Abstract
Objectives
To evaluate and validate cyst fluid CEA and amylase in differentiating 1) non-mucinous from mucinous pancreatic cystic lesions (PCLs), 2) benign mucinous from malignant mucinous PCLs, and 3) pseudocysts from non-pseudocysts (amylase only).
Methods
A retrospective analysis of patients with histologically confirmed PCLs from February 1996 to April 2007 was performed. Cyst fluid CEA (n=124) and/or amylase (n=91) were measured and correlated to cyst type.
Results
CEA levels (p = 0.0001), but not amylase, were higher in mucinous vs. non-mucinous cysts. The sensitivity, specificity and diagnostic accuracy of CEA ≥200 ng/ml for diagnosis of mucinous PCLs were 60%, 93%, and 72% respectively. CEA levels did not differentiate benign from malignant mucinous cysts. While amylase levels were higher in pseudocysts than non-pseudocysts (p=0.009), 54% of non-inflammatory PCLs had a level greater than 250 IU/L, including mucinous cystic neoplasms (MCN) (median 6,800, IQR 70 – 25,295). Malignant mucinous cysts had lower amylase levels than benign mucinous cysts (p = 0.0008).
Conclusion
Cyst fluid CEA and amylase levels are suggestive, but not diagnostic in differentiating pancreatic cystic lesions. Unlike CEA, amylase may help differentiate benign from malignant mucinous cysts. Novel biomarkers are needed.
Keywords: Pancreatic Cysts; Neoplasms, Cystic, Mucinous, and Serous; Biological Tumor Marker; Carcinoembryonic Antigen; Amylase
INTRODUCTION
Technological improvements and increased utilization of non-invasive imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) are identifying pancreatic cystic lesions (PCL) with increasing frequency.1, 2 Mucinous pancreatic cysts are clinically important because they represent precursor lesions to pancreatic adenocarcinoma. Since current treatment involves surgical resection, various tests have arisen to help predict which cysts are malignant or pre-malignant with a high likelihood of malignant transformation.3, 4
Previous studies have characterized the performance of cyst fluid markers including carcinoembyronic antigen (CEA) and amylase.5, 6 CEA has been proposed as a marker with high diagnostic accuracy in differentiating mucinous cysts with malignant potential (mucinous cystic neoplasm and intraductal papillary mucinous neoplasm) and non-mucinous benign cysts (serous cystic neoplasms and pseudocysts). Cyst fluid amylase levels may help differentiate pseudocysts from primary cysts. The primary objective of this study was to evaluate and validate the diagnostic performance of cyst fluid CEA and amylase in differentiating mucinous from non-mucinous pancreatic cysts, and benign mucinous from malignant mucinous cysts. Another objective for amylase was to determine whether levels could differentiate pseudocysts from non-pseudocysts.
METHODS
With approval of the Mayo Clinic Institutional Review Board, all patients from February 1996 to April 2007 seen at the Mayo Clinic- Rochester with 1) a pancreatic cyst, 2) cyst fluid CEA and/or amylase level, and 3) histological diagnosis as a gold standard for the cyst type, were identified. The medical charts of these patients were then retrospectively reviewed and clinical data were abstracted. Sources for histological diagnosis came mostly from surgical resections, but also included endoscopic tru-cut biopsies, or positive cytology by endoscopic fine needle aspiration. Non-diagnostic and normal cytological samples were not included. Histology was considered benign if there was no evidence of carcinoma in situ or invasive carcinoma. Differentiation into degrees of benign histological atypia was not included. The histological cyst types included in this analysis were mucinous cystic neoplasms (MCN), intraductal papillary mucinous neoplasms (IPMN), serous cystadenomas (SCA), pseudocysts (PC), pancreatic neuroendocrine tumors (Pancreatic-NET), and others. Branch duct, main duct, and mixed duct IPMN PCLs were included in one category. All cyst fluid analysis was performed in the Mayo Clinic Department of Pathology Clinical laboratory.
Descriptive statistics of each biomarker were performed and comparison of levels was performed between mucinous and non-mucinous cysts, and benign mucinous and malignant mucinous cysts. An additional comparison was performed between pseudocysts and non-pseudocysts for cyst fluid amylase levels. Based on the distributions of the cyst fluid levels, non-parametric assumptions were used for comparison testing (Wilcoxon rank-sum test). A receiver operator curve was generated to characterize the diagnostic performance of cyst fluid CEA to differentiate mucinous from non-mucinous cysts, and cyst fluid amylase to differentiate pseudocyst from non-pseudocyst.
Previous thresholds for cyst fluid CEA and amylase levels have been reported to be clinically useful.5, 6 These levels for CEA include a level less than 5 nanograms/milliliter (ng/ml) for non-mucinous cysts, and a level greater than 200 or 800 ng/ml for mucinous cysts. For amylase, a level less than 250 international units/liter (IU/L) is reportedly specific for non-pseudocysts. Using these thresholds, the sensitivity, specificity, and diagnostic accuracy of CEA and amylase were measured. Statistical analysis was performed using STATA 11.0 (College Station, TX).
RESULTS
A total of 126 patients were included in this study, where 104 underwent surgical resection. Of the remaining 22 patients with histology by tru-cut or cytology, 11 were cancer and 11 were non-cancer. Six out of these patients had at least 1 year of follow-up without evidence of cancer. The remaining 5 patients had a diagnosis of serous cystadenomas that correlated with recognized imaging of these types of cysts.
Of the total group with histology, 124 patients had a cyst fluid CEA level and 91 patients had a cyst fluid amylase level, and 89 patients had both. Of the 124 patients with cyst fluid CEA, there were 104 benign cysts- 21 MCN, 45 IPMN, 15 SCA, 20 PC, and 3 other cysts (a mixed serous mucinous cystadenoma, a lymphoepithelial cyst, and a case of polycystic disease). There were 20 malignant cysts- 15 mucinous cancers, 4 Pancreatic-NET, and 1 other (a solid cystic pseudopapillary tumor). Of the 91 patients with cyst fluid amylase level, there were 73 benign cysts- 16 MCN, 25 IPMN, 11 SCA, 19 PC, and 2 other cysts. There were 18 malignant cysts- 13 mucinous cancers, 4 Pancreatic-NET, and 1 other. Distribution of histological cyst type for each group is summarized in Table 1.
Table 1.
Distribution of histologically proven cyst types of patients with cyst fluid CEA and amylase, collected from February 1996 to April 2007.
| CEA (N = 124) |
Amylase (N = 91) |
|
|---|---|---|
| Benign Cysts | ||
| Mucinous Cystic Neoplasm (MCN) | 21 | 16 |
| Intraductal Papillary Mucinous Neoplasm (IPMN) | 45 | 25 |
| Serous Cystadenoma (SCA) | 15 | 11 |
| Pseudocyst (PC) | 20 | 19 |
| Other | 3 | 2 |
| Malignant Cysts | ||
| Mucin Cancers (includes PDAC*) | 15 | 13 |
| Pancreatic Neuroendocrine Tumor (Pancreatic-NET) | 4 | 4 |
| Other | 1 | 1 |
PDAC = pancreatic ductal adenocarcinoma
Carcinoembryonic Antigen (CEA)
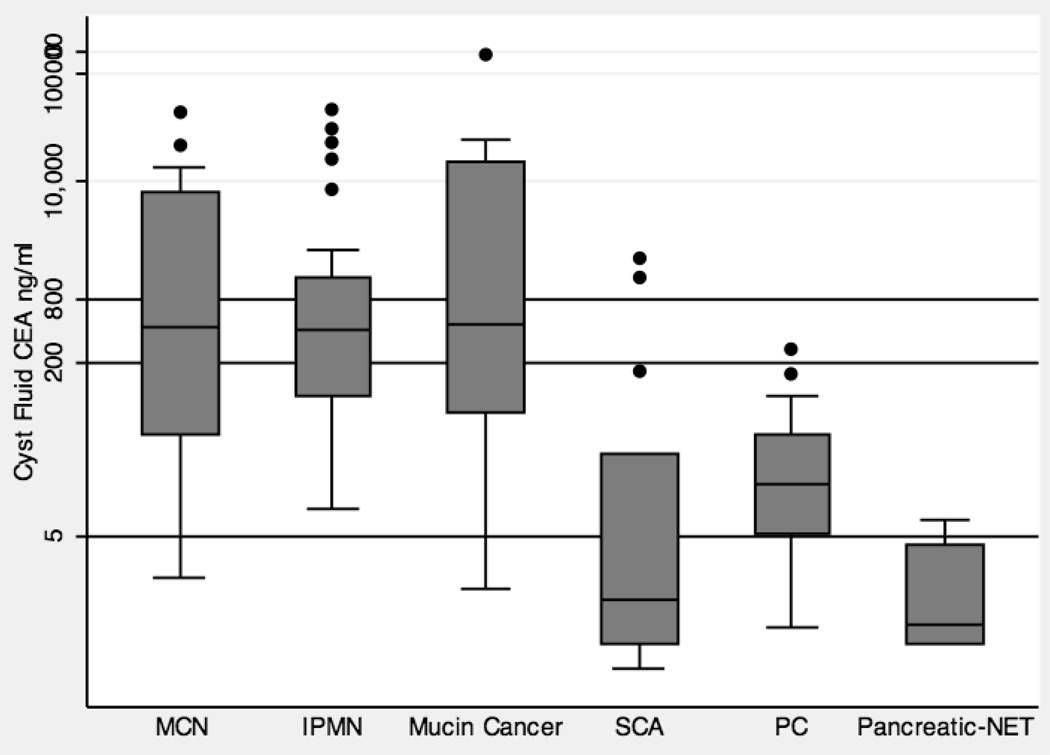
A box plot that graphically represents the distribution of cyst fluid CEA levels by cyst type is shown in Figure 1. The median (interquartile range, IQR) for benign PCLs including MCN, IPMN, SCA, and PC were 428 ng/ml (44 – 7,870), 414 ng/ml (102 – 1,223), 1.3 ng/ml (0.5 – 29), and 16 ng/ml (5.2 – 45) respectively. The median (IQR) for malignant mucin cancers, and Pancretic-NET PCLs were 458 ng/ml (71 – 14,990), and 0.8 (0.5 – 4) respectively.
Figure 1.
Box plot that graphically represents the distribution of cyst fluid CEA levels by cyst type. The y-axis is on a logarithmic scale (ng/ml). There are three lines that represent commonly used cutoff levels, 800 ng/ml, 200 ng/ml and 5 ng/ml.
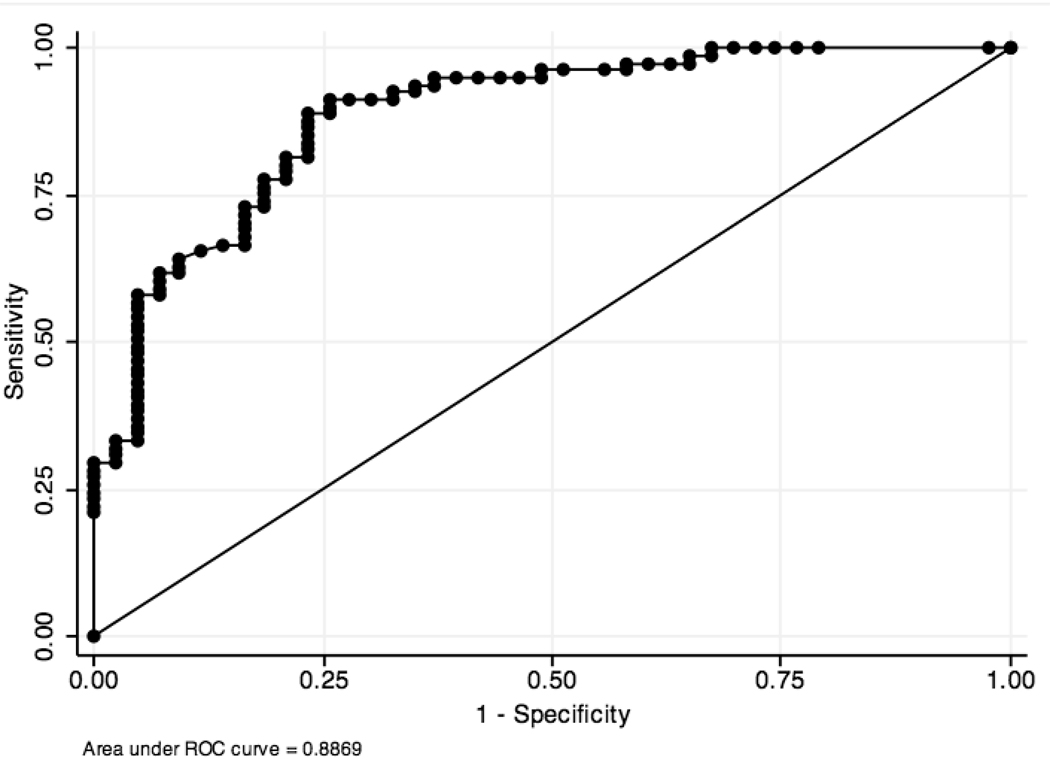
Table 2 summarizes the difference in CEA levels between the pre-specified cyst type categories. There was a significant difference between non-mucinous (median 7.2 ng/ml) and mucinous cysts (median 422.0 ng/ml) (p= 0.0001). A receiver operator curve (ROC) was constructed and this produced an area under the curve (AUC) of 0.89 (95% confidence interval (CI) 0.83 – 0.94) (Figure 2). The cutoff level that provided the highest diagnostic accuracy was 30 ng/ml. At this level, the sensitivity for mucinous cysts was 91% and the specificity was 74% with a diagnostic accuracy of 85%.
Table 2.
Comparison of median levels of each cyst fluid marker using different categories of cysts.
| CEA (ng/ml) | Amylase (IU/L) | |||
|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | |
| Non-Mucinous Cysts | 43 | 7 (1 – 36)* | 37 | 2,560 (103 – 17,000) |
| Mucinous Cysts | 81 | 422 (87 – 2,310) | 54 | 4,085 (68 –23,990) |
| Benign Mucinous Cysts | 66 | 418 (96 – 1,908) | 41 | 5,090 (110 – 31,500)* |
| Malignant Mucinous Cysts | 15 | 458 (71 – 14,990) | 13 | 60 (5 – 12,200) |
| Pseudocysts | 19 | 17,600 (7,000 – 29,500)* | ||
| Non-Pseudocysts | 72 | 876 (52 – 19,860) | ||
p < 0.05
Figure 2.
Receiver operator curve of the diagnostic performance of cyst fluid CEA to diagnose non-mucinous from mucinous cysts.
A CEA cutoff level of > 200 ng/ml for the diagnosis of mucinous PCLs had a sensitivity of 60%, specificity of 93%, and diagnostic accuracy of 72% (Figure 1). A CEA cutoff of > 800 ng/ml had a sensitivity of 38%, specificity of 95%, and diagnostic accuracy of 58% (Figure 1). The cause of decreased specificity included 1 out of 20 (5%) PC lesions that had a CEA > 200 ng/ml and 2 out of 15 (13%) SCA PCLs that had a CEA > 800 ng/ml. Cyst fluid CEA of < 5 ng/ml for the diagnosis of non-mucinous lesions (SCN, PC, Pancreatic-NET, and other cysts) had a sensitivity of 44%, specificity of 96%, and diagnostic accuracy of 78%. The sensitivity was low because 5 out of 15 (33%) SCA PCLs had CEA > 5 ng/ml with the highest level 1900 ng/ml. Only 5 out of 20 (25%) patients with a PC had a cyst fluid CEA < 5 ng/ml. Three (4%) mucinous cystic neoplasms had a CEA < 5 ng/ml including 1 malignant mucinous cyst with a CEA of 1.6 ng/ml.
There was no significant difference in cyst fluid CEA levels between benign mucinous cysts and malignant mucinous cysts (p=0.52). The median (IQR) CEA level for benign mucinous cysts and malignant mucinous cysts were 418 ng/ml (96 – 1,908) and 458 ng/ml (71 – 14,990) respectively.
Amylase
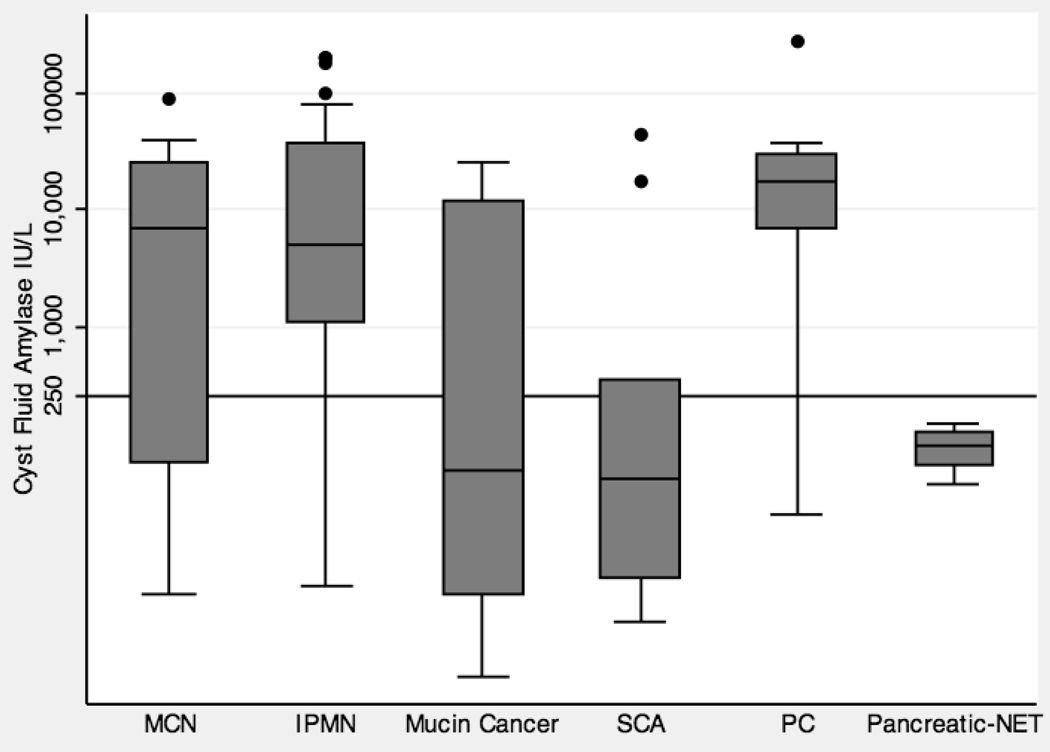
A box plot that graphically represents the distribution of cyst fluid amylase levels by cyst type is shown in Figure 3. The median (interquartile range, IQR) for benign PCLs including MCN, IPMN, SCA, and PC were 6,800 IU/L (70 – 25,295), 5,090 IU/L (1,119 – 38,290), 51 IU/L (7 – 349), and 17,600 IU/L (7,000 – 29,500) respectively. The median (IQR) for malignant mucin cancers and Pancreatic-NET PCLs were 60 IU/L (5 – 12,200), and 94 IU/L (65 – 124) respectively.
Figure 3.
Box plot that graphically represents the distribution of cyst fluid amylase levels by cyst type. The y-axis is on a logarithmic scale (IU/L). There is one line that represents the commonly used cutoff level of 250 IU/L.
Table 2 summarizes the difference in amylase levels between the pre-specified cyst type categories. There was no significant difference between non-mucinous (median (IQR) 2,560 IU/L (103 – 17,000)) and mucinous cysts (median (IQR) 4,085 IU/L (68 – 23,990)) (p= 0.71). A significant difference was observed between benign mucinous cysts and malignant mucinous cysts, and between pseudocysts and non-pseudocysts. The median (IQR) level for benign mucinous and malignant mucinous cysts was 5,090 IU/L (110 – 31,500) and 60 IU/L (5 – 12,200), respectively (p = 0.008). The median (IQR) level for pseudocysts and non-pseudocysts was 17,600 IU/L (7,000 – 29,500) and 876 IU/L (52 – 19,860), respectively (p = 0.009).
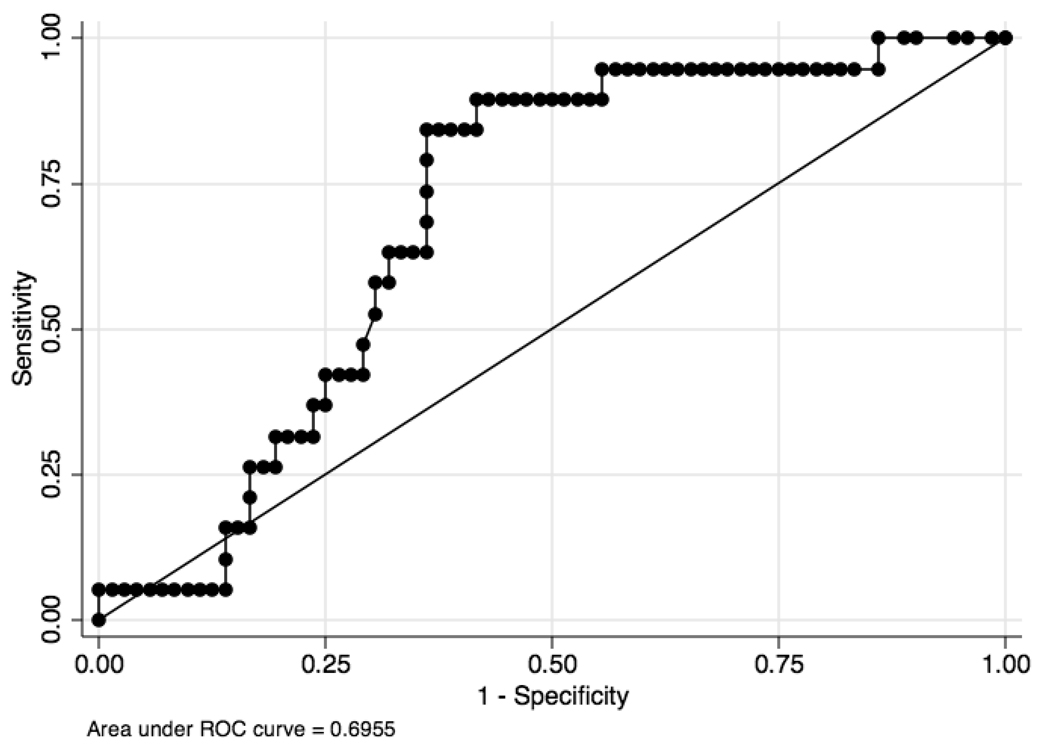
A receiver operator curve (ROC) of cyst fluid amylase levels for pseudocysts and non-pseudocysts produced an AUC of 0.69 (95% CI, 0.58 – 0.81) (Figure 4). The cutoff level that provided the highest combination of sensitivity, specificity and diagnostic accuracy was 5,680 IU/L. At this level, the sensitivity for pseudocysts was 84% and the specificity was 64% with a diagnostic accuracy of 68%. Using an amylase cutoff level of 250 IU/L, the sensitivity, specificity, and diagnostic accuracy for pseudocysts was 89%, 45%, and 54%, respectively. Seventeen of the 19 pseudocysts had a level greater than 250, but this was also seen in 54% of non-inflammatory PCLs. Further review of the 2 cases of pseudocysts with a low amylase (25 and 179) suggested that they were old and the diagnosis was not suspected pre-operatively.
Figure 4.
Receiver operator curve of the diagnostic performance of cyst fluid amylase levels to diagnose non-pseudocysts from pseudocysts.
Utility of Combined Analysis
Since a significant difference was observed in cyst fluid amylase levels between benign mucinous cysts and malignant mucinous cysts but not with cyst fluid CEA, a combined CEA and amylase approach was evaluated to determine if the malignant mucinous cysts could be more accurately diagnosed. There were 89 patients with both a cyst fluid CEA and amylase of which 12 patients (13%) had a malignant mucinous cyst. A cyst fluid CEA level of > 30 ng/ml and a cyst fluid amylase level of < 250 IU/L improved the diagnostic accuracy of malignant mucinous cysts from 46% to 78% compared with CEA > 30 ng/ml alone. This improvement was observed due to an increase in specificity (from 40% to 82%) despite a decrease in sensitivity (from 83% to 50%).
DISCUSSION
The clinical challenge in the management of pancreatic cysts is to identify those patients who should undergo pancreatic resection, a relatively complex surgical procedure associated with substantial morbidity, for early non-metastatic cancer and high-risk pre-cancerous cystic lesions while appropriately observing those patients with limited or no potential for malignant transformation. Mucin-producing cystic lesions that include mucinous cystic neoplasms and intraductal papillary mucinous neoplasms have malignant potential and are generally considered appropriate for surgical resection, while non-mucinous cysts such as serous cystadenomas and pseudocysts do not. Cystic pancreatic-NETs represent a rare malignant non-mucinous cyst.
Cyst fluid CEA and amylase have been evaluated for this purpose in earlier studies. Previous work by Brugge and colleagues evaluated various cyst fluid markers using 112 patients with histology and identified CEA as having the highest clinical utility in discriminating mucinous from non-mucinous cystic lesions.5 They reported that a level greater than 192 ng/ml had a sensitivity, specificity, and diagnostic accuracy of 73%, 84%, and 79%, respectively for mucinous cystic lesions. A later study incorporated these results into a pooled analysis with other smaller studies that evaluated CEA as a cyst fluid marker. This analysis identified cutoff levels of 5 ng/ml to be highly specific (95%) for non-mucinous cysts and 800 ng/ml to be highly specific (98%) for mucinous cysts. This analysis also pooled studies of cyst fluid amylase and found a level less than 250 U/L to be highly specific (98%) for non-pseudocysts.6
More recent studies have reported on CEA utility to diagnose mucinous and malignant cysts. Khalid and colleagues reported on 113 patients where 76 of them had an available cyst fluid CEA for comparison. Similar to earlier studies, they found a significant difference in CEA levels between mucinous and non-mucinous cysts. Using a cutoff of 192 ng/ml, they reported a similar sensitivity, specificity, and diagnostic accuracy (64%, 89%, and 77%, respectively) to the earlier study by Brugge and colleagues.7 Sawhney and colleagues studied cyst fluid CEA utility among 19 patients with histological conclusion and they reported CEA (cutoff 192 ng/ml) to have a sensitivity, specificity, and diagnostic accuracy of 82%, 100%, and 84%, respectively.8
This study presents a relatively large sample with a histological gold standard to evaluate the performance of cyst fluid CEA and amylase in the diagnosis of PCL’s. Similar to earlier findings, this study observed a significant difference in CEA levels between non-mucinous and mucinous cysts. Applying a similar cutoff of 200 ng/ml, a similar sensitivity (62%), specificity (93%), and diagnostic accuracy (73%) was appreciated. Similarly, using a cutoff of < 250 IU/L, amylase had a sensitivity of 45% and specificity of 89% to diagnose non-pseudocyst PCL’s. These results are fairly consistent with Van der Waaij and colleagues pooled analysis.6
This study supports earlier observations that CEA and amylase can be strongly suggestive, but not diagnostic in differentiating non-mucinous from mucinous cysts for CEA, and pseudocysts from non-pseudocysts for amylase. For example, a high pancreatic cyst fluid CEA (> 200) and a very low CEA (< 5) are suggestive but not diagnostic of mucinous and non-mucinous cystic neoplasms, respectively. Figure 1 highlights that while there are differences in CEA levels by cyst type, there is also substantial overlap. While an amylase less than 250 is helpful to exclude pseudocysts, levels above 250 were not specific for pseudocysts. In addition to benign IPMN PCLs, benign MCN lesions had a high level of cyst fluid amylase.
Some potentially novel observations from this study come from the analysis on cyst fluid amylase. Amylase levels are thought to be elevated in pseudocysts because of its presumed connection to the pancreatic ductal system. Since IPMN lesions are also characterized by being connected to the ductal system, one might also expect elevated amylase levels. MCN lesions on the other hand are characterized in part by their lack of ductal connection. The similarly elevated amylase levels observed in this study with IPMN lesions, however, suggest that there may be diminutive connections between the cyst and the ductal system. Another observation includes malignant mucinous cysts having a significantly lower amylase level than benign mucinous cysts (MCN plus IPMN lesions). It is unclear why this might be. One possible speculation may be that malignant transformation is associated with rapid uncontrolled cellular growth that occludes any microscopic ductal connections. This observation was not appreciated in the pooled analysis by Van de Waaij and colleagues.6 Further study of amylase in malignant mucinous cysts is warranted.
There are several limitations to this study that should introduce caution in accepting the results of these data. This study was retrospective and based from a single tertiary medical center. This introduces a referral and selection bias that may limit the generalizability of the results to other experiences. Furthermore, since most of the cases came from surgical resections, this is essentially a surgical series with inherent selection biases. Histologically, the outcomes of interest were limited to benign disease versus malignant disease, and the various degrees of dysplasia were not considered. IPMN lesions were not classified further into branch duct and main duct lesions for the purpose of this analysis. Further histological and clinical classifications might provider further insight into the performance of these tumor markers.
In conclusion, this study finds that current cyst fluid tumor markers such as CEA and amylase can help differentiate cyst lesions and support the management of pancreatic cystic lesions. These tumor markers may be particularly helpful in cases when in the context of other clinical and imaging data, the nature of the cyst remains indeterminate. The shortcomings however, of these biomarkers to better predict which cysts harbor malignancy or will become malignant argue for the need for new biomarkers. Studies using DNA analysis appear to be promising but require further study. Identifying other cyst fluid markers associated with cancer remains arguably attractive because of the easy accessibility of cyst fluid by endoscopic ultrasound.
Acknowledgments
Grant Support:
Walter G. Park was supported by NIH Training Grant 5T32DK007056.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekhar ALKS, Brown A, Pedrosa I. Prevalence of Incidental Pancreatic Cysts in the Adult Population. Pancreas. 2008;37 doi: 10.1038/ajg.2010.122. AB 495. [DOI] [PubMed] [Google Scholar]
- 3.Khalid A, Brugge W. ACG practice guidelines for the diagnosis and management of neoplastic pancreatic cysts. Am J Gastroenterol. 2007;102:2339–2349. doi: 10.1111/j.1572-0241.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 5.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 6.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 7.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Sawhney MS, Devarajan S, O'Farrel P, et al. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc. 2009;69:1106–1110. doi: 10.1016/j.gie.2008.08.015. [DOI] [PubMed] [Google Scholar]