Abstract
FUS, EWS, and TAF15 form the FET family of RNA-binding proteins whose genes are found rearranged with various transcription factor genes predominantly in sarcomas and in rare hematopoietic and epithelial cancers. The resulting fusion gene products have attracted considerable interest as diagnostic and promising therapeutic targets. So far, oncogenic FET fusion proteins have been regarded as strong transcription factors that aberrantly activate or repress target genes of their DNA-binding fusion partners. However, the role of the transactivating domain in the context of the normal FET proteins is poorly defined, and, therefore, our knowledge on how FET aberrations impact on tumor biology is incomplete. Since we believe that a full understanding of aberrant FET protein function can only arise from looking at both sides of the coin, the good and the evil, this paper summarizes evidence for the central function of FET proteins in bridging RNA transcription, processing, transport, and DNA repair.
1. Introduction
The Strange Case of Dr. Jekyll and Mr. Hyde, a novel by the Scottish poet Robert Luis Stevenson (1850–1894), screened multiple times worldwide, describes the struggle between the good and evil sides of one individual [1]. At daylight, Dr. Jekyll is an honorable member of the society, but when the light fades he turns into an evil beast. The coexistence of two faces of one individual has inspired more than poetry and psychology. The question of which circumstances favor the surfacing of one or the other and how it may be influenced is relevant to all areas of life, including economy, technology and medicine. Cancer unravels the “Hyde” side of genes and their biology, but we can learn about how to tame fierce Mr. Hyde by understanding the Dr. Jekyll behind, the normal function of cancer genes.
FET (FUS, EWS, TAF15) proteins are a ubiquitously expressed family of similarly structured proteins predominantly localizing to the nuclear [2]. FET genes have attracted broad attention since all known members are found involved in deleterious genomic rearrangements with transcription factor genes in a variety of human sarcomas and acute leukemias. Chimeric FET proteins are considered and mostly studied as aberrant transcription factors. This paper aims at summarizing the good sides of FET proteins and looking at the characteristics of aberrant FET proteins as Dr. Jekyll's second face which surfaces only upon gene rearrangement or mutation.
2. Dr. Jekyll
2.1. The FET Family of Proteins
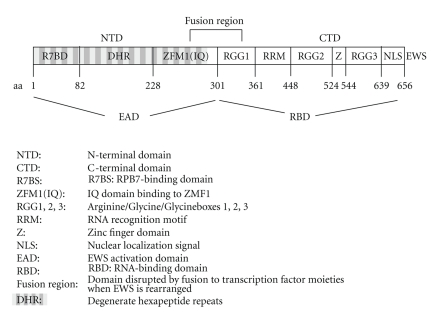
The prototype FET protein EWS was identified in 1992 as the gene product encoded by the Ewing's sarcoma breakpoint region 1 (EWSR1) on chromosome 22q12 constituting the first identified member of a family of putative RNA-binding proteins [3], including also FUS/TLS/Pigpen/hnRNP P2 [4–7], TAF15/hTAFII68/TAF2N/RPB56 [8, 9], and Drosophila Cabeza/SARFH [10, 11] that share distinct structural characteristics (Figure 1). This protein family is frequently referred to as the FET (previously TET) (FUS/TLS, EWS, TAF15) family of proteins. Our restricted knowledge about the molecular functions of FET proteins derives mainly from protein interaction studies which identified more than 30 associated proteins mostly as part of protein/RNA complexes [12] (Table 1). Of note, pull-down experiments using EWS as bait revealed that all three FET proteins interact with each other and are therefore likely to be part of the very same protein complexes. As demonstrated for EWS, the association with most interacting proteins depends on the presence of RNA and is destroyed upon RNaseA treatment (Table 1). The functional roles of interacting proteins suggest a general bridging role for FET proteins coupling RNA transcription, processing, transport, and DNA repair.
Figure 1.
Structure of the prototype FET protein EWS.
Table 1.
EWS interacting proteins: *not bound by methylated EWS; **not bound by methylated EWS upon RNaseA treatment.
| RNase A sensitive | |
|---|---|
| hnRNP A0 [12] | Pre-mRNA processing, RNA metabolism, RNA transport |
| hnRNP A1 [12] | Pre-mRNA processing, RNA metabolism, and RNA transport may modulate splice site selection |
| hnRNP A2B1 [12] | Pre-mRNA processing, RNA metabolism, RNA transport |
| hnRNP A3 [12] | Regulation of age-related gene expression, binds to telomeric RNA |
| hnRNP A/B [12] | Binds to multiprotein editosome complex |
| hnRNP A18* [12] | Stabilization of transcripts, genotoxic stress response, translational activator, binds to 3′UTR |
| hnRNP D0 [12] | Regulation of mRNA stability |
| hnRNP F [12] | Binds G-rich sequences |
| hnRNP G [12] | Regulation of splice site selection, DNA double-strand break repair |
| hnRNP H [12] | Pre-mRNA alternative splicing regulation |
| hnRNP H2 [12] | Involved in Fabray disease and X-linked agammaglobulinemia |
| hnRNP H3 [12] | Early heat shock-induced splicing arrest |
| hnRNP Q [12] | RNA stability, translationally coupled mRNA turnover |
| Small nuclear ribonucleoprotein Sm D3 [12] | Pre-mRNA splicing and small nuclear ribonucleoprotein biogenesis, histone 3′-end processing |
| U1 small nuclear ribonucleoprotein A* [12] | First snRNP to interact with pre-mRNA for the subsequent binding of U2 snRNP and the U4/U6/U5 tri-snRNP |
| Splicing factor, arginine/serine-rich 1* [12, 44] | Accuracy of splicing and regulation of alternative splicing |
| Splicing factor, arginine/serine-rich 3* [12] | Putative proliferation-/maturation-associated RNA processing |
| Splicing factor, arginine/serine-rich 9* [12] | Constitutive splicing |
| RRM containing coactivator activator [12] | Activation/modulation of nuclear receptors |
| Tubulin alpha ubiquitous chain [12] | Scaffold for cell shape and organelle movement |
| Vimentin1 | Organizer of a number of critical proteins involved in attachment, migration, and cell signaling |
|
| |
| RNase insensitive | |
|
| |
| Protein arginine N methyltransferase 1 [12] | Epigenetic regulation, signal transduction, DNA repair |
| Protein arginine N-methyltransferase 8 [63] | Localized at cell membrane |
| hnRNP M [12] | Splicing, selective recycling of immature GlcNAc-bearing, thyroglobulin molecules, potentially involved in signalling |
| hnRNP U [12, 43] | Binds double- and single-stranded RNA and DNA, binds pre-mRNA |
| FUS** [12] | This review |
| TAF15* [12] | This review |
| EWS* [64] | This review |
| RNA-dependent helicase p68 (DDX5) [12] | RNA-dependent ATPase, alteration of RNA secondary structure in splicing and translation initiation |
| RNA-dependent helicase p72 (DDX17) [12] | RNA-dependent ATPase, alteration of RNA secondary structure in splicing, and translation initiation |
| ATP-dependent RNA helicase A [12] | ATP-dependent unwinding of double-stranded RNA and DNA-RNA complexes, transcriptional regulation |
| ATP-dependent RNA helicase DHX36* [12] | Deadenylation and decay of mRNAs with 3′-UTR AU-rich elements |
| Elongation factor EF1 gamma [12] | Translation elongation, role in anchoring the translational complex to other cellular components |
| Elongation factor EF1 alpha [12] | Translation elongation, promotes aminoacyl-tRNA binding to ribosome |
| Dead box protein 3 X (DDX3X)* [12] | ATP-dependent RNA helicase |
| Tubuline beta-2 chain [12] | Scaffold for cell shape and organelle movement |
|
| |
| RNA dependence unknown: | |
|
| |
| RBP3 [29] | RNA Polymerase II component |
| TAF5 [29] | General transcription factor TFIID component |
| TAF7 [29] | General transcription factor TFIID component |
| TAF11 [29] | General transcription factor TFIID component |
| TAF13 [29] | General transcription factor TFIID component |
| Brn-3a [35] | Transcription factor |
| SF1 [47] | Splicing factor |
| YB1 [42] | Splicing factor |
| Survival motor neuron protein* [65] | Essential role in spliceosomal snRNP assembly in the cytoplasm and is required for pre-mRNA splicing in the nucleus |
| Serine threonine kinase receptor (STRAP) [33] | Inhibits transforming growth factor beta (TGF-beta) signaling |
| BARD1 [53] | DNA repair, mRNA maturation |
| Pyk2 [66] | Tyrosine kinase, signal transduction |
2.2. RNA Binding of FET Proteins
Several functional FET domains were defined (see Figure 1): the N-terminal domain is largely composed of a highly repetitive primary sequence containing multiple copies of a degenerate hexapeptide repeat motif similar to the C-terminal domain of RNA polymerase II. The C-terminal domain (CTD) contains a conserved nuclear import and retention signal (C-NLS) [13], a putative zinc-finger domain, and a conserved RNA recognition motif (RRM) flanked by 3 arginine-glycine-glycine (RGG) boxes [14] compatible with RNA binding of FET proteins. FUS has been demonstrated to bind preferentially to GGUG-containing RNAs [15]. EWS might have similar sequence specificity since it was demonstrated to bind strongly to both poly G and poly U, but not to poly A and poly C RNA, homopolymers [16]. Although it is only the zinc finger domain of FUS that makes physical contact with the GGUG motif, all three RGG boxes together with the RRM contribute to this activity [15]. Intriguingly, a recent study identified strong binding of FUS to human telomeric RNA [17] and to small low-copy-number RNAs tethered to the promoter of cyclin D1 [18]. Nothing is known about the RNA binding specificity TAF15.
2.3. A Role for FET Proteins in RNA Transcription
The FET N-terminal domain (NTD) resembles the activation domain of certain transcription factors such as SP-1 rich in glutamine and proline residues. When fused to a DNA-binding domain (DBD), as is the case in oncogenic FET derivatives, the NTD strongly activates reporter gene activity in a DNA-binding-dependent way [19–23]. The critical determinants for this transactivation activity are dispersed throughout the NTD [24], which is intrinsically disordered [25]. It is comprised of a variable number of a degenerate hexapeptide repeat motif (DHR) with the consensus SYGQQS, with homologies to the C-terminus of RNA polymerase II [3]. Mutation analysis of the EWS NTD revealed a critical dependence of the transactivation activity on the aromatic side chain of the conserved tyrosine residue present in the DHR [21].
The function of the NTD in the context of germline FET proteins remains largely unexplored. When included into artificial FET-DBD fusion proteins, the CTD inhibited transcriptional activation by the NTD [26]. More recent data demonstrated that the RGG motifs of the FET-CTD repress a range of transcriptional activation domains [27]. The context-dependent difference in the transactivation potential of the NTD might be explained by different structures and accessibility of the NTD for protein interactions in the presence and absence of the CTD [28]. Protein interaction between the very N-terminus of EWS and the RNA PolII holoenzyme component hsRPB7 was only observed for EWS-FLI1 and C-terminal truncated EWS, while interaction with hsRPB5 and hsRPB3 was restricted to germline EWS [29, 30]. EWS has been reported to support CREB-binding-protein-(CBP/p300-) dependent activation by the transcription factors HNF-4 and OCT-4 [31, 32] which is inhibited by the EWS-interacting protein STRAP (serine-threonine kinase receptor-associated protein) [33]. Similarly, FUS acts as a positive cofactor for NFkappaB-mediated transcription [34]. In contrast, EWS repressed BRN3A-dependent transcription [35].
All three FET proteins were found to associate with RNA polymerase II and subpopulations of the TFIID complex, respectively[8, 29, 36]. Consistent with an evolutionary conserved role of FET proteins in RNA transcription, SARFH was found to be associated with transcribed chromatin in Drosophila [10]. Interactions of the NTD with various transcription factors were described (FUS with steroid, thyroid hormone, and retinoid receptors [37], EWS with Brn3A and via CBP/p300 with HNF4 and OCT4 [31, 32, 35, 38]). Interestingly, EWS and FUS were found to bind directly to the proximal elements of the macrophage-specific promoter of the CSF-1 receptor (CSF1R) gene and also to high-affinity sites recognized by myeloid zinc finger protein 1 (Mzf1) suggesting a role in transcriptional start site selection of TATA-less promoters [39].
Besides their role in RNA-polymerase-II-mediated transcription, the recent finding of FUS repressing RNA polymerase III-dependent transcription of small untranslated RNAs implies a more general role for FET proteins in the orchestration of the transcriptome [40].
2.4. A Role for FET Proteins in mRNA Maturation
The RNA-binding specificity of FUS for the GGUG motif found in 5′splice sites suggests a role in RNA processing. EWS and FUS were identified within the same RNA-splicing complex together with polypyrimidine-tract-binding-protein-associated factor (PSF) [41]. In addition, EWS and FUS associate with a variety of splicing factors such as U1C, SR, SF1, and YB1 [15, 42–47]. Further, EWS NTD and FUS bind to novel RNA helicases [48, 49]. Moreover, interaction of the EWS NTD with BARD1, a protein playing an important role in the inhibition of RNA maturation at sites of stalled transcription upon DNA damage, was reported [31, 50–53]. Together, these results suggest that FET proteins couple RNA transcription to processing. The mechanism and specificity of this activity remain largely unknown.
2.5. A Role for FET Proteins in the Processing of Small Noncoding RNAs
EWS was recently identified in a protein complex with the nuclear RNase III DROSHA [54]. While DROSHA is known to be central to the cleavage of the pre-micro-RNA (miRNA) precursor from the primary miRNA transcript thereby initiating miRNA processing and transport to the cytoplasm, evidence for a functional role of the EWS containing DROSHA complex is missing. Therefore, a general role for EWS in the metabolism of noncoding RNAs remains to be demonstrated. Since about a quarter of miRNA genes are encoded in the introns of protein-coding genes [55], it is intriguing to speculate that EWS links not only transcription to RNA splicing but also to the generation of miRNAs from gene introns. This, so far hypothetical, activity may gain importance in the light of frequent negative posttranscriptional regulation of miRNA processing at the DROSHA level in cancer [56].
2.6. A Role for FET Proteins in RNA Transport
Consistent with their proposed function in gene regulation, FET proteins are mostly nuclear, localizing to inclusions such as the coiled body and the nucleolus (demonstrated for EWS, FUS, and pigpen in [9, 57, 58]). There is also evidence that FET proteins shuttle between the nucleus and the cytoplasm raising the possibility that they play a role in RNA transport [59, 60]. In mouse hippocampal neurons, FUS is localized to neuronal dendrites and, upon activation, translocate to the spines, where local translation takes place, carrying along specific mRNA transcripts [61, 62]. This finding implicates FET proteins in localizing cytoplasmic determinants for the local control of protein synthesis and secretion, at least in neurons.
For EWS, RNA binding, subcellular localization, and consequently transcriptional activity have been found to be regulated by extensive asymmetric dimethylation of the RGG motifs, mediated by protein arginine methyltransferases 1 and 8 (PRMT1, PRMT8) [67–70], which likely impacts on self-association of intact EWS required for nuclear localization [71, 72]. Extensively methylated EWS has even been identified on the cell surface [73]. So far, the functional relevance of these findings has yet to be determined.
2.7. A Role for FET Proteins in Genome Surveillance and DNA Repair
FUS deficiency in mice resulted in defective B-lymphocyte development and activation, high levels of chromosomal instability, and perinatal death [74]. EWS knock-out mice also displayed disrupted B-cell development and were extremely sensitive to ionizing radiation. Together with a defect in homologous recombination impairing meiosis and the observation of premature senescence of embryonic fibroblasts, these results suggest a role for EWS in recombination repair [75]. In the zebrafish, silencing of EWS genes during embryogenesis led to mitotic defects followed by p53-dependent apoptosis [76].
Consistent with the phenotype of FET deficiency in genetically modified mice, the interaction of EWS (and EWS-FLI1) with the BRCA1-associated ring finger domain protein BARD1 may point to a role of FET proteins in DNA double-strand break repair [53]. This hypothesis is strengthened by high genomic instability in FUS knock-out mice [74] and radiation sensitivity and impaired homologous recombination in EWS knockouts [75]. The recently discovered homologous DNA-strand-pairing activity of all four FET proteins may functionally contribute to this role [77].
Intriguingly, the RNA binding activity of FUS was reported to act as a sensor for DNA damage and to elicit transcriptional repression; as exemplified for cyclin D (CCND1) promoter regulation, DNA damage was demonstrated to induce the expression of single-stranded, low-copy-number ncRNA transcripts tethered to the 5′ regulatory regions of CCND1 which recruit FUS and allosterically modify it to bind to and repress CREB-binding protein (CBP) and p300 histone acetyltransferase activities [18].
Activation of gene transcription by many, if not all, sequence-specific transcription factors requires DNA-topoisomerase-II-beta-dependent, transient, site-specific dsDNA break formation [78]. One may speculate that the proposed role of FET proteins in recombination repair is linked to their association with transcription initiation complexes at promoter regions.
3. Mr. Hyde
3.1. The Role of FUS in Neurodegenerative Disease
Point mutations of FUS have recently been found in a subset of patients with familial amyotrophic lateral sclerosis (ALS), a neurodegenerative disorder destroying motoneurons [79, 80]. Previously, this disease has been associated with mutations in either superoxide dismutase 1 (SOD1) or TDP43 (43 kDa TAR DNA-binding domain protein). TDP43 is an essential nuclear RNA-binding protein that participates in transcriptional repression, exon splicing inhibition, and mRNA stabilization. The convergent phenotypes associated with FUS and TDP43 mutations suggest that they are part of the same machinery. In fact, TDP-43 and FUS were demonstrated to function in a biochemical complex to modulate expression of HDAC6, a recently identified mRNA substrate of TDP-43 [81].
3.2. The Oncogenic Function of FET Fusion Protein
The predominant type of FET gene aberrations is that of fusions to various transcription factor genes by which the FET RNA-binding domain is replaced by the DNA-binding domain of the transcription factor (Table 2). FET fusion proteins are capable of transforming cells in culture dependent on the cellular context. EWS-ETS fusions, for example, transform NIH3T3 and bone-marrow-derived mesenchymal progenitor cells, but not human or rat primary fibroblasts, mouse embryonic stem cells, or embryonic fibroblasts [102, 103]. The phenotype of tumors obtained in immunodeficient mice after transplantation of EWS-ETS-transformed NIH3T3 cells clearly differs from that obtained after transformation with other EWS-transcription factor fusions and resembles that of Ewing's sarcoma [104, 105]. In the xenograft model, the amino terminal portion of EWS, as well as FUS (and presumably also TAF15), is functionally interchangeable in the fusion protein, while the transcription factor moiety determines the tumor phenotype [7]. Functional interchangeability of the FET-NTD is also reflected in human sarcomas: both EWS-CHOP and FUS-CHOP characterize myxoid liposarcoma [5, 94], and EWS-NR4A3 and TAF15-NR4A3 are found in extraskeletal myxoid chondrosarcoma [106]. It was therefore hypothesized that FET fusion proteins affect differentiation programs by aberrant regulation of genes specifically recognized by the transcription factor DNA-binding moiety.
Table 2.
FET gene fusions in cancer. TF: transcription factor.
| Phenotype | FET partner | TF partner | TF type | Ref. |
|---|---|---|---|---|
| ESFT | ||||
| (85%) | EWS | FLI1 | ETS | [3] |
| (10%) | EWS | ERG | ETS | [82, 83] |
| (1%) | EWS | ETV1 | ETS | [84] |
| (1%) | EWS | ETV4 | ETS | [85, 86] |
| (1%) | EWS | FEV | ETS | [87] |
| (1%) | FUS | FEV | ETS | [25] |
| (1%) | FUS | ERG | ETS | [88] |
| ESFT-like | EWS | NFATC2 | rel related | [89] |
| Askin-like, CD99 neg. | EWS | ZNF278 | zinc finger | [90] |
| Bone sarcoma | EWS | POU5F1 | pou | [91] |
| Mucoepidermoid carcinaoma | EWS | POU5F1 | pou | [92] |
| Hidradenoma | EWS | POU5F1 | pou | [92] |
| EWS | PBX1 | homeobox | [92] | |
| Low-grade fibromyxoid sarcoma | FUS | CREB3L1 | Leucine zipper | [93] |
| Myxoid liposarcoma | EWS | DDIT3 | bZIP | [94] |
| FUS | DDIT3 | bZIP | [5] | |
| Clear cell sarcoma | EWS | ATF1 | bZIP | [95] |
| EWS | CREB1 | bZIP | [96] | |
| Desmoplastic SRCT | EWS | WT1 | zinc finger | [97] |
| Extraskeletal myxoid chondrosarcoma | EWS | NR4A3 | nuclear receptor | [98] |
| TAF15 | NR4A3 | nuclear receptor | [99] | |
| AML | FUS | ERG | ETS | [100] |
| cALL, AUL | EWS | ZNF384 | zinc finger | [101] |
| AML, ALL | TAF15 | TAF15 | zinc finger | [101] |
The best studied example in this respect is EWS-FLI1 in Ewing's sarcoma family tumors (ESFT). Using experimental knockdown of EWS-FLI1 in ESFT cell lines and comparison to primary tumours and normal tissues, signatures of the chimeric transcription factor on the ESFT transcriptome were defined [125, 130, 131]. An almost equal number of genes were found activated and repressed by EWS-FLI1. Of the approximately 600 to 800 significantly dysregulated genes, only a fraction is directly bound by EWS-FLI1 and many EWS-FLI1 bound genes do not show aberrant regulation (our unpublished observations). Over the years a number of directly EWS-FLI1-regulated genes have been characterized in ESFT (Table 3). It is interesting to note that almost all attempts to experimentally restore the presumed “normal” expression pattern of these targets in ESFT cell lines (by ectopic reexpression of EWS-FLI1 repressed genes and knockdown of EWS-FLI1-activated genes) resulted in reduced tumor cell growth in vitro and/or reduced tumorigenicity in vivo and in several cases enhanced chemosensitivity (Table 3). These results suggest that directly EWS-FLI1-regulated genes play essential roles in the establishment and/or the maintenance of the malignant phenotype of ESFT.
Table 3.
Validated direct EWS-FLI1 target genes.
| EWS-FLI1 activated genes | Consequences of target suppression |
|---|---|
| Id2 [107] | Not known |
| GLI1 [108] | Reduced anchorage independent growth [109] |
| VEGF [110] | Decreased osteolysis [111] |
| STYXL1 [112] | Not known |
| PLD2 [113] | Inhibition of PDGF BB signalling |
| PTPL1 [114] | Reduced growth and increased chemosensitivity [114] |
| CAV1 [115] | Reduced anchorage independent growth, reduced tumorigenicity |
| GSTM4 [116] | Abrogation of oncogenic transformation, increased chemosensitivity [116] |
| NR0B1 [117, 118] | Abrogation of oncogenic transformation [119] |
| EZH2 [120] | Reduced anchorage independent growth, reduced tumorigenicity [120] |
| AURKA, AURKB [121] | Not known |
| Tenascin C [122] | Not known |
|
| |
| EWS-FLI1 repressed genes | Consequences of target restoration |
|
| |
| TGFBR2 [123] | Loss of tumorigenicity [123] |
| CDKN1A [124] | Inhibition of cell growth [124] |
| IGFBP3 [125] | Inhibition of cell growth and motility [126] |
| FOXO1 [127] | Not known |
| DKK1 [128, 129] | Decreased tumorigenicity [128] |
Functional annotation of EWS-FLI1-regulated genes revealed that activated genes primarily annotate to proliferation-associated functions, while genes involved in developmental and differentiation processes are predominantly repressed [131], suggesting that EWS-FLI1 suppresses differentiation of the enigmatic ESFT precursor cell. In fact, sustained silencing of EWS-FLI1 restores the potential of ESFT cells to differentiate along adipogenic, neuronal, and osteogenic lineages [132], a feature shared with mesenchymal stem cells (MSC). Conversely, ectopic EWS-FLI1 expression blocks the differentiation potential of MSC and imposes an ESFT-like phenotype on them [103, 133, 134]. Consistent with the role of EWS-FLI1 in the disruption of developmental differentiation processes is the finding of skeletal malformations in mice expressing transgenic EWS-FLI1 in the mesenchymal lineage [135]. Similarly, the FUS-ERG fusion found in human myeloid leukemia with the t(16;21) translocation was demonstrated to block terminal differentiation of and confer a growth advantage to human myeloid progenitor cells [136].
Consistent with early in vitro data [19–21], activated genes showed an enrichment of ETS-binding motifs in their promoters while this motif was underrepresented in repressed genes [131]. This result suggests that gene repression regulating differentiation genes might be mediated by indirect mechanisms. One such mechanism involved in blocking osteogenic differentiation is interaction and interference of EWS-FLI1 with the master regulator of bone and cartilage development, RUNX2 [137]. RUNX2 was demonstrated to bind also to intact EWS and FUS [138]. A number of different transcription factor binding motifs overrepresented in the promoters of EWS-FLI1-repressed genes may be indicative of other protein interactions that remain to be defined. Additional mechanisms of gene repression downstream of EWS-FLI1 involve the activity of transcriptional repressors whose expression is upregulated by EWS-FLI1 such as NKX2.2 [139] or the epigenetic modifier EZH2 [120] and the regulation of microRNAs [140]. An alternative intriguing mechanism may involve the binding of EWS-FLI1 to microsatellites outside of promoter regions even at distances of several megabases from the transcriptional start sites [141–143]. While these elements can activate transcription when juxtaposed to a promoter, their activity and mechanism of action from distant sites remains elusive.
Interestingly, there is evidence that EWS and EWS-FLI1 form a fatal liaison in that genes targeted by the FLI1 DNA-binding domain encode for proteins that interact with the EWS N-terminal domain in both the intact EWS protein and the chimeric protein. This is the case for NR0B1, a protein known to form large complexes with the stem cell factors OCT3 and OCT4, as well as EWS [32, 144, 145]. Intriguingly, the translocation t(6;22)(p21;q12) found in some undifferentiated sarcomas and neoplasms of skin and salivary glands directly fuses OCT4 to EWSR1. Among EWS-FLI1-repressed genes is also hsa-mir-145, a microRNA targeting OCT4 and other stem cell factors and feeding back on EWS-FLI1 expression [133]. These findings provide evidence that EWS and EWS-FLI1 form a functional network in the regulation of tumor cell stemness.
3.3. A Transcription-Independent Role for the EWS-FLI1 Fusion Protein
The first indication that malignant transformation by FET fusion proteins may involve functions other than direct transcriptional activation of target genes recognized by the DBD came from functional dissection of the EWS-FLI1 fusion protein in NIH3T3 transformation assays. These studies suggested that the minimal transforming and the minimal transcriptional activation domains can be separated from each other [146]. Specifically, the 83 N-terminal amino acids were sufficient to transform NIH3T3 cells when fused to the FLI1 DBD. Protein interactions with this domain were found to be context dependent [28–30]. In addition, residual transforming activity of EWS-FLI1 was retained even when the FLI1-DBD was destroyed, suggesting a DNA-binding-independent function for the oncogenic fusion protein [147, 148]. Also, EWS-FLI1 was shown to inhibit the CBP-dependent transcriptional activity of the retinoid acid (RA) receptor RXR desensitizing cells to the differentiation and apoptosis inducing activity of RA by a mechanism unrelated to DNA binding [38].
Protein interaction studies revealed that the EWS-NTD and the FUS-NTD in the context of their oncogenic fusion proteins communicate with the same RNA processing factors as in germline EWS [42–44, 46, 47, 53] but interfere with serine arginine protein (SR) and YB1-mediated splicing [42, 44, 45]. In addition, it was demonstrated that EWS-FLI1, but not EWS, interfered with heterogeneous nuclear ribonucleoprotein A1-dependent 5′ splice site selection in an in vivo E1A splicing assay [149]. This result might possibly be explained by a dominant negative effect of EWS-FLI1 on the RNA processing function of EWS that remains to be investigated. In fact, we have previously demonstrated that EWS-FLI1 can interact with its germline counterpart [64]. Importantly, mutational analysis of EWS-FLI1 revealed that the ability to affect pre-mRNA splicing coincided with transforming activity [149]. These results suggest a role for EWS-FLI1 in RNA processing. However, this role may not be regarded as transcription independent. A recent study of transcriptional elongation of the direct EWS-FLI1 target gene cyclin D1 (CCND1) revealed that both EWS and EWS-FLI1 stimulate transcription of the gene, but elongation by EWS-FLI1 is significantly slowed down in comparison to EWS. As a result, expression of the oncogenic splice isoform D1b is favoured over the splice isoform D1a [150]. So far it remains unknown how many genes may be affected by this or a similar phenomenon.
3.4. EWS-FLI1 and Disrupted Tumor Suppression
FET fusion proteins are aberrantly expressed transcription factors driving cell proliferation. As such they impose oncogenic stress on the cell triggering the p53 checkpoint [151]. ESFT escape the oncogenic stress imposed by EWS-FLI1 by modulating p53 activity. Two mechanisms for this oncogenic property of EWS-FLI1 have recently been described: interference with tumor suppressive NOTCH signalling pathway activity through transcriptional regulation of autocrine NOTCH ligand expression [152] and direct interaction with p53 [153]. It should be noted, however, that the ability of EWS-FLI1 to modulate p53 activity is tissue dependent. In fibroblasts, EWS-FLI1 was demonstrated to elicit a p53-mediated cell-cycle arrest [151]. Most other cell types do not tolerate EWS-FLI1 expression at all and die in response to ectopic expression of the chimeric oncogene (for review [102]). The only tissue permissive to the oncogenic properties of EWS-FLI1 identified so far is mesenchymal stem cells [103]. The tissue-specific factors that steer the p53 response into the one (growth arrest/apoptosis) or the other (escape from oncogenic stress) direction remain to be elucidated.
There is also evidence for EWS-FLI1 interfering with the other central tumor suppressor pathway in oncogenesis: although the mechanism still remains to be defined, knockdown of EWS-FLI1 in ESFT cells leads to pRB-1 hypophosphorylation [154].
4. Getting Hold of Mr. Hyde
The development of small molecule inhibitors of biological macromolecules, originally in the context of chromosome translocations, has been pioneered by research on receptor tyrosine kinases. Here, the design of smart molecules is guided primarily by crystallography and structure/function analyses of the target proteins. For FET fusion proteins, this approach is not feasible because of the intrinsic disorder of their structure. However, recent landmark studies provided proof of principle for successful interference with protein interactions of intrinsically disordered proteins [155]. Guided by a peptide aptamer screen, a small molecule mimetic was described that competes with RNA helicase A for interaction with the EWS N-terminus in the context of the EWS-FLI1 fusion protein and slowed tumor formation in mice [156]. There is evidence from protein interaction studies that the faces of the EWS N-terminus look different in the context of the wildtype protein (Dr. Jekyll) and the transcription factor fusion protein (Mr. Hyde) [28–30]. Thus, there is hope that the evil culprit for the development and progression of several sarcomas and leukemias that is still hiding in the dark can be successfully targeted in the near future.
References
- 1.Stevenson RL. The Strange Case of Dr. Jekyll and Mr. Hyde. Penguin Books; 1886. [Google Scholar]
- 2.Andersson MK, Ståhlberg A, Arvidsson Y, et al. The multifunctional FUS, EWS and TAF15 proto-oncoproteins show cell type-specific expression patterns and involvement in cell spreading and stress response. BMC Cell Biology. 2008;9 doi: 10.1186/1471-2121-9-37. Article ID 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delattre O, Zucman J, Plougastel B, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359(6391):162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 4.Alliegro MC, Alliegro MA. A nuclear protein regulated during the transition from active to quiescent phenotype in cultured endothelial cells. Developmental Biology. 1996;174(2):288–297. doi: 10.1006/dbio.1996.0074. [DOI] [PubMed] [Google Scholar]
- 5.Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363(6430):640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 6.Rabbitts TH, Forster A, Larson R, Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nature Genetics. 1993;4(2):175–180. doi: 10.1038/ng0693-175. [DOI] [PubMed] [Google Scholar]
- 7.Zinszner H, Albalat R, Ron D. A novel effector domain from the RNA-binding protein TLS or EWS is required for oncogenic transformation by CHOP. Genes and Development. 1994;8(21):2513–2526. doi: 10.1101/gad.8.21.2513. [DOI] [PubMed] [Google Scholar]
- 8.Bertolotti A, Lutz Y, Heard DJ, Chambon P, Tora L. hTAF(II)68, a novel RNA/ssDNA-binding protein with homology to the pro-oncoproteins TLS/FUS and EWS is associated with both TFIID and RNA polymerase II. EMBO Journal. 1996;15(18):5022–5031. [PMC free article] [PubMed] [Google Scholar]
- 9.Hackl W, Lührmann R. Molecular cloning and subcellular localisation of the snRNP-associated protein 69KD, a structural homologue of the proto-oncoproteins TLS and EWS with RNA and RNA-binding properties. Journal of Molecular Biology. 1996;264(5):843–851. doi: 10.1006/jmbi.1996.0681. [DOI] [PubMed] [Google Scholar]
- 10.Immanuel D, Zinszner H, Ron D. Association of SARFH (sarcoma-associated RNA-binding fly homolog) with regions of chromatin transcribed by RNA polymerase II. Molecular and Cellular Biology. 1995;15(8):4562–4571. doi: 10.1128/mcb.15.8.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolow DT, Haynes SR. Cabeza, a Drosophila gene encoding a novel RNA binding protein, shares homology with EWS and TLS, two genes involved in human sarcoma formation. Nucleic Acids Research. 1995;23(5):835–843. doi: 10.1093/nar/23.5.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pahlich S, Quero L, Roschitzki B, Leemann-Zakaryan RP, Gehring H. Analysis of Ewing Sarcoma (EWS)-binding proteins: interaction with hnRNP M, U, and RNA-helicases p68/72 within protein-RNA complexes. Journal of Proteome Research. 2009;8(10):4455–4465. doi: 10.1021/pr900235t. [DOI] [PubMed] [Google Scholar]
- 13.Zakaryan RP, Gehring H. Identification and characterization of the nuclear localization/retention signal in the EWS proto-oncoprotein. Journal of Molecular Biology. 2006;363(1):27–38. doi: 10.1016/j.jmb.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Burd CG, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- 15.Lerga A, Hallier M, Delva L, et al. Identification of an RNA binding specificity for the potential splicing factor TLS. Journal of Biological Chemistry. 2001;276(9):6807–6816. doi: 10.1074/jbc.M008304200. [DOI] [PubMed] [Google Scholar]
- 16.Ohno T, Ouchida M, Lee L, Gatalica Z, Rao VN, Reddy ESP. The EWS gene, involved in Ewing family of tumors, malignant melanoma of soft parts and desmoplastic small round cell tumors, codes for an RNA binding protein with novel regulatory domains. Oncogene. 1994;9(10):3087–3097. [PubMed] [Google Scholar]
- 17.Takahama K, Kino K, Arai S, et al. Identification of RNA binding specificity for the TET-family proteins. Nucleic Acids Symposium Series. 2008;52(1):213–214. doi: 10.1093/nass/nrn108. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Arai S, Song X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454(7200):126–130. doi: 10.1038/nature06992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailly RA, Bosselut R, Zucman J, et al. DNA-binding and transcriptional activation properties of the EWS-FLI-1 fusion protein resulting from the t(11;22) translocation in Ewing sarcoma. Molecular and Cellular Biology. 1994;14(5):3230–3241. doi: 10.1128/mcb.14.5.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertolotti A, Bell B, Tora L. The N-terminal domain of human TAF(II)68 displays transactivation and oncogenic properties. Oncogene. 1999;18(56):8000–8010. doi: 10.1038/sj.onc.1203207. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Lee KAW. A repetitive element containing a critical tyrosine residue is required for transcriptional activation by the EWS/ATF1 oncogene. Oncogene. 2001;20(31):4161–4168. doi: 10.1038/sj.onc.1204522. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Lee HJ, Jun HJ, Kim J. The hTAF68-TEC fusion protein functions as a strong transcriptional activator. International Journal of Cancer. 2008;122(11):2446–2453. doi: 10.1002/ijc.23379. [DOI] [PubMed] [Google Scholar]
- 23.Prasad DDK, Ouchida M, Lee L, Rao VN, Reddy ESP. TLS/FUS fusion domain of TLS/FUS-erg chimeric protein resulting from the t(16;21) chromosomal translocation in human myeloid leukemia functions as a transcriptional activation domain. Oncogene. 1994;9(12):3717–3729. [PubMed] [Google Scholar]
- 24.Pan S, Ming KY, Dunn TA, Li KKC, Lee KAW. The EWS/ATF1 fusion protein contains a dispersed activation domain that functions directly. Oncogene. 1998;16(12):1625–1631. doi: 10.1038/sj.onc.1201671. [DOI] [PubMed] [Google Scholar]
- 25.Ng TL, O’Sullivan MJ, Pallen CJ, et al. Ewing sarcoma with novel translocation t(2;16) producing an in-flame fusion of FUS and FEV. Journal of Molecular Diagnostics. 2007;9(4):459–463. doi: 10.2353/jmoldx.2007.070009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li KKC, Lee KAW. Transcriptional activation by the Ewing’s sarcoma (EWS) oncogene can be cis-repressed by the EWS RNA-binding domain. Journal of Biological Chemistry. 2000;275(30):23053–23058. doi: 10.1074/jbc.M002961200. [DOI] [PubMed] [Google Scholar]
- 27.Alex D, Lee KAW. RGG-boxes of the EWS oncoprotein repress a range of transcriptional activation domains. Nucleic Acids Research. 2005;33(4):1323–1331. doi: 10.1093/nar/gki270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aryee DNT, Kreppel M, Bachmaier R, et al. Single-chain antibodies to the EWS NH terminus structurally discriminate between intact and chimeric EWS in Ewing’s sarcoma and interfere with the transcriptional activity of EWS in vivo. Cancer Research. 2006;66(20):9862–9869. doi: 10.1158/0008-5472.CAN-05-4042. [DOI] [PubMed] [Google Scholar]
- 29.Bertolotti A, Melot T, Acker J, Vigneron M, Delattre O, Tora L. EWS, but not EWS-FLI-1, is associated with both TFIID and RNA polymerase II: interactions between two members of the tet family, EWS and HTAF(II)68, and subunits of TFIID and RNA polymerase II complexes. Molecular and Cellular Biology. 1998;18(3):1489–1497. doi: 10.1128/mcb.18.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petermann R, Mossier BM, Aryee DNT, Khazak V, Golemis EA, Kovar H. Oncogenic EWS-Fli1 interacts with hsRPB7, a subunit of human RNA polymerase II. Oncogene. 1998;17(5):603–610. doi: 10.1038/sj.onc.1201964. [DOI] [PubMed] [Google Scholar]
- 31.Araya N, Hirota K, Shimamoto Y, et al. Cooperative interaction of EWS with CREB-binding protein selectively activates hepatocyte nuclear factor 4-mediated transcription. Journal of Biological Chemistry. 2003;278(7):5427–5432. doi: 10.1074/jbc.M210234200. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Rhee BK, Bae GY, Han YM, Kim J. Stimulation of Oct-4 activity by Ewing’s sarcoma protein. Stem Cells. 2005;23(6):738–751. doi: 10.1634/stemcells.2004-0375. [DOI] [PubMed] [Google Scholar]
- 33.Anumanthan G, Halder SK, Friedman DB, Datta PK. Oncogenic serine-threonine kinase receptor-associated protein modulates the function of ewing sarcoma protein through a novel mechanism. Cancer Research. 2006;66(22):10824–10832. doi: 10.1158/0008-5472.CAN-06-1599. [DOI] [PubMed] [Google Scholar]
- 34.Uranishi H, Tetsuka T, Yamashita M, et al. Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-κB p65-mediated transcription as a coactivator. Journal of Biological Chemistry. 2001;276(16):13395–13401. doi: 10.1074/jbc.M011176200. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GR, Latchman DS. The pro-oncoprotein EWS (Ewing’s Sarcoma protein) interacts with the Brn-3a POU transcription factor and inhibits its ability to activate transcription. Cancer Biology & Therapy. 2002;1(4):428–432. doi: 10.4161/cbt.1.4.23. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann A, Roeder RG. Cloning and characterization of human TAF20/15. Multiple interactions suggest a central role in TFIID complex formation. Journal of Biological Chemistry. 1996;271(30):18194–18202. doi: 10.1074/jbc.271.30.18194. [DOI] [PubMed] [Google Scholar]
- 37.Powers CA, Mathur M, Raaka BM, Ron D, Samuels HH. TLS (translocated-in-liposarcoma) is a high-affinity interactor for steroid, thyroid hormone, and retinoid receptors. Molecular Endocrinology. 1998;12(1):4–18. doi: 10.1210/mend.12.1.0043. [DOI] [PubMed] [Google Scholar]
- 38.Ramakrishnan R, Fujimura Y, Zou JP, et al. Role of protein-protein interactions in the antiapoptotic function of EWS-Fli-1. Oncogene. 2004;23(42):7087–7094. doi: 10.1038/sj.onc.1207927. [DOI] [PubMed] [Google Scholar]
- 39.Hume DA, Sasmono T, Himes SR, et al. The ewing sarcoma protein (EWS) binds directly to the proximal elements of the macrophage-specific promoter of the CSF-1 receptor (csf1r) gene. Journal of Immunology. 2008;180(10):6733–6742. doi: 10.4049/jimmunol.180.10.6733. [DOI] [PubMed] [Google Scholar]
- 40.Tan AY, Manley JL. TLS inhibits RNA polymerase III transcription. Molecular and Cellular Biology. 2010;30(1):186–196. doi: 10.1128/MCB.00884-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deloulme JC, Prichard L, Delattre O, Storm DR. The prooncoprotein EWS binds calmodulin and is phosphorylated by protein kinase C through an IQ domain. Journal of Biological Chemistry. 1997;272(43):27369–27377. doi: 10.1074/jbc.272.43.27369. [DOI] [PubMed] [Google Scholar]
- 42.Chansky HA, Hu M, Hickstein DD, Yang L. Oncogenic TLS/ERG and EWS/Fli-1 fusion proteins inhibit RNA splicing mediated by YB-1 protein. Cancer Research. 2001;61(9):3586–3590. [PubMed] [Google Scholar]
- 43.Knoop LL, Baker SJ. The splicing factor U1C represses EWS/FLI-mediated transactivation. Journal of Biological Chemistry. 2000;275(32):24865–24871. doi: 10.1074/jbc.M001661200. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Chansky HA, Hickstein DD. EWS·Fli-1 fusion protein interacts with hyperphosphorylated RNA polymerase II and interferes with serine-arginine protein-mediated RNA splicing. Journal of Biological Chemistry. 2000;275(48):37612–37618. doi: 10.1074/jbc.M005739200. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Embree LJ, Hickstein DD. TLS-ERG leukemia fusion protein inhibits RNA splicing mediated by serine-arginine proteins. Molecular and Cellular Biology. 2000;20(10):3345–3354. doi: 10.1128/mcb.20.10.3345-3354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Embree LJ, Tsai S, Hickstein DD. Oncoprotein TLS interacts with serine-arginine proteins involved in RNA splicing. Journal of Biological Chemistry. 1998;273(43):27761–27764. doi: 10.1074/jbc.273.43.27761. [DOI] [PubMed] [Google Scholar]
- 47.Zhang DI, Paley AJ, Childs G. The transcriptional repressor, ZFM1 interacts with and modulates the ability of EWS to activate transcription. Journal of Biological Chemistry. 1998;273(29):18086–18091. doi: 10.1074/jbc.273.29.18086. [DOI] [PubMed] [Google Scholar]
- 48.Sugiura T, Sakurai K, Nagano Y. Intracellular characterization of DDX39, a novel growth-associated RNA helicase. Experimental Cell Research. 2007;313(4):782–790. doi: 10.1016/j.yexcr.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Toretsky JA, Erkizan V, Levenson A, et al. Oncoprotein EWS-FLI1 activity is enhanced by RNA helicase A. Cancer Research. 2006;66(11):5574–5581. doi: 10.1158/0008-5472.CAN-05-3293. [DOI] [PubMed] [Google Scholar]
- 50.Kim HS, Li H, Cevher M, et al. DNA damage-induced BARD1 phosphorylation is critical for the inhibition of messenger RNA processing by BRCA1/BARD1 complex. Cancer Research. 2006;66(9):4561–4565. doi: 10.1158/0008-5472.CAN-05-3629. [DOI] [PubMed] [Google Scholar]
- 51.Kleiman FE, Manley JL. Functional interaction of BRCA1-associated BARD1 with polyadenylation factor CstF-50. Science. 1999;285(5433):1576–1579. doi: 10.1126/science.285.5433.1576. [DOI] [PubMed] [Google Scholar]
- 52.Kleiman FE, Manley JL. The BARD1-CstF-50 interaction links mRNA 3′ end formation to DNA damage and tumor suppression. Cell. 2001;104(5):743–753. doi: 10.1016/s0092-8674(01)00270-7. [DOI] [PubMed] [Google Scholar]
- 53.Spahn L, Petermann R, Siligan C, Schmid JA, Aryee DNT, Kovar H. Interaction of the EWS NH terminus with BARD1 links the Ewing’s sarcoma gene to a common tumor suppressor pathway. Cancer Research. 2002;62(16):4583–4587. [PubMed] [Google Scholar]
- 54.Gregory RI, Yan KP, Amuthan G, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 55.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294(5543):853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 56.Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes and Development. 2006;20(16):2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alliegro MC, Alliegro MA. Identification of a new coiled body component. Experimental Cell Research. 1997;231(2):386–390. [PubMed] [Google Scholar]
- 58.Zinszner H, Immanuel D, Yin Y, Liang FX, Ron D. A topogenic role for the oncogenic N-terminus of TLS: nucleolar localization when transcription is inhibited. Oncogene. 1997;14(4):451–461. doi: 10.1038/sj.onc.1200854. [DOI] [PubMed] [Google Scholar]
- 59.Calvio C, Neubauer G, Mann M, Lamond AI. Identification of hnRNP P2 as TLS/FUS using electrospray mass spectrometry. RNA. 1995;1(7):724–733. [PMC free article] [PubMed] [Google Scholar]
- 60.Zinszner H, Sok J, Immanuel D, Yin Y, Ron D. TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. Journal of Cell Science. 1997;110(15):1741–1750. doi: 10.1242/jcs.110.15.1741. [DOI] [PubMed] [Google Scholar]
- 61.Fujii R, Okabe S, Urushido T, et al. The RNA binding protein TLS is translocated to dendritic spines by mGluR5 activation and regulates spine morphology. Current Biology. 2005;15(6):587–593. doi: 10.1016/j.cub.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 62.Fujii R, Takumi T. TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines. Journal of Cell Science. 2005;118(24):5755–5765. doi: 10.1242/jcs.02692. [DOI] [PubMed] [Google Scholar]
- 63.Pahlich S, Zakaryan RP, Gehring H. Identification of proteins interacting with protein arginine methyltransferase 8: the Ewing sarcoma (EWS) protein binds independent of its methylation state. Proteins. 2008;72(4):1125–1137. doi: 10.1002/prot.22004. [DOI] [PubMed] [Google Scholar]
- 64.Spahn L, Siligan C, Bachmaier R, Schmid JA, Aryee DNT, Kovar H. Homotypic and heterotypic interactions of EWS, FLI1 and their oncogenic fusion protein. Oncogene. 2003;22(44):6819–6829. doi: 10.1038/sj.onc.1206810. [DOI] [PubMed] [Google Scholar]
- 65.Young PJ, Francis JW, Lince D, Coon K, Androphy EJ, Lorson CL. The Ewing’s sarcoma protein interacts with the Tudor domain of the survival motor neuron protein. Molecular Brain Research. 2003;119(1):37–49. doi: 10.1016/j.molbrainres.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Felsch JS, Lane WS, Peralta EG. Tyrosine kinase Pyk2 mediates G-protein-coupled receptor regulation of the Ewing sarcoma RNA-binding protein EWS. Current Biology. 1999;9(9):485–488. doi: 10.1016/s0960-9822(99)80214-0. [DOI] [PubMed] [Google Scholar]
- 67.Araya N, Hiraga H, Kako K, Arao Y, Kato S, Fukamizu A. Transcriptional down-regulation through nuclear exclusion of EWS methylated by PRMT1. Biochemical and Biophysical Research Communications. 2005;329(2):653–660. doi: 10.1016/j.bbrc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 68.Belyanskaya LL, Delattre O, Gehring H. Expression and subcellular localization of Ewing sarcoma (EWS) protein is affected by the methylation process. Experimental Cell Research. 2003;288(2):374–381. doi: 10.1016/s0014-4827(03)00221-0. [DOI] [PubMed] [Google Scholar]
- 69.Pahlich S, Bschir K, Chiavi C, Belyanskaya L, Gehring H. Different methylation characteristics of protein arginine methyltransferase 1 and 3 toward the Ewing Sarcoma protein and a peptide. Proteins. 2005;61(1):164–175. doi: 10.1002/prot.20579. [DOI] [PubMed] [Google Scholar]
- 70.Kim JD, Kako K, Kakiuchi M, Park GG, Fukamizu A. EWS is a substrate of type I protein arginine methyltransferase, PRMT8. International Journal of Molecular Medicine. 2008;22(3):309–315. [PubMed] [Google Scholar]
- 71.Shaw DJ, Morse R, Todd AG, Eggleton P, Lorson CL, Young PJ. Identification of a self-association domain in the Ewing’s sarcoma protein: a novel function for arginine-glycine-glycine rich motifs? Journal of Biochemistry. 2010;147(6):885–893. doi: 10.1093/jb/mvq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw DJ, Morse R, Todd AG, Eggleton P, Lorson CL, Young PJ. Identification of a tripartite import signal in the Ewing Sarcoma protein (EWS) Biochemical and Biophysical Research Communications. 2009;390(4):1197–1201. doi: 10.1016/j.bbrc.2009.10.120. [DOI] [PubMed] [Google Scholar]
- 73.Belyanskaya LL, Gehrig PM, Gehring H. Exposure on cell surface and extensive arginine methylation of EWS protein. Journal of Biological Chemistry. 2001;276(22):18681–18687. doi: 10.1074/jbc.M011446200. [DOI] [PubMed] [Google Scholar]
- 74.Hicks GG, Singh N, Nashabi A, et al. Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nature Genetics. 2000;24(2):175–179. doi: 10.1038/72842. [DOI] [PubMed] [Google Scholar]
- 75.Li H, Watford W, Li C, et al. Ewing sarcoma gene EWS is essential for meiosis and B lymphocyte development. Journal of Clinical Investigation. 2007;117(5):1314–1323. doi: 10.1172/JCI31222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azuma M, Embree LJ, Sabaawy H, Hickstein DD. Ewing sarcoma protein Ewsr1 maintains mitotic integrity and proneural cell survival in the zebrafish embryo. PLoS One. 2007;2(10) doi: 10.1371/journal.pone.0000979. Article ID e979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guipaud O, Guillonneau F, Labas V, et al. An in vitro enzymatic assay coupled to proteomics analysis reveals a new DNA processing activity for Ewing sarcoma and TAF(II)68 proteins. Proteomics. 2006;6(22):5962–5972. doi: 10.1002/pmic.200600259. [DOI] [PubMed] [Google Scholar]
- 78.Ju BG, Lunyak VV, Perissi V, et al. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science. 2006;312(5781):1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 79.Kwiatkowski TJ, Bosco DA, LeClerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 80.Vance C, Rogelj B, Hortobágyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SH, Shanware N, Bowler MJ, et al. ALS-associated proteins TDP-43 and FUS/TLS function in a common biochemical complex to coregulate HDAC6 mRNA. The Journal of Biological Chemistry. 2010;285(44):34097–34105. doi: 10.1074/jbc.M110.154831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sorensen PHB, Lessnick SL, Lopez-Terrada D, Liu XF, Triche TJ, Denny CT. A second Ewing’s sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nature Genetics. 1994;6(2):146–151. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- 83.Zucman J, Melot T, Desmaze C, et al. Combinatorial generation of variable fusion proteins in the Ewing family of tumours. EMBO Journal. 1993;12(12):4481–4487. doi: 10.1002/j.1460-2075.1993.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeon IS, Davis JN, Braun BS, et al. A variant Ewing’s sarcoma translocation (7;22) fuses the EWS gene to the ETS gene ETV1. Oncogene. 1995;10(6):1229–1234. [PubMed] [Google Scholar]
- 85.Kaneko Y, Yoshida K, Handa M, et al. Fusion of an ETS-family gene, EIAF, to EWS by t(17;22)(q12;q12) chromosome translocation in an undifferentiated sarcoma of infancy. Genes Chromosomes and Cancer. 1996;15(2):115–121. doi: 10.1002/(SICI)1098-2264(199602)15:2<115::AID-GCC6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 86.Urano F, Umezawa A, Hong W, Kikuchi H, Hata JI. A novel chimera gene between EWS and E1A-F, encoding the adenovirus E1A enhancer-binding protein, in extraosseous Ewing’s sarcoma. Biochemical and Biophysical Research Communications. 1996;219(2):608–612. doi: 10.1006/bbrc.1996.0281. [DOI] [PubMed] [Google Scholar]
- 87.Peter M, Couturier J, Pacquement H, et al. A new member of the ETS family fused to EWS in Ewing tumors. Oncogene. 1997;14(10):1159–1164. doi: 10.1038/sj.onc.1200933. [DOI] [PubMed] [Google Scholar]
- 88.Shing DC, McMullan DJ, Roberts P, et al. FUS/ERG gene fusions in Ewing’s tumors. Cancer Research. 2003;63(15):4568–4576. [PubMed] [Google Scholar]
- 89.Szuhai K, Ijszenga M, De Jong D, Karseladze A, Tanke HJ, Hogendoorn PCW. The NFATc2 Gene is involved in a novel cloned translocation in a ewing sarcoma variant that couples its function in immunology to oncology. Clinical Cancer Research. 2009;15(7):2259–2268. doi: 10.1158/1078-0432.CCR-08-2184. [DOI] [PubMed] [Google Scholar]
- 90.Mastrangelo T, Modena P, Tornielli S, et al. A novel zinc finger gene is fused to EWS in small round cell tumor. Oncogene. 2000;19(33):3799–3804. doi: 10.1038/sj.onc.1203762. [DOI] [PubMed] [Google Scholar]
- 91.Yamaguchi S, Yamazaki Y, Ishikawa Y, Kawaguchi N, Mukai H. EWSR1 is fused to POU5F1 in a bone tumor with translocation t(6;22) (p21;q12) Genes Chromosomes and Cancer. 2005;43(2):217–222. doi: 10.1002/gcc.20171. [DOI] [PubMed] [Google Scholar]
- 92.Möller E, Stenman G, Mandahl N, et al. POU5FI, encoding a key regulator of stem cell pluripotency, is fused to EWSRI in hidradenoma of the skin and mucoepidermoid carcinoma of the salivary glands. Journal of Pathology. 2008;215(1):78–86. doi: 10.1002/path.2327. [DOI] [PubMed] [Google Scholar]
- 93.Mertens F, Fletcher CDM, Antonescu CR, et al. Clinicopathologic and molecular genetic characterization of low-grade fibromyxoid sarcoma, and cloning of a novel FUS/CREB3L1 fusion gene. Laboratory Investigation. 2005;85(3):408–415. doi: 10.1038/labinvest.3700230. [DOI] [PubMed] [Google Scholar]
- 94.Panagopoulos I, Höglund M, Mertens F, Mandahl N, Mitelman F, Åman P. Fusion of the EWS and CHOP genes in myxoid liposarcoma. Oncogene. 1996;12(3):489–494. [PubMed] [Google Scholar]
- 95.Zucman J, Delattre O, Desmaze C, et al. EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nature Genetics. 1993;4(4):341–345. doi: 10.1038/ng0893-341. [DOI] [PubMed] [Google Scholar]
- 96.Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma—association with gastrointestinal location and absence of melanocytic differentiation. Clinical Cancer Research. 2006;12(18):5356–5362. doi: 10.1158/1078-0432.CCR-05-2811. [DOI] [PubMed] [Google Scholar]
- 97.Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Research. 1994;54(11):2837–2840. [PubMed] [Google Scholar]
- 98.Labelle Y, Zucman J, Stenman G, et al. Oncogenic conversion of a novel orphan nuclear receptor by chromosome translocation. Human Molecular Genetics. 1995;4(12):2219–2226. doi: 10.1093/hmg/4.12.2219. [DOI] [PubMed] [Google Scholar]
- 99.Sjögren H, Meis-Kindblom J, Kindblom LG, Åman P, Stenman G. Fusion of the EWS-related gene TAF2N to TEC in extraskeletal myxoid chondrosarcoma. Cancer Research. 1999;59(20):5064–5067. [PubMed] [Google Scholar]
- 100.Panagopoulos I, Aman P, Fioretos T, et al. Fusion of the FUS gene with ERG in acute myeloid leukemia with t(16;21)(p11;q22) Genes Chromosomes and Cancer. 1994;11(4):256–262. doi: 10.1002/gcc.2870110408. [DOI] [PubMed] [Google Scholar]
- 101.Martini A, La Starza R, Janssen H, et al. Recurrent rearrangement of the Ewing’s sarcoma gene, EWSR1, or its homologue, TAF15, with the transcription factor CIZ/NMP4 in acute leukemia. Cancer Research. 2002;62(19):5408–5412. [PubMed] [Google Scholar]
- 102.Kovar H. Context matters: the hen or egg problem in Ewing’s sarcoma. Seminars in Cancer Biology. 2005;15(3):189–196. doi: 10.1016/j.semcancer.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 103.Riggi N, Cironi L, Provero P, et al. Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Research. 2005;65(24):11459–11468. doi: 10.1158/0008-5472.CAN-05-1696. [DOI] [PubMed] [Google Scholar]
- 104.Teitell MA, Thompson AD, Sorensen PHB, Shimada H, Triche TJ, Denny CT. EWS/ETS fusion genes induce epithelial and neuroectodermal differentiation in NIH 3T3 fibroblasts. Laboratory Investigation. 1999;79(12):1535–1543. [PubMed] [Google Scholar]
- 105.Thompson AD, Teitell MA, Arvand A, Denny CT. Divergent Ewing’s sarcoma EWS/ETS fusions confer a common tumorigenic phenotype on NIH3T3 cells. Oncogene. 1999;18(40):5506–5513. doi: 10.1038/sj.onc.1202928. [DOI] [PubMed] [Google Scholar]
- 106.Sjögren H, Meis-Kindblom J, Kindblom LG, Åman P, Stenman G. Fusion of the EWS-related gene TAF2N to TEC in extraskeletal myxoid chondrosarcoma. Cancer Research. 1999;59(20):5064–5067. [PubMed] [Google Scholar]
- 107.Fukuma M, Okita H, Hata JI, Umezawa A. Upregulation of Id2, an oncogenic helix-loop-helix protein, is mediated by the chimeric EWS/ets protein in Ewing sarcoma. Oncogene. 2003;22(1):1–9. doi: 10.1038/sj.onc.1206055. [DOI] [PubMed] [Google Scholar]
- 108.Beauchamp E, Bulut G, Abaan O, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. Journal of Biological Chemistry. 2009;284(14):9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joo J, Christensen L, Warner K, et al. GLI1 is a central mediator of EWS/FLI1 signaling in Ewing tumors. PLoS One. 2009;4(10, article e7608) doi: 10.1371/journal.pone.0007608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing's sarcoma. Clinical Cancer Research. 2004;10(4):1344–1353. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 111.Guan H, Zhou Z, Cao Y, Duan X, Kleinerman ES. VEGF promotes the osteolytic bone destruction of ewing’s sarcoma tumors by upregulating RANKL. Oncology Research. 2009;18(2-3):117–125. doi: 10.3727/096504009789954627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siligan C, Ban J, Bachmaier R, et al. EWS-FLI1 target genes recovered from Ewing’s sarcoma chromatin. Oncogene. 2005;24(15):2512–2524. doi: 10.1038/sj.onc.1208455. [DOI] [PubMed] [Google Scholar]
- 113.Kikuchi R, Murakami M, Sobue S, et al. Ewing’s sarcoma fusion protein, EWS/Fli-1 and Fli-1 protein induce PLD2 but not PLD1 gene expression by binding to an ETS domain of 5′ promoter. Oncogene. 2007;26(12):1802–1810. doi: 10.1038/sj.onc.1209973. [DOI] [PubMed] [Google Scholar]
- 114.Abaan OD, Levenson A, Khan O, Furth PA, Üren A, Toretsky JA. PTPL1 is a direct transcriptional target of EWS-FLI1 and modulates Ewing’s Sarcoma tumorigenesis. Oncogene. 2005;24(16):2715–2722. doi: 10.1038/sj.onc.1208247. [DOI] [PubMed] [Google Scholar]
- 115.Tirado OM, Mateo-Lozano S, Villar J, et al. Caveolin-1 (CAV1) is a target of EWS/FLI-1 and a key determinant of the oncogenic phenotype and tumorigenicity of Ewing’s sarcoma cells. Cancer Research. 2006;66(20):9937–9947. doi: 10.1158/0008-5472.CAN-06-0927. [DOI] [PubMed] [Google Scholar]
- 116.Luo W, Gangwal K, Sankar S, Boucher KM, Thomas D, Lessnick SL. GSTM4 is a microsatellite-containing EWS/FLI target involved in Ewing’s sarcoma oncogenesis and therapeutic resistance. Oncogene. 2009;28(46):4126–4132. doi: 10.1038/onc.2009.262. [DOI] [PubMed] [Google Scholar]
- 117.Kinsey M, Smith R, Lessnick SL. NR0B1 is required for the oncogenic phenotype mediated by EWS/FLI in Ewing’s sarcoma. Molecular Cancer Research. 2006;4(11):851–859. doi: 10.1158/1541-7786.MCR-06-0090. [DOI] [PubMed] [Google Scholar]
- 118.Mendiola M, Carrillo J, García E, et al. The orphan nuclear receptor DAX1 is up-regulated by the EWS/FLI1 oncoprotein and is highly expressed in Ewing tumors. International Journal of Cancer. 2006;118(6):1381–1389. doi: 10.1002/ijc.21578. [DOI] [PubMed] [Google Scholar]
- 119.Kinsey M, Smith R, Iyer AK, McCabe ERB, Lessnick SL. EWS/FLI and its downstream target NR0B1 interact directly to modulate transcription and oncogenesis in Ewing’s sarcoma. Cancer Research. 2009;69(23):9047–9055. doi: 10.1158/0008-5472.CAN-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Richter GHS, Plehm S, Fasan A, et al. EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(13):5324–5329. doi: 10.1073/pnas.0810759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wakahara K, Ohno T, Kimura M, et al. EWS-Fli1 up-regulates expression of the aurora A and aurora B kinases. Molecular Cancer Research. 2008;6(12):1937–1945. doi: 10.1158/1541-7786.MCR-08-0054. [DOI] [PubMed] [Google Scholar]
- 122.Watanabe G, Nishimori H, Irifune H, et al. Induction of tenascin-C by tumor-specific EWS-ETS fusion genes. Genes Chromosomes and Cancer. 2003;36(3):224–232. doi: 10.1002/gcc.10153. [DOI] [PubMed] [Google Scholar]
- 123.Hahm KB, Cho K, Lee C, et al. Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nature Genetics. 1999;23(2):222–227. doi: 10.1038/13854. [DOI] [PubMed] [Google Scholar]
- 124.Nakatani F, Tanaka K, Sakimura R, et al. Identification of p21 as a direct target of EWS-Fli1 oncogenic fusion protein. Journal of Biological Chemistry. 2003;278(17):15105–15115. doi: 10.1074/jbc.M211470200. [DOI] [PubMed] [Google Scholar]
- 125.Prieur A, Tirode F, Cohen P, Delattre O. EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Molecular and Cellular Biology. 2004;24(16):7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benini S, Zuntini M, Manara MC, et al. Insulin-like growth factor binding protein 3 as an anticancer molecule in Ewing’s sarcoma. International Journal of Cancer. 2006;119(5):1039–1046. doi: 10.1002/ijc.21929. [DOI] [PubMed] [Google Scholar]
- 127.Yang L, Hu HM, Zielinska-Kwiatkowska A, Chansky HA. FOXO1 is a direct target of EWS-Fli1 oncogenic fusion protein in Ewing's sarcoma cells. Biochemical and Biophysical Research Communications. 2010;402(1):129–134. doi: 10.1016/j.bbrc.2010.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miyagawa Y, Okita H, Itagaki M, et al. EWS/ETS regulates the expression of the Dickkopf family in Ewing family tumor cells. PLoS One. 2009;4(2):p. e4634. doi: 10.1371/journal.pone.0004634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Navarro D, Agra N, Pestaña Á, Alonso J, González-Sancho JM. The EWS/FLI1 oncogenic protein inhibits expression of the Wnt inhibitor DICKKOPF-1 gene and antagonizes β-catenin/TCF-mediated transcription. Carcinogenesis. 2009;31(3):394–401. doi: 10.1093/carcin/bgp317. [DOI] [PubMed] [Google Scholar]
- 130.Hancock JD, Lessnick SL. A transcriptional profiling meta-analysis reveals a core EWS-FLI gene expression signature. Cell Cycle. 2008;7(2):250–256. doi: 10.4161/cc.7.2.5229. [DOI] [PubMed] [Google Scholar]
- 131.Kauer M, Ban J, Kofler R, et al. A molecular function map of Ewing’s sarcoma. PLoS One. 2009;4(4) doi: 10.1371/journal.pone.0005415. Article ID e5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tirode F, Laud-Duval K, Prieur A, Delorme B, Charbord P, Delattre O. Mesenchymal stem cell features of Ewing tumors. Cancer Cell. 2007;11(5):421–429. doi: 10.1016/j.ccr.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 133.Riggi N, Suvà ML, De Vito C, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes and Development. 2010;24(9):916–932. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riggi N, Suvà ML, Suvà D, et al. EWS-FLI-1 expression triggers a ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Cancer Research. 2008;68(7):2176–2185. doi: 10.1158/0008-5472.CAN-07-1761. [DOI] [PubMed] [Google Scholar]
- 135.Lin PP, Pandey MK, Jin F, et al. EWS-FLI1 induces developmental abnormalities and accelerates sarcoma formation in a transgenic mouse model. Cancer Research. 2008;68(21):8968–8975. doi: 10.1158/0008-5472.CAN-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pan J, Zou J, Wu DY, et al. TLS-ERG leukemia fusion protein deregulates cyclin-dependent kinase 1 and blocks terminal differentiation of myeloid progenitor cells. Molecular Cancer Research. 2008;6(5):862–872. doi: 10.1158/1541-7786.MCR-07-2070. [DOI] [PubMed] [Google Scholar]
- 137.Li X, McGee-Lawrence ME, Decker M, et al. The Ewing's sarcoma fusion protein, EWS-FLI, binds Runx2 and blocks osteoblast differentiation. Journal of Cellular Biochemistry. 2010;111(4):933–943. doi: 10.1002/jcb.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X, Decker M, Westendorf JJ. TEThered to Runx: novel binding partners for runx factors. Blood Cells, Molecules, and Diseases. 2010;45(1):82–85. doi: 10.1016/j.bcmd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Owen LA, Kowalewski AA, Lessnick SL. EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0001965. Article ID e1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kovar H. Downstream EWS/FLI1—upstream Ewing's sarcoma. Genome Medicine. 2010;2(1, article 8) doi: 10.1186/gm129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gangwal K, Lessnicke SL. Microsatellites are EWS/FLI response elements: genomic "junk" is EWS/FLI’s treasure. Cell Cycle. 2008;7(20):3127–3132. doi: 10.4161/cc.7.20.6892. [DOI] [PubMed] [Google Scholar]
- 142.Gangwal K, Sankar S, Hollenhorst PC, et al. Microsatellites as EWS/FLI response elements in Ewing’s sarcoma. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(29):10149–10154. doi: 10.1073/pnas.0801073105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guillon N, Tirode F, Boeva V, Zynovyev A, Barillot E, Delattre O. The oncogenic EWS-FLI1 protein binds in vivo GGAA microsatellite sequences with potential transcriptional activation function. PLoS One. 2009;4(3) doi: 10.1371/journal.pone.0004932. Article ID e4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun C, Nakatake Y, Akagi T, et al. Dax1 binds to Oct3/4 and inhibits its transcriptional activity in embryonic stem cells. Molecular and Cellular Biology. 2009;29(16):4574–4583. doi: 10.1128/MCB.01863-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang J, Rao S, Chu J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444(7117):364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 146.Lessnick SL, Braun BS, Denny CT, May WA. Multiple domains mediate transformation by the Ewing’s sarcoma EWS/FLI-1 fusion gene. Oncogene. 1995;10(3):423–431. [PubMed] [Google Scholar]
- 147.Jaishankar S, Zhang J, Roussel MF, Baker SJ. Transforming activity of EWS/FLI is not strictly dependent upon DNA-binding activity. Oncogene. 1999;18(40):5592–5597. doi: 10.1038/sj.onc.1202940. [DOI] [PubMed] [Google Scholar]
- 148.Welford SM, Hebert SP, Deneen B, Arvand A, Denny CT. DNA binding domain-independent pathways are involved in EWS/FLI1-mediated oncogenesis. Journal of Biological Chemistry. 2001;276(45):41977–41984. doi: 10.1074/jbc.M106757200. [DOI] [PubMed] [Google Scholar]
- 149.Knoop LL, Baker SJ. EWS/FLI alters 5′-splice site selection. Journal of Biological Chemistry. 2001;276(25):22317–22322. doi: 10.1074/jbc.M008950200. [DOI] [PubMed] [Google Scholar]
- 150.Sanchez G, Bittencourt D, Laud K, et al. Alteration of cyclin D1 transcript elongation by a mutated transcription factor up-regulates the oncogenic D1b splice isoform in cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(16):6004–6009. doi: 10.1073/pnas.0710748105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ban J, Bennani-Baiti IM, Kauer M, et al. EWS-FLI1 suppresses NOTCH-activated p53 in Ewing’s sarcoma. Cancer Research. 2008;68(17):7100–7109. doi: 10.1158/0008-5472.CAN-07-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li Y, Tanaka K, Fan X, et al. Inhibition of the transcriptional function of p53 by EWS-Fli1 chimeric protein in Ewing family tumors. Cancer Letters. 2010;294(1):57–65. doi: 10.1016/j.canlet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 153.Li Y, Tanaka K, Fan X, et al. Inhibition of the transcriptional function of p53 by EWS-Fli1 chimeric protein in Ewing family tumors. Cancer Letters. 2010;294(1):57–65. doi: 10.1016/j.canlet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 154.Hu H-M, Zielinska-Kwiatkowska A, Munro K, et al. EWS/FLI1 suppresses retinoblastoma protein function and senescence in Ewing's sarcoma cells. Journal of Orthopaedic Research. 2008;26(6):886–893. doi: 10.1002/jor.20597. [DOI] [PubMed] [Google Scholar]
- 155.Metallo SJ. Intrinsically disordered proteins are potential drug targets. Current Opinion in Chemical Biology. 2010;14(4):481–488. doi: 10.1016/j.cbpa.2010.06.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Erkizan HV, Kong Y, Merchant M, et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nature Medicine. 2009;15(7):750–756. doi: 10.1038/nm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]