Abstract
Effective prediction of future viral zoonoses requires an in-depth understanding of the heterologous viral population in key animal species that will likely serve as reservoir hosts or intermediates during the next viral epidemic. The importance of bats as natural hosts for several important viral zoonoses, including Ebola, Marburg, Nipah, Hendra, and rabies viruses and severe acute respiratory syndrome-coronavirus (SARS-CoV), has been established; however, the large viral population diversity (virome) of bats has been partially determined for only a few of the ∼1,200 bat species. To assess the virome of North American bats, we collected fecal, oral, urine, and tissue samples from individual bats captured at an abandoned railroad tunnel in Maryland that is cohabitated by 7 to 10 different bat species. Here, we present preliminary characterization of the virome of three common North American bat species, including big brown bats (Eptesicus fuscus), tricolored bats (Perimyotis subflavus), and little brown myotis (Myotis lucifugus). In samples derived from these bats, we identified viral sequences that were similar to at least three novel group 1 CoVs, large numbers of insect and plant virus sequences, and nearly full-length genomic sequences of two novel bacteriophages. These observations suggest that bats encounter and disseminate a large assortment of viruses capable of infecting many different animals, insects, and plants in nature.
The sum total of all viruses found in a species is called its virome (4), and in recent years, next-generation sequencing methodologies have been employed in a number of metagenomics studies designed to assess the viromes of different animal and plant species (41, 45, 47, 53, 67, 80, 81, 88, 89). In several cases, novel viruses were detected in many types of samples, including human blood (10), human diarrhea (29, 30, 36, 46, 58), human respiratory secretions (2, 3, 33, 88), equine feces (15), grapevines (20), and, most recently, bat guano (47). These studies have demonstrated that numerous viruses are naturally present in a given species and provide further evidence that many viral epidemics occur as the result of a cross-species transmission of viruses from one species to another.
Monitoring of animals harvested as bushmeat in sub-Saharan Africa has revealed that zoonotic viruses frequently spill over into the human populations that hunt, butcher, or manage the animals; and these results have provided significant insights into origins, geographic distributions, temperate limitations, and emergence potential of several important human pathogens (90). These studies, and others like them, have demonstrated that viruses can be relatively benign in a given host but have devastating consequences when they emerge into human populations. Several recent human epidemics have resulted from viruses that originated in zoonotic reservoirs, including the following: human immunodeficiency virus (HIV), which probably emerged from chimpanzees (32, 39, 54); H1N1 of 2009/2010, which likely originated in pigs and birds (5, 31, 71); and the severe acute respiratory syndrome coronavirus (SARS-CoV), which likely originated from the Chinese horseshoe bat (43, 64). Further studies assessing the worldwide distribution of bat coronavirus (BtCoV) has identified several novel CoVs in a variety of bat species (9, 16, 18, 26, 34, 43, 44, 50, 55, 57, 64, 74, 77, 91, 95). In fact, BtCoV sequences have greatly expanded CoV phylogeny from the three traditional groups known as group 1 (alphacoronaviruses; including human CoVs such as HCoV-229E and HCoV-NL63), group 2 (betacoronaviruses; including human CoVs such as SARS-CoV and HCoV-OC43), and group 3 (gammacoronaviruses; including avian CoVs).
In addition to CoVs, 12 of the 28 (∼43%) emerging viruses included on the NIAID list of emerging and reemerging pathogens in biodefense categories A to C (http://www.niaid.nih.gov/topics/emerging/pages/list.aspx) have been found in samples derived from multiple species of bats (13). Moreover, several other virus genera have been identified in bat samples, including coronaviruses, alphaviruses, lyssaviruses, rubulaviruses, flaviviruses, bunyaviruses, polyomaviruses, phleboviruses, oribiviruses, and orthoreoviruses, as well as uncharacterized viruses of the Arenaviridae, Picornaviridae, and Herpesviridae families (13). Given the depth of viral richness observed in bats, surprisingly little research has been conducted to determine which viruses are specific to bats, which viruses persistently infect different bat species, and which viruses are trafficked from one population to another with eventual dissemination to different species or reservoir hosts (12, 56, 75, 96). The one bat virome study published to date focused on bat guano obtained from a mixture of bat species in the southern United States (47).
Bats comprise the second largest order of mammals, with approximately 1,200 species making up nearly 25% of the ∼5,000 species in the class Mammalia (78, 79). Bats are further classified in the order Chiroptera, which is subdivided into two suborders: the Yinpterochiroptera (formerly Megachiroptera) which contains the megabats, and the Yangochiroptera (formerly Microchiroptera), which includes the majority of microbat families (13, 78, 79). Bat species occur on every continent, with the exception of Antarctica, and some microbat species migrate up to 1,287 km. In addition, the microbats are insectivorous, exhibit exceptionally long life spans of 25 to 35 years, and live in panmictic populations often comprised of millions of bats in densely packaged aggregates of greater than 300 bats per square foot (13).
In eastern North America, the most common microbats are from the family Vespertilionidae, genus Myotis, although big brown bats (Eptesicus fuscus) are the most commonly captured species during trapping and hibernacula surveys in Maryland (61). In addition, tricolored bats (Perimyotis subflavus) (61) and little brown myotis (Myotis lucifugus) are frequently captured from tunnels and caves during hibernaculum surveys in the state (6). Samples for this study were obtained by capturing individual bats at Indigo tunnel, which is an abandoned railroad tunnel located within the Ridge and Valley physiographic province in the Chesapeake and Ohio Canal National Historical Park near Little Orleans, Allegany County, MD. The tunnel is cohabitated by 7 to 10 different bat species. In 2009, samples were collected from seven different bat species during the summer roosting and fall swarm.
In this paper, we describe a preliminary study that focused on samples from 41 bats captured on a single sampling night, representing three bat species, including big brown bats, tricolored bats, and the little brown myotis. Fecal and oral samples were processed and grouped into six pools; viral RNA and DNA were extracted, reverse transcribed, bar coded, and amplified; and the cDNA was analyzed by 454 sequencing to assess the viral sequences present in each. Phylogenetic analyses demonstrated that an abundance of bacteriophage, plant, and insect viruses were present in all fecal samples. In addition, three fecal sample pools contained strong matches to at least three novel group 1 CoVs, and a single pool comprised of viral sequences extracted from oral swab samples contained multiple hits to novel betaherpesviruses.
MATERIALS AND METHODS
Study area.
Bat samples for this study were collected from the northeast entrance of Indigo tunnel located near Little Orleans, MD (GPS coordinates: easting 726475; northing 4391037). The tunnel was originally constructed in 1904 and was used primarily by the Western Maryland Railway. Use of the tunnel was discontinued in 1975 although the structure remains largely intact.
Bat sampling.
To capture the bats, two forest strainer harp traps (1.8 m by 2.3 m; Bat Conservation and Management, Carlisle, PA) were placed side by side in the tunnel entrance to catch bats entering or exiting the tunnel. The harp traps were completely surrounded with tarpaulin and bird netting to prevent bats from bypassing the traps. Captured bats were retrieved from the harp traps and held in paper sacks secured to a rope line for 10 to 15 min, allowing enough time for the excretion of fresh fecal boluses.
Sterile forceps were used to retrieve fecal boluses from the paper sacks, and these were placed into tubes containing 1 ml of universal transport medium (UTM; Copan Diagnostics, Inc., Murrieta, CA). We used miniature flocked swabs to acquire oral samples by placing the swab into the mouth cavity of each bat and gently rubbing the mucosa. Oral swabs containing bat saliva were immediately placed in 2 ml of UTM (Copan Diagnostics, Inc., Murrieta, CA) (26). All vials were labeled with a unique identifier for each bat, based upon the date (e.g., 12/Aug/2010 or Aug/12/2010), location, bat species, sex, reproductive condition, and age. Samples were stored in a cooler on ice while in the field and placed in storage at 4°C at the University of Maryland Center for Environmental Science (UMCES) Appalachian Laboratory. Each captured bat was also assessed to determine species, weight (g), forearm length (mm), wing score, sex, reproductive state, and age (49, 60). All captured bats were marked by hair clipping to facilitate identification of recaptures. Once the data were collected, the bat was released.
Precautions taken to prevent the spread of white nose syndrome.
Since white-nose syndrome (WNS), a deadly malady of hibernating bats, had been reported in hibernacula in nearby Pennsylvania, West Virginia, and Virginia, the sampling crew followed standard disinfection protocols based on recommendations put forth by the U.S. Fish and Wildlife Service in April 2008. The harp traps, holding bags, nets, and boots were sterilized at the end of each night with a 10% bleach solution. In addition, holding bags, boots, and leather gloves were also washed at the end of each week. Latex gloves were worn when bats were handled, and a new pair was used for each individual bat. All objects that each bat came in contact with were disposed of or immediately sterilized with a 10% bleach solution. A wing damage index was used to assess the wing condition of bats possibly affected by WNS (63). The presence of scar or necrotic tissue, flaking and/or dehydrated skin, and wing tears or other abnormalities was recorded and photographed.
Sample processing.
The fecal samples were vortexed to completely resuspend the fecal material into solution, and 1 ml of Hank's balanced salt solution (HBSS; Gibco, Auckland, New Zealand) was added to each sample, and samples were further vortexed to create a less viscous solution. The samples were then centrifuged at 10,000 × g using a benchtop microcentrifuge for 2 min, and supernatants were transferred to a fresh tube. This step was repeated twice. Samples were then pooled by species, sex, age, or sample type by adding 1 ml of each sample into the appropriately pooled sample tube. The remaining supernatants for each sample were divided into two tubes and stored at −80°C. The six pooled samples were then placed in a 10-ml syringes, and each pool was filtered through a 0.45-μm-pore-size syringe filter (Fisher Scientific, Pittsburgh, PA). The filtered supernatants were then brought up to volume using HBSS to fill Beckman SW41 Ultracentrifuge tubes (Beckman Coulter, Inc., Brea, CA) to capacity. Tubes were weighed, and HBSS was added such that all tubes weighed exactly the same. The samples were then centrifuged at 50,000 × g for 3 h at 10°C. Supernatants were poured into 10% bleach solution, and the pellet was resuspended in 100 μl of HBSS and frozen at −80°C until further processing could be performed.
Nuclease treatment and RNA extraction.
To reduce the amount of contaminating RNA and DNA present, each sample (116 μl) was treated with 14 U of DNase Turbo (Ambion, Austin, TX), 25 U of benzonase (Novagen, Darmstadt, Germany), and 20 U of RNase One (Promega, Madison, WI); samples were brought up to a final volume of 140 μl in 10× DNase buffer (Ambion, Austin, TX) and incubated at 37°C for 2 h. Samples were then immediately processed with a QiaAmp Viral RNA Mini Kit (Qiagen, Valencia, CA) using the manufacturer's protocol to extract viral RNA and DNA that were protected from nuclease digestion by the viral capsid (1). Viral RNA and DNA were eluted to a final volume of 60 μl and stored at −80°C prior to use.
Reverse transcription and single primer amplification.
Primers that added unique multiplex identification tags (bar codes) were ordered using the sequence-independent single primer amplification (SISPA) protocol, which has been described previously (3, 24, 25). Briefly, the bar code primers were designed as random hexamers containing novel 22-nucleotide (nt) tags. The primers used in this study have been published elsewhere (3, 24, 25). The primers containing the random hexamer were used to prime the reverse transcription reaction, using one unique bar code primer for each of the six pooled samples. The same 22-nt tag was used without the random hexamer extension to prime the PCR amplification stage.
Reverse transcription.
Viral RNA was reverse transcribed using a SuperScript III reverse transcriptase kit (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Briefly, 50 to 200 ng of RNA from each of the pooled samples and one bar code primer containing the random hexamer (10 μM) were incubated in the presence of dimethyl sulfoxide (DMSO) at 72°C for 5 min and then placed on ice. Dithiothreitol (DTT; 100 mM), deoxynucleoside triphosphates (dNTPs; 10 mM), RNAse Out (8 U; Invitrogen, Carlsbad, CA), and SuperScript III reverse transcriptase (100 U) were then added along with 5× First Strand buffer to bring the final reaction volume to 20 μl. This volume was incubated at 25°C for 10 min, at 50°C for 50 min, and then at 85°C for 10 min to deactivate the reverse transcriptase.
Klenow reaction.
A Klenow reaction was then performed to convert the cDNA into double-stranded DNA (dsDNA) with interspersed sequences of the 22-nt on one DNA strand. For this reaction, a Klenow polymerase (2.5 U; New England Biolabs, Ipswich, MA) was added to the cDNA reaction mixture, which was then incubated at 37°C for 60 min, followed by 75°C for 10 min.
Phosphatase and exonuclease treatment.
Phosphatase and exonuclease were used to remove phosphates and single-stranded bases from the newly synthesized dsDNA by adding 1 U of shrimp alkaline phosphatase ([SAP] USB, Cleveland, OH) and 2 U of exonuclease (USB, Cleveland, OH) to the samples at the end of the Klenow reaction along with phosphatase buffer and water to a final volume of 50 μl. The reaction mixture was then incubated at 37°C for 60 min, followed by 72°C for 15 min.
PCR amplification and DNA extraction.
Amplification was conducted using an Accuprime PCR Kit (Invitrogen, Carlsbad, CA) to ensure primer binding to specific template sequences. The reaction mixture contained 10 μl of the sample from the SAP-Exo reaction mixture, the primer specific for the bar code without the random hexamer portion (12.5 μM), Accuprime Taq polymerase (1 U), 10× Accuprime buffer 1, and water to a final volume of 50 μl. This mixture was then amplified using the following cycling conditions: 94°C for 2 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 68°C for 1 min, with a final 10 min at by 68°C. The PCRs were then analyzed by gel electrophoresis on a 1% agarose gel, and a DNA smear ranging from ∼0.5 to 1 kb was excised and extracted using a QiaQuick Gel Extraction Kit (Qiagen, Valencia, CA). Purified DNA samples were submitted for sequencing to the University of North Carolina (UNC) High Throughput Sequencing facility using Roche 454 Life Sciences FLX Titanium chemistry (454 Life Sciences of Roche, Branford, CT). The DNA concentration was determined by spectrophotometry for each of the six pooled samples, and equal concentrations of each pool were combined into a single tube that was submitted to the facility. However, the sequencing facility determined concentration using a fluorometer and determined the concentration was too low, and additional pool 1 sample was added to bring the final concentration of all pooled samples to ∼1 μg.
Assembly of sequence reads.
The large sequence files generated by 454 pyrosequencing were converted from the SFF format to Fasta format using the GS De Novo Assembler software from Roche (454 Life Sciences of Roche, Branford, CT). UNIX and PERL scripts were used to segregate the bar-coded reads into individual bins, allowing for a 2-nucleotide truncation from the read ends. The program Codon Code Aligner (CodonCode Corporation, Dedham, MA) was used to trim the bar code sequences from the ends of the reads. Contigs were generated de novo using three methods: (i) Codon Code Aligner (CodonCode Corporation, Dedham, MA) using default settings and running multiple iterations, (ii) Geneious (version 3.0; A. Drummond, J., B. Ashton, M. Cheung, J. Heled, M. Kearse, R. Moir, S. Stones-Havas, T. Thierer, and A. Wilson, Biomatters, Auckland, New Zealand) using the high-sensitivity assembly method with default settings, and (iii) DNAstar (DNASTAR, Inc., Madison, WI) using the NGEN assembler with default settings. Contigs analyzed in this study were manually corrected, and overlaps between reads were assessed to verify proper assembly by visualizing the assemblies and generating consensus sequences by removing spurious single nucleotide insertions that were likely sequence errors. Single base insertions that occurred a single time in a given column were removed, provided the inserted base was not present in the reference genome or in any other sequence reads. All contigs described in this report contained complete agreement across all read junctions.
Annotation of contigs.
Assembled contigs were then assessed for identity using a stand-alone version of the Basic Local Alignment Search Tool (BLAST) (14) downloaded from the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) or using BLAST as implemented in the Geneious package (Drummond et al., Biomatters, Auckland, New Zealand). Briefly, contig sequences were individually used to query the nonredundant (nr) nucleotide database and the viral RefSeq database (59). Contigs characterized in this study were verified using both search types, with E values set to reduce the number of random matches. Since search integrity is highly dependent upon the database used, contigs were annotated against the viral RefSeq database to identify potential matches to known viruses, and then these were verified by searching the larger and more comprehensive nonredundant nucleotide database. As general criteria, E values less than 10e−04 and bit scores greater than 40 were the established thresholds as nearly all hits meeting these criteria were corroborated by searching both databases. BLAST searches were conducted at the nucleotide level using blastn and discontiguous MegaBLAST and at the amino acid level using blastx and tblastX to translate either the query sequence, the database, or both. Contigs matching the same viral sequences were binned together for genome assembly. Contigs of interest were manually refined using either Codon Code Aligner or Geneious to generate consensus sequences that contained few or no ambiguities. Viral genomes were downloaded for each of the viruses that matched most closely to any of the contigs, and these genomes were used as reference sequences to guide assembly of full-length genomes. Coronavirus reference sequences were downloaded from PATRIC (http://patric.vbi.vt.edu/) (72), and all other viral reference sequences were obtained from GenBank (http://www.ncbi.nlm.nih.gov/) (8).
Comparison of contigs.
Contigs with similarity to known viruses were compared to a reference set of viral genomes representing the diversity within the viral family and genus of the virus most closely related to the contig. Initially, genomic sequences and contigs were compared to determine the region of the viral genome that matched to the contig, and these regions were extracted from the reference viral genomes along with 200 flanking nucleotides on either end to allow for insertion and deletion variations. The contig sequence and sequences from the reference set were then compared by multiple sequence alignment using Clustal X, version 2.011 (42), and MUSCLE (27) as implemented in the Geneious package. Contigs from different pools were individually compared to the reference genomes, and if contigs from different pools matched the same region in the viral reference set, multiple alignments were conducted including all of the related contigs. All alignments were manually refined and trimmed to represent a robust alignment of the novel contigs and the known reference sequences.
Phylogenetic analysis.
Maximum-likelihood phylogenetic trees were generated using the PhyML program (37) as implemented in the Geneious package. All trees were generated using the MtREV substitution model with estimated transition/transversion ratios for DNA models and the proportion of invariable sites fixed at zero. Boot strapping was conducted generating 100 boostrapped data sets, and a consensus tree was generated using Consensus from the Phylip package (28). Trees were visualized in the Geneious tree viewer (Drummond et al., Biomatters, Auckland, New Zealand), and the Seaview tool (35) was used for editing and rearranging branches. Alignments and trees were generated using either nucleotide sequences, amino acid sequences, or both.
Cloning of novel group 1 CoV replicase fragment.
Primers were designed based upon 454 sequence reads that assembled to the BtCoV-HKU2 genome, and these primers were used to amplify a ∼2,540-nucleotide fragment of the novel group 1 CoV replicase open reading frame 1ab (ORF1ab). Briefly, forward primer CoV_16486F (5-GCGTATGTGTTGTCTTGGTC-3) and reverse primer CoV_19013R (5-ACTAACTGCAACCCCGTTAA-3) were used in an attempt to amplify cDNA templates from each of the eight individual samples from pool 1. Each PCR mixture contained 2 μl of cDNA (derived from reverse transcribing viral RNA extracted from each sample), forward and reverse primers (10 mM), dNTPs (10 mM), Accuprime Taq polymerase (1 U; Invitrogen, Carslbad, CA), 10× buffer 1, and water to a final volume of 50 μl. These mixtures were then amplified using the following cycling conditions: 94°C for 5 min, followed by 40 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 3 min, with a final 10 min at 72°C. PCR fragments were excised, purified using a Qiagen gel extraction kit (Qiagen, Valencia, CA), cloned into the TopoXL vector (Invitrogen, Carlsbad, CA), grown up in competent TOP10 cells, screened by restriction digestion, and sequenced via Sanger sequencing at the UNC Genome Analysis core facility.
Nucleotide sequence accession numbers.
GenBank accession numbers for CoV genes used in phylogenies are HQ585081 to HQ585086. GenBank accession numbers for all other CoV sequences are HQ585087 to HQ585105. Those for all other viral genomes are HQ585106 to HQ585116.
RESULTS
Sampling.
Sampling was conducted from 24 July through 29 September 2009, at Indigo tunnel, alternating between the northeast and the southeast entrances. A total of 512 samples were collected from seven species of bats during this time, including 305 fecal samples, 130 DNA samples, 46 oral swabs, and 24 urine samples (Table 1). Forty-one of these samples were selected for this initial screen, and these included fecal and oral samples from three species including big brown bats, tricolored bats, and little brown myotis. All of these samples were collected during the night of 12 August to 13 August, and samples were pooled based upon species, sex, age, and sample type (Table 2) and sequenced by the University of North Carolina High Throughput Sequencing facility using the 454 Roche FLX Titanium chemistry.
TABLE 1.
Individual bat fecal samples collected in 2009
| Species | Common name | No. of bat samples collected by sex and age |
|||||
|---|---|---|---|---|---|---|---|
| Male |
Female |
Undetermined sexa | Total | ||||
| Adult | Juvenile | Adult | Juvenile | ||||
| Eptesicus fuscus | Big brown bat | 36 | 35 | 25 | 33 | 0 | 129 |
| Lasiurus borealis | Hoary bat | 0 | 1 | 0 | 0 | 0 | 1 |
| Lasiurus cinereus | Eastern red Bat | 0 | 1 | 0 | 0 | 0 | 1 |
| Myotis leibii | Eastern small-footed myotis | 3 | 13 | 2 | 5 | 0 | 23 |
| Myotis lucifugus | Little brown myotis | 24 | 15 | 11 | 9 | 2 | 61 |
| Myotis septentrionalis | Northern long-eared myotis | 17 | 7 | 7 | 9 | 1 | 41 |
| Perimyotis subflavus | Tricolored bat | 15 | 26 | 3 | 5 | 0 | 49 |
| Total | 95 | 98 | 48 | 61 | 3 | 305 | |
Bat escaped before sex could be determined.
TABLE 2.
Species and pools used in this study
| Pool no. | Size (no. of samples) | Date (mo/day/yr) | Species | Common name | Sexa | Ageb | Sample type |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 8/12/09 | Eptesicus fuscus | Big brown bat | F | J | Fecal |
| 2 | 5 | 8/12/09 | Eptesicus fuscus | Big brown bat | M | A | Fecal |
| 3 | 9 | 8/12/09 | Eptesicus fuscus | Big brown bat | M | J | Fecal |
| 4 | 7 | 8/12/09 | Myotis lucifugus | Little brown myotis | Mix | Mix | Fecal |
| 5 | 4 | 8/12/09 | Perimyotis subflavus | Tricolored bat | M | J | Fecal |
| 6 | 8 | 8/12/09 | Eptesicus fuscus | Big brown bat | F | J | Oral |
F, female, M, male; mix, mixed pool of males and females.
J, juvenile; A, adult; mix, mixed pool of adults and juveniles.
Next-generation sequencing results.
A total of 611,745 sequence reads were generated that contained one of the six unique bar code sequences, and these were segregated into bins based upon these unique sequences; the bar code tags were trimmed from the ends, and sequences shorter than 50 nt were removed, resulting in a total of 576,274 trimmed reads (Table 3). The average size of each trimmed read was 256 nt. Contigs were assembled for each bin, and >87% of the reads were assembled into contigs, with pool 1 containing the largest number of sequence reads (308,026) and contigs (4,044) (Table 3). Of note, pool 1 contained 4-fold more sequence reads than any of the other bar-coded samples, due to the additional DNA added to bring the pooled sample up to the required concentration (see Materials and Methods).
TABLE 3.
Overview of sequence reads and contigs
| Pool no. | Read data |
No. of contigs | Avg no. of reads/contig | ||||
|---|---|---|---|---|---|---|---|
| Total no. | Avg length (nt) | No. assembled | No. unassembled | % Assembleda | |||
| 1 | 308,026 | 263 | 268,811 | 39,215 | 87 | 4,044 | 66 |
| 2 | 70,275 | 237 | 63,061 | 7,214 | 90 | 1,135 | 55 |
| 3 | 26,741 | 241 | 24,032 | 2,709 | 90 | 966 | 24 |
| 4 | 76,580 | 228 | 62,153 | 14,427 | 81 | 1,111 | 55 |
| 5 | 44,838 | 253 | 40,774 | 4,064 | 91 | 886 | 46 |
| 6 | 49,814 | 237 | 44,653 | 5,161 | 90 | 641 | 69 |
| Total | 576,274 | 256 | 503,484 | 72,790 | 87 | 8,783 | 52.5 |
Rounded to the nearest whole number.
Initial characterization of the sequence reads.
Each bar code bin was formatted as a BLAST database, and the viral RefSeq database from NCBI containing all known viruses was used as input query to search each bar code pool database running tblastx on a local version of stand-alone BLAST (E-value cutoff of 10e−04). This strategy identified matches to known viruses, and a total of roughly 45,000 sequence reads from all pools (7 to 8%) contained sequence similarity to a variety of different viruses in the RefSeq database (Fig. 1) although many of these hits represented large numbers of reads that matched to one specific virus or viral gene. However, this preliminary assessment demonstrated that the virome of bats varies by species, age, and sample type (Fig. 1).
FIG. 1.
Distribution of sequence read matches by pool. The 576,274 sequence reads were compared to the viral RefSeq database using tblastx with an E-value limit of 10e−04 to determine which reads were associated with which viruses. Each pool described in Table 2 is represented as a pie chart, with the percentage of viruses for each category colored according to the key.
BLAST searches were also conducted using the nonredundant (nr) nucleotide database at NCBI and using programs blastn, blastx, and discontiguous MegaBLAST to identify sequences that matched to known viruses and to confirm the BLAST results using the RefSeq database. The strongest matches were to insect, plant, and bacteriophage sequences, and this initial search was used to identify potential reference genomes for viral genome assembly. However, many of the sequences were highly divergent at the nucleotide level, indicating that most of these viruses are novel, distantly related variants of known viruses. In some cases, translation of contigs to amino acid sequences provided greater reliability in finding matches and identifying sequence reads for building larger contigs. In addition to insect, plant, and bacteriophage sequences, several sequence reads were most closely related to group 1 BtCoVs.
Identification of novel coronavirus sequences.
Seventy-six individual sequence reads matched strongly to CoV sequences (Table 4). Pool 1 contained the majority of CoV-like sequences with 68 sequence reads, 62 of which formed 14 contigs (Table 4). Seven of these contigs matched most closely to group 1 BtCoV-HKU2, while the additional contigs were more closely related to other group 1 bat or human CoVs (HCoV) (Table 4). In pool 3, two additional contigs comprised of two and three reads, respectively, were unique and most closely related to HCoV-NL63 (Table 4). In addition, pool 2 had three CoV-like reads, two of which formed a contig that was unique and most closely related to group 1 CoV porcine epidemic diarrhea virus (PEDV), while the single sequence read was most closely related to BtCoV-HKU2 (Table 4). Interestingly, all of these viral sequences were significantly different from known CoVs, indicating that these are novel CoVs that we have named Appalachian Ridge CoV (ARCoV). These will be further designated in this study by appending the pool number from which they originated. Further, CoV sequences were found only in the big brown bat samples and were predominantly associated with juvenile bats.
TABLE 4.
Overview of CoV sequence reads and contigs
| Pool no. | Sequence no. | No. of reads | Length (nt) | Best match (virus) | Region | E value | Bit score |
|---|---|---|---|---|---|---|---|
| 1 | Pool1.C.1148 | 4 | 1,148 | HCoV-229E | Replicase | 7.32E−42 | 96.4 |
| 1 | Pool1.C.837 | 5 | 837 | BtCoV-512 | Nucleocapsid | 1.72E−39 | 91.8 |
| 1 | Pool1.C.676 | 4 | 676 | BtCoV-HKU2 | Replicase | 2.77E−101 | 378.2 |
| 1 | Pool1.C.454 | 2 | 454 | BtCoV-HKU2 | Replicase | 1.52E−77 | 198.6 |
| 1 | Pool1.239577 | 1 | 443 | BtCoV-512 | Replicase | 1.50E−23 | 79 |
| 1 | Pool1.C.414 | 12 | 414 | BtCoV-HKU2 | Replicase | 3.97E−19 | 87.7 |
| 1 | Pool1.287740 | 1 | 412 | PRCoV | Spike | 2.64E−22 | 98.2 |
| 1 | Pool1.C.412 | 4 | 412 | BtCoV-HKU2 | Replicase | 6.32E−25 | 106.9 |
| 1 | Pool1.C.368 | 8 | 368 | BtCoV-HKU2 | Replicase | 4.73E−39 | 106 |
| 1 | Pool1.C.362 | 4 | 362 | PEDV | Replicase | 2.19E−70 | 257.7 |
| 1 | Pool1.C.348 | 2 | 348 | BtCoV-512 | Replicase | 1.55E−29 | 86.3 |
| 1 | Pool1.C.328 | 4 | 328 | HCoV-229E | Replicase | 8.31E−40 | 84 |
| 1 | Pool1.293985 | 1 | 284 | HCoV-NL63 | Replicase | 4.03E−26 | 110.1 |
| 1 | Pool1.5088 | 1 | 270 | PEDV | Replicase | 5.07E−38 | 149.5 |
| 1 | Pool1.66604 | 1 | 265 | HCoV-229E | Replicase | 1.44E−35 | 100 |
| 1 | Pool1.C.265 | 5 | 265 | BtCoV-HKU2 | Membrane | 2.59E−34 | 137.2 |
| 1 | Pool1.C.264 | 2 | 264 | HCoV-229E | Replicase | 5.74E−20 | 89.5 |
| 1 | Pool1.265225 | 1 | 207 | PRCoV | Spike | 9.08E−23 | 98.2 |
| 1 | Pool1.C.149 | 3 | 149 | PEDV | Replicase | 1.21E−22 | 96.8 |
| 1 | Pool1.C.146 | 3 | 146 | BtCoV-HKU2 | Replicase | 1.15E−22 | 96.8 |
| 2 | Pool2.C.344 | 2 | 344 | PEDV | Membrane | 2.03E−39 | 84 |
| 2 | Pool2.21252 | 1 | 257 | BtCoV-HKU2 | Replicase | 2.42E−32 | 73 |
| 3 | Pool3.C.766 | 3 | 764 | HCoV-NL63 | Spike | 6.77E−42 | 84.9 |
| 3 | Pool3.C.450 | 2 | 450 | HCoV-NL63 | Membrane | 5.51E−38 | 150.4 |
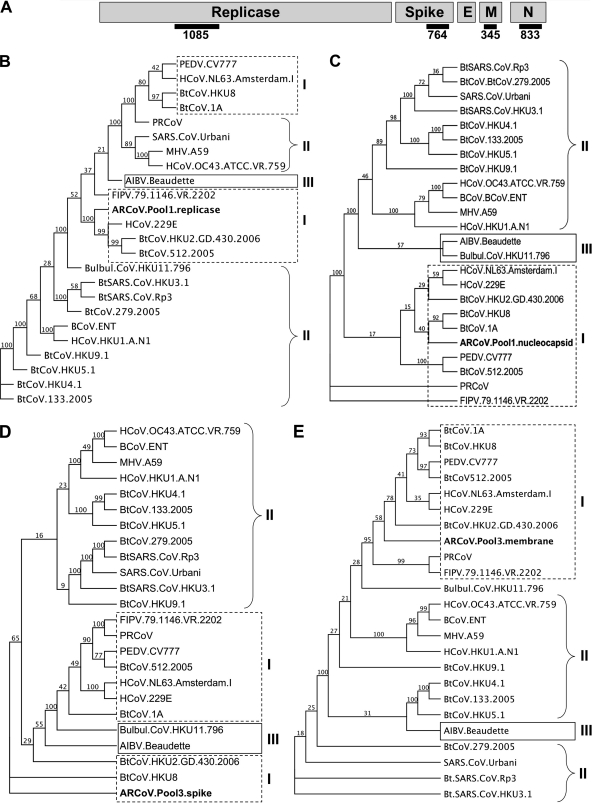
Multiple alignments were conducted for four contigs representing four different regions of the CoV genome, comparing novel viral sequences to CoV reference sequences acquired via download from the PATRIC website (Fig. 2A) (http://patric.vbi.vt.edu/). Maximum-likelihood trees were generated for each of the manually corrected multiple alignments, and in all cases, the novel CoV sequences found in this study were most closely related to group 1 CoVs (Fig. 2). A pool 1 contig that was 1,148 nt in length (designated contig Pool1.C.1148) was used to generate a multiple alignment with the reference set that spanned 1,085 nt of the ORF1a region of the replicase gene in the region of nonstructural protein 3 (nsp3) (Fig. 2B). This contig clustered most closely to HCoV-229E and shared 53.4% pairwise nucleotide identity with the replicase gene of this virus. Also from pool 1, contig Pool1.C.837 was used to generate a multiple alignment of the nucleocapsid gene based upon 833 nt (Fig. 2C). This ARCoV.Pool1.nucleocapsid fragment was closest to BtCoV-1A and shared 50.5% similarity to BtCoV-1A based upon pairwise nucleotide identity. While Pool1.C.1148 was most closely related to HCoV-229E and Pool1.C.837 was most closely related to BtCoV-1A, it is possible that these two contigs are from the same novel group 1 CoV.
FIG. 2.
Novel group 1 CoV phylogeny. At least three novel group 1 CoVs were identified in this study as CoV sequences were found in three pools (pools 1 to 3). (A) The CoV genome is comprised of five genes found in all CoVs, including the replicase that makes up the first two-thirds of the genome, and four structural genes including the spike (S), the envelope (E), the membrane (M), and the nucleocapsid (N) genes. Black bars represent the sequences identified and compared in this study. Maximum-likelihood analyses were conducted using the CoV contig sequences identified in this study in comparison to known CoV reference genomes. (B) Pool 1 contained a contig of 1,148 nt that was compared to other CoV reference genomes. An alignment of the most conserved 1,085 nt suggests that this contig is most closely related to group 1 HCoV-229E. (C) A second 833-nt contig in pool 1 matched most closely to the nucleocapsid gene of BtCoV-1A. (D) A 764-nt contig in pool 3 matched most closely to the spike gene of BtCoV-HKU8, a group 1 CoV. (E) A 450-nt contig in pool 3 was most closely related to the membrane gene of BtCoV-HKU2. Values at branch points represent bootstrap values based upon 100 replicates. Branches with values of <70 should be interpreted with caution. Trees are shown as proportional cladograms. Classic CoV group numbers are shown as roman numerals. Bat viruses are indicated with the prefix Bt. HCoV indicates human virus. MHV, murine hepatitis virus; FIPV, feline infectious peritonitis virus; AIBV, avian infectious bronchitis virus; FIPV, feline infectious peritonitis virus; BCoV, bovine coronovirus; PRCoV, porcine respiratory coronovirus.
In pool 3, two contigs were used to assess phylogeny with contig Pool3.C.766, used to create a 764-nucleotide fragment of the spike gene that was aligned to the reference set, and this contig was shown by phylogenetic analysis to be most closely related to BtCoV-HKU8 (41.1% pairwise identity at the nucleotide level) (Fig. 2D). Contig Pool3.C.450 was used to assess the membrane gene by comparing it to the reference set (Fig. 2E). This gene fragment was most closely related to the BtCoV-HKU2 membrane gene with 42.1% pairwise identity. Likewise, these contigs could be part of a second novel group 1 CoV associated with pool 3.
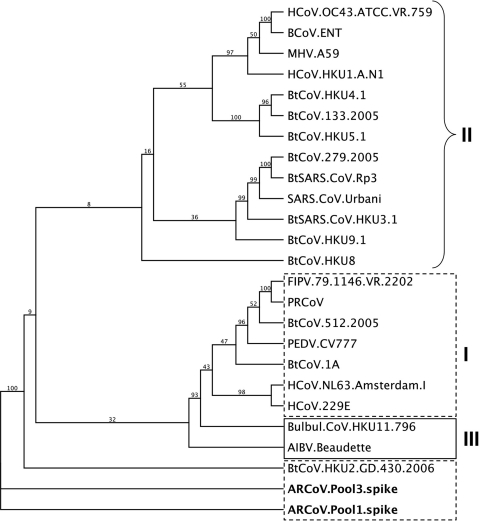
Interpool variation in the spike gene.
A sequence read of 412 nt in pool 1 (Pool1.287740) also matched to the same, albeit smaller, portion of the spike gene fragment identified in pool 3, indicating that two similar CoVs were found in both pools 1 and 3. This 412-nucleotide region shared a 96.4% pairwise similarity at the nucleotide level and 98.2% pairwise identity at the amino acid level between the pool 1 and pool 3 variants, and both were 41% similar at the nucleotide level to BtCoV-HKU2 (Fig. 3).
FIG. 3.
Variation between CoV spike genes found in different pools. A sequence read of 412 nt in length from pool 1, Pool 1.287740, matched to the larger spike gene fragment assembled from pool 3, indicating that two related CoVs were found in pools 1 and 3. This 412-nt region shared 96.4% pairwise similarity at the nucleotide level and 98.2% pairwise identity at the amino acid level between the pool 1 and pool 3 variants, and both were most closely related to BtCoV-HKU2. The tree was generated via maximum-likelihood analysis, with 100 bootstrap replicates, and is shown as a proportional cladogram.
These results indicate that at least three novel group 1 CoVs were detected in big brown bat fecal samples. No CoV sequences were present in samples analyzed for little brown myotis or tricolored bats, and no CoV sequences were detected in the saliva samples of big brown bats.
PCR amplification of novel group 1 CoV replicase fragment.
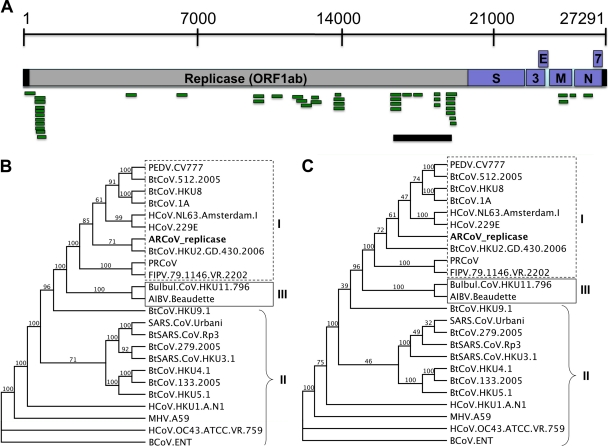
The BtCoV-HKU2 genome was used as a reference genome for assembling the 68 sequence reads identified in pool 1, and 45 of these reads mapped to this genome (Fig. 4A). Primers were designed using 454 reads that assembled to the genome, and PCR was conducted to fill in the gaps between 454 reads that mapped to the reference genome. One primer pair was used to successfully amplify 2,540 nt from genomic position 16486 to 19013 (based on BtCoV-HKU2 numbering), verifying that a novel CoV was present (Fig. 4A). Interestingly, pool 1 is comprised of eight juvenile female big brown bats, but only one of these samples was positive for this novel CoV. Sanger sequencing was used to determine the sequence of the PCR fragment, and the sequence was compared to the reference set of CoV genomes to determine its relatedness to other CoVs. Phylogenetic analysis suggests that the novel group 1 CoV in pool 1 is related to BtCoV-HKU2 and HCoV-229E (Fig. 4B and C).
FIG. 4.
Amplification of the replicase fragment of ARCoV from pool 1. (A) A total of 45 sequence reads derived from 454 sequencing of the pool 1 sample assembled to the BtCoV-HKU2 genome, and these were used to guide PCR amplification of a 2,540-nt fragment of ORF1ab of ARCoV. Green, 454 reads; blue, amplicons derived by PCR and Sanger sequencing; gray, replicase gene; pink, structural and accessory genes. (B) Phylogenetic analysis of the 2,540-nt fragment of the replicase shows that the ARCoV from pool 1 is most closely related to the group 1 CoVs, with its nearest neighbor being BtCoV-HKU2. (C) Phylogenetic analysis of the 833 amino acids suggests the ARCoV replicase sequence is most closely related to HCoV-229E. Trees are shown as proportional cladograms. Values at branch points represent bootstrap values based upon 100 replicates. Branches with values of <70 should be interpreted with caution. Classic CoV group numbers are shown as roman numerals.
Novel insect viruses from the Iflaviridae.
Several contigs from pools 1 to 5 (fecal samples) were determined to be strong matches (E value of <10e−20; bit scores of >100) to two related picorna-like viruses of insects. In fact, four out of the six pools contained differently sized contigs with an overlapping region of 1,449 nt that matched a similar region of known insect viruses. In all cases, the nucleotide and amino acid similarities were distant enough to suggest that these were likely to be novel insect viruses most closely related to two honeybee (Apis spp.) viruses: deformed wing virus (DWV) and sacbrood virus (SBV), both of which are members of the Iflaviridae family.
Phylogenetic analysis using the 1,449-nucleotide region demonstrated that these novel viruses clustered with other viruses of the Iflaviridae family, with all being most closely related to honeybee viruses (Fig. 5). While honeybee viruses were the closest relatives, the percent identity at the amino acid level ranged between 27.7% and 41.9%. This suggests that the insect viruses identified in the different samples were distant relatives of the honeybee viruses that probably infect an unidentified arthropod. In addition to this larger contig sequence, several other reads from all five pools had smaller matches to members of the Iflaviridae and the Dicistroviridae, with the closest relatives being the aphid and cricket lethal paralysis viruses (data not shown).
FIG. 5.
Novel insect viruses of the Iflaviridae family. Six unique 1,449-nt fragments from contigs assembled using sequence from four different pools (pools 1, 2 [n = 3], 4, and 5) were aligned to known insect viruses of the Iflaviridae and Dicistroviridae families and compared by maximum-likelihood analysis. Phylogenetic analysis demonstrated that these novel sequences clustered with other viruses of the Iflaviridae family, with all being most closely related to SBV and DWV, both of which are viruses of honeybees. Abbreviations are as follows: InV, insect virus; KBV, Kashmir bee virus; ALPV, aphid lethal paralysis virus; BQCV, black queen colony virus; CPV, cricket paralysis virus. Values at branch points represent bootstrap values based upon 100 replicates. Branches with values of <70 should be interpreted with caution. Scale bar represents 1 substitution per nucleotide position.
Detection of multiple novel plant viruses of the Tymoviridae family.
Two contigs from pool 3 and pool 5 contained regions of 1902 nt that matched most closely to the grapevine fleck virus (GFkV). In both cases, the contigs were assembled with a high degree of coverage, with the pool 3 contig comprised of 4,659 sequence reads and the pool 5 contig assembled using 364 sequence reads. Interestingly, these viruses shared 99.9% pairwise identity with one another and 56% pairwise nucleotide identity with GFkV. GFkV is primarily a graft-transmissible virus, without an insect vector, which suggests that the novel plant virus sequences are a distant relative of this virus. Phylogenetic analysis of this region demonstrated that the virus is similar to other plant viruses in the Tymoviridae family and that these are distinct viruses most closely related to the Marafivirus genus. In fact, phylogenetic analysis suggests that these novel plant viruses may represent a new genus of the Tymoviridae family (Fig. 6). Many other sequence reads from pools 1 to 5 also contained hits to many of the plant viruses in this figure (Fig. 6) and likely represent novel plant viruses of different plant species found in this region of Maryland.
FIG. 6.
Novel plant viruses of the Tymoviridae family. Two nearly identical plant virus sequences were identified in pool 1 and pool 3, and a 1,902-nt segment of each was most closely related to viruses of the Tymoviridae family. These viruses appear to form a new genus in the family as a sister clade to the Maculavirus genus. Abbreviations are as follows: PlV, plant virus; GFkV, grapevine fleck virus; CSDV, citrus sudden death-associated virus; OBDV, oat blue dwarf virus; KYMV, Kennedia yellow mosaic virus; OMV, okra mosaic virus; TYMV, turnip yellow mosaic virus; EMV, eggplant mosaic virus; PhyMV, Physalis mosaic virus. Numbers at branch points represent bootstrap values based upon 100 iterations. Scale bar represents 0.01 substitutions per nucleotide site.
Identification of novel bacteriophage sequences.
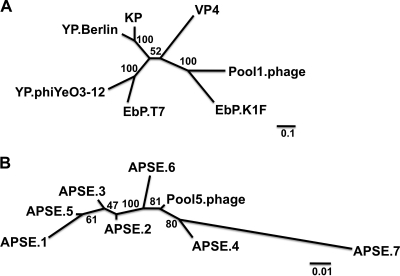
Several of the pools contained large contig sequences that matched different bacteriophages, with pool 1 containing several contigs that most closely matched to the enterobacteria phage K1F (EbP-K1F) of the Podoviridae family, the Autographivirinae subfamily, and the T7-like genus. EbP-K1F was used as a reference for assembling the reads from pool 1, and a total of 9,384 reads assembled to 88.3% of the EbP-K1F genome with a mean coverage of ∼82 reads. While phage evolution has been shown to be mosaic in nature and therefore not accurately represented by phylogenetics (38), we constructed a phylogenetic tree using the full-length DNA polymerase genes (2,115 nt) of the novel pool 1 phage and several reference genome sequences from the T7-like genus to demonstrate how closely related this novel sequence was to other viruses of the T7 lineage (Fig. 7A).
FIG. 7.
Two novel bacteriophage sequences identified in different pools. Several pools contained sequences that matched most closely to different bacteriophages. (A) In pool 1, a phage most closely related to enterobacteria phage (EbP) K1F was identified, and a phylogenetic tree was generated using maximum likelihood to compare the DNA polymerase gene of the novel pool 1 phage to that of other members of the T7-like genus. The tree demonstrates that the novel phage is most closely related to EbP-K1F. KP, Kluyvera phage Kvp1; VP, vibriophage VP4; YP, Yersinia phage. Values at branch points represent bootstrap values based upon 100 replicates. Branches with values of <70 should be interpreted with caution. Scale bar represents 0.1 substitutions per nucleotide site. (B) In pool 5, the predominant bacteriophage sequence was most closely related to Acyrthosiphon pisum secondary endosymbiont 1 (APSE-1) bacteriophage. The full-length terminase gene was used to compare the novel phage sequence to seven previously described APSE phage sequences. The phylogenetic analysis shows that the pool 5 APSE-like sequence is a novel bacteriophage most closely related to APSE-4 and APSE-6. Values at branch points represent bootstrap values based upon 100 replicates. Branches with values of <70 should be interpreted with caution. Scale bar represents 0.01 substitutions per nucleotide site.
In pool 5, the predominant bacteriophage sequence was most closely related to Acyrthosiphon pisum secondary endosymbiont 1 (APSE-1) bacteriophage, a group 1 virus of the Podoviridae family. A total of 289 sequence reads assembled to the APSE-1 reference genome, covering roughly 55% of the APSE-1 genome with a mean coverage of ∼2. Four contigs greater than 1,200 nt from four different regions of the genome were closely related to this phage, with nucleotide pairwise identities that ranged between 97 to 99%. The full-length terminase gene (1,184 nt) was used to compare the novel phage sequence from pool 5 to seven previously described APSE phage sequences (Fig. 7B) (21, 52). The phylogenetic analysis suggested that the pool 5 APSE-like sequence is a novel bacteriophage most closely related to APSE-4 and APSE-6.
Detection of herpesvirus in bat saliva.
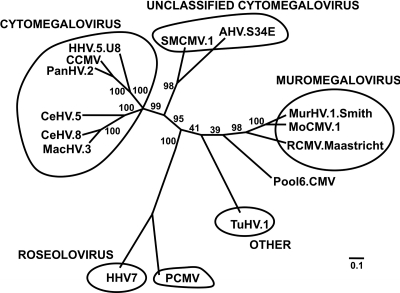
In the pool 6 sample, the predominant viral species were most closely related to genera within the Herpesviridae family, Betaherpesvirinae subfamily. A 798-nt contig was translated to a 266-amino acid sequence that was compared by multiple alignment and phylogenetics to several representative sequences of the Betaherpesvirinae subfamily. This sequence matched most closely to tupaiid herpesvirus 1 (TuHV-1) (53.9% pairwise identity), also known as tree shrew herpesvirus, for which a genus has not been assigned, and the rat cytomegalovirus (RCMV) of the Muromegalovirus genus (53.8% pairwise identity) (Fig. 8). Several additional contigs were similar to the Muromegalovirus genus, which is comprised of rat and mouse cytomegaloviruses.
FIG. 8.
Phylogeny of a novel herpesvirus found in pool 6. Several contigs from pool 6 matched most closely to viruses in a variety of genera of the Betaherpesvirinae subfamily. A 267-amino acid fragment found in pool 6 was aligned and compared to sequences of several reference viruses of the Betaherpesvirinae. Phylogenetic analyses indicated that this amino acid sequence may be from a novel bat cytomegalovirus that forms a new genera between Roseolovirus and Muromegalovirus. HHV, human herpesvirus; CCMV, chimpanzee cytomegalovirus; PanHV, panine herpesvirus; CeHV, cercopithecine herpesvirus; MacHV, macacine herpesvirus; PCMV, porcine cytomegalovirus; TuHV, tupaiid herpesvirus; RCMV, rat cytomegalovirus; MoCMV, mouse cytomegalovirus; MurHV, murid herpesvirus; AHV, atoline herpesvirus; SQCMV, squirrel monkey cytomegalovirus. Values at branch points represent bootstrap values based upon 100 replicates. Branches with values of <70 should be interpreted with caution. Scale bar represents 0.1 substitutions per nucleotide site.
DISCUSSION
From 1980 to 2007, 87 new pathogens were detected in humans, and 58 of these were viruses, including 45 single-stranded RNA viruses. Bunyavirus was the largest single virus family found in bats (94). These observations underscore the importance of characterizing the virome of as many bat species as possible, focusing particularly on bats that are frequently found in or near human habitats. The goal of this study was to characterize the viromes of three bat species that share a roost in an abandoned railroad tunnel in Maryland using next-generation sequencing technology to determine the entire population of viruses found in each species and to determine how viruses differ between bats segregated by species, sex, and age.
A set of 41 bat samples was selected and pooled based on important ecological factors including age (juvenile or adult), sex, and species. These parameters were selected for the following reasons: (i) juvenile bats captured in the summer and fall have not yet undergone hibernation and therefore may be subject to viruses that would be restricted by a decrease in body temperature; (ii) male and female bats of many microbat species live separately during the spring when females establish maternity colonies away from the male population, and these populations may encounter different viruses; (iii) different species forage in different areas, attract different ectoparasites, and frequently target different arthropods as food, providing different opportunities for viruses to infect different species. Therefore, five pools were established to assess viromes of fecal samples from adult male, juvenile male, and juvenile female big brown bats; a mixed population of little brown myotis; and juvenile male tricolored bats (Table 2). In addition, a single pool was established to examine the virome of oral secretions from juvenile female big brown bats (Table 2). The overall sequence results from each pool were compared to known virus sequences to determine a preliminary virome for each pooled sample, and these results indicated that the heterologous viral populations were largely different for each pool (Fig. 1). No emerging human viruses were detected in these samples although some sequence reads were distantly related to human viruses, including enteric viruses such as rotavirus, enterovirus, and CoVs. It is important to note that this analysis gives a preliminary overview of the differences in viromes between different species and sample types; however, a more comprehensive characterization of these viromes will require multiple sequencing runs of more individual bats and genome assembly and annotation to verify the viral populations associated with each species.
Novel group 1 CoVs identified in juvenile big brown bats.
SARS-CoV emerged from bats as a highly infectious and deadly disease caused by a CoV that crossed the species barrier from a zoonotic reservoir into the human population, which occurred in the wet markets of the Guangdong province of China (17). During the outbreak of 2002 to 2003, the overall mortality rate was over 8%, but for those over age 60, the death rate approached 60% (73). Although SARS-CoV has not reemerged since the original outbreak, the discovery of new human CoVs and SARS-like viruses in bats has been occurring with increasing frequency (9, 16, 18, 19, 26, 34, 43, 44, 50, 55, 57, 64, 65, 77, 92, 93, 95). In addition to SARS-CoV, two additional CoV cross-species transmission events have been documented, both of which resulted in human viruses that continue to circulate, including HCoV-OC43, a close relative of bovine CoV (BCoV) that crossed the bovine species barrier to infect humans approximately 120 years ago, as estimated by a theoretical molecular clock model (82), and HCoV-229E, which hypothetically emerged from African bats (55). Molecular clock analysis was also used to estimate that HCoV-229E emerged roughly 200 years ago, and the authors of this work suggest that all CoVs may have emerged from bats (55). Given that many CoVs continue to circulate in several different species of bats, including bats of the Old and New World, the next CoV spillover into humans or important domestic animals is only a matter of time and ecological opportunity.
CoV sequences have been found in the United States in the big brown bat (26) and in a Texas roost shared by Brazilian free-tailed bats (Tadarida brasiliensis), the cave myotis (Myotis velifer), evening bats (Nycticeius humeralis), and tricolored bats (47), but in both cases only small segments of the large replicase gene were recovered. In this study, we identified 76 CoV sequence reads that formed 17 contigs (Fig. 2 and Table 4), and these were most closely related to known group 1 CoVs, including BtCoV-HKU2 that was isolated from a Rhinolophus bat species in Hong Kong (44). Of note, the sequence identity of these CoV sequences to their closest CoV match ranged between 41.1 and 53.4% at the nucleotide level (38.7 to 89.1% at the amino acid level), suggesting that these are novel group 1 viruses that we have designated ARCoV. These sequence similarities are within the same range as the novel BtCoVs found in Southeast Asian and African bats (44, 55).
The ARCoV sequence was verified by reverse transcription-PCR (RT-PCR), using 454 sequence reads to design primers that successfully amplified a 2,540-nt fragment of ORF1ab (Fig. 4). We are currently using a primer-walking strategy in an attempt to ascertain the full-length CoV genomes from the different samples (21). Once an entire genome is derived, we will synthetically reconstruct the virus and determine if it replicates in bats (7).
CoVs were detected in three of the pools, indicating that at least three different CoVs were present, and pool 1 contained several contigs that matched more closely to different group 1 CoVs (Table 4 and Fig. 2), suggesting that more than one CoV may have been present in those samples. However, it is possible that these are all fragments of the same novel strain of CoV that is commonly associated with big brown bats. Other studies have found CoV coinfection in individual animals (40, 48); however, the study design precluded determining if this was the case in our samples. In addition, a similar section of the spike gene was found in pools 1 and 3, and these sequences were 98.2% identical at the amino acid level, indicating that a similar virus was found in two different pools of big brown bats (Fig. 3).
Novel viruses associated with arthropods of Cicadellidae family in the order Hemiptera.
In five of the pools (pools 1 to 5; all of the fecal samples) there was an abundance of sequences that matched most closely to viruses of insects and plants. Most of the plant virus homologs were related to known viruses vectored by arthropods that transmit the virus to plants. This is not surprising, given that there are 56 genera and 141 known families of plant viruses, and most depend upon an insect vector for transmission to other plants (11). The most common insect vectors to transmit plant viruses are known as the leafhoppers, which are a group of insects with piercing-sucking mouthparts and rows of spine-like setae (hairs) in their hind tibiae. There are over 20,000 species of leafhoppers, which belong to the family Cicadellidae in the order Hemiptera (Tree of Life Web Project [http://tolweb.org/Cicadellidae/10853/2005.07.15]). It has been estimated that in a single summer season the average maternity colony of approximately 150 bats can easily consume over 50,000 leafhoppers (86). This suggests that bats likely eat leafhoppers infected with important insect and/or plant viruses that are then shed in the bat feces. It is not known if these insect and plant viruses replicate in bats; however, the results of this study show that intact, perhaps infectious, virus does pass through the bat gastrointestinal tract, as evidenced by genome resistance to degradative nucleases.
The most common insect viruses observed in this study belonged to the picorna-like viruses of the Iflaviridae and Dicistroviridae families. Large segments of genomes (1,449 nt) most closely related to the DWV or SBV of the Iflaviridae were detected in four of the six pools (Fig. 5). Interestingly, DWV has been associated with honeybee colony collapse disorder (CCD) when the virus is transmitted by the ectoparasitic mite, Varroa destructor (23). SBV is also a virus of honeybees. However, since the sequence identity of these viruses is so low (less than 42% at the amino acid level) compared to DWV and SBV, we hypothesize that these are novel viruses of an unidentified arthropod.
Two nearly identical plant virus sequences were identified in pool 1 and pool 3 representing a 1,902-nucleotide segment that most closely matched viruses of the Tymoviridae family (Fig. 6). Interestingly, these viral homologs were also distant relatives, with roughly 56% pairwise identity to other viral sequences in the family, and these viruses likely form a new genus in the Tymoviridae family as a sister clade to the Maculavirus genus (Fig. 6).
Bacteriophage sequences suggest unexpected bacterial flora in bats.
The strongest matches to any viral sequences were bacteriophage sequences that were derived from bat fecal samples (pools 1 to 5). The populations of bacteriophage appeared to vary by pool; however, this may be due to sequence coverage and depth, and we will be investigating this observation more closely with additional samples. In pool 1, the predominant bacteriophage sequence matched most closely to EbP-K1F although portions of the sequence were more closely related to Yersinia phage Berlin (Fig. 7A, YP.Berlin). Sequence reads from this phage were assembled using the EbP-K1F genome as a reference, and the assembly aligned to 88.3% of the EbP-K1F genome, with a mean coverage of ∼82 reads. Phylogenetic analysis of the full-length DNA polymerase genes (2,115 nt) of the novel pool 1 phage in comparison to other bacteriophage of the T7-like genus indicated that the novel phage is distinctly different from EbP-K1F although this phage appears to be its closest relative (Fig. 7A). Given the mosaic nature of the bacteriophages (38), we speculate that this virus may contain genes from both the EbP-K1F and YP.Berlin parental strains, and we are currently completing the sequence of this genome using traditional PCR-based approaches.
EbP-K1F was originally isolated from human sewage in 1984 (83), and it is known to encode an endosialidase that allows it to infect Escherichia coli strains expressing the K1 antigen, which normally serves as a physical barrier to phage adsorption and aids in immune evasion (70). Such E. coli strains are often associated with human illnesses, including septicemia, urinary tract infections, and meningitis (69). YP.Berlin phage is known to infect the bacterium Yersinia pestis, the bacterium that causes bubonic plague in humans. To our knowledge, Y. pestis is not commonly found in animals in the eastern United States, and its primary reservoir is thought to be rodents in the western United States (http://www.cdc.gov/ncidod/dvbid/plague/).
In pool 5, the predominant viral sequences were most closely related to APSE-1 bacteriophage, which is a lambda-like phage of the Siphoviridae family, possessing a circular 36.5-kb dsDNA genome (66). APSE-1 infects and lyses a secondary endosymbiont of the pea aphid Acyrthosiphon pisum, which has also been found on black locust trees that are common in western Maryland. Interestingly, aphids rely upon a primary endosymbiont, such as Buchnera, to provide essential dietary nutrients, and many aphids also contain secondary symbionts, such as the enterobacteric “Candidatus Hamiltonella defensa,” which is a facultative symbiont that helps protect the aphid against entomopathogenic fungi and parasitoid wasps. The APSE phages are thought to be an obligate component of the “Ca. Hamiltonella” life cycle, and it has been proposed that APSE and “Ca. Hamiltonella” form a mutualistic symbiosis with Acyrthosiphon pisum, whereby toxin genes expressed by a particular APSE strain provide protection against eukaryotic parasites (21, 22, 52, 68).
The APSE phage found in this study is distinctly unique and most closely related to APSE-4 and APSE-6, when the full-length terminase genes of all seven known APSEs are compared (Fig. 7B). This phage did not contain the toxins CdtB and Stx associated with protection of the sweet pea aphid from parasites. Based upon the information known about APSE phage, we hypothesize that this is a novel APSE that was likely isolated from a strain of “Ca. Hamiltonella” and that both are probably mutualisitic symbionts of an unknown arthropod that was eaten by the bat. This observation suggests that bats may play an important role in distributing viruses and bacteria in the environment that might be beneficial to other organisms.
Identification of T7-like phage related to bacteriophages of bacterial species associated with human disease suggests that bats may be harboring and trafficking bacterial species known to be important human pathogens. To address this question, we are currently assessing the bacterial populations in these bat samples by 16S rRNA gene profiling of fecal DNA from the different species of bats collected in Maryland and elsewhere.
Detection of novel herpesviruses of the Betaherpesvirus genus.
The predominant viral species observed in the oral swab samples (pool 6) matched most closely to cytomegaloviruses of small mammals, including the rat cytomegalovirus and the tupaiid herpesvirus (TuHV). This phylogenetic tree was constructed using amino acid sequences because the nucleotide sequences were too distant to reliably align the sequences (Fig. 8). Phylogenetic analyses of the 267-amino-acid fragment found in pool 6 along with several reference viruses of the Betaherpesvirinae indicated that this amino acid sequence may be a novel bat cytomegalovirus that forms a new genus between Roseolovirus and Muromegalovirus (Fig. 8). Herpesviruses have been detected in bats in a number of studies (51, 62, 76, 84, 85, 87), and in European bats gamma- and betaherpesviruses were detected by pan-herpes consensus PCR (87). The predominant herpesviruses strains found in this study were more closely related to mammalian species besides bats, and we expect that different species of bats will contain different types of herpesviruses.
Adventures in metagenomics.
The use of 454 sequencing and other methods, as well as other emerging sequencing technologies that hold great promise for drastically reducing the cost of megasequencing, will continue to drive the discovery of novel viruses and help elucidate the complex viral ecology that plays a role in the emergence of infectious disease and in spread of virulence factors. In this study, we used 454 sequencing technology to analyze samples from 41 bats, generating over 600,000 sequence reads of over 250 nt in length. This abundance of sequence information provided many insights into the virome of bats and bat ecology; however, no full-length viral genomes were detected. These results were not unique, as our results were relatively similar to those obtained by Li et al., who recently conducted a study analyzing the guano of bats from the southwestern United States (47). However, we believe that virus discovery must focus upon deriving entire viral genomic sequences that can be used by the scientific community to characterize the viruses and compare them to others. Toward that end, we are employing traditional Sanger sequencing to fill in the gaps of several of our novel viral genomes. Additionally, we believe that individual bat-to-bat comparisons will allow us to elucidate the cross-species transmission dynamics between pathogen and host, as well as between different populations in a shared habitat.
We have encountered several issues with assembling the viral sequences in this study because in most cases the viral homologs are 50 to 60% identical at the nucleotide level to the closest sequence match in the database. This suggests that most sequences analyzed in this study belong to novel viruses, but there are no known reference sequences available to guide assembly. This problem has also been encountered with novel CoV sequences isolated from bats in China and elsewhere that have completely redefined the traditional CoV phylogeny because many of these viruses are too diverse to cluster into the traditional three group system (18, 44, 57, 64, 93).
To circumvent this problem, translated BLAST searches were used to identify matches at the amino acid level that did not assemble well at the nucleotide level, despite clear sequence conservation noted at the amino acid level. Tools to address divergent genome assembly are currently lacking, and development of new tools for assessing divergent genomes is essential. In addition to defining and characterizing the viromes of different bat species, our goal is to establish a reference set of viral genomes that have originated in bats that will allow future investigators access to these reference genomes to aid in genome assembly.
Acknowledgments
We thank the UNC High Throughput Sequencing Facility for help with 454 sequencing, J. Craig Venter Institute and David Spiro for help with bar-coding protocols, and Joseph Victoria and Eric Delwart for help with sample preparation protocols and reaction conditions. We thank the field technicians from the Appalachian Laboratory, including Angela Sjollema, Jennifer Saville, and Lisa Smith, who helped with sample collections.
This work was supported by NIH grant U54 AI057157 from the Southeastern Regional Center of Excellence for Emerging Infections and Biodefense.
The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published ahead of print on 6 October 2010.
REFERENCES
- 1.Allander, T., S. U. Emerson, R. E. Engle, R. H. Purcell, and J. Bukh. 2001. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. U. S. A. 98:11609-11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allander, T., T. Jartti, S. Gupta, H. G. Niesters, P. Lehtinen, R. Osterback, T. Vuorinen, M. Waris, A. Bjerkner, A. Tiveljung-Lindell, B. G. van den Hoogen, T. Hyypia, and O. Ruuskanen. 2007. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 44:904-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allander, T., M. T. Tammi, M. Eriksson, A. Bjerkner, A. Tiveljung-Lindell, and B. Andersson. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U. S. A. 102:12891-12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, N. G., J. L. Gerin, and N. L. Anderson. 2003. Global screening for human viral pathogens. Emerg. Infect. Dis. 9:768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias, C. F., M. Escalera-Zamudio, L. Soto-Del Rio Mde, A. G. Cobian-Guemes, P. Isa, and S. Lopez. 2009. Molecular anatomy of 2009 influenza virus A (H1N1). Arch. Med. Res. 40:643-654. [DOI] [PubMed] [Google Scholar]
- 6.Barbour, R. W., and W. H. Davis. 1969. Bats of America. University Press of Kentucky, Lexington, KY.
- 7.Becker, M. M., R. L. Graham, E. F. Donaldson, B. Rockx, A. C. Sims, T. Sheahan, R. J. Pickles, D. Corti, R. E. Johnston, R. S. Baric, and M. R. Denison. 2008. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc. Natl. Acad. Sci. U. S. A. 105:19944-19949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, and E. W. Sayers. 2009. GenBank. Nucleic Acids Res. 37:D26-D31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandao, P. E., K. Scheffer, L. Y. Villarreal, S. Achkar, N. Oliveira Rde, O. Fahl Wde, J. G. Castilho, I. Kotait, and L. J. Richtzenhain. 2008. A coronavirus detected in the vampire bat Desmodus rotundus. Braz. J. Infect. Dis. 12:466-468. [DOI] [PubMed] [Google Scholar]
- 10.Breitbart, M., and F. Rohwer. 2005. Method for discovering novel DNA viruses in blood using viral particle selection and shotgun sequencing. Biotechniques 39:729-736. [DOI] [PubMed] [Google Scholar]
- 11.Brunt, A. A., K. Crabtree, M. J. Dallwitz, A. J. Gibbs, L. Watson, and E. J. Zurcher. 20 August 1996, posting date. Plant viruses online: descriptions and lists from the VIDE database. University of Idaho, Moscow, ID.
- 12.Bunde, J. M., E. J. Heske, N. E. Mateus-Pinilla, J. E. Hofmann, and R. J. Novak. 2006. A survey for West Nile virus in bats from Illinois. J. Wildl. Dis. 42:455-458. [DOI] [PubMed] [Google Scholar]
- 13.Calisher, C. H., J. E. Childs, H. E. Field, K. V. Holmes, and T. Schountz. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19:531-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Camacho, C., G. Coulouris, V. Avagyan, N. Ma, J. Papadopoulos, K. Bealer, and T. L. Madden. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cann, A. J., S. E. Fandrich, and S. Heaphy. 2005. Analysis of the virus population present in equine faeces indicates the presence of hundreds of uncharacterized virus genomes. Virus Genes 30:151-156. [DOI] [PubMed] [Google Scholar]
- 16.Carrington, C. V., J. E. Foster, H. C. Zhu, J. X. Zhang, G. J. Smith, N. Thompson, A. J. Auguste, V. Ramkissoon, A. A. Adesiyun, and Y. Guan. 2008. Detection and phylogenetic analysis of group 1 coronaviruses in South American bats. Emerg. Infect. Dis. 14:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chinese SARS Molecular Epidemiology Consortium. 2004. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 303:1666-1669. [DOI] [PubMed] [Google Scholar]
- 18.Chu, D. K., J. S. Peiris, H. Chen, Y. Guan, and L. L. Poon. 2008. Genomic characterizations of bat coronaviruses (1A, 1B and HKU8) and evidence for co-infections in Miniopterus bats. J. Gen. Virol. 89:1282-1287. [DOI] [PubMed] [Google Scholar]
- 19.Chu, D. K., L. L. Poon, K. H. Chan, H. Chen, Y. Guan, K. Y. Yuen, and J. S. Peiris. 2006. Coronaviruses in bent-winged bats (Miniopterus spp.). J. Gen. Virol. 87:2461-2466. [DOI] [PubMed] [Google Scholar]
- 20.Coetzee, B., M. J. Freeborough, H. J. Maree, J. M. Celton, D. J. Rees, and J. T. Burger. 2010. Deep sequencing analysis of viruses infecting grapevines: virome of a vineyard. Virology 400:157-163. [DOI] [PubMed] [Google Scholar]
- 21.Degnan, P. H., and N. A. Moran. 2008. Diverse phage-encoded toxins in a protective insect endosymbiont. Appl. Environ. Microbiol. 74:6782-6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degnan, P. H., and N. A. Moran. 2008. Evolutionary genetics of a defensive facultative symbiont of insects: exchange of toxin-encoding bacteriophage. Mol. Ecol. 17:916-929. [DOI] [PubMed] [Google Scholar]
- 23.de Miranda, J. R., and E. Genersch. 2010. Deformed wing virus. J. Invertebr. Pathol. 103:S48-S61. [DOI] [PubMed] [Google Scholar]
- 24.Djikeng, A., R. Halpin, R. Kuzmickas, J. Depasse, J. Feldblyum, N. Sengamalay, C. Afonso, X. Zhang, N. G. Anderson, E. Ghedin, and D. J. Spiro. 2008. Viral genome sequencing by random priming methods. BMC Genomics 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djikeng, A., and D. Spiro. 2009. Advancing full length genome sequencing for human RNA viral pathogens. Future Virol. 4:47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominguez, S. R., T. J. O'Shea, L. M. Oko, and K. V. Holmes. 2007. Detection of group 1 coronaviruses in bats in North America. Emerg. Infect. Dis. 13:1295-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edgar, R. C. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 29.Finkbeiner, S. R., A. F. Allred, P. I. Tarr, E. J. Klein, C. D. Kirkwood, and D. Wang. 2008. Metagenomic analysis of human diarrhea: viral detection and discovery. PLoS Pathog. 4:e1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank, D. N., and N. R. Pace. 2008. Gastrointestinal microbiology enters the metagenomics era. Curr. Opin. Gastroenterol. 24:4-10. [DOI] [PubMed] [Google Scholar]
- 31.Fraser, C., C. A. Donnelly, S. Cauchemez, W. P. Hanage, M. D. Van Kerkhove, T. D. Hollingsworth, J. Griffin, R. F. Baggaley, H. E. Jenkins, E. J. Lyons, T. Jombart, W. R. Hinsley, N. C. Grassly, F. Balloux, A. C. Ghani, N. M. Ferguson, A. Rambaut, O. G. Pybus, H. Lopez-Gatell, C. M. Alpuche-Aranda, I. B. Chapela, E. P. Zavala, D. M. Guevara, F. Checchi, E. Garcia, S. Hugonnet, and C. Roth. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 33.Gaynor, A. M., M. D. Nissen, D. M. Whiley, I. M. Mackay, S. B. Lambert, G. Wu, D. C. Brennan, G. A. Storch, T. P. Sloots, and D. Wang. 2007. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 3:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gloza-Rausch, F., A. Ipsen, A. Seebens, M. Gottsche, M. Panning, J. Felix Drexler, N. Petersen, A. Annan, K. Grywna, M. Muller, S. Pfefferle, and C. Drosten. 2008. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg. Infect. Dis. 14:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gouy, M., S. Guindon, and O. Gascuel. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27:221-224. [DOI] [PubMed] [Google Scholar]
- 36.Greninger, A. L., C. Runckel, C. Y. Chiu, T. Haggerty, J. Parsonnet, D. Ganem, and J. L. DeRisi. 2009. The complete genome of klassevirus: a novel picornavirus in pediatric stool. Virol. J. 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guindon, S., and O. Gascuel. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696-704. [DOI] [PubMed] [Google Scholar]
- 38.Hatfull, G. F., D. Jacobs-Sera, J. G. Lawrence, W. H. Pope, D. A. Russell, C. C. Ko, R. J. Weber, M. C. Patel, K. L. Germane, R. H. Edgar, N. N. Hoyte, C. A. Bowman, A. T. Tantoco, E. C. Paladin, M. S. Myers, A. L. Smith, M. S. Grace, T. T. Pham, M. B. O'Brien, A. M. Vogelsberger, A. J. Hryckowian, J. L. Wynalek, H. Donis-Keller, M. W. Bogel, C. L. Peebles, S. G. Cresawn, and R. W. Hendrix. 2010. Comparative genomic analysis of 60 mycobacteriophage genomes: genome clustering, gene acquisition, and gene size. J. Mol. Biol. 397:119-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes, E. C. 2001. On the origin and evolution of the human immunodeficiency virus (HIV). Biol. Rev. Camb. Philos Soc. 76:239-254. [DOI] [PubMed] [Google Scholar]
- 40.Kottier, S. A., D. Cavanagh, and P. Britton. 1995. First experimental evidence of recombination in infectious bronchitis virus. Recombination in IBV. Adv. Exp. Med. Biol. 380:551-556. [DOI] [PubMed] [Google Scholar]
- 41.Kristensen, D. M., A. R. Mushegian, V. V. Dolja, and E. V. Koonin. 2010. New dimensions of the virus world discovered through metagenomics. Trends Microbiol. 18:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 43.Lau, S. K., P. C. Woo, K. S. Li, Y. Huang, H. W. Tsoi, B. H. Wong, S. S. Wong, S. Y. Leung, K. H. Chan, and K. Y. Yuen. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 102:14040-14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau, S. K., P. C. Woo, K. S. Li, Y. Huang, M. Wang, C. S. Lam, H. Xu, R. Guo, K. H. Chan, B. J. Zheng, and K. Y. Yuen. 2007. Complete genome sequence of bat coronavirus HKU2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology 367:428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letarov, A., and E. Kulikov. 2009. The bacteriophages in human- and animal body-associated microbial communities. J. Appl. Microbiol. 107:1-13. [DOI] [PubMed] [Google Scholar]
- 46.Li, L., J. Victoria, A. Kapoor, O. Blinkova, C. Wang, F. Babrzadeh, C. J. Mason, P. Pandey, H. Triki, O. Bahri, B. S. Oderinde, M. M. Baba, D. N. Bukbuk, J. M. Besser, J. M. Bartkus, and E. L. Delwart. 2009. A novel picornavirus associated with gastroenteritis. J. Virol. 83:12002-12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, L., J. G. Victoria, C. Wang, M. Jones, G. M. Fellers, T. H. Kunz, and E. Delwart. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 85:6955-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masters, P. S., and P. J. Rottier. 2005. Coronavirus reverse genetics by targeted RNA recombination. Curr. Top. Microbiol. Immunol. 287:133-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menzel, M. A., J. M. Menzel, S. B. Castleberry, J. Ozier, W. M. Ford, J. W. Edwards, and E. W. Pearson. 2002. Illustrated key to skins and skulls of bats in the Southeastern and Mid-Atlantic States. Research note NE-376. USDA Forest Service, Northeastern Research Station, Newtown Square, PA.
- 50.Misra, V., T. Dumonceaux, J. Dubois, C. Willis, S. Nadin-Davis, A. Severini, A. Wandeler, R. Lindsay, and H. Artsob. 2009. Detection of polyoma and corona viruses in bats of Canada. J. Gen. Virol. 90:2015-2022. [DOI] [PubMed] [Google Scholar]
- 51.Molnar, V., M. Janoska, B. Harrach, R. Glavits, N. Palmai, D. Rigo, E. Sos, and M. Liptovszky. 2008. Detection of a novel bat gammaherpesvirus in Hungary. Acta Vet. Hung. 56:529-538. [DOI] [PubMed] [Google Scholar]
- 52.Moran, N. A., P. H. Degnan, S. R. Santos, H. E. Dunbar, and H. Ochman. 2005. The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc. Natl. Acad. Sci. U. S. A. 102:16919-16926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng, T. F., C. Manire, K. Borrowman, T. Langer, L. Ehrhart, and M. Breitbart. 2009. Discovery of a novel single-stranded DNA virus from a sea turtle fibropapilloma by using viral metagenomics. J. Virol. 83:2500-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peeters, M., M. L. Chaix, and E. Delaporte. 2008. Genetic diversity and phylogeographic distribution of SIV: how to understand the origin of HIV. Med. Sci. (Paris) 24:621-628. (In French.) [DOI] [PubMed] [Google Scholar]
- 55.Pfefferle, S., S. Oppong, J. F. Drexler, F. Gloza-Rausch, A. Ipsen, A. Seebens, M. A. Muller, A. Annan, P. Vallo, Y. Adu-Sarkodie, T. F. Kruppa, and C. Drosten. 2009. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg. Infect. Dis. 15:1377-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pilipski, J. D., L. M. Pilipskl, and L. S. Risley. 2004. West Nile virus antibodies in bats from New Jersey and New York. J. Wildl. Dis. 40:335-337. [DOI] [PubMed] [Google Scholar]
- 57.Poon, L. L., D. K. Chu, K. H. Chan, O. K. Wong, T. M. Ellis, Y. H. Leung, S. K. Lau, P. C. Woo, K. Y. Suen, K. Y. Yuen, Y. Guan, and J. S. Peiris. 2005. Identification of a novel coronavirus in bats. J. Virol. 79:2001-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Preidis, G. A., and J. Versalovic. 2009. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology 136:2015-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pruitt, K. D., T. Tatusova, and D. R. Maglott. 2007. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 35:D61-D65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Racey, P. 2008. Reproductive assessment in bats, p. 31-45. In T. H. Kuntz (ed.), Ecological and behavioral methods in the study of bats. Smithsonian Institution Press, Washington, DC.
- 61.Raesly, R. L., and J. E. Gates. 1987. Winter habitat selection by north temperate cave bats. Am. Midl. Nat. 118:15-31. [Google Scholar]
- 62.Razafindratsimandresy, R., E. M. Jeanmaire, D. Counor, P. F. Vasconcelos, A. A. Sall, and J. M. Reynes. 2009. Partial molecular characterization of alphaherpesviruses isolated from tropical bats. J. Gen. Virol. 90:44-47. [DOI] [PubMed] [Google Scholar]
- 63.Reichard, J. D. 2008. Wing-damage index used for characterizing wing condition of bats affected by white-nose syndrome. National Speleological Society, Huntsville, AL. http://www.caves.org/WNS/BatWings.pdf.
- 64.Ren, W., W. Li, M. Yu, P. Hao, Y. Zhang, P. Zhou, S. Zhang, G. Zhao, Y. Zhong, S. Wang, L. F. Wang, and Z. Shi. 2006. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. J. Gen. Virol. 87:3355-3359. [DOI] [PubMed] [Google Scholar]
- 65.Rihtaric, D., P. Hostnik, A. Steyer, J. Grom, and I. Toplak. 2010. Identification of SARS-like coronaviruses in horseshoe bats (Rhinolophus hipposideros) in Slovenia. Arch. Virol. 155:507-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rohwer, F., and R. Edwards. 2002. The Phage Proteomic Tree: a genome-based taxonomy for phage. J. Bacteriol. 184:4529-4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roossinck, M. J., P. Saha, G. B. Wiley, J. Quan, J. D. White, H. Lai, F. Chavarria, G. Shen, and B. A. Roe. 2010. Ecogenomics: using massively parallel pyrosequencing to understand virus ecology. Mol. Ecol. 19(Suppl. 1):81-88. [DOI] [PubMed] [Google Scholar]
- 68.Sandstrom, J. P., J. A. Russell, J. P. White, and N. A. Moran. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217-228. [DOI] [PubMed] [Google Scholar]
- 69.Scholl, D., S. Adhya, and C. Merril. 2005. Escherichia coli K1's capsule is a barrier to bacteriophage T7. Appl. Environ. Microbiol. 71:4872-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scholl, D., and C. Merril. 2005. The genome of bacteriophage K1F, a T7-like phage that has acquired the ability to replicate on K1 strains of Escherichia coli. J. Bacteriol. 187:8499-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith, G. J., D. Vijaykrishna, J. Bahl, S. J. Lycett, M. Worobey, O. G. Pybus, S. K. Ma, C. L. Cheung, J. Raghwani, S. Bhatt, J. S. Peiris, Y. Guan, and A. Rambaut. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122-1125. [DOI] [PubMed] [Google Scholar]
- 72.Snyder, E. E., N. Kampanya, J. Lu, E. K. Nordberg, H. R. Karur, M. Shukla, J. Soneja, Y. Tian, T. Xue, H. Yoo, F. Zhang, C. Dharmanolla, N. V. Dongre, J. J. Gillespie, J. Hamelius, M. Hance, K. I. Huntington, D. Jukneliene, J. Koziski, L. Mackasmiel, S. P. Mane, V. Nguyen, A. Purkayastha, J. Shallom, G. Yu, Y. Guo, J. Gabbard, D. Hix, A. F. Azad, S. C. Baker, S. M. Boyle, Y. Khudyakov, X. J. Meng, C. Rupprecht, J. Vinje, O. R. Crasta, M. J. Czar, A. Dickerman, J. D. Eckart, R. Kenyon, R. Will, J. C. Setubal, and B. W. Sobral. 2007. PATRIC: the VBI PathoSystems Resource Integration Center. Nucleic Acids Res. 35:D401-D406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stadler, K., V. Masignani, M. Eickmann, S. Becker, S. Abrignani, H. D. Klenk, and R. Rappuoli. 2003. SARS—beginning to understand a new virus. Nat. Rev. Microbiol. 1:209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stockman, L. J., L. M. Haynes, C. Miao, J. L. Harcourt, C. E. Rupprecht, T. G. Ksiazek, T. B. Hyde, A. M. Fry, and L. J. Anderson. 2008. Coronavirus antibodies in bat biologists. Emerg. Infect. Dis. 14:999-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tajima, S., T. Takasaki, S. Matsuno, M. Nakayama, and I. Kurane. 2005. Genetic characterization of Yokose virus, a flavivirus isolated from the bat in Japan. Virology 332:38-44. [DOI] [PubMed] [Google Scholar]
- 76.Tandler, B. 1996. Cytomegalovirus in the principal submandibular gland of the little brown bat, Myotis lucifugus. J. Comp. Pathol. 114:1-9. [DOI] [PubMed] [Google Scholar]
- 77.Tang, X. C., J. X. Zhang, S. Y. Zhang, P. Wang, X. H. Fan, L. F. Li, G. Li, B. Q. Dong, W. Liu, C. L. Cheung, K. M. Xu, W. J. Song, D. Vijaykrishna, L. L. Poon, J. S. Peiris, G. J. Smith, H. Chen, and Y. Guan. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J. Virol. 80:7481-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teeling, E. C., M. S. Springer, O. Madsen, P. Bates, S. J. O'Brien, and W. J. Murphy. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307:580-584. [DOI] [PubMed] [Google Scholar]
- 79.Tudge, C. 2000. The variety of life: a survey and a celebration of all the creatures that have ever lived. Oxford University Press, Oxford, England.
- 80.Victoria, J. G., A. Kapoor, K. Dupuis, D. P. Schnurr, and E. L. Delwart. 2008. Rapid identification of known and new RNA viruses from animal tissues. PLoS Pathog. 4:e1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Victoria, J. G., A. Kapoor, L. Li, O. Blinkova, B. Slikas, C. Wang, A. Naeem, S. Zaidi, and E. Delwart. 2009. Metagenomic analyses of viruses in stool samples from children with acute flaccid paralysis. J. Virol. 83:4642-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vijgen, L., E. Keyaerts, E. Moes, I. Thoelen, E. Wollants, P. Lemey, A. M. Vandamme, and M. Van Ranst. 2005. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 79:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vimr, E. R., R. D. McCoy, H. F. Vollger, N. C. Wilkison, and F. A. Troy. 1984. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc. Natl. Acad. Sci. U. S. A. 81:1971-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe, S., K. Maeda, K. Suzuki, N. Ueda, K. Iha, S. Taniguchi, H. Shimoda, K. Kato, Y. Yoshikawa, S. Morikawa, I. Kurane, H. Akashi, and T. Mizutani. 2010. Novel betaherpesvirus in bats. Emerg. Infect. Dis. 16:986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe, S., N. Ueda, K. Iha, J. S. Masangkay, H. Fujii, P. Alviola, T. Mizutani, K. Maeda, D. Yamane, A. Walid, K. Kato, S. Kyuwa, Y. Tohya, Y. Yoshikawa, and H. Akashi. 2009. Detection of a new bat gammaherpesvirus in the Philippines. Virus Genes 39:90-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whitaker, J. O., Jr. 1993. Bats, beetles, and bugs: more big brown bats mean less agricultural pests. Bats 11:23. [Google Scholar]
- 87.Wibbelt, G., A. Kurth, N. Yasmum, M. Bannert, S. Nagel, A. Nitsche, and B. Ehlers. 2007. Discovery of herpesviruses in bats. J. Gen. Virol. 88:2651-2655. [DOI] [PubMed] [Google Scholar]
- 88.Willner, D., M. Furlan, M. Haynes, R. Schmieder, F. E. Angly, J. Silva, S. Tammadoni, B. Nosrat, D. Conrad, and F. Rohwer. 2009. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS One 4:e7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willner, D., R. V. Thurber, and F. Rohwer. 2009. Metagenomic signatures of 86 microbial and viral metagenomes. Environ. Microbiol. 11:1752-1766. [DOI] [PubMed] [Google Scholar]
- 90.Wolfe, N. D., C. P. Dunavan, and J. Diamond. 2007. Origins of major human infectious diseases. Nature 447:279-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Woo, P. C., S. K. Lau, Y. Huang, and K. Y. Yuen. 2009. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 234:1117-1127. [DOI] [PubMed] [Google Scholar]
- 92.Woo, P. C., S. K. Lau, K. S. Li, R. W. Poon, B. H. Wong, H. W. Tsoi, B. C. Yip, Y. Huang, K. H. Chan, and K. Y. Yuen. 2006. Molecular diversity of coronaviruses in bats. Virology 351:180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woo, P. C., M. Wang, S. K. Lau, H. Xu, R. W. Poon, R. Guo, B. H. Wong, K. Gao, H. W. Tsoi, Y. Huang, K. S. Li, C. S. Lam, K. H. Chan, B. J. Zheng, and K. Y. Yuen. 2007. Comparative analysis of twelve genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J. Virol. 81:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woolhouse, M., and E. Gaunt. 2007. Ecological origins of novel human pathogens. Crit. Rev. Microbiol. 33:231-242. [DOI] [PubMed] [Google Scholar]
- 95.Yuan, J., C. C. Hon, Y. Li, D. Wang, G. Xu, H. Zhang, P. Zhou, L. L. Poon, T. T. Lam, F. C. Leung, and Z. Shi. 2010. Intraspecies diversity of SARS-like coronaviruses in Rhinolophus sinicus and its implications for the origin of SARS coronaviruses in humans. J. Gen. Virol. 91:1058-1062. [DOI] [PubMed] [Google Scholar]
- 96.Zhang, H., X. Yang, and G. Li. 1998. Detection of dengue virus genome RNA in some kinds of animals caught from dengue fever endemic areas in Hainan Island with reverse transcription-polymerase chain reaction. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 12:226-228. (In Chinese.) [PubMed] [Google Scholar]