Abstract
Huntingtin-interacting protein 1-related (Hip1r) is highly expressed in gastric parietal cells, where it participates in vesicular trafficking associated with acid secretion. Hip1r-deficient mice have a progressive remodeling of the mucosa, including apoptotic loss of parietal cells, glandular hypertrophy, mucous cell metaplasia, and reduced numbers of zymogenic cells. In this study, we characterized gastric gland development in wild-type and Hip1r-deficient mice to define normal development, as well as the timing and sequence of the cellular transformation events in the mutant stomach. Postnatal (newborn to 8-wk-old) stomachs were examined by histological and gene expression analysis. At birth, gastric glands in wild-type and mutant mice were rudimentary and mature gastric epithelial cells were not apparent, although marker expression was detected for most cell lineages. Interestingly, newborns exhibited unusual cell types, including a novel surface cell filled with lipid and cells that coexpressed markers of mature mucous neck and zymogenic cells. Glandular morphogenesis proceeded rapidly in both genotypes, with gastric glands formed by weaning at 3 wk of age. In the Hip1r-deficient stomach, epithelial cell remodeling developed in a progressive manner. Initially, in the perinatal stomach, cellular changes were limited to parietal cell apoptosis. Other epithelial cell changes, including apoptotic loss of zymogenic cells and expansion of metaplastic mucous cells, emerged several weeks later when the glands were morphologically mature. Thus, parietal cell loss appeared to be the initiating event in Hip1r-deficient mice, with secondary remodeling of the other gastric epithelial cells.
Keywords: parietal cell, zymogenic cell, mucous cell, enteroendocrine cell, spasmolytic polypeptide-expressing metaplasia
the stomach is composed of a columnar epithelium organized into gastric glands that invaginate from surface pits down into the mesenchyme. These glands contain a number of epithelial cell types, which are continuously replenished throughout the life span of the organism. In the mouse stomach, the acid-secreting corpus contains four distinct cell lineages that arise from stem and progenitor cells in the isthmus region: surface mucous, parietal, enteroendocrine, and mucous neck-zymogenic. Each lineage forms differentiated secretory cells with distinctive migration properties and turnover rates to maintain homeostasis of the mature gastric gland. The cellular lineages in the corpus of the adult mouse stomach were described in a series of elegant ultrastructural studies performed by Karam and Leblond (13–17). These studies demonstrate that the surface mucous cells migrate from the progenitor cell zone up to the pit region lining the lumen. These cells secrete protective mucus and turn over every 3 days (15). The acid-secreting parietal cells migrate bidirectionally from the progenitor zone with a 54-day turnover rate and are a predominant cell type throughout the central region of the gland (13). The enteroendocrine cell lineage includes a number of different subtypes, including the histamine-secreting enterochromaffin like (ECL) cell, which is abundant in the base region, with an estimated turnover rate of 2–3 mo (17). Cells of the mucous neck-zymogenic cell lineage exhibit different features as they migrate from the stem cell zone through the neck region and into the base. In the neck region, the cells are intercalated among the parietal cells and express mucous cell markers mucin (Muc)-6 and trefoil factor 2 (TFF2) (1). Mucous neck cells migrate through the neck region over a 2-wk period into the base, whereupon they differentiate into digestive enzyme-secreting zymogenic cells with a life span of several months (16). At the neck-base boundary, rare transitional cells, which express markers of mucous neck and zymogenic cells, are present (16, 28).
Parietal cells appear to be critical regulators of cellular homeostasis in the gastric gland. Numerous studies have revealed that mouse mutants with parietal cell loss or dysfunction exhibit decreased numbers of zymogenic cells (8, 21, 31). Zymogenic cell loss is accompanied by expansion of a dysplastic cell type, i.e., spasmolytic polypeptide-expressing metaplasia (SPEM), which shares features with cells of the mucous neck-zymogenic lineage, including expression of TFF2 (25). SPEM can be rapidly induced, as demonstrated when mice are treated with the parietal cell toxin DMP 777. The parietal cell death induced by this protonophore leads to rapid loss of zymogenic cells within 1 day and expansion of the metaplastic TFF2-expressing SPEM lineage within 10 days (25). Parietal cells are known to secrete a variety of growth factors, including EGF family ligands (amphiregulin, heparin-binding EGF, and TGFα), parathyroid hormone-like hormone, sonic hedgehog, and VEGF-B (9, 10, 23, 38). Thus SPEM might result from the loss of a parietal cell-derived factor required for proper development of cells in the mucous neck-zymogenic lineage, although this parietal cell factor has not been identified. In addition, loss of parietal cells and reduced acid secretion are commonly associated with Helicobacter sp. infection and inflammation; thus inflammatory mediators may also play a role in the epithelial cell remodeling observed with parietal cell atrophy (12, 32). SPEM formation after parietal cell atrophy has been described in mice, as well as humans, in association with the cellular transformation events observed with the development of gastric cancer (35).
We recently described a mouse mutant that undergoes apoptotic death of parietal cells due to absence of the F-actin- and clathrin-binding protein Huntingtin-interacting protein 1-related (Hip1r). Hip1r is abundantly expressed in parietal cells, where it appears to play a critical role in the vesicular trafficking associated with acid secretion (8). Although Hip1r is largely parietal cell-specific in the oxyntic mucosa, Hip1r-deficient mice exhibited a multifaceted cellular transformation involving several different gastric epithelial cell lineages, including glandular hyperplasia with increased cellular proliferation, increased numbers of surface mucous cells, loss of zymogenic cells, and extensive SPEM. The alterations to the mucous neck and zymogenic cells are of particular interest in light of the parietal cell defect in this mutant (28). The reduced numbers of zymogenic cells and the appearance of SPEM may be due to the apoptotic loss of parietal cells (8).
The cellular transformation observed in adult Hip1r-deficient mice involves many cell types, with severity increasing with age, making it difficult to understand the underlying mechanisms for changes in individual lineages. Accordingly, this study focused on gastric cytodifferentiation in developing Hip1r-deficient stomach to understand the timing of the various lineage disruptions. Although the epithelial cell lineages in the adult stomach have been well established, the cellular composition of the developing mouse stomach is less well described. In rodents, gastric gland morphogenesis takes place after birth, with secretory function maturing during the first few postnatal weeks (7, 11, 18, 19). In this study, we examined the postnatal development of the major epithelial cell lineages in the oxyntic mucosa of wild-type (WT) and Hip1r-deficient mice from birth to 8 wk of age to encompass the critical timing of cellular differentiation of the gastric glands. The results support the hypothesis that parietal cell loss initiates the other cellular changes in the Hip1r-deficient stomach, consistent with the conclusion that parietal cells are critical for normal development and homeostasis of the gastric epithelium.
MATERIALS AND METHODS
Mice.
The Hip1r-deficient mice were on a mixed 129X1 and C57BL/6 strain background (10); WT mice were C57BL/6 strain. Mice were housed in ventilated and automated-watering cages under specific pathogen-free conditions. Preweaned [newborn (P0) to 3-wk-old] mice were freely fed before euthanasia, while older mice (5- to 8-wk-old) were fasted overnight with free access to water prior to use. Male and female mice were studied. Use of the mice was approved by The University of Michigan Committee on Use and Care of Animals.
Gastric acid content.
Stomachs were removed from 1-wk-old WT and Hip1r-deficient mice (n = 3–4) and cut along the greater curvature, and contents were collected by rinsing in 2 ml of 0.9% NaCl (pH 7.0). Acid content was measured by titration, as described elsewhere (22), and values were normalized to body weight (kg). The body weight of WT and Hip1r-deficient mice did not differ.
Histological analysis.
Stomachs were removed and fixed in 4% paraformaldehyde, as described previously (22). Paraffin sections (4 μm) were stained with hematoxylin and eosin (H&E) for evaluation of general histology. For Oil Red O staining, gastric cryosections were fixed in 4% paraformaldehyde for 5 min, and staining was performed using the Oil Red O solution and the propylene glycol method described by the manufacturer (H-504, Rowley Biochemical). Specific gastric cell types were identified by immunostaining for H+-K+-ATPase α-subunit (1:1,000 dilution of mouse monoclonal antibody; Medical and Biological Laboratories), Muc5AC (1:75 dilution of mouse monoclonal antibody; Novocastra), chromogranin A (CgA, 1:500 dilution of 94188/5, a gift from J. F. Rehfeld), EGF (1:10 dilution of mouse monoclonal antibody; Chemicon), TFF2 (1:100 dilution of heat shock protein-IgM; gift from Nicholas Wright, Barts and London School of Medicine and Dentistry), and intrinsic factor (IF, 1:2,000 dilution of rabbit anti-human IF; gift from David Alpers, Washington University, St. Louis, MO). Griffonia simplicifolia lectin II (GSII) staining (1:1,000 dilution of Alexa 488-conjugated GSII; Vector Laboratories) was also used for mucous neck cells. Primary antibody staining overnight (72 h for EGF) at 4°C, secondary antibody use, counterstaining, nuclear 4,6-diamidino-2-phenylindole dihydrochloride staining, and imaging by digital microscopy were performed as previously described (22).
Triple staining for IF, GSII, and H+-K+-ATPase α-subunit was performed by first immunostaining for IF (1:1,000 dilution) for 2 h at room temperature, rinsing in 0.01% Triton X-100 in PBS (TPBS), staining with GSII lectin for 1 h at room temperature, rinsing again in TPBS, and then immunostaining for H+-K+-ATPase α-subunit overnight at 4°C. Secondary antibody staining with donkey anti-rabbit Cy3 and donkey anti-mouse Alexa 647 was performed simultaneously (1:400 dilution; Jackson ImmunoResearch Labs and Invitrogen, respectively). Results were visualized using a confocal microscope (model FV500, Olympus).
Analysis of proliferation and apoptosis.
For analysis of proliferation, mice were injected (50 mg/kg ip) 1.5 h before euthanasia with 5-bromo-2-deoxyuridine (BrdU; Sigma) freshly prepared in normal saline (0.9% NaCl, pH 7.0). Proliferating cells were identified in paraffin sections by immunostaining for BrdU (1:50 dilution of mouse monoclonal antibody; Dako). Apoptotic parietal cells were identified by costaining for cleaved caspase-3 (1:50 dilution of rabbit monoclonal antibody; Cell Signaling) and H+-K+-ATPase α-subunit (1:100 dilution), as described elsewhere (8). Apoptotic zymogenic cells were identified by first immunostaining for IF (1:250 dilution of goat anti-human IF; gift from David Alpers) for 2 h at room temperature and then for cleaved caspase-3 overnight at 4°C. Secondary antibody staining with donkey anti-rabbit Cy3 and donkey anti-goat Cy2 was performed simultaneously (1:400 dilution; Jackson ImmunoResearch Labs). For identification of apoptotic mucous neck or transition cells, sections were triple-stained for cleaved caspase-3, IF, and GSII lectin by first immunostaining for caspase-3 overnight at 4°C, rinsing in 0.01% TPBS, and then incubating with IF for 2 h at room temperature, rinsing in 0.01% TPBS, and GSII staining for 1 h at room temperature. Secondary antibody staining with donkey anti-rabbit Alexa 555 and donkey anti-goat Alexa 647 was performed simultaneously (1:400 dilution; Invitrogen). Results were visualized by confocal microscopy.
Morphometric analysis.
Image J [version 1.34u, Wayne Rasband, National Institutes of Health (http://rsb.info.nig.gov/ij/)] was used to calculate epithelial area from five to nine field views per animal (n = 3 animals per age and genotype). The number of H+-K+-ATPase α-subunit-positive cells was counted, and data were expressed as number of positive cells per area of epithelium (μm2).
Quantitative RT-PCR measurement of mRNA abundance.
RNA was isolated from WT and Hip1r-deficient whole stomach (P0 and 1 wk old) or gastric corpus (3, 5, and 8 wk old, n = 3–9 per genotype), as previously described (22). RT reactions were performed with iScript (Bio-Rad) according to the manufacturer's instructions, and triplicates for each sample were amplified by PCR to measure specific mRNA concentrations (9). The primer sequences for PCR amplification are listed in Supplemental Table S1. Expression levels were normalized to the expression of Gapdh, which remained the same in WT and Hip1r-deficient samples. All quantitative RT-PCR (qRT-PCR) assays were validated by PCR of a dilution series to confirm appropriate quantitative amplification, and reaction specificity was confirmed by sequencing the amplicons.
Statistics.
GraphPad Prism software was used for statistical analysis and preparation of graphs. Quantitative data are presented as means ± SE and analyzed by Student's t-test to compare Hip1r-deficient with WT mice for each age group. P < 0.05 was considered significant.
RESULTS
Postnatal glandular morphogenesis.
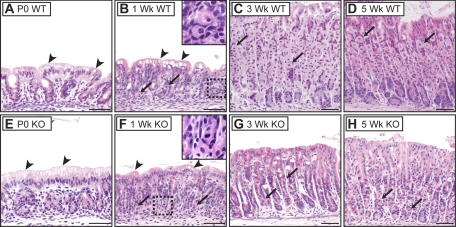
The gastric glands were rudimentary in newborn mouse stomach. Mature cell types, such as parietal cells and zymogenic cells, normally recognized by their distinctive H&E staining properties in adult stomach, were not apparent. Instead, the epithelium was composed of small glands of seemingly immature cells containing little cytoplasm, together with a distinctive surface cell type with abundant cytoplasm containing vacuoles and a basolaterally located nucleus (Fig. 1A). Proliferating cells in newborn stomach were scattered throughout the rudimentary glands, with the exception of surface cells, which were never observed to be BrdU-positive (Fig. 2A). The appearance of the newborn Hip1r-deficient stomach was similar to WT (Fig. 1E). This is in marked contrast to the extensive epithelial cell changes observed in the adult Hip1r-deficient mouse stomach (8).
Fig. 1.
Postnatal gastric epithelial cell development in wild-type (WT; A–D) and Huntingtin-interacting protein-1-related (Hip1r)-deficient (KO; E–H) mice. Paraffin sections from the corpus of newborn (P0; A and E), 1-wk-old (B and F), 3-wk-old (C and G), and 5-wk-old (D and H) mice were stained with hematoxylin-and-eosin (H&E). Glands are rudimentary at birth without identifiable mature cells until 3 wk of age. Immature parietal cells (arrows) are apparent by 1 wk. Insets: higher-magnification views of boxed regions in B and F. Note fewer and fragmented parietal cells in 3- and 5-wk-old Hip1r-deficient mouse stomach. Surface cells in young (P0 and 1-wk-old) mice contain vacuole-like structures (arrowheads). Scale bars, 50 μm.
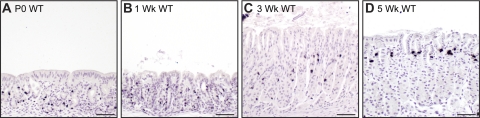
Fig. 2.
Gastric epithelial cell proliferation in WT mice during gastric gland development. Newborn (P0, A), 1-wk-old (B), 3-wk-old (C), and 5-wk-old (D) corpus was 5-bromo-2-deoxyuridine (BrdU) immunostained using diaminobenzidine (DAB) and counterstained with hematoxylin. Scattered proliferating cells were observed throughout rudimentary glands of immature stomach, with the exception of surface cells. Mature pattern of proliferation in the isthmus region was emerging at 3 wk of age. Scale bars, 50 μm.
Despite the prevalent cell proliferation observed in the newborn stomach, the stomach mucosa was similar in height at 1 wk of age, suggesting that as the overall size of the stomach grew, most newly formed cells contributed to increased numbers of gastric glands, rather than to increased gland height (Fig. 1, compare A with B). Some cellular maturation was apparent at 1 wk, with cells exhibiting parietal cell-like morphology, including centrally located nuclei and eosinophilic cytoplasmic staining, although these cells were very small compared with parietal cells in the mature stomach (Fig. 1, compare B with C, arrows). The unusual surface cell type was maintained at 1 wk, with increased numbers of cytoplasmic vacuoles. H&E staining appearance and BrdU incorporation were similar in the 1-wk-old Hip1r-deficient and WT mouse stomach (Fig. 1F; data not shown).
By 3 wk of age, WT mouse gastric glands had lengthened, with features of mature glands, including zonation into pit, isthmus, neck, and base regions. Mature-looking parietal cells were abundant in the midsection, and zymogenic cells were apparent at the base of the glands, as is normally observed in adult stomach (Fig. 1C). The unusual surface epithelial cells seen in young (P0 and 1-wk-old) stomach were replaced with cells that exhibited the characteristic morphology of normal adult surface mucous cells. Analysis of proliferation demonstrated that the proliferative isthmus zone was emerging at 3 wk of age, although some proliferating cells were still observed toward the base of the glands (Fig. 2C). The overall glandular morphology was similar in 5-wk-old WT mice, with further cellular maturation suggested by increased parietal cell size, expansion of zymogenic cells (Fig. 1D), and restriction of proliferating cells to the isthmus (Fig. 2D). Histological abnormalities were evident in H&E-stained Hip1r-deficient stomach by 3 wk of age. The mutant stomachs contained fewer and smaller parietal cells with irregular shapes (Fig. 1G, arrows). In addition, the surface mucous cell population appeared to be expanded in the mutant stomach. Both features progressed and were more prominent at 5 wk of age (Fig. 1H).
Unusual surface epithelium in young mice.
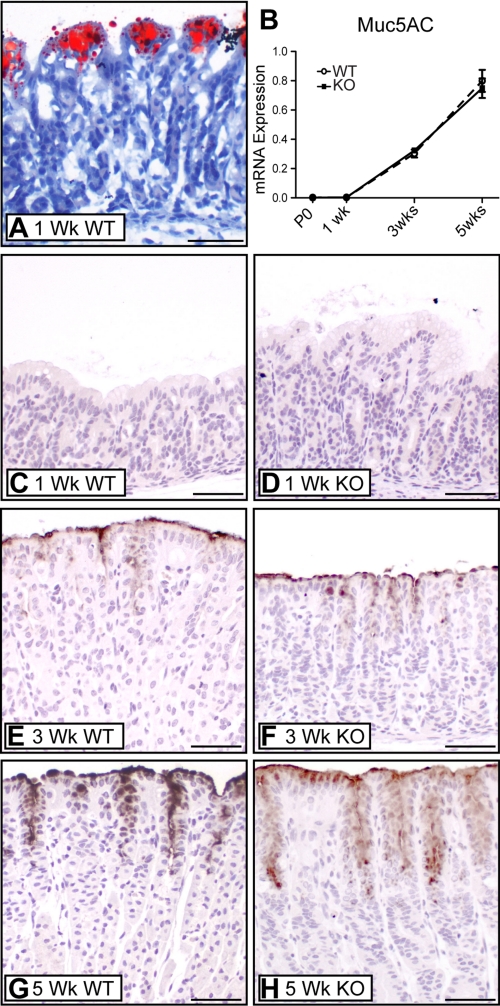
The unusual surface epithelium in young (P0 and 1-wk-old) mice was further studied to understand its relationship to the mature surface mucous cell lineage. Oil Red O staining revealed that the vacuoles observed in H&E-stained sections (Fig. 1, arrowheads) were filled with lipid, suggesting that these cells were absorbing dietary fats (Fig. 3A). The mature surface mucous cell marker Muc5AC was examined to define the timing of emergence of the mature cell type. Consistent with the distinct cellular morphology of the surface mucous cells, Muc5AC mRNA was not detected until 3 wk of age (Fig. 3B). This pattern was confirmed by immunostaining, with surface staining absent from young (P0 and 1-wk-old) mice and present at 3 wk of age (Fig. 3; data not shown). Activation of Muc5AC expression marks the timing of the morphological appearance of mature-looking surface mucous cells observed with H&E staining (Fig. 1C). Together, these results demonstrate that the surface epithelium of the newborn mouse stomach is composed of a distinct, possibly absorptive, lineage that is replaced by the mature mucous-secreting cells as the glands mature. Analysis of Hip1r-deficient mice showed a similar pattern of replacement of absorptive surface cells with Muc5AC-expressing cells, with maturation of the gastric glands at 3 wk of age (Fig. 3). In addition, as suggested from the H&E-stained sections, the Muc5AC-expressing surface mucous cells were expanded in 5-wk-old Hip1r-deficient mice (Fig. 3H). Although the histological data suggested increased Muc5AC protein staining in surface mucous cells in Hip1r-deficient mice, Muc5AC mRNA abundance was unchanged in the mutant mice (Fig. 3B), suggesting that RNA abundance and protein content do not directly correspond.
Fig. 3.
Unusual surface epithelium in young mice and emergence of mature gastric surface mucous cells upon maturation of gastric glands. A: Oil Red O-stained cryosection from 1-wk-old WT stomach. B: measurement of mucin-5AC (Muc5AC) mRNA abundance in WT and Hip1r-deficient mice by quantitative RT-PCR (qRT-PCR). Data (means ± SE) are shown in reference to Gapdh expression measured in the same samples (n = 3). C–H: Muc5AC-immunostained paraffin sections from 1-wk-old (C and D), 3-wk-old (E and F), and 5-wk-old (G and H) WT (C, E, and G) and Hip1r-deficient (D, F, and H) mice. DAB immunostaining with hematoxylin counterstaining. Note absence of staining at 1 wk of age and adult pattern of staining at 3 wk of age, with increased Muc5AC staining in Hip1r-deficient mice at 5 wk of age. Scale bars, 50 μm.
Parietal cell apoptosis in Hip1r-deficient mice.
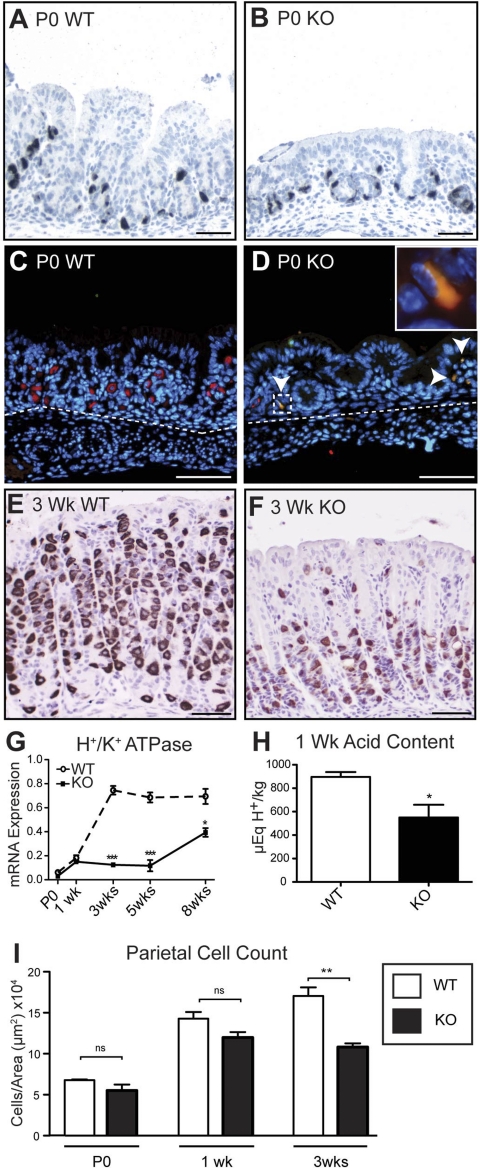
Immunostaining for the α-subunit of the H+-K+-ATPase proton pump was performed to examine parietal cell development in WT and Hip1r-deficient mice. Although parietal cells were not recognized in the H&E-stained newborn stomach, H+-K+-ATPase-immunopositive cells were observed (Fig. 4, A and B). However, the immunostained cells in the newborn stomach were very small and did not have the distinctive “fried egg” morphology characteristic of mature parietal cells. Numbers of H+-K+-ATPase-positive cells were similar in newborn Hip1r-deficient and WT mouse stomach. Parietal cells in adult Hip1r-deficient mice had previously been shown to undergo apoptotic cell death (8); thus we examined newborn stomach for apoptotic cells by coimmunostaining for activated caspase-3 and H+-K+-ATPase. Numerous costained apoptotic parietal cells were observed in the newborn Hip1r-deficient mouse stomach, while activated caspase-3-expressing cells were not observed in WT mice (Fig. 4, C and D). Apoptotic parietal cells were observed in Hip1r-deficient mouse stomach at all ages (data not shown).
Fig. 4.
Postnatal parietal cell maturation in WT and Hip1r-deficient mice. A–F: H+-K+-ATPase immunostaining of paraffin sections from WT (A, C, and E) and Hip1r-deficient (B, D, and F) mice. C and D: costaining for activated caspase-3 (green) and H+-K+-ATPase (red) showed apoptotic parietal cells in newborn Hip1r-deficient stomach (arrowheads). Inset: higher magnification of the boxed costained cell. 4,6-Diamidino-2-phenylindole dihydrochloride (DAPI) staining shows as blue. Dashed line, mucosal-submucosal boundary. E and F: H+-K+-ATPase immunostaining of paraffin sections from 3-wk-old WT and Hip1r-deficient mice. Scale bars, 50 μm. G: qRT-PCR measurement of H+-K+-ATPase α-subunit mRNA abundance in stomachs from different-aged mice. Data (means ± SE) are shown in reference to Gapdh expression measured in the same samples (n = 3). *P < 0.05; ***P < 0.001 vs. WT of the same age. H: H+ concentration in stomach contents of 1-wk-old WT and Hip1r-deficient mice. Data are means ± SE (n = 3–4). *P < 0.05. I: morphometric analysis of parietal cell number in WT and Hip1r-deficient mice at different ages, shown as numbers of H+-K+-ATPase-immunostained cells per mucosal tissue area. Data are means ± SE (n = 3 mice per age and genotype). ns, Not significant. **P < 0.01.
Reduced parietal cell numbers and fragmented morphology were apparent in 3-wk-old Hip1r-deficient mouse stomach (Fig. 4, E and F). The dynamic changes in parietal cell development and cell death were further examined by measurement of H+-K+-ATPase mRNA abundance. The results showed that expression increased with glandular maturation in WT stomach, with adult levels observed at 3 wk of age (Fig. 4G). In contrast, H+-K+-ATPase mRNA in Hip1r-deficient mice was markedly reduced, consistent with the apoptotic parietal cell loss. Morphometric analysis showed similar numbers of parietal cells in Hip1r-deficient and WT mice in immature (P0 and 1-wk-old) glands; however, parietal cell numbers were decreased at 3 wk of age, consistent with the profile of H+-K+-ATPase mRNA abundance (Fig. 4I). Although parietal cell numbers and H+-K+-ATPase mRNA abundance were similar to controls in 1-wk-old Hip1r-deficient mouse stomach, parietal cell function was impaired, as demonstrated by a significant reduction in acid content in the mutant stomach (Fig. 4H). Thus Hip1r plays a critical role in acid secretion in the immature parietal cells in very young mice.
Endocrine cell lineage development.
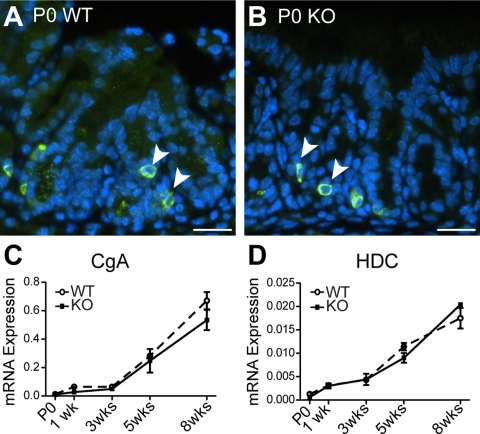
Staining for the pan-endocrine marker CgA demonstrated scattered cells at the base of the rudimentary glands in newborn mouse stomach, with apparently similar numbers of immunostained cells in WT and Hip1r-deficient mice (Fig. 5). Analysis of CgA mRNA expression showed modest expression in newborn through 3 wk of age, with a marked increase observed in 5- and 8-wk-old samples in WT and Hip1r-deficient stomach (Fig. 5C). Analysis of expression of the ECL cell-specific marker histidine decarboxylase showed a similar pattern, with marked increases in expression after 3 wk (Fig. 5D). These data suggest that enteroendocrine cells are formed in the newborn stomach, with marker expression reaching steady-state mature levels after the mice are ≥2 mo of age.
Fig. 5.
Postnatal maturation of enteroendocrine cells in WT and Hip1r-deficient mice. A and B: paraffin sections immunostained for pan-endocrine marker chromogranin A (CgA) (green; arrowheads) with DAPI (blue) nuclear stain. Scale bars, 20 μm. C and D: qRT-PCR measurement of CgA and histidine decarboxylase (HDC) mRNA abundance in stomachs from different-aged WT and Hip1r-deficient mice. Data (means ± SE) are shown relative to Gapdh expression (n = 3).
Mucous neck-zymogenic cell lineage development.
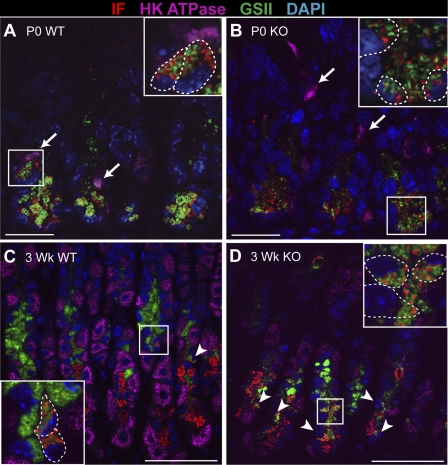
To characterize the mucous neck-zymogenic cell lineage during gastric glandular morphogenesis, we triple-stained the different-aged stomachs with GSII lectin and antibodies to IF and H+-K+-ATPase α-subunit to simultaneously detect markers for mucous neck, zymogenic, and parietal cells, respectively. The newborn stomach showed abundant staining of mucous neck cell (GSII lectin) and zymogenic cell (IF) markers at the base of the rudimentary glands (Fig. 6). Surprisingly, we observed only double-stained cells, instead of the single-positive mucous neck and zymogenic cells observed in the mature stomach. Thus the double-stained “transitional” cell appears to be the first zymogenic lineage cell type formed during gastric gland development. Confocal analysis of these double-stained cells in the newborn stomach revealed that GSII and IF were present in distinct vesicles (Fig. 6, insets). In contrast, H+-K+-ATPase was expressed in different cells (Fig. 6, arrows), demonstrating that the parietal and neck-zymogenic lineages were distinct. By 1 wk of age, single-positive cells expressing only GSII or IF were observed; however, numerous double-stained cells remained (data not shown). By 3 wk of age, the mature pattern, characterized by abundant cell populations staining with GSII or IF and only rare double-staining transitional cells, was observed in WT stomach (Fig. 6C, arrowheads).
Fig. 6.
Analysis of mucous neck-zymogenic cell lineage development in postnatal mouse stomach. Paraffin sections were triple-stained for the zymogenic cell marker intrinsic factor (IF, red), the parietal cell marker H+-K+-ATPase (magenta), and the mucous neck cell marker Griffonia simplicifolia (GSII) lectin (green) from WT (A and C) and Hip1r-deficient (B and D) newborn (P0) mice (A and B) and 3-wk-old mice (C and D). Zymogenic lineage cells from newborn mice exhibited complete colocalization of GSII lectin and IF. GSII and IF are stored within the same cell in separate granules. Arrows, parietal cells Arrowheads, GSII and IF double-positive transition cells. Insets: higher-magnification views of boxed regions. Dashed lines, cell boundaries determined by differential interference contrast microscopy. DAPI (blue) nuclear stain. Scale bars, 20 μm (P0) and 50 μm (3 wk).
The early neck-zymogenic lineage in the developing Hip1r-deficient mouse stomach was similar to that in the WT mouse stomach, with only GSII-IF double-stained cells detected in newborn stomach and single-stained mucous neck cell or zymogenic cells apparent by 3 wk of age (Fig. 6, B and D). However, the development of the mature mucous neck and zymogenic cells seemed stunted in the mutant stomach, with increased numbers of double-stained transition cells and fewer single IF-stained zymogenic cells in older (3- to 8-wk-old) mice (Fig. 6D; data not shown).
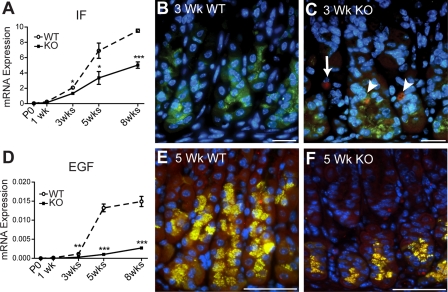
Analysis of IF mRNA abundance by qRT-PCR showed significantly reduced expression of this zymogenic cell marker in the Hip1r-deficient mouse stomach starting at 3 wk of age (Fig. 7A). Consistent with this result, histological staining showed decreased numbers of IF-stained cells in the Hip1r-deficient stomach (Figs. 6 and 7). To determine whether apoptotic zymogenic cell loss in Hip1r-deficient mice was a mechanism for fewer cells and lower IF expression, we costained for IF and activated caspase-3. Apoptotic zymogenic cells were detected in Hip1r-deficient mice at 3 wk of age (Fig. 7C, arrowheads), suggesting that cellular remodeling in the mutant mouse includes loss of zymogenic lineage cells by apoptosis. Cells costaining for IF and caspase-3 were observed in stomachs of older mutant (5- and 8-wk-old) mice as well, but not in stomachs of young (P0 and 1-wk-old) Hip1r-deficient mice (data not shown). Apoptotic zymogenic cells were never observed in WT stomach. To determine whether mucous neck or transition cells in Hip1r-deficient mice were also apoptotic, we triple-stained 3- and 5-wk-old stomachs for active caspase-3, IF, and GSII lectin. The results showed caspase costaining limited to IF-positive cells, but no caspase-3-positive GSII-labeled cells (data not shown). Thus only mature zymogenic cells, and not mucous neck or transition cells, were undergoing apoptotic cell death in the Hip1r-deficient mouse stomach.
Fig. 7.
Apoptotic loss of zymogenic cells in Hip1r-deficient mouse stomach. A: qRT-PCR measurement of IF mRNA abundance in WT and Hip1r-deficient mouse stomach. Data (means ± SE) are shown relative to Gapdh expression measured in the same samples (n = 3). *P < 0.05; ***P < 0.001 vs. WT of the same age. B and C: costaining for activated caspase-3 (red) and IF (green) showed apoptotic zymogenic cells in 3-wk-old Hip1r-deficient stomach (arrowheads). Other caspase-3-positive cells that did not costain (arrow) are likely to be parietal cells. Scale bars, 20 μm. D: qRT-PCR measurement of EGF mRNA abundance in WT and Hip1r-deficient mouse stomach. Data (means ± SE) are shown relative to Gapdh expression measured in the same samples (n = 3) **P < 0.01; ***P < 0.001 vs. WT of the same age. E and F: coimmunostaining of paraffin sections for EGF (red) and IF (green) showed colocalization in zymogenic cells in WT and Hip1r-deficient mice. DAPI (blue) nuclear stain. Scale bars, 50 μm.
Several different EGF family members are expressed in the gastric epithelium. We examined EGF ligands, including EGF, amphiregulin, betacellulin, heparin binding-EGF, and TGFα, as well as their receptor (EGFR), in WT and Hip1r-deficient mice at 5 wk of age by qRT-PCR measurement of mRNA abundance. With the exception of EGF, which was dramatically reduced in the Hip1r-deficient stomach, mRNA levels for the other ligands and the receptor were similar in the mutant and WT mice (Fig. 7D; data not shown). Further analysis of EGF mRNA abundance at all ages demonstrated abundant EGF expression in WT mice, with strong induction observed between 3 and 5 wk of age. In contrast, EGF expression in the mutant mouse was markedly reduced (Fig. 7D). The cellular source of EGF in the stomach had not been previously described; thus we stained gastric sections with an antibody to EGF and noted localization to cells in the base of the glands. Furthermore, coimmunostaining with antibodies to IF demonstrated that EGF is specifically expressed in zymogenic cells in the mouse stomach (Fig. 7E). Reduced number of EGF-expressing cells in Hip1r-deficient mice is consistent with the apoptotic loss of zymogenic cells in the mutant stomach (Fig. 7F).
Development of SPEM and glandular hypertrophy in Hip1r-deficient mice.
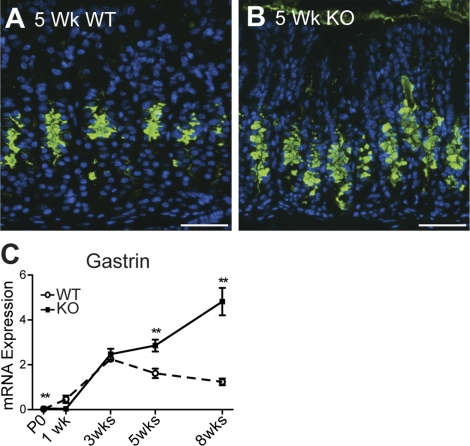
As Hip1r-deficient mice age, they develop a progressive mucous cell transformation (8). This aberrant mucous cell lineage shares features with the metaplastic lineage SPEM. We tested the timing of SPEM development in the Hip1r-deficient mice by immunostaining for TFF2. The results showed increased numbers of TFF2-positive cells emerging in the mutant stomach at 5 wk of age (Fig. 8). This feature was consistently observed in the more mature Hip1r-deficient mice, but not in mice younger than 5 wk of age. The delayed development of SPEM is consistent with the notion that this is a secondary response to the parietal cell changes.
Fig. 8.
Development of gastric metaplasia in Hip1r-deficient mice. A and B: immunostaining of paraffin sections from 5-wk-old WT (A) and Hip1r-deficient (B) mice for trefoil factor 2 (TFF2, green) with DAPI (blue) nuclear stain. Scale bars, 50 μm. C: qRT-PCR measurement of gastrin mRNA abundance in WT and Hip1r-deficient mouse antrum. Data (means ± SE) are shown relative to Gapdh expression measured in the same samples (n = 3–9). **P < 0.01 vs. WT of the same age.
Previous studies demonstrated increased proliferation and glandular hypertrophy in adult Hip1r-deficient mice (8). To determine the timing of these alterations in the mutant stomach, we examined gland length and proliferation in the various ages. Analysis of H&E-stained paraffin sections and measurement of proliferation by BrdU staining showed that these features did not emerge until after 5 wk of age (Figs. 1 and 2). Since we previously showed that hypergastrinemia was responsible for increased proliferation and glandular hypertrophy in 2- to 3-mo-old Hip1r-deficient mice (8), we measured gastrin mRNA levels by qRT-PCR to determine the timing of increased expression. Gastrin transcripts in WT stomach increased steadily after birth, achieving mature levels by 3 wk of age (Fig. 8C). In the Hip1r-deficient stomach, gastrin mRNA levels were similar to WT before weaning but differed markedly after 3 wk of age, with increased expression as the animals matured. The increased gastrin expression and subsequent increases in glandular proliferation induced by hypergastrinemia are also likely secondary responses to the loss of parietal cell acid secretion.
DISCUSSION
This study investigated the process of epithelial cell growth and differentiation in the postnatal mouse stomach to follow gastric gland formation in the acid-secreting corpus of WT and Hip1r-deficient mice. Gastric epithelial cytodifferentiation is initiated in mid-to-late gestation, with expression of gastric lineage markers first detected at embryonic day 15.5–16.5 in the mouse (26, 30). However, cells expressing differentiated cell markers remain immature for several days while the stomach mucosa undergoes active proliferation during the period of rapid organ growth. The newborn mouse stomach is composed of rudimentary glandular buds containing scattered, small cells that express low levels of the mRNA markers for three of the four mature lineages and stain for the parietal cell marker H+-K+-ATPase, the endocrine cell marker CgA, and the mucous neck-zymogenic lineage markers GSII lectin and IF. Although the cells in the rudimentary gastric glands of the perinatal mouse appear immature, there is modest differentiated cell function. Low levels of acid secretion and pepsin activity have been described in young rat stomach (7, 11), and in this study we detected changes in gastric acidity in the Hip1r-deficient mouse at 1 wk of age, consistent with reduced parietal cell function in the mutant.
The mouse stomach matures in the late suckling period, with glandular morphogenesis completed near the time of weaning at 3 wk of age (18, 19). This timing coincides with the general timing of maturation of digestive function in other gastrointestinal tissues, including salivary glands, intestine, and pancreas, with digestive enzymes commonly induced during the late suckling period (7). This timing in the mouse corresponds to the dietary transition at the time of weaning, although studies have implicated hormonal control by glucocorticoids and thyroxine, and not diet, as important regulators of the maturation process (7, 11). Although the basic cellular architecture of the gastric glands is established by 3 wk of age in the mouse, our analysis of lineage markers demonstrated that the timing of maturation for each of the four gastric lineages is not synchronous. A summary of our findings of the cellular development of WT stomach during the suckling period is presented in Table 1. Parietal cells are the first to mature, with adult levels of H+-K+-ATPase mRNA detected once the glands are formed at 3 wk of age. However, the other gastric lineages exhibited a slower developmental time frame, with mRNA levels for cell-specific markers reaching a plateau sometime around or after 8 wk of age. Thus studies of adult stomach should be performed on mice over 2 mo of age to ensure full maturity of the oxyntic mucosa.
Table 1.
Summary of epithelial cell development in mouse stomach during the suckling period
| Age |
||||
|---|---|---|---|---|
| Cell Lineage | Marker | P0 | 1 wk | 3 wk |
| Surface | ||||
| Mucous | Muc5AC | Absent | Absent | Increasing |
| Absorptive | Lipid droplets | Present | Present | Absent |
| Parietal | H+-K+-ATPase | Immature | Immature | Mature |
| Mucous neck/zymogenic | ||||
| Mucous neck | GSII lectin | Absent | Emerging | Present |
| Transition | GSII and IF | Present | Common | Rare |
| Zymogenic | IF, EGF | Absent | Emerging | Increasing |
| Endocrine | CgA | Few | Few | Increasing |
| Proliferating progenitor | BrdU | Scattered | Scattered | Isthmus region |
The cell changes were determined from analysis of mRNA abundance (determined by quantitative RT-PCR analysis) and by histochemical staining properties. Absent, mature cell type not observed; increasing, mRNA abundance for cell-specific marker not yet at adult steady-state level; immature, small cells that express cell-specific marker; scattered, proliferating cells scattered within the glands (not surface cells). P0, newborn; Muc5AC, mucin 5AC; GSII, Griffonia simplicifolia; IF, intrinsic factor; CgA, chromogranin A; BrdU, 5-bromo-2-deoxyuridine.
The gastric surface cell population undergoes a dramatic remodeling during the suckling period. Our analysis showed that the young (P0 and 1-wk-old) mouse stomach contains an apparently absorptive cell type that is replaced by mature surface mucous cells as glandular morphogenesis proceeds. Previous studies noted the presence of lipid droplets in the cytoplasm of gastric surface cells in suckling rats and mice, which appear to form upon absorption of medium-chain fatty acids (3, 6). How this absorptive surface cell type relates to the mature surface mucous cell lineage is not clear. A marker of the mature surface mucous cell type (Muc5AC) is not observed until after 1 wk of age, with replacement of the absorptive lineage by Muc5AC-expressing cells completed by 3 wk of age. The gastric surface epithelium in suckling mice likely facilitates nutrient absorption while intestinal digestive and absorptive function are known to be immature.
Comparison of the cellular composition of the developing Hip1r-deficient mouse stomach with that of the developing WT mouse stomach provided insight into the cellular events leading to the gastric epithelial cell transformation previously described for this mutant. Hip1r, together with Hip1, comprises a family of F-actin- and clathrin-binding proteins that function broadly in membrane trafficking (5). Hip1r is abundantly expressed in the gastric parietal cell, localizing to the acid-secreting canalicular membranes; accordingly, loss of Hip1r results in alterations in parietal cell vesicular trafficking and acid secretion (8). However, the cellular changes in the adult Hip1r-deficient mouse stomach are multifaceted, including glandular hypertrophy, loss of zymogenic cells, and expansion of an aberrant mucous neck cell population, in addition to dysfunction and apoptotic loss of parietal cells (8). In contrast to the severe epithelial cell changes observed in adult Hip1r-deficient mouse stomach, changes in the immature mutant stomach were initially limited to parietal cell apoptosis and reduced acid secretion; alterations in the other epithelial cell lineages were not apparent until later ages. These data, which are summarized in Table 2, suggest that the primary defect in the Hip1r-deficient mouse stomach is at the level of the parietal cell, with more widespread epithelial cell transformation developing over time as a consequence of parietal cell loss or dysfunction.
Table 2.
Summary of epithelial cell changes in Hip1r-deficient mouse corpus
| Cell Lineage | Immature Glands (P0–1 wk) | Mature Glands (3–5 wk) |
|---|---|---|
| Surface | Unchanged | Increased Muc5AC staining at 5 wk |
| Parietal | Apoptotic cells* | Fewer due to apoptotic cell death |
| Mucous neck/zymogenic | ||
| Mucous neck | Unchanged | Increased TFF2 staining at 5wk |
| Transition | Unchanged | Increased |
| Zymogenic | Unchanged | Fewer due to apoptotic cell death |
| Endocrine | Unchanged | Unchanged |
Cell lineage changes were assessed by comparison of cell type-specific mRNA abundance and histochemical staining properties of Huntingtin interacting protein 1-related (Hip1r)-deficient mice with wild-type mice. TFF2, trefoil factor 2.
Earliest detected cellular difference between Hip1r-deficient and wild-type stomach.
Parietal cells are critical for the development and maintenance of the mucous neck-zymogenic cell lineage. Mouse models with parietal cell loss show reduced numbers of zymogenic cells and expansion of an aberrant mucous neck cell population termed SPEM. These cellular changes have been observed in models of acute parietal cell loss resulting from the toxin DMP 777 (25), with parietal cell atrophy associated with Helicobacter infection (33) or parietal cell dysfunction resulting from autoimmune destruction (22) or apoptotic cell loss (8). The critical function provided by the parietal cell has not been described. However, the parietal cell is known to be the source of several different growth factors, including EGF ligands, parathyroid hormone-like hormone, and sonic hedgehog (10, 37). A recent report of the phenotype of parietal cell-specific loss of sonic hedgehog demonstrated a complex cellular phenotype that included reduced numbers of zymogenic cells and increased numbers of GSII-IF double-staining cells, both of which were observed in the Hip1r-deficient mouse (36). The similar cellular changes suggested that reduced sonic hedgehog resulting from parietal cell loss might be the mechanism of the mucous neck-zymogenic lineage changes in the Hip1r-deficient mouse. However, analysis of hedgehog pathway signaling in Hip1r-deficient mice by measurement of ligand (sonic and Indian hedgehog) and target (Gli1 and Patched1) gene expression did not show reduced levels (data not shown). Thus it is unlikely that reductions in hedgehog signaling are responsible for the secondary cellular remodeling subsequent to parietal cell dysfunction in the Hip1r-deficient mouse.
We observed increased surface mucous cell staining in Hip1r-deficient mice as they aged (>5 wk). Overexpression of the EGF ligand TGFα is known to induce an expansion of this cell population in transgenic mice and in patients with Ménétrier's disease (2, 4, 29). However, we determined that expression of TGFα or other EGF ligands is not increased in Hip1r-deficient mice, which, instead, exhibit a specific reduction in EGF resulting from the loss of zymogenic cells (Fig. 7). Previous studies by the Goldenring group (24, 27) demonstrated apparently normal gastric gland development in the basal state in mouse strains with reduced EGF receptor signaling due to loss of specific ligands or to a hypomorphic receptor mutation. However, some, but not all, of these mutant strains are more sensitive to SPEM induction after treatment with the parietal cell toxin DMP 777 (24, 27). Perhaps the loss of EGF in the Hip1r-deficient mice may contribute to the spontaneous development of SPEM that we observed. High levels of gastrin have also been demonstrated to induce surface mucous cell hyperplasia (20, 34). Thus our observation that increased gastrin expression occurred simultaneously with increased Muc5AC staining suggests that hypergastrinemia may be the primary cause of the expansion of this cell lineage.
Our study made the novel finding that the initial cell type formed during the development of the mucous neck-zymogenic lineage coexpresses markers of mucous neck cells (GSII lectin) and zymogenic cells (IF). Only GSII-IF double-staining cells were observed in the newborn mouse stomach, with single-positive cells starting to emerge by 1 wk of age. In contrast, adult stomach has been described to first form a progenitor cell population of mucous neck cells, which develop into zymogenic cells as they transition to the base of the gastric glands (16). The transition from mucous neck cell to zymogenic cell is marked by a cell type that expresses GSII and IF, similar to the immature cell we describe in the perinatal stomach (28). Interestingly, SPEM development also includes activation of double-staining cells (35). The relationship between the GSII-IF double-staining cells in the immature perinatal stomach, the double-staining transition cells in the adult, and the double-staining cells observed with SPEM are unknown. However, it is interesting to speculate that loss of a parietal cell factor may cause zymogenic cells to revert to a double-staining immature cell type as one aspect of SPEM development. We also observed apoptotic cell death of zymogenic cells in Hip1r-deficient mice, suggesting that cell death, as well as possible cellular dedifferentiation or transdifferentiation, may explain the zymogenic cell response to parietal cell dysfunction.
In summary, these studies have described the cellular changes associated with the development of the gastric glands in the mouse. We demonstrated that the immature perinatal stomach contains two unusual cell types: an apparently absorptive surface cell and GSII-IF double-positive precursor cells. The Hip1r-deficient mice exhibited parietal cell apoptosis at the earliest time point studied, demonstrating that this trafficking protein is critical for parietal cell survival. A progressive epithelial cell transformation, including SPEM, occurs in these mice as they age. Our analysis suggests that the epithelial cell transformation events are secondary to the loss or dysfunction of parietal cells, which supports the important role of the parietal cell in homeostasis of the gastric mucosa. Finally, our studies demonstrate that mRNA abundance for epithelial cell-specific markers does not reach mature, steady-state levels until well after gastric gland morphogenesis is complete, suggesting that the mouse stomach does not reach homeostasis until 2 mo of age.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant RO1-DK-078926 (L. C. Samuelson). Morphology Core support was provided by NIDDK Gastrointestinal Hormone Research Center Grant P30-DK-34933.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kelli VanDussen and Andrea Todisco for critical comments on the manuscript, Stephen Lentz for assistance with confocal microscopy, and Jonathan Ducastel and Katie Hamelink for maintenance of the mouse colonies.
REFERENCES
- 1.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol 325: 211–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dempsey PJ, Goldenring JR, Soroka CJ, Modlin IM, McClure RW, Lind CD, Ahlquist DA, Pittelkow MR, Lee DC, Sandgren EP, et al. Possible role of transforming growth factor-α in the pathogenesis of Menetrier's disease: supportive evidence form humans and transgenic mice. Gastroenterology 103: 1950–1963, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Egelrud T, Olivecrona T, Helander H. Studies on gastric absorption of lipids in the suckling rat. Scand J Gastroenterol 6: 329–333, 1971 [DOI] [PubMed] [Google Scholar]
- 4.Goldenring JR, Ray GS, Soroka CJ, Smith J, Modlin IM, Meise KS, Coffey RJ., Jr Overexpression of transforming growth factor-α alters differentiation of gastric cell lineages. Dig Dis Sci 41: 773–784, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Gottfried I, Ehrlich M, Ashery U. The Sla2p/HIP1/HIP1R family: similar structure, similar function in endocytosis? Biochem Soc Trans 38: 187–191, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Helander HF, Olivecrona T. Lipolysis and lipid absorption in the stomach of the suckling rat. Gastroenterology 59: 22–35, 1970 [PubMed] [Google Scholar]
- 7.Henning SJ. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol Gastrointest Liver Physiol 241: G199–G214, 1981 [DOI] [PubMed] [Google Scholar]
- 8.Jain RN, Al-Menhali AA, Keeley TM, Ren J, El-Zaatari M, Chen X, Merchant JL, Ross TS, Chew CS, Samuelson LC. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest 118: 2459–2470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RN, Brunkan CS, Chew CS, Samuelson LC. Gene expression profiling of gastrin target genes in parietal cells. Physiol Genomics 24: 124–132, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Jain RN, Samuelson LC. Differentiation of the gastric mucosa. II. Role of gastrin in gastric epithelial cell proliferation and maturation. Am J Physiol Gastrointest Liver Physiol 291: G762–G765, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Johnson LR. Functional development of the stomach. Annu Rev Physiol 47: 199–215, 1985 [DOI] [PubMed] [Google Scholar]
- 12.Kang W, Rathinavelu S, Samuelson LC, Merchant JL. Interferon-γ induction of gastric mucous neck cell hypertrophy. Lab Invest 85: 702–715, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec 236: 314–332, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236: 259–279, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec 236: 280–296, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec 236: 297–313, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 236: 333–340, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Karam SM, Li Q, Gordon JI. Gastric epithelial morphogenesis in normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 272: G1209–G1220, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Kataoka K, Sakano Y, Miura J. Histogenesis of the mouse gastric mucosa, with special reference to type and distribution of proliferative cells. Arch Histol Jpn 47: 459–474, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Konda Y, Kamimura H, Yokota H, Hayashi N, Sugano K, Takeuchi T. Gastrin stimulates the growth of gastric pit with less-differentiated features. Am J Physiol Gastrointest Liver Physiol 277: G773–G784, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem 271: 3671–3676, 1996 [PubMed] [Google Scholar]
- 22.Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol 290: G970–G979, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mills JC, Syder AJ, Hong CV, Guruge JL, Raaii F, Gordon JI. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci USA 98: 13687–13692, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology 132: 1804–1819, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol 288: G362–G375, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 signaling controls stomach morphogenesis. Dev Biol 303: 295–310, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa M, Nomura S, Varro A, Wang TC, Goldenring JR. Altered metaplastic response of waved-2 EGF receptor mutant mice to acute oxyntic atrophy. Am J Physiol Gastrointest Liver Physiol 290: G793–G804, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134: 211–222, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Sharp R, Babyatsky MW, Takagi H, Tagerud S, Wang TC, Bockman DE, Brand SJ, Merlino G. Transforming growth factor-α disrupts the normal program of cellular differentiation in the gastric mucosa of transgenic mice. Development 121: 149–161, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Spencer-Dene B, Sala FG, Bellusci S, Gschmeissner S, Stamp G, Dickson C. Stomach development is dependent on fibroblast growth factor 10/fibroblast growth factor receptor 2b-mediated signaling. Gastroenterology 130: 1233–1244, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Spicer Z, Miller ML, Andringa A, Riddle TM, Duffy JJ, Doetschman T, Shull GE. Stomachs of mice lacking the gastric H,K-ATPase α-subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem 275: 21555–21565, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1β promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology 138: 562–572, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology 114: 675–689, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918–1929, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer 12: 189–197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138: 550–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavros Y. The adventures of sonic hedgehog in development and repair. IV. Sonic hedgehog processing, secretion, and function in the stomach. Am J Physiol Gastrointest Liver Physiol 294: G1105–G1108, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, Gumucio G, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282: 33265–33274, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.