Abstract
A Brønsted base-catalyzed reaction of nitroalkanes with alkyl electrophiles provides indole heterocycles substituted at C3 bearing a sec-alkyl group with good enantioselectivity (up to 90% ee). Denitration by hydrogenolysis provides a product with equally high ee. An indolenine intermediate is implicated in the addition step, and surprisingly, water cosolvent was found to have a beneficial effect in this step, leading to a one-pot protocol for elimination/enantioselective addition using PBAM, a bis(amidine) chiral nonracemic base.
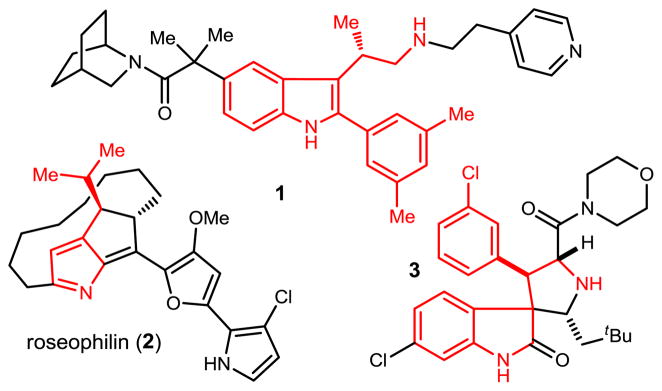
Indole and pyrrole-derived small molecules bearing an n-alkyl substituent at C3 are very common, but the emergence of small molecules exhibiting a sec-alkyl substituent at C3 is more recent. Compelling biological activity can be associated with these congeners (Figure 1): the gonadotropin releasing hormone antagonist developed by Merck (1),1 the antiobitic roseophilin (2),2 and inhibitors of MDM2 in the spirotryprostatin class (3).3
Figure 1.
Examples of biologically active indole- and pyrrole-derived heterocycles bearing a chiral C3-sec-alkyl substituent.
Synthetic methods that provide for functionalization of the C3 indole carbon do not often translate to the production of chiral nonracemic products, though an exception to this is the Friedel-Craft alkylation4 of indole where asymmetric versions have been reported.5 While both metal-based chiral complexes and organocatalysts have been used to catalyze the addition of indoles to nitro alkenes,6 there are currently no asymmetric additions to nonactivated α,β-substituted nitro alkenes, which would deliver highly substituted sec-alkyl substituents at the indole 3-position.7,8
In 2006, Petrini described an innovative solution to this structural motif using 3-(1-arylsulfonylalkyl) indole precursors to indolenine reactive intermediates.9 They have since reported a range of bases and nucleophiles to effect the elimination and subsequent addition.10 A variety of indolenine precursors have also been reported to be generated using both acidic11 and basic12 reaction conditions. Though a broad scope of additions has already been demonstrated (Grignard, nitroalkane, malonate, malononitriles), there have been very few asymmetric variations reported.13,14,15,16 More recently, a Brønsted-base catalyzed asymmetric addition of malononitrile using a cinchona based thiourea catalyst was reported with high selectivity.17 This has prompted us to report our findings on the catalytic, enantioselective Michael addition of arylnitromethanes to indolenine intermediates. In the context of BAM (Bis(AMidine)) catalysis,18 this is the first report of their use as chiral Brønsted bases.19
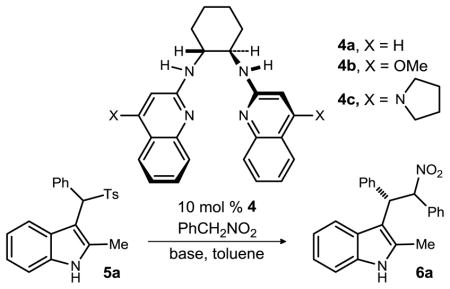
To gauge the reactivity of nitroalkanes with sulfone 5a using a BAM catalyst, the Petrini protocol was utilized for direct comparison. In this experiment using dichloromethane, conversion to the desired nitroalkane alkylation product (6a) was observed, but with low enantioselectivity (20% ee, Table 1, entry 1). Use of toluene improved selectivity to 60% ee (Table 1, entry 2). Attempts to improve enantioselection by lowering the temperature provided lower conversion and enantioselection (Table 1, entries 3–4), likely due to the heterogeneity of the reaction mixture and the sluggish elimination step. There was no change in diastereoselection throughout the reaction optimization process.20 Considering the possibility that the stoichiometric base might intervene during the addition as an achiral base, we examined several alternatives (Table 1, entries 5–7). Potassium fluoride (without alumina) provided a slight improvement while sodium and potassium carbonate provided similar enantioselection.
Table 1.
Optimization of enantioselective additions to sulfonylmethyl indoles.
 | |||||
|---|---|---|---|---|---|
| entry | catalyst | base | t(°C) | M | ee (%)b |
| 1c | 4c | KF/Alumina | rt | 0.2 | 20 |
| 2 | 4c | KF/Alumina | rt | 0.2 | 60 |
| 3 | 4c | KF/Alumina | −20 | 0.2 | 57 |
| 4 | 4c | KF/Alumina | −78 | 0.2 | 49 |
| 5 | 4c | KF | rt | 0.1 | 68 |
| 6 | 4c | Na2CO3 | rt | 0.1 | 61 |
| 7 | 4c | K2CO3 | rt | 0.2 | 70 |
| 8 | 4a | K2CO3 | rt | 0.2 | 56 |
| 9d | 4b | K2CO3 | rt | 0.2 | 73 |
| 10 | 4c | K2CO3 | rt | 0.1 | 78 |
| 11d | 4c | K2CO3 | rt | 0.1 | 81 |
All reactions were performed using 2 equivalents of phenylnitromethane on a 0.1 mmol scale and resulted in less than 1.5:1 dr material and between 30–80% yield.
Enantiomeric ratios were measured using chiral stationary phase HPLC and are reported for the major diastereomer.
Dichloromethane was used instead of toluene.
1 equivalent of phenylnitromethane was used.
Absolute stereochemistry assigned by correlation. See Supporting Information for details.
We briefly investigated BAM catalyst alternatives.19 The less Brønsted basic catalyst 4a (Table 1, entry 8) gave lower enantioselection while increasing the catalyst Brønsted basicity (4b) increased enantioselection (Table 1, entry 9).21 Our most Brønsted basic catalyst H,4PyrrolidineQuin-BAM (4c, PBAM) provided the highest enantioselection under the given conditions. Decreasing the concentration (Table 1, entry 10) and the equivalents of nucleophile (Table 1, entry 11) further improved enantioselection, thereby defining the optimal conditions.
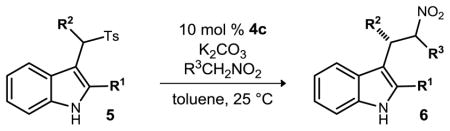
The substrate scope was investigated next (Table 2). Aryl rings bearing electron donating as well as electron withdrawing groups were tolerated (Table 2, entries 2–4), yielding the product with good enantioselection. Sterically hindered (Table 2, entry 5) and heterocyclic (Table 2, entry 6) substituents were tolerated, but a lowering of ee was observed. We were encouraged to see that selectivity remained relatively constant for the aliphatic analog tested (Table 2, entry 7). Though ester substituents gave slightly lower ee (Table 2, entries 8–9) we were encouraged by an improvement in diastereoselection to 3:1. Variations at the 2-position of the indole were also tolerated (Table 2, entries 10–11), but the lower enantioselection suggests the need for a steric influence at the indole C2. These observations translated to a pyrrole electrophile, as 5l (Table 2, entry 12) furnished 6l with good enantioselection. Electronic modifications of the nitroalkane pronucleophile (Table 2, entries 13–14) demonstrated that electron rich arylnitroalkanes result in improved enantioselection, whereas electron deficient aryl nitroalkanes provide substitution products with lower enantioselection. The use of nitroethane was also less selective (Table 2, entry 15).
Table 2.
Chiral base-catalyzed enantioselective nitroalkane alkylations.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | ee (%)b | yield(%)c (conv) | |
| 1 | Me | Ph | Ph | a | 81/81 | 78(85) |
| 2 | Me | pBrC6H4 | Ph | b | 82/75 | 74(76) |
| 3 | Me | pMeOC6H4 | Ph | c | 81/81 | 68(88) |
| 4 | Me | pF3CC6H4 | Ph | d | 84/74 | 71(78) |
| 5e | Me | oMePh | Ph | e | 76/70 | 76(95) |
| 6 | Me | 2Furyl | Ph | f | 53/44 | 69(99) |
| 7 | Me | nBu | Ph | g | 76/74 | 51(56) |
| 8d | Me | CO2Me | Ph | h | 66/47 | 47(52) |
| 9d,e | Me | CO2tBu | Ph | i | 65/66 | 55(70) |
| 10 | H | Ph | Ph | j | 40/0 | 65(68) |
| 11e | Ph | Ph | Ph | k | 74/72 | 49(52) |
| 12e | 2,5-Me-Pyrrole | Ph | Ph | l | 72/69 | 69(79) |
| 13 | Me | Ph | pMeOC6H4 | m | 89/85 | 52(77) |
| 14 | Me | Ph | pO2NC6H4 | n | 37/43 | 63(86) |
| 15e,f | Me | Ph | Me | o | 66/67 | 65(87) |
All reactions were performed on a 0.1 mmol scale using a standard 22 h reaction time and 7 equivalents of K2CO3. Diastereomeric ratios observed for crude reaction mixtures were measured by 1H NMR spectroscopy and ranged from 1:1 to 1.5:1 dr. See Supporting Information for complete details.
Enantiomeric ratios were measured using chiral stationary phase HPLC.
Isolated yields with conversions in parentheses.
3:1 dr observed.
72 h reaction time.
1.5 equivalents of nitroethane used.
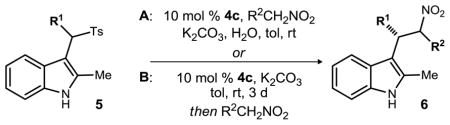
Experiments directed at probing the influence of base solubility revealed a beneficial water effect. We hypothesized that water might solubilize the inorganic base in a biphasic mixture, and in so doing, further limit the possible contribution of direct K2CO3-mediated substitution that would lead to racemic product formation. Furthermore, toluene sulfinate would also be solubilized by water. In a control experiment where water is used as solvent (no toluene), the product forms in high yield but as the racemate (Table 3, entry 1). However, when water was used as a cosolvent (2:1, toluene:water), dramatic increases in yield and enantioselection (Table 3, entry 2) were observed.
Table 3.
Chiral base-catalyzed enantioselective nitroalkane alkylations improved by a semiaqueous, one-pot protocol.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R1 | R2 | method | ee (%)b | yieldc | |
| 1d | Ph | Ph | water | a | 0/0 | 83 |
| 2 | A | a | 86/86 | 96 | ||
| 3 | B | a | 88/89 | 85 | ||
| 4 | pBrC6H4 | Ph | A | b | 87/86 | 84 |
| 5 | B | b | 88/86 | 94 | ||
| 6 | pMeOC6H4 | Ph | A | c | 85/85 | 92 |
| 7 | B | c | 87/88 | 66 | ||
| 8 | pF3CC6H4 | Ph | A | d | 84/76 | 83 |
| 9 | B | d | 88/78 | 83 | ||
| 10 | Ph | pMeOC6H4 | A | m | 87/87 | 72 |
| 11 | B | m | 90/75 | 82 | ||
| 12 | 2Furyl | Ph | A | f | 90/79 | 87 |
| 13 | B | f | 90/81 | 72 | ||
| 14 | nBu | Ph | A | g | 71/70 | 42 |
| 15 | B | g | 73/59 | 48 | ||
| 16e | CO2Me | Ph | A | h | 76/65 | 89 |
| 17e | B | h | 50/38 | 71 | ||
| 18 | Ph | Me | A | o | - | 0 |
| 19 | B | o | 10/10 | 68 | ||
All reactions were performed on a 0.1 mmol scale using a standard 22 h reaction time and 7 equivalents of K2CO3. Diastereomeric ratios observed for crude reaction mixtures were measured by 1H NMR spectroscopy and ranged from 1:1 to 1.5:1 dr. See Supporting Information for complete details.
Enantiomeric ratios were measured using chiral stationary phase HPLC.
Isolated yields.
Reaction run in water for 5 days.
3:1 dr observed.
In a second line of optimization, we hypothesized that the reactive indolenine intermediate might be formed fully in situ prior to the addition of pronucleophile (Table 3, Method B). This also provided the desired substitution products with similar enantioselection in most cases. Together, these methods suggest that similar indolenine intermediates are formed, leading to enantioselective product-forming pathways. Attenuations in enantioselection can be attributed, at least in part, to achiral base-promoted product formation. Table 3 compares these two methods across a range of substrates.
Both methods A and B gave high enantioselection for the various aryl analogs tested (Table 3, entries 4–9). Use of para-methoxy phenylnitromethane leads to an increase in ee for method A and B, but a significant difference in the major and minor diastereomers is observed in the latter (Table 3, entry 11). The furan analog saw a sharp increase in ee from the original method (53% ee/44% ee) to the new methods, as both gave 90% ee for the major diastereomer (Table 3, entries 12–13). The aliphatic analog (Table 3, entries 14–15) saw a small drop in ee from the original method, as well as in yield. The ester derivative (Table 3, entry 16) provided a small increase in ee while 3:1 dr was maintained. Unfortunately, the nitroethane addition product was produced with significantly lower ee for method B and did not produce any product for method A (Table 3, entries 18–19). The increased solubility of the nitroethane in water may have contributed to the low yield of the reaction.
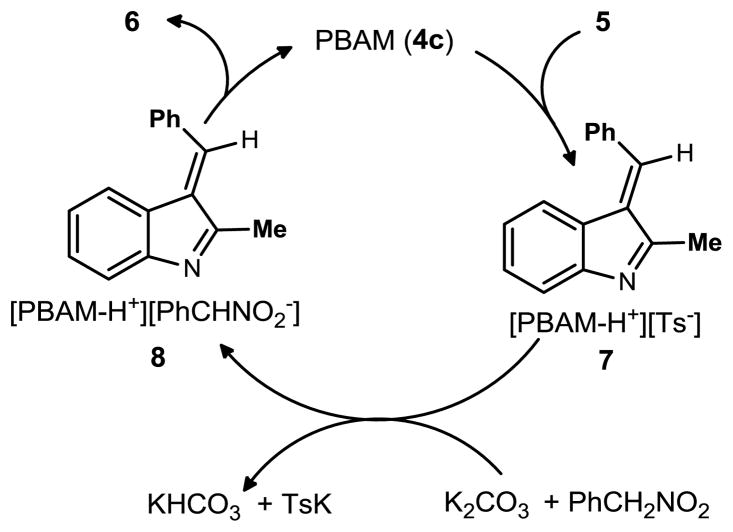
Using the data from all three methods and control experiments, a working mechanism is proposed in Scheme 2. We hypothesize that both PBAM and potassium carbonate can effect the elimination of toluene sulfinate. However, when only potassium carbonate is used the reaction is much slower (50% conversion after 22 h), leading to the conclusion that the organocatalyst is a more effective promoter of indolenine formation. If potassium carbonate regenerates PBAM from its sulfinic acid salt, the catalyst can then deprotonate the nitroalkane to form the nucleophilic nitronate. Alternatively, the PBAM-HTs–indolenine complex (7) could react with the potassium nitronate, PBAM-nitronic acid salt, or the simple nitronic acid enantioselectively. Based on the observations outlined above, we favor the first of these possibilities wherein the PBAM-nitronate salt reacts with the indolenine selectively.
Scheme 2.
Proposed cycle for chiral base catalysis of nitroalkane alkylations by an in situ-formed indolenine.
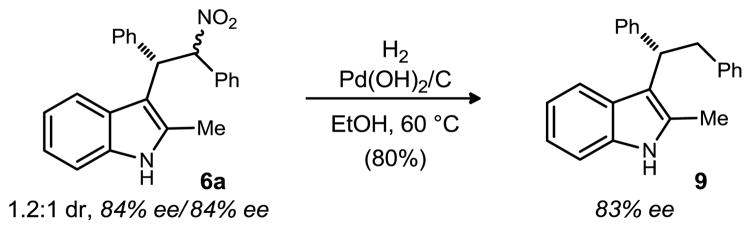
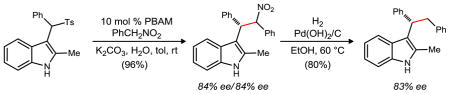
Nitroalkanes offer many opportunities for functionalization through reduction (tryptamine analogs), oxidation (Nef) and removal (denitration).22 Using modified conditions for the cleavage of benzylic nitro bonds by Carreira23 we were able to denitrate in high yield while enantioenrichment was conserved (Scheme 1). Denitration gives products (8) that would result from the enantioselective Friedel-Crafts reaction between indole and stilbene, a reaction not yet developed.
Scheme 1.
Convergence of diastereomeric nitroalkanes to a sec-alkyl 3-substituted indole with high ee.
In conclusion, an enantioselective Brønsted base catalyzed alkylation of nitroalkanes has been developed using indolenine electrophiles. The final protocol provides β-substituted tryptamine analogs and illustrates how these enantioselective reactions at room temperature can be improved by the addition of water.24
Supplementary Material
Acknowledgments
We are grateful to the NIH (GM 084333) and Amgen for financial support of this work, and the VICB for fellowship support (MCD).
Footnotes
Supporting Information Available Complete experimental details and analytical data for all new compounds.
References
- 1.Farr RN, Alabaster RJ, Chung JYL, Craig B, Edwards JS, Gibson AW, Ho GJ, Humphrey GR, Johnson SA, Grabowski EJJ. Tetrahedron: Asymmetry. 2003;14:3503–3515. [Google Scholar]
- 2.Isolation: Kayakawa Y, Kawakami K, Seto H, Furihata K. Tetrahedron Lett. 1992;33:2701–2704.rac-2 syntheses: Fuerstner A, Weintritt H. J Am Chem Soc. 1998;120:2817–2825.Harrington PE, Tius MA. Org Lett. 1999;1:649–651. doi: 10.1021/ol990124k.Robertson J, Hatley RJD. Chem Commun (Cambridge) 1999:1455–1456.Bitar AY, Frontier AJ. Org Lett. 2009;11:49–52. doi: 10.1021/ol802329y.Asymmetric syntheses of 2: Bamford SJ, Luker T, Speckamp WN, Hiemstra H. Org Lett. 2000;2:1157–1160. doi: 10.1021/ol005750s.Trost BM, Doherty GA. J Am Chem Soc. 2000;122:3801–3810.Boger DL, Hong J. J Am Chem Soc. 2001;123:8515–8519. doi: 10.1021/ja011271s.Biological activity: Fuerstner A. Angew Chem Int Ed. 2003;42:3582–3603. doi: 10.1002/anie.200300582.
- 3.Isolation and biological activity: Cui CB, Kakeya H, Osada H. Tetrahedron. 1996;52:12651–12666.Kondoh M, Usui T, Mayumi T, Osada H. J Antibiot. 1998;51:801–804. doi: 10.7164/antibiotics.51.801.Syntheses of 3: Edmondson SD, Danishefsky SJ. Angew Chem Int Ed. 1998;37:1138–1140. doi: 10.1002/(SICI)1521-3773(19980504)37:8<1138::AID-ANIE1138>3.0.CO;2-N.Edmondson S, Danishefsky SJ, Sepp-Lorenzino L, Rosen N. J Am Chem Soc. 1999;121:2147–2155.Overman LE, Rosen MD. Angew Chem Int Ed. 2000;39:4596–4599.Sebahar PR, Williams RM. J Am Chem Soc. 2000;122:5666–5667.Von NF, Danishefsky SJ. Angew Chem Int Ed. 2000;39:2175–2178. doi: 10.1002/1521-3773(20000616)39:12<2175::aid-anie2175>3.0.co;2-j.Bagul TD, Lakshmaiah G, Kawabata T, Fuji K. Org Lett. 2002;4:249–251. doi: 10.1021/ol016999s.Meyers C, Carreira EM. Angew Chem Int Ed. 2003;42:694–696. doi: 10.1002/anie.200390192.Miyake FY, Yakushijin K, Horne DA. Org Lett. 2004;6:4249–4251. doi: 10.1021/ol048311s.Marti C, Carreira EM. J Am Chem Soc. 2005;127:11505–11515. doi: 10.1021/ja0518880.Wang S, Ding K, Lu Y, Nikolovska-Coleska Z, Qiu S, Wang G, Qin D, Shangary S. WO/2006/091646. Preparation of spirotryprostatin A derivatives as inhibitors of MDM2. 2006 August 8;Trost BM, Stiles DT. Org Lett. 2007;9:2763–2766. doi: 10.1021/ol070971k.
- 4.For a recent review: Bandini M, Eichholzer A. Angew Chem Int Ed. 2009;48:9608–9644. doi: 10.1002/anie.200901843.
- 5.For recent reviews see: Jørgensen KA. Synthesis. 2003:1117–1125.Bandini M, Melloni A, Umani-Ronchi A. Angew Chem Int Ed. 2004;43:550–556. doi: 10.1002/anie.200301679.
- 6.Bandini M, Garelli A, Rovinetti M, Tommasi S, Umani-Ronchi A. Chirality. 2005;17:522–529. doi: 10.1002/chir.20189. [DOI] [PubMed] [Google Scholar]; Herrera RP, Sgarzani V, Bernardi L, Ricci A. Angew Chem Int Ed. 2005;44:6576–6579. doi: 10.1002/anie.200500227. [DOI] [PubMed] [Google Scholar]; Zhuang W, Hazell RG, Jørgensen KA. Org Biomol Chem. 2005;3:2566–2571. doi: 10.1039/b505220c. [DOI] [PubMed] [Google Scholar]; Jia YX, Zhu SF, Yang Y, Zhou QL. J Org Chem. 2006;71:75–80. doi: 10.1021/jo0516537. [DOI] [PubMed] [Google Scholar]; Lu SF, Du DM, Xu J. Org Lett. 2006;8:2115–2118. doi: 10.1021/ol060586f. [DOI] [PubMed] [Google Scholar]; Ganesh M, Seidel D. J Am Chem Soc. 2008;130:16464–16465. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]; Itoh J, Fuchibe K, Akiyama T. Angew Chem Int Ed. 2008;47:4016–4018. doi: 10.1002/anie.200800770. [DOI] [PubMed] [Google Scholar]; Liu H, Lu SF, Xu J, Du DM. Chem —Asian J. 2008;3:1111–1121. doi: 10.1002/asia.200800071. [DOI] [PubMed] [Google Scholar]; Yuan ZL, Lei ZY, Shi M. Tetrahedron: Asymmetry. 2008;19:1339–1346. [Google Scholar]; McKeon SC, Mueller-Bunz H, Guiry PJ. Eur J Org Chem. 2009:4833–4841. [Google Scholar]; Yokoyama N, Arai T. Chem Commun (Cambridge) 2009:3285–3287. doi: 10.1039/b904275j. [DOI] [PubMed] [Google Scholar]; Wang XF, Chen JR, Cao YJ, Cheng HG, Xiao WJ. Org Lett. 2010;12:1140–1143. doi: 10.1021/ol1001818. [DOI] [PubMed] [Google Scholar]
- 7.Additions of indole to α,β disubstituted nitrostyrenes: Bandini M, Melchiorre P, Melloni A, Umani-Ronchi A. Synthesis. 2002:1110–1114.Ballini R, Clemente RR, Palmieri A, Petrini M. Adv Synth Catal. 2006;348:191–196.Kusurkar R, Alkobati N, Gokule A, Chaudhari P, Waghchaure P. Synth Commun. 2006;36:1075–1081.Kantam ML, Laha S, Yadav J, Srinivas P. Synth Commun. 2009;39:4100–4108.Ye MC, Yang YY, Tang Y, Sun XL, Ma Z, Qin WM. Synlett. 2006:1240–1244.Habib PM, Kavala V, Raju BR, Kuo CW, Huang WC, Yao CF. Eur J Org Chem. 2009:4503–4514.An elegant asymmetric, tandem FC/Henry reaction: Arai T, Yokoyama N. Angew Chem Int Ed. 2008;47:4989–4992. doi: 10.1002/anie.200801373.
- 8.Enantioselective additions have been reported using nitroalkenes bearing an additional activating group (−CO2R): Sui Y, Liu L, Zhao JL, Wang D, Chen YJ. Tetrahedron. 2007;63:5173–5183.
- 9.Ballini R, Palmieri A, Petrini M, Torregiani E. Org Lett. 2006;8:4093–4096. doi: 10.1021/ol061604w. [DOI] [PubMed] [Google Scholar]
- 10.For a review: Palmieri A, Petrini M, Shaikh RR. Org Biomol Chem. 2010;8:1259–1270. doi: 10.1039/b919891a.For other works: Palmieri A, Petrini M. J Org Chem. 2007;72:1863–1866. doi: 10.1021/jo062538e.Ballini R, Palmieri A, Petrini M, Shaikh R. Adv Synth Catal. 2008;350:129–134.Palmieri A, Petrini M, Shaikh RR. Synlett. 2008;2008:1845–1851.Ballini R, Gabrielli S, Palmieri A, Petrini M. Adv Synth Catal. 2010;352:2459–2462.Marsili L, Palmieri A, Petrini M. Org Biomol Chem. 2010;8:706–712. doi: 10.1039/b919954c.
- 11.Cozzi P, Benfatti F, Zoli L. Angew Chem Int Ed. 2009;48:1313–1316. doi: 10.1002/anie.200805423. [DOI] [PubMed] [Google Scholar]; Campetella S, Palmieri A, Petrini M. Eur J Org Chem. 2009;2009:3184–3188. [Google Scholar]; Boas U, Brask J, Jensen KJ. Chem Rev. 2009;109:2092–2118. doi: 10.1021/cr068206r. [DOI] [PubMed] [Google Scholar]
- 12.Semenov BB, Granik VG. Pharm Chem J. 2004;38:287–310. [Google Scholar]; Semenov B, Smushkevich Y, Levina I, Kurkovskaya L, Lysenko K, Kachala V. Chem Heterocycl Compd. 2005;41:730–738. [Google Scholar]; Matsuo JI, Tanaki Y, Ishibashi H. Tetrahedron. 2008;64:5262–5267. [Google Scholar]
- 13.Proline catalyzed (enamine) additions: Shaikh R, Mazzanti A, Petrini M, Bartoli G, Melchiorre P. Angew Chem Int Ed. 2008;47:8707–8710. doi: 10.1002/anie.200803947.
- 14.Preliminary results using NHC: Li Y, Shi FQ, He QL, You SL. Org Lett. 2009;11:3182–3185. doi: 10.1021/ol9013238.
- 15.Enantioselective addition of N-Bz enamines to indolyl alcohols using a chiral phosphoric acid: Guo QX, Peng YG, Zhang JW, Song L, Feng Z, Gong LZ. Org Lett. 2009;11:4620–4623. doi: 10.1021/ol901892s.
- 16.Silver catalyzed diastereo- and enantioselective synthesis of tryptophans: Zheng BH, Ding CH, Hou XL, Dai LX. Org Lett. 2010;12:1688–1691. doi: 10.1021/ol100161n.
- 17.Jing L, Wei J, Zhou L, Huang Z, Li Z, Wu D, Xiang H, Zhou X. Chem —Eur J. 2010;16:10955–10958. doi: 10.1002/chem.201001662. [DOI] [PubMed] [Google Scholar]
- 18.For a recent review: Ting A, Goss J, McDougal N, Schaus S. Asymmetric Organocatalysis. Vol. 291. Springer; Berlin/Heidelberg: 2009. pp. 145–200.
- 19.BAM catalysis: Nugent BM, Yoder RA, Johnston JN. J Am Chem Soc. 2004;126:3418–3419. doi: 10.1021/ja031906i.Singh A, Yoder RA, Shen B, Johnston JN. J Am Chem Soc. 2007;129:3466–3467. doi: 10.1021/ja068073r.Shen B, Johnston JN. Org Lett. 2008;10:4397–4400. doi: 10.1021/ol801797h.Singh A, Johnston JN. J Am Chem Soc. 2008;130:5866–5867. doi: 10.1021/ja8011808.Davis TA, Wilt JC, Johnston JN. J Am Chem Soc. 2010;132:2880–2882. doi: 10.1021/ja908814h.Shen B, Makley DM, Johnston JN. Nature. 2010;465:1027–1032. doi: 10.1038/nature09125.
- 20.Material enriched in one diastereomer by column chromatography (4:1) was resubmitted to the reaction conditions to give material with the same diastereoselection (4:1).
- 21.Hess AS, Yoder RA, Johnston JN. Synlett. 2006:0147–0149. [Google Scholar]
- 22.Ono N. In: The Nitro Group in Organic Synthesis. Feuer H, editor. Wiley-VCH; New York: 2001. p. 1. [Google Scholar]
- 23.Fessard T, Motoyoshi H, Carreira E. Angew Chem Int Ed. 2007;46:2078–2081. doi: 10.1002/anie.200604263. [DOI] [PubMed] [Google Scholar]
- 24.For more on how water affects organic reactions: Ribe S, Wipf P. Chem Commun. 2001:299–307.Hayashi Y. Angew Chem Int Ed. 2006;45:8103–8104. doi: 10.1002/anie.200603378.Donahue MG, Hong KB, Johnston JN. Bioorg Med Chem Lett. 2009;19:4971–4973. doi: 10.1016/j.bmcl.2009.07.067.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.