Abstract
Serum and glucocorticoid-regulated kinase 2 (sgk2) is 80% identical to the kinase domain of sgk1, an important mediator of mineralocorticoid-regulated sodium (Na+) transport in the distal nephron of the kidney. The expression pattern and role in renal function of sgk2 are virtually uncharacterized. In situ hybridization and immunohistochemistry of rodent kidney coupled with real-time RT-PCR of microdissected rat kidney tubules showed robust sgk2 expression in the proximal straight tubule and thick ascending limb of the loop of Henle. Sgk2 expression was minimal in distal tubule cells with aquaporin-2 immunostaining but significant in proximal tubule cells with Na+/H+ exchanger 3 (NHE3) immunostaining. To ascertain whether mineralocorticoids regulate expression of sgk2 in a manner similar to sgk1, we examined sgk2 mRNA expression in the kidneys of adrenalectomized rats treated with physiological doses of aldosterone together with the glucocorticoid receptor antagonist RU486. Northern blot analysis and in situ hybridization showed that, unlike sgk1, sgk2 expression in the kidney was not altered by aldosterone treatment. Based on the observation that sgk2 is expressed in proximal tubule cells that also express NHE3, we asked whether sgk2 regulates NHE3 activity. We heterologously expressed sgk2 in opossum kidney (OKP) cells and measured Na+/H+ exchange activity by Na+-dependent cell pH recovery. Constitutively active sgk2, but not sgk1, stimulated Na+/H+ exchange activity by >30%. Moreover, the sgk2-mediated increase in Na+/H+ exchange activity correlated with an increase in cell surface expression of NHE3. Together, these results suggest that the pattern of expression, regulation, and role of sgk2 within the mammalian kidney are distinct from sgk1 and that sgk2 may play a previously unrecognized role in the control of transtubular Na+ transport through NHE3 in the proximal tubule.
Keywords: sgk, OKP, kidney tubule, sodium/hydrogen exchanger
the regulation of sodium (Na+) transport in the mammalian kidney is crucial for maintaining extracellular fluid volume and blood pressure in the face of changes in dietary Na+ content. A variety of transporters and channels are expressed along the nephron with marked axial heterogeneity, reflecting the distinct roles for ion transport in each of the different nephron segments. For example, the majority of Na+ reabsorption in the proximal tubule and thick ascending limb of the loop of Henle (TALH) is mediated via transporters that couple counter- and cotransport of Na+ with other solutes. Notable among these is Na+/H+ exchanger 3 (NHE3), which mediates bulk reabsorption of filtered NaCl and NaHCO3 in the proximal tubule (1). In contrast, the epithelial Na+ channel (ENaC) is expressed in the distal tubule and is a critical mediator of Na+ transport and blood pressure regulation (34, 43, 53, 54).
Serum and glucocorticoid regulated kinase 1 (sgk1), a serine-threonine kinase that stimulates ENaC-mediated Na+ transport, is regulated by mineralocorticoids in the kidney collecting duct (14, 48). Sgk1 mRNA has been identified in glomeruli, distal convoluted tubule (DCT), and cortical (CCD) and medullary collecting duct (MCD) (11, 14, 29). Immunoreactive protein expression of sgk1 in the kidney has been characterized independently by two groups: Loffing et al. (44) found sgk1 protein expression in DCT, connecting tubule, CCD, and MCD, while Alvarez de la Rosa et al. (3) detected sgk1 immunoreactive protein only in TALH, DCT, and CCD.
In addition to sgk1, two other sgk isoforms, sgk2 and sgk3, have been identified (41). Sgk2 and sgk3 share 80% homology with sgk1 in their kinase domains, and, like sgk1, are activated by 3′-phosphoinositide-dependent kinase-1 (PDK1), placing them under the regulation of the phosphoinositide 3′-kinase (PI 3-kinase) signaling pathway. The three sgk isoforms phosphorylate serine and threonine residues that lie within RXRXX(S/T) motifs (41). Despite these similarities, the sgk isoforms share only 50% amino acid homology in their COOH-terminal domains and <15% homology in their NH2-terminal domains (50). Kobayashi et al. (41) found sgk1 and sgk3 mRNAs in all tissues analyzed, whereas sgk2 mRNA was found selectively in tissues including the brain, pancreas, liver, and kidney (41). While sgk1 mRNA levels are regulated by serum and glucocorticoids, sgk2 and sgk3 mRNA levels are not (3, 41).
To date, few studies have directly addressed the cell-specific regulation or physiological function of sgk2 in the mammalian kidney. Several studies involving heterologous expression systems have demonstrated that sgk2 stimulates SMIT1 (37), EAAT2 (13), GluR6 (56), Kv1.3 (27), KCNE1 (21), Na+/K+-ATPase (55), and ENaC (22). However, in all of these studies, the stimulatory effect was not sgk2 specific; sgk1 or sgk3 were also capable of stimulating these channels or transporters. Without knowledge of the precise localization of sgk2 expression in the kidney, it is difficult to assess the physiological significance of these in vitro studies.
In this study, we used in situ hybridization of intact kidney and real-time RT-PCR of microdissected tubule segments to characterize the expression of sgk2 mRNA in the kidneys of adrenal-intact mice and rats. We also used immunohistochemistry of intact kidney and isolated kidney tubules to confirm that sgk2 immunoreactive protein is expressed with NHE3 in proximal tubule cells. We next hypothesized that mineralocorticoids might regulate expression of sgk2 in a manner similar to sgk1 in the kidney. We used Northern blot analysis and in situ hybridization to examine sgk2 mRNA expression in kidneys of adrenalectomized rats treated with physiological doses of aldosterone. Based on the knowledge that sgk2 is expressed in proximal tubule cells that also express NHE3, we hypothesized that sgk2 plays a role in modulating activity of NHE3 in opossum kidney (OKP) cells, a model system for NHE3-mediated Na+/H+ exchange in the proximal tubule. From these studies, we found the following: 1) sgk2 mRNA is expressed in proximal straight tubule (PST) and TALH in rat and mouse kidney; 2) sgk2 immunoreactive protein is expressed in rat proximal tubule cells that also express NHE3; 3) sgk2 mRNA expression is not stimulated by aldosterone in the kidneys of adrenalectomized rats; and 4) heterologous expression of sgk2 in OKP cells stimulates NHE3-mediated Na+/H+ exchange activity and cell surface expression of NHE3. Together, these findings suggest that the expression, regulation, and role of sgk2 within the mammalian kidney are distinct from sgk1 and that sgk2 regulates NHE3 activity in proximal tubule cells.
METHODS
Animals.
Male Sprague-Dawley rats (over 50 g) (Harlan, Indianapolis, IN) or C57BL/6 mice (over 20 wk old) were maintained on a regular chow diet with normal Na+ content. All procedures were in accordance with the Committees on Animal Research at the University of California, San Francisco and the University of Texas Southwestern Medical Center.
For studies examining the effects of mineralocorticoids on sgk2 expression, male Sprague-Dawley rats were sham-operated (control) or bilaterally adrenalectomized on the day before use and given overnight a 0.9% NaCl solution to drink. On the day of the experiment, the rats were first injected subcutaneously with 10 μg/kg RU486, a glucocorticoid receptor antagonist that blocks activation of the glucocorticoid receptor. Thirty minutes after the RU486 injection, rats were then injected with graded doses of aldosterone (0, 0.1, 0.3, 1.0, 3.0, or 10 μg/100 g body wt). Rats were euthanized 2 or 4 h after treatment; tissues were removed and processed for Northern blot and in situ hybridization studies.
Riboprobe preparation.
Rat sgk2 (nucleotides 196–793 of the open reading frame) was subcloned into a pBluescript KS expression vector (Stratagene, La Jolla, CA). [32P]dCTP-labeled rat sgk2 and sgk1 riboprobes were used for Northern blot analyses as described (11, 14). [33P]UTP-labeled (Amersham Biosciences, Piscataway, NJ) antisense sgk2 riboprobe was synthesized as described (11, 14) for radioisotopic in situ hybridization studies. Digoxygenin (DIG)-labeled riboprobe was generated with the DIG RNA Labeling Kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions for fluorescent in situ hybridization studies. Sense riboprobes for both types of in situ hybridization studies were used as negative controls.
In situ hybridization.
For radioisotopic in situ hybridization, frozen rat kidneys were sectioned (7–8 μm), thaw-mounted on SuperFrost slides (Fisher Scientific, Pittsburgh, PA), fixed, acetylated, and hybridized as previously described (11). Hybridization was performed in a hybridization solution containing 50% formamide and the appropriate antisense or sense riboprobe. After overnight hybridization, the slides were treated with ribonuclease A, washed to a final stringency of 0.5× SSC at 60°C, passed through an alcohol series, air-dried, and exposed to Hyperfilm MP (Amersham Biosciences). Slides were then dipped in Kodak emulsion, exposed in the dark at 4°C for 10 days, developed, and counterstained with hematoxylin-eosin.
Fluorescence in situ hybridization was performed with tyramide signal amplification (TSA) as previously described (28). Rats or mice were anesthetized with pentobarbital sodium (40–60 mg/kg) and perfused with chilled 0.9% NaCl, followed by chilled 1× PBS. Kidneys were bisected sagitally and fixed in 4% paraformaldehyde at 4°C overnight. Kidneys were rinsed several times with 1× PBS, embedded in paraffin, and sliced into 4- to 5-μm sections as described (28). Paraffin-embedded rat or mouse sections were postfixed with 4% paraformaldehyde, treated with proteinase K digestion, and acetylated. Sections were hybridized overnight using 200 ng/ml of DIG-labeled antisense mRNA or sense mRNA sgk2 riboprobe. Slides were washed to a final stringency of 0.1× SSC and then incubated in blocking buffer (Roche Applied Science) containing 5% normal rabbit IgG (DAKOCytomation, Carpinteria, CA). DIG-labeled riboprobes were detected by incubation with horseradish peroxidase-conjugated anti-DIG antibody (1:200, DAKOCytomation) followed by amplification with TSA-direct deposition of Cy3 (1:50, PerkinElmer Life Sciences, Boston, MA). The slides were then dehydrated and mounted with VectaShield (Vector Labs, Burlingame, CA). Images were obtained with a Carl Zeiss Axioskop 50 epifluorescence microscope (Carl Zeiss International, Thornwood, NJ) and processed using Metamorph version 6.2 (Universal Imaging, Downington, PA).
Immunohistochemistry of in situ hybridization sections.
Following fluorescent in situ hybridization as described above, slides were incubated in blocking solution (10% donkey serum/0.1% BSA in 1× PBS) before incubation with the antibody specific for the appropriate nephron segment marker. Goat anti-aquaporin-2 (AQP2) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:200; rabbit anti-Tamm-Horsfall (THP) antibody (Santa Cruz Biotechnology) was diluted 1:50; and mouse monoclonal anti-NHE3 antibody (clone 3H3 raised against opossum NHE3) (18, 19) was diluted 1:5. Sections were washed in 1× PBS and incubated with the appropriate secondary antibody (Texas red-conjugated donkey anti-goat, anti-rabbit, or anti-mouse IgG, Jackson Immunoresearch Laboratories, West Grove, PA) diluted at 1:500. Slides were rinsed in 1× PBS and mounted with VectaMount (Vector Labs).
Microdissection of rat tubules and real-time RT-PCR.
Male Sprague-Dawley rats (50–75 g) were anesthetized with pentobarbital (40–60 mg/kg), and their kidneys were removed, sliced coronally into several sections, and placed in chilled modified Hanks solution. One-millimeter nephron segments from glomeruli, proximal convoluted tubule (PCT), PST, TALH, and CCD were dissected manually and placed in lysis buffer containing guanidine thiocyanate and β-mercaptoethanol.
Total RNA from five tubules from each rat nephron segment was extracted according to the manufacturer's instructions (Absolutely RNA Microprep Kit, Stratagene). Reverse transcriptase reactions from each nephron segment were performed according to the manufacturer's instructions (Multiscribe RT, Applied Biosystems, Foster City, CA).
To verify the identities of each microdissected tubule segment, PCR primers of nephron segment-specific markers were generated using ABI Prism Primer Express Version 2.0 software (Applied Biosystems): 5′-CGAAGCCAGGGTGATACCC (rat nephrin) and 3′-CACAGACCAGTAACTCCCGT (rat nephrin) were used for the identification of glomeruli (36); 5′-AGCTCCAGCACCTCGACATC [rat Na+-dependent phosphate cotransporter 2A (NaPi 2A)] and 3′-CAAGCCAGAGGAGACCATGC (rat NaPi 2A) were used for the identification of PCT (17); 5′-AGACTGCGATCATGCTGGTG (rat sodium-glucose cotransporter-1; SGLT-1) and 3′-GGAAAGCAAACCCAGTCAGG (rat SGLT-1) were used for the identification of PST (42); 5′-GTGTCCAGGCCTCAGTGTCC (rat THP) and 3′-TCAGGAACCCCAAGTTGCTG (rat THP) were used for the identification of TALH (35); 5′-GCCACCTCCTTGGGATCTATT (rat AQP2) and 3′-GAGCGGGCTGGATTCATG (rat AQP2) were used for the identification of CCD (23). PCR primers were diluted for each reaction mixture using a SYBR Green PCR Master Mix kit according to the manufacturer's instructions (Applied Biosystems). Reactions, including no template controls, were performed in triplicate. Fluorescent changes during real-time quantitative PCR were measured with an ABI Prism 7700 sequence detector (Applied Biosystems). Thermal cycling parameters were the following: incubation at 95°C for 10 min followed by 40 cycles at 95°C for 15 s and 60°C for 60 s. To confirm specificity of an amplicon product, a dissociation curve was performed for each PCR reaction.
The following PCR primers were also designed and used for detecting gene amplification: 5′-CATTGGCAAAGGGAACTATGG (rat sgk2), 3′-AGTCAGACGGAGCCTTCTATGCA (rat sgk2); 5′-GGGACAACGTCCACCTTCTG (rat sgk1), 3′-AGGTTCTCCATAAGCAGCCG (rat sgk1); and 5′-TTTTTTATCTGCACTGCCAAGACT (rat cyclophilin A), 3′-CATGTGGTCTTTGGGAAGGTG (rat cyclophilin A). The following 6-carboxyfluorescin (FAM) TaqMan probes were also generated: 6-FAM-5′-AAGGTCCTACTGGCCAAG-MGBNFQ (rat sgk2); 6-FAM-5′-AGCCCTGAGTATCTC-MGBNFQ (rat sgk1); and 6-FAM-5′-AGTGGCTGGATGGCA-MGBNFQ (rat cyclophilin A). PCR primers and TaqMan probes were diluted for each reaction mixture using the TaqMan PCR kit according to the manufacturer's instructions (Applied Biosystems). Thermal cycling parameters were the same as described for real-time RT-PCR of nephron segment-specific markers. Specificity of each set of primers and probes was confirmed by BLAST search against GenBank and verified in standard PCR reactions using sgk2 and sgk1 expression plasmids as DNA templates.
Validation experiments were required to demonstrate that the efficiencies of target (sgk2 and sgk1) and normalizer reference (cyclophilin A) amplification were approximately equal so that the ΔΔCt method of analysis could be used, as described by the manufacturer (Applied Biosystems). Relative quantitation of sgk2 or sgk1 expression was calculated across microdissected nephron segments (glomeruli, PCT, PST, TALH, and CCD) using whole rat kidney as the calibrator reference.
Generation of sgk2 antibody.
Glutathione S-transferase (GST)-sgk2 fusion proteins were engineered, expressed, and purified on a glutathione-Sepharose column according to the GST purification system (Amersham Biosciences). Purified GST-sgk2 fusion protein was sent to Covance (Princeton, NJ) for production of rabbit polyclonal sgk2 antiserum. To validate antibody specificity for the sgk2 isoform, reticulocyte and OKP cell lysates expressing mouse sgk1–3 were resolved by 7.5% SDS-PAGE and then analyzed by immunoblotting with sgk2 antiserum diluted 1:5,000. Membranes were incubated with secondary anti-rabbit IgG horseradish peroxidase conjugate (Amersham Biosciences) diluted 1:5,000 and processed as described (57). Membranes were stripped and reblotted with anti-GAPDH antibody diluted 1:20,000 (Chemicon International, Temecula, CA) for detection of GAPDH as a protein loading control.
Kidney tissue and single-tubule preparation.
Rats were anesthetized with pentobarbital sodium (40–60 mg/kg) and perfused with 2.5% paraformaldehyde for tissue fixation. Kidneys were removed and postfixed in 4% paraformaldehyde at 4°C. PST were microdissected from kidneys of rats on a regular chow diet. Tubules were embedded in gelatin and fixed with 4% paraformaldehyde in 4°C. Three-micrometer frozen sections of kidney tissue and tubule preparations were cut with a cryostat microtome as described (7).
Sgk2 and NHE3 immunohistochemistry.
Tissue and single-tubule frozen sections were washed with PBS followed by incubation with 0.1% Triton X-100. Sections were then blocked with a blocking solution (PBS, 1.5% BSA, 10% goat serum) and then incubated with the primary antibody [anti-sgk2 rabbit polyclonal antibody 1:40 or anti-NHE3 rabbit polyclonal antibody 1566 (6) 1:300 diluted in blocking solution] in 4°C overnight. After washing with PBS, the sections were incubated with FITC-labeled secondary antibody (for NHE3) or rhodamine-coupled secondary antibody (for sgk2). After additional PBS washes, sections were mounted and analyzed by epifluorescence microscopy with Nomarski optics.
Isolation of RNA and Northern blot analysis.
Total RNA was extracted from kidneys of adult rats by homogenization in TRIzol reagent (Invitrogen, Carlsbad, CA). Homogenates were extracted with chloroform, and RNA was precipitated from the aqueous phase with isopropanol. RNA pellets were washed in 70% ethanol, dried, and dissolved in water. Northern blot analysis was performed as described (11, 14). Briefly, formaldehyde agarose gels were loaded with 15 μg/lane of total RNA, transferred, and hybridized with an anti-sense [32P]dCTP-labeled rat sgk2. All filters were rehybridized with an anti-sense [32P]dCTP-labeled riboprobe complementary to DNA for rat sgk1 (as a positive control for aldosterone induction) or rat cyclophilin A (as a loading control) as described (11, 12).
OKP cell culture and transfection.
Wild-type mouse sgk1 and sgk2 open reading frames were subcloned into pCDNA3 expression vectors (Invitrogen). Expression vectors for kinase-dead forms (sgk1 K127M and sgk2 K64M; both mutations prevent ATP binding to the sgk catalytic sites) and constitutively active (CA) forms (sgk1 S422D and sgk2 S356D; substitution of aspartate for serine residue mimics phosphorylation and subsequent activation by PDK1) of sgk1 and sgk2 were generated by PCR-based mutagenesis.
OKP cells are a clonal subline of the opossum kidney cell line originally described by Cole et al. (15). OKP cells were seeded on glass coverslips as previously described (4). To examine the functional effects of sgk1 and sgk2 on NHE3 activity, transient transfection of OKP cells was performed using LipofectAMINE 2000 (Invitrogen) with the following sgk1 and sgk2 plasmid constructs: wild-type, kinase-dead, CA, and vector control (pCDNA3 expression vector alone). Transfection efficiency of OKP cells was assessed by transfection with pEGFP-C1 vector (Clontech, Palo Alto, CA) followed by visualization with a fluorescent microscope (Carl Zeiss International); only cell batches with transfection efficiencies >50% were used. Transfected OKP cells were switched to serum-free media for 48 h before measurement of Na+/H+ exchange activity.
Measurement of intracellular pH and Na+/H+ exchange activity.
Continuous measurement of intracellular pH (pHi) was accomplished using the intracellularly trapped pH-sensitive dye BCECF (Molecular Probes, Eugene, OR) as described previously (4). Transfected OKP cells were loaded with acetoxymethyl ester of BCECF, and pHi was calibrated using K+/nigericin as described (2). Na+/H+ exchange activity was measured as the initial rate of the Na+-dependent pHi increase (pHi/dt) after an acid load in the absence of CO2/HCO3−; results were expressed relative to empty vector control. Comparisons were always made between cells derived from the same passage and transfected on the same day before performance of the Na+/H+ exchange assay.
Cell surface biotinylation of NHE3.
Confluent OKP cells in 6-mm plates were rinsed with ice-cold PBS, and surface proteins were biotinylated by incubating cells at 4°C with 1.5 mg/ml sulfo-NHS-SS-Biotin (ThermoScientific, Rockford, IL) in buffer containing 10 mM triethanolamine (pH 7.4), 2 mM CaCl2, and 150 mM NaCl. After labeling, plates were washed at 4°C with quenching buffer (PBS containing 1 mM MgCl2, 0.1 mM CaCl2, and 100 mM glycine). Cells were then lysed in RIPA buffer with protease inhibitors. Lysates were cleared by centrifugation, and the supernatants were diluted to 2.0 mg/ml of protein with RIPA buffer. Cell lysates of equivalent amounts of protein were equilibrated overnight at 4°C with NeutrAvidin agarose beads (ThermoScientific). Beads were washed sequentially with solutions A [50 mM Tris·HCl (pH 7.4), 100 mM NaCl, and 5 mM EDTA], B [50 mM Tris·HCl (pH 7.4) and 500 mM NaCl], and C (50 mM Tris·HCl, pH 7.4).
Biotinylated proteins were released by heating to 95°C with 2.5× loading buffer, separated by SDS-PAGE (10% gel), and electrophoretically transferred to polyvinylidene difluoride membranes. Membranes were probed overnight at 4°C with the 3H3 monoclonal mouse anti-opossum NHE3 antiserum, which has been characterized to specifically label NHE3 by immunoblotting (25). The membranes were then washed in PBS containing 0.05% Tween 20, incubated with a horseradish peroxidase-labeled anti-mouse secondary antibody and visualized by enhanced chemiluminescence.
Statistics.
All results are reported as means ± SE. Statistical analyses for all pairwise multiple comparisons of Na+/H+ exchange activity and cell surface NHE3 assays in OKP cells were performed using ANOVA with Bonferroni's adjustment. Differences were considered to be significant at P values <0.05.
RESULTS
Sgk2 is expressed in the proximal tubule and TALH.
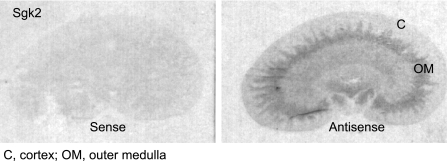
Radioisotopic in situ hybridization of kidneys from adrenal-intact rats revealed that sgk2 mRNA expression was restricted to the medullary rays of the cortex and the outer stripe of the outer medulla and was very low in the outer cortex, inner medulla, and papilla (Fig. 1). Inspection of emulsion-dipped sections counterstained with hematoxylin-eosin revealed that sgk2 expression was expressed in the larger, more intensely eosinophilic proximal tubule cells of the medullary rays of the cortex and outer medulla (not shown).
Fig. 1.
Serum and glucocorticoid-regulated kinase 2 (Sgk2) is expressed in the medullary rays of the cortex (C) and the outer stripe of the outer medulla (OM) in kidney sections from adrenal-intact rats. Sgk2 mRNA expression in the kidney from adrenal-intact rats was measured by radioisotopic in situ hybridization. Longitudinal frozen sections (7–8 μm thick) were cut from rat kidneys and hybridized with sgk2 antisense RNA probes. Sense controls showed absence of hybridization signal.
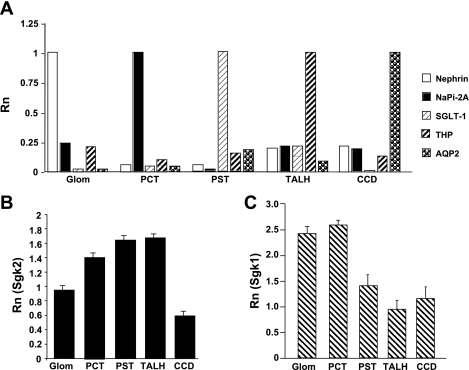
Since the pattern of expression for sgk2 by in situ hybridization was distinct from sgk1, we next performed real-time RT-PCR on total RNA extracted from microdissected nephron segments from rat kidney as an independent assessment of sgk2 expression in the nephron. The kidneys used for microdissection were from adrenal-intact male Sprague-Dawley rats maintained on a regular chow diet. Successful microdissection of glomeruli, PCT, PST, TALH, and CCD was verified by demonstrating that each of the tubule segments expressed appropriate nephron segment-specific markers (Fig. 2A).
Fig. 2.
Relative expression of sgk2 is distinct from sgk1 in microdissected nephron segments from adrenal-intact rats. A: validation of successful microdissection of rat nephron segments was confirmed by amplification of segment-specific markers. Real-time RT-PCR was performed for rat nephrin, sodium-dependent phosphate cotransporter 2A (NaPi 2A), sodium-glucose cotransporter-1 (SGLT-1), Tamm-Horsfall protein (THP), and aquaporin-2 (AQP2) on RNA extracted from microdissected glomeruli (Glom), proximal convoluted tubule (PCT), proximal straight tubule (PST), thick ascending limb of the loop of Henle (TALH), and cortical collecting duct (CCD) tubule segments of kidneys from rats maintained on a normal salt diet. Values are expressed as reporter signal fluorescence (Rn) at a set cycle threshold (Ct) normalized to the specific marker for each of the tubule segments. B: real-time RT-PCR quantitation of sgk2 expression in microdissected rat nephron segments. Quantitation of sgk2 expression was made relative to whole kidney (calibrator reference) using the ΔΔCt method of analysis (see methods). C: real-time RT-PCR quantitation of sgk1 expression in microdissected rat nephron segments. Quantitation of sgk1 expression was made relative to whole kidney. Experiments were performed a total of 4 times (with each run done in triplicate with no template controls) from 4 separate animals on different days with similar results.
Sgk2 was expressed in all microdissected nephron segments. Relative to sgk2 expression levels in whole kidney, higher levels of sgk2 expression were observed in the PST and TALH, and lower levels of expression were observed in the glomerulus and CCD (Fig. 2B). In contrast to sgk2, sgk1 was expressed more prominently in glomeruli and PCT relative to whole kidney (Fig. 2C). From these localization studies, we hypothesized that the pattern of sgk2 expression reflected a role of sgk2 in regulating a population of transporters expressed in the proximal tubule and TALH.
Sgk2 is coexpressed in proximal tubule cells expressing NHE3.
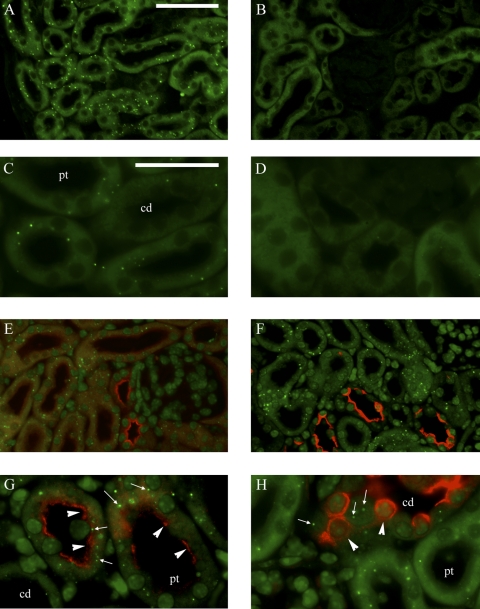
To more accurately determine the specific cell types that express sgk2, we performed in situ hybridization using fluorescent-labeled probes for sgk2 mRNA in combination with immunohistochemical staining for nephron segment-specific markers in both rat and mouse kidney. Figure 3 shows representative images of sgk2 mRNA expression in the mouse kidney. To identify the kidney cell types that express sgk2, we concurrently immunostained in situ hybridization sections for NHE3 and THP. It has been reported that both rat (6) and mouse (7) kidneys express NHE3, with highest levels in PCT and TALH, low levels in PST, and not in CD. In tubules where NHE3 expression is strongest (early PCT or S1 segment), sgk2 expression was moderate. In tubules where NHE3 immunostaining is less pronounced but still prominent (late PCT or S2 segment), sgk2 expression was more pronounced, so that there was significant co-localization of sgk2 with NHE3 immunoreactivity [Fig. 3, E (×20 magnification) and G (×63 magnification)]. In the PST or S3 segment, where NHE3 expression tapers off in the deep cortex and in the outer stripe of the outer medulla, sgk2 expression persisted. Sgk2 expression was also apparent in TALH, as determined by its colocalization with both NHE3 and THP immunoreactivity (not shown).
Fig. 3.
Sgk2 mRNA expression overlaps with Na+/H+ exchanger 3 (NHE3) immunoreactivity in proximal tubules of adrenal-intact mice. Fluorescent in situ hybridization was performed of sgk2 in mouse kidney. Sections were hybridized with sgk2 antisense (A and C) or sense (B and D) riboprobes and costained with antibodies against NHE3 (E and G) or AQP2 (F and H). Renal cortex is shown in all panels. Sgk2-specific signal appears as punctate green dots (A and C), the typical pattern seen with tyramide signal amplification, which is not observed in sections incubated with sgk2 sense riboprobes (B and D). G: sgk2 antisense hybridization signal (arrows) colocalizing with NHE3 immunoreactivity, as represented by red staining in the PCT (arrowheads). H: cell that hybridizes with antisense sgk2 (arrows) but lacks AQP2 immunoreactivity, while an adjacent principal cell shows AQP2 immunostaining (arrowheads) but lacks antisense sgk2 hybridization. pt, Proximal tubule; cd, collecting duct. Magnification: ×20 (A, B, E, and F; bar = 100 μm) and ×63 (C, D, G, and H; bar = 200 μm).
Sgk2 signal was low in CCD, MCD, and papilla in both mouse and rat kidneys. In the mouse cortex [Fig. 3, F (×20 magnification) and H (×63 magnification)], sgk2 expression appeared only in tubule cells that lack AQP2 immunoreactivity (a marker of principal cells of CCD and MCD). In mouse and rat medulla, sgk2 expression was also not detectable in tubule cells that display AQP2 immunostaining (not shown). Taken together, in situ hybridization with radioactive or fluorescent-labeled probes suggest that sgk2 is expressed in the more distal segments of the proximal tubule and TALH, where it colocalizes with NHE3 expression.
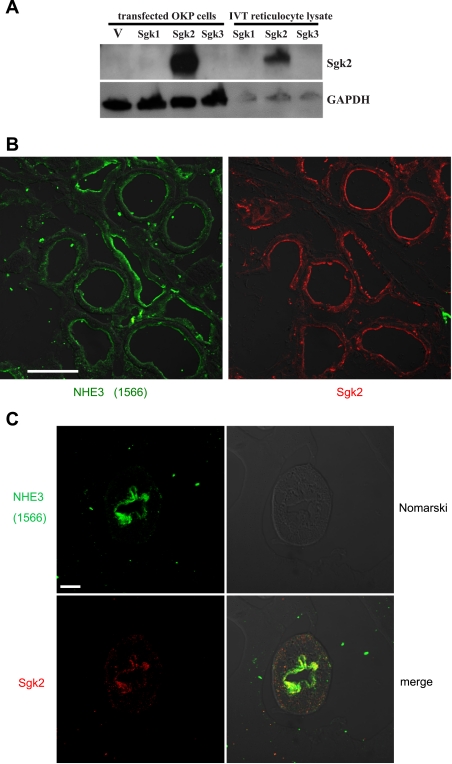
To further characterize the colocalization of sgk2 and NHE3 expression in proximal tubule cells, we investigated sgk2 and NHE3 immunoreactive protein expression in rat kidney tissue and single tubule preparations. We generated a rabbit polyclonal sgk2 antibody and used Western blotting to test the ability of the antiserum to recognize sgk2. Figure 4A shows that the sgk2 antibody only recognized a 37-kDa band from in vitro transcribed/translated reticulocyte lysates and from OKP cell lysates expressing mouse sgk2. We did not observe any immunoreactive bands in lysates expressing mouse sgk1, sgk3, or pCDNA3 expression vector (Fig. 4A), confirming the specificity of the sgk2 antibody. We next examined the distribution of sgk2 and NHE3 expression in successive kidney sections from adrenal-intact rats. As described previously (6), NHE3 staining with antiserum 1566 was detected in the apical membrane of the proximal tubule. The sgk2 antibody labeled proximal tubule segments that also showed NHE3 immunoreactivity (Fig. 4B). We also examined the cellular localization of sgk2 and NHE3 in single microdissected tubules. As described previously (7), NHE3 was observed as a brush-border protein in the PST. Sgk2 was also detected at or near the apical membrane in the PST (Fig. 4C), confirming that sgk2 and NHE3 are expressed together in proximal tubule cells.
Fig. 4.
Sgk2 and NHE3 immunoreactivity overlap in kidney proximal tubule cells of adrenal-intact rats. A: rabbit polyclonal sgk2 antiserum is specific for heterologously expressed sgk2 protein. Opossum kidney cell (OKP) or in vitro translated (IVT) reticulocyte lysates expressing mouse sgk1, sgk2, sgk3, and pcDNA3 expression control vector (V) were resolved by SDS-PAGE and immunoblotted with sgk2 antiserum. Sgk2 antiserum only recognized a single band at 37 kDa, consistent with the molecular mass of sgk2 protein, in OKP cell or reticulocyte lysates expressing sgk2 protein. Membranes were stripped and reblotted with anti-GAPDH antibody for detection of GAPDH as a protein loading control. B: rat kidneys were perfusion fixed and stained with anti-NHE3 or anti-sgk2 as described in methods. Proximal tubules demonstrate both NHE3 and sgk2 immunoreactivity. Bar = 50 μm. C: microdissected proximal straight tubules stained for NHE3 and sgk2 immunohistochemistry as described in methods. Sgk2 immunoreactivity is localized at or near the apical membrane, where NHE3 immunoreactivity is also apparent. Bar = 10 μm. Images in B and C were taken using Nomarski optics.
Sgk2 is not an aldosterone-responsive isoform of sgk in the rat kidney.
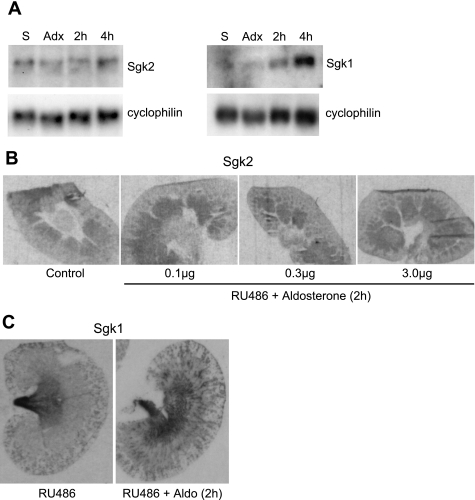
Sgk1 is a key aldosterone-induced gene involved in the control of ENaC-mediated Na+ transport in the kidney. Because sgk2 is a close relative of sgk1, we investigated whether sgk2 was also stimulated by aldosterone. Northern blots were performed on rat kidney homogenates from adrenalectomized rats treated with aldosterone (1 μg/100 g body wt). Aldosterone at 2 or 4 h after subcutaneous injection did not enhance levels of sgk2 expression in the kidney (Fig. 5A), whereas sgk1 mRNA was strongly induced in the kidney by aldosterone at both time points.
Fig. 5.
Sgk2 mRNA is not regulated by aldosterone in rat kidney homogenates or in rat kidney sections. A: Northern blot analysis for sgk2 mRNA in kidney homogenates of adrenalectomized rats injected subcutaneously with aldosterone. Male rats were pretreated with 10 μg RU486 for 30 min before aldosterone was injected (1 μg/100 g body wt). Kidneys were harvested from each rat (n = 5–8/group) at 2 (2h) and 4 (4h) h after aldosterone injection. Blots were hybridized with probes for sgk2 and sgk1 mRNA to assess respective levels in kidney homogenates. Cyclophilin mRNA served as RNA loading controls. The blot shown is representative of 3 independent experiments. S, sham operated; Adx, adrenalectomized. B: sgk2 mRNA expression by radioisotopic in situ hybridization of kidneys from adrenalectomized rats treated with RU486 and graded doses of aldosterone (0.1–3 μg/100 g body wt). Autoradiograms of whole rat kidneys harvested 2 h after aldosterone injection. No change in sgk2 expression is apparent in any part of the kidney in response to aldosterone treatment. Four sections per animal (n = 5–8/group) were analyzed. C: autoradiograms of whole rat kidneys show robust induction of sgk1 by aldosterone treatment (2 h) in the cortex, and, more generally, in the medulla.
Although Northern blot analysis did not show aldosterone regulation of sgk2 in kidney homogenates at either 2 or 4 h, the possibility that aldosterone could focally regulate sgk2 expression in specific nephron segments still remained. To determine spatiotemporal regulation of sgk2, we performed in situ hybridization of sgk2 on kidneys from adrenalectomized rats treated with graded doses of aldosterone (0.1–10 μg/100 g body wt). All rats were pretreated with the glucocorticoid receptor antagonist RU486 to ensure that only the mineralocorticoid receptor was activated with aldosterone treatment. At all doses, aldosterone at 2 or 4 h after injection failed to alter sgk2 expression in the kidney, remaining at levels similar to those of sham controls (Fig. 5B). In contrast, at all doses tested, aldosterone enhanced the levels of hybridization to sgk1 mRNA over the collecting tubules of both cortex and outer medulla at 2 and 4 h after injection (Fig. 5C). Together, these data strongly suggest that, unlike sgk1, sgk2 is not an aldosterone-responsive gene in rat kidney.
Activated sgk2, but not sgk1, stimulates NHE3-mediated Na+/H+ activity in OKP cells.
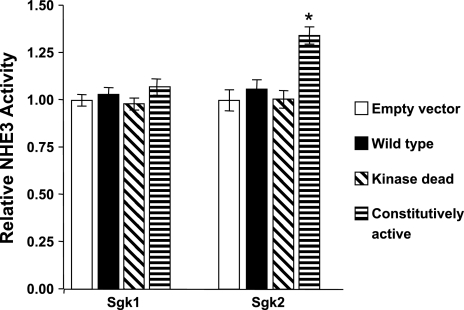
Since sgk2 expression in proximal tubule colocalized with NHE3 immunoreactivity, we next compared the effects of sgk1 and sgk2 on NHE3-mediated Na+/H+ exchange activity in OKP cells. OKP cells are derived from the opossum proximal tubule and are a well-established model system for the study of NHE3-mediated Na+/H+ exchange activity (4, 5, 15, 38). Constitutively active sgk2 (CA-sgk2) stimulated endogenous Na+/H+ exchange activity by >30% compared with OKP cells transfected with wild-type sgk2, kinase-dead sgk2, or control vector (P < 0.001) (Fig. 6). In contrast, Na+/H+ exchange activity was not affected by heterologous expression of wild type, kinase-dead, or CA-sgk1 (Fig. 6).
Fig. 6.
Activated sgk2, but not sgk1, stimulates NHE3-mediated Na+/H+ exchange activity. OKP cells were transfected with empty vector, wild-type, kinase-dead, or constitutively active expression vector constructs for sgk1 or sgk2. Values are derived from Na+/H+ exchange activity for each of the sgk isoforms and expressed relative to empty vector control, means ± SE (n = 16 for sgk1 comparisons, n = 15 for sgk2 comparisons). Transfection efficiency was ∼60% for each batch of experiments as assessed by cotransfected pEGFP-C1 expression vector. *P < 0.001 for constitutively-active sgk2 vs. empty vector control.
Activated sgk2 increases cell surface expression of NHE3 in OKP cells.
One possible mechanism for stimulation of Na+/H+ exchange activity by activated sgk2 involves changes in trafficking between cell surface and intracellular NHE3. Transfection of CA-sgk2 stimulated surface expression of NHE3 in OKP cells in a dose-dependent manner (Fig. 7). This increase in cell surface NHE3 was not associated with an increase in total cellular NHE3 protein abundance, suggesting that activated sgk2 increases the proportion of NHE3 that is present at the cell surface.
Fig. 7.
Activated sgk2 increases cell surface NHE3 expression. OKP cells were transfected with empty vector or constitutively active sgk2 at the indicated concentrations. Surface NHE3 antigen was quantified as biotin-accessible and Neutravidin-precipitated NHE3 from whole cell lysates measured by immunoblot. A: representative immunoblot. B: summary of results from 6 different experiments with quantification (means ± SE) of NHE3 band intensities in the biotinylated fraction relative to that in empty vector. IB, immunoblotting; IP, immunoprecipitation; WCL, whole cell lysate. *P < 0.05.
DISCUSSION
In the present study, we used a combination of in situ hybridization, immunohistochemistry, and real-time RT-PCR techniques to analyze renal expression of sgk2 in adrenal-intact and adrenalectomized rats treated with aldosterone. We also compared the functional effects of two sgk isoforms on the modulation of NHE3, a key Na+ transporter in the proximal tubule. To our knowledge, this is the first study to document sgk2 expression in the kidney and to demonstrate that sgk2 has a stimulatory effect on NHE3.
We found that sgk2 has a distinct expression pattern in the rodent kidney, which was particularly evident with in situ hybridization experiments: sgk2 was expressed in the medullary rays of the cortex and the outer stripe of the outer medulla. This expression pattern is strikingly different from that of sgk1. Comparison of sgk1 and sgk2 mRNA expression across microdissected nephron segments measured by real-time RT-PCR revealed that highest levels of sgk2 expression were found in PCT, PST, and TALH, while the highest levels of sgk1 expression were found in glomeruli and PCT. Notably, sgk2 expression was lowest in glomeruli and CCD. With in situ hybridization experiments, we only observed sgk2 mRNA hybridization in CCD cells that did not express AQP2, suggesting that sgk2 might be expressed only in intercalated cells of the CD. The distribution of sgk1 expression that we observed also agrees with previous reports showing that sgk1 is expressed in nephron segments other than the distal nephron, including glomeruli and proximal tubule (11, 14, 29). Of note, sgk1 expression in the proximal tubule has been observed by real-time RT-PCR (44); our localization studies confirmed that the PCT expresses sgk1 in significant quantities in kidneys obtained from adrenal-intact rats maintained on a normal-salt diet.
Surprisingly, despite being closely related to sgk1, sgk2 is not an aldosterone-inducible gene in the kidney. Previous reports have suggested that aldosterone does not regulate sgk2 expression in cultured cells (41, 49). Alvarez de la Rosa et al. (3) have demonstrated that aldosterone does not stimulate sgk2 expression in the kidneys of adrenal-intact rats, although the authors were unable to detect significant expression of sgk2 mRNA in the rat kidney. To isolate the specific effects of mineralocorticoid action on the kidney, we assessed for aldosterone induction of sgk2 regulation in the kidneys of adrenalectomized rats. We also used in situ hybridization studies to evaluate possible regional changes in sgk2 expression after aldosterone induction. Physiological doses of aldosterone did not stimulate sgk2 expression by either Northern blot analysis or in situ hybridization in adrenalectomized rats, indicating that the regulation of sgk2 is likely to be completely different from that of sgk1.
Since we observed by real-time RT-PCR a significant amount of sgk2 expression in microdissected proximal tubule segments, we used both in situ hybridization and immunohistochemistry techniques to demonstrate that sgk2 mRNA and protein expression co-localized with NHE3 immunoreactivity in proximal tubule cells. Based on these findings, we compared the effects of sgk1 and sgk2 on Na+/H+ exchange in OKP cells, a cell line derived from the proximal tubule and exhibiting NHE3-mediated Na+/H+ exchange activity (4, 5, 16, 30, 38, 39, 58, 61). OKP cells do not express NHE1 or NHE2, as shown by pharmacological inhibitor studies (47) or RT-PCR (Moe OW, unpublished observations). In this model system, we found that sgk2, but not sgk1, stimulated Na+/H+ exchange activity. Moreover, this increase in Na+/H+ exchange activity was associated with an increase in cell surface NHE3 expression. For these experiments, we used CA-sgk isoform mutants, which substitute an acidic amino acid residue that mimics PI 3-kinase-dependent activation (40, 41, 51). PI 3-kinase appears to stimulate NHE3 activity in cultured cells through redistribution of NHE3 from endosomal compartments to the plasma membrane (20). In addition, insulin, a hormone dependent on PI 3-kinase activity, enhances NHE3 total/cell surface abundance and activity in OKP cells (38). Since sgk2 kinase activity is also controlled by PI 3-kinase (40), sgk2 potentially functions as an effector for insulin-mediated stimulation of NHE3 activity. Consistent with this notion, the stimulatory effect of sgk2 is on par with that of insulin on Na+/H+ antiporter activity in OKP cells (38).
It is significant that CA-sgk1 did not stimulate Na+/H+ exchange activity in OKP cells. Yun et al. (60) have reported that sgk1, in concert with NHERF2, stimulates NHE3 activity in PS120 fibroblasts. This effect required association of sgk1 with NHERF2 (through its PDZ domain), and the authors suggested NHERF2 may act as a scaffold protein for sgk1 and NHE3 interaction (60). It is possible that our OKP cell line does not express adequate NHERF2, and therefore sgk1 fails to stimulate NHE3. Fuster et al. (24) also did not find an effect of CA or kinase-dead sgk1 on baseline NHE3 activity, although kinase-dead sgk1 partially blocked chronic stimulation of NHE3 by insulin. It is notable that sgk2 lacks a PDZ interaction domain and hence would not likely interact with NHERF2 to regulate NHE3; nevertheless, the underlying mechanistic basis for sgk2-mediated stimulation of NHE3, under conditions by which sgk1 does not, remains to be determined. Based on our NHE3 surface expression studies in OKP cells, we propose that activated sgk2 stimulates an increase in the residence time of NHE3 at the cell surface, where it can mediate Na+/H+ exchange.
Further insight into the in vivo function of sgk2 will be attained through the characterization of genetically manipulated mouse models. For example, sgk1 knockout mice display moderate aldosterone resistance that is manifest only on low-sodium (21a, 59) or high-potassium (31) diets, while targeted disruption of mineralocorticoid receptor (9, 10) α-ENaC (32, 33), β-ENaC (46, 52), and γ-ENaC (8) in mice results in severe renal salt wasting, hyperkalemia, and early death. This suggests other compensatory mechanisms for salt reabsorption can occur in sgk1 knockout mice that prevent severe renal salt wasting; indeed, micropuncture studies of the sgk1 knockout mouse reveal an increase in fractional Na+ reabsorption in the proximal tubule (59). Sgk2 may play a major role in this compensatory process through its stimulatory action on NHE3 in the proximal tubule and TALH. In contrast, sgk3 is not likely to substantially contribute to this compensatory process because sgk3 knockout mice do not exhibit salt wasting (45). Moreover, sgk1/sgk3 double knockout mice show the same degree of salt wasting as sgk1 knockout mice (26).
Although several groups have evaluated the ability of sgk2 to stimulate ion transport using cultured cells or Xenopus laevis oocytes, the physiological significance of these studies is unclear. We have demonstrated that regulation of sgk2 expression in the rat kidney is completely different from regulation of sgk1 expression, which is responsive to aldosterone stimulation. We have also delineated the expression pattern of sgk2 in the rodent kidney and examined the role of sgk2 in regulating NHE3. Our results suggest that sgk2 acts predominantly in the proximal tubule and TALH where it may function to regulate NHE3 activity, while sgk1 acts in the distal nephron to regulate ENaC-mediated Na+ transport. Thus sgk2 likely plays an important and distinct role in the regulation of transtubular Na+ transport in the proximal tubule.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants F32-DK065361 and K08-DK-073487 (A. C. Pao), R01-DK080787 (A. Bhargava), R01-DK48481 (O. W. Moe), and R01-DK56695 (D. Pearce), the American Heart Association Texas Affiliate 03225098Y (F. Di Sole), and Seed Funds from the Charles and Jane Pak Center of Mineral Metabolism and Clinical Research (X. Shao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We appreciate the helpful assistance of E. Abdul Salam for preparation of reagents for OKP cell culture and BCECF assays; I. A. Bobulescu and D. Fuster for expert assistance with BCECF assays; and V. Bhalla and J. A. McCormick for critical evaluation of the experiments and manuscript.
REFERENCES
- 1.Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev 70: 79–114, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Alpern RJ. Mechanism of basolateral membrane H+/OH−/HCO3− transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol 86: 613–636, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez de la Rosa D, Coric T, Todorovic N, Shao D, Wang T, Canessa CM. Distribution and regulation of expression of serum- and glucocorticoid-induced kinase-1 in the rat kidney. J Physiol 551: 455–466, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambuhl P, Amemiya M, Preisig PA, Moe OW, Alpern RJ. Chronic hyperosmolality increases NHE3 activity in OKP cells. J Clin Invest 101: 170–177, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambuhl PM, Yang X, Peng Y, Preisig PA, Moe OW, Alpern RJ. Glucocorticoids enhance acid activation of the Na+/H+ exchanger 3 (NHE3). J Clin Invest 103: 429–435, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Bacic D, Kaissling B, McLeroy P, Zou L, Baum M, Moe OW. Dopamine acutely decreases apical membrane Na/H exchanger NHE3 protein in mouse renal proximal tubule. Kidney Int 64: 2133–2141, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B. Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest 102: 1634–1640, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA 95: 9424–9429, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger S, Bleich M, Schmid W, Greger R, Schutz G. Mineralocorticoid receptor knockout mice: lessons on Na+ metabolism. Kidney Int 57: 1295–1298, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Bhargava A, Fullerton MJ, Myles K, Purdy TM, Funder JW, Pearce D, Cole TJ. The serum- and glucocorticoid-induced kinase is a physiological mediator of aldosterone action. Endocrinology 142: 1587–1594, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bhargava A, Meijer OC, Dallman MF, Pearce D. Plasma membrane calcium pump isoform 1 gene expression is repressed by corticosterone and stress in rat hippocampus. J Neurosci 20: 3129–3138, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4–2 is impacted by SGK kinases. J Neurochem 97: 911–921, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci USA 96: 2514–2519, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole JA, Forte LR, Krause WJ, Thorne PK. Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am J Physiol Renal Fluid Electrolyte Physiol 256: F672–F679, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem 275: 31601–31608, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Custer M, Lotscher M, Biber J, Murer H, Kaissling B. Expression of Na-Pi cotransport in rat kidney: localization by RT-PCR and immunohistochemistry. Am J Physiol Renal Fluid Electrolyte Physiol 266: F767–F774, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Di Sole F, Babich V, Moe OW. The calcineurin homologous protein-1 increases Na+/H+ -exchanger 3 trafficking via ezrin phosphorylation. J Am Soc Nephrol 20: 1776–1786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Sole F, Cerull R, Babich V, Casavola V, Helmle-Roth C, Burckhardt G. Short- and long-term A3 adenosine receptor activation inhibits the Na+/H+ exchanger NHE3 activity and expression in opossum kidney cells. J Cell Physiol 216: 221–233, 2008 [DOI] [PubMed] [Google Scholar]
- 20.D'Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem 273: 2035–2043, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Embark HM, Bohmer C, Vallon V, Luft F, Lang F. Regulation of KCNE1-dependent K+ current by the serum and glucocorticoid-inducible kinase (SGK) isoforms. Pflügers Arch 445: 601–606, 2003 [DOI] [PubMed] [Google Scholar]
- 21a.Fejes-Tóth G, Frindt G, Náray-Fejes-Tóth A, Palmer LG. Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol 294: F1298–F1305, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Friedrich B, Feng Y, Cohen P, Risler T, Vandewalle A, Broer S, Wang J, Pearce D, Lang F. The serine/threonine kinases SGK2 and SGK3 are potent stimulators of the epithelial Na+ channel alpha,beta,gamma-ENaC. Pflügers Arch 445: 693–696, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Fuster DG, Bobulescu IA, Zhang J, Wade J, Moe OW. Characterization of the regulation of renal Na+/H+ exchanger NHE3 by insulin. Am J Physiol Renal Physiol 292: F577–F585, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530–F538, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick JA, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F. Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol 290: R945–R950, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Henke G, Maier G, Wallisch S, Boehmer C, Lang F. Regulation of the voltage gated K+ channel Kv1.3 by the ubiquitin ligase Nedd4–2 and the serum and glucocorticoid inducible kinase SGK1. J Cell Physiol 199: 194–199, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou J, Speirs HJ, Seckl JR, Brown RW. Sgk1 gene expression in kidney and its regulation by aldosterone: spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol 13: 1190–1198, 2002 [PubMed] [Google Scholar]
- 30.Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem 276: 26906–26915, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet 12: 325–328, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Hummler E, Barker P, Talbot C, Wang Q, Verdumo C, Grubb B, Gatzy J, Burnier M, Horisberger JD, Beermann F, Boucher R, Rossier BC. A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism. Proc Natl Acad Sci USA 94: 11710–11715, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hummler E, Horisberger JD. Genetic disorders of membrane transport. V. The epithelial sodium channel and its implication in human diseases. Am J Physiol Gastrointest Liver Physiol 276: G567–G571, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Ishidate T, Ward HJ, Hoyer JR. Quantitative studies of tubular immune complex formation and clearance in rats. Kidney Int 38: 1075–1084, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Klaus F, Palmada M, Lindner R, Laufer J, Jeyaraj S, Lang F, Boehmer C. Up-regulation of hypertonicity-activated myo-inositol transporter SMIT1 by the cell volume-sensitive protein kinase SGK1. J Physiol 586: 1539–1547, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klisic J, Hu MC, Nief V, Reyes L, Fuster D, Moe OW, Ambuhl PM. Insulin activates Na+/H+ exchanger 3: biphasic response and glucocorticoid dependence. Am J Physiol Renal Physiol 283: F532–F539, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Klisic J, Zhang J, Nief V, Reyes L, Moe OW, Ambuhl PM. Albumin regulates the Na+/H+ exchanger 3 in OKP cells. J Am Soc Nephrol 14: 3008–3016, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J 339: 319–328, 1999 [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344: 189–197, 1999 [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J Biol Chem 269: 12032–12039, 1994 [PubMed] [Google Scholar]
- 43.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system: possible role of SGK. Am J Physiol Renal Physiol 280: F675–F682, 2001 [DOI] [PubMed] [Google Scholar]
- 45.McCormick JA, Feng Y, Dawson K, Behne MJ, Yu B, Wang J, Wyatt AW, Henke G, Grahammer F, Mauro TM, Lang F, Pearce D. Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell 15: 4278–4288, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald FJ, Yang B, Hrstka RF, Drummond HA, Tarr DE, McCray PB, Jr, Stokes JB, Welsh MJ, Williamson RA. Disruption of the beta subunit of the epithelial Na+ channel in mice: hyperkalemia and neonatal death associated with a pseudohypoaldosteronism phenotype. Proc Natl Acad Sci USA 96: 1727–1731, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moe OW, Miller RT, Horie S, Cano A, Preisig PA, Alpern RJ. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest 88: 1703–1708, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naray-Fejes-Toth A, Canessa C, Cleaveland ES, Aldrich G, Fejes-Toth G. sgk is an aldosterone-induced kinase in the renal collecting duct: effects on epithelial Na+ channels. J Biol Chem 274: 16973–16978, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Naray-Fejes-Toth A, Helms MN, Stokes JB, Fejes-Toth G. Regulation of sodium transport in mammalian collecting duct cells by aldosterone-induced kinase, SGK1: structure/function studies. Mol Cell Endocrinol 217: 197–202, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Pao AC, McCormick JA, Li H, Siu J, Govaerts C, Bhalla V, Soundararajan R, Pearce D. NH2 terminus of serum and glucocorticoid-regulated kinase 1 binds to phosphoinositides and is essential for isoform-specific physiological functions. Am J Physiol Renal Physiol 292: F1741–F1750, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J 18: 3024–3033, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, Rossier BC, Hummler E. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci USA 96: 1732–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Schafer JA. Abnormal regulation of ENaC: syndromes of salt retention and salt wasting by the collecting duct. Am J Physiol Renal Physiol 283: F221–F235, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Setiawan I, Henke G, Feng Y, Bohmer C, Vasilets LA, Schwarz W, Lang F. Stimulation of Xenopus oocyte Na+,K+ATPase by the serum and glucocorticoid-dependent kinase sgk1. Pflügers Arch 444: 426–431, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Strutz-Seebohm N, Seebohm G, Shumilina E, Mack AF, Wagner HJ, Lampert A, Grahammer F, Henke G, Just L, Skutella T, Hollmann M, Lang F. Glucocorticoid adrenal steroids and glucocorticoid-inducible kinase isoforms in the regulation of GluR6 expression. J Physiol 565: 391–401, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Barbry P, Maiyar AC, Rozansky DJ, Bhargava A, Leong M, Firestone GL, Pearce D. SGK integrates insulin and mineralocorticoid regulation of epithelial sodium transport. Am J Physiol Renal Physiol 280: F303–F313, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Wiederkehr MR, Di Sole F, Collazo R, Quinones H, Fan L, Murer H, Helmle-Kolb C, Moe OW. Characterization of acute inhibition of Na/H exchanger NHE-3 by dopamine in opossum kidney cells. Kidney Int 59: 197–209, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D. Impaired renal Na+ retention in the sgk1-knockout mouse. J Clin Invest 110: 1263–1268, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem 277: 7676–7683, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999 [DOI] [PubMed] [Google Scholar]