Abstract
The acquisition of invasiveness in ovarian cancer (OC) is accompanied by the process of epithelial-to-mesenchymal transition (EMT). The MUC4 mucin is overexpressed in ovarian tumors and has a role in the invasiveness of OC cells. The present study was aimed at evaluating the potential involvement of MUC4 in the metastasis of OC cells by inducing EMT. Ectopic overexpression of MUC4 in OC cells (SKOV3-MUC4) resulted in morphological alterations along with a decreased expression of epithelial markers (E-cadherin and cytokeratin (CK)-18) and an increased expression of mesenchymal markers (N-cadherin and vimentin) compared with the control cells (SKOV3-vector). Also, pro-EMT transcription factors TWIST1, TWIST2 and SNAIL showed an upregulation in SKOV3-MUC4 cells. We further investigated the pathways upstream of N-cadherin, such as focal adhesion kinase (FAK), MKK7, JNK1/2 and c-Jun, which were also activated in the SKOV3-MUC4 cells compared with SKOV3-vector cells. Inhibition of phospho-FAK (pFAK) and pJNK1/2 decreased N-cadherin expression in the MUC4-overexpressing cells, which further led to a significant decrease in cellular motility. Knockdown of N-cadherin decreased the activation of extracellular signal-regulated kinase-1/2 (ERK1/2), AKT and matrix metalloproteinase 9 (MMP9), and inhibited the motility in the SKOV3-MUC4 cells. Upon in vivo tumorigenesis and metastasis analysis, the SKOV3-MUC4 cells produced significantly larger tumors and demonstrated a higher incidence of metastasis to distance organs (peritoneal wall, colon, intestine, stomach, lymph nodes, liver and diaphragm). Taken together, our study reveals a novel role for MUC4 in inducing EMT through the upregulation of N-cadherin and promoting metastasis of OC cells.
Keywords: MUC4, EMT, E-cadherin, N-cadherin, metastasis and ovarian cancer
Introduction
Epithelial-to-mesenchymal transition (EMT) is a trans-differentiation process characterized by coordinated molecular and cellular changes defined by a reduction in cell–cell adhesion and loss of apical–basolateral polarity (Thiery, 2002). The gain of mesenchymal markers and the loss of epithelial markers, the hallmarks of EMT, as well as altered morphological features associated with this process inducing increased motility and invasion of cancer cells (Thiery, 2002; Ahmed et al., 2007). EMT is also involved in cancer progression and metastasis and it is probable that a common molecular mechanism is shared by these processes (Thiery, 2002; Ahmed et al., 2007). EMT occurs during ovarian cancer (OC) progression in response to various stimuli. However, the underlying mechanisms are not well established in OC.
The MUC4 mucin is a large glycoprotein that frequently displays an altered expression in several cancers (Andrianifahanana et al., 2001; Carraway et al., 2001; Hollingsworth and Swanson, 2004; Chaturvedi et al., 2007; Ponnusamy et al., 2008). An overexpression of MUC4 mRNA has been reported in OC (Giuntoli et al., 1998; Lopez-Ferrer et al., 2001) and in an earlier study, we have shown that MUC4 is aberrantly expressed in >90% of malignant ovarian tumors with very low to an undetectable expression in the normal ovary (Chauhan et al., 2006). These observations suggest that MUC4 may have a major role in the pathogenesis of OC. The domain organization of MUC4 suggests that it is a membrane-anchored protein containing two subunits, MUC4α (an extra-cellular mucin-type glycoprotein subunit) and a MUC4β transmembrane subunit with a short cytoplasmic tail (Nollet et al., 1998; Moniaux et al., 1999). The juxtamembrane domain of the MUC4 has also been suggested to induce the metastatic cellular characteristics and signaling by interacting with epidermal growth factor receptors (that is, HER2 via any of its three epidermal growth factor domains; Chaturvedi et al., 2007; Ponnusamy et al., 2008). Further, we have reported that MUC4 activates the focal adhesion kinase (FAK) in pancreatic and OC cells (Chaturvedi et al., 2007; Ponnusamy et al., 2008). In our previous studies, we have observed MUC4-induced phenotypic variation and signaling alteration in pancreatic cancer and OC cells (Chaturvedi et al., 2007; Ponnusamy et al., 2008), which encourages us to further look into its role in EMT and cadherin alterations. Cadherins are transmembrane glycoproteins belonging to a large family of calcium-dependent cell–cell adhesion molecules that have important roles in maintaining normal histoarchitechture, cell polarity, embryonic development and signal transduction (Behrens, 1994; Chen et al., 1997; Hazan et al., 2004). The altered expression of E-cadherin and N-cadherin has been shown to strongly affect motility, aggregation, invasive and adhesive properties of a large variety of cells (Kondo et al., 1998; Auersperg et al., 1999; Hazan et al., 2000). The upregulation of N-cadherin has also been shown to promote tumor growth, invasion and metastasis, and knockdown of c-Jun prevents N-cadherin upregulation and limited tumor growth and invasion in a mouse model for pancreatic cancer (Shintani et al., 2008).
The current study was designed to test the hypothesis that MUC4 signaling promotes EMT, thereby increasing the metastatic nature of human OC cells. Our results suggest that MUC4 could be a potential candidate for inducing EMT by altering the expression/activation of metastasis promoting signaling molecules.
Results
Overexpression of the MUC4 mucin in human OC cells
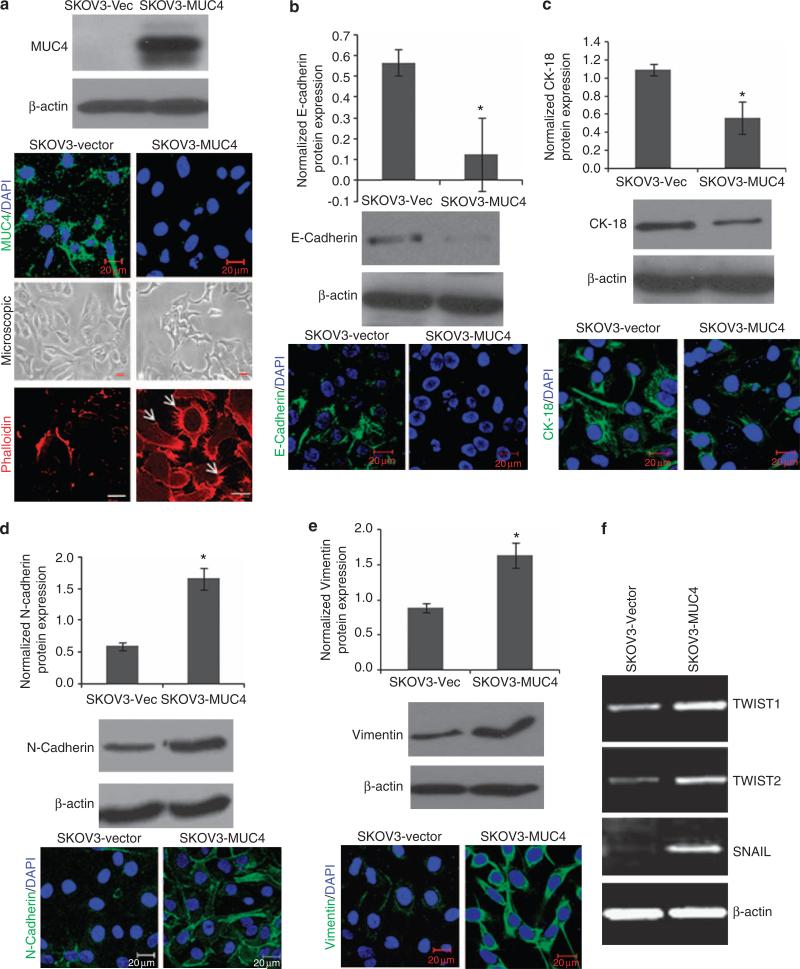
We have generated a MUC4 gene construct (~9 kb) to overcome the transfection-associated problems owing to the large size (27 kb) of MUC4 mRNA, and investigated the biological function and effect of MUC4 expression (Moniaux et al., 2007; Ponnusamy et al., 2008). MUC4-negative OC cell lines, SKOV3 and OVCAR3, were selected for analyzing the role of MUC4 in OC. The MUC4 construct is similar to the wild-type MUC4 except for the tandem repeat region, which is 10% the size of the wild-type allele (Moniaux et al., 2007; Ponnusamy et al., 2008). The expression of MUC4 in stable cell transfectants was evaluated by immunoblotting and confocal analysis using a MUC4 antibody (8G7) that recognizes an epitope in the tandem repeat domain of MUC4. This antibody recognizes a MUC4 protein band of ~322 kDa, specifically in MUC4-transfected cells (SKOV3-MUC4 and OVCAR3-MUC4) and not in the vector-transfected cells (SKOV3-vector and OVCAR3-vector; Figure 1a and Supplementary Figure 1). The expression and subcellular localization of MUC4 was further confirmed by immunofluorescence and confocal microscopy. A total of 90% of the SKOV3-MUC4-transfected cells showed localization of MUC4 in both the plasma membrane and cytoplasm (Figure 1a) MUC4 is undetectable in the vector-transfected SKOV3 cells (Figure 1a).
Figure 1.
MUC4 overexpression alters cell morphology and epithelial phenotype marker expression. (a) Immunoblotting analysis of ectopic MUC4 expression in SKOV3 cells (SKOV3-MUC4) and control SKOV3 cells (SKOV3-vector). First panel showed the confocal immunofluorescence analysis for MUC4 in SKOV3-vector and SKOV3-MUC4 cells (scale bar-20 μm; fluorescein isothiocyanate (FITC) for MUC4 and 4′-6-diamidino-2-phenylindole (DAPI) for nuclear staining). Middle panel showed the phase contrast microscopic picture of MUC4-overexpressing SKOV3 cells and control cells (original magnification × 200, scale bar-0.8 mm). The last panel showed the phalloidin-rhodamine (phalloidin-RITC) staining of actin–cytoskeleton variation in both empty vector- and MUC4-transfected SKOV3 cells (scale bar-20 μm). MUC4-overexpressed SKOV3 cells were associated with the presence of more microspikes, lamellopodia- and filopodia-like cellular projections (arrows) with dense actin concentrated at the cellular protrusions compared with the empty vector control cells. (b, c) Immunoblotting analysis showed significantly decreased expression of epithelial markers E-cadherin (*P = 0.021) and CK-18 (*P = 0.01) in SKOV3-MUC4 cells compared with SKOV3-vector cells. Confocal microscopy showed increased staining of E-cadherin and CK in the SKOV3-vector cells and diminished staining in SKOV3-MUC4 cells (scale bar-20 μm). (FITC-conjugated goat anti-mouse IgG for secondary antibody and DAPI was used for nuclear staining.) (d, e) Immunoblotting analysis showed the significantly increased expression of mesenchymal marker N-cadherin (*P = 0.017) and vimentin (*P = 0.04) in SKOV3-MUC4 cells compared with SKOV3-vector cells. Confocal microscopy showed increased staining of N-cadherin and vimentin in the SKOV3-MUC4 cells and decreased staining in SKOV3-vector cells (scale bar-20 μm). (FITC-conjugated goat anti-mouse IgG for secondary antibody and DAPI was used for nuclear staining.) (f) Reverse transcriptase–PCR analysis for the EMT key transcription factors TWIST1, TWIST2 and SNAIL in both SKOV3-MUC4 and SKOV3-vector cells. β-actin was used as a control.
MUC4 overexpression induces a mesenchymal phenotype in OC cells
We further analyzed the morphology and changes in the actin–cytoskeleton of MUC4-expressing SKOV3 cells. Phase contrast microscopy image revealed that the cells underwent a morphological change, from cobblestone-like cell morphology to an elongated and spindle-like morphology (mesenchymal phenotype), with reduced cell–cell contact (Figure 1a) compared with vector-transfected cells. Polymerization of globular actin leads to the formation of long fibrous molecules called filamentous actin (Cunningham et al., 1992). The localization and distribution of filamentous actin were analyzed by phalloidin staining in both MUC4-overexpressing and vector-transfected SKOV3 cells. Confocal microscopy of the phalloidin-stained cells also confirmed the presence of filopodia, lammelopodia and microspikes in the MUC4-transfected cells (Figure 1a). The vector-transfected SKOV3 cells showed less staining and did not show any cellular outgrowth (Figure 1a).
MUC4 expression alters the expression of EMT markers in OC cells
To investigate the mechanism underlying the morphological changes, we examined the expression of well-known epithelial markers (E-cadherin (CDH1), cytokeratin (CK)-18) and mesenchymal markers (N-cadherin (CDH2), and vimentin (VIM)) in the MUC4- and vector-transfected SKOV3 cells. Western blot analysis showed that the expression of E-cadherin and CK-18 was significantly decreased (Figures 1b and c), whereas that of N-cadherin and vimentin was significantly increased (Figures 1d and e) in the MUC4-transfected SKOV3 cells compared with the vector control cells. Immunofluorescence analysis revealed an E-cadherin staining restricted to the cell–cell contacts in the SKOV3-vector cells, although a complete disappearance of E-cadherin at the cell–cell junction was observed in the MUC4-transfected SKOV3 cells (Figure 1b). CK-18 expression in the SKOV3-vector cells was strong and exhibited a filamentous pattern, whereas it was significantly less in SKOV3-MUC4 cells (Figure 1c). In contrast, N-cadherin (Figure 1d) and vimentin (Figure 1e) showed increased staining in the MUC4-transfected SKOV3 cells as compared with control cells. The decreased expression of E-cadherin and increased expression of N-cadherin were also seen in OVCAR3 cells transiently transfected with MUC4, suggesting that the observed effects are not limited to a single OC cell line (Supplementary Figure 1). These results demonstrate that the expression of EMT-associated markers is increased in a coordinated manner in MUC4-overexpressing OC cells.
Further, the mRNA expression of TWIST1, TWIST2 and SNAIL, key transcription factors that promote EMT, was significantly increased in the SKOV3-MUC4 cells compared with vector-transfected cells (Figure 1f), suggesting that MUC4 expression induces EMT in OC cells.
MUC4 activates the FAK pathway to upregulate N-cadherin expression via JNK1/2 in SKOV3 cells
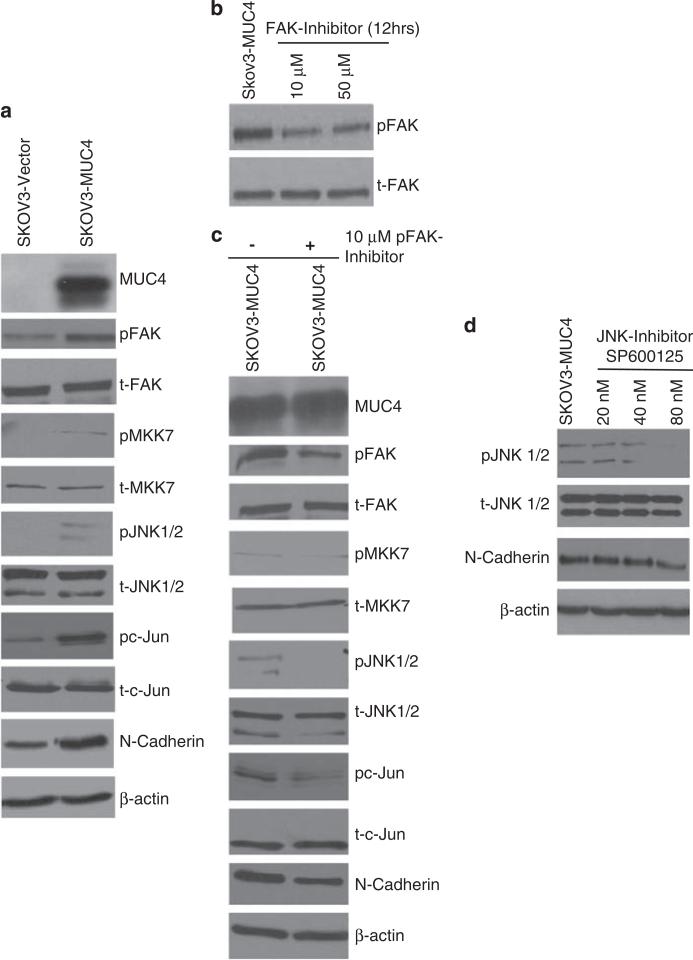
We further analyzed the mechanism of MUC4-induced N-cadherin upregulation in OC cells. We observed an increase in the activated form of FAK (Y397) in the MUC4-transfected SKOV3 and OVCAR3 cells compared with the vector-transfected cells, (Figure 2a; Supplementary Figure 1) although the level of total FAK remained unchanged (Figure 2a). We also analyzed the expression MKK7, JNK1/2 and c-Jun, which are the downstream signaling molecules induced upon FAK activation. MUC4-induced activation of FAK led to the activation of MKK7, JNK1/2 and c-Jun in the MUC4-transfected cells (Figure 2a). The level of the total protein, however, remained unchanged. These results suggest that MUC4 activates FAK and its downstream signaling and thus may lead to the upregulation of N-cadherin in OC cells.
Figure 2.
MUC4 expression induces upregulation of N-cadherin via FAK signaling. (a) Immunoblot analysis showed an increase in the activation of FAK, MKK7, JNK1/2, c-Jun and upregulate N-cadherin in SKOV3-MUC4 cells as compared with vector control cells. The total form of FAK, MKK7, JNK1/2, c-Jun molecules remains unchanged in both cell lines. β-actin was used as a loading control. (b) Treatment with pFAK inhibitor: Immunobloting analysis of pFAK and FAK in cells treated with 10 and 50 μm FAK inhibitor (FAK inhibitor-14 (1,2,4,5-benzenetetraamine tetrahydrochloride)-treated SKOV3-MUC4 cells. (c) Western blot analysis showed that the inhibition of pFAK reduces the activation of FAK, MKK7, JNK1/2, c-Jun and decreases the expression of N-cadherin. The total form of FAK, MKK7, JNK1/2, c-Jun molecules remains the same. β-actin was used as a loading control. (d) Inhibition of pJNK1/2: immunoblotting analysis showing the expression of pJNK, JNK and N-cadherin in JNK1/2 inhibitor (SP600125; 20, 40 and 80 nm)-treated SKOV3-MUC4 cells. β-actin was used as a loading control.
Inhibition of phospho-FAK (pFAK) and pJNK1/2 decreases the level of N-cadherin and its downstream signaling
FAK was inhibited with a small molecule inhibitor-14 (1,2,4,5-benzenetetraamine tetrahydrochloride), which targets the Tyr397 phosphorylation site on FAK (Golubovskaya et al., 2008). By using different doses (10 and 50 μm) of FAK inhibitor-14 for 12 h, we observed a concentration-dependent decrease in the activation of FAK in MUC4-transfected SKOV3 cells (Figure 2b). All other FAK downstream signaling molecules (MKK7, JNK1/2 and c-Jun) were also analyzed under similar conditions. MKK7, JNK1/2 and c-Jun molecules showed decreased activation in the FAK inhibitor-treated SKOV3-MUC4 cells compared with untreated cells (Figure 2c). The total form of MKK7, JNK1/2 and c-Jun, however, remained unchanged (Figure 2c). Interestingly, N-cadherin expression was also significantly decreased in the FAK –inhibitor-treated SKOV3-MUC4 cells compared with the untreated cells (Figure 2c). To confirm that the FAK activity leads to upregulation of N-cadherin level via activation of the JNK1/2 pathway, we inhibited the activation of JNK1/2 by treating the cells with SP600125, a JNK1/2 inhibitor. On treatment with increasing doses (20, 40 and 80 nm) of JNK1/2 inhibitor for 12 h, MUC4-transfected SKOV3 cells showed a progressive decrease in the activation of JNK1/2 (Figure 2d) associated with a reduction in the expression of N-cadherin in SKOV3-MUC4 cells (Figure 2d). These results suggest that MUC4-induced activation of FAK and its downstream effectors, MKK7, JNK1/2 and c-Jun, are involved in the induction of N-cadherin in OC cells.
MUC4 decreases aggregation and increases clonogenicity of SKOV3 cells
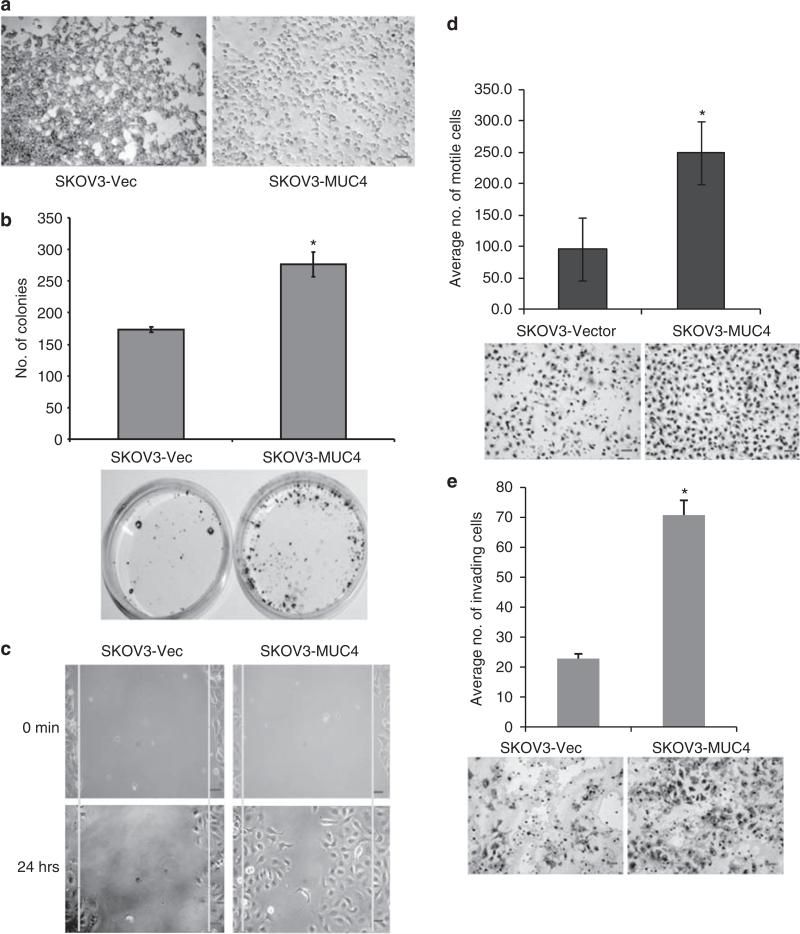
To analyze the functional activity of MUC4 in OC cells, we primarily performed in vitro hanging drop cell aggregation and colony forming assays. The MUC4-expressing cells showed less aggregation whereas the vector-transfected SKOV3 cells exhibited large and tightly attached cell aggregates after 12 h incubation in hanging drop analysis (Figure 3a). It is a well-known factor that cancer cells possess an increased efficiency of colonization than the normal cells. The colony-forming efficiency of SKOV3-MUC4 and vector-transfected cells was investigated by seeding 500 cells in a 10 mm culture dish and counting visible colonies after 3 weeks. MUC4-overexpressing cells showed a significantly higher number of colonies (*P = 0.029) than the vector-transfected cells (Figure 3b).
Figure 3.
MUC4 decreases the aggregation and increases the colonogenecity, motility and invasiveness of SKOV3 cells. (a) Aggregation assay: a reduced cellular aggregation was observed in MUC4-overexpressing SKOV3 cells, whereas vector-transfected cells (SKOV3-vector) showed bigger and tight cell aggregates (original magnification × 100, scale bar-0.8 mm). (b) Colony forming assay: MUC4-transfected SKOV3 cells formed a significantly greater number of (*P = 0.029) colonies than the control cells (original magnification × 40, scale bar-0.8 mm). (c) Wound healing assay was performed to visualize the differences in motility of MUC4- and vector-transfected SKOV3 cell lines (original magnification × 100, scale bar-0.8 mm). (d) Boyden's chamber motility assay for both MUC4- and empty vector-transfected SKOV3 cells. Cell motility was observed to be significantly (*P = 0.0001) increased in MUC4-transfected SKOV3 cells (original magnification × 100, scale bar-0.8 mm). (e) Matrigel-coated Boyden's chamber invasion assay. SKOV3-MUC4 cells exhibited a significant increase in invasiveness compared with the SKOV3-vector cells (*P = 0.00055; original magnification × 100, scale bar-0.8 mm).
MUC4 induces motility and the invasive property of SKOV3 cells
We further performed a wound healing assay to qualitatively observe the effect of MUC4-induced motility in SKOV3 cells. The scratch was made in 90% confluence of MUC4- and vector-transfected SKOV3 cells. After allowing the cells to move into the scratch wound for 12 h, we observed an increase in the number of cells that moved into the scratch wound in the MUC4-transfected cells compared with vector-transfected cells (Figure 3c). The quantitative cellular motility was also analyzed in both cells using Boyden's chamber assay. MUC4-transfected cells showed a significant (*P = 0.0001) increase in motility when compared with the vector-transfected SKOV3 cells (Figure 3d). The morphological variation and decreased aggregation in MUC4-overexpressed cells may induce significant cell motility. The presence of structures like lammelopodia, filopodia and microspikes may be a cause of increased motility in the MUC4-transfected cells. To investigate the invasion capacity of MUC4-transfected SKOV3 cells, an in vitro invasion assay was performed. The MUC4-transfected SKOV3 cells showed a highly significant (*P = 0.000055) increase in the number of invading cells compared with vector-transfected cells (Figure 3e).
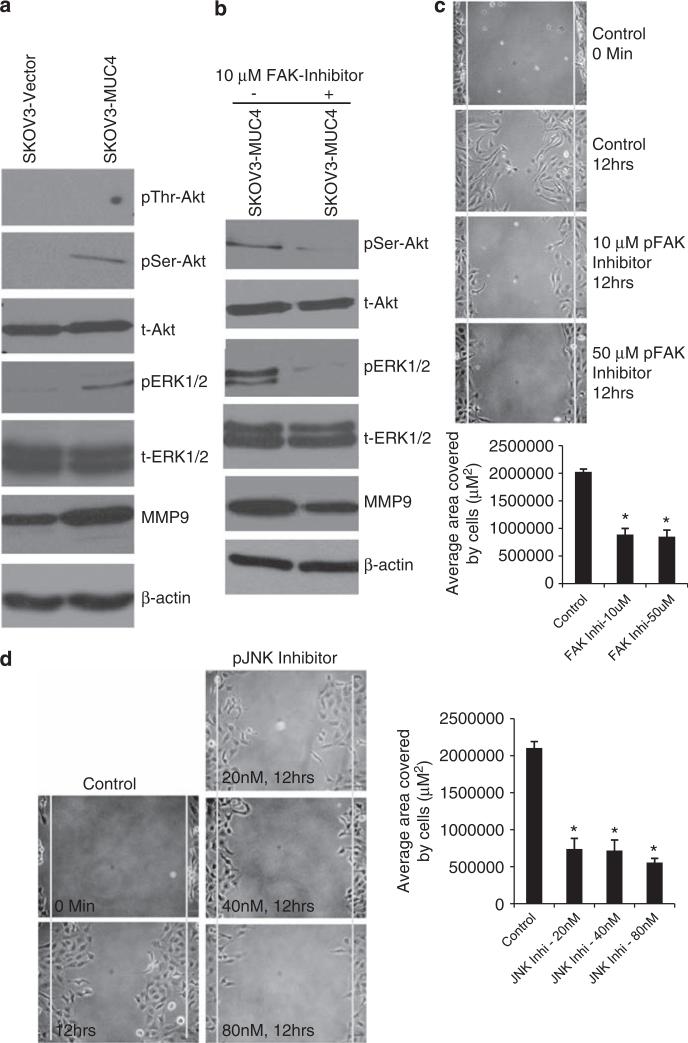
Effect of MUC4 on Akt, extracellular signal-regulated kinase-1/2 (ERK1/2) signaling and matrix metalloproteinase 9 (MMP9) expression in OC cells
The effect of MUC4 on Akt, ERK1/2 signaling and also on MMP9 was analyzed by immunoblotting. The MUC4-overexpressing SKOV3 and OVCAR3 cells showed a significant increase in the activated Akt (Ser473), ERK1/2, although the total levels of these proteins remained unchanged (Figure 4a; Supplementary Figure 1). The threonine (308) phosphorylation of Akt did not show any activation in both MUC4- and vector-transfected SKOV3 cells (Figure 4a). We also observed an increased level of MMP9 expression in MUC4-transfected but not in vector-transfected OC cells (Figure 4a).
Figure 4.
Analysis of N-cadherin downstream signaling pathways in MUC4-expressing and control cells. (a) Western blot analysis of N-cadherin downstream signaling molecules showed increased activation of serine (473) Akt, ERK1/2 and increased expression of MMP9 in MUC4-overexpressed cells. No variation was seen in threonine (Y308) phosphorylated Akt. The total form of Akt and ERK1/2 were unchanged in both the SKOV3-derived sublines. β-actin was used as a loading control. (b) Western blot analysis showed that pFAK inhibition also reduces the phosphorylation of serine (473) Akt, ERK1/2 and decreases the expression of MMP9 compared with the mock control. (c, d) Wound healing assay: The scratch was made across the cell monolayer in pFAK (1,2,4,5-benzenetetraamine tetrahydrochloride) and pJNK (SP600125) inhibitor-treated SKOV3-MUC4 cells with mock control. The migration of cells was measured (μm2) in treated and untreated cells. Significant migration of cells was analyzed using two-tailed Student's t-test. A P-value of <0.05 was considered statistically significant. (FAK Inhi-10 mm, *P <0.0004; FAK Inhi-50 μm, *P <0.0003), (JNK1/2 Inhi-20 nm, *P <0.005; JNK1/2 Inhi-40 nm, *P <0.0002; JNK1/2 Inhi-80 nm, *P <0.0001) (original magnification × 100, scale bar-0.8 mm).
The aforementioned results suggest that MUC4 upregulates N-cadherin through FAK and its downstream signaling molecules MKK7, JNK1/2 and c-Jun (Figures 2b and c). We further analyzed Akt, ERK1/2 and MMP9 molecules in FAK-inhibited samples. The downregulation of phospho-serine (473) Akt and phospho ERK1/2 in the FAK-inhibited cells (Figure 4b) suggests that these molecules act downstream of FAK and N-cadherin signaling.
Inhibition of pFAK and pJNK1/2 decreases the motility
Scratch assays were performed in both FAK inhibitor- and JNK1/2 inhibitor-treated MUC4-overexpressing SKOV3 cells. Significant inhibition of motility was observed in the FAK inhibitor- and JNK1/2 inhibitor-treated cells compared with the untreated cells (Figures 4c and d). These results suggest that MUC4-induced activation of FAK and JNK1/2 leads to the increased motility of OC cells.
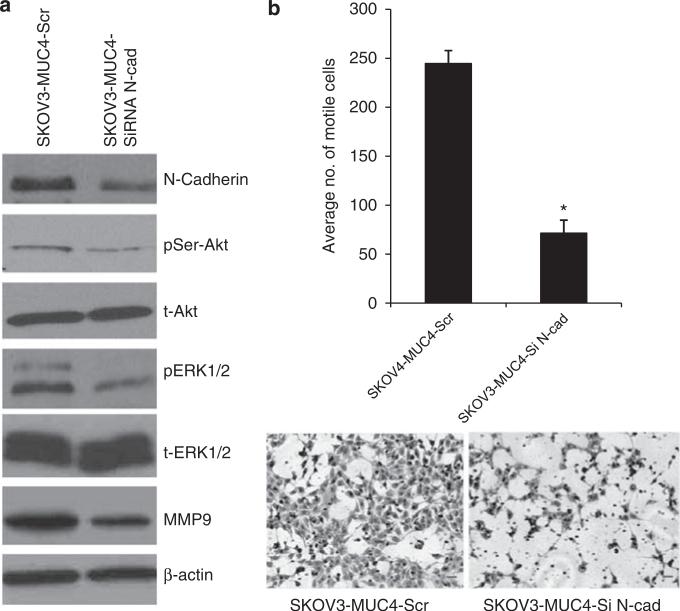
Knockdown of N-cadherin reduces the motility in SKOV3-MUC4 cells
To further establish the importance of N-cadherin in MUC4-induced motility, we silenced the expression of N-cadherin in SKOV3-MUC4 cells using N-cadherinspecific small interfering RNA (siRNA) oligos. N-cadherin was 50% downregulated in siRNA-treated cells compared with scramble RNA interference-treated cells (Figure 5a). The downregulation of N-cadherin was associated with a decreased activation of Akt (Ser473), ERK1/2 and decreased expression of MMP9 when compared with the scramble RNA interference-treated cells (Figure 5a). The total form of Akt, ERK1/2 remained unchanged in both the cells. Additionally, we also performed a motility assay in the N-cadherin siRNA- and scramble siRNA-treated SKOV3-MUC4 cells. A significant (*P = 0.004) reduction in motility was observed in the N-cadherin siRNA-treated SKOV3-MUC4 cells compared with those treated with scrambled RNA interference. These results confirmed that MUC4 induces the motility and invasion through Akt, ERK1/2 and MMP9 pathways in OC cells.
Figure 5.
Knockdown of N-cadherin in MUC4-overexpressing cells reduces their motility. (a) The transient knockdown of N-cadherin using three pooled siRNA oligos for 48 h in SKOV3-MUC4 cells. Western blot analysis showed a downregulation of N-cadherin in siRNA-treated SKOV3-MUC4 cells compared with scramble RNA interference (RNAi)-treated SKOV3-MUC4 cells. The phosphorylated (Ser) Akt and pERK1/2 were reduced in N-cadherin knockdown cells compared with control cells. The total form of Akt and ERK, however, remained unchanged. MMP9 expression was also reduced in the N-cadherin knockdown cells. β-actin was used as a loading control. (b) Motility assay in N-cadherin knockdown SKOV3-MUC4 cells. SKOV3-MUC4 cells were treated with N-cadherin siRNA and after 24 h were trypsinized for the motility assay as described previously. There was a significant decrease in motility in the N-cadherin siRNA-treated cells compared with the scramble RNAi-treated cells (*P = 0.004; original magnification × 100, scale bar-0.8 mm).
Overexpression of MUC4 induces tumorogenecity and metastasis of SKOV3 cells
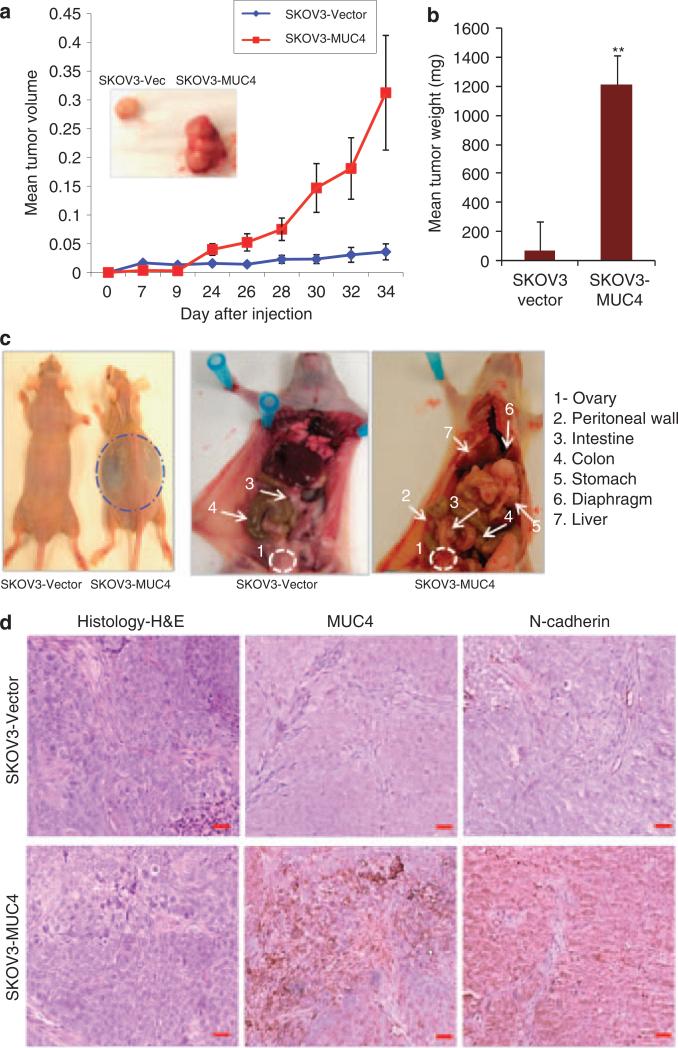
To test the hypothesis that MUC4 has a role in tumor development and metastasis, we compared the tumorigenicity and metastatic potential of MUC4-transfected SKOV3 cells with vector-transfected cells by in vivo analysis. SKOV3-MUC4 and SKOV3-vector cells were injected subcutaneously (s.c.) into athymic nude mice (six animals per group). All mice were killed and tumors were dissected at the end of the experiment. Tumor size was measured every alternate day up to 34 days. The mean tumor volumes produced by the vector- and MUC4-transfected SKOV3 cells are represented graphically (Figure 6a). A comparison of the tumor weight and volume between the vector- and MUC4-transfected SKOV3 cells showed a significantly higher tumor volume and weight (P = 0.00001) in the mice injected with the MUC4-transfected SKOV3 cells compared with those injected with control cells (Figure 6b).
Figure 6.
Analysis of tumor growth and metastasis in the MUC4-transfected OC cells. (a, b) In vivo tumorigenesis assay for SKOV3-MUC4 and SKOV3-vector cells with NUDE/SCID mice following subcutaneous implantation. Measurement of tumor volumes indicated that SKOV3-MUC4 cells formed significantly larger tumors as compared with control cells. Inset shows a representative image for tumors developed in SKOV3-vector- and SKOV3-MUC4-injected mice. Tumor weight was significantly higher (**P = 0.00001) in the SKOV3-MUC4 cells compared with SKOV3-vector cells. (c) In vivo metastasis analysis for SKOV3-MUC4 and SKOV3-vector cells with NUDE/SCID mice. The SKOV3-MUC4 cells injected mice showed more ascitic fluid accumulation. The ovary was found to develop tumors in both groups of mice. The SKOV3-vector-injected mice showed fewer incidence of metastasis in distant organ sites, whereas SKOV3-MUC4-injected mice showed metastatic deposits in the peritoneal wall, small intestine, colon, stomach, liver and diaphragm. (d) Hematoxylin and eosin staining of tumors produced by i.p. injection of SKOV3-vector and SKOV3-MUC4 cells. The immunohistochemical analysis of MUC4 and N-cadherin showed increased reactivity in the SKOV3-MUC4 tumors compared with SKOV3-vector tumors (original magnification × 200, scale bar-0.8 mm).
To examine the effect of MUC4 on metastasis, a total of eight immunodeficient mice were intraperitoneally (i.p.) injected with SKOV3-vector and SKOV3-MUC4 cells in two different sets of experiments and killed after thirty-second day of the experiment. Tumors were observed in the ovaries of both SKOV3-vector- and SKOV3-MUC4-injected mice. Mice injected with the MUC4-overexpressing cells showed greater ascitic fluid accumulation in the peritoneal cavity than in control mice (Figure 6c). The incidence of metastases present in the distant organs, including the liver, pelvic peritoneal wall, diaphragm, lymph nodes, stomach, colon and intestine, were documented (Figure 6c and Table 1).
Table 1.
Incidence of metastasis for SKOV3-vector and SKOV3-MUC4-injected nude mice (n = 8)
| Cell lines | Liver | Pelvic peritoneal wall | Diaphragm | Lumph nodes | Stomach | Colon | Intestine |
|---|---|---|---|---|---|---|---|
| SKOV3-vector | 0/8 (0%) | 1/8 (12.5%) | 0/8 (0%) | 0/8 (0%) | 0/8 (0%) | 2/8 (25%) | 3/8 37.5%) |
| SKOV3-MUC4 | 8/8 (100%) | 8/8 (100%) | 6/8 (75%) | 6/8 (62.5%) | 8/8 (100%) | 7/8 (87%) | 7/8 (87%) |
| < P-value | 0.022* | 0.087 | 0.05* | 0.05* | 0.022* | 0.22 | 0.42 |
The incidence of metastasis was compared between the SKOV3-vector and SKOV3-MUC4 cells injected mice by the χ2-test. A *P-value of 00.05 was considered statistically significant.
indicates the significance of metastatic incidences between two groups.
Most of the mice injected with MUC4-expressing SKOV3 cells (n = 8) developed metastases (large metastatic nodules) at multiple sites, whereas the mice injected with the control cells (n = 8) exhibited metastatic (fewer and smaller metastatic lesions) deposits only at a few sites (Table 1). The incidence of liver (P = 0.022), diaphragm (P = 0.05), lymph node (P = 0.05), stomach (P = 0.02) and pelvic peritoneal wall (P = 0.082) metastasis was significantly higher in the SKOV3-MUC4 cells injected mice compared with the SKOV3-vector-injected mice. The incidence of colon (P = 0.22) and intestinal (P = 0.42) metastasis was not significantly different between the two groups of mice (Table 1). The tumor was dissected from the peritoneum and stained with hematoxylin and eosin for both MUC4-transfected and control SKOV3 cells injected mice (Figure 6d). It was also stained for MUC4 and N-cadherin antibodies. The immunoreactivity of MUC4 and N-cadherin was higher in SKOV3-MUC4-injected tumors as compared with SKOV3-vector-injected mice tumors (Figure 6d).
Discussion
This study has three salient findings. First, MUC4 overexpression induces an elongated and spindle-like morphology (mesenchymal phenotype) and significant alteration of EMT markers in OC cells. Second, MUC4 activates the FAK pathway and upregulates the expression of N-cadherin in OC cells. Third, MUC4-induced N-cadherin is involved in the metastasis of OC cells. To the best of our knowledge, this is the first report suggesting the novel role of MUC4 in the induction of EMT and metastasis in OC cells.
EMT is associated with a loss of epithelial and gain of mesenchymal characteristics, resulting in increased invasive potential in epithelial cancer (Thiery, 2002; Ahmed et al., 2007). Previous studies have shown that MUC4 is overexpressed in many carcinomas, including ovarian carcinoma (Boman et al., 2001; Chauhan et al., 2006). A recent study by our group suggested that MUC4 has a major role in pancreatic and OC cell motility, in part, by altering downstream signaling (Chaturvedi et al., 2007; Ponnusamy et al., 2008). The current study addresses the importance of MUC4 in induction of mesenchymal phenotype and investigated the underlying molecular mechanism. Our result indicates that MUC4-transfected SKOV3 cells showed mesenchymal phenotype with phalloidin staining. Similarly, a previous report had shown that the phalloidin staining helps to clearly visualize the alterations of actin–cytoskeleton rearrangement and morphological variation (Theriault et al., 2007). Further, the loss of epithelial markers E-cadherin and CK-18, (Ahmed et al., 2007; Gravdal et al., 2007; Kolosionek et al., 2009) and gain of mesenchymal markers N-cadherin and vimentin (Thiery, 2002; Ahmed et al., 2007; Gravdal et al., 2007; Kolosionek et al., 2009) are the hallmarks of EMT. In addition to the morphological changes, overexpression of MUC4 resulted in a similar loss of epithelial markers and a gain of mesenchymal markers, suggesting that MUC4 is actively involved in the EMT process in OC cells (Figure 1). A recent study revealed that another mucin, MUC1, is involved in inducing the EMT process by engaging it with various signaling modules (Horn et al., 2009) and also supports our finding of mucin-mediated EMT. Furthermore, the EMT associated key transcription factors TWIST1, TWIST2 and SNAIL (Kang and Massague, 2004; Kudo-Saito et al., 2009) also showed an increased expression in the MUC4-transfected cells (Figure 1). These results also suggest that MUC4 induces EMT in OC cells.
Based on a recent finding of FAK-mediated upregulation of N-cadherin in the pancreatic cancer cell line (Shintani et al., 2008), we further elucidated the mechanism underlying MUC4-induced upregulation of N-cadherin through the activation of the FAK signaling pathway in human OC cells (Figure 2). This finding provides mechanistic support to our previous study (Chauhan et al., 2006; Ponnusamy et al., 2008), which indicated that MUC4 overexpression is associated with increased metastatic potential and a poor prognosis in OC. Our results in the present study clearly demonstrated that the overexpression of MUC4 induces activation of FAK and its downstream molecules, such as MKK7, JNK1/2 and c-Jun, and hence upregulates N-cadherin in OC cells. These results confirmed, for the first time, that MUC4 induces the upregulation of N-cadherin expression through FAK and the JNK1/2 pathway in OC cells. In addition, recent reports have clearly established a switch between E-cadherin to N-cadherin expression during the EMT process in various epithelial cancers (Maeda et al., 2005; Gravdal et al., 2007).
EMT is thought to be a key step in tumor metastasis via the induction of a highly invasive phenotype, and its molecular mechanisms have been extensively studied (Thiery, 2002). MUC4 overexpression induces dramatic variation in OC cell morphology and decreases aggregation, and increases colony formation, motility and invasion, suggesting that MUC4 may promote the metastatic property in OC cells. In one of our earlier studies, we showed that MUC4 caused an alteration in motility and aggregation of pancreatic and OC cells (Singh et al., 2004; Ponnusamy et al., 2008). The aberrant overexpression of MUC4 on the entire cell surface in cancer cells may constantly engage itself in stimulating the downstream molecules and upregulates N-cadherin expression, and thereby induce the EMT and promote the metastatic behavior of the OC cells.
Furthermore, we observed an increase in the activation of Akt (Ser473), ERK1/2 and an increased expression of MMP9 in MUC4-transfected SKOV3 cells, which is decreased when N-cadherin is silenced in the same cells, leading to decreased motility (Figure 5). A previous report lends support to our finding that in the presence of N-cadherin, FGF2 causes sustained activation of the MAPK-ERK pathway, leading to MMP9 transcriptional upregulation and enhanced cellular invasion (Suyama et al., 2002). In our study, the increased phosphorylation of ERK1/2 may lead to an upregulation of MMP9 and induce the invasion of OC cells. In addition, the activation of Akt (Ser473) could also be involved in the survival and migration of OC cells (Ponnusamy et al., 2008). Interestingly, the inhibition of pFAK and pJNK1/2 in MUC4-overexpressed OC cells resulted in a significant decrease in cell migration, suggesting that MUC4-induced activation of FAK and the subsequent increase in N-cadherin expression may be the cause of increased motility in OC cells. A study by Hazan et al. (1997) reported that N-cadherin expression is significantly higher in more invasive, less differentiated cancer cell lines that lacked E-cadherin expression (Hazan et al. 1997).
The induction of N-cadherin with MUC4 expression also correlated with an increased incidence of tumor metastasis in vivo, suggesting a role of MUC4 in the potentiation of tumor metastasis. In vivo metastasis of tumor cells is an extremely complicated process that involves several consecutive events (that is, EMT, detachment of tumor cells from primary site, intravasation into blood stream, evasion of immune surveillance, adherence to vascular endothelial cells of distant organs and finally extravasation into tissues followed by MET) (Fidler, 1990; Folkman, 1990). Membrane-bound mucins are considered to be the important determinants of the cell's adhesive properties and therefore its metastatic potential, even in cases where the level of known adhesion molecules remain unchanged (Sommers, 1996; Truant et al., 2003). Our in vivo tumorigenesis and metastasis analysis showed that there is a significant increase in tumor formation and incidence of metastasis in the liver, peritoneal wall, diaphragm, lymph nodes, stomach and also more ascitic fluid accumulation in MUC4-overexpressing OC cells. Further the tumors from MUC4-expressing cells showed increased immunoreactivity for MUC4 and N-cadherin compared with control tumors. These in vivo results confirmed that MUC4 induces N-cadherin expression in vivo and this may be involved in the metastasis of OC.
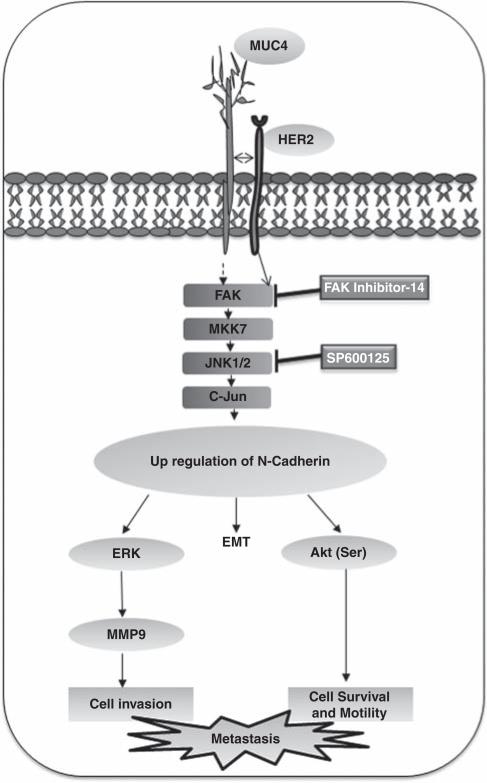
In conclusion, we provide the first evidence that MUC4 induces EMT by altering the levels of epithelial and mesenchymal markers in OC cells. The underlying mechanism involves disruption of E-cadherin expression, increased activation of FAK, MKK7, JNK1/2 and c-Jun that further leads to an upregulation of N-cadherin. N-cadherin further may activate downstream effectors like ERK1/2, Akt leading to the increased expression of MMP9, which may be the primary cause of decreased aggregation, increased motility and invasiveness of OC cells (Figure 7). The in vivo results also confirmed that MUC4 increases the tumorigenicity and metastatic nature of OC cells through the induction of N-cadherin. Overall, our results strongly suggest for the first time that MUC4 induces EMT and has a determinative role in regulating the growth, motility, invasiveness and metastatic potential of OC cells by upregulating the expression of N-cadherin via the FAK signaling pathway.
Figure 7.
Schematic representation of the proposed mechanism of MUC4-induced EMT and metastasis of OC. MUC4 induces the FAK activation either directly (unknown mechanism) or by interacting with HER2 cause the activation of MKK7, JNK1/2 and c-Jun and leading to upregulation of N-cadherin. The upregulation of N-cadherin induces the EMT process in OC cells. Furthermore, the N-cadherin downstream signaling of Akt, ERK1/2 and MMP9 may be the primary cause for an increased motility and invasion in MUC4-expressing OC cells.
Materials and methods
Generation of MUC4 construct
We generated a MUC4 gene construct to overcome the transfection-associated problems owing to its large size and to investigate the biological function and effect of MUC4 expression in OC cells. (Moniaux et al., 2007; Ponnusamy et al., 2008). The resultant MUC4 complementary DNA was sub-cloned into the pSecTag-C vector for further transfection studies.
Cell culture and transfection procedure
SKOV3 and OVCAR3 cells were procured from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modied Eagle's medium and RPMI medium, respectively, supplemented with 10% fetal calf serum and antibiotics. The MUC4 gene construct, along with the empty vector control pSecTag-C, were transfected in SKOV3 OC cells by Fugene (Invitrogen, Carlsbad, CA, USA), following the manufacturer's protocol. Transfected cells were selected in the medium containing 200 μg/ml zeocin (30 days) and the drug resistance (zeo +) clones (three from the empty vector, five from the MUC4 gene construct transfected) were selected from different plates and studied after expansion. OVCAR3 cells were used for the transient transfection of MUC4 and vector control plasmids for 72 h. The transient knockdown (48 h) of N-cadherin was carried out in SKOV3-MUC4 cells using a pool of three siRNA oligos specific for N-cadherin and with control oligos (Santa Cruz Biotechnologies, Santa Cruz, CA, USA).
Immunoblot assay
SKOV3-derived cell lines were processed for protein extraction and western blotting using standard procedures. SDS-agarose (2%) gel electrophoresis was performed for MUC4 using 20 μg protein samples under reducing conditions. For E-cadherin, N-cadherin, CK-18, vimentin, FAK, MKK7, JNK1/2, c-Jun, ERK, Akt, MMP9 and β-actin expression, SDS–polyacrylamide gel electrophoresis (10%) was performed under similar conditions. Resolved proteins were transferred on to the polyvinylidene fluoride membrane and immunoblot assay was performed for the above mentioned proteins (for details see Supplementary Information).
Confocal immunofluorescence microscopy
MUC4- and vector-transfected SKOV3 cells were grown on sterilized cover slips for 20 h and MUC4, E-, N-cadherin, CK-18 and vimentin antibodies were used for immunofluorescence analysis (detailed procedure there in Supplementary Information).
Inhibition of pFAK and pJNK1/2 in SKOV3-MUC4 cells
SKOV3-MUC4 cells (3 × 106) were seeded and, after 12 h, cells were treated with small molecule inhibitor-14 (1,2,4,5-benzenetetraamine tetrahydrochloride) for 12 h (Golubovskaya et al., 2008) in different doses (10 and 50 μm). The inhibition of pJNK1/2 was also carried out in MUC4-overexpressed SKOV3 cells. The pJNK1/2 inhibitor SP600125 (20, 40 and 80 nm) was used to treat SKOV3-MUC4 cells for 12 h. After the inhibition of pFAK and pJNK1/2, cells were used to extract the protein for further western blot analysis.
Aggregation assay
Aggregation assay was carried out with SKOV3-vector and SKOV3-MUC4 cells (detailed procedure there in Supplementary Information).
Wound healing assay
The scratch was made across the cell monolayer in OC cells. The cells were allowed to incubate for 12 h and images taken in six different fields. After 12 h, the migration of cells was measured (μm2) by using DatInf Measure setup Wizard software (http://tucows.texasonline.net). The significant migration of cells was analyzed using two-tailed Student's t-test. A P-value of <0.05 was considered statistically significant.
Cell motility and invasion assay
We have performed motility and invasion assays using chamber with monolayer-coated polyethylene teraphthalate membranes (six-well insert, pore size of 8 μm; Becton Dickinson, Franklin Lakes, NJ, USA) and Matrigel-coated membrane inserts (BD Biosciences, Bedford, MA, USA) for both SKOV3-vector and SKOV-MUC4 cells respectively (detailed procedure given in Supplementary Information).
Colony forming assay
Cells were seeded in triplicate at 500 cells/10-cm dishes in complete medium. After 2 weeks of growth, the cells were fixed and stained with crystal violet stain (0.1%, w/v in 20 nm 4-morpholinepropanesulfonic acid; Sigma Chemicals, St Louis, MO, USA), and the grossly visible colonies were counted. All experiments were repeated at least three times. Plating efficiency was determined as the number of colonies formed divided by the total number of cells plated.
In vivo tumor growth and metastasis
To test the tumorigenicity, the MUC4-transfected SKOV3 cells, along with the control cells were subcutaneously injected into immunodeficient female mice (n = 6 for each group) and tumor growth was followed for 34 days. To analyze the effect of the overexpression of MUC4 on the metastatic property of SKOV3 cells, we have performed intraperitoneal injection (i.p.) in immunodeficient female mice (n = 8 for each group). We have observed distant organ metastasis on thirty-second day after the implantation of the tumor cells (detailed procedure in Supplementary Information).
Histological and immunohistochemical analysis
The tumors were dissected from i.p. injected animals (in both SKOV3-vector and SKOV3-MUC4 cells) and fixed in 4% paraformaldehyde in phosphate-buffered saline overnight and subsequently embedded in paraffin wax. Sections were cut at a thickness of 4 μm and stained with hematoxylin and eosin for histological analysis. Immunohistochemical analysis was also performed for MUC4 and N-cadherin in tumors developed from both type of cells (SKOV3-vector and SKOV3-MUC4 cells) as described previously (Moniaux et al., 2004; Singh et al., 2004; detailed procedure in Supplementary Information).
Statistical analysis
Different statistical analysis (two-tailed Student's t-test and two-tail Fisher's exact test) was performed for these studies and details are given in Supplementary Information.
Supplementary Material
Acknowledgements
This work are supported by the grants from the National Institutes of Health (CA78590, CA111294, CA127297, CA133774 and CA131944), Department of Defense (BC074639) and the Susan G Komen Foundation (KG070826). We thank Ms Kristi L Berger for editing the paper. The authors acknowledge the invaluable technical support from Mr Erik Moore and Mrs Kavita Mallya. We also thank Janice A Tayor and James R Talaska of the confocal laser scanning microscope core facility at the UNMC for their support.
Abbreviations
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FAK
focal adhesion kinase
- FBS
fetal bovine serum
- SMC
sialo mucin complex
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213:581–588. doi: 10.1002/jcp.21240. [DOI] [PubMed] [Google Scholar]
- Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, et al. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci USA. 1999;96:6249–6254. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J. Cadherins as determinants of tissue morphology and suppressors of invasion. Acta Anat (Basel) 1994;149:165–169. doi: 10.1159/000147572. [DOI] [PubMed] [Google Scholar]
- Boman F, Buisine MP, Wacrenier A, Querleu D, Aubert JP, Porchet N. Mucin gene transcripts in benign and borderline mucinous tumours of the ovary: an in situ hybridization study. J Pathol. 2001;193:339–344. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH798>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Price-Schiavi SA, Komatsu M, Jepson S, Perez A, Carraway CA. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–337. doi: 10.1023/a:1011327708973. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- Chauhan SC, Singh AP, Ruiz F, Johansson SL, Jain M, Smith LM, et al. Aberrant expression of MUC4 in ovarian carcinoma: diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Mod Pathol. 2006;19:1386–1394. doi: 10.1038/modpathol.3800646. [DOI] [PubMed] [Google Scholar]
- Chen H, Paradies NE, Fedor-Chaiken M, Brackenbury R. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J Cell Sci. 1997;110:345–356. doi: 10.1242/jcs.110.3.345. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, et al. Actin-binding protein requirement for cortical stability and efficient locomotion. Science. 1992;255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- Folkman J. Endothelial cells and angiogenic growth factors in cancer growth and metastasis. Introduction. Cancer Metastasis Rev. 1990;9:171–174. doi: 10.1007/BF00046358. [DOI] [PubMed] [Google Scholar]
- Giuntoli RL, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–5550. [PubMed] [Google Scholar]
- Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, et al. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–7416. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. A switch from E-cadherin to N-cadherin expression indicates epithelial to mesenchymal transition and is of strong and independent importance for the progress of prostate cancer. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Kang L, Whooley BP, Borgen PI. N-cadherin promotes adhesion between invasive breast cancer cells and the stroma. Cell Adhes Commun. 1997;4:399–411. doi: 10.3109/15419069709004457. [DOI] [PubMed] [Google Scholar]
- Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann NY Acad Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Horn G, Gaziel A, Wreschner DH, Smorodinsky NI, Ehrlich M. ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumor cells induced by truncated MUC1. Exp Cell Res. 2009;315:1490–1504. doi: 10.1016/j.yexcr.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F, et al. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell. 2009;20:4751–4765. doi: 10.1091/mbc.E09-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Kohno N, Yokoyama A, Hiwada K. Decreased MUC1 expression induces E-cadherin-mediated cell adhesion of breast cancer cell lines. Cancer Res. 1998;58:2014–2019. [PubMed] [Google Scholar]
- Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer A, Alameda F, Barranco C, Garrido M, de Boloś C. MUC4 expression is increased in dysplastic cervical disorders. Hum Pathol. 2001;32:1197–1202. doi: 10.1053/hupa.2001.28938. [DOI] [PubMed] [Google Scholar]
- Maeda M, Johnson KR, Wheelock MJ. Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci. 2005;118:873–887. doi: 10.1242/jcs.01634. [DOI] [PubMed] [Google Scholar]
- Moniaux N, Chaturvedi P, Varshney GC, Meza JL, Rodriguez-Sierra JF, Aubert JP, et al. Human MUC4 mucin induces ultra-structural changes and tumorigenicity in pancreatic cancer cells. Br J Cancer. 2007;97:345–357. doi: 10.1038/sj.bjc.6603868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert JP. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- Moniaux N, Varshney GC, Chauhan SC, Copin MC, Jain M, Wittel UA, et al. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–261. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- Nollet S, Moniaux N, Maury J, Petitprez D, Degand P, Laine A, et al. Human mucin gene MUC4: organization of its 5′-region and polymorphism of its central tandem repeat array. Biochem J. 1998;332:739–748. doi: 10.1042/bj3320739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK. MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells. Br J Cancer. 2008;99:520–526. doi: 10.1038/sj.bjc.6604517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–630. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- Sommers CL. The role of cadherin-mediated adhesion in breast cancer. J Mammary Gland Biol Neoplasia. 1996;1:219–229. doi: 10.1007/BF02013645. [DOI] [PubMed] [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/s1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28:1153–1162. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Truant S, Bruyneel E, Gouyer V, De WO, Pruvot FR, Mareel M, et al. Requirement of both mucins and proteoglycans in cell-cell dissociation and invasiveness of colon carcinoma HT-29 cells. Int J Cancer. 2003;104:683–694. doi: 10.1002/ijc.11011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.