Abstract
Huntington's disease (HD) is an inherited, progressive and ultimately fatal neurodegenerative disorder that is characterized by psychiatric, cognitive and motor symptoms. Among the pathways implicated in HD are those involving mitogen-activated protein kinase signaling and particularly the Ras-extracellular signal-regulated kinase (ERK) cascade. Studies in both cells and animal models suggest that ERK activation might provide a novel therapeutic target for the treatment of HD but compounds that specifically activate ERK are few. To test the hypothesis that pharmaceutical activation of ERK might be protective for HD, a polyphenol, fisetin, which was previously shown to activate the Ras-ERK cascade, was tested in three different models of HD: PC12 cells expressing mutant Httex1 under the control of an inducible promoter, Drosophila expressing mutant Httex1 and the R6/2 mouse model of HD. The results indicate that fisetin can reduce the impact of mutant huntingtin in each of these disease models. Prompted by this observation, we determined that the related polyphenol, resveratrol, also activates ERK and is protective in HD models. Notably, although more than a dozen small molecule inhibitors of ERK activation are in clinical trials, very few small molecule activators of ERK signaling are reported. Thus, fisetin, resveratrol and related compounds might be useful for the treatment of HD by virtue of their unique ability to activate ERK.
INTRODUCTION
Huntington's disease (HD) is a late-onset, progressive and fatal neurodegenerative disorder for which there is, at present, no cure. It is caused by the expansion of a trinucleotide repeat that encodes an abnormally long polyglutamine tract in the huntingtin (Htt) protein. The identification of the disease-causing mutation has allowed the development of a number of cellular and animal models of HD and these have been used to elucidate the mechanisms underlying disease development and progression (reviewed in 1).
Among the pathways implicated in HD are those involving mitogen-activated protein kinase (MAPK) signaling and particularly the Ras-extracellular signal-regulated kinase (ERK) cascade (2). Although both protective and deleterious roles have been proposed for ERK activation in neuronal cells (3–5), recent studies using mutant-Htt-expressing nerve cells provide strong evidence that activation of ERK provides neuroprotection, while specific inhibition of ERK activation enhances cell death (2). More recently, neuroprotective compounds identified using a neuronal cell culture model of HD in combination with a library of 1040 biologically active compounds were shown to prevent cell death by inhibiting mitochondrial function resulting in the activation of ERK and Akt signaling with the ERK pathway playing the major role (6). Furthermore, reduced signaling by growth factors such as brain-derived neurotrophic factor (BDNF) and EGF-1 (7–11) that activate the Ras-ERK cascade has been found in HD models and patients. Together, these results suggest that ERK activation might provide a novel therapeutic approach to prevent neuronal dysfunction in HD.
The Ras-ERK cascade is classically activated by growth factors or neurotrophic factors such as BDNF or EGF-1. These factors initiate a complex signaling cascade leading to the activation of Ras, Raf and MAPK/ERK kinase (MEK), a dual specificity kinase that activates ERK via phosphorylation on both threonine and tyrosine residues. However, because these factors are proteins, their clinical use has been limited by difficulties in delivery to the brain and unsuitable pharmacokinetics (12). An alternative approach is to identify small molecules that can substitute for growth factors. We recently showed that the flavonoid fisetin can activate the Ras-ERK cascade in nerve cells (13,14) and activation of this signaling pathway is associated with the neuroprotective, neurotrophic and cognition-enhancing effects of fisetin (13,14). Interestingly, HD in both rodents and humans is characterized by deficits in learning and memory (15,16), two functions in which ERK plays a critical role (17). We have also recently shown that a related polyphenol, resveratrol, is effective at suppressing HD pathology in a Drosophila model of HD, and that this suppression does not involve activation of sirtuins (18,19).
Combining these observations, we sought to test the hypothesis that fisetin and related polyphenols such as resveratrol, might be useful for the treatment of HD by activating the ERK pathway. To this end, we tested fisetin in three different models of HD: PC12 cells expressing mutant Httex1 under the control of an inducible promoter, Drosophila expressing mutant Httex1 and the R6/2 mouse model of HD. We also tested whether the protective effect of the related polyphenol, resveratrol, could be accounted for by activation of the ERK pathway using both pharmacologic and genetic manipulations. The results indicate that fisetin can reduce the impact of mutant huntingtin in each of these disease models and that both fisetin and resveratrol activate the ERK pathway, thus suggesting that polyphenols and/or their derivatives might be useful for the treatment of HD.
RESULTS
The polyphenol fisetin protects PC12 cells from mutant huntingtin expression
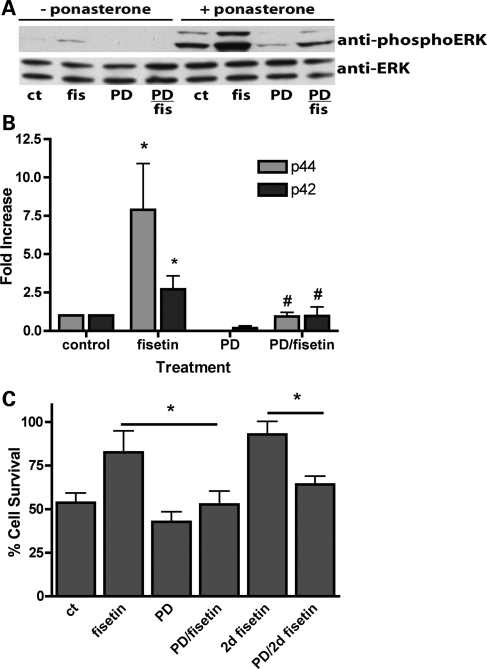
Induction of mutant Htt (Httex1-103QP-EGFP) by treatment of PC12/HttQ103 cells with ponasterone (PA) results in the death of ∼45% of the cells within 72 h (Fig. 1) (19). As shown in Figure 1A, treatment with fisetin at the time of Httex1-103QP induction increases cell survival in a dose-dependent manner with a maximal effect seen between 5 and 10 μm. Fisetin did not alter the formation of EGFP-tagged Httex1-103QP aggregates (Fig. 1B) or the overall level of Httex1-103QP-EGFP expression (Fig. 1C).
Figure 1.
(A) Dose-dependent effect of fisetin on the survival of PC12/HttQ103 cells. Cells were un-induced or induced with 5 μm PA in N2 medium to express the entire Htt exon 1 fused to EGFP. Such cells typically form Htt protein aggregates (B) and die within 3–4 days. Fisetin was added to the un-induced cells or the induced cells at the time of induction. After 72h, cell survival was measured by the MTT assay. The results are plotted as the percentage cell survival in induced cells relative to un-induced cells and are the average ± SD of four independent experiments. (B) PC12/HttQ103 cells induced with 5 μm PA in the absence (control) or presence (+fisetin) of 10 μm fisetin were examined after 48 h by fluorescence microscopy to assess the presence and localization of Httex1-103QP-EGFP. Fluorescent aggregates were counted in five random fields from both control and fisetin-treated cells. (C) PC12/HttQ103 cells were induced with 5 μm PA in the absence (control) or presence (fisetin) of 10 μm fisetin. After 48 h, cell extracts were prepared and equal amounts of protein were analyzed by SDS–PAGE and immunoblotting with antibodies to GFP and actin as a loading control. A representative blot is shown. Similar results were obtained in three independent experiments. The average GFP signal from the fisetin-treated cells, quantified by densitometry, normalized to actin and then normalized to the levels of GFP in the absence of fisetin, was plotted as ±SD.
Protection by fisetin is dependent on ERK activation
An earlier study (2) showed that expression of mutant huntingtin in PC12 cells resulted in the activation of multiple MAPKs including both ERK and c-Jun N-terminal kinases (JNKs). ERK activation was specifically implicated in neuroprotection (2). Given that fisetin was previously shown to induce ERK activation in PC12 cells (20) as well as primary neurons (14) and hippocampal slices (13), we asked if it could activate ERK, as indicated by an increase in phosphorylation, in the PC12/HttQ103 cells. Httex1-103QP itself induces modest ERK activation in the PC12/HttQ103 cells and this is enhanced by treatment with fisetin (Fig. 2A and B). Importantly, inhibition of ERK activation by co-treatment with the MEK inhibitor PD98059 completely eliminates the effects of fisetin on ERK activation (Fig. 2A and B) and on cell survival (Fig. 2C), suggesting that fisetin is acting on the MAPK pathway itself. Interestingly, treatment with 10 μm fisetin 24 h after the induction of Httex1-103QP (2d fisetin) provided an equal or greater amount of protection indicating that fisetin (Fig. 2C) can also reduce the effects of Httex1-103QP already present in cells. This result further supports the conclusion that the effect of fisetin is not on suppression of Httex1-103QP synthesis.
Figure 2.
(A) Effect of fisetin and Httex1-103QP-EGFP on ERK phosphorylation in PC12/HttQ103 cells. PC12/HttQ103 cells were un-induced or induced with 5 μm PA in N2 medium. Fisetin (10 μm) and/or PD98059 (40 μm) was added to the un-induced cells or the induced cells at the time of induction as indicated. After 24 h, cell extracts were prepared and equal amounts of protein were analyzed by SDS–PAGE and immunoblotting with antibodies to phospho-ERK and total ERK. A representative blot is shown. Although fisetin induces ERK phosphorylation in both the absence and presence of Httex1-103QP-EGFP, the effect is much stronger in the presence of Httex1-103QP-EGFP. Similar results were obtained in four independent experiments. (B) The average phosphoprotein signal from the PA-treated samples in the blots in (A), quantified by densitometry, normalized to total ERK and then normalized to the levels in the absence of fisetin or PD98059, was plotted as ±SD. Asterisk (*) denotes fisetin-treated versus control, P < 0.001; Number sign (#) denotes PD + fisetin versus fisetin, P < 0.001 (Unpaired t test). (C) ERK phosphorylation promotes survival in PC12/HttQ103 cells. PC12/HttQ103 cells were un-induced or induced with 5 μm PA in N2 medium. Fisetin (10 μm) and/or PD98059 (PD, 40 μm) was added to the un-induced cells or the induced cells at the time of induction as indicated. After 72 h, cell survival was measured by the MTT assay. In some experiments, fisetin was added 24 h after the induction of Httex1-101QP-EGFP expression with PA (2d fisetin). The results are plotted as the % cell survival of induced cells relative to un-induced cells and are the average ± SD of five independent experiments. Asterisk (*) indicates significantly different from fisetin alone, P < 0.001 (unpaired t test).
Fisetin reduces JNK activation in PC12/HttQ103 cells
JNK was also shown to be activated by Httex1-103QP expression in the PC12 cell model (2) and, opposite to ERK activation, contributed to cell death. In contrast to its effects on ERK activation, fisetin reduced JNK activation, as indicated by a decrease in JNK phosphorylation in the PC12/HttQ103 cells (Fig. 3A and B). Activation of JNK by Httex1-103QP expression in the PC12/HttQ103 cells (Fig. 3A and B) correlated with the activation of caspase 3 (Fig. 3C) (2), which is thought to contribute to cell death (19). Consistent with its effects on JNK activation and cell survival, fisetin also significantly reduced caspase 3 activation (Fig. 3C). Together these results suggest that fisetin could be useful for selective activation of ERK and thus for the treatment of HD.
Figure 3.
(A) Effect of fisetin and Httex1-103QP-EGFP on JNK phosphorylation. PC12/HttQ103 cells were un-induced or induced with 5 μm PA in N2 medium. Fisetin (10 μm) was added to the un-induced cells or the induced cells at the time of induction as indicated. After 24h, cell extracts were prepared and equal amounts of protein were analyzed by SDS–PAGE and immunoblotting with antibodies to phospho-JNK and total JNK1. A representative blot is shown. Similar results were obtained in three independent experiments. (B) The average phosphoprotein signal from the PA-treated samples in the blots in (A), quantified by densitometry, normalized to total JNK and then normalized to the level in the absence of fisetin, was plotted as ±SD. Asterisk (*) indicates fisetin-treated versus control, P < 0.02 (unpaired t test). (C) Effect of fisetin and Httex1-103QP-EGFP on caspase 3 activation in the PC12/HttQ103 cells. Cells were un-induced or induced with 5 μm PA in N2 medium. Fisetin was added to the un-induced cells or the induced cells at the time of induction. After 48 h, caspase 3 activity was measured by caspase 3/7 Glo kit. The results are the average ± SD of three independent experiments. Asterisk (*) indicates fisetin-treated versus control, P < 0.02 (unpaired t test).
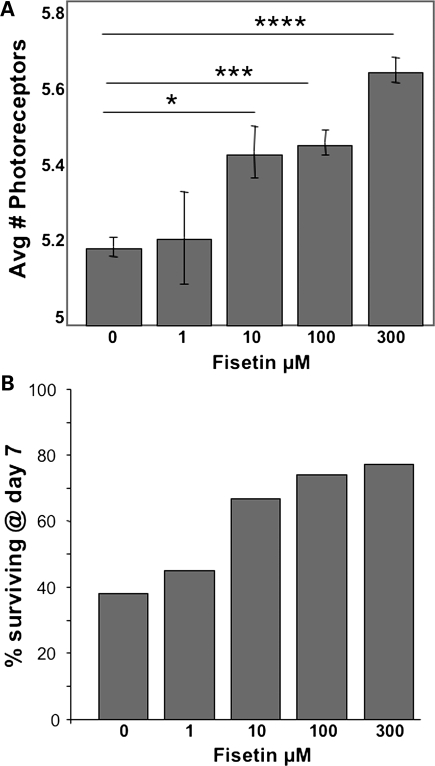
Fisetin suppresses pathology in a Drosophila model of HD
To test the potential efficacy of fisetin treatment in vivo, we used a Drosophila model of HD (21) where expression of mutant human Httex1 in neuronal cells leads to HD-like symptoms (22). Flies expressing pathogenic human Htt (w elav:Gal4/w; P{UAS-Httex1p Q93}/+) Httex1p Q93) were harvested shortly after eclosion and placed on standard fly food containing various concentrations of fisetin. Flies were transferred to fresh food every day and after 7 days were examined for neuronal degeneration by the pseudopupil technique (Fig. 4A) and survival (Fig. 4B). By day 7, the blinded pseudopupil analysis of neurodegeneration showed a dose-dependent increase in photoreceptor neuron survival when flies were fed fisetin-containing food (Fig. 4A). At 300 μm, fisetin showed the least neurodegeneration with ∼25% rescue when compared with the control. Further, only about 38% of untreated flies were still alive by day 7, while vials containing siblings reared on fisetin-containing food showed increasing levels of survival in a dose-dependent manner (i.e. up to 77% surviving at 300 μm) (Fig. 4B). Thus, fisetin shows promise in suppressing mutant Htt pathology in a Drosophila model of HD.
Figure 4.
Suppression of pathology in a Drosophila model of HD. (A) Dose-dependent rescue of photoreceptor neuron degeneration by increasing concentrations of fisetin: *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001 (unpaired t test). (B) The number of eclosed Httex1p Q93-challenged animals surviving until day 7 increases in a dose-dependent fashion with increasing concentrations of fisetin. A chi-square analysis of this dataset suggests an association between survival and fisetin treatment (P < 0.025).
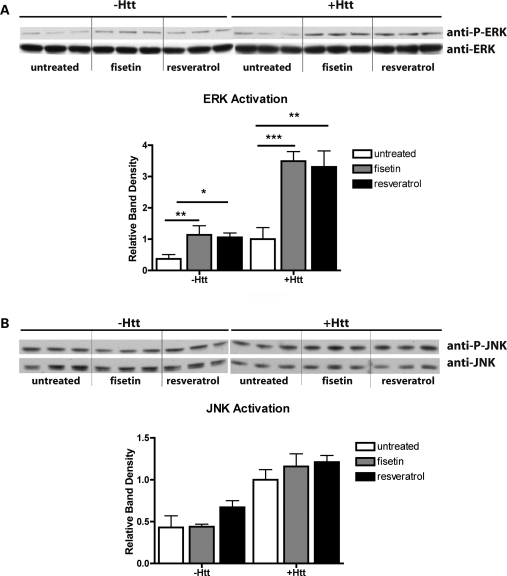
Fisetin and resveratrol increase ERK activation in Drosophila
Given our observations with respect to fisetin and ERK activation in the PC12/HttQ103 cells, we sought to determine if the in vivo effects of fisetin in flies are also mediated by ERK activation. In addition, our previous observations that the structurally related polyphenol, resveratrol, also suppresses neurodegeneration in this fly model by an unknown mechanism (18) prompted us to compare the effects of these two drugs on ERK activation in flies. Heads from untreated, fisetin-treated and resveratrol-treated non-Htt-expressing controls (i.e. non-Htt-expressing siblings, (w/Y; P{UAS-Httex1p Q93}/+) and mutant Htt-expressing flies (w elav:Gal4/w; P{UAS-Httex1p Q93}/+) were analyzed for ERK phosphorylation following 19.5 h of exposure to the compounds. Western blots of three independent extracts each were analyzed for total ERK and phospho-ERK (Fig. 5A). In contrast to the PC12/HttQ103 cells, only a single ERK band is seen in the fly head extracts. ERK phosphorylation and thereby activation was very low in the untreated heads from control flies and was significantly increased by both fisetin and resveratrol treatment (Fig. 5A). While ERK phosphorylation was higher in the heads from untreated mutant Htt-expressing flies when compared with heads from untreated non-expressing control flies, both fisetin and resveratrol still significantly increased ERK phosphorylation (Fig. 5A). To better understand the selectivity of these treatments with respect to JNK activation, western blots of the same head extracts were analyzed for total JNK and phospho-JNK (Fig. 5B). In contrast to the results with ERK, in the same head extracts, JNK phosphorylation was slightly higher in the heads from untreated mutant Htt flies when compared with the heads from untreated non-Htt-expressing flies (Fig. 5B). Furthermore, neither fisetin nor resveratrol showed any significant effects on the phosphorylation of JNK (Fig. 5B).
Figure 5.
Effect of fisetin and Htt on ERK (A) or JNK (B) phosphorylation in Drosophila. Wild-type (-Htt) or Httex1p Q93-challenged (+Htt) flies were fed a normal diet (untreated) or a diet supplemented with fisetin (300 μm) or resveratrol (300 μm) for 19.5 h before harvesting. Three separate collections of heads were isolated, extracts prepared and equal amounts of protein were analyzed by SDS–PAGE and immunoblotting with antibodies to phospho-ERK and total ERK (A) or phospho-JNK and total JNK (B). A representative blot is shown. Since fisetin induces ERK phosphorylation in both the absence and presence of Httex1p Q93 but the effect is stronger in the presence of Httex1p Q93, the exposure of the Httex1p Q93 phospho-ERK blot was for half the time as the exposure of the wild-type phospho-ERK blot. The average phosphoprotein signal from the samples as quantified by densitometry, normalized to total ERK or total JNK and then normalized to the level in the Httex1p Q93 flies fed control diet, was plotted ±SD. *P < 0.05; **P < 0.01; ***P < 0.001 (unpaired t-test).
Genetically altering ERK activation affects survival of HD flies
Given the positive results with both fisetin and resveratrol on suppressing neuronal pathology and activating ERK, we sought to genetically confirm whether activation of ERK is a valid therapeutic target for suppressing HD-mediated neurodegeneration. The general strategy for testing involves comparing Httex1p Q93-challenged animals to their siblings in which one copy of a particular gene of interest has been eliminated. Reducing the level of ERK protein by half (i.e. heterozygous for the Drosophila ERK gene, rolled which is 81% identical to human ERK1/2) had no effect on the survival or neuronal degeneration of Htt-challenged Drosophila (i.e. w elav:Gal4/w; rl/+; P{UAS-Httex1p Q93}/+) (Fig. 6A and B). In contrast, a 50% reduction in the level of Drosophila MEK, Dsor (66% identical to human MEK1), decreased the survival of Httex1p Q93-challenged animals (i.e. w elav:Gal4 +/w Dsor1; P{UAS-Httex1p Q93}/+) (Fig. 6A). These observations are consistent with the idea that ERK levels are not limiting but that ERK activation is protective to mutant Htt-challenged neurons. To further test this, we sought to genetically increase the levels of activated ERK by reducing the doses of the ERK phosphatase genes, Protein Tyrosine Phosphatase-ERK/Enhancer of Ras1 gene (PTP-ER) and microtubule star (mts), two phosphatases known to act on ERK (23,24). Reducing the dose of each of these had a positive impact on the number of animals surviving to adulthood (eclosion) and on the number of photoreceptor neurons surviving (i.e. w elav:Gal4/w; mts/+; P{UAS-Httex1p Q93}/+ and w elav:Gal4/w; PTP-ER/+; P{UAS-Httex1p Q93}/+) (Fig. 6A and B), although only the rescue afforded by reducing the PTP-ER gene met the criteria for statistical significance. Consistent with the postulated opposing actions of ERK and JNK activation, a 50% reduction of JNK (i.e. heterozygous for the Drosophila JNK gene basket, bsk which is 79% identical to hJNK) provided a modest increase in survival and longevity of Httex1p Q93-challenged animals (Supplemental Material, Fig. S1A and B). Although increased survival of neurons with a 50% reduction in JNK dose did not reach a statistically robust level (Fig. 6B), inhibition of JNK activation with SP600125 did exhibit a robust, dose-dependent increase in neuronal survival (Supplemental Material, Fig. S1C). Together, these observations suggest that polyphenols such as fisetin and resveratrol can be neuroprotective in an HD setting by selectively activating ERK.
Figure 6.
Genetically elevated ERK activation is protective for Drosophila challenged with expanded Httex1. Siblings from crosses of flies expressing Elav-driven pathogenic Htt fragments that were either normal or heterozygous for an ERK or JNK-related gene of interest were compared for survival to adulthood (A). The number of progeny falling in the resulting genotypic classes were scored and the effects on huntingtin-induced lethality were calculated as relative viability scores (the ratio of the number of mutant and non-mutant Htt-expressing flies divided by the ratio of the number of mutant and non-mutant Htt non-expressing flies). The line at 100% indicates the eclosion of Htt-challenged flies against which siblings with reduced levels of the indicated tester genes are compared. The statistical significance of relative eclosion was assessed by Chi-square testing; triple asterisks (***) indicates P < 0.001. The number of progeny (n) was greater than 1500 in all cases. The Drosophila symbol of each gene is listed above the corresponding name of the mammalian protein (ERK P'ase and PP2A cat are the ERK tyrosine phosphatase and catalytic subunit of the PP2A Ser/Thr phosphatase). (B) The number of photoreceptor neurons surviving in expanded Httex1-challenged flies that are heterozygous for several of these mutations is shown. Black bars indicate photoreceptor counts in control animals with two doses of the tester gene, while the white bars represent counts in siblings that are heterozygous for a mutation of the tester gene (e.g. bsk, rl, PTP-ER or mts). Control animals are recognized by the CyO balancer which does not affect rhabdomere numbers when heterozygous. Reduction of the ERK tyrosine phosphatase, PTP-ER, demonstrated significant improvement in neuronal survival. Statistical significance was assessed by unpaired t-test; asterisk (*) indicates P < 0.05.
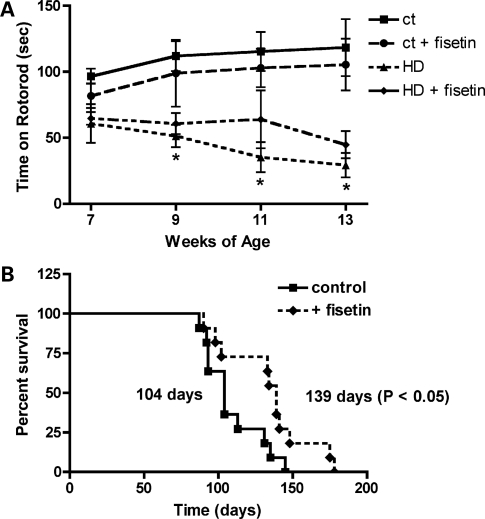
Fisetin improves rotorod performance and increases lifespan in the R6/2 mouse model of HD
Given the positive results with fisetin in both the cell- and Drosophila-based assays, we decided to test the effect of fisetin in a mammalian model of HD, the R6/2 mouse. This transgenic mouse line expresses human mutant exon 1 of huntingtin with a highly expanded repeat from the mouse Htt promoter, and has been widely used as a model for testing novel therapeutic approaches to the treatment of HD [e.g. (25–27)]. Since the overall goal was to determine if oral administration of fisetin could be useful for the treatment of HD, fisetin was fed to genotyped R6/2 mice and their wild-type littermates in the food at 0.05% beginning at ∼6 weeks of age. The mice were tested on the rotorod from ∼7 to 13 weeks of age and survival was followed. At the time of acquisition of the animals, rotorod performance was already impaired in the R6/2 mice when compared with their wild-type littermates (Fig. 7A). However, the performance declined significantly more rapidly in animals on the control diet when compared with those on the fisetin diet (Fig. 7A). Similarly, as shown in Figure 7B, while the median lifespan of the R6/2 mice on the control diet was 104 days, that of fisetin-fed mice was increased by ∼30% to 134 days.
Figure 7.
Fisetin treatment decreases motor impairment and mortality in R6/2 mice. R6/2 mice on 0.05% fisetin or control diet (n = 15) were monitored for (A) rotorod performance and (B) mortality. Fisetin improved rotorod performance at 9, 11 and 13 weeks (P < 0.01) and survival (P < 0.05) (unpaired t test).
DISCUSSION
It has been suggested that polyphenols, such as fisetin and resveratrol, may extend lifespan by activating the enzyme Sir2 (28,29) and that this property of polyphenols may be useful in disease settings such as neurodegeneration (30,31). However, others report no effects of resveratrol on the lifespan of Drosophila or worms (32), and a number of studies indicate that it does not directly activate SIRT1 (18,33–36) leaving these potentially beneficial compounds in search of a mechanism. The studies reported here show that fisetin can promote survival in both in vitro and in animal models of HD. Furthermore, our studies with both PC12 cells and a Drosophila model of HD strongly suggest that the activation of the Ras-ERK cascade by fisetin (13,14,20) plays a key role in its ability to promote cellular, neuronal and organismal survival in the face of Htt challenge. These observations are further supported by the studies in Drosophila where fisetin, as well as resveratrol, increased ERK phosphorylation. Genetic manipulation of ERK activation levels provides independent evidence validating ERK activation as therapeutic in the HD setting. These results suggest that ERK activation might be a common target of polyphenolic compounds that are neuroprotective in HD.
ERK and JNK are often described in a Ying–Yang relationship, where JNK activation promotes cell death while ERK promotes survival and many manipulations that affect one often affect the other. Thus, agents that can preferentially activate ERK could be therapeutically desirable. Our finding that fisetin enhances ERK activation while not enhancing JNK activation in PC12 cells and Drosophila is encouraging. The observations that both fisetin and resveratrol are neuroprotective in both mammalian cells (31; this study) and Drosophila (18; this study) coupled with our finding that both compounds activate ERK in Drosophila suggest that enhanced ERK phosphorylation is key to the beneficial effects of these polyphenols. This conclusion is further supported by the observation that the neuroprotective effects of the mixed lineage kinase inhibitors, CEP-11004 and CEP-1347, in models of HD were associated with ERK activation rather than with JNK inhibition (26).
The pathology of HD is complex and appears to affect multiple cellular functions including transcription, protein modification and processing, oxidative stress and mitochondrial function (reviewed in 1). Thus, it has been suggested that drug combinations may provide the best approach for treating the disease (37). We and others have shown that in addition to their ability to activate the Ras-ERK cascade, polyphenols have a number of other activities that might make them particularly useful for the treatment of HD. For example, both fisetin and resveratrol have direct antioxidant activity and can also increase the intracellular levels of glutathione, the major intracellular antioxidant (38–40). In addition, both fisetin and resveratrol can induce several transcription factors associated with the protection of nerve cells from stress including Nrf2 (14,39,41). Both these polyphenols can also increase proteasome activity (42,43) and fisetin has been found to maintain ATP levels in the presence of toxic stress (44). Thus, although much of the protection afforded by these polyphenols could be accounted for by their effects on ERK activation shown in this study, both fisetin and resveratrol appear to behave like multiple-target drugs having many of the beneficial characteristics of drug combinations that would be desirable in HD and that could be used without the need to establish the parameters of co-treatment.
Compounds that are active following oral administration are likely to be the most useful for the treatment of HD since treatment is likely to be necessary for an extended time given the slow development of the disease. Thus, we incorporated fisetin into the food for our mouse study and this approach resulted in significant benefits to the R6/2 mice. In contrast, a recent study with resveratrol which used gavage in an undefined vehicle (45) to treat the N171-82Q transgenic mouse model of HD failed to show any improvement in either motor performance or survival. These results suggest that oral administration via food as opposed to gavage may have a significant impact on the therapeutic benefits of polyphenols.
Fisetin and resveratrol are small, orally available molecules (13,46,47) that can cross the blood–brain barrier (48,49) and maintain the ERK signaling pathway in an activated state that our studies indicate will help preserve brain function in HD. These properties make this class of compounds potentially attractive therapeutic agents.
MATERIALS AND METHODS
Chemicals
Fisetin was purchased from Indofine Chemical Company. For the mouse studies, it was incorporated into the food at 0.05% by Harlan/Teklad. Periodic extraction and testing confirmed its stability under these conditions. Resveratrol was purchased from Sigma.
Cell culture
An ecdysone-inducible PC12 cell line (PC12/HttQ103) containing the huntingtin protein exon 1 fused to EGFP (HttQ103 = Httex1-103QP-EGFP) (19) was obtained from Leslie Thompson (UC Irvine). The cells were grown in DMEM/high glucose-containing 10% fetal calf serum (Hyclone), 5% horse serum (Invitrogen) and 1% penicillin/streptomycin. Httex1-103QP-EGFP expression was induced by treating with 5 μm PA (Sigma) in N2 (Invitrogen) medium for the indicated times. For cell survival studies, the cells were plated at ∼50% confluency in 35 mm dishes. After 24 h, the medium was replaced with N2 medium alone or N2 + PA. In some cases, 10 μm fisetin and/or 40 μm PD98059 (Sigma) were included. The cells were treated for 72 h and then processed for the MTT assay as described (50).
SDS–PAGE and immunoblotting
For sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), PC12/HttQ103 cells were plated at the same density as used for the cell death assays and harvested 1–2 days after induction of Httex1-103QP-EGFP. For SDS–PAGE analysis of fly heads, extracts were prepared by feeding adult flies overnight (19.5 h) on food containing 300 µM of either fisetin or resveratrol. Frozen flies were vortexed to release the heads and homogenized (10 heads/30 μl) in ice-cold lysis buffer [1% Triton X-100, 150 mm NaCl, 50 mm Tris, 1 mm EDTA, protease inhibitor mixture (Roche), pH 7.2]. The samples were separated using 10% Criterion XT Precast Bis–Tris gels (Biorad, Hercules, CA, USA). Proteins were transferred to nitrocellulose membranes and the measurement of the quality of protein, electrophoresis and transfer checked by staining with Poinceau S. Membranes were blocked with 5% skim milk in TBS-T (20 mm Tris buffer pH 7.5, 0.5 M NaCl, 0.1% Tween 20) for 2 h at room temperature and incubated overnight at 4°C in the primary antibody diluted in 5% bovine serum albumen in TBS/0.05% Tween 20. The primary antibodies used were: rabbit anti-phospho-MAPK (no. 9101, 1/5000), mouse anti-phospho-MAPK (no. 9106, 1/1000), rabbit anti-MAPK (no. 9102, 1/1000), mouse anti-phospho-JNK (no. 9255, 1/2000), rabbit anti-JNK (no. 9252, 1/1000) and HRP-anti-actin (no. 5125; 1/40,000) from Cell Signaling; mouse anti-JNK1 (no. 15701A, 1/500) from PharMingen and rabbit anti-GFP (no. sc8334, 1/1000) from Santa Cruz Biotechnology. Subsequently, blots were washed in TBS/0.05% Tween 20 and incubated for 1h at room temperature in horseradish peroxidase-goat anti-rabbit or goat anti-mouse (Biorad) diluted 1/5000 in 5% skim milk in TBS/0.1% Tween 20. After additional washing, protein bands were detected by chemiluminescence using the Super Signal West Pico Substrate (Pierce). Each PC12 western blot was repeated at least three times with independent protein samples. The fly brain western blots utilized head lysate preparations from three separate experiments and were repeated twice.
Caspase 3 activity
PC12/HttQ103 cells were plated in white 96-well plates at the same density as used for the cell death assays and either un-induced or induced with PA for 2–3 days in the absence or presence of 10 μm fisetin. The dish was removed from the incubator and an equal volume of Caspase 3/7 Glo (Promega) was added to each well. After 60 min, the luminescence was read on a luminometer (Molecular Devices) and the level of caspase activity determined relative to un-induced cells grown in the absence or presence of fisetin.
Fly culture
Flies were reared at 25°C on standard cornmeal-glucose-yeast Drosophila medium supplemented with varying concentrations of fisetin or resveratrol. Virgin female flies from the w; P{UAS-Httex1p Q93}4F1 transgenic line (21) were mated with the pan-neuronal elav driver w; {w+mW.hs = GawB}elavC155. Emerging flies were harvested <6 h post-eclosion and raised at 25°C.
For genetic manipulation of ERK or JNK levels, siblings from crosses of flies expressing Elav-driven pathogenic Htt that were either normal or heterozygous for an ERK or JNK-related gene of interest were compared for lethality and/or for survival or longevity. Genetic interaction tests were performed by crossing w; P{UAS-Httex1p Q93}4F1 virgins to males hemizygous for the elav>GAL driver and also heterozygous for a mutation of the gene of interest over a chromosome with an appropriate dominant marker (Cy or Sb for the second or third chromosome, respectively). To test the X chromosomal Dsor1 allele, elav>GAL4/Y; P{UAS-Httex1p Q93}4F1/Sb males were crossed to Dsor1[G42]/FM7a virgins. In the next generation, flies of different genotypes were counted and relative eclosion rates calculated. Fly strains carrying the mutant alleles bsk1, rolled10a, Dsor1G42, PTP-ERXE-2776 and mtsXE-2258 were obtained from the Bloomington Drosophila Stock Center and references describing each mutant can be found at flybase.org (stock numbers: bsk = #3088; rl = #742; Dsor = #7131; PTP-ER = #5765; mts = #5684; note: all second-chromosome balancers were changed to simple CyO for experimental manipulations).
Pseudopupil analysis
Flies were transferred to fresh food every day, and assayed for neurodegeneration at 7 days post-eclosion using the pseudopupil technique (21). Seven-day-old flies were decapitated and heads mounted in a drop of nail polish on a microscopic slide. The head was then covered with immersion oil and examined under Nikon EFD-3/Optiphot-2 scope with 50X oil objective. At least 200 ommatidia in 8–12 flies were examined and the number of visible rhabdomeres was counted for each.
Mouse studies
R6/2 mice with a CBA/C57Bl6 background (51) were obtained from Jackson Laboratory at 42–45 days of age. Three different cohorts of mice were used for these studies for a total of 15 R6/2 mice and an equivalent number of age- and strain-matched controls. Upon arrival, mice were housed five per cage under standard conditions with ad libitum access to food and water. The mice were started on control chow or chow containing 0.05% fisetin shortly after arrival at Salk. The mice consumed the same amount of chow in the absence or presence of fisetin. Motor performance was assessed bi-weekly shortly since arrival (42–45 days) until 91 days of age. During testing, the mice were placed on a rotorod accelerating from 0 to 50 rpm over 180 s. Each mouse had three separate trials at 180 s each and latency to fall from the apparatus was recorded. The three results were averaged and recorded. All procedures were approved by the Salk Institute Institutional Animal Care and Use Committee.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG online.
FUNDING
This work was supported in part by the National Institutes of Health [NS045283 to J.L.M. and AG025337 to P.M. and David Schubert].
ACKNOWLEDGEMENTS
This work was made possible in part through access to the National Drosophila Stock Center in Bloomington, IN, USA.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Zuccato C., Valenza M., Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol. Rev. 2010;90:905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 2.Apostol B.L., Illes K., Pallos J., Bodai L., Wu J., Strand A., Schweitzer E.S., Olson J.M., Kazantsev A., Marsh J.L., et al. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum. Mol. Genet. 2006;15:273–285. doi: 10.1093/hmg/ddi443. [DOI] [PubMed] [Google Scholar]
- 3.Colucci-D'Amato L., Perrone-Capone C., di Porzio U. Chronic activation of ERK and neurodegenerative diseases. Bioessays. 2003;25:1085–1095. doi: 10.1002/bies.10355. [DOI] [PubMed] [Google Scholar]
- 4.Cheung E.C., Salck R.S. Emerging role for ERK as a key regulator of neuronal apoptosis. SciSTKE. 2004;251:PE45–PE53. doi: 10.1126/stke.2512004pe45. [DOI] [PubMed] [Google Scholar]
- 5.Chu C.T., Levinthal D.J., Kulich S.M., Chalovich E.M., DeFranco D.B. Oxidative neuronal injury. The dark side of ERK1/2. Eur. J. Biochem. 2004;271:2060–2066. doi: 10.1111/j.1432-1033.2004.04132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma H., Cheng R., Voisine C., Hart A.C., Stockwell B.R. Inhibitors of metabolism rescue cell death in Huntington's disease models. Proc. Natl Acad. Sci. USA. 2007;104:14525–14530. doi: 10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song C., Perides G., Liu Y.F. Expression of full-length polyglutamine-expanded huntingtin disrupts growth factor receptor signaling in rat pheochromocytoma (PC12) cells. J. Biol. Chem. 2002;277:6703–6707. doi: 10.1074/jbc.M110338200. [DOI] [PubMed] [Google Scholar]
- 8.Lievens J.-C., Rival T., Iche M., Chneiweiss H., Birman S. Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum. Mol. Genet. 2005;14:713–724. doi: 10.1093/hmg/ddi067. [DOI] [PubMed] [Google Scholar]
- 9.Gines S., Bosch M., Marco S., Gavalda N., Diaz-Hernandez M., Lucas J.J., Canals J.M., Alberch J. Reduced expression of the TrkB receptor in Huntington's disease mouse models and in human brain. Eur. J. Neurosci. 2006;23:649–658. doi: 10.1111/j.1460-9568.2006.04590.x. [DOI] [PubMed] [Google Scholar]
- 10.Strand A.D., Baquet Z.C., Aragaki A.K., Holmans P., Yang L., Cleren C., Beal M.F., Jones L., Kooperberg C., Olson J.M., et al. Expression profiling of Huntington's disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J. Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharami K., Xie Y., An J.J., Tonegawa S., Xu B. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington's disease phenotypes in mice. J. Neurochem. 2008;105:369–379. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy Y.S., Gilgun-Sherki Y., Melamed E., Offen D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs. 2005;19:97–127. doi: 10.2165/00063030-200519020-00003. [DOI] [PubMed] [Google Scholar]
- 13.Maher P., Akaishi T., Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc. Natl Acad. Sci. USA. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdo J., Schubert D., Maher P. Glutathione production is regulated via distinct pathways in stressed and non-stressed cortical cultures. Brain Res. 2008;1189:12–22. doi: 10.1016/j.brainres.2007.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spires T.L., Hannan A.J. Molecular mechanisms mediating pathological plasticity in Huntington's disease and Alzheimer's disease. J. Neurochem. 2007;100:874–882. doi: 10.1111/j.1471-4159.2006.04275.x. [DOI] [PubMed] [Google Scholar]
- 16.Gil J.M., Rego A.C. Mechanisms of neurodegeneration in Huntington's disease. Eur. J. Neurosci. 2008;27:2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 17.Sweatt J.D. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Pallos J., Bodai L., Lukacsovich T., Purcell J.M., Steffan J.S., Thompson L.M., Marsh J.L. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington's disease. Hum. Mol. Genet. 2008;17:3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aiken C.T., Tobin A.J., Schweitzer E.S. A cell-based screen for drugs to treat Huntington's disease. Neurobiol. Dis. 2004;16:546–555. doi: 10.1016/j.nbd.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Sagara Y., Vahnnasy J., Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J. Neurochem. 2004;90:1144–1155. doi: 10.1111/j.1471-4159.2004.02563.x. [DOI] [PubMed] [Google Scholar]
- 21.Steffan J.S., Bodai L., Pallos J., Poelman M., McCampbell A., Apostol B.L., Kazantsev A., Schmidt E., Zhu Y.Z., Greenwald M., et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413:739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- 22.Marsh J.L., Thompson L.M. Can flies help humans treat neurodegenerative diseases. Bioessays. 2004;26:485–496. doi: 10.1002/bies.20029. [DOI] [PubMed] [Google Scholar]
- 23.Karim F.D., Rubin G.M. PTP-ER, a novel tyrosine phosphatase, functions downstream of Ras1 to downregulate MAP kinase during Drosophila eye development. Mol. Cell. 1999;3:741–750. doi: 10.1016/s1097-2765(01)80006-x. [DOI] [PubMed] [Google Scholar]
- 24.Silverstein A.M., Barrow C.A., Davis A.J., Mumby M.C. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl Acad. Sci. USA. 2002;99:4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saydoff J.A., Garcia R.A.G., Browne S.E., Liu L., Sheng J., Brenneman D., Hu Z., Cardin S., Gonzalez A., von Bostel R.W., et al. Oral uridine pro-drug PN401 is neuroprotective in the R6/2 and N171–82Q mouse models of Huntington's disease. Neurobiol. Dis. 2006;24:455–465. doi: 10.1016/j.nbd.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Apostol B.L., Simmons D.A., Zuccato C., Illes K., Pallos J., Casale M., Conforti P., Ramos C., Roarke M., Kathuria S., et al. CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice. Mol. Cell. Neurosci. 2008;39:8–20. doi: 10.1016/j.mcn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Yang L., Calingasan N.Y., Wille E.J., Cormier K., Smith K., Ferrante R.J., Beal M.F. Combination therapy with Coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson's and Huntington's diseases. J. Neurochem. 2009;109:1427–1439. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.-L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 29.Wood J.G., Rogina B., Lavu S., Howitz K.T., Helfand S.L., Tatar M., Sinclair D.A. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 30.Sinclair D.A. Sirtuins for healthy neurons. Nat. Genet. 2005;37:339–340. doi: 10.1038/ng0405-339. [DOI] [PubMed] [Google Scholar]
- 31.Parker J.A., Arango M., Abderrahmane S., Lambert E., Tourette C., Catoire H., Neri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat. Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 32.Bass T.M., Weinkove D., Houthoofd K., Gems D., Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech. Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E.A., Caldwell S.D., Napper A., Curtis R., DiStefano P.S., Fields S., et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 34.Borra M.T., Smith B.C., Denu J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 35.Beher D., Wu J., Cumine S., Kim K.W., Lu S.-C., Atangan L., Wang M. Resveratrol is not a direct activator of Sirt1 enzyme activity. Chem. Biol. Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 36.Pacholec M., Chrunyk B., Cunningham D., Flynn D., Griffith D., Griffor M., Loulakis P., Pabst B., Qiu X., Stockman B., et al. SRT1720, SRT2183, SRT1460 and resveratrol are not direct activators of Sirt1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agrawal N., Pallos J., Slepko N., Apostol B.L., Bodai L., Chang L.-W., Chiang A.-S., Thompson L.M., Marsh J.L. Identification of combinatorial drug regimens for treatment of Huntington's disease using Drosophila. Proc. Natl Acad. Sci. USA. 2005;102:3777–3781. doi: 10.1073/pnas.0500055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishige K., Schubert D., Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001;30:433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 39.Maher P. A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic. Res. 2006;40:1105–1111. doi: 10.1080/10715760600672509. [DOI] [PubMed] [Google Scholar]
- 40.de Almeida L.M., Pineiro C.C., Leite M.C., Brolese G., Tramontina F., Feoli A.M., Gottfried C., Goncalves C.A. Resveratrol increases glutamate uptake, glutathione content and S100B secretion in cortical astrocyte cultures. Cell. Mol. Neurobiol. 2007;27:661–668. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kode A., Rajendrasozhan S., Caito S., Yang S.R., Megson I.L., Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 42.Maher P. The flavonoid fisetin promotes nerve cell survival from trophic factor withdrawal by enhancement of proteasome activity. Arch. Biochem. Biophys. 2008;476:139–144. doi: 10.1016/j.abb.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 43.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J. Biol. Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 44.Maher P., Salgado K.F., Zivin J.A., Lapchak P.A. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho D.J., Calingasan N.Y., Wille E., Dumont M., Beal M.F. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp. Neurol. 2010;225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Shia C.-S., Tsai S.-Y., Kuo S.-C., Hou Y.-C., Chao P.-D.L. Metabolism and pharmacokinetics of 3,3′,4′,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone and 7-hydroxyflavone and antihemolysis effects of fisetin and its serum metabolites. J. Agric. Food Chem. 2009;57:83–89. doi: 10.1021/jf802378q. [DOI] [PubMed] [Google Scholar]
- 47.Meng X., Maliakal P., Lu H., Lee M.J., Yang C.S. Urinary and plasma levels of resveratrol and quercetin in humans, mice and rats after injection of pure compounds and grape juice. J. Agric. Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 48.Rivera F., Urbanavicius J., Gervaz E., Morquio A., Dajas F. Some aspects of the in vivo neuroprotective capacity of flavonoids: bioavailability and structure-activity relationship. Neurotox. Res. 2004;6:543–553. doi: 10.1007/BF03033450. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q., Xu J., Rottinghaus G.E., Simonyi A., Lubahn S., Sun G.Y., Sun A.Y. Resveratrol protects against global cerebral ischemia injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 50.Maher P., Hanneken A. Flavonoids protect retinal ganglion cells from oxidative stress-induced death. Invest. Ophthalmol. Vis. Sci. 2005;46:4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- 51.Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S.W., et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.