Abstract
Since the pioneering work of Henry Pickering Bowditch in the late 1800s to early 1900s, cardiac muscle contraction has remained an intensely studied topic for several reasons. The heart is located centrally in our body, and its pumping motion demands the attention of the observer. The contraction of the heart encompasses a complex interplay of mechanical, chemical, and electrical properties, and its function can thus be studied from any of these viewpoints. In addition, diseases of the heart are currently killing more people in the Westernized world than any other disease. When combined with the increasing emphasis of research to be clinically relevant, this contributes to the heart remaining a topic of continued basic and clinical investigation. Yet, there are significant aspects of cardiac muscle contraction that are still not well understood. A big complication of the study of cardiac muscle contraction is that there exists no equilibrium among many of the important governing parameters, which include pre- and afterload, intracellular ion concentrations, membrane potential, and velocity and direction of movement. Thus the classic approach of perturbing an equilibrium or a steady state to learn about the role of the perturbing factor in the system cannot be unambiguously interpreted, since each of the parameters that govern contraction are constantly changing, as well as constantly changing their interaction with each other. In this review, presented as the 54th Bowditch Lecture at Experimental Biology meeting in Anaheim in April 2010, I will revisit several governing factors of cardiac muscle relaxation by applying newly developed tools and protocols to isolated cardiac muscle tissue in which the dynamic interactions between the governing factors of contraction and relaxation can be studied.
Keywords: muscle, rabbit, calcium transient, frequency, sarcomere length, kinetics
henry pickering bowditch was the first to describe several important aspects of heart muscle contraction that are still being investigated today. In his 1871 publication, “Ueber die Eigenthuemlichkeiten der Reizbarheit, welche die Muskelfasern des Herzens zeigen,” roughly translated as “Properties of excitability of cardiac muscle fibers,” he made several fundamental observations (6). He showed that, in principle, the heart muscle, unlike skeletal muscle, has an all-or-nothing excitation mode, that when stimulated at a fixed frequency and amplitude, adaptation of contraction occurs (Treppe effect) and that the force of contraction of cardiac muscle increases when the frequency of stimulation increases. The latter two subjects we still study in my laboratory, and thus it is a truly special honor for me to have been awarded the opportunity to give the 54th Bowditch Lecture. The past 140 years have allowed several generations of scientists to develop a wide array of tools that we now have available to study cardiac muscle contractile function. These new techniques and tools have allowed us to now start investigating how those biological principles uncovered by Bowditch are governed at the molecular level. In the 1871 work by Bowditch, the focus was on the strength of contraction of the heart muscle. Meanwhile, we know that not only the strength but also the speed of both contraction and relaxation are very important in governing contractile function. A reduced speed of contraction and relaxation is a central phenotypical finding in many cardiomyopathies, specifically those pathological situations that include components of diastolic dysfunction (15, 40). In this review lecture, I will revisit the contractile properties of cardiac muscle, focusing on relaxation, and how these properties are governed, using recent studies from my laboratory performed with tools developed over the past decades by others and us. As our main model, we will use isolated, intact small, linear trabeculae dissected from the ventricle of the rabbit heart. The intact nature of this preparation preserves all calcium-handling properties and allows us to stimulate the muscle electrically with various rates and patterns (57), as well as apply drug treatments and measure intracellular calcium transients. These trabeculae contain all major cell types found in the ventricle (4, 27) and exist of cardiomyocytes, endocardial- and vascular endothelial cells, and fibroblasts. They contain most, if not all, aspects of contractile regulation, including ejection properties (9, 27), while the linearity of trabeculae make them suitable for mechanical studies of the sarcomeric myofilaments (53). The rabbit, unlike the mouse or rat, has a composition of myofilament isoforms (34) that is very similar to humans, and critical aspects of excitation-contraction (EC) coupling, such as the relative contribution of the sarcoplasmic reticulum (SR) to overall calcium handling, is also very similar to human EC coupling (36).
In the following four parts that comprise this review, I will 1) revisit frequency-dependent control of relaxation, 2) revisit length-dependent control of relaxation, 3) discuss the coupling between contraction and relaxation, and 4) touch base on the effect of impaired relaxation in cardiac hypertrophy.
Frequency-Dependent Control of Cardiac Muscle Relaxation
Frequency-dependent activation is one of the main regulatory systems used by the heart to increase cardiac output. In addition to the increased number of beats per time unit that results in an increase in cardiac output per se, the force of contraction increases as the heart rate goes up. This positive force-frequency relationship (FFR) occurs in all mammals (28), albeit that the magnitude of the effect is larger in bigger mammals such as humans, dogs, and rabbits, compared with small animals such as rats and mice (14, 28). Although there are reports published in which a negative force-frequency is attributed to mice and rats, these generally result from cold temperature and/or the use of a range of frequencies that are well outside the in vivo range of the mouse or rat and/or the use of preparations that are too thick to adequately oxygenate at high pacing rates. The latter factor, the effect of muscle dimensions on force-frequency behavior, was first shown by Schouten and ter Keurs (44) who observed that the FFR was no longer negative when thin preparations were used. Outside the in vivo range, the regulation of contractile strength and kinetics do not necessarily reflect those of the in vivo situation, and processes that are not rate limiting in vivo may become the regulatory step(s) under nonphysiological conditions. As a result, depending on the frequencies and experimental conditions used, a negative or biphasic FFR can be obtained (28, 33, 36). This is even true for the large mammals: if frequencies well below the in vivo range are used, even rabbits, humans, and dogs can show a biphasic or even a negative FFR. While within the human heart rate range the force of contraction can double, in rats generally only a small increase in contraction is observed, whereas in mice this increase in contractile force is even less (14, 20). Thus mice and rats mainly increase their cardiac output by the number of contractions, whereas in larger mammals an increased force of contraction plays a much more significant role. The underlying molecular events show that the increase in force development is mainly due to an increase in the intracellular calcium transient (18). This calcium transient is increased mainly because of the fact that per time unit, more calcium influxes take place, and this resets the intracellular calcium homeostasis to a level where the calcium transient is higher in amplitude. This in turn will activate the thin filament more, resulting in larger numbers of active cross bridges, generating more force. In addition, the relative contribution of the various calcium handling processes (i.e., SR uptake vs. transmembrane extrusion) between species is responsible for several of these differences observed (36, 38).
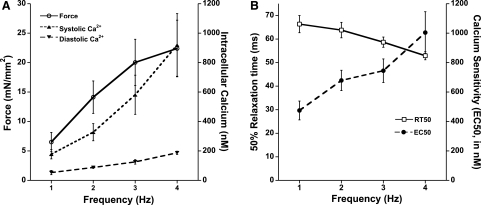
Not only does the heart beat more forcefully when the rate of contraction increases, but the kinetics of the contraction and relaxation also speed up. This process is also known as frequency-dependent acceleration of relaxation. This process occurs in all mammals (28) and does not show a biphasic or negative effect for smaller mammals, even when assessed outside the in vivo range for the species used. An assessment of intracellular calcium transients shows that when the frequency of contraction increases, the calcium transient is not only larger in amplitude (Fig. 1A) but also abbreviated (33, 50). The increased kinetics are therefore generally thought to be the cause of frequency-dependent acceleration of relaxation (10, 43); as calcium declines faster, relaxation of force can occur faster. However, the increase in the extracellular calcium transient is substantial, and despite the faster calcium transient kinetics, at any given time during the cardiac twitch, the absolute calcium concentration is often higher (54). In addition, diastolic calcium rises very significantly when the stimulation rate is increased (54). In fact, in many cases the diastolic calcium concentration at a high stimulation rate can exceed the systolic calcium level at a low stimulation rate. Yet, the muscle relaxes fully even at this high diastolic calcium level, a level that causes near-maximal force to be developed at low stimulation rates. This is not an isolated finding. Reviewing, for instance, work on isolated myocytes at different pacing rates shows a similar phenomenon, a large increase in diastolic calcium concentration at higher pacing rates that often exceeds systolic calcium concentrations assessed in the same preparation at a low stimulation rate (46).
Fig. 1.
A: active force development increases in isolated rabbit cardiac trabeculae when frequency of stimulation is increased, resulting in a positive force-frequency relationship. Both diastolic and systolic calcium concentrations increase when frequency increases, whereas the calcium transient amplitude (systolic minus diastolic values) also increases. B: time from peak force to 50% relaxation (RT50) declined when frequency of stimulation is increased, whereas myofilament calcium sensitivity decreases with frequency. Data were recorded at optimal length and at 37°C and modified from Varian and Janssen (54).
Conceptually, how then does this muscle or myocyte relax properly at high stimulation rates? These findings initiated a postulation of the hypothesis that when the frequency of a stimulation increases, the myofilament responsiveness to the activating calcium ions decreases. Previous studies have investigated whether the frequency of stimulation impacts on myofilament calcium sensitivity but have rendered inconclusive results. A major difficultly with the assessment of myofilament calcium sensitivity is that such assessments are typically done in demembranized (i.e., skinned) preparations (8, 19). These preparations are devoid of a functional membrane and can thus not be electrically stimulated. A second difficulty in the assessment of calcium sensitivity is that in twitch-contracting muscle, there exists no equilibrium between the intracellular calcium concentration and developed force, and previous studies have not been able to actually assess myofilament calcium sensitivity in intact twitching muscle under near physiological conditions. In the past few years, we developed and refined a protocol that to a large extent can overcome these two major technical difficulties. Using high concentrations of extracellular potassium in the superfusate of the muscle, we can slowly depolarize this intact twitch-contracting muscle (56), and a “potassium contracture” develops (21). During the development of this contracture, force slowly rises until it reaches maximal tension. When the muscle is then superfused with a normal Krebs-Henseleit solution, the membrane potential redevelops and the muscle returns to the earlier steady state. This protocol is fully reversible (56), and thus repeatable, without adverse effects on the force or kinetics of the twitch-contracting muscle. While the muscle is loaded with a calcium indicator, such as fura-2, calcium and force can be assessed simultaneously (56). Because the potassium contracture, compared with a twitch, is very slow (i.e., 20–25 s vs. 200–400 ms, respectively), the intracellular calcium concentration and the developed force are in pseudoequilibrium. This is confirmed by the fact that when calcium is plotted versus force, the resulting phase-plane loop is completely closed, indicating a uniform, hysteresis-free relationship between force and calcium, unlike the normal twitch force-calcium relationship that shows a very much open loop (29, 33). We thus obtain a steady-state relationship between calcium and force, and this relationship can be fitted with a standard Hill equation from which EC50 (calcium sensitivity), slope, and maximal force can be mathematically derived, completely analogous to the analysis of a force-pCa curve obtained in skinned muscles or myocytes. Using this protocol we assessed myofilament calcium sensitivity in muscles contracting at different frequencies (Fig. 1B). We found that when frequency increases, myofilament calcium sensitivity indeed substantially decreased (54). From this we concluded that at an increased frequency of stimulation, the desensitization of the myofilaments for activator calcium appears to play at least a part in the observed increase in relaxation.
Given this result, the next question that arises is how this frequency-dependent decrease in myofilament calcium sensitivity is mediated at the molecular level. From past studies it is well known that a phosphorylation of troponin I (TnI) that occurs as a result of β-adrenergic stimulation decreases calcium sensitivity (32). We thus initially focused on the phosphorylation of myofilament proteins, such as TnI, as the potential underlying mechanism of frequency-dependent myofilament desensitization. Muscles contracting at different frequencies were flash frozen in the setup, and a pro-Q diamond stain protein analysis was performed. We observed that at 4 Hz, the phosphorylation of both TnI and myosin light chain-2 (MLC-2) was higher than at 1 Hz, whereas it was equal to isoproterenol treatment (at 1 Hz) (54); the latter intervention is known to almost fully phosphorylate TnI. Further experiments (unpublished) indicate that this increased phosphorylation is likely mediated via a Ser-Thr kinase: staurosporine inhibited frequency-dependent changes in TnI and MLC-2 phosphorylation (K. D. Varian and P. M. L. Janssen, unpublished). The frequency-dependent change in myofilament calcium responsiveness may thus allow the relaxation of the myocardium when the diastolic calcium rises during high-frequency stimulation. The underlying mechanism involves phosphorylation of myofilament proteins, likely via the activation of a Ser-Thr kinase. Future studies would be needed to identify the specific pathways, as well as unravel the relative contribution of different target proteins, which may not be limited to the initially observed TnI and MLC-2.
In summary, from this part of my presentation I conclude that 1) when frequency increases, the decline of the force accelerates and that 2) myofilament calcium sensitivity decreases with increasing frequency, likely involving myofilament phosphorylation, and these observations correlate with acceleration of relaxation.
Length-Dependent Control of Cardiac Muscle Relaxation
Since the pioneering work of Frank (12) and Starling (47), the mechanism of how contractile strength of the heart is modulated by the length of the cardiac fibers has been a topic of intense investigation. Most initial studies into the length-dependent activation were focused on the length dependency of the developed force or pressure, and it has since become well known that the increase in force at a greater muscle/sarcomere length is accompanied by a slowing of the relaxation (1, 26, 53). This is in sharp contrast to frequency-dependent activation, where an increase in force occurred with a concurrent speeding up of relaxation (see Frequency-Dependent Control of Cardiac Muscle Relaxation). Although sarcomere length control can be imperative for specific analysis (9, 23, 53), the slowing down of contraction and relaxation is observed with increasing length in isometric cardiac muscles as well (2), where there is unavoidable central segment shortening, as well as when the sarcomeres are held constant using laser diffraction-based length feedback (23). Most of the previous studies on length-dependent activation were performed at room temperature and in smaller mammals, with the rat being the animal of choice for most biophysical experiments. We set out to revisit length-dependent activation while taking advantage of developed tools and protocols that allow us to simultaneously assess force and intracellular calcium, in a large mammal, and at frequencies and temperatures closely mimicking in vivo physiological settings.
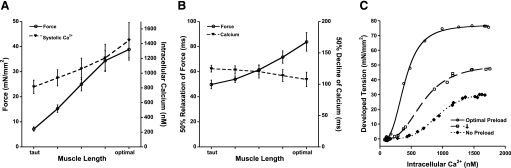
We observed (Fig. 2A) that in rabbit ventricular muscle, at 37°C, the force of contraction increased greatly when the overall muscle length was increased from near slack to optimal (39), where optimal muscle length was defined as the length where a further increase in length would no longer result in a further increase in active developed force. While the increase in the force of contraction resulted in a decrease in the speed of relaxation (Fig. 2B), the effect on the intracellular calcium transient was much less pronounced. As muscle length increased, the peak of the calcium transient increased less pronounced, whereas the time from peak calcium to 50% decline accelerated, in contrast to force decline that decelerated. The increase in calcium transient amplitude has been attributed to a slow process that occurs over minutes after an increase in length. Indeed, the data we collected were indeed obtained after the muscle had reached a new steady state. From our previous work in rats, we know that an instantaneous change in length does not result in an immediate increase of the calcium transient (23). In fact, when sarcomere length was clamped in that study, the first contraction actually showed a depressed, not enhanced, calcium transient, which we attributed to a combination of the identical calcium transient minus the increase calcium now bound to troponin C at the higher length, resulting in a net decrease of the measured calcium transient. The increase of force production and prolonged relaxation is thus not primarily due to a change in excitation properties but rather in downstream, myofilament-based effects.
Fig. 2.
A: active force development increases in isolated rabbit cardiac trabeculae when length of the muscle is increased. The amplitude of the intracellular calcium transient also increases but is less pronounced. B: RT50 of the force transient is slowed when muscle length is increased, whereas the 50% decline in the intracellular calcium transient is slightly accelerated. C: assessment of calcium sensitivity shows that at higher length, the muscle becomes more sensitive to calcium, whereas maximal force production is increased. Data were recorded at a stimulation frequency of 2 Hz at 37°C and modified from Monasky et al. (39) and Monasky et al. (37).
At steady state, we show there is a dissociation between the rate of calcium decline and the force decline, suggesting that the rate of cytosolic calcium removal does not solely dictate the rate of force decline. Myofilament calcium sensitivity has been shown by many studies to be increased when cardiac muscle length increases (1, 30). Thus, to study and quantify whether in the rabbit and under near-physiological conditions this calcium sensitization after a change in length occurs, we employed the potassium-contracture technique (56) described in the first part of this presentation. At three different lengths, we assessed myofilament calcium sensitivity (Fig. 2C) and observed a very robust increase in calcium sensitivity, from about 1,500 nM at a taut length to 700 nM at optimal length, whereas the maximal force in this example increased from about 30 to 75 mN/mm2 (37). Thus, in this study we show for the first time that in intact rabbit myocardium under near-physiological conditions, calcium sensitivity robustly increases when the length of the cardiac muscle is increased. The EC50 values for calcium sensitivity may appear low compared with those generally obtained in skinned fiber or skinned cell preparation studies that have investigated the Frank-Starling effect (11, 31, 59). This low EC50 value is, however, consistent with a previous report in which myofilament calcium sensitivity was compared in intact and skinned preparations where too it was observed that in intact muscles the calcium sensitivity is significantly higher (13). In addition, the EC50 in the 500 nM range would fit well with the quantitative calcium transient data obtained in general. When we compared our value of 653 ± 120 nM at optimal length compared with the value of 679 ± 69 nM obtained in the first project under nearly identical conditions, we see these values are very close, indicating the reproducibility of this novel assessment protocol.
Processes that slowly alter myocardial force after a change in preload have commonly been explained by a change in ion homeostasis that consequently occurs. However, Cazorla and colleagues (7) showed that stretch can also affect the phosphorylation status of proteins within the myofilament maxtrix and that such phosphorylations have functional consequences for contractile output. Thus we continued to investigate whether, in parallel to the first project presented, the phosphorylation of myofilament proteins potentially plays a role in the observed changes in relaxation. While contracting in the setup, we doused muscles with liquid nitrogen to preserve the phosphorylation status as best as possible. Muscles were frozen either while contracting either at very low (near zero) preload or at optimal preload. We found that at high length, tropomyosin, TnI, and MLC-2 were more phosphorylated than at lower preload. The amounts of tissue available from a single trabecula is very small, typically only 10–50 μg, and thus in combination with the pro-Q diamond staining used, which does not always reveal all targets clearly, we can at this point not exclude that there exist additional targets, nor can we at this stage quantify the magnitude of the increase in phosphorylation. We proceeded to use an antibody approach to further investigate the myofilament protein phosphorylation. Our initial effort was directed to TnI; this protein has multiple phosphorylation sites and is shown to be of great significance in the modification of myofilament calcium sensitivity (45). Using antibodies directed against TnI phosphorylated at serines 22 and 23, we showed that at least part of the increased phosphorylation detected by the pro-Q diamond method stemmed from the serines-22/23 of TnI. Interestingly, an increase in TnI phosphorylation at these sites is often, but not always, correlated with a decrease in myofilament calcium sensitivity, rather than the observed increase. Since the assessments were done at steady state, where calcium handling had been augmented as part of the slow force response (58), we cannot exclude and it may be even likely that the augmented calcium transients are responsible for the increase in TnI phosphorylation. We can only speculate that the possible underlying molecular mechanism is similar to that observed for frequency-dependent changes in myofilament responsiveness.
In summary, from this part of my presentation, I conclude that 1) under physiological conditions, changes in length dissociate the decline of the calcium transient from force relaxation, making myofilament properties rate limiting; 2) relaxation is not the same as calcium transient decline; 3) under physiological conditions, myofilament calcium sensitivity increases when the length of the sarcomeres increases; and 4) the steady-state force-length relationship is tuned at steady state by processes involving the phosphorylation of sarcomeric proteins.
Contraction-Relaxation Coupling
In the third part of the presentation, I am going to focus on the kinetics of contraction and relaxation of cardiac muscle. Although the amplitude of force development by the myofilaments is a major determinant of cardiac function, the kinetics at which the contractions take place are important as well. We now know that in several cardiac pathologies the force of contraction is relatively well preserved, resulting in a preserved ejection fraction (5). However, in these pathologies, the kinetics of relaxation are slow, resulting in a low end-diastolic ventricular volume, which then even with a forceful contraction is depriving the body of sufficient cardiac output (15). We deduct from the first two parts of this review that force both increases with frequency and length (or preload) but that in case of frequency the relaxation significantly speeds up, whereas with length it significantly slows down. This thus strongly indicates that the level of force per se does not directly determine the kinetics of a contraction. Furthermore, previous studies by us (25, 29, 39) and others (48, 52) have indicated that myofilament properties play an important role in the governing of contraction kinetics, but the balance between the kinetics of contraction with those of the kinetics of relaxation have not been closely examined.
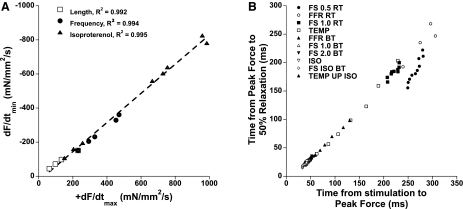
We examined, initially via retrospective analysis, data from mouse studies in which we recorded twitch contractions obtained at all three main modulating mechanisms of contractile force generation: length-dependent activation, frequency-dependent activation, and β-adrenergic stimulation (22). For each mechanism, a minimum of four different conditions (either lengths, frequencies, or concentrations of isoproterenol) were recorded. The twitch contraction data, obtained at steady state, were then analyzed on their time to peak tension (TTP), the time from peak tension to 50% relaxation (RT50), active developed force, and the maximal positive and negative derivatives of force (dF/dtmax and dF/dtmin, respectively). When we compared, per protocol, dF/dtmin versus dF/dtmax, we found not only that they correlated very tightly with a typical r2 of >0.96 but also that the slopes of this correlation were constant between the protocols and that this correlation dissected both axes at or near zero. An example of data from a single experiment is shown in Fig. 3A. Thus, regardless of the experimental condition, the ratio between dF/dtmin and dF/dtmax was constant for any measured twitch. When we then assessed this balance for multiple muscles from mice of the same strain, they again were remarkably constant, showing little muscle-to-muscle variation. We proceeded to analyze a larger population of data that we had collected over the past 8 years (20, 24, 49, 50) and found that for all but one strain (i.e., in 12 out of 13 strains analyzed), this correlation of dF/dtmin and dF/dtmax was not significantly different. Although it may be expected to be similar in several strains of control mice, in the 13 strains analyzed, several had a well-documented alteration of EC coupling or exhibited severe cardiac pathology. For example, mice where the expression of the SR calcium ATPase was reduced by ∼40% because of heterozygous knockout show a reduced rate of calcium resequestration into the SR, resulting in a slow calcium transient decline (42). Yet, the ratio of dF/dtmin and dF/dtmax obtained (0.77 ± 0.03) was similar to the respective control mice (0.77 ± 0.04). In a murine line with the opposite phenotype, where the faster sarco(endo)plasmic reticulum Ca2+-ATPase isoform 1a isoform was expressed through transgenesis (35) and exhibits faster calcium uptake into the SR and faster calcium transient decline, the ratio (0.81 ± 0.02) was again similar to normal nontransgenic mice. Thus, despite vast changes in the critical elements of EC coupling, the ratio of dF/dtmin and dF/dtmax was not affected. Additional mice, including a mouse model of Duchenne's muscular dystrophy (mdx mice) and mice in which not only dystrophin but also the analog utrophin was knocked out and show severe muscle pathophysiology (24), this balance was unaffected (ratios of 0.86 ± 0.04 and 0.78 ± 0.04, respectively). In all these mice, data were collected at different lengths, frequency, as well as at different concentrations of isoproterenol. Further analysis of mice in which only two of these mechanisms were investigated, too, showed that the ratio between dF/dtmin and dF/dtmax was not affected by other genetic interventions, including the loss of lysosome-associated membrane protein-2 (51), overexpression of sarcolipin (3), ablation of sarcolipin (not published), and other wild-type mice (C57BL/6, C57BL10, and FVBN, not published). Similar results, in all strains, were obtained when not comparing peak dF/dt but compared time from stimulation to peak tension (TTP) to time from peak tension to 50% relaxation (RT50). In one of these studies (50), we simultaneously recorded intracellular calibrated calcium transients. From that data, we gained further confirmation that this balance is not primarily affected by EC coupling. Where the calcium transient balance between RT50 and TTP of the calcium transient changed greatly (for instance from 2.45 to ∼1.4 when frequency was increased from 2 to 10 Hz), the balance of this parameter was unchanged in the resulting force tracings (0.54 to 0.52). This overall analysis of these strains led us to conclude that this kinetic balance is a parameter not primarily influenced by EC coupling changes and more likely is a structural property of the sarcomere that is virtually unaffected by normal regulatory mechanisms.
Fig. 3.
A: correlation of the maximal speeds of contraction and relaxation in an isolated murine trabecula (wild-type C57 mouse), assessed under conditions where length, frequency, or concentration of the β-adrenergic agonist isoproterenol was varied. Data recorded at 37°C. dF/dtmax and dF/dtmin, maximum positive and negative derivatives of force, respectively. B: correlation of speed of relaxation and speed of contraction in rat trabeculae (average data of n = 10 trabeculae) at various conditions. FS, Frank-Starling relationship, muscle contracted at 4 different lengths (0.5, 1, or 2 stimulation frequency); RT, room temperature (held at 22.5°C); BT, body temperature (held at 37.5°C); Temp, 1 Hz, optimal length at various temperature (in 2.5°C steps) between RT and BT; Iso, various concentrations of isoproterenol; Temp Up ISO, maximal isoproterenol concentration at different temperatures; FFR, force-frequency relationship. Because the slowest data (low dF/dt) are bundled and to convey that the correlation holds true for timing parameters in general rather than only dF/dt, we plotted the same data now correlating time from stimulation to peak tension to time from peak tension to RT50. All data except for those at low preload, 0.5 Hz, and at the lowest temperature (●) deviated significantly (P < 0.05, analysis of variance) from the mean correlation. Data modified from Janssen (22).
Further evidence that this kinetic balance is a structural property was gained by the exception observed in the analysis of the above-mentioned total of 19 strains; there was one strain that significantly deviated from the average ratio of dF/dtmin and dF/dtmax (0.82 ± 0.02) from all groups. When we analyzed mice in which the myosin-binding protein C (MyBP-C) was truncated and did not functionally express MyBP-C (41), they showed a highly significant different ratio of 0.61 ± 0.07, in sharp contrast to normal littermates of the same study that had a ratio of 0.89 ± 0.04. Still, within this group, the correlation between dF/dtmin and dF/dtmax was very tight, with an r2 of 0.98. This indicates that the changes in length, frequency, or presence of isoproterenol do not affect the correlation between balance dF/dtmin and dF/dtmax, only the slope of the relationship in this strain. The location of MyBP-C within the sarcomeric matrix and specifically whether or not it binds to actin are currently incompletely understood, but it is generally seen as part of the thick filament structure. This would thus fit well with the hypothesis that this kinetic unifier is located/has to do with the sarcomeric structure.
The above data were all examined at body temperature in the mouse. However, an investigation of the contractile properties in isolated muscles have been done in rats and at room temperature. To examine whether this kinetic balance is preserved when temperature is lowered, we performed a similar analysis of contraction and relaxation kinetics on our 2002 study in rat trabeculae (29), where we assessed all of the above conditions at body temperature, but in addition assessed seven different temperatures, as well as different frequencies and length at room temperature. The derived correlation was very tight (see Fig. 3B), with the ratio of dF/dtmin over dF/dtmax being 0.85 ± 0.04, which is nearly identical to the average for the mouse, which was 0.84 ± 0.02 when the MyPB-C mouse was excluded. Within all the rat muscle data, there was, however, one outlying protocol: data at low frequency (0.5 Hz) and low preload and at low temperature (22.5°C) showed a consistently lower ratio of dF/dtmin and dF/dtmax than all the other protocols. Note that this protocol is also the one furthest removed from the physiological situation compared with all other assessments. This finding may have implications for the interpretation of the studies on isolated myocytes regarding contraction-relaxation coupling, since they often are assessed without preload, at low frequency, and at room temperature.
In summary, from this part of my presentation I conclude that 1) force kinetics are tightly coupling contraction speed to relaxation speed, and the underlying calcium transient shape does not uncouple this relationship; 2) modulation of structural myofilament properties, however, can change the slope of this contraction-relaxation coupling but not delinearize this relationship; 3) only if mechanical load, normal frequency, or physiological temperature are not used, this relationship can deviate from the overall linear correlation; and 4) for assessment for contractile properties, it may be advised to avoid the combination of cold, slow, and nonloaded conditions for the assessment of contractile properties by making at the very least one of these experimental conditions physiologically relevant.
Impaired Relaxation in Cardiac Hypertrophy
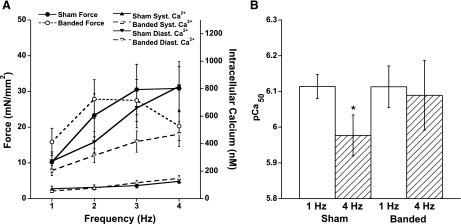
In this fourth and last part of this lecture I want to share recent data collected on cardiac contraction-relaxation coupling in a model where relaxation impairment is prominent in a clinical setting. In the hypertrophic heart, a slow relaxation often manifests as one of the critical parameters that contribute to the depressed function (15, 17). We showed in part 1 that frequency-dependent activation impacts myofilament calcium sensitivity and postulate that in a pathological situation, a deregulation of this physiological mechanism may be a possible contributor to the pathophysiology. We again used the rabbit as our model species and used a pulmonary artery banding (PAB) surgical intervention to induce right ventricular hypertrophy (55). Ten weeks postsurgery, in this model the right ventricle has significantly hypertrophied, as had the right atrium (16), whereas both the left ventricle and atrium were not affected. On the protein expression level, the PAB rabbit model showed an increased expression of atrial natriuretic peptide and the sodium-calcium exchanger and a decreased level of the SR calcium ATPase (55). Functionally, the FFR had become blunted (Fig. 4A) and relaxation of isolated muscles was significantly slower compared with sham-operated controls. In this model, we then investigated the myofilament calcium sensitivity using our potassium-contracture protocol described in part 1.
Fig. 4.
A: trabeculae isolated from pulmonary artery-banded rabbits show a blunted FFR, whereas the systolic (Syst) calcium concentration increases less pronounced with increasing frequency compared with muscles isolated from sham-operated rabbits. Diastolic (Diast) calcium concentration rose with frequency in both groups. B: myofilament calcium sensitivity significantly (*P < 0.05) decreased in muscles from sham-operated rabbits but remained unchanged in pulmonary artery-banded muscles when frequency of stimulation was increased from 1 to 4 Hz. All data were obtained at steady state and at optimal length at 37°C. Data modified from Varian et al. (55).
Our first observation was that the sham-operated animals functionally behaved the same as in the control experiments in control, unoperated rabbits used in part 1; as frequency was increased, myofilament calcium sensitivity decreased significantly. This indicated that the effects of the surgery itself after 10 wk had no significant impact on the functional assays conducted. In sharp contrast, after 10 wk of PAB, the frequency-dependent decrease in calcium sensitivity was nearly completely lost in these trabeculae (Fig. 4B). Thus the compensatory decrease in calcium sensitivity did not occur when the muscle was stimulated at a higher frequency. The overall time between the two stimulations is, of course, reduced at a higher heart rate, and this restriction, when combined with the observed impaired acceleration of relaxation, resulted in a significant elevation of diastolic force (55). The hypertrophied muscle was not able to fully relax, causing an active diastolic tension. Although the results from isometrically contracting muscle can quantitatively not be directly translated in the behavior of the whole ventricle, it stands to reason that this elevated diastolic tension in the myocardial tissue elevates diastolic filing pressure. This would fit well with the histological finding of a hypertrophied atrium in this model (16), likely caused by pressure-overload hypertrophy.
On the molecular level, we investigated the phosphorylation level of proteins previously identified as potential players in this mechanism. In line with the functional findings, the increase in TnI phosphorylation seen in normal rabbit myocardium with elevated heart rate was no longer observed. At this point, we can only speculate about the signaling mechanism behind this phenomenon. Future studies aimed at identifying the involved kinases and/or proteases, as well as a more comprehensive study on potential posttranslational modification of myofilament targets, are needed to further understand the governing of relaxation of the myocardium.
Clinically, the relaxation of the cardiac muscle is increasingly gaining attention. Whereas several decades ago heart failure was mainly seen as a loss of contractile force, developments in imaging technology and in other aspects of cardiac function assessment currently indicate that more than half of the patients suffering from heart failure exhibit some degree of diastolic dysfunction. Moreover, a significant subset of patients have a normal ejection fraction, but because of impaired filling caused by a slow relaxation of the cardiac muscle, their ventricle simply contains not enough blood, so a normal ejection fraction still results in an impaired cardiac output.
In summary, from this part of my presentation I conclude that 1) frequency-mediated myofilament desensitization is impaired in the hypertrophied myocardium, likely contributing to impaired relaxation, and 2) phosphorylation of myofilament targets is impaired at a higher frequency, likely contributing or leading to diastolic dysfunction.
Summary
I will conclude by repeating the key findings of our studies these past few years. I hope to have shown that cardiac muscle relaxation is not a mere cessation of contraction but an actively regulated physiological process. We showed that 1) FFR is in part mediated at the molecular level by a desensitization of myofilaments that facilitate accelerated relaxation; 2) the decline of the calcium transient does not directly reflect relaxation: sarcomeric properties are paramount in governing relaxation of the heart muscle; 3) relaxation and contraction speed are intrinsically coupled over the full range of physiological perturbations; only the most nonphysiological conditions can uncouple this relationship; and 4) in a model of cardiac hypertrophy, frequency-mediated myofilament desensitization is impaired, likely leading/contributing to impaired relaxation and diastolic dysfunction.
GRANTS
This studies in this presentation were supported by National Heart, Lung, and Blood Institute Grants R01-HL-746387 and KO2-HL-083957 (to P. M. L. Janssen) and American Heart Association Established Investigator Award 0740040N (to P. M. L. Janssen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
I thank the American Physiological Society and especially Dr. Zucker for giving me the honor of being a Bowditch Lecturer and the opportunity to present my work, and I sincerely thank my mentors, colleagues, students, and technicians, all who, past or present, contributed in some form to the work presented here.
REFERENCES
- 1.Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol 17: 821–840, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol (Lond) 327: 79–94, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babu GJ, Bhupathy P, Petrashevskaya NN, Wang H, Raman S, Wheeler D, Jagatheesan G, Wieczorek D, Schwartz A, Janssen PM, Ziolo MT, Periasamy M. Targeted overexpression of sarcolipin in the mouse heart decreases sarcoplasmic reticulum calcium transport and cardiac contractility. J Biol Chem 281: 3972–3979, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bers DM. Ca2+ sparks. Jumping the gap from the cell to cardiac muscle. Circ Res 81: 636–638, 1997 [PubMed] [Google Scholar]
- 5.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin 4: 23–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowditch HP. Ueber die Eigenthuemlichkeiten der Reizbarkeit, welche die Muskelfasern des Herzens zeigen. Ber Sachs Ges (Akad) Wiss 23: 652–689, 1871 [Google Scholar]
- 7.Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J 19: 88–90, 2005 [DOI] [PubMed] [Google Scholar]
- 8.de Tombe PP. Altered contractile function in heart failure. Cardiovasc Res 37: 367–380, 1998 [DOI] [PubMed] [Google Scholar]
- 9.de Tombe PP, Little WC. Inotropic effects of ejection are myocardial properties. Am J Physiol Heart Circ Physiol 266: H1202–H1213, 1994 [DOI] [PubMed] [Google Scholar]
- 10.De Santiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol 34: 975–984, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: impact of sarcomere length. Am J Physiol Heart Circ Physiol 282: H1055–H1062, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Frank O. Zur Dynamik des Herzmuskels. Z Biol 32: 370–447, 1895 [Google Scholar]
- 13.Gao WD, Backx PH, Azan-Backx M, Marban E. Myofilament Ca2+ sensitivity in intact versus skinned rat ventricular muscle. Circ Res 74: 408–415, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Georgakopoulos D, Kass D. Minimal force-frequency modulation of inotropy and relaxation of in situ murine heart. J Physiol 534: 535–545, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman W. Diastolic dysfunction and congestive heart failure. Circulation 81: III1–III7, 1990 [PubMed] [Google Scholar]
- 16.Gupta SC, Varian KD, Bal NC, Abraham JL, Periasamy M, Janssen PM. Pulmonary artery banding alters the expression of Ca2+ transport proteins in the right atrium in rabbits. Am J Physiol Heart Circ Physiol 296: H1933–H1939, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwathmey JK, Morgan JP. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res 57: 836–843, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest 85: 1599–1613, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison SM, Bers DM. Influence of temperature on the calcium sensitivity of the myofilaments of skinned ventricular muscle from the rabbit. J Gen Physiol 93: 411–428, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiranandani N, Raman S, Kalyanasundaram A, Periasamy M, Janssen PM. Frequency-dependent contractile strength in mice over- and underexpressing the sarco(endo)plasmic reticulum calcium-ATPase. Am J Physiol Regul Integr Comp Physiol 293: R30–R36, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Holubarsch C. Force generation in experimental tetanus, KCl contracture, and oxygen and glucose deficiency contracture in mammalian myocardium. Pflügers Arch 396: 277–284, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol 299: H1092–H1099, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen PM, de Tombe PP. Uncontrolled sarcomere shortening increases intracellular Ca2+ transient in rat cardiac trabeculae. Am J Physiol Heart Circ Physiol 272: H1892–H1897, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Janssen PM, Hiranandani N, Mays TA, Rafael-Fortney JA. Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 289: H2373–H2378, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Janssen PM, Honda H, Koiwa Y, Shirato K. The effect of diastolic vibration on the relaxation of rat papillary muscle. Cardiovasc Res 32: 344–350, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Janssen PM, Hunter WC. Force, not sarcomere length, correlates with prolongation of isosarcometric contraction. Am J Physiol Heart Circ Physiol 269: H676–H685, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Janssen PM, Lehnart SE, Prestle J, Lynker JC, Salfeld P, Just H, Hasenfuss G. The trabecula culture system: a novel technique to study contractile parameters over a multiday time period. Am J Physiol Heart Circ Physiol 274: H1481–H1488, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol 43: 523–531, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janssen PM, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol 282: H499–H507, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res 58: 755–768, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res 90: 59–65, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Kranias EG, Solaro RJ. Phosphorylation of troponin I and phospholamban during catecholamine stimulation of rabbit heart. Nature 298: 182–184, 1982 [DOI] [PubMed] [Google Scholar]
- 33.Layland J, Kentish JC. Positive force- and [Ca2+]i-frequency relationships in rat ventricular trabeculae at physiological frequencies. Am J Physiol Heart Circ Physiol 276: H9–H18, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Ling E, O'Brien PJ, Salerno T, Ianuzzo CD. Effects of different thyroid treatments on the biochemical characteristics of rabbit myocardium. Can J Cardiol 4: 301–306, 1988 [PubMed] [Google Scholar]
- 35.Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL, Loukianova T, Grupp G, Lytton J, Walsh RA, Periasamy M. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res 83: 889–897, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Maier LS, Bers DM, Pieske B. Differences in Ca2+-handling and sarcoplasmic reticulum Ca2+-content in isolated rat and rabbit myocardium. J Mol Cell Cardiol 32: 2249–2258, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Monasky MM, Biesiadecki BJ, Janssen PM. Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions. J Mol Cell Cardiol 48: 1023–1028, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monasky MM, Janssen PM. The positive force-frequency relationship is maintained in absence of sarcoplasmic reticulum function in rabbit, but not in rat myocardium. J Comp Physiol [B] 179: 469–479, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Monasky MM, Varian KD, Davis JP, Janssen PM. Dissociation of force decline from calcium decline by preload in isolated rabbit myocardium. Pflügers Arch 456: 267–276, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Morgan JP, Erny RE, Allen PD, Grossman W, Gwathmey JK. Abnormal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation 81: III21–III32, 1990 [PubMed] [Google Scholar]
- 41.Palmer BM, Georgakopoulos D, Janssen PM, Wang Y, Alpert NR, Belardi DF, Harris SP, Moss RL, Burgon PG, Seidman CE, Seidman JG, Maughan DW, Kass DA. Role of cardiac myosin binding protein C in sustaining left ventricular systolic stiffening. Circ Res 94: 1249–1255, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem 274: 2556–2562, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Picht E, De Santiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol 42: 196–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schouten VJ, ter Keurs HE. The force-frequency relationship in rat myocardium. The influence of muscle dimensions. Pflügers Arch 407: 14–17, 1986 [DOI] [PubMed] [Google Scholar]
- 45.Solaro RJ, van der Velden J. Why does troponin I have so many phosphorylation sites? Fact and fancy. J Mol Cell Cardiol 48: 810–816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 45: 32–43, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Starling EH. The Lineacre lecture on the Law of the heart. London: Longmans Green, 1918 [Google Scholar]
- 48.Stehle R, Iorga B. Kinetics of cardiac sarcomeric processes and rate-limiting steps in contraction and relaxation. J Mol Cell Cardiol 48: 843–850, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Stull LB, Hiranandani N, Kelley MA, Leppo MK, Marban E, Janssen PM. Murine strain differences in contractile function are temperature- and frequency-dependent. Pflügers Arch 452: 140–145, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Stull LB, Leppo M, Marban E, Janssen PM. Physiological determinants of contractile force generation and calcium handling in mouse myocardium. J Mol Cell Cardiol 34: 1367–1376, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Stypmann J, Janssen PM, Prestle J, Engelen MA, Kogler H, Lullmann-Rauch R, Eckardt L, von Figura K, Landgrebe J, Mleczko A, Saftig P. LAMP-2 deficient mice show depressed cardiac contractile function without significant changes in calcium handling. Basic Res Cardiol 101: 281–291, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Takagi T, Koiwa Y, Kikuchi J, Honda H, Hoshi N, Butler JP, Takishima T. Diastolic vibration improves systolic function in cases of incomplete relaxation. Circulation 86: 1955–1964, 1992 [DOI] [PubMed] [Google Scholar]
- 53.ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res 46: 703–714, 1980 [DOI] [PubMed] [Google Scholar]
- 54.Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292: H2212–H2219, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Varian KD, Kijtawornrat A, Gupta SC, Torres CA, Monasky MM, Hiranandani N, Delfin DA, Rafael-Fortney JA, Periasamy M, Hamlin RL, Janssen PM. Impairment of diastolic function by lack of frequency-dependent myofilament desensitization rabbit right ventricular hypertrophy. Circ Heart Fail 2: 472–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varian KD, Raman S, Janssen PM. Measurement of myofilament calcium sensitivity at physiological temperature in intact cardiac trabeculae. Am J Physiol Heart Circ Physiol 290: H2092–H2097, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Varian KD, Xu Y, Torres CA, Monasky MM, Janssen PM. A random cycle length approach for assessment of myocardial contraction in isolated rabbit myocardium. Am J Physiol Heart Circ Physiol 297: H1940–H1948, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Lewinski D, Stumme B, Maier LS, Luers C, Bers DM, Pieske B. Stretch-dependent slow force response in isolated rabbit myocardium is Na+ dependent. Cardiovasc Res 57: 1052–1061, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Wannenburg T, Janssen PM, Fan D, de Tombe PP. The Frank-Starling mechanism is not mediated by changes in rate of cross-bridge detachment. Am J Physiol Heart Circ Physiol 273: H2428–H2435, 1997 [DOI] [PubMed] [Google Scholar]