Abstract
This study examined the dose-related efficacy of disulfiram for treating cocaine dependence in methadone-stabilized cocaine dependent participants.
Design
One hundred sixty-one cocaine-and opioid-dependent volunteers were entered into a 14-week, double blind, randomized, placebo-controlled clinical trial at two sites.
Methods
Participants were stabilized on methadone during weeks 1–2 and received disulfiram at 0, 62.5, 125 or 250 mg/day during weeks 3–14. All participants also received weekly cognitive behavioral therapy. Thrice-weekly urine samples and weekly self-reported drug use assessments were obtained.
Results
Baseline subject characteristics, retention and drug use did not differ across groups. Outcome analyses were performed on those who participated beyond week 2. Opioid positive urine samples and self-reported opioid use did not differ by treatment group. The prevalence of alcohol use was low prior to and during the trial and did not differ by treatment group. Cocaine-positive urines increased over time in the 62.5 and 125 mg disulfiram groups and decreased over time in the 250 mg disulfiram and placebo groups (p<0.0001). Self-reported cocaine use increased in the 125 mg disulfiram group relative to the other three treatment groups (p=0.04).
Conclusions
Disulfiram may be contraindicated for cocaine dependence at doses less than 250 mg/day. Whether disulfiram at higher doses is efficacious in reducing cocaine use in dually cocaine and opioid dependent individuals needs to be determined.
Keywords: cocaine dependence, opioid dependence, disulfiram, clinical trial
1. Introduction
Cocaine dependence is a major public health problem, with more than 1.8 million US persons aged 12 or older (SAMSHA, 2007) and about 4 million Europeans aged 15–64 yrs (EMDCDDA, 2009) reporting cocaine use during the past year. Comorbid cocaine dependence is also a major problem among methadone maintenance programs, where rates of cocaine use may exceed 50% (Black et al., 1987; Condelli et al., 1991; Dobler-Mikola et al., 2005; Meandzija et al., 1994). Cocaine use during methadone maintenance has been associated with several medical (Bovasso and Cacciola, 2003; Bux et al., 1995; MacGowan et al., 1997; Meandzija et al., 1994; Wolf et al., 2004), psychiatric (Bovasso et al., 2003; Compton et al., 1995; Disney et al., 2005; El-Bassel et al., 2004; Grella et al., 1995; Hartell et al., 1995; King et al., 2001; Kosten et al., 1988; Ledgerwood et al., 2002; Magura et al., 1998; Magura et al., 2002) and legal (Bovasso and Cacciola, 2003; Grella et al., 1995; Hunt et al., 1986) problems, as well as greater attrition (Magura et al., 1998) and poorer treatment outcomes (Williamson et al, 2006).
Dopamine is avidly transported by both dopamine and norepinephrine transporters and cocaine blocks the activity of these transporters (Carboni et al., 1990; Pacholczyk et al., 1991; Tanda et al., 1997), thereby enhancing the action of dopamine. However, a number of innovative pharmacological approaches targeting these mechanisms have been evaluated to determine whether they are effective in reducing cocaine use and have been shown to have limited success (de Lima et al., 2002; Lima et al., 2002; Lima et al., 2003; Soares et al., 2004). The novel pharmacotherapy disulfiram has shown some initial promise in treating cocaine dependence in both non opioid-dependent (Carroll et al., 1993, 1998, 2004) and opioid-dependent cocaine abusers (George et al., 2000; Petrakis et al., 2000). Disulfiram was developed for alcohol dependence, but alcohol is a significant problem in most cocaine abusers who use alcohol to attenuate negative effects of cocaine or cocaine withdrawal and possibly enhance and extend cocaine euphoria through the effects of cocaethylene, which is an active metabolite of cocaine and alcohol (Jatlow et al., 1991; McCance-Katz et al., 1993, 1995; Carroll et al., 1993; Gawin & Kleber, 1986). Alcohol can also become a powerful conditioned cue for cocaine use through repeated pairings with cocaine and alcohol's disinhibiting effects that impair users' judgment and control over cocaine use (Higgins et al., 1996). Thus, disulfiram was initially examined as a treatment for cocaine dependence because of the prevalence of alcohol use in the cocaine dependent population (e.g., Carroll et al., 1993).
Nevertheless, disulfiram has been found to alter the pharmacokinetics of cocaine, increasing plasma cocaine concentrations along with cocaine-associated negative effects and cardiovascular responses in some patients, suggesting that disulfiram also directly impacts the behavioral response to cocaine (Hameedi et al., 1995; McCance-Katz et al., 1998a,b). Although adverse events associated with disulfiram were rarely observed during the disulfiram clinical trials in cocaine-dependent patients (Carroll et al., 2004; Carroll et al., 1998; Carroll et al., 1993; George et al., 2000; Petrakis et al., 2000), the potential for an adverse interaction between disulfiram and either cocaine or alcohol appears dosage dependent. However, disulfiram increases cocaine plasma levels, but not cardiovascular or subjective responses, at doses as low as 125 mg, suggesting that doses below 250 mg may be efficacious in facilitating cocaine abstinence with greater safety (McCance-Katz et al., 1998a, b; McCance, 2000). Thus, the present study explored the dose-related efficacy of disulfiram up to 250 mg/day in dually cocaine- and opioid-dependent patients. Our hypothesis was that disulfiram would facilitate cocaine abstinence relative to placebo in a dose-related manner.
2. Methods
2.1 Participants
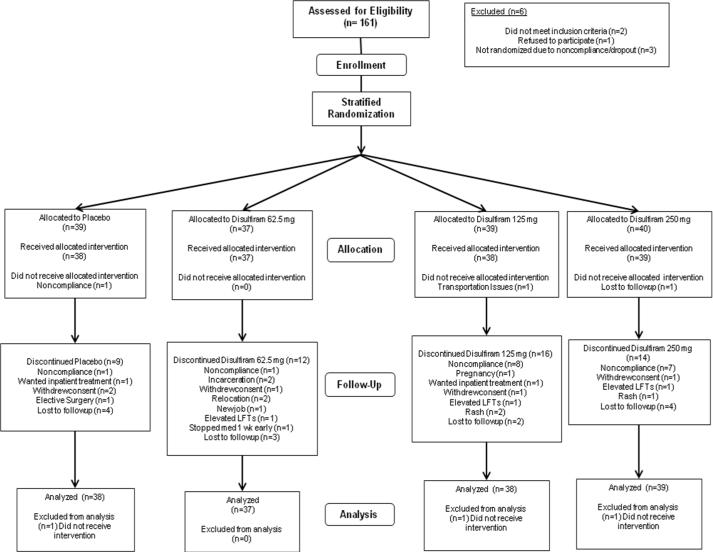
One hundred sixty-one individuals seeking opioid maintenance treatment were recruited from the greater West Haven (VAHCS; N=126; 3/2001 to 3/2004) or Little Rock (UAMS; N=35; 6/2005 to 10/2006) area (see Figure 1). Each gave written informed consent, as approved by the Yale Human Investigations Committee, Veterans Affairs Connecticut Human Studies Subcommittee (VAHCS) and UAMS Institutional Review Board (UAMS). Each participant (aged 18–65 years) currently used cocaine with at least weekly self-reported use during the month preceding study entry, and had either laboratory confirmation of opioid use during the month prior to study entry or manifested opioid withdrawal. Participants met DSM-IV criteria for opioid and cocaine dependence. Exclusion criteria included current alcohol physical dependence, abnormal liver function (with laboratory enzyme levels greater than three times normal), active hepatitis, hypertension, a current cardiac condition, occult coronary artery disease, high risk of cardiovascular disease, seizure disorders, other significant medical condition contraindicating disulfiram or methadone treatment, history of schizophrenia, bipolar disorder, other psychotic disorders, current suicidality or homicidality, current use of a prescribed psychotropic medication that could not be discontinued, current use of metronidazole or clotrimazole, benzodiazepine-positive urine toxicology screen, and pregnancy or breastfeeding.
Figure 1.
Flow diagram of subject progress through the phases of the randomized clinical trial.
2.2 Research Design and Procedures
In this 14-week, randomized, double blind clinical trial, methadone-stabilized (weeks 1–2) cocaine- and opioid-dependent individuals were randomly assigned to receive disulfiram at one of the following doses during wks 3–14: 0, 62.5, 125, or 250 mg/day (Figure 1). The data manager performed the randomization using a computerized urn randomization program (Wei et al., 1988), balancing groups on age, sex, race and Cocaine Selective Severity Assessment score (CSSA; Kampman et al., 1998; Mulvaney et al., 1999). Only the research pharmacist and data manager were aware of the medication condition.
Participants attended clinic at either the West Haven Veterans Affairs Healthcare System (VAHCS) or University of Arkansas for Medical Sciences Substance Abuse Treatment Clinic (UAMS) 6 days/wk (M-S) to complete study tasks, undergo counseling and receive study medications. On Saturday, participants received study medications to take on Sunday.
Methadone dispensing procedures occurred in accordance with manufacturer recommendations (Physicians' Desk Reference, 1998). Participants ingested methadone in a colored liquid daily at the dispensing window under observation of the dispensing nurse (except on Sundays, for which take home doses were provided). Participants initially received 25 mg of methadone. This dose was increased by 5 mg/day until participants received an initial maintenance dose of 60 mg/day (day 1 of week 2). The maintenance dose was adjusted as necessary thereafter, depending upon withdrawal symptoms or side effects. In order to obtain a relatively homogeneous sample, the methadone maintenance dose ranged from 40–100 mg/day. During stabilization onto methadone (weeks 1–2), placebo (microcrystalline cellulose) was suspended in the methadone. Then during weeks 3–14, either crushed disulfiram tablets or placebo (i.e., 300 mg of microcrystalline cellulose) was suspended in the methadone solution. Participants were advised to avoid alcohol or alcohol-containing products. At the beginning of week 15, disulfiram or placebo was no longer suspended in the methadone. At the end of the study, participants either transferred to a local methadone program after a 2-week washout period or underwent detoxification from methadone over a 4–6 week period.
All participants received weekly, manual-driven, individual cognitive behavioral therapy (Carroll, 1996). Participants were discharged for missing three consecutive methadone doses, missing four consecutive therapy sessions or having benzodiazepine-positive urine screens for 3 weeks. Subjects discharged from the study were tapered off methadone and referred to an appropriate local treatment program.
2.3 Assessments
At intake, the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1995), the Addiction Severity Index (ASI; McLellan et al., 1980), and the CSSA were completed. The primary outcome of interest was cocaine use, as determined by urine toxicology results and self reports. Secondary outcomes included retention, opioid use and adverse events. Supervised urine samples were obtained thrice weekly and tested for the presence of cocaine metabolite (benzoylecgonine) and other drugs using an Olympus AU 640 Emit system (Olympus America Inc., Melville, NY; VAHC) or Hitachi 717 Automated Analyzers (Boehringer Mannheim Corp., Indianapolis, IN; UAMS), with a cut-off concentration of 300 ng/ml. Self-report assessments of cocaine (e.g., days during which cocaine was used; dimes of cocaine used, with a dime roughly equivalent to $10 street worth of cocaine or crack cocaine) and other drug use were obtained weekly using instruments developed in previous studies (Kosten et al., 2003; Oliveto et al., 2005). Participants were monitored at every visit for adverse symptoms. The blind was assessed by asking participants what they believed they were receiving. At the UAMS site, the research nurse also reported what she believed the participants were receiving.
2.4 Training of Raters
The raters had previous experience in clinical rating and interviewing and at least a Bachelor's level education or its equivalent in experience. Under supervision, each rater received one month of training on the ASI, SCID and DSM-IV. Training included observation of interviews and ratings, co-rating, and interviewing with another experienced staff member present. In order to conduct interviews for this study, it was required that the rater complete three consecutive conjoint interviews on which DSM IV diagnoses were in complete agreement with those of more experienced raters. After training, reliability was periodically spot-checked.
2.5 Data Analyses
Kaplan-Meier survival analysis was performed to test for differences in retention among medication groups. Differences in baseline subject characteristics across medication groups or between the two sites were determined using Analysis of Variance (ANOVA) or its non-parametric analogue, Wilcoxon or Kruskal-Wallis test for continuous variables and Pearson Chi Square tests for categorical variables with medication group and then with site as factors. Differences in drug use during week 2 (baseline use) as well as drug use during the disulfiram phase of the study (weeks 3–14) were analyzed using parametric or non-parametric ANOVA, depending on the distribution of the outcome variable, with medication group as the factor.
Cocaine use data were analyzed using a random coefficient regression model, also known as hierarchical linear model (HLM) with medication group and study week as factors to determine whether results differentially changed over time across medication groups (Bryk et al., 1987; Gibbons et al., 1993). These analyses were performed in SAS PROC GLIMMIX, an HLM modeling program within SAS in order to determine whether patients improve differentially across conditions (Bryk and Raudenbush, 1987; Gibbons et al., 1993). This approach of modeling repeated measures is specifically designed for unbalanced repeated measures designs with missing data, allowing for intra-subject serial correlation and unequal variance and covariance structures over time. Solution of these problems, common to clinical trial data, is accomplished by incorporating available trend data for each individual with information on the behavior of the group from which the subject is drawn. Because individuals were stabilized onto methadone during weeks 1–2 and disulfiram administration started at the beginning of week 3, all available data from weeks 2–14 were used in the analyses. Week 2 was used as baseline because all participants underwent methadone induction during week 1 and reached the initial methadone maintenance dose (60 mg/day) by the beginning of week 2. Thus, only data for those who participated past week 2 were included in the analyses.
Because self-reported cocaine use count data were very non-normal and skewed towards zero, the data were fit with the appropriate error distribution term (i.e., a Poisson, Negative Binomial or Gamma) and appropriate log link function. For each model, contrasts were performed for placebo versus each dose. For all analyses, p < 0.05 was used to infer statistical significance. SAS software (SAS System for Windows Version 9.2, SAS Institute Inc., Cary, NC, USA) was employed.
3.0 Results
3.1 Retention, Adverse Events, Missing Data and Blind Assessment
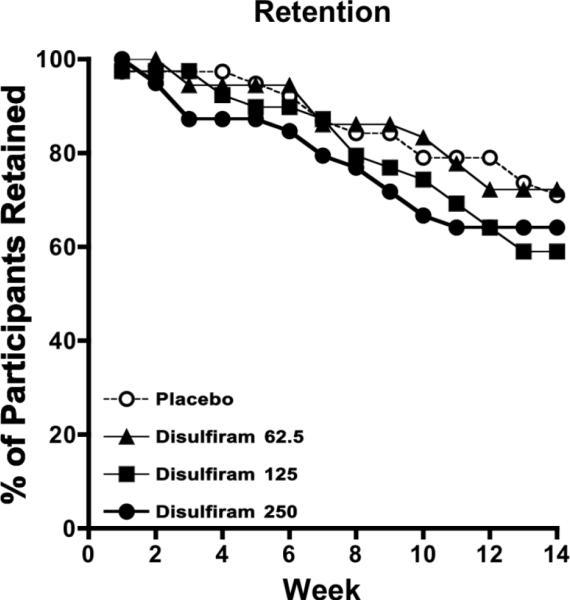
Retention rates did not differ among medication groups (Kaplan-Meier χ2= 2.82, df=3, p=0.42; Figure 2), with 104 completers (64.8%). Average duration of participation ranged from 11.1–12.2 wks. Fifty-four participants did not complete the protocol for reasons specified in figure 1. Forty-nine instances of adverse events occurred, of which nine were deemed study-related (Table 1). All symptoms resolved upon decreasing methadone dose, terminating disulfiram administration, or without intervention in the case of the alcohol-disulfiram reactions. The overall percentage of missing urine samples out of the total possible (i.e., 39 urine samples) ranged from 8.8 (18.6) to 17.0 (27.4) in the placebo and 250 mg disulfiram groups, respectively, and did not differ significantly across groups (χ2=2.75, df=3, p=0.43). Groups did not differ with respect to the percentage of participants reporting whether they thought they were receiving placebo or disulfiram, with 43.9% and 56.1% of the overall sample believing they were receiving placebo and disulfiram, respectively (χ2=4.43, df=3, p=0.22). Similarly, research staff members at the UAMS site reported believing that 50% participants overall were on either placebo or disulfiram and no differences across groups occurred (χ2=3.60, df=3, p=0.31), although several cells had less than the expected cell count and so the accuracy of this statistical result is questionable.
Figure 2.
Weekly percentage of participants retained in each of the four treatment groups across the 14-week trial: placebo (open circles), disulfiram at 62.5 mg/day (closed triangles), disulfiram at 125 mg/day (closed squares), and disulfiram at 250 mg/day (closed circles).
Table 1.
Summary of study-related adverse events.
| Subject ID | Adverse Event Description | Disulfiram Dose (mg/day) |
|---|---|---|
| 015 | Rash | 250 |
| 061 | Increased liver function | 125 |
| 066 | Rash | 125 |
| 094 | Increased liver functions | 250 |
| 106 | Increased liver functions | 62.5 |
| 128 | Arthritic-like symptoms in hands; swollen and painful (history of response to methadone) | 125 |
| 142 | Nausea and vomiting (reportedly ate cake that may have had alcohol in it) | 125 |
| 147 | Chills, nausea, extreme vomiting and fever (took medicine containing alcohol) | 125 |
| 150 | Constipation/bloody stools | 250 |
3.2 Demographics and Baseline Measures
Of the 158 enrolled into the study proper, six participants did not participate beyond week 2 (Figure 1). Table 2 shows the demographic data for the 152 participants who actually received disulfiram or placebo. Groups generally did not differ in terms of subject characteristics, except for race; however, this result was problematic because there were fewer than expected participants in several cells, making the chi-square test unreliable.
Table 2.
Summary of group characteristics
| Disulfiram Dose (mg/day) | |||||||
|---|---|---|---|---|---|---|---|
| Measure | 0 | 62.5 | 125 | 250 | Test Stat. | df | p value |
| N (West Haven/Little Rock) | 38 (28/10) | 37 (32/5) | 38 (30/8) | 39 (33/6) | χ2= 2.47 | 3 | 0.48 |
| Age: yrs(std) | 36.1(9.1) | 38.4(8.6) | 36.8(9.5) | 34.1(9.2) | F= 1.41 | 3 | 0.24 |
| Gender: (%M) | 65.8 | 48.7 | 55.3 | 66.7 | χ2= 3.5 | 3 | 0.32 |
| Race: C/AA/HO | 26/3/9 | 28/7/2 | 35/3/0 | 29/3/7 | χ2= 16.3 | 6 | 0.01 |
| CSSA: Score(std) | 32.0(17.9) | 26.0 (13.6) | 29.9(19.4) | 27.1(20.0) | χ2=2.62 | 3 | 0.45 |
| Marital: NM/M/O | 20/8/10 | 17/7/13 | 21/5/12 | 25/6/8 | χ2=3.7 | 6 | 0.72 |
| Income: $/mo (std) | 957(1725) | 754(1022) | 773(917) | 1053(1824) | χ2=0.54 | 3 | 0.91 |
| Current SCID diagnoses (%): | |||||||
| Cocaine dependence | 100.0 | 97.3 | 97.4 | 100.0 | χ2=2.08 | 3 | 0.49 |
| Opioid dependence | 100.0 | 100.0 | 100.0 | 100.0 | ---- | ---- | NA |
| Alcohol dependence | 15.8 | 18.9 | 23.7 | 23.1 | χ2=0.97 | 3 | 0.81 |
| Depression | 23.7 | 21.6 | 29.0 | 25.6 | χ2=0.58 | 3 | 0.90 |
| ASI Drug Use Data: | |||||||
| No. days used cocaine in past 30 days(std) | 18.5(9.97) | 15.6(9.9) | 15.4(8.8) | 18.9(8.7) | χ2=4.20 | 3 | 0.24 |
| No. days used heroin in past 30 days(std) | 17.5(14.2) | 24.4(11.0) | 23.4(11.7) | 22.6(11.8) | χ2=5.30 | 3 | 0.15 |
| No. days used other opioids in past 30 days(std) | 7.3(11.8) | 4.4(9.4) | 5.3(9.2) | 4.0(9.3) | χ2=2.36 | 3 | 0.50 |
| No. days used alcohol in past 30 days(std) | 4.3 (8.62) | 1.84 (2.73) | 4.10 (6.54) | 2.41 (4.73) | χ2=1.08 | 3 | 0.78 |
Race: C=Caucasian, AA=African American, HO=Hispanic or other
Marital: NM=never married, M=married, O=other
The primary opioid of choice differed across sites, with heroin used significantly more often at VAHCS (F=423.2, df=1, p<0.001) and other opioids used more often at UAMS (F=190.2, df=1, p<0.001). Current alcohol dependence was diagnosed more often at VACHS (24.5% versus 3.5%; χ2=6.3, df=1, p=0.01). However, the proportion of participants enrolled at each site did not differ across treatment groups (see table 2).
3.3. Opioid Use Treatment Outcomes
The percentage of opioid positive urine screens did not differ across groups during either baseline (wk 2) or the disulfiram phase (wks 3–14) of the study (Table 3). Average self-reported heroin use or opioid pill use did not differ across groups at either baseline or during weeks 3–13 of the study. Neither methadone maintenance dose nor the number of missed doses differed across groups during the disulfiram phase of the study.
Table 3.
Summary of Selected Treatment Outcome Measures
| Disulfiram Dose (mg/day) | |||||||
|---|---|---|---|---|---|---|---|
| Measure | 0 | 62.5 | 125 | 250 | Test Stat. | df | p value |
| N | 38 | 37 | 38 | 39 | |||
| Methadone Maintenance: | |||||||
| Mean methadone dose (mg/day; wks 3–14) | 69.2(11.6) | 70.0(12.4) | 68.4(15.8) | 69.5(14.4) | F=0.09 | 3 | p=0.96 |
| Mean no. missed doses (wks 3–14) | 0.29(0.56) | 0.22(0.30) | 0.61(1.48) | 0.34(0.58) | χ2=3.17 | 3 | p=0.36 |
| Opioid Use: | |||||||
| Mean % opioid-positive screens (wk 2) | 59.6(41) | 68.5(42) | 56.1(45) | 71.8(40) | χ2=3.46 | 3 | p=0.32 |
| Mean % opioid-positive screens (wks 3–14) | 49.2(35) | 51.7(40) | 52.4(39) | 58.1(36) | χ2=1.10 | 3 | p=0.78 |
| Mean no. bags of heroin used/wk (wk 2) | 7.2(14.4) | 9.6(24.0) | 5.8(9.6) | 7.5(13.8) | χ2=1.65 | 3 | p=0.65 |
| Mean no. bags of heroin used/wk (wks 3–14) | 1.1(3.2) | 3.5(5.4) | 2.4(2.8) | 3.0(4.6) | χ2=2.24 | 3 | p=0.53 |
| Mean no. pills opioids used/wk (wk 2) | 1.08(3.59) | 1.08(3.39) | 0.95(3.28) | 0.63(3.4) | χ2=2.30 | 3 | p=0.51 |
| Mean no. pills opioids used/wk (wks 3–14) | 0.90(1.96) | 0.26(1.18) | 0.27(0.61) | 0.20(0.66) | χ2=6.11 | 3 | p=0.11 |
| Alcohol Use: | |||||||
| Mean drinks alcohol used/wk (wk 2) | 0.02 (0.16) | 0.03 (0.17) | 0.05 (0.33) | 0.84 (3.81) | χ2=2.13 | 3 | p=0.54 |
| Mean drinks alcohol used/wk (wks 3–14) | 0.24 (0.82) | 0.06 (0.13) | 0.16 (0.66) | 1.01 (3.93) | χ2=3.07 | 3 | p=0.38 |
3.4. Alcohol Use Treatment Outcomes
During week 2, the mean reported number of alcoholic beverages was less than 1 drink/day and did not differ across groups (Table 3). Overall, self-reported alcohol use continued to remain low and did not differ across groups during weeks 3–14. In addition, none of the disulfiram groups showed a change in alcohol use over time relative to the placebo group (p>0.2; data not shown).
3.5 Effects of Disulfiram Dose on Cocaine Use
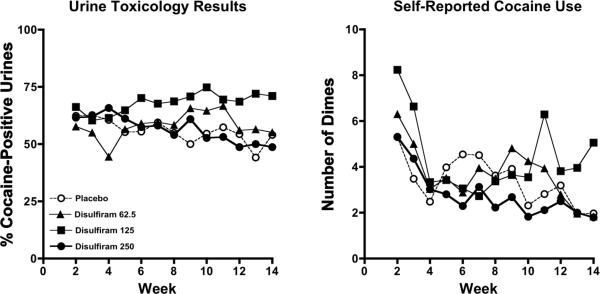
Cocaine use during the trial, in terms of the percentage of urine samples positive for cocaine and the number of participants with urine samples negative and positive for cocaine, is shown in figure 3 and table 4, respectively. During week 2, cocaine positive urine screens did not differ across groups, with the percentage of urines positive for cocaine ranging from 57.7 (48) to 66.2(43) in the 62.5 and 125 mg disulfiram groups, respectively (χ2=0.49, df=3, p=0.92; see figure 3, left panel). During weeks 2–14, cocaine-positive urine screens increased over time in the 62.5 (t=3.74, df=4806, p=0.0002; slope=0.08) and 125 (t=5.45, df=4806, p<0.0001; slope=0.18) mg disulfiram groups relative to placebo (slope=−.05), but similarly decreased over time in the 250 mg disulfiram and placebo groups (t=−1.33, df=4806, p=0.18; slope=−0.10 and −0.05, respectively; Figure 3).
Figure 3.
Weekly percentage of urine toxicology screens positive for cocaine (left panel) and weekly self-reported number of dimes of cocaine used (right panel) for the four treatment groups across the 14-week trial: placebo (open circles), disulfiram at 62.5 mg/day (closed triangles), disulfiram at 125 mg/day (closed squares), and disulfiram at 250 mg/day (closed circles).
Table 4.
The number of participants with urine samples that either tested all negative (neg) or had at least one positive (pos) test, respectively, for cocaine/cocaine metabolite within a given week.
| Placebo | Disulfiram 62.5 | Disulfiram 125 | Disulfiram 250 | |||||
|---|---|---|---|---|---|---|---|---|
| Study Week | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos |
| 2 | 11 | 27 | 13 | 24 | 9 | 29 | 10 | 29 |
| 3 | 12 | 26 | 14 | 23 | 12 | 25 | 11 | 25 |
| 4 | 12 | 26 | 16 | 20 | 12 | 26 | 11 | 25 |
| 5 | 15 | 23 | 12 | 24 | 10 | 26 | 13 | 23 |
| 6 | 14 | 23 | 13 | 22 | 6 | 28 | 13 | 23 |
| 7 | 12 | 25 | 8 | 25 | 7 | 27 | 11 | 24 |
| 8 | 11 | 23 | 9 | 23 | 9 | 24 | 13 | 20 |
| 9 | 12 | 21 | 10 | 22 | 8 | 24 | 10 | 22 |
| 10 | 11 | 22 | 9 | 23 | 6 | 25 | 13 | 18 |
| 11 | 10 | 22 | 7 | 24 | 7 | 23 | 9 | 18 |
| 12 | 11 | 20 | 10 | 18 | 6 | 20 | 10 | 16 |
| 13 | 16 | 15 | 10 | 16 | 7 | 18 | 12 | 14 |
| 14 | 12 | 17 | 8 | 18 | 6 | 17 | 11 | 15 |
During the course of the trial, the number of participants with all cocaine-free urine samples within a given week essentially did not change over time in the placebo and disulfiram 250 groups, but decreased over time in the disulfiram 62.5 and 125 groups (table 4). The number of participants with at least one cocaine-positive urine sample decreased similarly over time in the placebo, disulfiram 125 and disulfiram 250 groups, but essentially did not change or slightly increased in the disulfiram 62.5 group.
Overall, self-reported cocaine use did not differ across groups at baseline, with the number of dimes used per week ranging from 3.5 (4.9) to 6.6 (9.4) in the placebo and 125 mg disulfiram groups, respectively (χ2=1.52, df=3, p=0.67). Nevertheless, self-reported cocaine use increased over the course of the trial in the 125 mg disulfiram group (t=2.02, df=954.1, p=0.04; slope=0.013), but did not differ over time in the 62.5 (t=1.17, df=1068, p=0.24; slope=−0.009) and 250 (t=−1.28, df=1076, p=0.20; slope=−0.074) mg disulfiram groups relative to placebo (slope=−0.04; Figure 3, right panel).
4.0 Discussion
We did not expect that disulfiram would be associated with an exacerbation of cocaine use at doses below 250 mg/day. This exacerbation may be at least partly due to the nonspecific nature of disulfiram's effects. Disulfiram inhibits not only aldehyde dehydrogenases (Faiman, 1979; Gibbons et al., 1993; Haley, 1979; Wright et al., 1990) but also dopamine beta-hydroxylase (DBH) and other esterases (Faiman, 1979; Haley, 1979; Honjo et al., 1969), including microsomal carboxylesterases and plasma cholinesterase (Faiman, 1979) which are the primary pathways for cocaine metabolism (Stewart et al., 1979). Disulfiram's complex pharmacology at doses ranging from 62.5 to 500 mg includes increasing cocaine plasma concentration in some cocaine-experienced participants (Baker et al., 2007; Hameedi et al., 1995; McCance-Katz et al., 1998a,b), prolonging cocaine-induced cardiovascular effects (Hameedi et al., 1995; McCance-Katz et al., 1998a,b) reducing cocaine-induced positive subjective effects (Baker et al., 2007) and enhancing cocaine-induced negative subjective effects (Hameedi et al., 1995; McCance-Katz et al., 1998a,b). However, disulfiram reportedly is a more potent inhibitor of aldehyde dehydrogenase and esterases than of DBH (Karamanakos et al., 2001). DBH is thought to impact the self-reported effects of cocaine through its regulation of the dopamine-norepinephrine ratio in the brain (Stanley et al., 1997). Thus, disulfiram at these lower doses may be prolonging the behavioral effects of cocaine through cholinesterase inhibition without significantly altering the quality of the cocaine-induced subjective aversive or craving effects that would be related to DBH inhibition. Without altering cocaine's aversive or craving effects, disulfiram-induced prolongation of cocaine's actions may sustain and enhance priming effect for more cocaine use. Although the mechanism for this disulfiram-induced increase in cocaine use is speculative, these results suggest that prescribing disulfiram at doses lower than 250 mg/day may be contraindicated in cocaine dependent individuals.
Although the finding that disulfiram at 250 mg/day did not alter cocaine use relative to placebo is inconsistent with results in non opioid-dependent cocaine abusers (Carroll et al., 2004; Carroll et al., 1998), these results are somewhat similar to prior reports in opioid-dependent cocaine abusers (Cubells et al., 2004b; George et al., 2000; Petrakis et al., 2000). For instance, in a 12-week randomized clinical trial (Petrakis et al., 2000), 67 opioid addicts who were stable in methadone maintenance treatment and met criteria for cocaine dependence were randomized to receive disulfiram or placebo, with medication placed directly in subjects' methadone to assure compliance. There was a significant effect of disulfiram on self-reported quantity and frequency measures of self-reported cocaine use, although whether urine toxicology screens for cocaine metabolites differed significantly between disulfiram and placebo conditions was not reported. Moreover, alcohol use was minimal for both the disulfiram and placebo groups during treatment, and the significant effect of disulfiram occurred, whether or not subjects were alcohol users at baseline. Meanwhile, a small, randomized, double blind, placebo-controlled trial examining the efficacy of disulfiram (250 mg/day) in 15 buprenorphine-maintained cocaine abusers showed that the degree of abstinence from cocaine was significantly greater on disulfiram (250 mg/day) than placebo (7.8 +/− 2.6 wks vs. 3.3 +/− 0.5 wks; p < 0.05) (George et al., 2000). However, in a larger, randomized clinical trial in which buprenorphine-maintained cocaine abusers were treated with disulfiram (250 mg/day) vs. placebo, disulfiram treatment was superior to placebo on self-report measures but not urine drug screen results (Cubells et al., 2004a).
The apparently less robust efficacy of disulfiram in dually cocaine- and opioid-dependent populations may be because abusers who are cocaine but not opioid dependent are seeking treatment for their cocaine abuse, while the majority of dually cocaine- and opioid-dependent individuals are seeking treatment for their opioid abuse. Thus, primary cocaine abusers may be more motivated to stop their cocaine use than those who are also opioid dependent, thereby requiring higher doses of disulfiram to be effective in the latter group. It is also well known that those dependent on more than one substance are often more difficult to treat than those dependent on just one drug of abuse (Dutra et al., 2008). In any case, more research is necessary to determine the relative efficacy of disulfiram in these populations, including examining doses higher than 250 mg.
There are several limitations to this trial. First, the principal investigator moved to a different university during the trial, and the study was conducted at two sites. Although an equal proportion of participants were assigned to each medication group and generally no significant site differences occurred in outcomes across medication groups, the potential for unknown site differences impacting treatment response cannot be ruled out. Second, although opioid use appeared to diminish relative to that observed during week 2 of the study, approximately 50% of urine samples were still positive for opioids during the trial, indicating that opioid use was still relatively high over the course of the study. Although these results are consistent with other clinical trials involving opioid maintenance in dually cocaine- and opioid-dependent populations (Grabowski et al., 2004; Petrakis et al., 2000), this study protocol restricted participants to no more than 100 mg of methadone per day, and some patients appeared to need larger methadone doses to discontinue their opioid use. Indeed, methadone dose was slightly, but significantly, correlated with opioid-positive urines overall (Pearson R=0.24, p=0.0028), although when this association was examined within treatment group, it was only significant in the disulfiram 125 mg group (Pearson R=0.38, p=0.02). Cocaine-positive urine samples also showed a positive correlation with opioid-positive urines (Pearson R=0.18, p=0.02), with the disulfiram 62.5 and 125 mg treatment groups showing a positive association between these outcomes (Pearson R=0.42, p=0.009). These findings suggest a complex interaction among opioid use, cocaine use and disulfiram dose. Future studies should consider not imposing a particular range of methadone doses, but rather follow methadone clinic guidelines in this regard to allow for greater generalization of findings.
Third, due to the skewed nature of the data, nonparametric tests were employed to analyze differences in mean use, which decreased the power to detect differences in overall use. Fourth, whether disulfiram differentially impacted alcohol use could not be discerned due to the reportedly low levels of alcohol consumed during the study. Nevertheless, the blind appeared adequate, even though participants could have tested the blind by ingesting alcohol. In addition, the fact that similar results were obtained across a rural and more urban site suggest that these results have applicability to the cocaine abusing methadone patient population in general, although the methadone dose constraint does limit applicability to some degree. Be that as it may, the present findings suggest that disulfiram at doses lower than 250 mg/day may be contraindicated for treating cocaine dependence. Given the low incidence of adverse events, their independence from disulfiram dose and the need for more randomized clinical trials to determine whether disulfiram has efficacy for treating cocaine dependence in general (Pani et al., 2010), examining the efficacy of disulfiram at doses higher than 250 mg/day may clarify the dose-related efficacy of disulfiram in this population.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A preliminary report of this work was presented at the Annual Meeting of American College of Neuropsychopharmacology on December 15, 2004 in San Juan, PR and the Annual Meeting of the College on Problems of Drug Dependence on June 20, 2007 in Quebec City, Quebec. Trial Registration: Clinicaltrials.gov identifier NCT00218608.
References
- Baker JA, Jatlow P, McCance-Katz EF. Disulfiram effects on responses to intravenous cocaine administration. Drug Alcohol Depend. 2007;87:202–209. doi: 10.1016/j.drugalcdep.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JL, Dolan MP, Penk WE, Robinowitz R, DeFord HA. The effect of increased cocaine use on drug treatment. Addict Behav. 1987;12:289–292. doi: 10.1016/0306-4603(87)90042-6. [DOI] [PubMed] [Google Scholar]
- Bovasso G, Cacciola J. The long-term outcomes of drug use by methadone maintenance patients. J Behav Health Serv Res. 2003;30:290–303. doi: 10.1007/BF02287318. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application if hierarchical linear models to assessing change. Psychological Bull. 1987;101:147–158. [Google Scholar]
- Bux DA, Lamb RJ, Iguchi MY. Cocaine use and HIV risk behavior in methadone maintenance patients. Drug Alcohol Depend. 1995;37:29–35. doi: 10.1016/0376-8716(94)01058-s. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Cognitive-Behavioral Coping Skills Treatment for Cocaine Dependence. Yale University Psychotherapy Development Center; New Haven, CT: 1996. [Google Scholar]
- Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61:264–272. doi: 10.1001/archpsyc.61.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–727. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54:199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- Compton WM, Lamb RJ, Fletcher BW. Results of the NIDA treatment demonstration grants' cocaine workgroup: characteristics of cocaine users and HIV risk behaviors. Drug Alcohol Depend. 1995;37:1–6. doi: 10.1016/0376-8716(94)01061-o. [DOI] [PubMed] [Google Scholar]
- Condelli WS, Fairbank JA, Dennis ML, Rachal JV. Cocaine use by clients in methadone programs: significance, scope, and behavioral interventions. J Subst Abuse Treat. 1991;8:203–212. doi: 10.1016/0740-5472(91)90040-h. [DOI] [PubMed] [Google Scholar]
- Cubells JF, Charwarski MC, George TP, Schottenfeld RS. DBH genotype in disulfiram treatment for cocaine dependence. Neuropsychopharmacology. 2004a;29:S72. [Google Scholar]
- Cubells JF, Zabetian CP. Human genetics of plasma dopamine beta-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004b;174:463–476. doi: 10.1007/s00213-004-1840-8. [DOI] [PubMed] [Google Scholar]
- de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: a systematic review. Addiction. 2002;97:931–949. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- Disney ER, Kidorf M, King VL, Neufeld K, Kolodner K, Brooner RK. Prevalence and correlates of cocaine physical dependence subtypes using the DSM-IV in outpatients receiving opioid agonist medication. Drug Alcohol Depend. 2005;79:23–32. doi: 10.1016/j.drugalcdep.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Dobler-Mikola A, Hattenschwiler J, Meili D, Beck T, Boni E, Modestin J. Patterns of heroin, cocaine, and alcohol abuse during long-term methadone maintenance treatment. J Subst Abuse Treat. 2005;29:259–265. doi: 10.1016/j.jsat.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Gilbert L, Frye V, Wu E, Go H, Hill J, Richman BL. Physical and sexual intimate partner violence among women in methadone maintenance treatment. Psychol Addict Behav. 2004;18:180–183. doi: 10.1037/0893-164X.18.2.180. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug addiction (EMCDDA) Annual Report 2009: The State of the Drugs Problem in Europe. Publications Office of the European Union; Luxembourg: 2009. [Google Scholar]
- Faiman MD. Biochemistry and Pharmacology of Ethanol. Plenum Press; New York: 1979. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington DC: 1995. [Google Scholar]
- George TP, Chawarski MC, Pakes J, Carroll KM, Kosten TR, Schottenfeld RS. Disulfiram versus placebo for cocaine dependence in buprenorphine-maintained subjects: a preliminary trial. Biol Psychiatry. 2000;47:1080–1086. doi: 10.1016/s0006-3223(99)00310-8. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, Elkin I, Waternaux C, Kraemer HC, Greenhouse JB, Shea MT, Imber SD, Sotsky SM, Watkins JT. Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of Depression Collaborative Research Program dataset. Arch Gen Psychiatry. 1993;50:739–750. doi: 10.1001/archpsyc.1993.01820210073009. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Grella CE, Anglin MD, Wugalter SE. Cocaine and crack use and HIV risk behaviors among high-risk methadone maintenance clients. Drug Alcohol Depend. 1995;37:15–21. doi: 10.1016/0376-8716(94)01059-t. [DOI] [PubMed] [Google Scholar]
- Haley TJ. Disulfiram (tetraethylthioperoxydicarbonic diamide): a reappraisal of its toxicity and therapeutic application. Drug Metab Rev. 1979;9:319–335. doi: 10.3109/03602537908993897. [DOI] [PubMed] [Google Scholar]
- Hameedi FA, Rosen MI, McCance-Katz EF, McMahon TJ, Price LH, Jatlow PI, Woods SW, Kosten TR. Behavioral, physiological, and pharmacological interaction of cocaine and disulfiram in humans. Biol Psychiatry. 1995;37:560–563. doi: 10.1016/0006-3223(94)00361-6. [DOI] [PubMed] [Google Scholar]
- Hartell DM, Schoenbaum EE, Selwyn PA, Kline J, Davenny K, Klien RS, Friedland GH. Heroin use during methadone maintenance treatment: the importance of methadone doe and cocaine use. Amer J Publ Hlth. 1995;85:83–88. doi: 10.2105/ajph.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Roll JM, Bickel WK. Alcohol pretreatment increases preference for cocaine over monetary reinforcement. Psychopharmacology. 1996;123:1–8. doi: 10.1007/BF02246274. [DOI] [PubMed] [Google Scholar]
- Honjo T, Netter KJ. Inhibition of drug demethylation by disulfiram in vivo and in vitro. Biochem Pharmacol. 1969;18:2681–2683. doi: 10.1016/0006-2952(69)90201-9. [DOI] [PubMed] [Google Scholar]
- Hunt D, Spunt B, Lipton D, Goldsmith D, Strug D. The costly bonus: cocaine related crime among methadone treatment clients. Adv Alcohol Subst Abuse. 1986;6:107–122. doi: 10.1300/J251v06n02_08. [DOI] [PubMed] [Google Scholar]
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, Roth RH. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 1991;48:1787–1794. doi: 10.1016/0024-3205(91)90217-y. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Karamanakos PN, Pappas P, Stephanou P, Marselos M. Differentiation of disulfiram effects on central catecholamines and hepatic ethanol metabolism. Pharmacol Toxicol. 2001;88:106–110. [PubMed] [Google Scholar]
- King VL, Kidorf MS, Stoller KB, Carter JA, Brooner RK. Influence of antisocial personality subtypes on drug abuse treatment response. J Nerv Ment Dis. 2001;189:593–601. doi: 10.1097/00005053-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained paintents. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. Antecedents and consequences of cocaine abuse among opioid addicts. A 2.5-year follow-up. J Nerv Ment Dis. 1988;176:176–181. doi: 10.1097/00005053-198803000-00006. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Downey KK. Relationship between problem gambling and substance use in a methadone maintenance population. Addict Behav. 2002;27:483–491. doi: 10.1016/s0306-4603(01)00187-3. [DOI] [PubMed] [Google Scholar]
- Lima AR, Lima MS, Soares BG, Farrell M. Carbamazepine for cocaine dependence. Cochrane Database Syst Rev. 2002:CD002023. doi: 10.1002/14651858.CD002023. [DOI] [PubMed] [Google Scholar]
- Lima MS, Reisser AA, Soares BG, Farrell M. Antidepressants for cocaine dependence. Cochrane Database Syst Rev. 2003:CD002950. doi: 10.1002/14651858.CD002950. [DOI] [PubMed] [Google Scholar]
- MacGowan RJ, Fichtner RR, Swanson N, Collier C, Kroliczak A, Cole G. Factors associated with client-reported HIV infection among clients entering methadone treatment. AIDS Educ Prev. 1997;9:205–217. [PubMed] [Google Scholar]
- Magura S, Nwakeze PC, Demsky SY. Pre- and in-treatment predictors of retention in methadone treatment using survival analysis. Addiction. 1998;93:51–60. doi: 10.1046/j.1360-0443.1998.931516.x. [DOI] [PubMed] [Google Scholar]
- Magura S, Rosenblum A, Fong C, Villano C, Richman B. Treating cocaine-using methadone patients: predictors of outcomes in a psychosocial clinical trial. Subst Use Misuse. 2002;37:1927–1955. doi: 10.1081/ja-120016225. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry. 1998a;43:540–543. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P. Disulfiram effects on acute cocaine administration. Drug Alcohol Depend. 1998b;52:27–39. doi: 10.1016/s0376-8716(98)00050-7. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology (Berl) 1993;111:39–46. doi: 10.1007/BF02257405. [DOI] [PubMed] [Google Scholar]
- McCance EF. 2000. Unpublished data.
- McCance EF, Price LH, Kosten TR, Jatlow PI. Cocaethylene: pharmacology, physiology and behavioral effects in humans. J Pharmacol Exp Ther. 1995;274:215–223. [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Meandzija B, O'Connor PG, Fitzgerald B, Rounsaville BJ, Kosten TR. HIV infection and cocaine use in methadone maintained and untreated intravenous drug users. Drug Alcohol Depend. 1994;36:109–113. doi: 10.1016/0376-8716(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. J Subst Abuse Treat. 1999;16:129–135. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Oliveto A, Poling J, Sevarino KA, Gonsai KR, McCance-Katz EF, Stine SM, Kosten TR. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depend. 2005;79:157–165. doi: 10.1016/j.drugalcdep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Vacca R, Amato L, Vecchi S, Davoli M. Disulfiram for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2010;(Issue 1) doi: 10.1002/14651858.CD007024.pub2. Art. No.: CD007024. DOI: 10.1002/14651858.CD007024.pub2. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Carroll KM, Nich C, Gordon LT, McCance-Katz EF, Frankforter T, Rounsaville BJ. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95:219–228. doi: 10.1046/j.1360-0443.2000.9522198.x. [DOI] [PubMed] [Google Scholar]
- Physicians' Desk Reference . Medical Economics Company, Inc.; Montvale, NJ: 1998. [Google Scholar]
- Soares BG, Lima MS, Reisser AA, Farrell M. Dopamine agonists for cocaine dependence. Cochrane Database Syst Rev. 2004:CD003352. doi: 10.1002/14651858.CD003352. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Li B, Bonhaus DW, Johnson LG, Lee K, Porter S, Walker K, Martinez G, Eglen RM, Whiting RL, Hegde SS. Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase. Br J Pharmacol. 1997;121:1803–1809. doi: 10.1038/sj.bjp.0701315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ, Inaba T, Lucassen M, Kalow W. Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clin Pharmacol Ther. 1979;25:464–468. doi: 10.1002/cpt1979254464. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Office of Applied Studies. DHHS; Rockville, MD: 2003. Results from the 2002 National Survey on Drug Use and Health: National Findings. (NHSDA Series H-22). Publication No. SMA 03-3836. [Google Scholar]
- Tanda G, Pontieri FE, Frau R, Di Chiara G. Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci. 1997;9:2077–2085. doi: 10.1111/j.1460-9568.1997.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teesson M. The effect of persistence of cocaine use on 12-month outcomes for the treatment of heroin dependence. Drug Alcohol Depend. 2006;81:293–300. doi: 10.1016/j.drugalcdep.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Wolf BC, Lavezzi WA, Sullivan LM, Flannagan LM. Methadone-related deaths in Palm Beach County. J Forensic Sci. 2004;49:375–378. [PubMed] [Google Scholar]
- Wright C, Moore RD. Disulfiram treatment of alcoholism. Am J Med. 1990;88:647–655. doi: 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]