Abstract
Poly(dimethylsiloxane) (PDMS) microstructures have been widely used in bio-microelectromechanical systems (bio-MEMS) for various types of analytical, diagnostic and therapeutic applications. However, PDMS-based soft lithographic techniques still use conventional microfabrication processes to generate a master mold, which requires access to clean room facilities and costly equipment. With the increasing use of these systems in various fields, the development of benchtop systems for fabricating microdevices is emerging as an important challenge in their widespread use. Here we demonstrate a simple, low-cost and rapid method to fabricate PDMS microstructures by using micropatterned poly(ethylene glycol) diacrylate (PEGDA) master molds. In this method, PEGDA microstructures were patterned on a glass substrate by photolithography under ambient conditions and by using simple tools. The resulting PEGDA structures were subsequently used to generate PDMS microstructures by standard molding in a reproducible and repeatable manner. The thickness of the PEGDA microstructures was controllable from 15 to 300 μm by using commonly available spacer materials. We also demonstrate the use of this method to fabricate microfluidic channels capable of generating concentration gradients. In addition, we fabricated PEGDA microstructures by photolithography from the light generated from commonly available laminar cell culture hood. These data suggest that this approach could be beneficial for fabricating low-cost PDMS-based microdevices in resource limited settings.
1. Introduction
Polydimethylsiloxane (PDMS) microstructures have been widely used for nano- and microscale applications in bio-microelectromechanical systems (bio-MEMS) [1]. This is because PDMS is mechanically robust, optically clear, chemically inert and stable [2]. Typically, PDMS-based microfabrication requires master molds patterned by photolithography with SU-8 photoresist and silicon. This conventional photolithography process requires clean room facilities and costly equipment such as a spin coater, a mask aligner and a wet bench. This requirement has been an obstacle for the widespread use of PDMS-based devices by biologists [3, 4].
Due to the need for simple, low-cost and rapid microfabrication techniques for creating PDMS microstructures, the development of simplified methods to fabricate master molds may be beneficial. Many simplified microfabrication techniques have been developed for creating PDMS structures. These include printed circuit technology [5], laser printed thermoplastic film [6], wax printing [7], ice patterning [8] and liquid molding [9]. However, these methods have a number of limitations. For example, the printing circuit method uses a toxic copper etching solution and additional etching and rinsing steps. For the laser printing method, shrinkage after thermal treatment hampers the specific dimension of the structure. The wax printing process relies on specialized printers and results in features of transparency film that may be rough. Ice patterning requires additional steps (evaporation and freezing) and is limited to fabricating dome-shaped molds. Finally, the liquid molding method is not well suited for fabricating structures over 100 μm thickness due to the surface tension of the liquid.
Photocrosslinkable polymers have been widely used in microengineered cell patterning and encapsulation [10–13]. In particular, poly(ethylene glycol) diacrylate (PEGDA) has been applied commonly to fabricate a microwell array [14, 15], encapsulate cells in hydrogels [16] and generate cell-laden microgels for tissue engineering [17]. Furthermore, photolithographically patterned PEGDA hydrogel microstructures have been shown to have resolutions comparable to conventional photoresist-based patterns [18] making them potentially applicable as master molds for generating PDMS microstructures.
In this study, we develop a method to micropattern PDMS by using photolithographically generated PEGDA-based microstructures as a templating material. In particular, we develop a simplified photolithography method that can generate PEGDA (and the resulting PDMS) microstructures with thicknesses ranging from 15 to 300 μm. The proposed technique did not require expensive clean room facilities and can generate structures with good resolution by using common equipment in most biological laboratories. By assembling a photomask and UV irradiator, micropatterned PEGDA master molds were prepared within several minutes for PDMS replication. In addition, gradient-forming microfluidic channels were fabricated with direct applications in biological processes. Given the ease with which this process can be adapted for benchtop fabrication, it may be of benefit for a broad range of users.
2. Materials and methods
2.1. Materials
PEGDA, 3-(trimethoxysilyl)propyl methacrylate (TMSPMA), 2,2-dimethoxy-2-phenyl-acetophenone (DMPA), sodium hydroxide and absolute ethanol were purchased from Sigma-Aldrich (Wisconsin, USA). PDMS (Sylgard 184, Dow Corning), glass slides and glass coverslips were purchased from Fisher Scientific (Philadelphia, USA), and the printed photomask was purchased from CADart (Washington, USA). The UV light source (Omnicure S2000) with filter (wavelength: 320–500 nm) was manufactured at EXFO Photonic Solutions Inc. (Ontario, Canada). The thickness of spacers was measured with an electronic digital micrometer (Marathon Watch Company Ltd, Ontario, Canada).
2.2. Substrate preparation
The surface of glass slides (25 × 75 mm2, thickness = 1 mm) was acrylated to increase the adhesion of micropatterns [18]. In brief, the glass slides were treated with 10% sodium hydroxide solution for 1 h, rinsed with distilled water and dried under N2 gas. The glass slides were stacked and wetted with TMSPMA and incubated at 80 °C overnight. The coated glass slides were then washed three times with ethanol, dried under N2 gas and stored at room temperature prior to use. All the glass substrates were used within 7 days after TMSPMA surface treatment.
2.3. Fabrication of PEGDA master molds
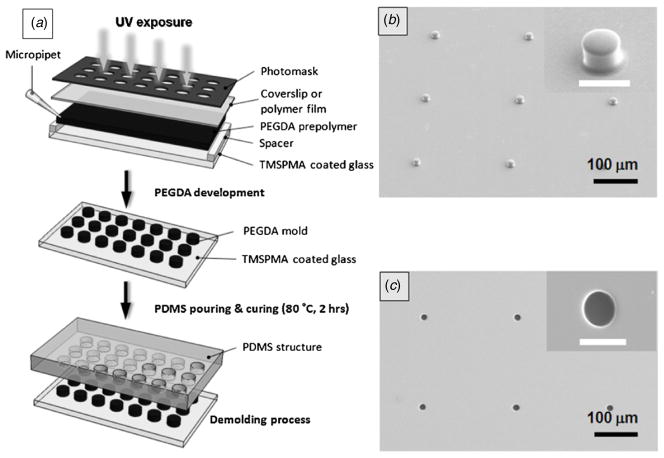
The schematic of the method to fabricate micropatterned PEGDA structures as template molds for the PDMS microstructure is shown in figure 1(a). The printed film photomask, coverslip, spacer and TMSPMA-coated glass slide were assembled by stacking. The PEGDA prepolymer solution was loaded in the space between the coverslip and the TMSPMA-coated glass slide. Light (350–500 nm) was then illuminated on the assembly for several seconds. A prepolymer solution was prepared by mixing 5 mL of PEGDA (MW 258 Da, 99%) with 150 mg of DMPA (99%) photoinitiator. After photocrosslinking, micropatterned PEGDA molds were developed using the ethanol–water solution (70:30) to remove non-crosslinked prepolymers and dried with N2 blowing. Several minutes were required for one cycle of the photolithographic PEGDA master mold fabrication process (stacking, PEGDA solution loading, UV exposure, developing and N2 drying).
Figure 1.
Micropatterned PEGDA template for the fabrication of PDMS microstructures. (a) Photolithography of PEGDA on the TMSPMA-coated glass slide. TMSPMA-coated glass substrates were stacked under a photomask with spacers and coverslip, filled with PEGDA and UV crosslinked. After developing with the ethanol–water solution, the PDMS prepolymer was poured on the glass and thermally cured. (b) Scanning electron microscopy image of crosslinked PEGDA microstructures with the 25 μm diameter circle photomask and polyethylene wrap film spacers. (c) PDMS microwell array replicated from the PEGDA micromold of (b). Insets are magnified images (scale bar = 20 μm).
2.4. Thickness measurements
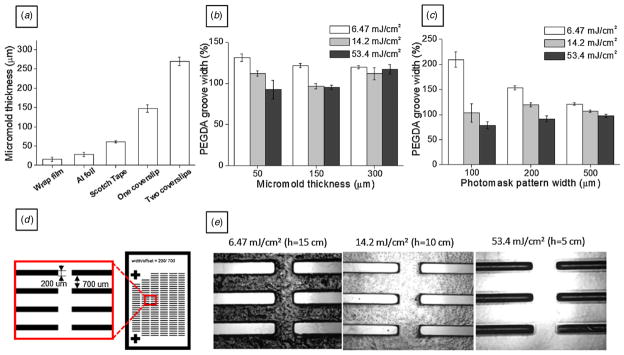
The thickness of spacer was measured with an electronic digital micrometer. To measure PEGDA microstructures, a photomask with striped micropatterns was assembled and polymerized on non-treated glass slide substrates and PEGDA micromolds were detached with a razor blade. Images of PEGDA micromolds were taken with an Eclipse TE2000-U optical microscope (Nikon, Japan) and analyzed with ImageJ software (NIH, USA). At least three samples were prepared for each spacer condition and ten pictures were taken at different locations for each sample. Measured data are shown in figure 2 with mean ± SD.
Figure 2.
(a) Measurement of the PEGDA micromold thickness fabricated by using various spacers. PEGDA master molds showed a controlled and reproducible height. (b) Groove size of the PEGDA master mold crosslinked from the 200 μm width stripe photomask under different spacers (Scotch tape: 50 μm, one coverslip: 150 μm and two coverslips: 300 μm) and UV energy. (c) PEGDA micromold groove width under varying UV doses from the original stripe width of 100, 200 and 500 μm in the photomask. The spacer was one layer of coverslip. (d) 200 μm width stripe photomask used for PEGDA patterns. (e) PEGDA pattern generated using the 200 μm width stripe photomask. PEGDA patterns were produced with controlled UV energy (6.47, 14.2 and 53.4 mJ cm−2). The UV energy was controlled with the distance from the UV source to the photomask (h = 15, 10 and 5 cm).
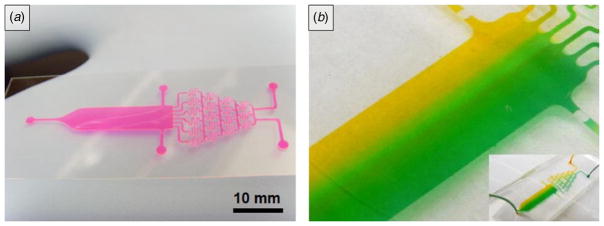
The effect of spacer thickness on the resolution of PEGDA micropatterns was studied by using a photomask patterned with 200 μm wide patterns (figure 2(d)). UV light illumination was controlled by changing the distance between the UV light source and the photomask. The resulting light intensities were 6.47, 13.2 and 53.4 mJ cm−2 for the distances of 5, 10 and 15 cm, respectively. The variation in the resulting dimensions of the polymerized PEGDA micropatterns was measured and compared with photomasks with various-width stripe features (width: 50, 100, 250 and 500 μm) in different thickness spacers (thickness: 50, 150 and 300 μm). Finally, to demonstrate the feasibility of our proposed method for fabricating microfluidic chips, a PDMS microfluidic gradient generator was made by using a single spacer of 150 μm thick coverslips (figure 4).
Figure 4.
Fabrication of PDMS microfluidic channels from the PEGDA generated microstructures. (a) PEGDA micromold of a microchannel fabricated on the glass substrate, colored with Rhodamine B, and (b) PDMS microfluidic channel fabricated from (a) showing the laminar flow in the microchannel, colored with green and yellow dyes. The channel width in the gradient region is 4.8 mm.
2.5. PDMS replication and microfluidic device fabrication
Micropatterned PEGDA master molds were replicated with PDMS elastomer. PDMS was prepared by mixing 10:1 weight ratio of prepolymer and crosslinking agent, degassed under vacuum, poured on the patterns, and thermally cured at 80 °C for 2 h. Demolded PDMS was bonded on bare glass slides after oxygen plasma treatment for microchip fabrication.
2.6. Scanning electron microscopy
To analyze the samples by using scanning electron microscopy (SEM), PEGDA master molds were rinsed with 70% of ethanol in water, dried under N2 gas and kept in vacuum for 24 h. Prior to SEM imaging, the samples were sputter coated with palladium–platinum alloy target material with the ion current of 40 mA for 80 s. The surface morphology of the PEGDA master mold and replicated PDMS was observed with a field emission scanning electron microscope (FE SEM) Ultra 55 (Carl Zeiss, Inc., NY, USA) with an acceleration voltage of 5 kV.
3. Results and discussion
3.1. PEGDA master mold fabrication
We used the tough mechanical properties of PEGDA structures along with the ease with which they can be micropatterned for fabricating PDMS structures for BioMEMS applications. Figure 1(b) is a SEM image of arrayed microposts from photopatterned PEGDA. PEGDA micromolds were patterned with a 20 μm diameter circle photomask of 15 μm target thickness by using a polyethylene wrap film (GLAD, CA, USA) as the spacer. Figure 1(c) shows the SEM image of the PDMS microstructure replicated from the PEGDA master mold in figure 1(b). As can be seen, the microstructures generated using this technique show good pattern fidelity and can be transferred to the resulting PDMS structures.
Figure 2 shows the effects of various parameters on the resulting PEGDA and PDMS structures. As can be seen, micropatterns with heights ranging from 15 to 300 μm were fabricated by using commonly available materials as spacers (see table 1). The PEGDA micromold height could be controlled in a relatively wide range from 15.8 ± 5.35 μm with one layer of wrap film spacer to 269 ± 11.1 μm by using two stacked coverslips. During PEGDA precursor solution filling, coverslips were bent due to capillary forces when the space between the coverslip and the glass slide was incompletely filled. This capillary force-induced bending may be a reason for thickness differences of the resulting PEGDA structures from the measured spacer thickness. But the deviation of the PEGDA micromold height can be minimized by filling with sufficient volume of PEGDA prepolymer solution. As shown in figure 2(a), the thickness of the PEGDA could be easily changed in a reproducible manner by using commonly available materials. The manual placing of a soft spacer, like polyethylene wrap film, may result in the deviation of the PEGDA micromold height from the spacer thickness. For reproducible fabrication of micropatterns of less than 10 μm thickness, conventional spin coater-based microfabrication technology may be advantageous as has been demonstrated previously [18].
Table 1.
Measured thickness of spacers and PEGDA mold after UV crosslinking (mean ± SD).
| Spacer | Spacer thickness (A, n = 15), μm | PEG height (B, n = 100), μm |
|---|---|---|
| Polyethylene wrap film | 14.3 ± 5.1 | 15.8 ± 5.3 |
| Aluminum foil | 18 ± 1.7 | 28.1 ± 5.4 |
| Scotch tape | 48.8 ± 2.0 | 61.2 ± 2.7 |
| Coverslip | 155 ± 1.6 | 148 ± 9.4 |
| Two coverslips | 311 ± 2.9 | 269 ± 11 |
The optimal UV condition and resulting relative pattern size were 14.2 mJ cm−2 and 103.4 ± 18.4% for 100 μm. For 200 μm and 500 μm width mask patterns, the measured pattern sizes at the optimal UV energy with 53.4 mJ cm−2 were 91.5 ± 5.7% and 97.4 ± 2.8%, respectively (figure 2(b)). Figure 2(c) shows the effect of height on the PEGDA pattern size under different UV energy conditions. In these experiments, the photomask pattern width was fixed to 200 μm and the UV energy was changed (6.47, 14.2 and 53.4 mJ cm−2). For conditions in which the PEGDA micromold height was 50 μm, the width of the polymerized PEGDA grooves decreased from 131 ± 4.45% to 92.5 ± 11.3% of the size of the photomask patterns as the UV energy was increased from 6.47 mJ cm−2 to 53.4 mJ cm−2. This indicates that, as expected, the degree of polymerization increased with illuminated UV energy, resulting in a decrease in the PEGDA groove width as shown in figure 2(e). UV energy for patterning can be changed according to different photoinitiator concentration (supplementary image S1 available from stacks.iop.org/BF/2/045001/mmedia). The UV exposure time for the same-sized pattern was decreased as photoinitiator concentration increased. For a photomask with a 320 μm width and 150 μm thickness spacer, shorter exposure time resulted in larger groove width reflecting insufficient crosslinking for all photoinitiator concentrations.
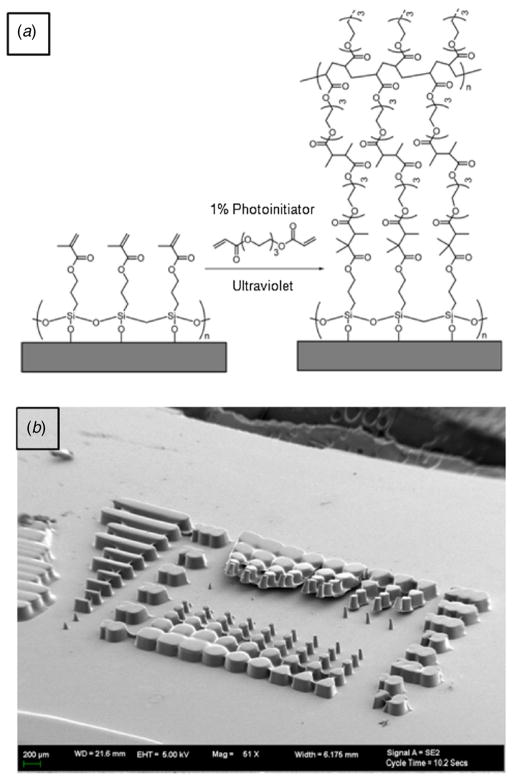
3.2. Stability of PEGDA master molds
PEGDA master molds were stable without changing their structures on the TMSPMA-coated substrates and were resistant to wear after multiple rounds of replications with PDMS. The acrylic functional group of TMSPMA on the substrates and PEGDA were crosslinked during UV exposure, which enhanced the stability of polymerized PEGDA on glass substrates (figure 3(a)). Acrylation of the substrates with TMSPMA improved adhesion and stabilized the micropattern layer on the glass as well as Si wafers [18]. In contrast, PEGDA layers formed on non-treated glass substrates were easily detached from the substrate during drying and PDMS curing processes (figure 3(b)).
Figure 3.
(a) UV crosslinking of PEGDA with surface bound TMSPMA. (b) Delamination of PEGDA micromolds from the bare glass surface.
Low molecular weight (LMW) PEGDA with 258 Da was more stable for micropatterning compared to high molecular weight (HMW) PEGDA. HMW PEGDA (over 1000 Da) structures, both under hydrated and dehydrated conditions, were easily detached from the substrates. This was partially due to the swelling of the structures during washing processes. In contrast, LMW PEGDA (under 1000 Da) did not show significant swelling or shrinking during development and PDMS curing steps. The PDMS microfluidic pattern replicated from PEGDA micromolds did not show a significant difference in function compared to conventional photoresist-based products. In addition, PEGDA replicated PDMS structures could be plasma treated for adhesion to glass surfaces similar to PDMS cured on the Si wafer. These microstructures could be used to generate microfluidic channels capable of generating concentration gradients with potential applications in biological systems (figure 4) [19, 20].
3.3. Advantages of a PEGDA master mold for PDMS fabrication
The proposed method can be used to fabricate soft polymer-based microfluidic devices using a PEGDA master mold without spin-coating and time-consuming baking processes as well as high-cost microfabrication facilities. Compared to conventional photoresist micropatterning, the PEGDA photolithographic method has several advantages such as simplicity, usage of non-toxic materials, easy control of thickness, cost effectiveness and convenience.
The first key advantage of this method over traditional SU-8-based methods for fabricating PDMS structures is its simplicity. The proposed process only requires four easy steps: the stacking of substrate glasses with a photomask, PEGDA loading, photo illumination and washing. Thus this process can be performed in less than 5 min. The TMSPMA coating step takes a relatively long time, but a large number of glass slides can be prepared in one process. Secondly, the reagents and developer used in the process are less toxic than those used in conventional processes [21–23]. For example, PEGDA is less toxic in comparison to conventional photoresists and can be used for cell encapsulation [17, 21, 24, 25]. PEGDA is water and alcohol soluble and unreacted macromers can be removed with water during the developing step. Thirdly, the thickness control of PEGDA micromolds is easily adjustable. Micropatterns with various thicknesses can be fabricated with commonly available spacers. In a conventional photoresist process, the spin coating speed must be adjusted to control pattern thickness, which should be optimized to the photoresist’s viscosity. This is an iterative process for an initial setup of fabrication facility. In this study, pattern thickness control can be easily achieved by inserting different spacers of known thickness. The specific thickness micropattern ranging from 15 to 300 μm could be easily achieved by combining these spacers properly. Furthermore, the proposed method does not require a spin coater, aligners, photoresists, chemical developers and chemical hood set up in clean room facilities, which are necessary for a conventional micropatterning process [6, 26].
Despite its advantages, PEGDA-based photolithography has potential limitations in fabricating high-aspect-ratio structures compared to conventional photolithography using thick-negative photoresist (e.g. SU-8). This limitation is mainly caused by unparallel UV light which is not well collimated from the UV source. If a well-collimated UV source of aligner is used for exposure, the resolution and aspect ratio of structures will improve [27]. Overall, we propose that PEGDA-based photolithography for PDMS microstructure fabrication has a potential for rapid prototyping and fabricating a low-cost PDMS for general applications where high-resolution and high-aspect-ratio structures are not required. Additionally, several seconds of ultrasonication will improve the sharpness of the final developed structure.
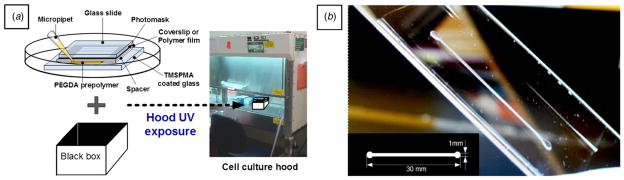
Furthermore, patterning of PEGDA using common UV light sources (e.g. cell culture hood UV, UV illuminator for PCR-gel analysis, etc) was performed using the same stacking procedure as the previous experiment. Longer exposure time was employed because intensities of those pieces of equipment are lower compared to UV irradiator. In this patterning test, millimeter-scale PEGDA structures were well crosslinked and developed under normal UV light sources. A fabricated PEGDA channel exposed under cell culture hood UV (wavelength: 253.7 nm) for 30 min is shown in figure 5. However, it was difficult to achieve microscale patterns using normal UV light sources. As expected, this can be explained by unparallel and scattered UV light. Aside from the limited resolution, it shows the feasibility and potential of PEGDA-based photolithography in biological and chemical applications using the UV light source of common equipment in the laboratory.
Figure 5.
(a) Schematic representation of the experimental setup for PEGDA patterning using a common UV light source. The black box located under the petri dish was used to prevent light reflecting from the bottom. (b) Photograph of the PEGDA channel mold exposed under cell culture hood UV for 30 min. The inserted mask image shows the dimension of the channel (length: 30 mm, width: 1 mm and diameters of inlet/outlet: 2 mm). Two coverslips (spacer thickness: 300 μm) were used as a spacer in this case.
As an alternative to high-resolution printed film photomask used in this paper, office transparency films printed by a regular laser printer can also be used to fabricate the PEGDA master mold using the same procedure. However, there are limits in the resolution and roughness along the pattern’s outline. The resolution and roughness depend on the resolution of the printer (regular office printer: 600 dpi or 1200 dpi) as well as the surface and transparency of films.
4. Conclusion
In this paper, we demonstrated the benchtop photolithographic fabrication of PEGDA templates for the molding of PDMS microstructures. PEGDA micromolds were prepared in several minutes and replicated to PDMS microstructures. Micromold thickness control with commonly available spacers was effective and reproducible for 15–300 μm thick PEGDA micromolds. With this technique, PDMS microfluidic chips capable of generating fluidic gradients were also fabricated. Also, PEGDA-based photolithography was performed by using the UV light source from common laboratory equipment. The proposed technique is simple, convenient, non-toxic and cost effective compared to conventional clean room facility-based high-end fabrication methods. With its simplicity, this technique has a strong potential as an alternative choice for benchtop microfabrication.
Supplementary Material
Acknowledgments
This paper was supported by the National Institutes of Health (EB007249; DE019024; HL092836), National Science Foundation (DMR0847287), the Institute for Soldier Nanotechnology and the US Army Corps of Engineers. WYS was partially supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-357-D00099).
Footnotes
Online supplementary data available from stacks.iop.org/BF/2/045001/mmedia
(Some figures in this article are in colour only in the electronic version)
Contributor Information
Sang-Hoon Lee, Email: dbiomed@korea.ac.kr.
Ali Khademhosseini, Email: alik@rics.bwh.harvard.edu.
References
- 1.Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem. 2003;75:6544–54. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- 2.Xia Y, Whitesides GM. Soft lithography. Ann Rev Mater Sci. 1998;28:153–84. [Google Scholar]
- 3.Andersson H, Berg A. Microfabrication and microfluidics for tissue engineering: state of the art and future opportunities. Lab Chip. 2004;4:98–103. doi: 10.1039/b314469k. [DOI] [PubMed] [Google Scholar]
- 4.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480–7. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudarsan AP, Ugaz VM. Printed circuit technology for fabrication of plastic-based microfluidic devices. Anal Chem. 2004;76:3229–35. doi: 10.1021/ac035411n. [DOI] [PubMed] [Google Scholar]
- 6.Grimes A, Breslauer DN, Long M, Pegan J, Lee LP, Khine M. Shrinky-Dink microfluidics: rapid generation of deep and rounded patterns. Lab Chip. 2008;8:170–2. doi: 10.1039/b711622e. [DOI] [PubMed] [Google Scholar]
- 7.Kaigala GV, Ho S, Penterman R, Backhouse CJ. Rapid prototyping of microfluidic devices with a wax printer. Lab Chip. 2007;7:384–7. doi: 10.1039/b617764f. [DOI] [PubMed] [Google Scholar]
- 8.Park JY, Hwang CM, Lee SH. Ice-lithographic fabrication of concave microwells and a microfluidic network. Biomed Microdevices. 2009;11:129–33. doi: 10.1007/s10544-008-9216-1. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Wang Q, Qin J, Lin B. A facile ‘liquid-molding’ method to fabricate PDMS microdevices with 3-dimensional channel topography. Lab Chip. 2009;9:1200–5. doi: 10.1039/b818721e. [DOI] [PubMed] [Google Scholar]
- 10.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, III, Langer R, Burdick JA. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79:522–32. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 11.Chiu DT, et al. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proc Natl Acad Sci USA. 2000;97:2408–13. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda J, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27:5259–67. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci USA. 2009;106:16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29:752–63. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdick J, Anseth K. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–23. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 17.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci USA. 2008;105:9522–7. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revzin A, et al. Fabrication of poly(ethylene glycol) hydrogel microstructures using photolithography. Langmuir. 2001;17:5440–7. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- 19.Chung BG, et al. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip. 2005;5:401–6. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 20.Burdick JA, Khademhosseini A, Langer R. Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir. 2004;20:5153–6. doi: 10.1021/la049298n. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, et al. Integrating sensing hydrogel microstructures into micropatterned hepatocellular cocultures. Langmuir. 2009;25:3880. doi: 10.1021/la803635r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuttelman C, Benoit D, Tripodi M, Anseth K. The effect of ethylene glycol methacrylate phosphate in PEG hydrogels on mineralization and viability of encapsulated hMSCs. Biomaterials. 2006;27:1377–86. doi: 10.1016/j.biomaterials.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Yan J, Sun Y, Zhu H, Marcu L, Revzin A. Enzyme-containing hydrogel micropatterns serving a dual purpose of cell sequestration and metabolite detection. Biosens Bioelectron. 2009;24:2604–10. doi: 10.1016/j.bios.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Tsang V, et al. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 25.Zguris J, Itle L, Koh W, Pishko M. A novel single-step fabrication technique to create heterogeneous poly (ethylene glycol) hydrogel microstructures containing multiple phenotypes of mammalian cells. Langmuir. 2005;21:4168–74. doi: 10.1021/la0470176. [DOI] [PubMed] [Google Scholar]
- 26.Feng R, Farris R. Influence of processing conditions on the thermal and mechanical properties of SU8 negative photoresist coatings. J Micromech Microeng. 2003;13:80–8. [Google Scholar]
- 27.Bogdanov A, Peredkov S. Use of SU-8 photoresist for very high aspect ratio x-ray lithography. Microelectron Eng. 2000;53:493–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.