Abstract
Measuring serum androgen levels in women has been challenging due to limitations in method accuracy, precision sensitivity and specificity at low hormone levels. The clinical significance of changes in sex steroids across the menstrual cycle and lifespan has remained controversial, in part due to these limitations. We used validated liquid chromatography tandem mass spectrometry(LC-MS/MS) assays to determine testosterone (T) and dihydrotestosterone (DHT) along with estradiol (E2) and estrone (E1) levels across the menstrual cycle of 31 healthy premenopausal females and in 19 postmenopausal females. Samples were obtained in ovulatory women in the early follicular phase (EFP), midcycle and mid luteal phase (MLP).
Overall, the levels of T, DHT, E2 and E1 in premenopausal women measured by LCMS/MS were lower overall than previously reported with immunoassays. In premenopausal women, serum T, Free T, E2, E1 and SHBG levels peaked at midcycle and remained higher in the MLP, whereas DHT did not change. In postmenopausal women, T, free T, SHBG and DHT were significantly lower than in premenopausal women, concomitant with declines in E2 and E1. These data support the hypothesis that the changes in T and DHT that occur across the cycle may reflect changes in SHBG and estrogen, whereas in menopause, androgen levels decrease. LC-MS/MS may provide more accurate and precise measurement of sex steroid hormones than prior immunoassay methods and can be useful to assess the clinical significance of changes in T, DHT, E2 and E1 levels in females.
Keywords: sex hormones, liquid chromatography mass spectrometry, menopause, menstrual cycle, testosterone, estradiol
Introduction
Immunoassays to measure sex steroid hormones have presented a technical challenge to both researchers and health care providers. Historically, testosterone (T) assays were designed to measure levels in men and may not be accurate nor precise for use in women where levels are usually 10–20-fold lower(1, 2). With declining use of radioimmunoassays (RIA) and the advent of radioimmunometric and other immunoassay methods, the sensitivity, specificity and accuracy of many T measurements have been questioned (3), as reviewed in a recent Endocrine Society position statement (2). In addition, estradiol (E2), present in low quantities after the menopause, can be difficult to measure as well (4, 5). Santen et al. compared estradiol levels by RIA with mass spectrometry and contend that the metabolites of estrogen may interfere with testing by RIA and elevate estradiol (4). The development of accurate and sensitive androgen and estrogen assays are important to define reference ranges in women and men for the diagnosis of hormone deficiency or excess states. In addition, precise assays are important for monitoring hormonal therapy to ensure that supplemental treatment results in levels within the physiological range.
Prior studies using RIA have suggested that T levels in women are highest between days 8–18 of the menstrual cycle and lowest in the early follicular phase and late luteal phase (6–9). In addition, the “normal range” of testosterone levels in women did not account for varying degrees of hirsutism, acne, BMI, presence of polycystic ovarian syndrome or medications that could alter androgen levels (10, 11). Although many commercial assays list the “normal range” of T in women to be up to 90 ng/dl (conversion factor to SI units 3.47), from a clinical standpoint, most experts have argued that a testosterone above 50ng/dl is often associated with clinical hyperandrogenism, such as acne and/or hirsutism (12). A task force appointed by the Endocrine Society published a position statement emphasizing that T values change with age and disease status as well as time of day and concluded that current normative values for women by direct immunoassays across the lifespan are not accurate(2). They suggested that RIA after extraction and chromatography, although more accurate and sensitive than direct assays, needed further validation. The requirement to correct for recovery may affect precision.(2)
Given these limitations, scholars have debated whether true alterations in serum testosterone occur with menopause. Most studies suggest that peak T levels occur in the second decade and then decline with age(13), but they do not abruptly fall in naturally menopausal women due to preservation of theca cell function (14, 15). A cross sectional study by Davison et al. of 1423 women showed no effect of menopause on T levels, but did show a decline dependent on age (14, 16). Surgical menopause, however, results in abrupt decreases in T, by as much as 50% (17–19). To reexamine the changes in sex steroids in women with validated LC-MS/MS methods, we simultaneously measured T, dihydrotestosterone (DHT), (20) E2 and estrone (E1) in pre and post menopausal women screened for absence of signs or symptoms of androgen.
Experimental
Study participants
Healthy female volunteers, ages 20–79 were recruited locally with flyers approved by the Institutional Review Board of the University of Colorado. 31 premenopausal women (ages 23–49) and 19 post menopausal women (ages 42 to 72) were enrolled. Postmenopausal women were not on hormone therapy and were categorized into two groups: less than 5 years (n=9) or more than 5 years after menopause (n=10). Menopause was defined as no menstrual cycle for more than 6 months. A telephone screening provided a review of inclusion and exclusion criteria including: current and prior medical conditions, medication use, menstrual history, and symptoms of hyperandrogenism. Subjects were excluded for any acute or chronic illness, known pituitary or ovarian dysfunction, abnormal liver function, use of oral contraceptives, narcotics, glucocorticoids and/or any medications known to alter sex hormone levels or pituitary function. Premenopausal women were excluded for history of irregular cycles, as well as current or past medical treatment for acne or hirsutism.
All participants provided written informed consent. Blood samples were obtained between 8 and 10 am, centrifuged and sera stored in aliquots at −80 °C until assayed. Height and weight were measured and body mass index (BMI) was calculated. Premenopausal women were sampled three times. The first sample was drawn during the early follicular phase (EFP) of their cycle (Days 1–5). At the initial visit, subjects were given a LH surge detection kit (www.medimpex.com) to monitor morning urine samples 3–5 days prior to the expected day of ovulation. After detection of the LH surge, a second blood sample was obtained within 48 hours (midcycle). The third sample was drawn in ovulating women during the mid luteal phase (MLP), obtained 7–10 days after ovulation. Women who did not detect ovulation were excluded from additional sampling after the EFP (N=10). Post menopausal women were sampled once. Eighteen women had undergone natural menopause, and one woman had undergone surgical oopherectomy.
Hormone Measurements
All hormones except LH, FSH and SHBG were measured in de-identified serum samples by the General Clinical Research Center Core Laboratory and the Endocrine Research Laboratory at Harbor-UCLA Medical Center and Los Angeles Biomedical Research Institute. Serum T and DHT concentrations were measured simultaneously with a validated LC-MS/MS method previously described without modifications (20). Testosterone and DHT (>99% pure Sigma–Aldrich, St. Louis, MO) were used as calibration standards. 1,2 deuterated (D2)-testosterone (>98% pure, Cambridge Isotope Laboratories, Inc., Andover, MA) and 19, 19, 19 trideuterated (D3)-dihydrotestosterone synthesized by Dr. Barry Dent (> 98% pure, Wellington, New Zealand) were used as the internal standard for T and DHT measurements respectively. Calibration standards and test samples were prepared for T and DHT LC-MS/MS using a liquid/liquid extraction twice with 2 mL ethyl acetate: hexanes (3:2 volume: volume). LC-MS/MS were performed with a Shimadzu HPLC system (Columbia, MD) attached to an Applied Biosystems API5000 LC-MS/MS (Foster City, CA) equipped with a TurboIon Spray source. There was no interference of T in the DHT assay and vice versa. The calibration standards showed a linear response from 1 ng/dL (0.35 nmol/L) to 2000 ng/dL (69.3 nmol/L) for T and to 1000 ng/dL (34.4 nmol/L) for DHT. The within and between run precision was less than 5 % for both T and DHT. The recovery of samples spiked with the steroids was between 100 to 113% for T and 98 to 107 % for DHT. The lower limit of quantification (LLOQ) was 2 ng/ 0.069 nmol/L for both steroids determined as the lowest concentration of steroids that can be detected in serum spiked with the analyte with a recovery CV of < 20% (21). Comparison of the results of T and DHT by LC-MS/MS versus RIA has been previously described (22). The reference ranges for the female (n=133 cycling females) were 9.5 to 58.2 ng/dL (0.33 to 2.0 nmol/L) for T and 2.6 to 26.5 ng/dL (.09 to 9.2 nmol/L) for DHT. Free testosterone levels were calculated as described at www.issam.ch (23).
Serum E2 and E1 concentrations were measured simultaneously with a newly developed and validated LC-MS/MS method which was a modification of the method reported by Nelson et al. (24). The major difference from the reported method was in the sample preparation and omission of derivatization. The new method did not employ the densyl chloride derivatization used by Nelson et al.(24). Estradiol and estrone (>99% pure Sigma Aldrich, St. Louis, MO) were used as calibration standards. 2,4,16,16-deuterated (D4)-E2 and E1 (>95% pure, Cambridge Isotope Laboratories, Inc., Andover, MA) were used as the internal standard for E2 and E1 measurements. Calibration standards and test samples were prepared for LC-MS/MS. First the proteins in the sera were precipitated using acetonitrile. The supernatant was dried and reconstituted with phosphate buffered saline and then extracted with 2ml of diethyl ether. LC-MS/MS were performed with a Shimadzu HPLC system (Columbia, MD) attached to an Applied Biosystems API5000 LC-MS/MS (Foster City, CA) equipped with a TurboIon Spray source. There was no interference of E2 in the E1 assay and vice versa. The calibration standards showed a linear response from 1.0pg/ml to 1000pg/ml of E2, and 5.0pg/ml to 5000pg/ml for E1. Analysis of four pools with E2 concentrations ranging from about 10pg/ml to 500pg/ml and for E1 from 140pg/ml to 1000pg/ml for E1, showed the within run precision range to be from 2.78% to 3.58% for E2 and 1.92% to 6.10% for E1. The between run precision for E2 (computed over 27 runs) ranged from 3.7% to 9.8% for E2 and 3.8% to 13.2% for E1. The recovery of samples spiked with the steroids was between 84.4% to 129% with a mean recovery of 101.8% for E2 and 84.2% to 132.6% with a mean recovery of 98.7% for E1. The LLOQ was 2pg/ml for E2 and 5pg/ml for E1. The reference ranges for the female with blood samples collected during the follicular phase (n=35 cycling females) were 7.9 to 189.6 pg/ml for E2 and 21.7 to 234.3 pg/ml for E1. Serum E2 measured by LC-MS/MS and RIA demonstrated good correlation with a correlation coefficient of r = 0.9872 with an intercept of 11.7 pg/mL. When the differences were plotted against the average of the two methods, serum E2 levels below the mean value of 40pg/ml, were lower than corresponding levels from the RIA method. At higher concentrations (>40pg/ml), however, LC-MS/MS results tended to be higher. Comparison between E1 by LC-MS/MS and RIA were not done.
LH, FSH and SHBG levels were measured using validated methods as previously described (25, 26). Serum FSH and LH were measured by the highly sensitive and specific fluoroimmunometric (FIA) assays with reagents provided by Delfia (Wallace, Gaithersburg, MD). The intra-assay coefficient of variations for LH and FSH were 3.9% and 5.4%, respectively; and the inter-assay variations for LH and FSH were 6.8% and 9.8%, respectively (adult normal male range: LH 1.3–8.1 IU/L; FSH 1.4–9.5 IU/L). For both LH and FSH assays, the LOQ was determined to be 0.1 IU/L. SHBG was measured by FIA with reagents provided by Delfia (Wallace, Gaithersburg, MD). The LOQ was determined to be 0.5 nmol/L. The intra- and inter-assay coefficients were 2.3% and 6.1%, respectively. The normal range for this assay is 10.8 to 46.6 nmol/L.
Statistical Analysis
Premenopausal women
Differences in the hormone levels across the menstrual cycle were assessed using a mixed effects model (i.e., repeated measures ANOVA). The final mixed effects model was adjusted for age and body mass index (BMI). A Tukey adjustment was used for pairwise comparisons between different periods in the menstrual cycle. Only subjects with complete data were in the analysis of change over time given that subjects with missing data usually had only EFP data (N=15). In addition, one outlier subject with E2 levels >400 at midcycle and MLP was excluded from analysis. All outcome measures were natural-log transformed prior to analysis, with the exception of free T, which was analyzed on its natural scale.
Postmenopausal women
Two-sample t-tests were used to assess whether hormone levels differed between postmenopausal women <5 years from menopause and ≥5 years from menopause. Because one-third of postmenopausal DHT values were below the detection limit of the assay (<0.069 nmol/L ) (5 in <5 yr post menopause and 2 in ≥5 yr post menopause), we applied two methods of analysis: 1) all values below the detection limit were set equal to just below the detection limit (1.95 ng/dL; assay lower limit of quantification was 2ng/dL) and 2) a truncated distribution method described by Huges (27) and implemented in PROC LIFEREG in SAS v9.1, which more appropriately accounts for variation in the data. The results were consistent with both methods so only the results using the first method of analysis are presented. General linear models (ANCOVA) were used to adjust for age and BMI in the final analyses. When comparing the pre- and postmenopausal women, the EFP samples were used for the premenopausal group whether or not the women ovulated (N=29). Two sample t-tests were used to compare the pre and postmenopausal groups. Final analysis included adjustment for BMI in a general linear model. For all analyses a two-sided p-value less than 0.05 was considered statistically significant.
Results
Steroid hormone levels in women across the menstrual cycle and after menopause
Premenopausal women
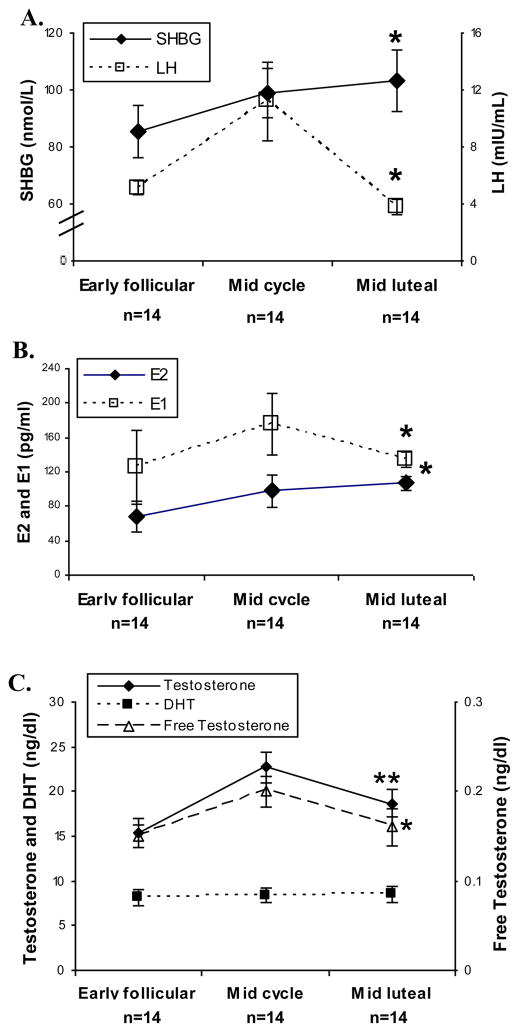
As expected, average LH levels increased at midcycle concomitant with significant increases in E1, E2, and SHBG, which remained high in the MLP (Table 1; Figure 1A and 1B[PL1]). Average T levels significantly changed over the menstrual cycle (p<0.001) (Figure 1C), with midcycle levels approximately 50% higher than those in EFP, 22.7 +/− 1.7 and 15.4 +/− 1.6 ng/dl respectively (p<0.001). The average MLP level of T was 18.7 +/− 1.5 ng/dl, approximately 25% higher than that at EFP (p=0.056), but the midcycle and MLP T levels were not different (p=0.073). This pattern remained consistent after adjusting for age and BMI. Average free T was .20 +/− .017 ng/dl at midcycle, 35% higher than EFP at .15 +/− 0.013 (p<0.013), and did not change from midcycle to MLP (p=0.063). In contrast to T and free T, DHT levels did not change significantly across the menstrual cycle (p=0.59).
Table 1.
Mean and SEM’s of age, BMI, and hormone levels for phases of the cycle for premenopausal women with complete data (n=14).
| Measure | EFP | Midcycle | MLP | P-valuea |
|---|---|---|---|---|
| Age, yr | 35.6 (2.1) | |||
| BMI[C4] (kg/m2) | 23.5 (1.4) | |||
| T[CW5] (ng/dl) | 15.4 (1.6) | 22.7 (1.7) | 18.7 (1.5) | 0.0003 |
| DHT (ng/dl) | 8.1 (0.85) | 8.4 (0.75) | 8.5 (0.88) | 0.59 |
| SHBG (nmol/l) | 85.3 (9.2) | 99.0 (8.8) | 103.4 (10.7) | 0.0011 |
| E2 (pg/ml) | 68.1 (18.6) | 98.1 (19.0) | 106.8 (8.3) | 0.0037 |
| E1 (pg/ml) | 125.4 (43.7)* | 175.8 (36.3) | 134.1 (9.6) | 0.0098 |
| FreeT (ng/dl) | 0.15 (0.013)* | 0.20 (0.017) | 0.16 (0.021) | 0.013 |
| LH (IU/L) | 5.1 (0.50) | 11.2 (2.8) | 3.8 (0.56) | 0.0020 |
| FSH (IU/L) | 6.5 (0.58) | 6.9 (0.70) | 3.0 (0.45) | <0.0001 |
EFP=Early follicular phase and MLP=Mid luteal phase
P-values are for the overall test that any two groups differ.
Bolded values indicate that the period significantly (p<0.05) differs from the other two periods of the cycle.
Figure 1.
Panel A
LH and SHBG levels during the EFP, midcycle and MLP) measured by fluoroimmunometric assay. (*p<0.01)
Panel B
E2 and E1 levels during the EFP, midcycle and MLP measured by LCMS/MS. (*p<0.01)
Panel C
T, free T and DHT levels during the EFP, midcycle and MLP. T and DHT were measured by LCMS/MS (**p<0.001 and p=0.59). Free T was calculated as described in methods. (*p<0.01).
Postmenopausal women compared to premenopausal women
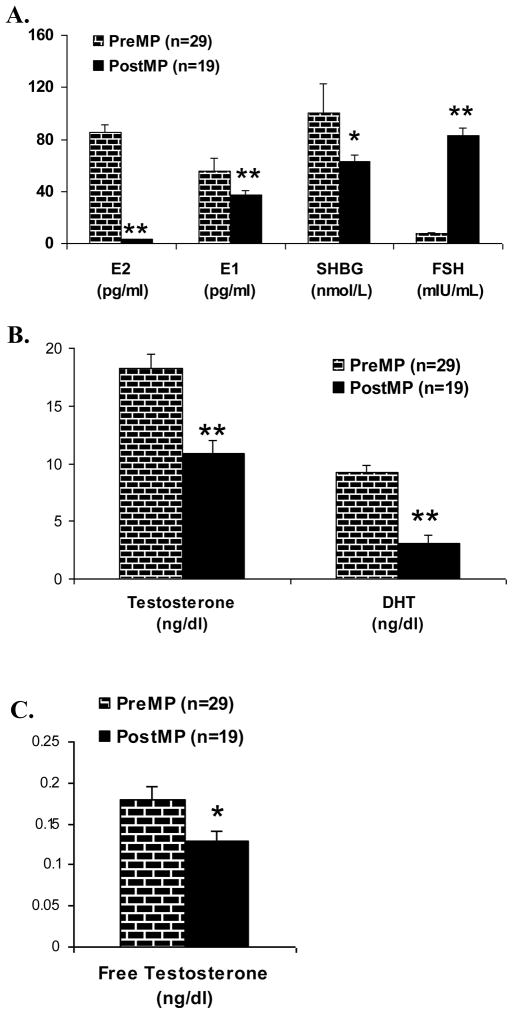
As expected, average LH and FSH levels were significantly higher in postmenopausal women compared to premenopausal women (p<0.001; Table 2 and Figure 2A). All post menopausal women in the <5 year category had elevated FSH ( range 34–118 IU/L). E2 levels were 10 times higher in premenopausal than postmenopausal women (p<0.001; Figure 2A). E1 levels were 100.6 +/− 21.8 pg/ml, approximately two times higher in premenopausal women compared to postmenopausal women. (p<0.029; Figure 2A). Testosterone levels in premenopausal women were 1.5 times the average level of postmenopausal women (p<0.001; Figure 2B). In contrast to the lack of change seen across the menstrual cycle, DHT levels decreased by more than half in postmenopausal women (p<0.001; Figure 2B). As expected, free T levels were also lower in postmenopausal compared to premenopausal women (p=0.013; Figure 2C). The results remained consistent after adjusting for BMI.
Table 2.
Mean and SEM’s of the age, BMI and hormones levels for EFP for all premenopausal women and all postmenopausal women.
| Measure | Premenopause | Postmenopause | |
|---|---|---|---|
| EFP (N=29) | <5 years (N=9) | ≥5 years (N=10) | |
| Age, yr | 34.6 (2.1) | 53.0 (2.4) | 59.3 (5.60) |
| BMI[C6] (kg/m2) | 24.8 (0.98) | 25.7 (3.2) | 25.2 (3.7) |
| T (ng/dl) | 18.3 (1.2) | 11.3 (1.8) | 10.5 (1.3) |
| DHT (ng/dl) | 9.2 (0.67) | 2.6 (0.8) | 3.5 (0.5) |
| SHBG (nmol/l) | 85.4 (6.0) | 57.1 (7.2) | 67.7 (8.4) |
| E2 (pg/ml) | 55.4 (10.3) | 4.9 (1.3)a | 1.3 (0.30) |
| E1 (pg/ml) | 100.6 (21.8) | 39.8 (6.5) | 35.0 (3.7) |
| FreeT (ng/dl) | 0.18 (0.015) | 0.14 (0.023) | 0.12 (0.01) |
| LH (IU/L) | 5.4 (0.35) | 44.8 (5.5) | 37.2 (3.7) |
| FSH (IU/L) | 7.6 (0.88) | 85.9 (9.4) | 81.0 (6.9) |
Bolded values in the EFP column indicate that premenopausal women significantly differ compared to postmenopausal women.
There is a significant difference (p<0.05)between women <5 and ≥5 years postmenopausal.
Figure 2.
Panel A
E2, E1, SHBG and FSH levels during EFP in premenopausal women compared to postmenopausal women. E2 and E1 were measured by LCMS/MS (**p<0.0001). SHBG and FSH levels were measured by fluroimmunometric assay (*p<0.05 and **p<0.0001).
Panel B
T and DHT levels during EFP in premenopausal women compared to postmenopausal women. T and DHT were measured by LCMS/MS (**p<0.001).
Panel C
Free T during EFP in premenopausal women compared to postmenopausal women. Free T was calculated as described in methods. (*p<0.05).
With the exception of E2, sex hormone levels did not significantly differ between women <5 and ≥5 years after menopause (Table 2). E2 levels were more than ≥50% lower in women >5 years after menopause at 1.3 +/− 0.3 pg/ml when compared to women <5 years after menopause at 4.9 +/− 1.3 pg/ml (p<0.001). Again, results did not change after adjusting for BMI.
Discussion
In this study, we used validated LC-MS/MS methods to measure T, DHT, E2 and E1 levels to assess changes in androgen and estrogen levels in pre and postmenopausal women. In the postmenopausal female, accuracy and sensitivity at the lower levels are particularly important. LC-MS/MS specifically measures each steroid hormone without any interference from structurally similar steroids and are not affected by non specific binding to the primary antibody in immunoassays. Thus, it is anticipated that hormone levels will be lower using LC-MS/MS because of the lack of interference. Overall, serum levels of these sex hormones were lower when measured with mass spectrometry when compared with RIA, confirming recent reports by others in men and women (4, 28). Others have suggested that precise measurement of post menopausal estradiol levels may be useful in predicting fracture risk as well as breast cancer (4). LC-MS/MS provides more accurate and precise T measurements in the lower range than many radioimmuno- or immunometric assays (2, 29–31). These data suggest that prior studies defining “low T” states may have been limited by their lack of sensitivity to accurately measure low levels in women.
In premenopausal women, our data confirmed earlier reports based on RIA that indicated T levels peak at mid cycle. These changes in total T levels across the menstrual cycle reflect those of SHBG and E2 levels, which progressively increase across the follicular phase However, the peak absolute concentrations were lower in our data. Our highest T levels occurred midcycle at 22.7 +/− 1.7 ng/dl. (Table 1) A recent study by Salameh et al. using high turbulence LC-MS/MS gave the upper limit of normal for women aged 18–29 as 44ng/dL (30). They included 235 women who were “normal” by self report and did not specifically exclude women with clinical or biochemical hyperandrogenism and/or anoulvation, which may explain lower levels in our population.
At present, no direct free T assay by LC-MS/MS is available and the commercially available direct free T assay measurements are inadequate and often reflect total T(1). Cappola and colleagues measured free T in post menopausal women by equilibrium dialysis method with mean levels of 2.8 +/− 2.5 pg/ml (32). The range of their normal levels was broad, from 0.3 to 20.6 pg/ml, but 60% of subjects had a BMI>25. These assays, however, are more technically challenging and are generally limited to research laboratories. A similar mid cycle peak was also noted in a study using equilibrium dialysis examining free T across the cycle of healthy females by Sinha-Hikim et al.(33). In our study, we calculated free T using a previously validated formula (34) and a similar but very modest trend to peak mid cycle was noted for free T (.20 +/−.017. (Table 1)). Calculation of free T is reported to correlate well with measurements by equilibrium dialysis(2, 34). However, with the myriad of factors that modulate SHBG in many disease conditions, it is unclear if the calculation of free T always reflects bioavailable T activity (35). Development of direct free T measurements by LC-MS/MS would confirm or refute the trend we and others have noted.
In contrast to T and free T, DHT levels did not change across the menstrual cycle, but did decrease significantly with menopause. DHT levels in the post menopausal female have not previously been reported by LC-MS/MS methods. These data suggest that the changes seen in total T over the menstrual cycle may be predominantly reflective of an estrogen-induced effect to increase levels of SHBG, consistent with the modest effect on free T, and no effect on DHT levels. In contrast, a consistent decrease in both androgens occurs with menopause. We interpret this cautiously, as many have questioned the importance of circulating levels of DHT in women, suggesting that serum androgen levels may not reflect the effects seen at the tissue level (36). Since DHT levels in women are quite low (only 1/10 of T levels), it is not surprising that the relevance of these low values has not been explored using the traditional and relatively insensitive commercial radioimmunoassay. DHT is produced in individual tissue compartments from 5 α reduction (5 α-reductase 1 and 2) of testosterone and from androstenedione after conversion to 5 α-androstane-3,17-dione (36). In men, DHT levels have been shown to play a role in balding at the level of the hair follicle, and inhibitors of 5 α-reductase are used for its treatment (37). However, in females, the role of DHT is less clear. In our study, despite improved assay sensitivity, one-third of the postmenopausal women had undetectable DHT levels. The significant decrease in serum DHT levels in menopausal women in this study suggests that levels do decline with cessation of ovarian function and raises the issue as to whether some postmenopausal women may be relatively androgen insufficient. Future studies are needed to assess if DHT levels measured by LCMS/MS will be useful in different physiologic and pathophysiologic states of androgen deficiency or excess.
Our findings describing changes in E1, E2 and SHBG with menopause are consistent with earlier findings by RIA (38, 39). E2 is converted to E1 in the presence of hydroxysteroid dehydrogenase in fat (40) and therefore BMI correlates more with E1 than E2. SHBG also decreases with increasing BMI (39)and with menopause (38, 39). Others have reported an increase in E1/E2 ratio during the perimenopause (39) and some have advocated using the ratio of E1/E2 to monitor the menopausal transition (39). Kushnir et al. reported post menopausal ranges by LCMS/MS, for E1 and E2 as 3–32 pg/ml and 2–21 pg/ml respectively, however they did not delineate the changes in E1 occurring over the course of a menstrual cycle in their premenopausal samples (41). Whether more sensitive E1 assays will be useful in addition to E2 levels in clinical medicine remains to be tested.
Strengths of our study were that the subjects were carefully screened as a healthy population free of androgen excess. In addition, normal menstrual cycles were documented in 21 of our 31 premenopausal women. Ovulation kits confirmed that the sampling in premenopausal women was performed accurately at the midcycle peak. One third of subjects were non-ovulatory (10/31; 32%) and were excluded from sampling after EFP. This could be due to several reasons. Many healthy ovulatory women have some non-ovulatory cycles over the course of a year (42). There may have been variability in subject interpretation of the LH surge kit despite attempts at training, and some women may have had anovulatory disorders that were not detected by our screening procedures. Measurement of progesterone in the luteal phase would have clarified this issue but was not available.
There are several advantages and limitations to LC-MS/MS measurement of sex hormones when compared with immunoassays(30, 43). LC-MS/MS is useful for small molecules and is highly sensitive. Limitations are that the initial instruments cost is higher than the automated assays, although the cost per sample can be low. In addition, the LC-MS/MS approach requires processing of samples by extraction and a higher level of technical training to utilize the equipment. However, the accuracy at low levels and ability to run multiple analyses on the same sample will likely decrease costs and increase clinical utility (43). Additional studies are needed in normal patient populations and those with hormone excess and deficient states to further characterize the clinical utility of these new approaches to the measurement of sex steroids.
research highlights.
Levels of T, DHT, E2 and E1 in premenopausal women measured by LCMS/MS are lower overall than reported with immunoassays.
In premenopausal women, testosterone and free testosterone peak at midcycle but DHT does not change.
In postmenopausal women, T, free T, SHBG and DHT are significantly lower than in premenopausal women.
Changes in T and DHT across the cycle may reflect changes in SHBG and estrogen, whereas in menopause, androgen levels decrease.
LC-MS/MS may provide more accurate measurement of sex steroid hormones than prior immunoassay methods.
Acknowledgments
We thank Gabriela Molina for her assistance in patient recruitment. We thank Steve Shiraishi and Andrew Leung at Harbor-UCLA Medical Center and Los Angeles Biomedical Research Center for their help with the development of LC-MS/MS methods, the staff at the General Clinical Research Center Core Laboratory (MO1 RR00425) and the Endocrine Research Laboratory for their assistance in the assays and Kayla Carstens at the UCD Adult CTRC Core Laboratory for her technical expertise.
Supported by VA Merit to MEW and MO1 00425 to the General Clinical Research Center at Harbor-UCLA Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 89(2):534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 2.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 3.Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 49(8):1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- 4.Santen RJ, Lee JS, Wang S, Demers LM, Mauras N, Wang H, Singh R. Potential role of ultra-sensitive estradiol assays in estimating the risk of breast cancer and fractures. Steroids. 73(13):1318–1321. doi: 10.1016/j.steroids.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. doi: 10.1016/j.jsbmb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril. 77(Suppl 4):S83–88. doi: 10.1016/s0015-0282(02)02965-5. [DOI] [PubMed] [Google Scholar]
- 7.Salonia A, Pontillo M, Nappi RE, Zanni G, Fabbri F, Scavini M, Daverio R, Gallina A, Rigatti P, Bosi E, Bonini PA, Montorsi F. Menstrual cycle-related changes in circulating androgens in healthy women with self-reported normal sexual function. J Sex Med. 5(4):854–863. doi: 10.1111/j.1743-6109.2008.00791.x. [DOI] [PubMed] [Google Scholar]
- 8.Massafra C, De Felice C, Agnusdei DP, Gioia D, Bagnoli F. Androgens and osteocalcin during the menstrual cycle. J Clin Endocrinol Metab. 84(3):971–974. doi: 10.1210/jcem.84.3.5512. [DOI] [PubMed] [Google Scholar]
- 9.Judd HL, Yen SS. Serum androstenedione and testosterone levels during the menstrual cycle. J Clin Endocrinol Metab. 36(3):475–481. doi: 10.1210/jcem-36-3-475. [DOI] [PubMed] [Google Scholar]
- 10.Abraham GE. Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J Clin Endocrinol Metab. 39(2):340–346. doi: 10.1210/jcem-39-2-340. [DOI] [PubMed] [Google Scholar]
- 11.Krug R, Pietrowsky R, Fehm HL, Born J. Selective influence of menstrual cycle on perception of stimuli with reproductive significance. Psychosom Med. 56(5):410–417. doi: 10.1097/00006842-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Guay A, Jacobson J, Munarriz R, Traish A, Talakoub L, Quirk F, Goldstein I, Spark R. Serum androgen levels in healthy premenopausal women with and without sexual dysfunction: Part B: Reduced serum androgen levels in healthy premenopausal women with complaints of sexual dysfunction. Int J Impot Res. 16(2):121–129. doi: 10.1038/sj.ijir.3901176. [DOI] [PubMed] [Google Scholar]
- 13.Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab. 80(4):1429–1430. doi: 10.1210/jcem.80.4.7714119. [DOI] [PubMed] [Google Scholar]
- 14.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 90(7):3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 15.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 92(8):3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 16.Spencer JB, Klein M, Kumar A, Azziz R. The age-associated decline of androgens in reproductive age and menopausal Black and White women. J Clin Endocrinol Metab. 92(12):4730–4733. doi: 10.1210/jc.2006-2365. [DOI] [PubMed] [Google Scholar]
- 17.Judd HL, Lucas WE, Yen SS. Effect of oophorectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. Am J Obstet Gynecol. 118(6):793–798. doi: 10.1016/0002-9378(74)90490-6. [DOI] [PubMed] [Google Scholar]
- 18.Lobo RA. Androgens in postmenopausal women: production, possible role, and replacement options. Obstet Gynecol Surv. 56(6):361–376. doi: 10.1097/00006254-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 85(2):645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 20.Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin Chem. 54(11):1855–1863. doi: 10.1373/clinchem.2008.103846. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. PharmRes. 24(10):1962–1973. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 22.Shiraishi S, Lee PW, Leung A, Goh VH, Swerdloff RS, Wang C. Simultaneous measurement of serum testosterone and dihydrotestosterone by liquid chromatography-tandem mass spectrometry. Clin Chem. 54(11):1855–1863. doi: 10.1373/clinchem.2008.103846. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 24.Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 50(2):373–384. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- 25.Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 85(12):4500–4510. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Wang XH, Nelson AL, Lee KK, Cui YG, Tong JS, Berman N, Lumbreras L, Leung A, Hull L, Desai S, Swerdloff RS. Levonorgestrel implants enhanced the suppression of spermatogenesis by testosterone implants: comparison between Chinese and non-Chinese men. J Clin Endocrinol Metab. 91(2):460–470. doi: 10.1210/jc.2005-1743. [DOI] [PubMed] [Google Scholar]
- 27.Huges MD. Analyses and design issues using censored biomarker measurements with an example of viral load measurements in HIV clinical trials. Statistics in Medicine. :193171–3191. doi: 10.1002/1097-0258(20001215)19:23<3171::aid-sim619>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Hsing AW, Stanczyk FZ, Belanger A, Schroeder P, Chang L, Falk RT, Fears TR. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol Biomarkers Prev. 16(5):1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- 29.Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 149(1):63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 75(2):169–175. doi: 10.1016/j.steroids.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, Meikle AW. Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin Chem. 52(1):120–128. doi: 10.1373/clinchem.2005.052167. [DOI] [PubMed] [Google Scholar]
- 32.Cappola AR, Ratcliffe SJ, Bhasin S, Blackman MR, Cauley J, Robbins J, Zmuda JM, Harris T, Fried LP. Determinants of serum total and free testosterone levels in women over the age of 65 years. J Clin Endocrinol Metab. 92(2):509–516. doi: 10.1210/jc.2006-1399. [DOI] [PubMed] [Google Scholar]
- 33.Sinha-Hikim I, Arver S, Beall G, Shen R, Guerrero M, Sattler F, Shikuma C, Nelson JC, Landgren BM, Mazer NA, Bhasin S. The use of a sensitive equilibrium dialysis method for the measurement of free testosterone levels in healthy, cycling women and in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 83(4):1312–1318. doi: 10.1210/jcem.83.4.4718. [DOI] [PubMed] [Google Scholar]
- 34.Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab. 89(2):525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- 35.Sartorius G, Ly LP, Sikaris K, McLachlan R, Handelsman DJ. Predictive accuracy and sources of variability in calculated free testosterone estimates. Ann Clin Biochem. 46(Pt 2):137–143. doi: 10.1258/acb.2008.008171. [DOI] [PubMed] [Google Scholar]
- 36.Stanczyk FZ. Diagnosis of hyperandrogenism: biochemical criteria. Best Pract Res Clin Endocrinol Metab. 20(2):177–191. doi: 10.1016/j.beem.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Vierhapper H, Maier H, Nowotny P, Waldhausl W. Production rates of testosterone and of dihydrotestosterone in female pattern hair loss. Metabolism. 52(7):927–929. doi: 10.1016/s0026-0495(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 38.Gershagen S, Doeberl A, Jeppsson S, Rannevik G. Decreasing serum levels of sex hormone-binding globulin around the menopause and temporary relation to changing levels of ovarian steroids, as demonstrated in a longitudinal study. Fertil Steril. 51(4):616–621. doi: 10.1016/s0015-0282(16)60609-x. [DOI] [PubMed] [Google Scholar]
- 39.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 61(1–2):67–77. doi: 10.1016/j.maturitas.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Deslypere JP, Verdonck L, Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab. 61(3):564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- 41.Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, Bunker AM, Meikle AW. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol. 129(4):530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- 42.Prior JC, Vigna YM, Schechter MT, Burgess AE. Spinal bone loss and ovulatory disturbances. N Engl J Med. 323(18):1221–1227. doi: 10.1056/NEJM199011013231801. [DOI] [PubMed] [Google Scholar]
- 43.Vogeser M, Parhofer KG. Liquid chromatography tandem-mass spectrometry (LC-MS/MS)--technique and applications in endocrinology. Exp Clin Endocrinol Diabetes. 115(9):559–570. doi: 10.1055/s-2007-981458. [DOI] [PubMed] [Google Scholar]