HIV-1 preferentially infects M. tuberculosis-specific CD4+ T cells due to their increased production of IL-2.
Abstract
HIV-1 infection results in the progressive loss of CD4 T cells. In this study, we address how different pathogen-specific CD4 T cells are affected by HIV infection and the cellular parameters involved. We found striking differences in the depletion rates between CD4 T cells to two common opportunistic pathogens, cytomegalovirus (CMV) and Mycobacterium tuberculosis (MTB). CMV-specific CD4 T cells persisted after HIV infection, whereas MTB-specific CD4 T cells were depleted rapidly. CMV-specific CD4 T cells expressed a mature phenotype and produced very little IL-2, but large amounts of MIP-1β. In contrast, MTB-specific CD4 T cells were less mature, and most produced IL-2 but not MIP-1β. Staphylococcal enterotoxin B–stimulated IL-2–producing cells were more susceptible to HIV infection in vitro than MIP-1β–producing cells. Moreover, IL-2 production was associated with expression of CD25, and neutralization of IL-2 completely abrogated productive HIV infection in vitro. HIV DNA was found to be most abundant in IL-2–producing cells, and least abundant in MIP-1β–producing MTB-specific CD4 T cells from HIV-infected subjects with active tuberculosis. These data support the hypothesis that differences in function affect the susceptibility of pathogen-specific CD4 T cells to HIV infection and depletion in vivo, providing a potential mechanism to explain the rapid loss of MTB-specific CD4 T cells after HIV infection.
HIV infection is characterized by the progressive depletion of CD4 T helper cells, eventually leading to AIDS and the onset of various opportunistic diseases. Highly active antiretroviral therapy interrupts HIV replication and leads to increases in CD4 T cell numbers and function, with associated clearance of many opportunistic infections. Data indicate that ongoing viral replication within CD4 T cells is among the most important parameters driving the massive, systemic depletion of memory CD4 T cells during acute immunodeficiency virus infection (Li et al., 2005; Mattapallil et al., 2005) and the subsequent slow depletion of CD4 T cells during chronic disease. Although CD4 T cell–mediated delayed type hypersensitivity reactions are lost during disease progression (Blatt et al., 1993; Raszka et al., 1996), it is largely unclear whether depletion of particular pathogen-specific CD4 T cells equates with susceptibility to given opportunistic infectious diseases. Some data suggest that this might be the case; e.g., cytomegalovirus (CMV) end organ disease is associated with and preceded by the loss of CMV-specific CD4 T cell responses (Komanduri et al., 1998; Bronke et al., 2005). Whether different pathogen-specific CD4 T cell populations are being differentially affected by HIV and what pathogenic mechanisms contribute to the depletion of individual pathogen-specific CD4 T cells is unknown.
Pathogen-specific α/β (αβ) CD4 T cells play a central role in the orchestration of adaptive immune responses and are important for protective immunity to many microbial pathogens, including Mycobacterium tuberculosis (MTB; Caruso et al., 1999; Gallegos et al., 2008). CD4 T cells are a crucial component of the response to acute MTB infection in the mouse model (Mogues et al., 2001). Upon antigen recognition, MTB-specific CD4 T cells secrete IFN-γ and TNF, which activate infected macrophages and contribute to the containment of intraendosomal bacilli (Cooper et al., 1993; Flynn et al., 1995; Kaufmann and McMichael, 2005) and to the formation of bactericidal granulomas (Ray et al., 2009). Hence, MTB-specific CD4 T cells are thought to be of central importance in the efficient control of MTB infection and prevention of further dissemination to extrapulmonary sites.
Active pulmonary and extrapulmonary tuberculosis (TB) are among the most commonly observed opportunistic infectious diseases in HIV-infected subjects within MTB endemic areas, and pulmonary TB is frequently the first manifestation of AIDS in such regions (http://www.who.int/tb/challenges/hiv/faq/en/, accessed on Dec third 2009). Within areas of high TB incidence, the risk of developing active TB is significantly increased, even during the first year after HIV infection when total CD4 T cell counts are still quite high (Kaufmann and McMichael, 2005; Sonnenberg et al., 2005). TB was shown to be the cause of death in almost 50% of HIV seropositive South African gold miners (Murray et al., 2007). Furthermore, evidence of active and disseminated TB was found in almost 50% of post-mortem autopsies conducted on HIV-infected Kenyan subjects (Rana et al., 2000). In the absence of HIV infection or other immunodeficiencies, MTB is reasonably well controlled, and only ∼10% of MTB-exposed individuals develop active TB disease. Thus, the dramatic increase in active TB associated with HIV infection suggests that MTB-specific immunity might be particularly vulnerable to HIV-associated immune damage.
In stark contrast to pulmonary TB, which frequently occurs in HIV-infected subjects with relatively high CD4 T cell counts, CMV-associated end organ disease typically affects AIDS patients only after CD4 counts have fallen to very low levels. Although these pathogens differ substantially, MTB and CMV share a range of similarities. Both cause persistent or latent infections that are tightly controlled by the adaptive immune system in healthy individuals, but cause life threatening disease in immunocompromised states. Importantly, pathogen-specific CD4 T cells play important roles in the control of both infections (Komanduri et al., 1998, 2001; Caruso et al., 1999; Gamadia et al., 2003; Bronke et al., 2005; Gallegos et al., 2008).
MTB-specific CD4 T cell responses are depleted in peripheral blood early after HIV infection, whereas they do not change appreciably over a 6–12-mo time interval in HIV-negative subjects with latent MTB infection (Geldmacher et al., 2008). During chronic HIV infection, MTB-specific CD4 T cell responses are significantly decreased in the periphery and bronchoalveolar lavage (BAL; Geldmacher et al., 2008; Kalsdorf et al., 2009). In contrast, CMV-specific CD4 T cells are often detectable even late into chronic HIV infection (Waldrop et al., 1997). This difference provides an opportunity to study cellular parameters associated with the rapid depletion or persistence of pathogen-specific CD4 T cells after HIV infection and to further elucidate the mechanisms underlying the differential rates of depletion of various pathogen-specific CD4 T cells. A better understanding of such mechanisms might help to elucidate general mechanisms of HIV pathology and to define parameters that can protect vaccine-induced HIV-specific CD4 T cells from preferential infection and depletion upon exposure to HIV.
RESULTS
MTB-specific and CMV-specific CD4 T cell responses after HIV infection
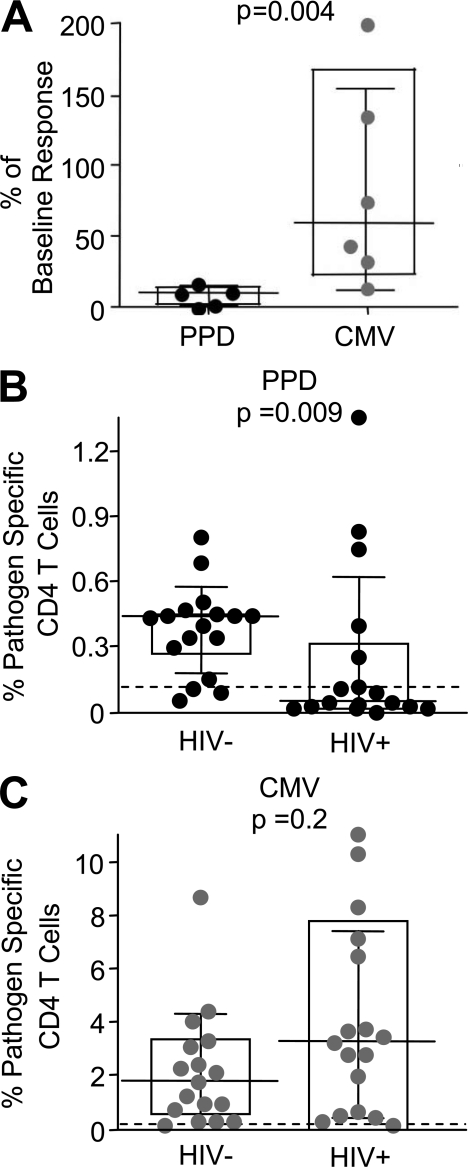
To determine the effect of HIV on different pathogen-specific CD4 T cells in the periphery, we first compared the frequencies of MTB- and CMV-specific CD4 T cell responses shortly before, early after, and during chronic HIV infection in subjects without evidence of active TB or CMV disease. In 5 Tanzanian subjects who acquired HIV infection during the study, MTB- and CMV-specific responses were detectable at 3 mo before the first HIV seropositive study visit. During this baseline visit, the median frequencies of MTB- and CMV-specific memory CD4 T cells were 0.18% (range, 0.1–0.8) and 0.51% (range, 0.36–2.6), respectively. The median frequency of MTB-specific CD4 T cells decreased by 89.4% (Fig. 1) within 6–12 mo after HIV seroconversion. MTB-specific CD4 T cells completely disappeared in four of five subjects, and decreased by more than fivefold in the fifth subject. We had previously demonstrated that MTB-specific CD4 T cells do not change appreciably over a 6–12-mo time interval in HIV− subjects with latent MTB infection (Geldmacher et al., 2008). In contrast, CMV-specific CD4 T cells were still detectable after 6–12 mo in all subjects, with a median decrease of only 40.6%, despite recent HIV infection. In four subjects, the frequencies of CMV-specific CD4 T cells were also determined during the first HIV seropositive visit and/or at 3 or 6 mo thereafter, but were always detectable (unpublished data). The absolute decline in MTB- and CMV-specific CD4 T cells after HIV infection was also quantified in subjects with available CD4 T cell counts before and early after HIV infection. MTB-specific CD4 T cells declined from a median of 1,092 cells/ml (n = 4; range, 572–2,525) to a median of 29 cells/ml (range, 0–427), and CMV-specific CD4 T cells declined from a median of 3,298 cells/ml (n = 5; range, 2,316–6,300) to a median of 1,184 cells/ml (range, 1,061–5,457). Collectively, these data demonstrate that the rate of in vivo CD4 T cell depletion early after HIV infection can differ depending on the antigen specificity of the response.
Figure 1.
MTB- and CMV-specific CD4 T cells are lost at different rates after HIV infection. (A) The frequency of MTB-specific (black, 5 PPD responding subjects, who remained TB asymptomatic) and CMV-specific memory CD4 T cell responses (gray; n = 6 CMV responding subjects who remained CMV disease free) at 6–12 mo after HIV seroconversion as the percentage of baseline response detected at 3 mo before the first HIV-seropositive follow up in latently infected subjects. The frequency of MTB- or CMV-specific CD4 T cell responses in chronically HIV-infected subjects (median time since HIV infection >3 yr; n = 17) and a HIV− control group (n = 17) is shown in B and C, respectively. The limit of detection is indicated. Memory CD4 T cells were defined by expression of CD27 and CD45RO. IFN-γ+ memory CD4 T cells were detected after in vitro stimulation of PBMCs with PPD or whole inactivated CMV virus by intracellular cytokine staining. Statistical analysis was performed using the Mann-Whitney test.
The effect of HIV infection on the frequencies of MTB- and CMV-specific CD4 T cells was also determined in a cross-sectional analysis of HIV-infected and uninfected Tanzanian subjects (n = 17 HIV+, 17 HIV−; Fig. 1, B and C). All subjects were clinically asymptomatic for MTB or CMV infection. HIV-infected subjects had a median CD4 T cell count of 640 cells/µl (range: 109–758). In line with previous studies (Waldrop et al., 1997), CMV-specific CD4 T cells were detectable in most samples, and the median magnitude of the response was actually higher in HIV+ than HIV− subjects (3.32 versus 1.72%, respectively). In contrast, HIV infection was associated with significantly reduced magnitudes (P = 0.009) and reduced prevalence of detectable MTB-specific responses. 6 of 17 HIV+ subjects had detectable MTB-specific CD4 T cell responses compared with 14 of 17 in HIV− subjects. Collectively, these data demonstrate that early and chronic untreated HIV infection is associated with severe depletion of MTB-specific CD4 T cell responses, whereas CMV-specific CD4 T cell responses tend to persist. These data suggest that MTB-specific CD4 T cells are more susceptible than CMV-specific CD4 T cells to in vivo HIV infection and deletion.
Phenotype and function of MTB- and CMV-specific CD4 T cells
To investigate factors that might be associated with the different rates of CD4 T cell decline, we first assessed expression of the HIV coreceptor, CCR5, on MTB- and CMV-specific CD4 T cells. Although CCR5 was present on the CD4 T cells of both specificities, there was no difference in the expression of CCR5 (percentage or mean fluorescent intensity) between CD4 T cells of the two specificities (unpublished data).
We next examined multiple other surface maturation markers and several functional characteristics within the following groups of pathogen-specific CD4 T cells: (a) MTB- and CMV-specific CD4 T cells in HIV− subjects in the absence of active TB disease and (b) MTB-specific CD4 T cells in association with active TB disease in HIV+ subjects (Fig. 2). Cellular maturation was studied using the differentiation markers CD27, CD45RO, and CD57, which discriminate between naive (CD27+CD45RO−), central memory-like (CD27+CD45RO+), effector memory-like (CD27−CD45RO+), and terminally differentiated (CD57+) CD4 T cells (Brenchley et al., 2003).
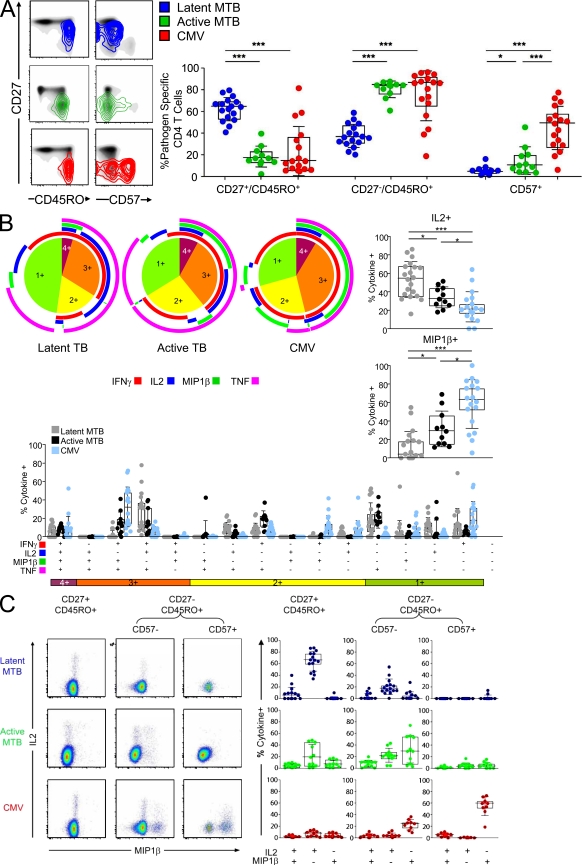
Figure 2.
Differences in cellular maturation between MTB- and CMV-specific CD4 T cells are diminished during active TB disease and are associated with diametrically opposed production of IL-2 and MIP-1β. (A) Surface expression of the maturation markers CD27, CD45RO, and CD57 on total CD4 T cells (black) is shown for representative subjects as a density plot overlay. MTB-specific CD4 T cells in the absence (blue, HIV−) or presence (green, HIV+) of active TB disease and for CMV-specific CD4 T cells (red). The percentage of CD27+CD45RO+, CD27−CD45RO+, and CD57+ subsets within MTB- and CMV-specific CD4 T cells from HIV− subjects (blue, n = 17) and HIV+ subjects with active TB (green, n = 11; bottom). (B) Flow cytometric analysis of IFN-γ, IL-2, MIP-1β, and TNF production within pathogen-specific CD4 T cells. PBMCs from subjects with latent MTB infection (defined by positive response to region of difference 1 [RD1] antigens) or from HIV+ subjects with active TB were stimulated with MTB-antigens (PPD or a mix of PPD and RD1 peptide pools) or CMV whole antigen. The pie charts show the fraction of cells with 1, 2, 3, or 4 functions. The color-coded circles indicate the proportion of the 4, 3, 2, and 1 functional responses response that are contributed by the single cytokines IFN-γ (red), IL-2 (blue), Mip-1β (green), and TNF (pink). The fraction of cells that produce MIP-1β (right top) or IL-2 (bottom right) is shown as percentage of total cytokine-positive CD4 T cells. The median is indicated. Further delineation of the 16 different possible cytokine combinations is shown (bottom). (C) Intracellular staining of IL-2 (y axis) and MIP-1β (x axis) within CD27+CD45RO+, CD27−CD45RO+CD57−, and CD27−CD45RO+CD57+ CD4 T cells after stimulation with PPD or CMV is shown for one representative subject (left) and for all studied responses further delineated by IL-2 and MIP-1β production and presented as percentage of total cytokine-positive response (right). Statistical analysis was performed using the Mann-Whitney test (***, P < 0.0005; **, P < 0.005; *, P < 0.05).
In HIV− subjects with latent TB, the majority of MTB-specific CD4 T cells had a CD27+CD45RO+ phenotype (median 64%; Fig. 2 A). A minority had a CD27−CD45RO+ phenotype (median 36%). CD57 expression was not detectable or insignificant. In contrast, MTB-specific CD4 T cells in HIV+ subjects with active TB were phenotypically distinct and were characterized by down-regulation of CD27 (median 83% were CD45RO+CD27−; P < 0.001) and increased expression of CD57 (median 9.5%; P < 0.05). CMV-specific CD4 T cells also lacked CD27 expression (median 85.6% were CD45RO+CD27−) and showed even more frequent expression of CD57 (median 48.4%).
To determine whether cell maturation was associated with functional changes within pathogen-specific CD4 T cells, we analyzed expression of IFN-γ, IL-2, TNF, and MIP-1β within the three different groups of CD4 T cells and delineated the functions according to their maturational phenotype. As shown in Fig. 2 B, diametrically opposed patterns of IL-2 and MIP-1β production were detected in MTB-specific CD4 T cells among HIV− TB asymptomatic subjects and CMV-specific CD4 T cells (right). Large fractions of CMV-specific CD4 T cells produced MIP-1β (median, 62%), but few produced IL-2 (median, 20%). In contrast, most MTB-specific CD4 T cells in asymptomatic infection produced IL-2 (P < 0.001; median, 54%), but not MIP-1β (P < 0.001; median, 3%). Differences in MIP-1β and IL-2 production occurred throughout the 16 possible cytokine combinations (bottom). No such differences were detected for TNF or IFN-γ production (unpublished data). MTB-specific CD4 T cells in HIV+ subjects with active TB were functionally distinct from those in HIV− subjects during asymptomatic TB infection. The median fraction of MIP-1β+ cells was higher (29%; P < 0.05), whereas the median fraction of IL-2+ cells was lower (32%; P < 0.05) in HIV+ subjects with active TB as compared with the response in HIV− subjects with latent TB. Most notably, the subset of three functional cells that produced MIP-1β, IFN-γ, and TNF, but no IL-2, which is characteristic of the CMV-specific CD4 T cell response, was virtually absent from MTB-specific CD4 T cells during latent TB, but present during active TB.
Whether these distinct functional properties were associated with different cellular maturation was then analyzed by comparing pathogen-specific IL-2 and MIP-1β production by CD27+CD45RO+, CD27−CD45RO+CD57−, and CD27−CD45RO+CD57+ CD4 T cells. A representative dot plot (left) for IL-2 and MIP-1β production for each group and for all studied responses (right) is shown in Fig. 2 C. Independent of the pathogen specificity, a CD45RO+CD27− phenotype was associated with more MIP-1β+ and fewer IL-2+, cells. Large fractions of CD27−CD45RO+CD57+ CD4 T cells were only present within the CMV-specific response, and almost all expressed MIP-1β exclusively. Virtually none of the CD27−CD45RO+CD57+ cells produced IL-2, irrespective of specificity. Together, these data suggest that the capacity to produce IL-2 is reduced, whereas the capacity to produce MIP-1β is increased, with maturation of pathogen-specific CD4 T cells.
Effect of HIV infection on CD4 T cell function and phenotypes in vivo
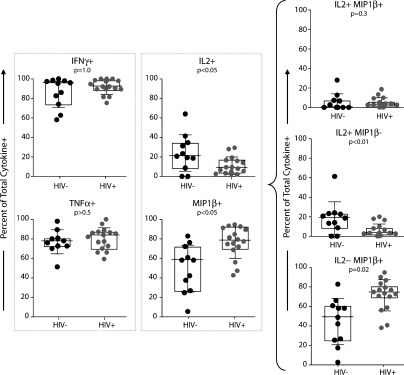
Less IL-2 production and greater MIP-1β production among MTB-specific CD4 T cells in HIV+ versus HIV− subjects, and between MTB and CMV-specific CD4 T cells (Fig. 2), is consistent with the hypothesis that HIV preferentially infects and depletes IL-2–producing CD4 T cells and is partially inhibited from infecting and depleting MIP-1β–producing CD4 T cells in vivo. We further addressed this possibility by comparing the proportion of CMV-specific, cytokine-producing cells among HIV+ and HIV− subjects (Fig. 3). There was no difference in the proportion of IFN-γ– or TNF-producing CMV-specific CD4 T cells in HIV+ and HIV− subjects. However, there was a significantly smaller proportion of IL-2–producing cells (P < 0.05; Fig. 3) and a significantly greater proportion of MIP-1β–producing cells (P < 0.05, Fig. 3) in HIV+ versus HIV− subjects. This difference was reflected in cells that produced MIP-1β but not IL-2 and produced IL-2 but not MIP-1β, but not in the cells that produced both effector molecules simultaneously (Fig. 3, right). Hypothetically, the decline in MTB-specific CD4 T cells could also be explained by selective anergy within the CD27+ memory CD4 compartment or selective depletion of this compartment after HIV infection. However, the functional capacity of the CD27+ memory CD4 compartment upon staphylococcal enterotoxin B (SEB) stimulation was not compromised after HIV infection and the proportion of CD27+ memory CD4 compartment remained constant after HIV infection in six HIV seroconverters (unpublished data). These data are therefore consistent with the hypothesis that with HIV infection in vivo, IL-2–producing cells are more likely to get infected and depleted than MIP-1β–producing cells.
Figure 3.
HIV infection is characterized by increased fractions of MIP-1β+ and decreased fractions of IL-2+ CMV-specific CD4 T cells. (left) The fraction of IFN-γ+, TNF+, MIP-1β+, or IL-2+ among total cytokine positive CD4 T cells (y axis) is shown for HIV− (n = 11) and HIV+ subjects (n = 16). (right) The fraction of IL-2+MIP-1β+, IL-2+MIP-1β−, and IL-2−MIP-1β+ among total cytokine-positive CD4 T cells. PBMCs were stimulated overnight with whole inactivated CMV and the background (unstimulated control) was subtracted. Statistical analysis was performed using the Mann-Whitney test.
IL-2 dependence of HIV infection in vitro
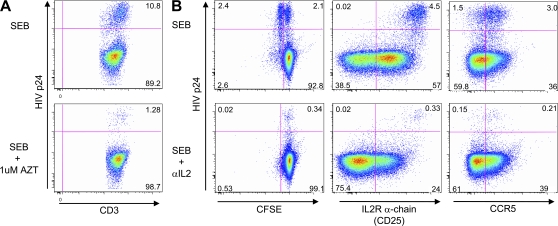
The in vitro inhibitory effects of CCR5 ligands on HIV infection have been previously described (Cocchi et al., 1995; Kinter et al., 1996; Paxton et al., 1996). To explore whether endogenous IL-2 production augments in vitro HIV infection, we performed HIV infection experiments in the presence or absence of a neutralizing anti–IL-2 antibody. SEB-stimulated PBMCs were infected with the CCR5-tropic HIV strain BAL, and CD4 T cells were analyzed for HIV p24 content in the context of cell division and CD25 and CCR5 surface expression (in the absence of exogenously added IL-2). We also attempted stimulation with purified protein derivative (PPD) in this experimental set up. However, the frequency of responding cells was so low that there were too few events in which to accurately measure HIV infection. The p24 staining in this system was the result of active viral replication, and not retained p24 from the virus inoculum, as it was inhibited by the addition of the reverse transcription inhibitor azidothymidine (AZT; Fig. 4 A). In the absence of the IL-2 neutralizing antibody, 48% of dividing cells (CFSElow), but only 2.2% of the cells that had not undergone cell division were p24+ (Fig. 4 B, top). Virtually all of these p24+ cells expressed CD25, demonstrating that productive HIV infection occurred within cells that could respond to IL-2 signaling. Neutralization of IL-2 resulted in a 12.5-fold reduction of p24+ cells and completely abrogated cell division (Fig. 4 B, bottom). The fraction of CD25+ cells was reduced by 60% (Fig. 4 B, bottom), whereas the fraction of CCR5+ cells was unaffected by the anti–IL-2 treatment, indicating that neutralization of IL-2 did not affect HIV replication through modulation of the coreceptor, but by affecting post-entry events associated with cell division. These data demonstrate that productive HIV-infection of SEB-stimulated CD4 T cells is associated with CD25 expression, cell cycle induction, and the production of IL-2 upon TCR engagement.
Figure 4.
Productive HIV-1 infection of SEB-responding CD4 T cells is inhibited by in vitro neutralization of IL-2. (A) Productive HIV infection of CD4 T cells was analyzed after gating on CD4low T cells and staining for HIV-p24 (y axis). Shown are dot plots from samples stimulated in the presence or absence of 1 µM AZT. (B) Dot plots from samples stimulated in the presence (bottom) or absence (top) of a neutralizing anti–IL-2 antibody comparing CFSE-fluorescence intensity (left), surface CD25 (middle), and CCR5 (right) with HIV-p24 staining. PBMC samples were stimulated for 24 h with SEB and then infected for another 24 h with the CCR5 tropic HIV-1 strain BAL.
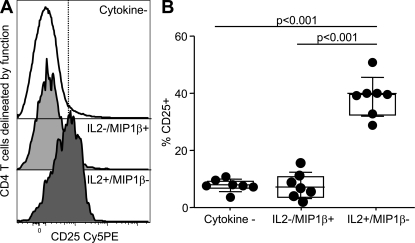
We next analyzed the expression of CD25 on IL-2+MIP-1β− and IL-2−MIP-1β+ CD4 T cells after 6 h SEB stimulation. Fig. 5 A shows a single experiment, and Fig. 5 B shows the composite results from seven experiments. These data demonstrate that a median of 39.5% of IL-2+MIP-1β−, but only 6.6% of MIP-1β+IL-2− CD4 T cells express CD25 after stimulation. The expression of CD25 on the MIP-1β+IL-2− CD4 T cells is the same as on all other nonresponding memory CD4 T cells, suggesting that IL-2 is specifically and intimately linked to expression of its high-affinity receptor, CD25.
Figure 5.
The capacity to secrete IL-2 by antigen-specific CD4 T cells is associated with increased CD25 expression. (A) Representative histogram of the CD25 expression on cytokine-negative, MIP-1β+IL-2−, and MIP-1β−IL-2+ CD4 T cells after 6 h of SEB stimulation of PBMCs. (B) The fraction of CD25+ CD4 T cells among the same subsets (n = 7). Statistical analysis was performed using the Mann-Whitney test.
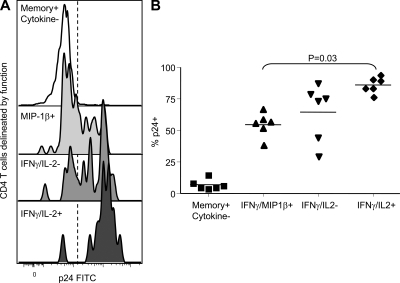
We next analyzed in vitro HIV infection of SEB-stimulated CD4 T cells according to their expression pattern of MIP-1β, IFN-γ, and IL-2 (Fig. 6). Within the responding CD4 T cell populations, we were able to gate upon cells with cytokine profiles that match either CMV- (MIP-1β+IL-2−) or MTB-specific (MIP-1β−IL-2+) CD4 T cells. CD4 T cells with a cytokine profile matching that of MTB-specific T cells had higher HIV infection rates as compared with those matching CMV-specific T cells (P = 0.03).
Figure 6.
In vitro HIV infection of cytokine-expressing cells. (A) Representative histograms of the HIV p24 staining from CD4 T cells delineated by intracellular staining for MIP-1β, IFN-γ, and IL-2 and (B) the fraction of p24+ CD4 T cells delineated by intracellular staining for MIP-1β, IFN-γ, and IL-2 from six independent experiments. PBMC samples were stimulated for 24h with SEB and then infected for another 24 h with the CCR5 tropic HIV-1 strain BAL. Productive HIV infection of CD4 T cells was analyzed after gating on CD4low T cells and staining for HIV-p24.
Cytokine expression delineates in vivo infection history of MTB-specific T cells
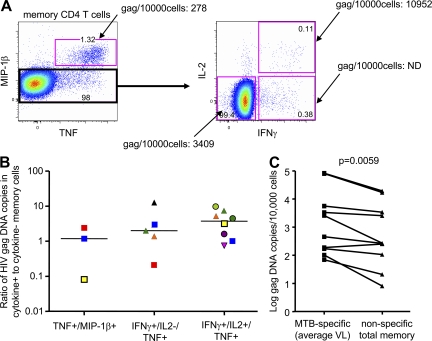
To determine in vivo cellular infection rates within MTB-specific and nonspecific memory CD4 T cells, we stimulated PBMCs from HIV/MTB coinfected subjects with a mix of PPD, early secretory antigenic target 6 (ESAT6), and culture filtrate protein 10 (CFP10), sorted the CD4 T cells according to cytokine and chemokine expression (Fig. 7 A), and quantified the HIV gag DNA within the different subsets. Despite relatively low numbers of sorted cytokine/chemokine+ cells, we were able to quantify proviral DNA in 16 responding MTB-specific CD4 T cell populations from 10 HIV-infected subjects with active TB. We first compared the number of HIV gag DNA copies within MTB-specific, cytokine/chemokine+ CD4 T cell samples with the nonresponding CD4 memory T cell compartment (CD27+CD45RO+, CD27−CD45RO+, and CD27−CD45RO− subsets). In 9 of 10 subjects, the number of HIV gag DNA copies detected in MTB-specific CD4 T cells was higher compared with total memory CD4 T cells (median 2.5-fold increase; P < 0.01; Fig. 7 C). Next, we determined whether MTB-specific CD4 T cells expressing different patterns of IL-2 and MIP-1β contained different amounts of HIV gag DNA. MTB-specific CD4 T cells that exclusively expressed either MIP-1β or IL-2 were virtually nonexistent. The median number of HIV gag DNA copies per 10,000 cells was 1,426.5 for IFNγ+IL-2+MIP-1β−TNF+ (range 109–127,500 mean), 538 for IFNγ+IL-2−MIP-1β−TNF+ (range, 29–34,167), 315 for MIP-1β+TNF+ (range, 278–6,136), and 268 for cytokine negative memory CD4 T cells (range, 8–19,370). To reduce the inherent patient-to-patient variability in the HIV gag DNA copy numbers, and overcome the fact that many of the sorted samples contained too few cells to register a signal in the PCR assay, the results were expressed as a ratio of HIV gag DNA copies in cytokine+/cytokine− cells (Fig. 7 B). Consistent with the aforementioned raw data calculations, the IL-2–producing MTB-specific CD4 T cells tended to harbor greater numbers of HIV gag copies per cell as compared with the IL-2− and MIP-1β+ cells, although this trend was not statistically significant within this small number of subjects. Together, these data support the hypothesis that the functional characteristics, and in particular the capacity to secrete IL-2 but not MIP-1β upon MTB-specific stimulation, are associated with increased susceptibility of MTB-specific CD4 T cells to HIV infection.
Figure 7.
In vivo HIV gag DNA in MTB-specific CD4 T cells. (A) Gating/sorting strategy used to sort different memory CD4 T cell populations delineated by IFN-γ, IL-2, and MIP-1β production. MIP-1β− memory CD4 T cells were further delineated into IFNγ+IL-2+TNF+, IFNγ+IL-2−TNF+, and cytokine-negative memory CD4 T cells. The HIV gag DNA/10,000 cells determined within these populations is indicated. (B) The ratio of HIV gag copies/10,000 cells detected within cytokine-positive to cytokine-negative memory T cells from 16 cytokine-positive CD4 T cell populations sorted from 10 HIV+ subjects (different symbols) with active TB. (C) The mean number of gag copies/10,000 cells detected in MTB-specific CD4 T cells or total memory CD4 T cells is shown for each subject. Memory CD4 T cells were defined by expression of CD45RO and CD27. The PBMCs were stimulated overnight with a mix of PPD and RD1 peptide pools and further analyzed as described in the Materials and methods section. Gag DNA within different CD4 T cell populations of the same subject was quantified during the same RT-PCR run. The statistical analysis in C was performed using the Wilcoxon-rank-matched pairs test.
DISCUSSION
We have previously demonstrated that MTB-specific CD4 T cells are depleted from the periphery early after HIV infection, whereas in HIV− subjects the frequencies of MTB-specific CD4 T cells do not change appreciably over time (Geldmacher et al., 2008). In contrast, CMV-specific CD4 T cells are frequently detectable in the periphery during chronic HIV infection (Waldrop et al., 1997) and contain comparatively little proviral DNA (Douek et al., 2002). In this study, we detected significantly higher levels of HIV gag DNA in MTB-specific CD4 T cells than in total memory CD4 cells in the periphery. The higher levels of HIV gag DNA within MTB-specific CD4 T cells (ratio of 2.5 to total memory CD4 T cells) are comparable to those observed in HIV-specific CD4 T cells (ratio 3.4; Douek et al., 2002). Brenchley et al. (2006) have previously shown that HIV-specific CD4 T cells are present at very low frequencies in most HIV+ individuals and that direct HIV infection of virus-specific CD4 T cells reduces their life span in vivo. In this context, these data support the hypothesis that direct HIV infection of MTB-specific CD4 T cells contributes to their depletion, possibly compromising the generation of effective immune responses to MTB in HIV-infected subjects. We thus explored which functional and phenotypic characteristics might account for the higher infection rates and more rapid depletion of peripheral MTB-specific CD4 T cells after HIV infection and tested the hypothesis that differences in specific functions might contribute to differential HIV infection of antigen-specific CD4 T cells in vivo.
We have previously shown that MTB-specific CD4 T cells express comparatively high levels of the HIV-coreceptor CCR5. However, CCR5 expression was similar on MTB- and CMV-specific CD4 T cells (unpublished data) indicating that coreceptor expression alone could not account for any difference in rates of depletion after HIV infection between MTB- and CMV-specific CD4 T cells. Instead, we found significant differences in the capacity of MTB- and CMV-specific CD4 T cells to produce IL-2 and the CCR5 ligand MIP-1β. CMV-specific CD4 T cells tended to produce MIP-1β but not IL-2, and displayed an effector memory phenotype and this functional profile that was even more pronounced during chronic HIV infection, whereas MTB-specific CD4 T cells produced IL-2 but not MIP-1β, and were less mature. Cells producing IL-2 in the absence of MIP-1β were more susceptible to productive HIV infection within SEB-activated CD4 T cells in vitro (Fig. 6) and this functional profile within MTB-specific CD4 T cells was associated with a greater frequency of HIV gag DNA in vivo (Fig. 7). These observations together with the striking differences in the depletion dynamics between MTB- and CMV-specific CD4 T cells early after HIV infection suggest that these differences in function contribute to differential cellular susceptibility to HIV infection and depletion of these two cell populations, and the associated differences in disease onset caused by these opportunistic pathogens.
The capacity of CCR5 ligands to block HIV replication in vitro is well established (Cocchi et al., 1995, Kinter et al., 1996). Indeed, CD4 T cells with a relative resistance to CCR5-tropic HIV strains are capable of producing comparatively high amounts of such CCR5 ligands, including MIP-1β (Paxton et al., 1996). We have recently shown that CMV-specific CD4 T cells that produce MIP-1β and MIP-1α contain ∼10-fold less HIV gag DNA than CMV-specific CD4 T cells that do not produce these chemokines (Casazza et al., 2009). These data indicate that autocrine production of MIP-1β protects CD4 T cells from HIV infection in vivo. Thus, the lack of MIP-1β production by the less mature MTB-specific CD4 T cells might contribute to their increased susceptibility to HIV infection and subsequent depletion. In contrast, the production of MIP-1β by the more mature CMV-specific CD4 T cells is likely to contribute to their relative resistance to HIV infection (Douek et al., 2002; Casazza et al., 2009), and thus to their persistence after HIV infection. Our observation that HIV infection is associated with further increased proportions of MIP-1β+ but decreased proportions of IL-2+ CMV-specific CD4 T cells is in line with these findings and with our hypothesis that the functional profile of pathogen-specific CD4 T cells might influence their susceptibility to HIV. Indeed, as a common theme, the tendency of more differentiated CD4 T cells to produce MIP-1β could explain the previously described low HIV infection rates within CD57+ CD4 T cells in vivo (Brenchley et al., 2003).
IL-2 signaling pathway and HIV infection
Whereas MTB-specific CD4 T cells lacked the capacity to produce MIP-1β in individuals with latent MTB infection, they had a remarkable capacity to produce IL-2. IL-2 and its high-affinity IL-2 receptor α-chain CD25, whose expression rapidly increases upon T cell receptor engagement, are important in driving T cells into the cell cycle. In addition to being critical for T cell proliferation, IL-2 may also positively impact upon HIV reverse transcription (Zack et al., 1990) by increasing the concentration of dNTPs in CD25+ CD4 T cells. Indeed, CD25 expression and productive HIV infection appear to be linked; in vitro depletion of CD25+ CD4 T cells efficiently blocks viral replication (Finberg et al., 1991;, Ramilo et al., 1993; Chou et al., 1997). CD25 expression on CD4 T cells correlates with PPD-induced HIV replication (Goletti et al., 1996), and it was recently demonstrated that productive HIV-1 infection within lymphoid tissue explants occurred predominantly in CD4 T cells expressing CD25 (Biancotto et al., 2008). In vivo, CD25+ CD4 T cells contain up to sevenfold more immunodeficiency virus during acute infection, a phenomenon that was particularly apparent within lymph nodes (Mexas et al., 2008). Our observations that in vitro neutralization of IL-2 reduced CD25 up-regulation, abrogated antigen-specific CD4 T cell proliferation, and almost completely abrogated productive HIV infection of CD4 T cells supports a central role for the IL-2–CD25 signaling pathway in the productive infection of antigen-specific CD4 T cells. Our finding that peripheral MTB-specific CD4 T cells with the capacity to produce IL-2, but not MIP-1β, contained the highest amount of HIV gag DNA in vivo supports the conclusion that IL-2 expression by MTB-specific CD4 T cells leads to their increased susceptibility to HIV infection and depletion. However, despite the large numbers of PBMCs used for a single experiment (3 × 107), the number of sorted MTB-specific CD4 T cells with different functional profiles was often too low to detect HIV DNA, and larger studies will be needed to expand upon this finding. The capacity to produce IL-2 in the absence of MIP-1β might create a microenvironment that very efficiently drives the replication of HIV within MTB-specific CD4 T cells. Indeed, the enormous potential of MTB-specific immune responses to contribute to an explosive replication of a single virus quasi species in vivo has been demonstrated within simian immunodeficiency virus (SIV)–infected Rhesus macaques; up to 35% of viral quasi species in PBMCs were closely related to the single major species that was isolated at the site of the tuberculin skin test (Cheynier et al., 1998).
Impact of function and phenotype on HIV infection
Activated, CD25-expressing CD4 T cells that are HIV infected have been shown to preferentially undergo apoptosis in lymphoid tissue explants (Biancotto et al., 2008), and SIV infection of CD4 T cells at mucosal sites can reach extremely high frequencies during acute infection (Mattapallil et al., 2005). Our observations support the hypothesis that MTB-specific IL-2+MIP-1β− CD4 T cells might represent a pool of CD4 T cells that are highly susceptible to HIV infection and may become rapidly depleted upon HIV infection before they can further differentiate into effector cells, a general mechanism that has been proposed previously for the depletion of memory CD4 T cells (Grossman et al., 2006; Okoye et al., 2007). In this scenario, the few MTB-specific CD4 T cells that avoid HIV infection could go on to mature further, and in the process gain expression of MIP-1β and lose IL-2 expression, simultaneously making them less susceptible to HIV infection. The resulting shift from IL-2 expression to MIP-1β expression concomitant with loss of CD27 and gain of CD57 expression that is predicted by this model is exactly what we observed in MTB- and CMV-specific CD4 T cells in individuals who were infected with HIV (Fig. 2 C). Collectively, our observations support the hypothesis that antigen-specific IL-2 production, particularly in the absence of CCR5 ligand MIP-1β, contributes to the productive HIV infection and subsequent depletion of MTB-specific CD4 T cells early after HIV infection.
During clinically latent TB, the majority of MTB-specific CD4 T cells expressed CD27 and can be considered as having a central memory-like phenotype (CCR7 was not measured); however, they also expressed higher levels of CCR5 compared with the total memory compartment (Geldmacher et al., 2008). In contrast, CMV-specific CD4 T cells had a highly differentiated effector memory-like phenotype. HIV infection was associated with a significantly increased fraction of senescent, CD57+ cells (Brenchley et al., 2004) and MIP-1β+ cells, and a reduced fraction of cells with the capacity to produce IL-2. Similarly, in HIV patients with active TB, MTB-specific CD4 T cells were characterized by a more mature CD27low phenotype, a detectable fraction of CD57+ and MIP-1β+ CD4 T cells, and a reduced capacity to produce IL-2. These differences might also be driven by the strength and persistence of T cell receptor engagement and thus different patterns of pathogen reactivation might contribute to the phenotype and function of pathogen-specific CD4 T cell responses and also to their persistence or reappearance after HIV infection; indeed CMV viral replication can be detected in almost ∼20% of HIV infected subjects (Griffin et al., 2008, Slyker et al., 2009) and MTB reactivation might account for the change in phenotype and function in subjects with active as compared with latent TB (Streitz et al., 2007). However, CD4 T cells targeting cleared antigens, such as the flu or the tetanus toxin, and with the capacity to produce IL-2 are also disproportionally depleted (Tilton et al., 2007) upon antiretroviral treatment interruption, suggesting that this phenomenon cannot be solely explained by enhanced stimulation, TCR driven maturation and associated changes in functionality. Thus, increased HIV infection among those cells still capable of producing IL-2 might preferentially deplete these less mature, central memory-like CD4 T cells.
Because our study focuses on the phenotype and function of antigen-specific cells within the periphery, we are unable to address the impact of antigen-specific T cell functional changes, as they occur after antigen exposure in other tissues, nor can we rule out specific migration of cells with certain phenotypes or functions as an explanation for the changes we observe. However, other published data suggest that MTB-specific CD4 T cells are depleted also from the BAL of HIV infected, TB asymptomatic subjects from a TB endemic area (Kalsdorf et al., 2009).
Implications for vaccines
Preferential infection of vaccine-induced, HIV-specific CD4 T cells might reduce efficiency of vaccine-induced immunity. Our results help to define cellular parameters associated with HIV infection and either depletion or persistence of antigen-specific CD4 T cells. These findings could help define the type of CD4 response one would hope to induce with a HIV vaccine. Indeed, just recently it has been shown that vaccination with replication competent, recombinant CMV was able to induce differentiated, MIP-1β–producing HIV-specific T cells that were associated with protection against SIV challenge (Hansen et al., 2009).
In conclusion, our data suggest that specific functional and maturational characteristics of MTB-specific CD4 T cells might contribute to increased HIV susceptibility and their subsequent depletion after HIV infection. Strong antigenic stimulation during active TB might obscure this depleting effect, despite high infection (and turnover) rates of MTB-specific memory CD4 T cells, and at the same time might drive phenotypic and functional changes that confer partial cellular resistance to HIV infection. These results suggest that MTB-specific adaptive immunity is particularly vulnerable to HIV-associated immune damage.
MATERIALS AND METHODS
Study subjects.
Subjects from three cohorts were included in this study. Most were part of a large, well-characterized, high-risk cohort of female bar workers (HISIS) enrolled in a prospective study of HIV-1 infection in the Mbeya region of Southwest Tanzania, which is described in detail elsewhere (Riedner et al., 2003; Herbinger et al., 2006). Subjects with evidence of active TB were enrolled at the NIMR-Mbeya Medical Research Program (NIMR-MMRP) Tuberculosis Clinic in collaboration with the National Tuberculosis and Leprosy Program of Tanzania (NTLP). The purpose and the procedures of the study were explained thoroughly to potential participants. Only persons who gave voluntarily written informed consent in the presence of a witness were enrolled. Acid Fast Bacilli smear positive TB patients were referred to the MMRP Tuberculosis clinic. Active TB was diagnosed by examination and culture (BACTEC MGIT; BD) of three separate sputum samples per patient. HIV status was determined using HIV 1/2 STAT-PAK (Chembio Diagnostic Systems) and positive results were confirmed using Enzygnost Anti-HIV 1/2 Plus ELISA (Siemens). Patients eligible for TB treatment were referred to the NTLP for treatment according to national guidelines. Patients diagnosed with HIV infection were referred to the Southern Highland Care and Treatment Program. None of the subjects evaluated in the current study were on active antiretroviral therapy.
In addition, blood was obtained from HIV-positive antiretroviral therapy naive Ghanaian patients with suspected TB attending the HIV clinic of Koforidua Hospital, Ghana, after informed consent. Ethical clearance was granted by the Institutional Review Board of the Noguchi Memorial Institute for Medical Research, NMIMR-IRB CPN 039/06-07. HIV infection was confirmed using the Determine HIV-1/2 test (Abbott) and the First Response HIV 1–2.0 test (Premier Medical Corporation Ltd).
PBMCs from these patient cohorts were isolated, cryopreserved, stored in liquid nitrogen, and used in subsequent immunological assays. Use of these patient materials was reviewed and approved by the ethics committees of all partners in compliance with national guidelines and institutional policies.
Conjugated antibodies.
The following antibodies were used: IFN-γ–FITC, IL-2-allophycocyanin (APC), MIP-1β-PE, CD3-Cy7APC, CCR5-Cy7PE and CD25-Cy5PE (BD), CD27-Cy5PE and CD45RO-Texas red-PE (Beckman Coulter), and CD4-PECy5.5 (Invitrogen) and anti-HIV p24/p55 antibody (PE or FITC; clone KC57; Beckman Coulter). CD8-quantum dot (QD) 655, CD57-QD565, and TNF-Alexa Fluor 680 were conjugated in accordance with standard protocols (http://drmr.com/abcon/index.html).
Antigens.
For cell stimulations, PPD (Statens Serum Institut) was used at a final concentration of 10 µg/ml. 2 µl of undiluted CMV grade 2 antigen (Microbix) was used to stimulate PBMC. Peptides 15 aa in length and overlapping by 11 aa were designed for ESAT6 (AF420491.1) and CFP10 (AAC83445) by using the PeptGen peptide generator from the HIV Molecular Immunology Database (http://www.hiv.lanl.gov/content/immunology/) and were used at a final concentration of 2 µg/ml/peptide. For sorting experiments, MTB-specific CD4 T cells were stimulated overnight with a mix of PPD, ESAT6, and CFP10 peptides to maximize detection of the MTB-specific T cell population.
Stimulation and flow cytometric analysis.
Previously cryopreserved PBMCs were recovered by thawing and washing twice in Benzonase (4 µl of 25 kU Benzonase in 20 ml; Novagen) containing 37°C complete media (CM; 10% heat inactivated fetal calf serum, 100 U/ml penicillin G, 100U/ml streptomycin sulfate, and 1.7 mm sodium glutamine). Cell stimulation was performed in CM in the presence of 1 µg/ml each of anti-CD28 and anti-CD49d (BD). Duration of stimulation varied in different assays. For the detection of MTB- and CMV-specific CD4 T cell responses and analysis of their functionality and cell surface phenotype, PBMCs were stimulated with specific antigens for 2 h before adding Brefeldin A (BFA; Sigma-Aldrich), and then further incubated overnight (14–18 h; 6 h for analysis of CD25 expression) at 37°C. For in vitro HIV infection experiments, PBMCs were stimulated for 48 h and BFA was added 4 h before flow cytometric analysis. Staining was performed using a modified version of a previously described method (Betts et al., 2006). After stimulation, PBMCs were washed once with PBS and stained with Vivid/Aqua (Invitrogen; Perfetto et al., 2006). Anti-CCR5 antibody was then added for 10 min at room temperature in the dark. Subsequently, surface proteins were stained for 20 min. The cells were permeabilized using the Cytofix/Cytoperm kit (BD), after which they were stained for CD3, IFN-γ, MIP-1β, IL-2, and TNF. Cells were then washed and fixed with 1% paraformaldehyde. Cells were analyzed with a modified flow cytometer (LSR II; BD). Between 300,000 and 1,000,000 total events were collected from each sample. Electronic compensation was conducted with antibody capture beads (BD) stained separately with the individual antibodies used in the test samples. Flow cytometry data were analyzed using FlowJo (version 8.8.3; Tree Star, Inc.). For polychromatic analysis, initial gating for each sample set used a forward scatter area versus a forward scatter height plot to gate out cell aggregates. The cells were then gated through a forward scatter area versus a side scatter height plot to isolate small lymphocytes. A CD3 versus Vivid/Aqua plot was then used to remove dead cells. The cutoff point for positive CD4 T cell responses was 2-fold the negative control and at least 0.1% cytokine positive after subtraction of the background.
Virus growth and characterization.
CCR5-tropic HIV-1 BAL was grown on phytohemagglutinin (PHA; Sigma-Aldrich)-stimulated PBMCs in CM supplemented with IL-2 (Chiron). HIV p24 antigen in cell culture supernatants was monitored by ELISA (Beckman Coulter). Virus was harvested at the peak of infection, on either day 4 or 7. Viruses were concentrated by ultracentrifugation at 30,000 rpm for 70 min at 4°C, and virus pellets were resuspended in fresh RMPI at 10–50× to obtain clean, concentrated virus stocks. Control concentrated conditioned supernatant from uninfected PHA-stimulated T cells was collected as a control. The viral titers were determined by sensitive 14-d end-point titration assays using PHA- and IL-2–stimulated PBMC, and listed as 50% tissue culture infectious doses per ml (TCID50/ml), as described previously (Mascola et al., 1996). The final titer of the stock of HIV Bal was 2.6 × 106 TCID50/ml.
In vitro HIV infection of primary CD4 T cells.
Previously cryopreserved PBMCs were thawed for co-culture, washed thoroughly, and labeled with 0.25 µM CFSE fluorescent dye (Invitrogen). CFSE-labeled cells were washed thoroughly and resuspended at 2 × 106 cells/tube in CM and stimulated for 24 h with 1 µg/ml SEB at 37°C. Cells were then washed, resuspended in 200 µl of HIV-1 BAL stock (0.26 multiplicity of infection), and incubated at 37°C for 2 h. The residual virus was then washed off and cells were resuspended in 1 ml of CM with 1 µg/ml SEB, and then incubated at 37°C for an additional 22 h. 10 µg/ml BFA (Sigma-Aldrich) was added to all tubes during the last 4 h before immediate analysis via flow cytometry. Stimulation was performed in the presence or absence of 1 µM AZT or 10 µg/ml anti–IL-2 antibody (BD). In some experiments, CFSE was not used, and HIV infection was detected by staining for p24 antigen.
Cell sorting.
PBMCs from 20 HIV+ subjects with active TB were thawed, washed twice in the presence of 50 U/ml benzonase, and resuspended at 3 × 106 cells/ml in CM. Costimulatory antibodies (αCD28 and αCD49d; 1 µg/ml final concentration) were added to cells before splitting them into different tubes for overnight stimulation at 37°C with either MTB antigens or no antigen. BFA (10 µg/ml) was added to all tubes 2 h after stimulation. After incubation, cells were washed and stained with pretitrated surface antibodies as described in Stimulation and flow cytometric analysis. Cytokine-producing CD4 T cells were sorted with a modified FACSAria flow cytometer (BD) by gating tightly on live memory CD3+CD4+ T cells. MIP-1β+TNF+ memory CD4 T cells were then sorted and all other memory CD4 T cells were further delineated and sorted according to IFN-γ and IL-2 staining into IFNγ+IL-2+MIP-1β−, IFNγ+IL-2−MIP-1β−, and cytokine negative memory CD4 T cells. Between 193 and 50,000 fixed memory CD4 T cells from each of the four different populations were collected, depending on the number of PBMCs available and the response level for each individual. Cells were then stored at −80°C before gag DNA analysis.
Quantification of HIV gag viral DNA.
HIV DNA was quantified by qPCR with an ABI7700 (PerkinElmer) similar to a previously described protocol (Douek et al., 2002). HIV gag primers and probe were optimized for detection and quantification of East African subtype A and C strains. Gag primer position and sequences were as follows, 783gag, forward, 5′-GAGAGAGATGGGTGCGAGAGCGTC-3′ (Tm>60), 895gag, reverse, 5′-CTKTCCAGCTCCCTGCTTGCCCA-3′ (Tm>60); FAM-labeled probe 844gagPr, 5′-ATTHGBTTAAGGCCAGGGGGAARGAAAMAAT-3′. Sites where base sequences differed among HIV isolates were made degenerate and are noted as follows: where degenerate bases are denoted as R (A or G), M (A or C), K (G or T), H (A or C or T), and B (C, G, or T). To quantify the cell number in each reaction mix, the human albumin gene copy number was also assessed by qPCR. Albumin primer and probe sequences were as follows: hAlb, forward, 5′-TGCATGAGAAAACGCCAGTAA-3′; hAlb, reverse, 5′-ATGGTCGCCTGTTCACCAA-3′; and hAlbProbe, 5′-FAM-TGACAGAGTCACCAAATGCTGCACAGAA-BHQ1-3′. Sorted T cells were lysed in 0.1 mg/ml proteinase K buffered with Tris-Cl for 1 h at 56°C, and then for 10 min at 95°C to inactivate the enzyme. 5 µl of lysate was used in a total reaction volume of 25 µl containing 12.5 µM primers, 5 µM probe, a 10-mM concentration of each deoxynucleoside triphosphate, 3.5 mM MgCl2, 1.25 mM blue 636 reference dye, and 0.625 U platinum Taq in the supplied buffer. Reaction conditions included 5-min activation at 95°C, followed by 15 s at 95°C and 1 min at 60°C for 45 cycles. Quantification was generated using standard curves for HIV-1 gag and albumin. Cell-associated gag DNA from different T cell subsets from the same subjects were quantified during the same PCR run to assure comparability of the results.
Statistical analysis.
Data analyses were performed using Prism version 4.0 software (GraphPad, Inc.). Comparisons of two groups were performed using the Mann-Whitney test. Comparisons of paired groups were performed using the Wilcoxon matched pairs test. Tests used for statistical analysis are described in the figure legends.
Acknowledgments
We thank Mr. Weston Assisya for the coordination of field studies and Jutta Jung for coordinating TB diagnosis in Mbeya, Tanzania. We would also like to thank Drs. John Tetteh, Sampson F. Ofori, and Mike Ofori for donor recruitment and sample collection in Ghana. We also thank all donors for their contribution to the study.
This study has been supported by the European Commission through grants ICA_CT-1999-10007, ICA_CT-2002-10048, and E.C. DGVIII – AIDCO - SANTE/2006/129-931 and a Netherlands Organisation for Scientific Research WOTRO Grant (01.53.2004.025).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- AZT
- azidothymidine
- BAL
- bronchoalveolar lavage
- BFA
- Brefeldin A
- CFP10
- culture filtrate protein 10
- CM
- complete media
- CMV
- cytomegalovirus
- ESAT6
- early secretory antigenic target 6
- MTB
- Mycobacterium tuberculosis
- PHA
- phytohemagglutinin
- PPD
- purified protein derivative
- RD1
- region of difference 1
- SEB
- staphylococcal enterotoxin B
- SIV
- simian immunodeficiency virus
- TB
- tuberculosis
References
- Betts M.R., Nason M.C., West S.M., De Rosa S.C., Migueles S.A., Abraham J., Lederman M.M., Benito J.M., Goepfert P.A., Connors M., et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 107:4781–4789 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancotto A., Iglehart S.J., Vanpouille C., Condack C.E., Lisco A., Ruecker E., Hirsch I., Margolis L.B., Grivel J.C. 2008. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 111:699–704 10.1182/blood-2007-05-088435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt S.P., Hendrix C.W., Butzin C.A., Freeman T.M., Ward W.W., Hensley R.E., Melcher G.P., Donovan D.J., Boswell R.N. 1993. Delayed-type hypersensitivity skin testing predicts progression to AIDS in HIV-infected patients. Ann. Intern. Med. 119:177–184 [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Karandikar N.J., Betts M.R., Ambrozak D.R., Hill B.J., Crotty L.E., Casazza J.P., Kuruppu J., Migueles S.A., Connors M., et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 101:2711–2720 10.1182/blood-2002-07-2103 [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Hill B.J., Ambrozak D.R., Price D.A., Guenaga F.J., Casazza J.P., Kuruppu J., Yazdani J., Migueles S.A., Connors M., et al. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160–1168 10.1128/JVI.78.3.1160-1168.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J.M., Ruff L.E., Casazza J.P., Koup R.A., Price D.A., Douek D.C. 2006. Preferential infection shortens the life span of human immunodeficiency virus-specific CD4+ T cells in vivo. J. Virol. 80:6801–6809 10.1128/JVI.00070-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronke C., Palmer N.M., Jansen C.A., Westerlaken G.H., Polstra A.M., Reiss P., Bakker M., Miedema F., Tesselaar K., van Baarle D. 2005. Dynamics of cytomegalovirus (CMV)-specific T cells in HIV-1-infected individuals progressing to AIDS with CMV end-organ disease. J. Infect. Dis. 191:873–880 10.1086/427828 [DOI] [PubMed] [Google Scholar]
- Caruso A.M., Serbina N., Klein E., Triebold K., Bloom B.R., Flynn J.L. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407–5416 [PubMed] [Google Scholar]
- Casazza J.P., Brenchley J.M., Hill B.J., Ayana R., Ambrozak D., Roederer M., Douek D.C., Betts M.R., Koup R.A. 2009. Autocrine production of beta-chemokines protects CMV-Specific CD4 T cells from HIV infection. PLoS Pathog. 5:e1000646 10.1371/journal.ppat.1000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheynier R., Gratton S., Halloran M., Stahmer I., Letvin N.L., Wain-Hobson S. 1998. Antigenic stimulation by BCG vaccine as an in vivo driving force for SIV replication and dissemination. Nat. Med. 4:421–427 10.1038/nm0498-421 [DOI] [PubMed] [Google Scholar]
- Chou C.S., Ramilo O., Vitetta E.S. 1997. Highly purified CD25- resting T cells cannot be infected de novo with HIV-1. Proc. Natl. Acad. Sci. USA. 94:1361–1365 10.1073/pnas.94.4.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F., DeVico A.L., Garzino-Demo A., Arya S.K., Gallo R.C., Lusso P. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 270:1811–1815 10.1126/science.270.5243.1811 [DOI] [PubMed] [Google Scholar]
- Cooper A.M., Dalton D.K., Stewart T.A., Griffin J.P., Russell D.G., Orme I.M. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243–2247 10.1084/jem.178.6.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek D.C., Brenchley J.M., Betts M.R., Ambrozak D.R., Hill B.J., Okamoto Y., Casazza J.P., Kuruppu J., Kunstman K., Wolinsky S., et al. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 417:95–98 10.1038/417095a [DOI] [PubMed] [Google Scholar]
- Finberg R.W., Wahl S.M., Allen J.B., Soman G., Strom T.B., Murphy J.R., Nichols J.C. 1991. Selective elimination of HIV-1-infected cells with an interleukin-2 receptor-specific cytotoxin. Science. 252:1703–1705 10.1126/science.1904628 [DOI] [PubMed] [Google Scholar]
- Flynn J.L., Goldstein M.M., Chan J., Triebold K.J., Pfeffer K., Lowenstein C.J., Schreiber R., Mak T.W., Bloom B.R. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 2:561–572 10.1016/1074-7613(95)90001-2 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., Pamer E.G., Glickman M.S. 2008. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 205:2359–2368 10.1084/jem.20080353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamadia L.E., Remmerswaal E.B., Weel J.F., Bemelman F., van Lier R.A., Ten Berge I.J. 2003. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 101:2686–2692 10.1182/blood-2002-08-2502 [DOI] [PubMed] [Google Scholar]
- Geldmacher C., Schuetz A., Ngwenyama N., Casazza J.P., Sanga E., Saathoff E., Boehme C., Geis S., Maboko L., Singh M., et al. 2008. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J. Infect. Dis. 198:1590–1598 10.1086/593017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goletti D., Weissman D., Jackson R.W., Graham N.M., Vlahov D., Klein R.S., Munsiff S.S., Ortona L., Cauda R., Fauci A.S. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 157:1271–1278 [PubMed] [Google Scholar]
- Griffin E., Krantz E., Selke S., Huang M.L., Wald A. 2008. Oral mucosal reactivation rates of herpesviruses among HIV-1 seropositive persons. J. Med. Virol. 80:1153–1159 10.1002/jmv.21214 [DOI] [PubMed] [Google Scholar]
- Grossman Z., Meier-Schellersheim M., Paul W.E., Picker L.J. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289–295 10.1038/nm1380 [DOI] [PubMed] [Google Scholar]
- Hansen S.G., Vieville C., Whizin N., Coyne-Johnson L., Siess D.C., Drummond D.D., Legasse A.W., Axthelm M.K., Oswald K., Trubey C.M., et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293–299 10.1038/nm.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbinger K.H., Gerhardt M., Piyasirisilp S., Mloka D., Arroyo M.A., Hoffmann O., Maboko L., Birx D.L., Mmbando D., McCutchan F.E., Hoelscher M. 2006. Frequency of HIV type 1 dual infection and HIV diversity: analysis of low- and high-risk populations in Mbeya Region, Tanzania. AIDS Res. Hum. Retroviruses. 22:599–606 10.1089/aid.2006.22.599 [DOI] [PubMed] [Google Scholar]
- HTTP.WWW.WHO.INT/TB/CHALLENGES/HIV/FAQ/EN/. Accessed on Dec 3rd 2009. Frequently asked questions about TB and HIV.
- Kalsdorf B., Scriba T.J., Wood K., Day C.L., Dheda K., Dawson R., Hanekom W.A., Lange C., Wilkinson R.J. 2009. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am. J. Respir. Crit. Care Med. 180:1262–1270 10.1164/rccm.200907-1011OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S.H., McMichael A.J. 2005. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat. Med. 11:S33–S44 10.1038/nm1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter A.L., Ostrowski M., Goletti D., Oliva A., Weissman D., Gantt K., Hardy E., Jackson R., Ehler L., Fauci A.S. 1996. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous beta-chemokines and the viral inductive effects of other endogenous cytokines. Proc. Natl. Acad. Sci. USA. 93:14076–14081 10.1073/pnas.93.24.14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komanduri K.V., Viswanathan M.N., Wieder E.D., Schmidt D.K., Bredt B.M., Jacobson M.A., McCune J.M. 1998. Restoration of cytomegalovirus-specific CD4+ T-lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat. Med. 4:953–956 10.1038/nm0898-953 [DOI] [PubMed] [Google Scholar]
- Komanduri K.V., Feinberg J., Hutchins R.K., Frame R.D., Schmidt D.K., Viswanathan M.N., Lalezari J.P., McCune J.M. 2001. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J. Infect. Dis. 183:1285–1289 10.1086/319683 [DOI] [PubMed] [Google Scholar]
- Li Q., Duan L., Estes J.D., Ma Z.M., Rourke T., Wang Y., Reilly C., Carlis J., Miller C.J., Haase A.T. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- Mascola J.R., Louder M.K., Surman S.R., Vancott T.C., Yu X.F., Bradac J., Porter K.R., Nelson K.E., Girard M., McNeil J.G., et al. 1996. Human immunodeficiency virus type 1 neutralizing antibody serotyping using serum pools and an infectivity reduction assay. AIDS Res. Hum. Retroviruses. 12:1319–1328 10.1089/aid.1996.12.1319 [DOI] [PubMed] [Google Scholar]
- Mattapallil J.J., Douek D.C., Hill B., Nishimura Y., Martin M., Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 434:1093–1097 10.1038/nature03501 [DOI] [PubMed] [Google Scholar]
- Mexas A.M., Fogle J.E., Tompkins W.A., Tompkins M.B. 2008. CD4+CD25+ regulatory T cells are infected and activated during acute FIV infection. Vet. Immunol. Immunopathol. 126:263–272 10.1016/j.vetimm.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogues T., Goodrich M.E., Ryan L., LaCourse R., North R.J. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271–280 10.1084/jem.193.3.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J., Sonnenberg P., Nelson G., Bester A., Shearer S., Glynn J.R. 2007. Cause of death and presence of respiratory disease at autopsy in an HIV-1 seroconversion cohort of southern African gold miners. AIDS. 21:S97–S104 10.1097/01.aids.0000299416.61808.24 [DOI] [PubMed] [Google Scholar]
- Okoye A., Meier-Schellersheim M., Brenchley J.M., Hagen S.I., Walker J.M., Rohankhedkar M., Lum R., Edgar J.B., Planer S.L., Legasse A., et al. 2007. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 204:2171–2185 10.1084/jem.20070567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton W.A., Martin S.R., Tse D., O’Brien T.R., Skurnick J., VanDevanter N.L., Padian N., Braun J.F., Kotler D.P., Wolinsky S.M., Koup R.A. 1996. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat. Med. 2:412–417 10.1038/nm0496-412 [DOI] [PubMed] [Google Scholar]
- Perfetto S.P., Chattopadhyay P.K., Lamoreaux L., Nguyen R., Ambrozak D., Koup R.A., Roederer M. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods. 313:199–208 10.1016/j.jim.2006.04.007 [DOI] [PubMed] [Google Scholar]
- Ramilo O., Bell K.D., Uhr J.W., Vitetta E.S. 1993. Role of CD25+ and CD25-T cells in acute HIV infection in vitro. J. Immunol. 150:5202–5208 [PubMed] [Google Scholar]
- Rana F.S., Hawken M.P., Mwachari C., Bhatt S.M., Abdullah F., Ng’ang’a L.W., Power C., Githui W.A., Porter J.D., Lucas S.B. 2000. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J. Acquir. Immune Defic. Syndr. 24:23–29 [DOI] [PubMed] [Google Scholar]
- Raszka W.V., Moriarty R.A., Ottolini M.G., Waecker N.J., Ascher D.P., Cieslak T.J., Fischer G.W., Robb M.L. 1996. Delayed-type hypersensitivity skin testing in human immunodeficiency virus-infected pediatric patients. J. Pediatr. 129:245–250 10.1016/S0022-3476(96)70249-4 [DOI] [PubMed] [Google Scholar]
- Ray J.C., Flynn J.L., Kirschner D.E. 2009. Synergy between individual TNF-dependent functions determines granuloma performance for controlling Mycobacterium tuberculosis infection. J. Immunol. 182:3706–3717 10.4049/jimmunol.0802297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedner G., Rusizoka M., Hoffmann O., Nichombe F., Lyamuya E., Mmbando D., Maboko L., Hay P., Todd J., Hayes R., et al. 2003. Baseline survey of sexually transmitted infections in a cohort of female bar workers in Mbeya Region, Tanzania. Sex. Transm. Infect. 79:382–387 10.1136/sti.79.5.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyker J.A., Lohman-Payne B.L., Rowland-Jones S.L., Otieno P., Maleche-Obimbo E., Richardson B., Farquhar C., Mbori-Ngacha D., Emery V.C., John-Stewart G.C. 2009. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS. 23:117–124 10.1097/QAD.0b013e32831c8abd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg P., Glynn J.R., Fielding K., Murray J., Godfrey-Faussett P., Shearer S. 2005. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J. Infect. Dis. 191:150–158 10.1086/426827 [DOI] [PubMed] [Google Scholar]
- Streitz M., Tesfa L., Yildirim V., Yahyazadeh A., Ulrichs T., Lenkei R., Quassem A., Liebetrau G., Nomura L., Maecker H., et al. 2007. Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis. PLoS One. 2:e735 10.1371/journal.pone.0000735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton J.C., Luskin M.R., Johnson A.J., Manion M., Hallahan C.W., Metcalf J.A., McLaughlin M., Davey R.T., Jr, Connors M. 2007. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. J. Virol. 81:2713–2725 10.1128/JVI.01830-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop S.L., Pitcher C.J., Peterson D.M., Maino V.C., Picker L.J. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Invest. 99:1739–1750 10.1172/JCI119338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J.A., Arrigo S.J., Weitsman S.R., Go A.S., Haislip A., Chen I.S. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 61:213–222 10.1016/0092-8674(90)90802-L [DOI] [PubMed] [Google Scholar]