Abstract
Hypoxia stimulates pulmonary artery smooth muscle cell (PASMC) proliferation. Recent studies have implicated an important role for microRNAs (miRNAs) in hypoxia-mediated responses in various cellular processes, including cell proliferation. In this study, we investigated the role of microRNA-21 (miR-21) in hypoxia-induced PASMC proliferation and migration. We first demonstrated that miR-21 expression increased by ∼3-fold in human PASMC after 6 h of hypoxia (3% O2) and remained high (∼2-fold) after 24 h of hypoxia. Knockdown of miR-21 with anti-miR-21 inhibitors significantly reduced hypoxia-induced cell proliferation, whereas miR-21 overexpression in normoxia enhanced cell proliferation. We also found that miR-21 is essential for hypoxia-induced cell migration. Protein expression of miR-21 target genes, specifically programmed cell death protein 4 (PDCD4), Sprouty 2 (SPRY2), and peroxisome proliferator-activated receptor-α (PPARα), was decreased in hypoxia and in PASMC overexpressing miR-21 in normoxia and increased in hypoxic cells in which miR-21 was knocked down. In addition, PPARα 3′-untranslated region (UTR) luciferase-based reporter gene assays demonstrated that PPARα is a direct target of miR-21. Taken together, our findings indicate that miR-21 plays a significant role in hypoxia-induced pulmonary vascular smooth muscle cell proliferation and migration by regulating multiple gene targets.
Keywords: lentiviral pri-miR overexpression, anti-miR inhibitor, pulmonary vascular smooth muscle
mirnas are single-stranded RNA molecules of about 21–23 nucleotides in length that regulate gene expression by interacting with the 3′-untranslated regions (UTR) of specific mRNA targets and inhibiting translation (3). In humans, there are currently ∼772 miRNAs listed in the Sanger miRNA registry miRBase 12.0 (1, 24–26), and according to published estimates there are 1,000 or more human miRNAs that target up to 30% of the human genome (36). It is also predicted that on an average, a single miRNA can have more than 100 targets (5). MiRNAs play important roles in the regulation of diverse cellular processes, including proliferation, differentiation, and apoptosis (10, 15), in cardiac (52, 53, 59), skeletal (11), and smooth muscle (13). MicroRNA-143 (miR-143) and miR-145 have been shown to regulate proliferation and differentiation of vascular smooth muscle cells (SMC), thus linking the function of miRNAs to SMC plasticity and fate determination (13, 44).
From recent reports on hypoxia-regulated miRNAs (7, 14, 18, 20, 29, 32–34), miR-210 has been identified as being the most upregulated in hypoxia. MiR-21 is also highly upregulated in hypoxia in tumors and tumor cell lines (61, 62), suggesting that it may have an important role in cell growth and proliferation. Interestingly, a variety of miRNAs can be downregulated by hypoxia (27, 29).
Chronic hypoxia is a well-known trigger of pulmonary vascular remodeling resulting in pulmonary hypertension, which is associated with SMC proliferation (40, 60), but the cellular and molecular mechanisms involved in these proliferative responses are still not completely understood. A recent study demonstrated increased expression of miR-451 and miR-30c in models of pulmonary arterial hypertension (PAH) in rats induced by monocrotaline and chronic hypoxia and differential expression of miR-21 and let-7a in the two models (8). It has also been reported that miR-17-5 and miR-20a, via the STAT3-miR-17/92-bone morphogenetic protein (BMP) receptor type II (BMPR2) pathway, play a role in the regulation of BMPR2, a key determinant of most cases of idiopathic familial pulmonary hypertension (6). Nevertheless, information on miRNA regulation of pulmonary artery SMC is limited at this time. The objective of this study was to investigate the role of miR-21 in hypoxia-induced proliferation and migration in human pulmonary artery SMCs (HPASMCs).
MATERIALS AND METHODS
Cell culture.
HPASMCs were obtained from Lonza (cat. no. CC-0237) and maintained in SmGM-2 BulletKit media (Lonza) containing 5% FBS, growth factors, and antibiotics. Cells were cultured in a humidified incubator with a constant supply of 5% CO2 at 37°C. Cells were subcultured at subconfluence and used for experiments at passages 5–10.
Normoxia and hypoxia conditions.
Cells were made hypoxic by placing them in a special hypoxia incubator into which a gas mixture of 5% CO2 and balance nitrogen was used to keep oxygen concentrations within the chamber at 3%. For normoxia experiments, cells were incubated in a humidified incubator with a constant supply of air (21% O2) with 5% CO2 at 37°C. Based on our previous studies (43), the Po2 in the cell medium was ∼30 Torr during hypoxia and ∼100 Torr during normoxia.
Quantitative RT-PCR.
Total RNA was extracted using TRIzol reagent (Invitrogen), treated with TURBO DNase I (Applied Biosystems) to remove genomic DNA contamination, and quantified using the NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). Identical starting concentrations of total RNA were used for all samples treated for normoxia/hypoxia. Total RNA was then reverse-transcribed to cDNA using a miScript Reverse Transcription Kit (Qiagen). Change in expression of various miRNA levels was determined quantitatively using quantitative RT-PCR (qRT-PCR). The miScript SYBR Green PCR Kit (Qiagen) containing a QuantiTect SYBR Green PCR Master Mix and the miScript Universal Primer along with the miRNA-specific primer was used for the detection of mature miRNAs. Amplification and detection of the PCR products was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems). U6 primers were used as internal controls for RNA template normalization since they did not show any difference in expression levels under normoxic or hypoxic conditions (data not shown).
MiR-21 inhibition.
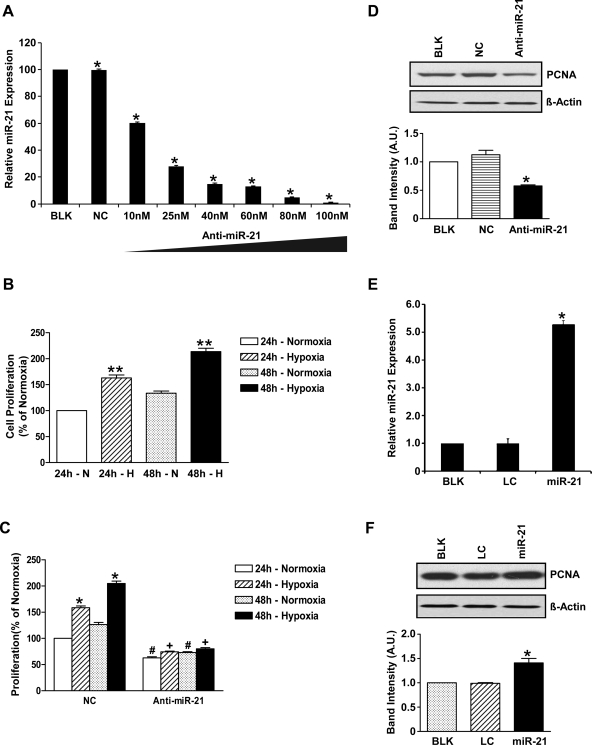
We used an anti-miR-21 inhibitor purchased from Ambion (Applied Biosystems) to inhibit miR-21 in HPASMCs. An anti-miR negative inhibitor was used as a negative control. Cells at ∼70% confluence after overnight culture on 60-mm petri dishes were transfected with vehicle (phosphate-buffered saline), negative inhibitor, or anti-miR-21 inhibitors by Lipofectamine 2000 (Invitrogen). After 6-h transfection, fresh medium was added, and the cells were cultured for 24 h and then exposed to either hypoxic or normoxic conditions for 24 h. The effectiveness of the exogenously introduced miRNA inhibitor against miR-21 was first determined by using varying concentrations of the inhibitor ranging from 10 to 100 nM. Based on the amount of inhibition of miR-21 expression at different inhibitor concentrations, 60 nM inhibitor was chosen for all miR-21 inhibition experiments (Fig. 2A).
Fig. 2.
MiR-21 plays a role in HPASMC proliferation. A: miR-21 expression at different concentrations of anti-miR-21 inhibitor oligonucleotides. Data are shown as means ± SE (n = 3). *P < 0.05 vs. “blank” control HPASMCs (BLK). NC, negative control HPASMCs transfected with control inhibitor oligonucleotides; anti-miR-21, HPASMCs transfected with anti-miR-21 inhibitor oligonucleotides. B: cell proliferation measured as percentage of normoxia. Hypoxia induced cell proliferation in control HPASMCs after 24- and 48-h hypoxia. **P < 0.005 compared with 24-h normoxia. C: there is a significant increase in cell proliferation in hypoxia at 24 and 48 h compared with normoxic cells with negative inhibitor (NC) at the same time points; *P < 0.05. When miR-21 was inhibited, cell proliferation was significantly decreased even in normoxia after 24 and 48 h; #P < 0.05 vs. normoxia NC. Hypoxia in anti-miR-21 cells after 24 and 48 h resulted in a small but significantly lower increase in cell proliferation compared with untreated cells (approximately 7 and 11%, respectively; +P < 0.05). D: miR-21 inhibition resulted in 50% reduction in PCNA expression as depicted in the scanned Western blot. β-Actin was used as loading control. *P < 0.05 in the anti-miR-21 cells compared with controls. A.U., arbitrary units. E: miR-21, lentiviral HPASMCs overexpressing miR-21. Data shown are means ± SE (n = 3); *P < 0.05 vs. controls [BLK and lentiviral control (LC) HPASMCs]. F: scanned Western blot and densitometric measurements showing increased PCNA expression in miR-21-overexpressing cells confirming that miR-21 plays a role in HPASMC proliferation. Data shown are means ± SE (n = 3); *P < 0.05 vs. controls (BLK and LC).
Lentiviral pri-miR-21 overexpression.
HPASMCs overexpressing miR-21 were generated using the Lenti-X lentiviral expression system (Clontech). We used a Lenti-X HT Packaging System in which Lenti-X expression vector containing an enhanced green fluorescent protein (EGFP) reporter gene followed by primary (pri-) miR-21 sequence was cotransfected along with a Lenti-X HT Packaging Mix into the 293T Cell Line using Lipofectamine 2000. The pri-miR-21 was amplified from human genomic DNA with the forward primer 5′-CACCTCGAGCCTTTAGGAGCATTATGAGC-3′ and reverse primer 5′-GAGAATTCATCCTCCCTCCATACTGCTG-3′. The PCR product size was 402 bp. Lentiviral supernatants produced by the transfected packaging cells were then used to infect and transduce target cells (HPASMCs) along with Polybrene (4 μg/ml). MiR-21-overexpressing cells were selected with 1.5 μg/ml puromycin. All experiments with miR-21-overexpressing cells involved the use of appropriate lentiviral negative controls (control lentiviral cells expressing EGFP without miRNA sequence) and uninfected HPASMC controls.
Western immunoblot analysis.
Cell lysates were prepared from cells exposed to hypoxia or normoxia. Total protein from cells was isolated using cell lysis buffer (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% IGEPAL, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate) containing protease and phosphatase inhibitor cocktails (Sigma-Aldrich) and centrifuged, the supernatants were collected, and protein concentration was determined using a conventional Coomassie Bradford protein assay kit (Bio-Rad). Equal amounts of total protein (∼50 μg) from cells were subjected to SDS-PAGE on 4–12% Tris-glycine gels (Invitrogen) and transferred to nitrocellulose membrane. Membranes were blocked for 1 h at room temperature in Tris-buffered saline with 0.05% Tween 20 (TBST) containing 5% nonfat powdered milk and probed with primary antibody in TBST with 5% nonfat powdered milk overnight at 4°C. In all cases, a secondary antibody labeled with horseradish peroxidase (Jackson ImmunoResearch) was used at dilutions of 1:10,000 for 1 h at room temperature, and the protein bands were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce). The relative band intensities were quantified by densitometry using NIH ImageJ software (National Institutes of Health) and normalized with image densities of β-actin that were used as loading controls. The primary antibodies used for this study included rabbit polyclonal anti-human PCNA (1:2,000 dilution; Proteintech Group), rabbit polyclonal anti-human bestrophin 3 (BEST3; 1:1,000 dilution; FabGennix), rabbit polyclonal anti-human β-actin (1:2,000 dilution), rabbit polyclonal anti-human peroxisome proliferator-activated receptor-α (PPARα; 1:1,000 dilution), mouse monoclonal anti-human programmed cell death protein 4 (PDCD4; 1:1,000 dilution), and rabbit polyclonal anti-human Sprouty 2 homolog of Drosophila (SPRY2; 1:1,000 dilution), all from Santa Cruz Biotechnology.
Cell growth and proliferation assay.
HPASMC proliferation was determined by in vitro cell counting and PCNA immunoblotting. To study the effect of miR-21 inhibition on hypoxia-induced cell proliferation, three groups of transfected cells were used, blank control group (vehicle), negative control group (transfected with control miRNA oligonucleotide), and anti-miR-21 inhibitor group. For studying the effect of miR-21 overexpression on hypoxia-induced cell proliferation, three groups of cells were used, uninfected HPASMC control group, lentiviral control group (expressing EGFP alone), and miR-21 group (overexpressing miR-21). Equal numbers of cells were used, and cells were counted both before and after normoxia and hypoxia treatments. Cells were starved in SmGM-2 medium containing 0.2% FBS for 16 h to achieve quiescence. The medium was then replaced with complete medium and normoxia or hypoxia exposure was carried out for 24 and 48 h. For cell counting, cells were detached by trypsin and resuspended in a measured volume of complete medium and then counted using a standard Neubauer chamber (hemocytometer). PCNA Western blot studies were performed (as described in Western immunoblot analysis). All experiments were performed in triplicate.
Wound-healing migration assay.
HPASMCs were grown to confluence on 60-mm petri dishes. To study the effect of miR-21 inhibition on hypoxia-induced cell migration, the same three groups of cells were used as described earlier for proliferation experiments. Cells were growth-arrested for 16 h in SmGM-2 containing 0.2% FBS (starvation medium). A scratch was made in the cell monolayer, medium was replaced by complete medium, baseline (0-h time point) images were captured, and the dishes were then exposed to hypoxia or normoxia. Cell migration was assessed at 24 h by counting the number of cells that had migrated across the scratch (43), and this number was normalized to the scratch area. Data were expressed as percentage of control. Cell migration data were obtained from three independent wound-healing experiments.
Boyden chamber migration assay.
Cell migration was also studied with an 8-μm pore size Transwell chemotaxis cell migration assay kit (Corning Life Sciences). As described previously for proliferation experiments involving miR-21 inhibitors, we used the same three groups of transfected cells (vehicle, negative control inhibitor, and anti-miR-21 inhibitor) for these experiments. Briefly, the cells were first treated with normoxia or hypoxia and then harvested, and 1 × 105 cells were plated into each well of the migration chamber, with experiments performed in triplicate for each condition. SmGM-2 medium with FBS was added to the bottom wells and incubated for 24 h at 37°C in normoxia or hypoxia. Migration assay was also performed with transfected cells. Cells that did not migrate were gently discarded from the top. The migration chamber plate was then removed, and the cells on the top of the membrane were removed using cotton swabs. Cells that had migrated to the bottom were fixed in methanol for 2 min. They were stained with 0.5% crystal violet in 2% methanol for 2 min. The inserts were rinsed in water, and five random microscopic fields at ×200 magnification were counted in each filter using a calibrated ocular grid. Experiments were carried out in triplicate. Data are expressed as the average number of cells per field ± SE and expressed as percentage of control.
3′-UTR luciferase assay.
A 653-bp fragment of PPARα 3′-UTR, which contains the putative binding site for miR-21, was amplified from human genomic DNA by PCR with the primers 5′-GAGAATTCGTGGCCTGTCTTCCCATTCACC-3′ (forward) and 5′-GGTCTAGAGGGCTCTTCTACGCAAACCTGG-3′ (reverse). After double digestion with EcoR I and Xba I restriction enzymes, PPARα-3′-UTR was inserted into a modified pGL3 control with corresponding sites (pGL3-PPARα-3′-UTR construct). A mutant construct was generated by site-directed mutagenesis using PCR with primers of 5′-TCTTAAAAACAAACAAACAAAAAAAAAATCTGTTAGAATTCGTATCAAAATGCAGCTGTTGTTTTGTTTTTGGCTCACT-3′ (forward) and 5′-AGTGAGCCAAAAACAAAACAACAGCTGCATTTTGATACGAATTCTAACAGATTTTTTTTTTGTTTGTTTGTTTTTAAGA-3′ (reverse). The mutant construct (pGL3-PPARα-3′-UTRmut) was confirmed by DNA sequence analysis. HEK-293A cells were plated (8 × 104 cells/well) in 24-well plates. Twenty nanograms of pGL3-PPARα-3′-UTR or pGL3-PPARα-3′-UTRmut construct was cotransfected with 2 ng of a Renilla luciferase expression vector construct, pRL-TK (Promega), and 200 ng of miR-21 overexpression vector or its control vector without any miRNA insert. Luciferase assays were performed 48 h after transfection using the dual-luciferase reporter assay system (Promega), and luciferase activity was measured using a GloMax-Multi Detection System (Promega). Firefly luciferase activity was normalized to Renilla luciferase expression for each sample.
Statistical analysis.
All data shown are mean values of at least three experiments, each performed in triplicate, with standard errors (SE). Comparisons between two groups or among multiple groups were made using the Student's t-test and one-way ANOVA. We used GraphPad Prism 4 statistics software package and NIH ImageJ program for data analysis. A P value <0.05 was considered statistically significant.
RESULTS
Hypoxia induces miR-21 expression.
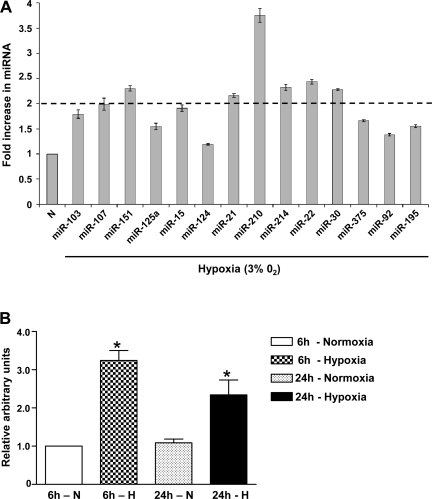
To first determine which miRNAs had increased expression during hypoxia in HPASMCs, we exposed HPASMCs to hypoxia for 24 h and profiled the expression of 14 selected miRNAs, including miR-103, miR-107, miR-124, miR-125a, miR-15, miR-151, miR-21, miR-210, miR-214, miR-22, miR-30, miR-375, miR-92, and miR-195, by qRT-PCR with specific primers for individual miRNAs. Figure 1A depicts the fold change of these selected miRNAs in hypoxia. MiR-210 showed the highest increase (∼3.5-fold), whereas miR-107, miR-151, miR-21, miR-214, miR-22, and miR-30 demonstrated ∼2-fold increase after 24-h hypoxia compared with normoxic controls. A time course study demonstrated an ∼3-fold induction of miR-21 expression after only 6 h of hypoxia, and the increased expression levels persisted at ∼2-fold even after 24 h of hypoxia (Fig. 1B).
Fig. 1.
Selected microRNAs (miRNAs) that are induced by hypoxia (H) in human pulmonary artery smooth muscle cells (HPASMCs). A: the expression of a selected group of hypoxia-inducible miRNAs was validated in HPASMCs by quantitative RT-PCR. The graph depicts the fold increase in individual miRNA expression in hypoxia compared with normoxia (N) after normalizing with U6 controls. A 2-fold increase in expression (dotted line) was considered significant. B: a >3-fold increase in microRNA-21 (miR-21) expression after 6 h of hypoxia persisted at >2-fold levels after 24 h of hypoxia. Data are shown as relative arbitrary units, means ± SE (n = 3). *P < 0.05 compared with normoxia for the same time point.
MiR-21 plays a role in hypoxia-induced HPASMC proliferation.
Figure 2A is a dose-response curve demonstrating inhibition in miR-21 expression in HPASMCs at different anti-miR-21 inhibitor oligonucleotide concentrations. A 40% reduction in miR-21 expression was seen with as little as 10 nM anti-miR-21 inhibitor and ∼70% reduction at 25 nM anti-miR-21 inhibitor concentration. A maximum reduction in miR-21 expression of ∼90% was seen with 80 nM anti-miR-21 inhibitor concentrations.
Figure 2B shows that hypoxia induced an ∼63% increase in cell proliferation after 24 h and ∼68% increase after 48 h in untreated HPASMC.
Figure 2C shows that cells with negative inhibitor also exhibited increased proliferation after 24 and 48 h of hypoxia by 59 and 78%, respectively (P < 0.05). MiR-21 inhibition in normoxia resulted in ∼37 and ∼53% reduction in cell proliferation at 24 and 48 h, respectively (P < 0.05), and cell proliferation in hypoxia increased by a significantly smaller amount after 24 and 48 h (∼7% after 24-h and ∼11% after 48-h hypoxia; P < 0.05). These data show that miR-21 inhibition reduces cell proliferation in normoxia and almost completely abolishes hypoxic induction of cell proliferation.
Figure 2D shows a 50% reduction in PCNA protein expression in anti-miR-21 cell lysates (0.577 ± 0.019 vs. 1.126 ± 0.077 arbitrary densitometric units; P < 0.05), indicating that inhibition of miR-21 leads to reduced SMC proliferation.
Figure 2E shows a sixfold increase in miR-21 expression in miR-21-overexpressing SMCs (P < 0.05).
Figure 2F shows that miR-21 overexpression also increased PCNA protein expression in HPASMCs by 50%, indicating that miR-21 overexpression enhances HPASMC proliferation.
Effect of miR-21 inhibition on hypoxia-induced HPASMC migration.
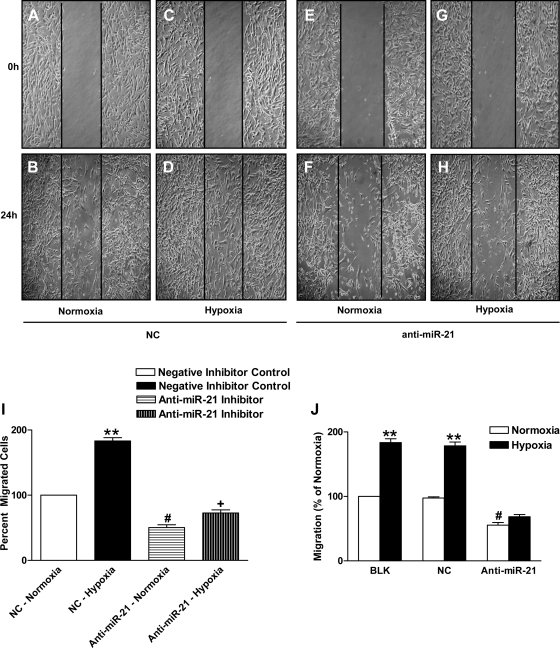
Since vascular SMC proliferation and migration are known to contribute to vascular remodeling in pulmonary hypertension (23, 50), we studied the effect of miR-21 inhibition on hypoxia-induced HPASMC migration using the wound-healing model/scratch assay (Fig. 3, A–I). Hypoxia caused an ∼83% increase in cell migration (198.0 ± 6.42 cells in hypoxia vs. 108.0 ± 1.85 cells in normoxia; P < 0.005). Figure 3I shows that miR-21 inhibition resulted in ∼49% reduction in cell migration after 24 h of normoxia (54.33 ± 4.40 vs. 108.0 ± 1.85; P < 0.05). In anti-miR-21-treated cells, cell migration increased by only ∼22% after 24-h hypoxia (P < 0.05). These results suggest that inhibition of miR-21 resulted in reduced cell migration in normoxia and significantly reduced hypoxia-induced increase in cell migration. To strengthen our findings from the wound-healing model, we studied cell migration using the Boyden chamber method. Figure 3J represents a bar plot for the Boyden chamber assay showing increased cell migration by ∼83% in hypoxia (202.33 ± 4.05 cells in hypoxia vs. 110.33 ± 2.02 cells in normoxia; P < 0.005). Figure 3J shows that miR-21 inhibition resulted in ∼49% reduction in cell migration after 24-h normoxia (61.33 ± 5.81 vs. 110.33 ± 2.02 cells in normoxia; P < 0.05) and almost completely abolished hypoxia-induced increase in HPASMC migration (increase from 61.33 ± 5.81 to 75.66 ± 4.481 cells; ∼6% increase compared with ∼83% increase in control cells). These data indicate that miR-21 plays a role in SMC migration in normoxia but is essential for hypoxia-induced increase in pulmonary vascular SMC migration.
Fig. 3.
MiR-21 inhibition decreases HPASMC migration in hypoxia. A–H: miR-21 inhibition led to reduced HPASMC migration as measured by wound-healing migration assay. A–D represent the negative control group, and E–H represent the anti-miR-21 group. A, B, E, and F represent the normoxia group, and C, D, G, and H represent the hypoxia group. I: cell migration of negative inhibitor control HPASMCs after 24-h hypoxia increased by 83%. **P < 0.005 vs. control group. Cells with anti-miR-21 showed a 49% decrease in migration in normoxia compared with NC; #P < 0.05 vs. NC normoxic group. Hypoxia increased migration by only 22% in the anti-miR-21-treated cells; +P < 0.05 compared with cell migration in normoxia. J: HPASMC migration (percentage of normoxia) assayed using the Boyden chamber method showed increase in cell migration in hypoxia; **P < 0.005 vs. normoxia BLK and NC group. MiR-21 inhibition reduced SMC migration in normoxia, #P < 0.05, different from BLK and NC normoxia groups. Hypoxia did not increase migration in miR-21-inhibited cells significantly. Data are shown as means ± SE (n = 3).
Effect of hypoxia on miR-21 predicted targets.
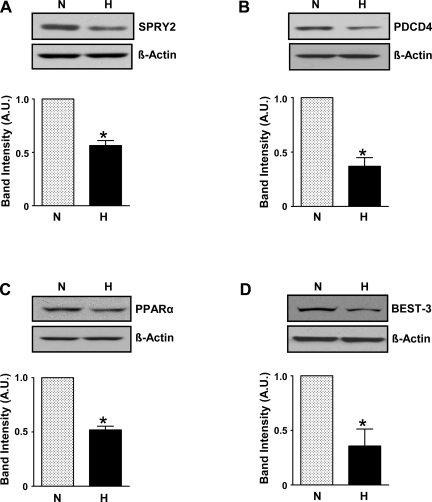
We performed an in silico search for potential miR-21 targets using TargetScan, PicTar, and MiRanda target prediction algorithms. Some known (antiproliferative) targets were found, namely PDCD4 and SPRY2. We also found other predicted targets such as the nuclear receptor, PPARα, and BEST3, a Ca2+-activated Cl− channel, which may be involved in cell proliferation. We measured protein levels of these four miR-21 targets in cell lysates obtained from HPASMCs exposed to hypoxia for 24 h. As shown in Fig. 4, SPRY2, PDCD4, PPARα, and BEST3 were reduced in hypoxia-treated HPASMC compared with normoxia control. Densitometric analyses of the protein bands normalized to β-actin bands showed a reduction in protein levels of SPRY2 by ∼50%, P < 0.05; PDCD4 by ∼60%, P < 0.05; PPARα by ∼50%, P < 0.05; and BEST3 by ∼70%, P < 0.05.
Fig. 4.
Hypoxia reduces protein expression of miR-21-predicted targets. A–D depict reduced protein expression of miR-21 target proteins, viz., SPRY2, or Sprouty homolog 2 (A), PDCD4, or programmed cell death protein 4, a proapoptotic factor (B), PPARα, or peroxisome proliferator-activated receptor-α, a nuclear receptor (C), and BEST3, or bestrophin 3, a Ca2+-activated Cl− channel (D). Data are shown as means ± SE (n = 3); *P < 0.05 vs. normoxia.
Effect of miR-21 overexpression on targets involved in proliferation.
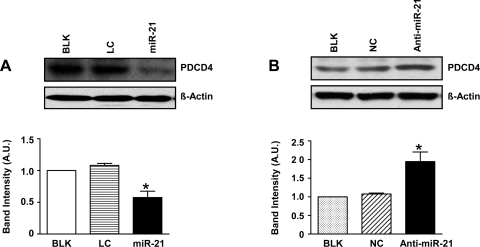
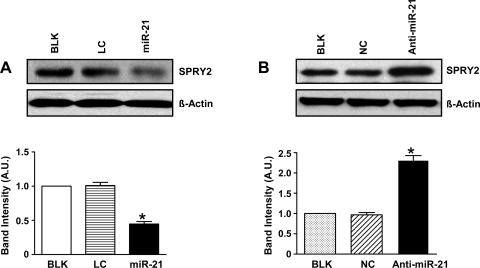
We investigated the effect of miR-21 overexpression on expression of these proteins involved in proliferation. MiR-21 overexpression in normoxia decreased PDCD4 protein expression significantly (Fig. 5A; P < 0.05). The fact that miR-21 targets PDCD4 in hypoxia was further confirmed by inhibition of miR-21 in hypoxic HPASMC, which resulted in an ∼2-fold increase in PDCD4 protein levels (Fig. 5B; P < 0.05). Figure 6 represents scanned Western blots and densitometric analyses depicting the effect of miR-21 overexpression in normoxia (Fig. 6A) and inhibition in hypoxia (Fig. 6B) on SPRY2 protein expression levels. MiR-21 overexpression in normoxia resulted in a decrease in SPRY2 protein expression by ∼60% (Fig. 6A; P < 0.05). MiR-21 regulation of SPRY2 was further confirmed by miR-21 inhibition in hypoxia, which resulted in ∼2.5-fold increase in SPRY2 protein expression (Fig. 6B; P < 0.05).
Fig. 5.
MiR-21 targets PDCD4 in HPASMC. A: scanned Western blot and densitometric measurements showing that miR-21 overexpression in normoxia decreased protein levels of PDCD4. B: Western blot analysis showed that miR-21 inhibition in hypoxia resulted in increased PDCD4 expression compared with BLK and NC. Data are represented as means ± SE (n = 3); *P < 0.05 vs. controls BLK and LC for A and BLK and NC for B, respectively.
Fig. 6.
MiR-21 targets SPRY2 in HPASMC. A: scanned Western blot and densitometric measurements showing reduced protein levels of SPRY2 after miR-21 overexpression in normoxia. B: Western blot analysis showed that in hypoxia, miR-21 inhibition caused increased SPRY2 protein expression compared with controls. Data are shown as means ± SE (n = 3); *P < 0.05 vs. controls BLK and LC for A and BLK and NC for B, respectively.
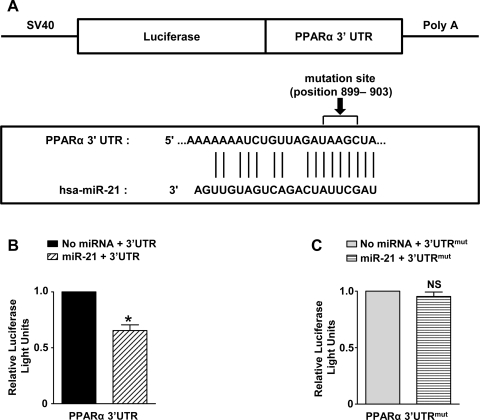
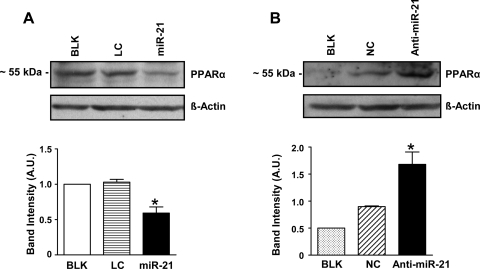
Since target prediction algorithms indicated a predicted consequential pairing between miR-21 and the 3′-UTR region of PPARα (Fig. 8A), we studied the effect of miR-21 overexpression in normoxia on PPARα protein expression levels. MiR-21 overexpression resulted in significantly reduced basal protein expression levels of PPARα as shown in Fig. 7A (P < 0.05). Inhibition of miR-21 in HPASMCs using anti-miR-21 inhibitors during hypoxia resulted in ∼2-fold increase in PPARα expression (Fig. 7B; P < 0.05). To investigate whether miR-21 directly regulates PPARα expression, a luciferase assay was set up in which the 3′-UTR of the PPARα gene (Fig. 8A) was inserted downstream of a luciferase open reading frame (pGL3-PPARα-3′-UTR). As a control, we deleted the sequence complementary to the miR-21 seed sequence in this latter construct. The luciferase constructs were transfected into HEK-293 cells with a plasmid carrying a constitutive expression cassette for miR-21 or a control sequence. A significant decrease in luciferase activity was observed in the construct bearing an intact miR-21 binding site when compared with the control (Fig. 8B; P < 0.05). The decrease in luciferase activity was lost on mutation of the putative binding site for miR-21 by site-directed mutagenesis (Fig. 8C; P = not significant). Based on these findings, we conclude that PPARα is another direct target of miR-21. Our studies employing miR-21 gain and loss-of-function experiments did not result in any significant difference in BEST3 protein expression levels (results not shown), implying that BEST3 regulation in hypoxia is probably not mediated via miR-21.
Fig. 8.
A: representative image of the conserved miR-21 binding site in the 3′-untranslated region (3′-UTR) of PPARα along with the mutation site. B: luciferase reporter analysis in HEK-293 cells using a PPARα 3′-UTR assay showed direct binding by miR-21 to the 3′-UTR-region of PPARα, suggesting that PPARα could be a direct miR-21 target. Data are shown as means ± SE (n = 3); *P < 0.05 with cells containing miR-21 and 3′-UTR vs. control containing only 3′-UTR without miRNA. C: mutation in 3′-UTR of PPARα abolished binding/interaction with miR-21. SV40, simian virus 40; Poly A, a stretch of adenine nucleotides added to the 3′ end (tail) of the luciferase construct via a process called polyadenylation; hsa, Homo sapiens; mut, mutant; NS, not significant.
Fig. 7.
MiR-21 targets PPARα, a predicted miR-21 target. A: Western blot analysis showed that miR-21 overexpression in normoxia resulted in reduced PPARα protein expression. B: Western blot analysis showed that in hypoxia, miR-21 inhibition caused increased PPARα protein expression compared with controls. Data are shown as means ± SE (n = 3); *P < 0.05 vs. controls BLK and LC for A and BLK and NC for B, respectively.
DISCUSSION
MiR-21 has been well-studied in various cancer cells (37, 54, 61, 62), however, little is known about its expression and function in HPASMCs in hypoxia. We have shown for the first time that miR-21 expression is induced by hypoxia as early as 6 h and remains fairly high even after 24 h of hypoxia in HPASMC. A recent study of PAH using chronically hypoxic and monocrotaline-injected rats reported that miR-21 is downregulated in rat lungs after monocrotaline injection but not after hypoxic exposure (8). These results imply that the miRNAs modulated by hypoxia may differ based on experimental conditions (in vivo vs. in vitro studies), cell type, and the severity and duration of the hypoxic stress (7, 17, 29, 34). Our results demonstrate that at least after acute hypoxia, miR-21 expression increases in pulmonary artery SMC in culture. Our studies also demonstrate that miR-21 plays an important role in hypoxia-mediated pulmonary artery SMC proliferation.
It has been reported that migration of proliferating vascular SMCs from the medial to the luminal side of the vessel is an important event in vascular remodeling (9, 45, 47). We (43) have previously reported that hypoxia increases cell migration in ovine fetal pulmonary artery SMCs via cGMP-dependent protein kinase PKG. In this study, we have shown that miR-21 plays a role in migration of HPASMCs in hypoxia. Similar studies in AGS gastric cancer lines (58) have shown that miR-21 regulates gastric cancer cell migration in vitro. Thus it can be concluded that the upregulation of miR-21 in hypoxia is important for both HPASMC proliferation and migration.
Identifying miRNA targets is a challenge. Based on published literature, we tested the effect of hypoxia on two known miR-21 targets related to cell proliferation and migration. We found that PDCD4 and SPRY2 were downregulated after hypoxic exposure. Overexpression of miR-21 in normoxia led to decreased expression of these proteins, and knockdown of miR-21 in hypoxia increased their expression, suggesting that they are targeted by miR-21 in HPASMC. In this study, we also determined whether miR-21 regulates some other known targets involved in cell proliferation. Using three miRNA target prediction algorithms, we predicted that miR-21 may target PPARα and BEST3. Although protein expression levels of both PPARα and BEST3 were downregulated in hypoxia, only PPARα was confirmed as a probable target by Western blotting and 3′-UTR reporter assay. Since miRNAs bind through partial sequence homology to the 3′-UTR of their target genes, a single miRNA can regulate multiple targets (5). Thus we have shown for the first time that at least three target genes, PDCD4, SPRY2, and PPARα, are regulated by miR-21 in HPASMCs in hypoxia. PDCD4 is a recently discovered tumor suppressor protein that inhibits protein synthesis by suppression of translation initiation. Regulation of PDCD4 by miR-21 has been investigated by several groups in cancer cells (2, 19, 39, 62). Two recent reports have emphasized the protective effect of miR-21 via its target gene, PDCD4, against hydrogen peroxide-induced injury in cardiac myocytes (12) and apoptosis in aortic SMCs (38). It has been reported that transforming growth factor-β (TGF-β) and BMP signaling promote an increase in miR-21 expression, and in vascular SMCs PDCD4 is a functional target of miR-21 involved in the BMP-mediated induction of SMC markers (16). Cordes et al. (13) recently showed that miR-145 and miR-143 promote differentiation and repress proliferation of SMCs. Furthermore, they demonstrated a reduction in miR-143 and miR-145 expression in neointimal lesion after vascular injury in concordance with an earlier report (12). Based on these reports, it is interesting to speculate that under basal normoxic conditions, miR-21 may function along with other miRNAs such as miR-143 and miR-145 (13) in regulating SMC contractility. However, under hypoxic stress, a key trigger of pulmonary vascular remodeling, elevated miR-21 levels may regulate multiple targets in mediating a proproliferative response in SMCs. In our study, we have provided evidence that upregulation of miR-21 in hypoxia promotes HPASMC proliferation, and this could possibly occur by its suppression of PDCD4. Sprouty was initially discovered as an inhibitor of FGF signaling (28), and this effect was shown by knockdown of SPRY2 in mouse lungs (51). Sayed et al. (46) recently reported that miR-21 targets SPRY2 in mouse neonatal cardiocytes, which is consistent with the finding that SPRY2 overexpression inhibits cell migration (55) and miR-21 enhances cell proliferation and migration (41). Our results suggest that increased expression of miR-21 in hypoxia leads to downregulation of SPRY2, thereby enhancing HPASMC proliferation and migration.
A recent study reported that phosphatase and tensin homolog (PTEN) and B-cell lymphoma 2 family of apoptosis regulator proteins (Bcl-2) are targeted by miR-21 in aortic vascular SMC proliferation and neointimal lesion formation (31), suggesting the interplay of multiple miR-21 targets in the regulation of vascular SMC proliferation. PPARα belongs to the PPARs, members of the nuclear receptor superfamily of ligand-activated transcription factors (TF), which include two other members, PPARβ and PPARγ. They are involved in lipid metabolism and homeostasis (35) and are known to play a role in vascular disease by regulating vascular SMCs (35, 57). Studies by Zahradka et al. (56) have established that PPARα activation blocks cyclin-dependent kinase 2 (cdk2) phosphorylation, a key event in the cell cycle progression from G1 to S phase, eventually leading to inhibition of DNA synthesis, cell migration, and neointimal hyperplasia. It has also been reported that PPARα activation suppresses G1-to-S cell cycle progression by increasing the expression of the cyclin-dependent kinase inhibitor p16INK4a (21). The same group recently identified telomerase as the key downstream target for the antiproliferative effects of PPARα in vascular SMCs and also demonstrated that PPARα interferes with a p16/pRB/E2F-dependent transcriptional activation of telomerase (22).
Hypoxia has been shown to influence PPARα regulation by inhibiting the PPARα-retinoid X receptor (RXR) gene regulatory pathway in cardiac myocytes (30). Two other reports have implicated HIF-1 in the inhibition of PPARα expression in hypoxia (42) and also in hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of PPARα/RXR (4). We have shown that hypoxia decreases PPARα protein expression in HPASMCs. Our data with HPASMCs overexpressing miR-21 demonstrated a novel miRNA-mediated transcriptional regulation of PPARα (a putative miR-21 target) expression via direct binding of miR-21 to its 3′-UTR as shown by in vitro luciferase-based reporter gene assays. However, there is a report by Shah et al. (48) that PPARα itself regulates a let-7c miRNA-mediated signaling cascade, which is critical for PPARα agonist-induced liver proliferation and tumorigenesis. This phenomenon of cross talk between TF and miRNAs (both involved in gene regulation) has been discussed by Shalgi et al. (49), wherein they have provided new insights on the architecture of the combined transcriptional-posttranscriptional regulatory network. A significant fraction of this regulatory network consists of miRNA-TF coregulators in which the TF appears to regulate the miRNA or to be regulated by the miRNA forming diverse feed-forward loops. Although PPARα regulation clearly needs further investigation, we can conclude that it could be posttranscriptionally downmodulated by hypoxia via miR-21.
In summary, we have demonstrated that the hypoxic upregulation of miR-21 in HPASMCs has a functional role in HPASMC proliferation and migration and has also implicated unique miR-21 targets, PDCD4 and SPRY2 (known for their antiproliferative and antimigratory effects in vascular SMCs), in these processes via their posttranscriptional suppression/downregulation by miR-21. In addition, we have demonstrated that miR-21 may also target nuclear receptor PPARα (a known antiproliferative target and TF). Based on our findings, we speculate that miR-21 regulation of multiple targets will ultimately contribute to a better understanding of the molecular mechanisms involved in pulmonary artery SMC proliferation and vascular remodeling in hypoxia and to the development of new treatment modalities for vascular diseases such as pulmonary hypertension.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants HL-059435 and HL-075187 to J. U. Raj.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Guofei Zhou, Dr. Aarti Raghavan, Zhixin Wang, and Laura Bach for helpful discussions and technical assistance. We are also grateful to Dr. Evgeny Pilipenko for kindly providing us with HepG2 cells (control) for our experiments.
REFERENCES
- 1.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. RNA 9: 277–279, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27: 2128–2136, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Belanger AJ, Luo Z, Vincent KA, Akita GY, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. Biochem Biophys Res Commun 364: 567–572, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA-target recognition. PLoS Biol 3: e85, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 104: 1184–1191, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14: 1340–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, Denby L, Dempsie Y, Long L, Morrell NW, Baker AH. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 30: 716–723, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Casscells W. Migration of smooth muscle and endothelial cells. Critical events in restenosis. Circulation 86: 723–729, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science 303: 83–86, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Liu X, Zhang S, Lin Y, Yang J, Zhang C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J Mol Cell Cardiol 47: 5–14, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corn PG. Hypoxic regulation of miR-210: shrinking targets expand HIF-1's influence. Cancer Biol Ther 7: 265–267, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 122: 6–7, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454: 56–61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donker RB, Mouillet JF, Nelson DM, Sadovsky Y. The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod 13: 273–279, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem 283: 1026–1033, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros D, Weber BL, Simon C, Coukos G, Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther 7: 255–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gizard F, Amant C, Barbier O, Bellosta S, Robillard R, Percevault F, Sevestre H, Krimpenfort P, Corsini A, Rochette J, Glineur C, Fruchart JC, Torpier G, Staels B. PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest 115: 3228–3238, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gizard F, Nomiyama T, Zhao Y, Findeisen HM, Heywood EB, Jones KL, Staels B, Bruemmer D. The PPARalpha/p16INK4a pathway inhibits vascular smooth muscle cell proliferation by repressing cell cycle-dependent telomerase activation. Circ Res 103: 1155–1163, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 283: L354–L363, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res 32: D109–D111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34: D140–D144, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154–D158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimbellot JS, Erickson SW, Mehta T, Wen H, Page GP, Sorscher EJ, Hong JS. Correlation of microRNA levels during hypoxia with predicted target mRNAs through genome-wide microarray analysis. BMC Med Genomics 2: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. sprouty Encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell 92: 253–263, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1: e116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huss JM, Levy FH, Kelly DP. Hypoxia inhibits the peroxisome proliferator-activated receptor alpha/retinoid X receptor gene regulatory pathway in cardiac myocytes: a mechanism for O2-dependent modulation of mitochondrial fatty acid oxidation. J Biol Chem 276: 27605–27612, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 100: 1579–1588, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ 15: 667–671, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Kulshreshtha R, Ferracin M, Negrini M, Calin GA, Davuluri RV, Ivan M. Regulation of microRNA expression: the hypoxic component. Cell Cycle 6: 1426–1431, 2007 [PubMed] [Google Scholar]
- 34.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol 27: 1859–1867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144: 2201–2207, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Li J, Huang H, Sun L, Yang M, Pan C, Chen W, Wu D, Lin Z, Zeng C, Yao Y, Zhang P, Song E. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res 15: 3998–4008, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem 284: 7903–7913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27: 4373–4379, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Marsboom G, Archer SL. Pathways of proliferation: new targets to inhibit the growth of vascular smooth muscle cells. Circ Res 103: 1047–1049, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133: 647–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol 166: 7543–7548, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Negash S, Narasimhan SR, Zhou W, Liu J, Wei FL, Tian J, Raj JU. Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia. Am J Physiol Heart Circ Physiol 297: H304–H312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neppl RL, Wang DZ. Smooth(ing) muscle differentiation by microRNAs. Cell Stem Cell 5: 130–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. MicroRNA-21 targets Sprouty2 and promotes cellular outgrowths. Mol Biol Cell 19: 3272–3282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res 58: 427–444, 1986 [DOI] [PubMed] [Google Scholar]
- 48.Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol 27: 4238–4247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shalgi R, Lieber D, Oren M, Pilpel Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput Biol 3: e131, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol 59: 89–144, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol 9: 219–222, 1999 [DOI] [PubMed] [Google Scholar]
- 52.van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest 117: 2369–2376, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun 388: 539–542, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Yigzaw Y, Cartin L, Pierre S, Scholich K, Patel TB. The C terminus of sprouty is important for modulation of cellular migration and proliferation. J Biol Chem 276: 22742–22747, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Zahradka P, Wright B, Fuerst M, Yurkova N, Molnar K, Taylor CG. Peroxisome proliferator-activated receptor alpha and gamma ligands differentially affect smooth muscle cell proliferation and migration. J Pharmacol Exp Ther 317: 651–659, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zahradka P, Yurkova N, Litchie B, Moon MC, Del Rizzo DF, Taylor CG. Activation of peroxisome proliferator-activated receptors alpha and gamma1 inhibits human smooth muscle cell proliferation. Mol Cell Biochem 246: 105–110, 2003 [PubMed] [Google Scholar]
- 58.Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest 88: 1358–1366, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129: 303–317, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Zhou W, Dasgupta C, Negash S, Raj JU. Modulation of pulmonary vascular smooth muscle cell phenotype in hypoxia: role of cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 292: L1459–L1466, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1). J Biol Chem 282: 14328–14336, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res 18: 350–359, 2008 [DOI] [PubMed] [Google Scholar]