available on the effect of BPA on the proper functioning of ovarian antral follicles in the adult mouse model. Thus, this study tested the hypothesis that postnatal exposure to BPA inhibits growth and steroidogenesis of adult ovarian antral follicles in mice.
MATERIALS AND METHODS
Chemicals.
BPA powder (99%) was purchased from Sigma-Aldrich (St Louis, MO). A stock solution of BPA was dissolved and diluted in dimethyl sulfoxide (DMSO) (Sigma-Aldrich) to achieve various BPA treatment concentrations (1.3, 13.3, and 133 mg/ml) for final working concentrations of 1.0, 10, and 100 μg of BPA per milliliter of culture media (4.4, 44, and 440μM BPA, respectively). Additionally, using these treatment concentrations allowed each working concentration to contain the same volume of chemical and vehicle (0.75 μl BPA:DMSO per milliliter of culture media).
The concentrations chosen for the cultures were based on concentrations used in previous studies (Can et al., 2005; Lee et al., 2007; Lenie et al., 2008; Mlynarcikova et al., 2009; Vandenberg et al., 2007; Watson et al., 2007; Xu et al., 2002; Zhou et al., 2008). BPA concentrations were also chosen based on studies showing the effects of BPA on ovarian cells. For example, BPA exposure between 100 fM and 100μM for 24–72 h results in an increase in apoptosis and G2-to-M arrest in cultured mouse ovarian granulosa cells (Xu et al., 2002). The selected concentrations of BPA are relevant to current regulatory levels set for BPA. The lowest observable adverse effect level (LOAEL) is 50 mg/kg/day. This equates to 210.6μM. The doses used in the experiments were 4.4, 44, and 440μM (or 1, 10, and 100 μg/ml), encompassing the LOAEL concentration.
5-Pregnen-3β-ol-20-one (Pregnenolone; pregn) powder was purchased from Sigma-Aldrich. A stock solution of pregn was prepared in DMSO for a final concentration in culture of 10 μg/ml.
Animals.
Adult, cycling female FVB mice were purchased from Jackson Laboratory (Bar Harbor, ME) and allowed to acclimate to the facility for at least 5 days before use. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility. Food (Harlan Teklad 8626) and water were provided for ad libitum consumption. Temperature was maintained at 22 ± 1°C, and animals were subjected to 12-h light-dark cycles. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
In vitro follicle culture.
Female FVB mice were euthanized on postnatal day 32 and their ovaries removed using aseptic technique. Antral follicles were mechanically isolated from the ovary based on relative size (250–400 μm), cleaned of interstitial tissue using fine watchmaker forceps (Gupta et al., 2006; Miller et al., 2005), and individually placed in wells of a 96-well culture plate and covered with unsupplemented α-minimal essential medium (α-MEM) prior to treatment. Sufficient numbers of antral follicles for statistical power were isolated from unprimed mouse ovaries; follicles from two to three mice were isolated per experiment providing approximately 20–40 antral follicles from each mouse. Each experiment contained a minimum of 8–16 follicles per treatment group. Doses of vehicle control (DMSO), BPA (4.4, 44, and 440μM), pregn (10 μg/ml), pregn + BPA (44μM), and pregn + BPA (440μM) were individually prepared in supplemented α-MEM. Supplemented α-MEM was prepared with: 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, and 5.5 ng/ml selenium), 100 U/ml penicillin, 100 mg/ml streptomycin, 5 IU/ml human recombinant follicle-stimulating hormone (Dr A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, Torrance, CA), and 5% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA) (Gupta et al., 2006; Miller et al., 2005). An equal volume of chemical was added for each dose to control for the amount of vehicle in each preparation (0.75 μl/ml of media: BPA treatments; 1.0 μl/ml of media: DMSO and pregn treatments). Antral follicles were cultured for 120 h in an incubator supplying 5% CO2 at 37°C.
Analysis of follicle growth.
Follicle growth was examined at 24-h intervals by measuring follicle diameter on perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. Follicle diameter measurements were averaged among treatment groups and plotted to compare the effects of chemical treatments on growth over time. Data were presented as percent change over time.
Analysis of hormone levels.
Media was collected after 120 h of follicle culture and subjected to ELISA for measurement of estradiol, estrone, testosterone, androstenedione, dehydroepiandrosterone sulfate (DHEA-S), and progesterone levels. ELISA kits and reagents were obtained from ALPCO Diagnostics (estrone, testosterone, androstenedione, progesterone, and DHEA-S) and Diagnostics Research Group (estradiol). The assays were run using the manufacturer’s instructions. All samples were run in duplicate and all intra- and interassay coefficients of variability were less than 10%.
Analysis of quantitative real-time PCR.
Female FVB mouse antral follicles were cultured as describedabove for 120 h. At the end of culture, follicles were collected and snap frozen at −80°C for quantitative real-time PCR (qPCR) analysis. Total RNA was extracted from follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. Reverse transcriptase generation of complementary DNA (cDNA) was performed with 0.3–1 μg of total RNA using an iScript RT Kit (Bio-Rad Laboratories, Inc., Hercules, CA). qPCR was conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) and accompanying software (CFX Manager Software) according to the manufacturer’s instructions. The CFX96 quantifies the amount of PCR product generated by measuring a dye (SYBR Green) that fluoresces when bound to double-stranded DNA. A standard curve was generated from five serial dilutions of one of the samples, thus allowing analysis of the amount of cDNA in the exponential phase. Specific qPCR primers for the genes of interest and annealing temperatures are listed in Table 1, along with GenBank accession numbers (Akingbemi et al., 2003; Hernandez-Ochoa et al., 2010; Pakarainen et al., 2005; Sha et al., 1996; Weihua et al., 2000). qPCR analysis was performed using 2 μl cDNA, forward and reverse primers (5 pmol) for steroidogenic acute regulatory protein (StAR), cytochrome P450 side-chain cleavage (P450scc), 3β-hydroxysteroid dehydrogenase (3β-HSD), 17β-hydroxysteroid dehydrogenase (17β-HSD), cytochrome P450 aromatase (Cyp19), cytochrome P450 17 α-hydroxylase/17,20 lyase (Cyp17α), or β-actin, in conjunction with a SsoFast EvaGreen Supermix qPCR Kit (Bio-Rad Laboratories). An initial incubation of 95°C for 10 min was followed by denaturing at 94°C for 10 s, annealing from 56 to 61°C for 10 s, and extension at 72°C for 10 s, for 40 cycles (β-actin), followed by final extension at 72°C for 10 min. A melting curve was generated at 55–90°C to monitor the generation of a single product. The software also generated a standard curve. β-Actin was used as reference gene for each sample. Final values were calculated and expressed as the ratio normalized to β-actin. All analyses were performed in duplicate for at least three separate experiments.
TABLE 1.
Primers used in Quantitative Real-Time Polymerase Chain Reactions (qPCR)
| Gene name | Gene symbol | Primer sequences |
Annealing temperature | GenBank accession # | |
| Forward | Reverse | ||||
| Beta-actin | Actb | F: GGGCACAGTGTGGGTGAC | R: CTGGCACCACACCTTCTAC | 56°C | NM_007393 |
| Steroidogenic acute regulatory protein | StAR | F: CAGGGAGAGGTGGCTATGCA | R: CCGTGTCTTTTCCAATCCTCTG | 57°C | NM_007810 |
| Cytochrome P450 cholesterol side-chain cleavage | Cyp11a1 | F: AGATCCCTTCCCCTGGCGACAATG | R: CGCATGAGAAGAGTATCGACGCATC | 60°C | NM_008293 |
| 3b-Hydroxysteroid dehydrogenase 1 | HSD3b1 | F: CAGGAGAAAGAACTGCAGGAGGTC | R: GCACACTTGCTTGAACACAGGC | 59.5°C | NM_008293 |
| Cytochrome P450 aromatase | Cyp19a1 | F: CATGGTCCCGCAAACTGTGA | R: GTAGTAGTTGCAGGCACTTC | 56°C | NM_007810 |
| Cytochrome P450 steroid 17-a-hydroxylase 1 | Cyp17a1 | F: CCAGGACCCAAGTGTGTTCT | R: CCTGATACGAAGCACTTCTCG | 56°C | NM_007809 |
| 17B-hydroxysteroid dehydrogenase 1 | HSD17b1 | F: ACTGTGCCAGCAAGTTTGCG | R: AAGCGGTTCGTGGAGAAGTAG | 58°C | NM_010475 |
Statistical analysis.
Data were expressed as means ± SEM, and multiple comparisons between experimental groups were made using ANOVA followed by Tukey’s post hoc comparison. Tests for trend were analyzed using linear regression analyses for the overall effect of BPA concentration (continuous variable). At least three separate experiments were conducted for each treatment prior to data analysis. Statistical significance was assigned at p ≤ 0.05.
RESULTS
Effect of BPA on Follicle Growth
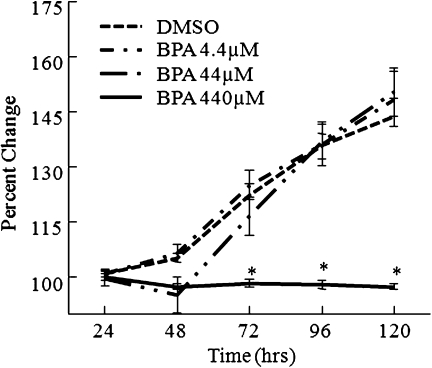
Exposure to BPA (440μM) significantly decreased antral follicle growth compared with DMSO controls beginning at 72 h, and this decrease in follicle growth remained throughout the 120-h culture (Fig. 1). No significant differences in follicle growth were observed between follicles exposed to DMSO, BPA (44μM), or BPA (4.4μM).
FIG. 1.
Effect of BPA exposure of FVB mice antral follicle growth. Antral follicles were mechanically isolated from FVB mice and exposed in vitro to BPA (4.4–440μM) for 120 h. Growth of follicles was monitored during culture and recorded in micrometers and reported as percent change over time. The graph represents means ± SEMs from at least three separate experiments. Line with asterisk (*) is significantly different from controls (n = 8–16 follicles per treatment per experiment from at least three separate experiments; p ≤ 0.05).
Effect of BPA on Sex Steroid Hormone Production
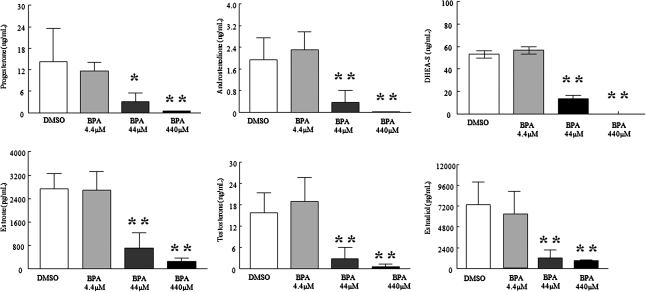
BPA (440μM) exposure for 120 h significantly decreased estradiol, estrone, testosterone, androstenedione, DHEA-S, and progesterone levels produced by the follicles compared with DMSO (Fig. 2). BPA (44μM) exposure for 120 h significantly decreased estradiol, estrone, testosterone, androstenedione, DHEA-S, and progesterone levels produced by the follicles. No significant differences in steroid levels were found between DMSO and 4.4μM BPA.
FIG. 2.
Effect of BPA exposure on antral follicle hormone production. After exposure of antral follicles to DMSO control or BPA (4.4–440μM) for 120 h in vitro, media was collected and subjected to various hormone measurements by ELISA. These graphs represent the means ± SEMs from separate experiments. A single asterisk (*) denotes a significant p value for overall effect of BPA concentration as the continuous variable using linear regression. A double asterisk (**) denotes a significant p value from the DMSO control via ANOVA, post hoc Tukey’s Honestly Significant Difference (test) (n = 3–4; p ≤ 0.05).
Effect of BPA on Gene Expression
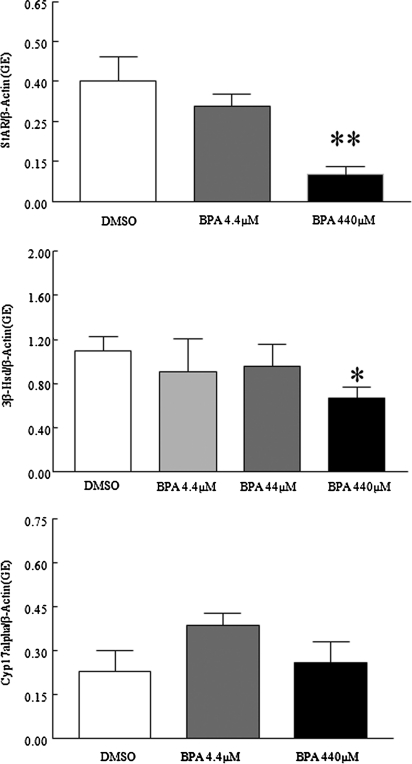
Because BPA decreased steroidogenesis in the cultured antral follicles, studies were conducted to see if it did so by decreasing the expression of enzymes required for steroidogenesis. Specifically, levels of steroidogenic enzymes StAR, 3β-HSD, and Cyp17α were compared in control and BPA-treated follicles. Only StAR messenger RNA (mRNA) expression levels were significantly decreased following exposure to BPA44μM and BPA440μM compared with DMSO controls (Figs. 3 and 6; BPA-only treated follicles). Exposure to BPA440μM, however, resulted in a significant trend for decreased 3β-HSD expression compared with controls. Furthermore, BPA44μM and BPA440μM significantly decreased P450scc mRNA expression levels compared with DMSO controls (Fig. 6; BPA-only treated follicles).
FIG. 3.
Effect of BPA exposure on StAR, 3β-HSD, and Cyp17α mRNA expression levels. After exposure of antral follicles to DMSO control of BPA (4.4–440μM) for 120 h in vitro, the follicles were collected and subjected to qPCR analysis for StAR, 3β-HSD, and Cyp17α mRNA expression levels. All values were normalized to β-actin as a loading control. Graph represents means ± SEMs from separate experiments. A single asterisk (*) denotes a significant p value for overall effect of BPA concentration as the continuous variable using linear regression. A double asterisk (**) denotes a significant p value from the DMSO control via ANOVA, post hoc Tukey’s HSD (n = 8–16 follicles per treatment per experiment from three separate experiments; p ≤ 0.05).
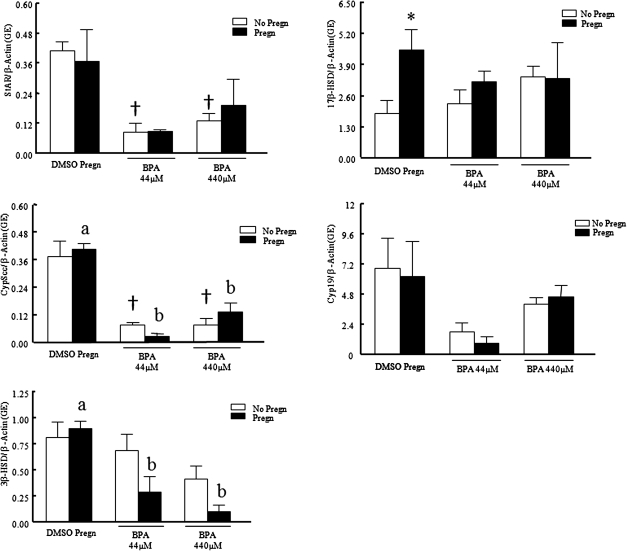
FIG. 6.
Effect of pregnenolone cotreatment with BPA on steroidogenic enzyme mRNA expression levels. After exposure of antral follicles to DMSO control, BPA (4.4–440μM) pregnenolone (10 μg/ml) or pregnenolone, and BPA (44–440μM) for 120 h in vitro, the follicles were collected and subjected to qPCR analysis for StAR, P450scc, 3β-HSD, Cyp19, and 17β-HSD mRNA expression levels. All values were normalized to β-actin as a loading control. Graph represents means ± SEMs from separate experiments. Asterisks indicate a significant difference between no pregnenolone and pregnenolone groups; symbols (†) indicate a significant difference between BPA and control groups without pregnenolone; letters indicate a significant difference between BPA and control groups with pregnenolone groups (n = 3–4; p ≤ 0.05).
Effect of Pregnenolone Cotreatment on BPA-Treated Follicles
The BPA-induced inhibition of steroidogenesis and follicle growth could be the result of decreased StAR and P450scc levels in the follicles because these are rate-limiting enzymes for the estradiol biosynthesis pathway. Because addition of pregnenolone bypasses this disrupted enzyme, studies were conducted to investigate whether cotreatment of follicles with pregnenolone and BPA would protect follicles from the toxic effects of BPA on follicle growth and steroidogenesis.
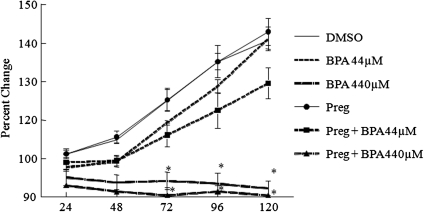
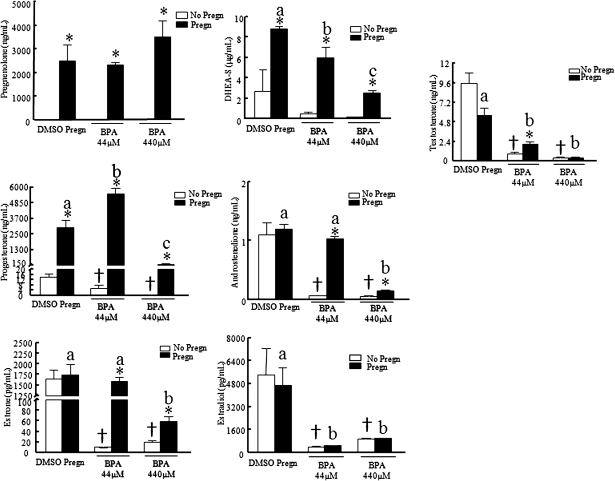
Addition of pregnenolone to the supplemented media did not protect follicles from BPA-induced follicle growth inhibition (Fig. 4). However, cotreatment of follicles with pregnenolone and BPA did provide some protection against inhibition of steroidogenesis (Fig. 5). Pregnenolone cotreatment with BPA increased pregnenolone, progesterone, and DHEA-S levels compared with DMSO controls and maintained androstenedione and estrone levels similar to DMSO controls. Pregnenolone cotreatment with BPA, however, did not increase levels of testosterone and estradiol production compared with DMSO controls. Specifically, progesterone levels were 2.9 ± 0.4 ng/ml with BPA44μM only and 14.1 ± 2.5 ng/ml with DMSO only, but progesterone levels were 5502.1 ± 387.2 ng/ml with pregn + BPA44μM. Androstenedione levels were 0.07 ± 0.003 ng/mL with BPA44μM only and 1.1 ± 0.2 ng/ml with DMSO only, but androstenedione levels were 1.0 ± 0.04 ng/ml with pregn + BPA44μM. Estradiol levels were 378.1 ± 54.5 pg/ml with BPA44μM only, 5417.8 ± 1849.9 pg/ml with DMSO only, and 465.6 ± 11.2 pg/ml with pregn + BPA44μM.
FIG. 4.
Effect of pregnenolone cotreatment with BPA on antral follicle growth. Antral follicles were mechanically isolated from FVB mice and exposed in vitro to BPA (4.4–440μM), pregnenolone (10 μg/ml) or pregnenolone, and BPA (44–440μM) for 120 h. Growth of follicles was monitored during culture and recorded in micrometers and reported as percent change. The graph represents means ± SEMs from at least three separate experiments. Lines with asterisks (*) are significantly different from DMSO controls (n = 8–16 follicles per treatment per experiment from at least three separate experiments; p ≤ 0.05).
FIG. 5.
Effect of pregnenolone cotreatment with BPA on antral follicle hormone production. After exposure of antral follicles to DMSO control, BPA (4.4–440μM) pregnenolone (10 μg/ml) or pregnenolone, and BPA (44–440μM) for 120 h in vitro, media was collected and subjected to various hormone measurements by ELISA. These graphs represent the means ± SEMs from separate experiments. Asterisks (*) indicate a significant difference between no pregnenolone and pregnenolone groups; symbols (†) indicate a significant difference between BPA and control groups without pregnenolone; letters indicate a significant difference between BPA and control groups with pregnenolone groups (n = 3–4; p ≤ 0.05).
Effect of Pregnenolone + BPA on Gene Expression
Because levels of pregnenolone, DHEA-S, progesterone, androstenedione, and estrone were protected from BPA-induced inhibition in the pregnenolone-treated groups compared with their nonpregnenolone counterparts, gene expression levels of the enzymes responsible for their metabolism along the biosynthesis pathway were measured. Addition of pregnenolone treatment did not protect the follicles from BPA-induced alterations in steroidogenic enzyme gene expression (Fig. 6). Specifically, StAR mRNA expression levels were 0.09 ± 0.04 genomic equivalents (ge) with BPA44μM only and 0.41 ± 0.04 ge with DMSO, but StAR mRNA expression levels were 0.09 ± 0.006 ge with pregn + BPA44μM. P450scc mRNA expression levels were 0.08 ± 0.01 ge with BPA44μM only and 0.37 ± 0.07 ge with DMSO only, but P450scc mRNA expression levels were 0.03 ± 0.13 ge with pregn + BPA44μM.
DISCUSSION
Using an in vitro follicle culture system, we have shown that BPA inhibits follicle growth and decreases hormone production in mouse ovarian antral follicles. Furthermore, although cotreatment with pregnenolone did not protect follicles from BPA-induced inhibition of follicle growth, it partially protected follicles from BPA-induced inhibition of steroidogenesis.
Sex steroid hormone production is required for proper development of antral follicles (Cain et al., 1995). These hormones produced in the ovary have an autocrine effect on the growth and development of follicles. Estrogens, such as estradiol, stimulate follicle growth and protect the follicle from atresia (Quirk et al., 2004). Progestins, such as progesterone, have an inhibitory effect and induce atresia, although the specific actions of progesterone on the follicle are not currently well understood (Fukuda et al., 1980). Androgens, such as androstenedione, testosterone, and dehydrotestosterone, have been reported to both stimulate follicle growth and induce atresia in antral follicles (Drummond, 2006).
Hormone production is dependent on the components of the estradiol biosynthesis pathway. More specifically, production of downstream hormones in the pathway, such as estradiol, is dependent on the availability of upstream hormones, such as progestins and androgens. All the hormones within the biosynthesis pathway are dependent on the availability of the lipid precursor, cholesterol. Furthermore, the rate-limiting step of the estradiol biosynthesis pathway is the transport of cholesterol by StAR from lipid droplets in the theca cells into the inner matrix of the mitochondria. Therefore, without this transport, cholesterol cannot be converted to pregnenolone via P450scc, and further hormone metabolism and production in the antral follicles is inhibited. Our results suggest that both StAR and P450scc mRNA expression are inhibited following exposure to BPA. This would prevent cholesterol uptake into the mitochondria and metabolism to pregnenolone, explaining the decrease in hormone production following exposure to BPA in our study.
To try and bypass these enzymes, we added pregnenolone to the culture. We hypothesized that the pregnenolone addition would protect the follicles from inhibited growth and hormone production. Exposing the follicles concurrently to pregnenolone and BPA did not prevent inhibition of follicle growth but protected hormone production in the theca cells, although not necessarily in the granulosa cells. These data support our finding that StAR and P450scc mRNA levels are inhibited by BPA. Thus, cholesterol uptake into the mitochondria and metabolism to pregnenolone is inhibited, impairing hormone production in the antral follicles. Without adequate hormone levels, late antral follicle growth and ovulation will be impaired, as well as further hormone production within the follicles, thus affecting fertility.
Our results support previous work, indicating that BPA exposure affects progesterone production using porcine granulosa cells incubated for 24–72 h, although this previous work was done in isolated granulosa cells not the whole follicle as in our study (Mlynarcikova et al., 2005). Another study using isolated granulosa and theca-interstitial cells investigated hormone production from gonadotropin-primed follicular cells. This study found exposure to BPA between 10−7 and 10−5M increased StAR, P450scc, and 3β-HSD mRNA and protein levels and increased testosterone and progesterone production, but it decreased estradiol levels and Cyp19 mRNA expression levels (Zhou et al., 2008). Our results suggest the opposite, indicating BPA exposure decreases StAR and P450scc mRNA levels and decreases testosterone and progesterone production. The reasons for the different results from our study and the previous study could stem from species differences. We used mice, whereas the other study used rats. Furthermore, differences in results could stem from the fact that we used isolated, intact whole follicles, the functional unit of the ovary, whereas the previous study used isolated granulosa and theca-interstitial cells.
Our data indicate that BPA affects steroidogenesis in the granulosa and theca cells, beginning by downregulating StAR and later, affecting granulosa-theca cell communication including hormone diffusion from the theca into the granulosa cells. This could be why pregnenolone cotreatment restores hormone levels in the theca to at least DMSO control levels following exposure to BPA, but why hormones levels in the granulosa cells, such as testosterone and estradiol, are still decreased compared with DMSO controls. Malfunctioning granulosa cells could lead to impaired follicle growth in antral follicles because the proliferation of these cells is responsible for the increasing size of the developing follicles. Furthermore, hormone production could be impaired because the granulosa cells are the only cells in the antral follicle able to aromatize androgens produced in the theca into estrogens (Drummond, 2006).
In conclusion, this study shows that the estradiol biosynthetic pathway in the ovary is a possible target for BPA action in the mouse. Impaired steroid biosynthesis could affect functioning of the endocrine system throughout the body, meriting further research in animal models. Furthermore, as the mouse endocrine system is similar to the human in many regards, further research in human models is also warranted.
FUNDING
National Institute of Health (R01 ES019178, P20 ES 018163).
Acknowledgments
The authors would like to acknowledge the members of the Flaws’ lab for their support and input throughout the project.
References
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144:84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- Cain L, Chatterjee S, Collins TJ. In vitro folliculogenesis of rat preantral follicles. Endocrinology. 1995;136:3369–3377. doi: 10.1210/endo.136.8.7628372. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Semiz O, Cinar O. Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol. Hum. Reprod. 2005;11:389–396. doi: 10.1093/molehr/gah179. [DOI] [PubMed] [Google Scholar]
- Drummond AE. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 2006;4:16–26. doi: 10.1186/1477-7827-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Vogt E, Cukurcam L, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat. Res. 2008;651:82–92. doi: 10.1016/j.mrgentox.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Katayama K, Tojo S. Inhibitory effect of progesterone on follicular growth and induced superovulation in the rat. Arch. Gynecol. 1980;230:77–87. doi: 10.1007/BF02108599. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol. Sci. 2006;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Hartshorne GM. In vitro culture of ovarian follicles. Rev. Reprod. 1997;2:94–104. doi: 10.1530/ror.0.0020094. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ochoa I, Barnett-Ringgold KR, Dehlinger SL, Gupta RK, Leslie TC, Roby KF, Flaws JA. The ability of the aryl hydrocarbon receptor to regulate ovarian follicle growth and estradiol biosynthesis in mice depends on stage of sexual maturity. Biol. Reprod. 2010;83:698–706. doi: 10.1095/biolreprod.110.087015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma A, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstibestrol on female mouse reproduction. Reprod. Toxicol. 2002;16:122. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Hunt P, Koehler K, Susiarjo M, Hodges C, Ilagan A, Voigt R, Thomas S, Thomas B, Hassold T. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Kang J, Katayama Y, Kondo F. Biodegradation or metabolism of bisphenol A: from microorganisms to mammals. Toxicology. 2006;217:81–90. doi: 10.1016/j.tox.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lee YM, Seong MJ, Lee JW, Lee YK, Kim TM, Nam SY, Kim DJ, Yun YW, Kim TS, Han SY, et al. Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen. J. Vet. Sci. 2007;8:27–38. doi: 10.4142/jvs.2007.8.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat. Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfield CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol. Sci. 2005;88:213–221. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- Mlynarcikova A, Kolena J, Fickova M, Scsukova S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol. Cell. Endocrinol. 2005;244:57–62. doi: 10.1016/j.mce.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Mlynarcikova A, Nagyova E, Fickova M, Scsukova S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicology. 2009;23:371–377. doi: 10.1016/j.tiv.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007;24:253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi K, Watanabe H. Measurement of bisphenol A in human urine using liquid chromatography with multi-channel coulometric electrochemical detection. J. Chromatogr. B. 2002;780:365–370. doi: 10.1016/s1570-0232(02)00547-0. [DOI] [PubMed] [Google Scholar]
- Pakarainen T, Zhang FP, Nurmi L, Poutanen M, Huhtaniemi I. Knockout of luteinizing hormone receptor abolishes the effects of follicle-stimulating hormone on preovulatory maturation and ovulation of mouse graafian follicles. Mol. Endocrinol. 2005;19:2591–2602. doi: 10.1210/me.2005-0075. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J. Anim. Sci. 2004;82:E40–E52. doi: 10.2527/2004.8213_supplE40x. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Sha J, Baker P, O'Shaughnessy PJ. Both reductive forms of 17 beta-hydroxysteroid dehydrogenase (types 1 and 3) are expressed during development in the mouse testis. Biochem. Biophys. Res. Commun. 1996;222:90–94. doi: 10.1006/bbrc.1996.0702. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillet T. Bisphenol A, chapter 2: new data shed light on exposure, potential bioaccumulation. Environews. 2009;117:A210. doi: 10.1289/ehp.117-a210b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–932. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72:124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5936–5941. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, Takai Y, Matsumi H, Koga K, Taketani Y, et al. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem. Biophys. Res. Commun. 2002;292:456–462. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008;283:12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]