Abstract
Past therapies for the treatment of obesity have typically involved pharmacological agents usually in combination with a calorie-controlled diet. This paper reviews the efficacy and safety of pharmacotherapies for obesity focusing on drugs approved for long-term therapy (orlistat), drugs approved for short-term use (amfepramone [diethylpropion], phentermine), recently withdrawn therapies (rimonabant, sibutamine) and drugs evaluated in Phase III studies (taranabant, pramlintide, lorcaserin and tesofensine and combination therapies of topiramate plus phentermine, bupropion plus naltrexone, and bupropion plus zonisamide). No current pharmacotherapy possesses the efficacy needed to produce substantial weight loss in morbidly obese patients. Meta-analyses support a significant though modest loss in bodyweight with a mean weight difference of 4.7 kg (95% CI 4.1 to 5.3 kg) for rimonabant, 4.2 kg (95% CI 3.6 to 4.8 kg) for sibutramine and 2.9 kg (95% CI 2.5 to 3.2 kg) for orlistat compared to placebo at ≥12 months. Of the Phase III pharmacotherapies, lorcaserin, taranabant, topiramate and bupropion with naltrexone have demonstrated significant weight loss compared to placebo at ≥12 months. Some pharmacotherapies have also demonstrated clinical benefits. Further studies are required in some populations such as younger and older people whilst the long term safety continues to be a major consideration and has led to the withdrawal of several drugs.
1. Introduction
Management strategies for weight reduction in obese individuals include physical interventions such as exercise, diet, and surgery, behavioural therapies, and pharmacological treatments. These strategies may be used alone or in combination for greater efficacy. Most randomized controlled trials (RCTs) evaluating pharmacotherapies include a calorie- controlled diet, and some also encourage participants to increase their physical activity.
Drugs used to induce weight loss may reduce appetite or increase satiety, reduce the absorption of nutrients, or increase energy expenditure. Weight loss with pharmacotherapies is generally modest, that is, usually 2 to 7.9 kg more than that achieved with placebo treatment [1]. In the past drug therapies available have included thyroid hormone, dinitrophenol and amphetamines, followed by amphetamine analogues, aminorex, and the fenfluramines [1]. More recently a number of newer agents have been trialed though only orlistat and sibutramine were approved for long-term use (≥24 weeks). Following the recent withdrawal of sibutramine this leaves only orlistat (Table 1).
Table 1.
Drugs used for weight loss in obesity.
| Drug | Introduced | Mechanism of action | Status |
|---|---|---|---|
| Dinitrophenol | 1930s | Increases metabolic rate | Withdrawn—risk of neuropathy and cataracts |
| Amphetamines: dexamphetamine, methamphetamine | 1936 | Appetite suppression | Banned, restricted or discouraged—dependency and abuse potential, cardiovascular adverse effects |
| Amphetamine-like analogues: Phentermine, diethylpropion, phenylpropanolamine | 1959-US | Appetite suppression | Diethylpropion—available for short-term use (≤12 weeks) |
| Phentermine—available for short-term use (≤12 weeks) in some countries, withdrawn 2000 (UK) | |||
| Phenylpropanolamine-withdrawn 2000—increased risk haemorrhagic stroke | |||
| Aminorex | 1965 | Appetite suppression | Withdrawn 1968—pulmonary hypertension |
| Mazindol | 1970s | Appetite suppression | Discontinued 1993—Australia |
| Fenfluramine | 1963-Europe 1973-US | Appetite suppression | Withdrawn 1997—valvular heart disease, pulmonary hypertension |
| Dexfenfluramine | 1985-Europe 1996-US | Appetite suppression | Withdrawn 1997—valvular heart disease, pulmonary hypertension |
| Orlistat | 1998-Europe and US | Decreased fat absorption | Also available over-the-counter in several countries |
| Sibutramine | 1997-US 2001-Europe | Appetite suppression | Temporarily withdrawn 2002 Italy-concerns of raised risk of heart attacks and strokes |
| Increase in contraindications 2010-US, Australia | |||
| Suspension of market authorization 2010 | |||
| Rimonabant | 2006-Europe | Withdrawn 2009—potential of serious psychiatric disorders | |
Amongst the drugs marketed for weight loss there have been several instances of market withdrawal due to serious adverse events. The agents involved include dinitrophenol, aminorex, the fenfluramines, phenylpropanolamine and most recently rimonabant. Other drugs such as the amphetamines are severely restricted due to their abuse potential. Fenfluramine and dexfenfluramine were recalled from the world market in 1997 due to concerns of an increased prevalence of valvular heart disease, and the possible association with primary pulmonary hypertension [2–6]. In April 2000, the European Medicines Agency (EMEA) recommended the withdrawal of several weight loss drugs from the market including phentermine, amfepramone (diethylpropion) and mazindol due to an unfavourable risk to benefits ratio [7]. This was followed by the voluntary withdrawal of medications containing phenylpropanolamine due to reports of haemorrhagic stroke in women [8] (Table 1).
Rimonabant was approved as an adjunct to diet and exercise for the treatment of obese or overweight patients by the EMEA in 2006. However the FDA never approved its use in the US due to serious safety concerns. Then in January 2009, the EMEA withdrew market authorisation for rimonabant in all countries of the European Union due to an increased risk of psychiatric adverse events, including depressed mood disorders, anxiety, and suicidal ideation [9–11]. Concern was recently raised regarding the safety of sibutramine, following earlier reports of increased systolic and diastolic blood pressure and heart rate [10]. With this concern in mind, the safety was investigated in patients with a history of cardiovascular disease in the Sibutramine Cardiovascular Outcomes Trial (SCOUT). The release of preliminary results from SCOUT led to the compulsory inclusion of contraindications and precautions in the US and Australian product information, whilst the EMEA recommended total suspension of market authorisation for the drug in Europe [12–16]. Following the subsequent publication of the SCOUT study [17] the FDA considered whether to severely restrict access to the sibutramine or remove it from the market. Sibutramine was subsequently withdrawn by the manufacturer.
Some drugs which had demonstrated positive weight loss potential such as taranabant have been abandoned during late phase clinical trials due to unacceptable adverse events. Whilst axokine, a ciliary neurotrophic factor that was administered as a daily subcutaneous injection, was abandoned due to the low percentage of responders as a result of the development of antibodies in the majority of patients taking the drug [18].
The efficacy and safety of long-term drug therapy is a very important consideration in the management obesity which often requires ongoing therapy to achieve and maintain the weight loss. This paper provides a review of the efficacy and safety of drug therapies for weight loss with at least six months of patient follow-up focusing on randomised controlled trials (RCTs) published over the last 4 years of recent past and current pharmacotherapies, as well as those in late phase clinical trials.
2. Measuring Effectiveness of Drug Therapy
There are some challenges in establishing the medium and longer-term efficacy of pharmacotherapies designed to induce weight loss. These include the continuance of patients throughout the entire study duration and the likelihood that patients who report more weight loss will be more likely to complete the study. In an effort to control bias from this source the use of last observation carried forward is commonly used to approximate weight loss for the patients withdrawing from a study [7, 19, 20].
There is also some controversy as to which primary outcome measures are best to evaluate the efficacy of drug therapies, that is, absolute weight loss (in excess of placebo), percentage weight loss, percentage of patients achieving ≥5% or ≥10% weight loss of initial weight, BMI, or waist circumference (WC). The length of time over which weight loss is sustained is also important which implies prolonged follow-up, at least twelve months or if possible longer. In studies involving children, the BMI appears to be the most appropriate measure of effectiveness [21]. Secondary efficacy endpoints are increasingly reported especially in more recent studies, and these include clinical measures such as blood pressure, glycaemic control (blood glucose or HbA1C levels) and cholesterol levels [14, 22, 23].
3. Past Drug Therapies and Current Approved Drugs
Drugs that have been prescribed or evaluated for obesity may reduce fat absorption or regulate satiety via their action on serotonin, noradrenergic or dopaminergic or the cannabinoid receptor systems in the brain (Table 2) [1, 3, 24–26].
Table 2.
Central mechanisms of action of anti-obesity pharmacotherapies.
| Central Subsystem | Drugs targets | Possible receptor subtypes involved |
|---|---|---|
| Monoamine system (indirect agonists and subtype selective receptor antagonists) | Single therapies | |
| (i) Dex/fenfluramine (WD), fluoxetine | (i) 5HT | |
| (ii) Phentermine/Diethylpropion (ST) | (ii) DA, NA | |
| (iii) Sibutramine | (iii) α1, β1, β3 adrenergic and 5HT2B/C | |
| (iv) Bupropion | (iv) DA, NA | |
| (v) Tesofensine | (v) DA, NA, 5HT | |
| (vi) Lorcaserin | (vi) 5HT2C | |
| Opioid system (μ-opioid receptor antagonist) | (i) Naltrexone | (i) μ-opioid |
| (ii) Topiramate | (ii) AMPA/kainite glutamate* | |
| (iii) Zonisamide | (iii) 5HT, DA* | |
| Cannabinoid system | Single therapies: | |
| (i) Rimonabant (WD) | (i) CB1 | |
| (ii) Taranabant (DC) | (ii) CB1 | |
| Monoamine/Opioid system | Bupropion/naltrexone | (i) DA, NA/μ-opioid |
| Bupropion/zonisamide | (ii) DA, NA/5HT, DA* | |
| Neuropeptide Y/Agouti-related peptide system | Pramlintide/metreleptin | (i) Calcitonin receptor*/Leptin receptor |
5HT: serotonergic, DA: dopaminergic, NA: noradrenergic, WD:withdrawn; DC: phase III trials discontinued; ST: short term; *: unknown; AMPA: α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate.
3.1. Amphetamines and Amphetamine-Like Analogues
Amphetamines and amphetamine-like analogues (phentermine, diethylpropion, phenylpropanolamine) are indirect-acting sympathomimetic agents that act by releasing noradrenaline (NA) from presynaptic vesicles in the lateral hypothalamus [1]. Mazindol, a related but discontinued drug, blocks the reuptake of NA by presynaptic neurons (Table 2) [1]. The increase in NA concentration within the synaptic cleft results in the stimulation of β2-adrenergic receptors and a resultant inhibition of appetite.
There is little data from large randomized controlled trials (RCTs) relating to the long-term efficacy or safety of amphetamines and amphetamine-like analogues, especially when used as monotherapy. These drugs have limited use in the routine management of obesity and are not currently approved for long-term use. Phentermine has been available since the late 1950s and is approved for short-term use in the US and Australia (Table 2). It has been evaluated as both monotherapy and as combination therapy though not in large-scale studies [27, 28]. A 36-week RCT in 108 overweight women demonstrated a mean weight loss of 12.2 kg (13%) with phentermine (30 mg daily) compared to 4.8 kg (5.2%) with placebo (P < .001). Phentermine has been used in combination with fenfluramine and with fluoxetine [29]. Combination therapy with phentermine (15 mg) and fenfluramine (60 mg), demonstrated significantly more weight loss than placebo in a 28-week RCT (15.5% versus 4.9%, P < .001) [28]. Phentermine is currently under evaluation in combination with topiramate and with pramlintide (see Drug Monotherapies and Combination Therapies in Clinical Development).
3.1.1. Diethylpropion (amfepramone)
another amphetamine-like analogue has been available for weight loss since the early 1960s; however there are few if any RCTs of its long-term use especially with large sample sizes [30, 31]. Diethylpropion (75 mg daily) demonstrated significantly greater weight loss in a small 24-week study of 20 patients than placebo (11.6 kg versus 2.5 kg, P < .01) [31]. Recently, diethylpropion (50 mg twice a day) was shown to be more effective than placebo in a small 6-month RCT with 69 obese adult patients (9.3 kg [95% CI 7–11.5 kg] versus 3.1 kg [95% CI 1.8–4.3 kg], P < .0001) [32]. Greater than 5% weight loss was achieved in 67.6% of diethylpropion patients and 25.0% of those receiving placebo (P = .0005). After further 6 months during an open label period of the study patients who were originally in the diethylpropion group lost a mean of 10.1 kg (95% CI 7.5–12.8). The most common side effects were dry mouth and insomnia (P = .02 and P = .009, respectively). These were experienced in the first 3 months but become less apparent with continuing treatment [32].
3.2. Fenfluramines
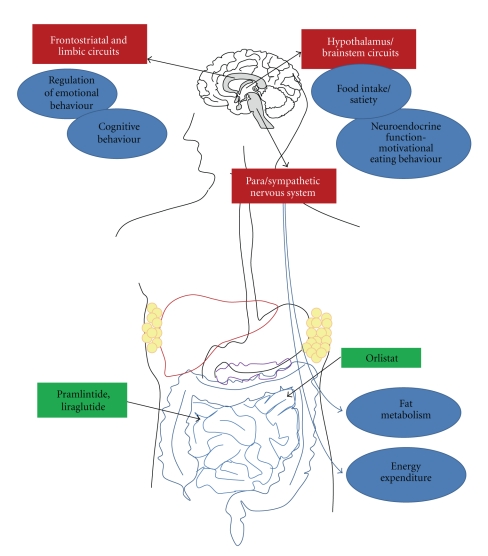
Fenfluramine and dexfenfluramine elevate serum levels of serotonin (5HT) in the central nervous system by stimulating 5HT release and inhibiting its reuptake (Figure 1). Increased levels of 5HT appear to stimulate the hypothalamus, which controls satiation as well as mood, sleep, body temperature and other vital functions. These agents also activate melanocortin 4 receptors that in turn stimulate activation of 5-HT2C receptors, producing an increased release of 5HT within the hypothalamic-pituitary-adrenal axis which is claimed to lead to hypophagia and anorexia [33–36].
Figure 1.
Overview of central and peripheral functions associated with anti-obesity pharmacotherapies.
A meta-analysis of RCTs with fenfluramine and dexfenfluramine demonstrated higher weight loss than placebo following up to 12 months of treatment. The greatest efficacy was shown following 3 months treatment, 3.7 kg weight loss [37].
Although RCTs with fenfluramines (fenfluramine and dexfenfluramine), either alone [38, 39] or with phentermine [40], demonstrated significant weight-loss, they were withdrawn from the market due to increased reports of valvular heart disease and primary pulmonary hypertension [2, 3, 29, 41–43] ). The prevalence rates of both valvular heart disease and primary pulmonary hypertension were higher following longer exposure to the fenfluramines [3].
3.3. Antidepressants
3.3.1. Fluoxetine, Bupropion
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI) that augments 5HT within the central nervous system has been prescribed off-label for weight loss. Although significant weight loss was reported with 60 mg of this agent in short-term studies of 6–8 weeks, with maximum weight loss achieved at 12–20-weeks, this is followed by a regain in bodyweight [44–47]. Most RCTs have not shown a significant difference when fluoxetine was compared to placebo at 52 weeks [46, 48]. Significantly greater weight loss has however been demonstrated at 8 months when fluoxetine was used in combination with dexfenfluramine (13.4 versus 6.2 kg with placebo) [49]. In clinical practice fluoxetine 10–20 mg has been used with phentermine (i.e., phen-pro or phen-flu) but there are no RCTs of either the long-term efficacy or safety of this combination [50]. A retrospective chart review suggested this combination is not as effective as fenfluramine with phentermine [51]. Fluoxetine generally has a tolerable safety profile with reported adverse events of headache, asthenia, nausea, diarrhoea, somnolence, insomnia, nervousness, sweating, and tremor [47].
3.3.2. Bupropion
is another antidepressant which inhibits reuptake of dopamine (DA) and noradrenaline (NA) resulting in a loss of appetite and decreased food intake [52] and modest weight loss in obese people [53–56]. The efficacy of bupropion as a sustained release (SR) formulation was demonstrated at 48 weeks in obese patients [53]. Weight loss was dose dependent with 7.5% initial weight loss for subjects taking 300 mg bupropion-SR and 8.6% with 400 mg [53]. Bupropion-SR was generally well tolerated, and weight loss was maintained at 48 weeks. A meta-analysis of weight loss treatments which included 5 bupropion studies reported a mean weight loss of 2.8 kg (95% CI, 1.1 to 4.5 kg) at 6 to 12 months with bupropion compared to placebo [56] (Table 3). Although bupropion is not approved for weight loss, it has been used off-label and is currently under evaluation as combination therapy with naltrexone, a μ-opioid receptor antagonist and zonisamide, a GABA receptor activator (see Drug Monotherapies and Combination Therapies under Investigation).
Table 3.
Comparative efficacy of pharmacotherapy from recent meta-analyses of long-term studies in adults (12 months or more).
| Drug | No. of studies | Total subjects | Mean weight difference (kg) (95% CI) | Reference |
|---|---|---|---|---|
| Rimonabant | 4 | Placebo: ~1600 Rimonabant: ~2500 | 4.7 (4.1, 5.3) | [9, 57, 58] |
| Orlistat | 14 | Placebo: 4509 Orlistat: 4948 |
2.9 (2.5, 3.2) | [57, 58] |
| Sibutramine | 7 | Placebo: 699 Sibutramine: 837 | 4.2 (3.6, 4.8) | [57, 58] |
| Sibutramine Orlistat | 5 | Sibutramine: 229 Orlistat: 249 | 3.4 (2.3, 4.6) | [59] |
| Bupropion* | 5 | Bupropion: 618 Placebo: 344 |
2.8 kg (1.1 to 4.5 kg) | [56] |
CI: confidence interval; * 6 to 12 month studies.
Note: another meta-analysis of 5 studies with rimonabant compared to placebo, did not provide mean weight difference in kg, however the odds ratio was 1.07 (95% CI 0.9, 1.3) [60].
3.4. Orlistat
Orlistat (a gastrointestinal lipase inhibitor) is a synthetic drug derived from a naturally occurring lipase inhibitor. It does not directly act on appetite as other obesity pharmacotherapies, rather it decreases fat absorption by binding to pancreatic lipase, the principle enzyme that hydrolyses triglyceride (Table 2) (Figure 1) [26]. A detailed review of the efficacy of orlistat treatment in obesity has previously been described [1]. The long-term efficacy of orlistat (120 mg three times daily) for weight loss has been demonstrated in several RCTs of 2- to 4- year therapy compared to placebo [61–64], as well as improvements in blood pressure, insulin resistance, and serum lipid levels [57, 64–66]. Several systematic reviews in adults [56, 57, 67–70] and a systematic review with 2 short-term studies in adolescents [71] demonstrated significantly more weight loss with orlistat than placebo, 6.2 kg (95% CI, 1.7 to 14.0 kg).
The most commonly experienced side effects of orlistat are gastrointestinal and include diarrhoea, flatulence, bloating, abdominal pain, and dyspepsia [25, 66, 70]. Recently, severe liver injury has been reported. The FDA received 32 reports of serious liver injury in patients using orlistat between 1999 and October 2008, including 6 cases of liver failure [72]. This prompted the FDA to undertake a review of the safety of orlistat treatment. The review identified a total of 13 cases of severe liver injury (12 foreign reports with orlistat 120 mg and a US report with the lower dose over-the-counter product [orlistat 60 mg]) and in May 2010 led to a label revision and the addition of a warning of severe liver injury.
3.5. Sibutramine
Sibutramine, a 5HT and NA uptake inhibitor, was originally developed as an antidepressant and subsequently found to reduce appetite [26]. It has 2 active metabolites, which inhibit NA and 5HT uptake (and to a lesser extent DA) without any direct effect on neuronal NA, DA and 5HT release. It has been suggested that sibutramine has a dual action to facilitate weight loss, an anorectic effect suggested to be mediated through the central α1 and β1 adrenergic receptors and thermogenic effects through β3 adrenergic receptors peripherally [73].
Maximal weight loss occurs by 6 months with sibutramine treatment [74, 75] and was dose related [74, 76, 77]. Sibutramine has consistently demonstrated significantly more weight loss than placebo in several RCTs with ≥1 year of therapy [1, 74, 75, 78–80]. Systematic reviews which included 7 sibutramine RCTs reported 4.3 kg (95% CI: 3.6 kg to 4.9 kg) or 4.6% (95% CI: 3.8% to 5.4%) greater weight loss than placebo [58, 70] (Table 3). There was ≥10% weight loss in 18% (95% CI: 11% to 25%) more sibutramine patients than placebo [58, 70]. Attrition rates in sibutramine studies were approximately 30%–40% [58]. In RCTs of 3 to 12 months that compared sibutramine and orlistat, the weighted mean difference in weight loss was 2.2 kg (95% CI 0.5–3.9) in favour of sibutramine [59]. A systematic review in adolescents which included 5 short-term studies has demonstrated significantly more weight loss with sibutramine than placebo, 5.3 kg (95% CI, 3.5 to 7.2 kg) [71].
Although treatment with sibutramine has resulted in lowered concentrations of cholesterol and triglycerides, blood pressure and pulse rate may be increased [57]. Increases in diastolic blood pressure (DBP) with sibutramine were reported in 2 meta-analyses, one in hypertensive patients which included 2 studies where the weighted mean difference was +3.2 mm Hg (95%CI +1.4 to +4.9 mm Hg) [66], whilst another reported a placebo-controlled change in DBP of +1.7 (95% CI 0.7, 2.6) and a small nonsignificant change in systolic BP (+0.5 mm Hg, 95% CI −1.1, 2.1) [81]. Although sibutramine may reduce body weight by a similar amount as orlistat in hypertensive patients, it does not have the same beneficial effects on BP [65].
Weight loss was significantly greater at 1 year when sibutramine was combined with lifestyle modification (10.8% ± 10.2%, mean ± SD, P < .05) and diet (16.5% ± 8.0%, P < .05) than when sibutramine was used alone (4.1% ± 6.3%) [82]. Although the addition of orlistat to sibutramine therapy does not appear to enhance weight loss [83, 84], combination therapy with the amylin analogue pramlintide is producing promising results [85] (see Titled Drug Monotherapies and Combination Therapies under Investigation).
Apart from increases in BP and heart rate the most common side-effects reported with sibutramine are dry mouth, constipation, and headache [57, 66].
Following the report of two sibutramine-related deaths in Britain and serious side effects in France, the EMEA demanded a long-term trial in patients at high risk of cardiovascular disease hence the Sibutramine Cardiovascular Outcome trial (SCOUT) was initiated [86, 87]. SCOUT is a double-blind, randomized, placebo-controlled outcome trial in 10,742 overweight or obese patients at high-risk for cardiovascular disease that commenced recruitment in December 2002. Of the total patients 97% had cardiovascular disease, 88% had hypertension, and 84% had type 2 diabetes [88]. Until recently the only published results from SCOUT were from the 4–6-week lead in period [13–15, 87, 89]. At 6 weeks there was a significant reduction in body weight (2.2 kg), waist circumference (2.0 cm), systolic (3.0 mm Hg) and diastolic blood pressure (1.0 mm Hg) with sibutramine treatment, however pulse rate was increased by 1.5 bpm (all P < .001) [89]. Results were similar for the diabetic patients in the study, that is, a 2.1 kg decrease in weight and decrease in blood pressure by 3.5/1.0 mm Hg with sibutramine compared to placebo [14]. A total of 9,800 patients were followed up for six years. The preliminary data released in late 2009, suggested that sibutramine was associated with a higher rate of CV events than placebo [90], whilst data from a FDA early communication indicated that there was an increased rate of CV events (heart attacks, strokes, resuscitated cardiac arrest, CV death) in patients with cardiovascular disease and diabetes (11.9% placebo, 13.9% sibutramine, hazard ratio 1.18, 95% CI 1.02–1.35, P = .023) [91]. The EMEA concluded that the benefits of sibutramine did not outweigh the risks and recommended that all marketing authorisations for medicines containing sibutramine should be suspended throughout Europe [10]. The FDA initially allowed sibutramine to be available, but asked for stronger warnings on the product labels [92]. The warning recommended that sibutramine should not be used by people who have a history of stroke or heart attacks and uncontrolled high blood pressure. The recent publication of the SCOUT study which had a mean follow-up period of 3.4 years reported a large number of patients that discontinued treatment (40.2% sibutramine, 42.3% placebo), a higher risk of cardiovascular outcome with sibutramine (11.4% versus 10%, hazard ratio 1.16 95% CI 1.03–1.31, P = .02). [17] In particular there was a higher rate of nonfatal MI and nonfatal stroke for sibutramine (4.1% and 2.6%, resp.) than placebo (3.2% and 1.9%).
A 3-year prospective observational study of 15,686 patients prescribed sibutramine in New Zealand has not demonstrated a higher risk of death from a cardiovascular event [93]. The FDA is currently reviewing the potential benefits and risks of sibutramine [94].
3.6. Rimonabant
Rimonabant, an endocannabinoid receptor (subtype 1) blocker, was developed as a result of observations on the appetite stimulation associated with recreational cannabis use (Table 2). The drug has a range of both central and metabolic peripheral effects and had also been investigated for smoking cessation [26, 95].
Attrition rates in a pooled study of 5,580 patients without diabetes and 1,047 patients with diabetes taking rimonabant 20 mg daily for one year and a hypocaloric diet were approximately 40% [96]. In the nondiabetic patient subgroup, rimonabant reduced body weight by 6.5 kg compared to placebo (P < .001). Weight-loss of ≥5% was achieved in 50.8% of the treatment group, and waist circumference was reduced by 6.4 cm compared to placebo (P < .001) (Table 4) [96]. There was an improvement in glycaemic control in diabetic patients with a reduction in mean HbA1C levels of 0.6% (P < .001) [96]. Discontinuation due to side-effects occurred in 13.8% of rimonabant patients and in 7.2% of placebo patients. The most commonly experienced adverse events were gastrointestinal disorders, mood alterations with depressive symptoms, anxiety, dizziness, nausea, and upper respiratory tract infections.
Table 4.
Recent randomised controlled trials of weight loss therapies with 6-month followup.
| Drug | No. subjects | Outcomes | Serious adverse events | Reference | |||
|---|---|---|---|---|---|---|---|
| Absolute weight loss (kg) (95% CI) | ≥5% weight loss | ≥10% weight loss | Change in WC (cm) | ||||
| Bupropion (400 mg) plus Naltrexone | Placebo-85 | 0.9 ± 0.5 | 15% | 2% | −0.9 ± 0.9 | Nausea: 3.5% P, 28.1% BN16, 39.7% BN32, 41% BN48 | [22] |
| N 48 mg-56 B-60 | 1.1 ± 0.7 | 10% | 2% | −3.8 ± 1.2 | |||
| BN16 mg-64 | 2.6 ± 0.6 | 26% | 7% | −2.9 ± 1.1 | |||
| 5.1 ± 0.6P < .05 | 52%, P < .05 | 17%, P < .05 | −3.7 ± 1.1, P < .05 | ||||
| BN 32 mg-63 | 5.1 ± 0.6P < .05 | 51%, P < .05 | 19%, P < .05 | −4.6 ± 1.0, P < .05 | |||
| BN 48 mg-61 | 4.0 ± 0.6P < .05 | 39%, P < .05 | 15%, P < .05 | −4.7 ± 1.2, P < .05 | |||
| Bupropion (120 mg/360 mg) + Zonisamide (360 mg/360 mg) | 729 (total) | 1.4∧ P | 15%∧ P | 4%∧ P | NA | NA | [97] |
| 3.2∧ Z120 5.3∧ Z360 |
27%∧ Z120 44%∧ Z360 | 9%∧ Z120 18%∧ Z360 |
|||||
| 2.3∧ B360 | 21%∧ B360 | 11%∧ B360 | |||||
| 6.1∧ ZB120 P < .001 7.5∧ ZB360 P < .001 |
47%∧ ZB120 (P < .05) 60%∧ ZB360 (P < .05) | 25%∧ ZB120 P < .05 32%∧ ZB360 P < .05 |
|||||
| Rimonabant | P-417 | 0.5 (0.3, 1.3) | NR | NR | 1.0 (−0.2, 1.9) | Psychiatric: 28.4% P, 43.4% R, P < .001 | [98] |
| R-422 | 4.3 (3.5, 5.1) P < .001 | 4.5 (−3.7, −5.4), P < .001 | Severe psychiatric: 3.5% P, 4.8% R, P = .52 | ||||
| Rimonabant* | 3165 (total) | Placebo 1.6 R 6.5 |
19.7% 50.8%, P < .001 |
7.8% 27%, P < .001 |
6.4 2.5 |
Depression: 0.8% P, 1.9% R, NS Anxiety: 0.3 P, 1.0% R, NS | [96] |
| Tesofensine | Placebo-52 | 2.2 (0.9, 3.5) | 13 (29%) | 3 (7%) | −2.4 (0.7, 4.2) | Anger and hostility: 4% P, 14.3% T 1 mg, P = .018 | [99] |
| T 0.25 mg-52 | 6.7 (5.4, 8.0), P < .0001 | 29 (59%) | 17 (35%) | −5.9 (4.0, 7.8), P = .007 | Increased confusion: P-NA, | ||
| T 0.5 mg-50 | 11.3 (9.9, 12.7), P < .0001 | 41 (87%) | 25 (53%) | –9·4 (7.7, 11.0), P < .0001 | T 0.5 mg P = .015, | ||
| T 1.0 mg-49 | 12·8 (11.6, 14.1), P < .0001 | 42 (91%) | 34 (74%) | –9·3 (7.6, 11.0), P < .0001 | T 1 mg P = .0003 | ||
Absolute weight loss: weight loss from baseline; NS: not significant; NR: not recorded; WC: waist circumference; P: placebo; B: bupropion; N: naltrexone; Z: zonisamide; R: rimonabant; T: tesofensine; NA: not available; *pooled non-diabetic patients; #mean ± standard deviation; ∧estimated absolute weight based on mean baseline weight of 100 kg.
Four large Rimonabant in Obesity and Related Metabolic Disorders (RIO) Phase III studies (RIO-Europe, RIO-North America, RIO-Diabetes, RIO-Lipids) were included in two meta-analyses and a systematic review to investigate the efficacy and safety of rimonabant in improving cardiovascular and metabolic risk factors in overweight patients [9, 60] (Table 3). Compared with placebo, rimonabant (20 mg) produced a 4.9 kg (95% CI 4.3, 5.0) greater reduction in body weight as well as improvements in waist circumference (−3.84 cm, 95% CI −4.26, −3.42), high-density lipoprotein cholesterol, triglyceride levels, and systolic and diastolic BP [60]. A subsequent meta-analysis which included the 4 RIO studies provided evidence of the likelihood of experiencing serious side effects with rimonabant [9]. The odds ratio (OR) for depression was 2.51 (95% CI, 1.23–5.12) and 3.03 (95%, 1.09–8.42) for anxiety [9]. A systematic review and meta-analysis reported that the 20 mg rimonabant dose was associated with an increased risk of adverse events (RR 1.35; 95% CI 1.17–1.56), increased discontinuation rate (RR 1.79; 95% CI 1.35–2.38), and psychiatric (RR 2.35; 95% CI 1.66–3.34), and nervous system adverse events (RR 2.35; 95% CI 1.49–3.70) [100]. The number needed to harm (NNH) for psychiatric adverse events was 30 [100]. In a comparison with other pharmacotherapies the risk ratios for discontinuation in RCTs due to adverse events were significantly elevated for rimonabant (2.00; 95% CI 1.66–2.41) and orlistat (1.59; 95% CI 1.21–2.08), but not sibutramine (0.98, 95% CI 0.68–1.41) [20]. The risk difference was largest for rimonabant (7%, 95% CI: 5%–9%; NNH 14, 95% CI: 11–19) compared with placebo, followed by orlistat (3%, 1%–4%; NNH 39, 95% CI: 25–83), while no significant difference was seen for sibutramine (0.2%, 95% CI: −3% to 4%; NNH 500).
In late 2008, the manufacturers of rimonabant announced that all clinical research studies would be stopped permanently. This announcement followed a decision by the EMEA to withdraw marketing of the drug as the risks especially of psychiatric side effects were considered to outweigh the drug's benefits [101].
3.7. Systematic Reviews Comparing Several Drug Therapies
Several meta-analyses and systematic reviews have demonstrated that pharmacotherapy in combination with a low calorie diet and in some cases exercise generally results in a maximum weight reduction at six months of 1–9.6 kg, maintenance of weight loss with continued therapy, and a regain in weight after drug therapy is discontinued [7, 30]. The largest mean effect sizes were demonstrated with amphetamines, fenfluramines and sibutramine, though no drug demonstrated clear superiority [30, 58] and most of the drugs have been prescribed for a limited duration. A systematic review which included 14 RCTs with orlistat, 7 RCTs of sibutramine and 4 RCTs with rimonabant compared to placebo, reported 2.9 kg greater weight loss with orlistat than placebo, 4.2 kg for sibutramine and 4.7 kg for rimonabant (Table 3). Patients on active drug therapy were significantly more likely to achieve ≥5% and ≥10% weight loss [57]. Continuation on treatment was a problem with attrition rates averaging 30%–40% within 12 months [57].
In adolescents a meta-analyses of RCTs with orlistat and sibutramine demonstrated a mean decrease in weight between the intervention and control groups of 5.25 kg (95% CI: 3.03–7.48) after a minimum follow-up of 6 months [71]. Systemic reviews of pharmacotherapy for overweight and obese children, adolescents, and older adults only include a limited number of mainly short-term studies [21, 102–104] hence, there is a lack of high-quality evidence to support the efficacy and safety of drug therapy in these populations.
4. Drug Monotherapies and Combination Therapies under Investigation
Some already marketed drugs (that are approved for other indications) and several new agents are currently being evaluated for the management of obesity [25, 105, 106]. These include tesofensine, a pharmacological agent that targets the inhibition of NA, DA, and 5HT reuptake and, liraglutide a glucagon-like peptide-1 analog and lorcaserin the selective serotonin 2C (5-HT2C) receptor agonist (Table 2). There are also several combination drug therapies in Phase III trials including bupropion and naltrexone, bupropion and zonisamide, phentermine and topiramate, and pramlintide and metreleptin. Some drugs that were in late phase trials such as axokine, a naturally occurring re-engineered human protein known as cilary neurotrophic factor, taranabant a CB1R inverse agonist, and ecopipam a selective dopamine D1/D5 antagonist have been abandoned, the latter two due to an increase in psychiatric adverse events. There are also some weight loss medications that have previously been used in the management of diabetes that are being evaluated for weight loss, that is, pramlintide, liraglutide, and exenatide.
4.1. Pramlintide
Pramlintide, a synthetic analog of the pancreatic hormone amylin, was originally used for the treatment of type 1 and 2 diabetes. It has been associated with reduced, appetite, food intake and enhanced satiety through delayed gastrointestinal motility and is currently under investigation as a potential treatment for obesity [25, 105]. In a 16-week dose escalation RCT 3.7% mean weight loss was demonstrated with pramlintide 240 μg given as a subcutaneous (SC) injection compared to placebo (P < .001) and ≥5% weight loss was achieved in 31% of patients (P < .001) [107]. In obese patients participating in a 4-month RCT of pramlintide at doses of 120, 240, and 360 μg administered two or three times a day, followed by a single blind extension to 1 year, weight loss was regained in the placebo group but maintained or continued in all but the pramlintide 120 μg twice daily arm [108]. Nausea was the most common adverse event.
4.2. Glucagon-Like Peptide-1 (GLP1) Analogues: Liraglutide, Exenatide
Liraglutide and exenatide are glucagon-like peptide-1 (GLP1) analogues developed and approved for the treatment of type 2 diabetes (Table 2) [109]. Phase III trials of liraglutide have demonstrated beneficial weight loss in obese patients. These analogues have a dual mechanism of action, that is, on the gastrointestinal (GI) tract and the brain. Signals from the GI tract are sent to the brain to increase the secretion of leptin, resulting in suppressed appetite, energy intake and a delay in gastric emptying. A key benefit with long-term use of liraglutide and exenatide is a decrease in HbA1c levels and systolic BP [110, 111]. A recent 20 week dose-ranging RCT of liraglutide (1.2, 1.8 mg, 2.4 mg, 3.0 mg) in comparison with orlistat (120 mg) treatment in 564 nondiabetic obese patients demonstrated a mean weight loss of 4-8 kg, 5.5 kg, 6.3 kg, and 7.2 kg, resp. compared with 2·8 kg with placebo and 4·1 kg with orlistat (P = .003 for 1.2 mg, P < .0001 for 1.8–3.0 mg liraglutide) [112]. Higher doses of liraglutide (2.4 and 3.0 mg) demonstrated significantly greater mean weight loss than orlistat. The most common adverse events with liraglutide were nausea and vomiting, but these were not significantly different to the placebo group. Patients treated with liraglutide also showed a significant reduction in blood pressure and the prevalence of prediabetes (84%–96%).
Exenatide is currently only in Phase II trials [113] for obesity but early results from an open-label study have demonstrated weight loss as well as an improvement in glycemic control [114].
4.3. Taranabant
Taranabant a cannabinoid CB-1 receptor (CB1R) inverse agonist which reduces appetite and increases energy expenditure has been evaluated for the treatment of obesity [115]. It demonstrated greater weight loss with higher doses in a 12 week RCT that assessed its safety and efficacy. Four Phase III trials have been published, two assessed the risk/benefit profile of low and high doses and one included patients with type 2 diabetes [23, 116–118]. Mean weight loss after 1 year of taranabant was 5.0 kg with the 0.5 mg dose, 5.2 kg with the 1mg, 6.4 kg with the 2 mg compared to 1.4 kg for placebo (all P < .001) [118] Significantly more patients achieved ≥5% and ≥10% loss of baseline body weight with taranabant than placebo (P < .001 for all doses) (Table 5). Approximately 80% of patients from each taranabant dose group experienced one or more adverse events [118].
Table 5.
Recent randomised controlled trials of weight loss therapies with 12-month followup.
| Drug | No. subjects | Outcomes | Serious adverse events | Reference | |||
|---|---|---|---|---|---|---|---|
| Absolute weight loss (kg) (95% CI) | ≥5% weight loss | ≥10% weight loss | Change in WC (cm) | ||||
| Lorcaserin | Placebo-716 | 2.16 ± 0.14∧ | 20.3% | 7.7% | 3.9 ± 0.2 | Headache 2% P, 0.8% L | [120] |
| L-883 | 5.81 ± 0.16 P < .001 | 47.5% P < .001 | 22.6% P < .001 | 6.8 ± 0.2P < .001 | Dizziness 0.8% P, 0.1% L | ||
| Taranabant | Placebo-417 | 2.6 (1.8, 3.3) | 27.2% | 8.4% | −3.1 (2.3, 3.9) | Nausea 6.5% P, 16.7% TB 2 mg, 21.4% TB 4 mg, P < .001 | [31] |
| TB 2 mg-415 | 6.6 (5.9, 7.4), P < .001 | 56.5%, P < .001 | 27.9%, P < .001 | −7.0 (6.1, 8.1), P < .001 | Vomiting 3.4% P, 8.4 TB 4 mg, P < .01 | ||
| TB 4 mg-414 | 8.1 (7.4, 8.9), P < .001 | 64.2%, P < .001 | 35.8%, P < .001 | −7.5 (6.7, 8.3), P < .001 | Diarrhoea 7.2% P, 12.3 TB 2 mg, P < .05, 13.7% TB 4 mg, P < .01 Anxiety 3.4 P, 9.9 TB 4 mg, P < .01 |
||
| Taranabant | Placebo-196 | +1.7 (0.8, 2.7) | 62.2% | NR | −0.7 (−0.3, 1.8) | Irritability TB 1 mg and 2 mg, P ≤ .038 | [116] |
| TB 0.5 mg-196 | 0.1 (−1.0, 0.8), P < .007 | 71.8% | NR | −1.5 (−2.6, −0.5) | |||
| TB 1 mg-196 | 0.6 (−1.5, 0.4), P < .007 | 78%, P < .05 | NR | −2.3 (−3.4, −1.3) | |||
| TB 2 mg-196 | 1.2 (−2.1, −0.3), P < .007 | 83.3%, P < .001 | NR | −2.4 (−3.4, −1.3) | |||
| Taranabant | Placebo-137 | 1.4 (−0.5, −2.4) | 24.3% | 7.3% | −3.0 (−1.9, −4.1) | Psychiatric-17.7% P, 28.8% TB1mg, 29%TB 2 mg, P ≤ .038 | [118] |
| TB 0.5 mg-141 | 5.0 (−4.0, −5.9), P < .001 | 44.2, P < .001 | 21.3, P < .001 | −5.6 (−4.5, −6.6), P < .001 | |||
| TB 1 mg-138 | 5.2 (−4.2, −6.2), P < .001 | 45.3, P < .001 | 18.2, P < .001 | −5.7 (−4.6, −6.7), P < .001 | |||
| TB 2 mg-277 | 6.4 (−5.7, −7.2), P < .001 | 53, P < .001 | 28, P < .001 | −6.9 (−6.1, −7.7), P < .001 | |||
| Bupropion/naltrexone* | Placebo-202 | 7.3% ± 0.9% | 60.4% | 30.2% | −6.8 (5.3, 8.3) | Nausea 10.5% P, 34.1% BN P < .001 | [121] |
| BN 360/32–591 | 11.5% ± 0.6%, P < .001 | 80.4%, P < .001 | 55.2%, P < .001 | −10.0 (9.0, 10.9), P < .001 | Dizziness 4.5% P, 14.6% BN, P < .001 | ||
| Bupropion/naltrexone | B-60 | 2.7 ± 0.9 | 33% | 12% | NA | NA | [22] |
| BN 16 mg-64 | 5.0 ± 0.9 | 50% | 22% | ||||
| BN 32 mg-63 | 6.1 ± 0.8, P < .05 | 51% | 25% | ||||
| BN 48 mg-61 | 4.6 ± 0.9 | 39% | 20% | ||||
| Pramlintide | Placebo-63Pramlintide-61 | 2.1 ± 0.9 3.6 ± 0.7 |
3% 28% |
11% 36% |
NR | Nausea 0% P, 30% Pramlintide | [85] |
| Topiramate | Placebo-55 T−54 |
2.5 ± 3.1 6.0 ± 5.2, P < .001 |
19% 50%, P < .001 |
2% 20%, P < .001 |
−2.3 ± 4.7 −4.2 ± 5.7, P = .078 |
Paraesthesia −0% P, 28% TPsychiatric −11% P, 33% T | [122] |
| Topiramate/phentermine | P-498 | 1.6 | 17% | NA | NA | NA | [123] |
| TP 3.75/23 mg-234 | 5.1, P < .0001 | 45%, P < .0001 | |||||
| TP15/92 mg-498 | 11, P < .0001 | 67%, P < .0001 | |||||
| Topiramate/phentermine | P-979 | 1.8 | 21% | NA | NA | NA | [123] |
| TP 7.5/46 mg-488 | 8.4, P < .0001 | 62%, P < .0001 | |||||
| TP15/92 mg-981 | 10.4, P < .0001 | 70%, P < .0001 | |||||
Absolute weight loss: weight loss from baseline; NS: not significant, NA: not available; L: lorcaserin; NB: naltrexone/bupropion, TB: taranabant; T: topiramate controlled release; TP: topiramate/phentermine; ∧mean ± standard error; *both groups also received intensive behavior modification.
A study using higher doses (2 mg, 4 mg, and 6 mg) achieved greater mean weight loss at 1 year of treatment which persisted to 2 years (Table 5) [23]. Although weight loss with the highest dose of 6 mg proved to be the most efficacious after 1 year of treatment, the adverse events were significantly increased with increasing doses particularly serious psychiatric events which included depression, depressive mood, anxiety, anger, and aggression [23]. The odds ratios for suicidality with increasing doses of taranabant after 1 year treatment were 1.74 (95% CI 0.87–3.51) with the 2 mg dose, 2.16 (95% CI 1.10–4.25) for 4 mg, and 2.34 (95% CI 1.11–4.96) with the 6 mg. Hence, only the lower doses (2 mg and 4 mg) were used for the remainder of the study.
The overall safety and efficacy profile of taranabant from the Phase III trials did not support its further development in the treatment of obesity, and clinical trials were ceased [23, 117, 119].
4.4. Lorcaserin
Lorcaserin is a selective serotonin 2C receptor agonist (5-HT2C), sharing characteristics similar to fenfluramines, which acts through another serotonin receptor (5-HT2B) that has been associated with cardiac valvular disease [124] (Table 2).
Recent clinical trials with lorcaserin have demonstrated effective weight loss compared to placebo along with a good safety profile [125, 126]. Results from two recently presented pivotal Phase III trials, BLOOM (Behavioral modification and Lorcaserin for Overweight and Obesity Management) and BLOSSOM (Behavioral modification and Lorcaserin Second Study for Obesity Management) indicated greater weight loss with lorcaserin than with placebo (Table 5) [120, 127, 128]. In these RCTs, 6380 non-diabetic patients aged 18–66 years with a BMI 27–45 kg/m2 were treated for 52 weeks with lorcaserin 10 mg twice daily or with placebo. Using the pooled data from these two trials, weight loss at 52 weeks decreased by 5.8% in the lorcaserin group and 2.5% in the placebo group (P < .0001) [127]. Weight loss was similar amongst males and females but was higher in Caucasian patients than African American patients or Hispanic patients and patients >50 years lost more weight than younger patients. Average weight loss at 1 year in the BLOOM study was 5.8 ± 0.2 kg with lorcaserin and 2.2 ± 0.1 kg with placebo ones (P < .001) with 47.5% and 20.3% loosing ≥5% of their body weight (Table 5) [120]. Weight loss was maintained in 67.9% of lorcaserin patients in year 2 and 50.3% of placebo (P < .001) (Table 6) [120]. After 52 weeks of lorcaserin treatment, changes in lipid and glucose values were more favourable in responders than nonresponders, and twice as many patients responded to lorcaserin as placebo (i.e., ≥5% body weight loss in 47.1% lorcaserin patients and 22.6% placebo) [128]. The most frequent adverse events reported were headache, dizziness and nausea, but these were not significantly different between treatment groups (Table 5). There was no increase in the rate of cardiac valvulopathy after 2-year treatment with lorcaserin [120].
Table 6.
Recent randomised controlled trials of weight loss therapies with 2-years followup.
| Drug | No subjects | Outcomes | Serious adverse events | Reference | |||
|---|---|---|---|---|---|---|---|
| Absolute weight loss (kg) (95% CI) | ≥5% weight loss | ≥10% weight loss | Change in WC (cm) | ||||
| Lorcaserin | P-684 | 3.0% ± 0.2% | 50.3% | 7.7% | 4.3 ± 0.2 | NS | [120] |
| L-564 | 7.0% ± 0.2% | 67.9% | 22.6% | 8.1 ± 0.2 | |||
| P < .001 | P < .001 | P < .001 | P < .001 | ||||
| Taranabant | P-244 | 1.4 (0.3, 2.5) | 30.3% | 13.4 | −2.7 (1.5, 3.8) | NS | [23] |
| TB 2 mg-264 | 6.4 (5.3, 7.4), P < .001 |
59.6, P < .001 | 33, P < .001 | −6.3 (5.2, 7.4) P < .05 | |||
| TB 4 mg-260 | 7.6 (6.5, 8.7), P < .001 |
64.8, P < .001 | 37.9, P < .001 | −7.0 (5.9, 8.1), P < .01 | |||
Absolute weight loss = weight loss from baseline; WC: waist circumference, NR: not recorded, TB: taranabant, NS: not significant; P: placebo; L: lorcaserin.
Although the recently published study indicated lorcaserin was safe and moderately effective, there was a high dropout rate [120]. Lorcaserin was submitted for FDA approval however in September 2010 the advisors recommended against approval as they did not consider that the potential benefits of the drug outweighed the risks. In particular they claimed that patients on lorcaserin did not achieve the percentage point criterion set by the FDA. The FDA which usually takes the advice of its committees is expected to decide in October whether to approve lorcaserin.
4.5. Tesofensine
Tesofensine is another novel pharmacological agent which inhibits the uptake of presynaptic NA, DA, and 5HT (Table 2, Figure 1). Tesofensine was discovered to decrease weight in patients receiving the drug for the treatment of Alzheimer's and Parkinson's disease [129]. Investigators performed a dose-dependent analysis in obese patients for 14 weeks, demonstrating a mean change in weight loss for tesofensine doses of 0.125 mg, 0.25 mg, 0.5 mg and 1 mg of 2.1%, 8.2%, 14.1%, and 20.9%, resp. [129]. Of the total obese patients in the study, 32.1% achieved a ≥5% weight loss with tesofensine, (P < .001 for 0.25, 0.5, and 1.0 mg versus placebo). No effect on blood pressure was observed, but there were increases in heart rate with increasing dose.
Further evidence was demonstrated in another 24-week Phase IIb randomised dose-dependent tesofensine trial in 203 obese individuals, with 79% of participants completing the study [99]. Weight loss was dose dependant with 4.5% weight loss (0.25 mg), 9.2% (0.5 mg), and 10.6% (1.0 mg) and was greater than that achieved with diet and placebo (P < .0001) (Table 4). The drug was well tolerated with no significant increases in systolic or diastolic blood pressure however, heart rate was increased by 7.4 beats/min in the middose group (P = .0001).
4.6. Naltrexone
Naltrexone, a high affinity and long-acting opioid receptor antagonist which was originally produced for the treatment of opioid and alcohol dependence, decreased food intake and led to weight loss in former narcotic addicts. The role of opioid receptors in eating behaviour was initially demonstrated following the administration of naloxone to rats resulting in a significant reduction in short-term food intake by blocking β-endorphin (Table 2) [130]. In RCTs naltrexone (an analogue of naloxone) has not consistently demonstrated statistically significant weight loss in obese and lean subjects [131–134].
4.7. Bupropion Plus Naltrexone (Contrave)
Bupropion was combined with the naltrexone following the recognition that naltrexone blocks β-endorphin mediated pro-opiomelanocortin (POMC) autoinhibition to sustain α-MSH release, whilst bupropion (through DA receptors) activates POMC neurons and enhances the release of the anorexiant neuropeptide α-MSH in the hypothalamus [22, 135, 136]. The bupropion-naltrexone combination is said to tackle the motivation/reinforcement that food brings (DA effect) and the pleasure/palatability of eating (opioid effect) [137].
A 24 week dose ranging study of naltrexone/bupropion-SR did not demonstrate increased weight loss with increasing doses of naltrexone (weight loss for 16 mg dose was 4.62% [95% CI: −6.24 to −2.99, P < .001], for 32 mg dose 4.65% [95% CI: −6.20 to −3.09, P < .001], and for the 48 mg dose 3.53% [95% CI: −5.15 to −1.90, P < .001]) (Table 4) [22]. Nevertheless, weight-loss was maintained in a 24-week extended period.
An open-label 24-week study demonstrated that naltrexone 32 mg SR/bupropion-SR 360 mg resulted in significant improvements in depressive symptoms in addition to weight loss and improved control of eating in overweight and obese women with major depression [138]. Depression scores as measured with the Montgomery-Asberg Depression Rating Scale decreased from an average of 23.7 at baseline to 10.5 (consistent with mild depression) at week 12 (P < .001) and 8.4 (consistent with remission) at week 24 (P < .001).
Several Phase III trials have been conducted in both diabetic and non-diabetic patients including COR-I, COR-II, COR-BMOD and COR-Diabetes [105, 121, 139–141]. COR-Diabetes was a 56-week RCT of 505 overweight or obese patients with type 2 diabetes (Hb A1C levels 7% to 10%, mean 8.0%) randomized to naltrexone 32 mg SR/bupropion 360 mg SR or placebo [140]. The naltrexone/bupropion patients lost significantly more weight (5.0% versus 1.8%, P < .001) at 56 weeks [140] with 44.5% of patients achieving ≥5% loss of body weight compared to 18.9% on placebo. Greater improvement in glycemic control was achieved in the treatment group with average baseline HbA1C reduced by 0.6% compared to 0.1% for placebo. The investigators noted that over 44% of treated patients achieved the American Diabetes Association treatment target of <7% for HbA1C compared to 26% of placebo patients (P < .001).
This drug combination has generally been welltolerated in most patients (Table 5). Nausea was the most frequent adverse event, and this occurred more frequently with higher naltrexone doses. A new drug application has been submitted for review by the FDA with the outcome expected in December 2010.
4.8. Bupropion Plus Zonisamide
The combination of bupropion with the epilepsy agent, zonisamide has been evaluated in three Phase II trials [97, 142–144]. The mechanism of action for zonisamide has not been fully characterised, however it has demonstrated biphasic DA and 5HT activity [142, 145]. The potential of zonisamide in the management of obesity was demonstrated in a small RCT where zonisamide patients experienced significantly more weight lost than those on placebo [145]. A 24-week RCT of bupropion 300 mg combined with zonisamide 400 mg achieved greater weight loss (9.2%) than either drugs alone (bupropion 6.6%, zonisamide 3.6%) or placebo (0.4%) [143]. Similar results were observed in a randomised open-label study [142]. Weight loss in a 24 week multicentre RCT with either drug alone and different combinations of zonisamide SR with bupropion SR were 1.4% with placebo, 3.2% with zonisamide SR 120 mg, 5.3% with zonisamide SR 360 mg, 2.3% with bupropion SR 360 mg, 6.1% with zonisamide SR 120 mg/bupropion SR 360 mg, and 7.5% for zonisamide SR 360 mg/bupropion SR 360 mg with ≥5% weight loss in 15%, 27%, 44%, 21%, 47%, 60%, respectively, [97]. The most frequent adverse events reported were headache, nausea and insomnia.
Weight loss with zonisamide and bupropion appears to be greater than that observed with the bupropion/naltrexone combination over the same period of treatment [22].
4.9. Topiramate Plus Phentermine (Qnexa)
Topiramate is a GABA agonist and an approved antiepileptic drug which has been trialed as monotherapy for weight loss [1]. It acts as an appetite suppressant that has been suggested to influence kainate/α-amino-3-hydroxy-5-methylisoxozole-4-propionicacid glutamate receptors, voltage-gated sodium channels, and γ-aminobutyric acid-A activity [146], however the exact mechanism of action for weight loss is not known (Table 2). Several RCTs demonstrated greater weight loss with topiramate monotherapy than placebo with continued weight loss throughout the duration of the study [1]. However concerns regarding central and peripheral nervous system adverse effects led to Phase III trials of topiramate being halted and topiramate being reformulated. As the sustained release formulation did not have better tolerability trials were discontinued in December 2004.
The combination of controlled release low dose topiramate with low dose phentermine has recently been shown to be effective for weight loss treatment [147]. A 28-week RCT using phentermine with topiramate (92 mg/15 mg and 46 mg/7.5 mg doses) demonstrated a 9.2% weight loss compared to a 6.4% weight loss with topiramate alone, 6.1% for phentermine alone and 1.7% for placebo [123]. The tolerance and safety of this drug combination are being evaluated in several Phase III trials (EQUATE, EQUIP, CONQUER). In July 2010 an FDA advisory committee agreed that the phentermine/topiramate combination was effective in reducing weight loss however it refused to endorse a recommendation for the treatment of obesity due to safety concerns which included increased heart rate, possible birth defects, and psychiatric problems (depression, suicidal thoughts, impaired memory and concentration) [148]. The final FDA determination on the drug combination is expected in late October 2010.
4.10. Pramlintide Combination Therapies
Pramlintide has been combined with recombinant methyl human leptin (metreleptin), an adipocyte-derived hormone involved in long-term signalling of adiposity and energy intake [149]. In early trials this combination of an amylin and a leptin agonist has demonstrated greater weight loss than either drug alone [148, 149]. Weight loss with pramlintide/metreleptin was 12.7% ± 0.9% (mean ± SE) to week 20 compared with 8.4% ± 0.9% for pramlintide (P < .001) and 8.2% ± 1.3% for metreleptin (P < .01) [149]. Pramlintide is also being evaluated in combination with sibutramine and phentermine [85]. In a 24-week open-label study weight loss was in subjects taking pramlintide and sibutramine was 11.1% ± 1.1% (mean ± SE), 11.3% ± 0.9% for those taking pramlintide plus phentermine, 3.7% ± 0.7% with pramlintide alone, and 2.2% ± 0.7% with placebo (P < .001) [85]. Common side effects experienced with combination treatments were nausea and increased heart rate [85]. There was a significant increase in heart rate and blood pressure with the combination of pramlintide and sibutramine (3.1 ± 1.2 beats/min, P < .05; 2.7 ± 0.9 mm Hg, P < .01) and pramlintide with phentermine (4.5 ± 1.3 beats/min, P < .01; 3.5 ± 1.2 mm Hg, P < .001). Pramlintide is also being investigated with exenatide, the GLP-1 agonist used for the treatment of obesity in diabetic and non-diabetic patients [113].
5. Conclusion
Pharmacological interventions in addition to lifestyle changes (diet and physical activity) and in some cases behavioural modifications are used to promote weight loss. At present, only two drugs are currently approved and available for the long-term treatment of obesity—orlistat and sibutramine. However, there are several drugs and combination drug therapies undergoing Phase III trials that may be approved in the next few years. Pharmacotherapies have demonstrated a significant though modest decrease in weight compared to placebo over 1-2 years. Unfortunately weight loss following pharmacological intervention is not sustained when therapy is discontinued with individuals regaining some or all of the weight that was originally lost.
Obesity is often considered a chronic disease, hence it requires long-term therapy. Currently, there is a lack of high quality evidence from long-term studies of both the efficacy and safety of pharmacological interventions for obesity. Serious safety concerns have resulted in the withdrawal of some drugs that had originally received market approval whilst other drugs have been abandoned during Phase III evaluation. An increase in psychiatric disorders following Phase III studies (RIO-Europe, RIO-North America, RIO-Diabetes and RIO-Lipids) with rimonabant treatment resulted in its withdrawal from the European market two years after its approval. Orlistat treatment is associated with troublesome side effects such as diarrhoea, flatulence, bloating, abdominal pain, and dyspepsia which may not be acceptable to some patients on long-term treatment whilst the recent concerns of severe liver disease have led to a review of its safety. Long-term treatment with sibutramine is associated with a positive though modest efficacy profile and a low risk profile for neuropsychiatric adverse events; however we will need to wait for the publication of the full results of the SCOUT study to determine if there is an increase rate of CV events in patients with cardiovascular disease and diabetes.
Among the drugs in late phase trials, lorcaserin appears to be a potential candidate for long-term treatment in obesity due to its demonstrated efficacy and tolerable safety profile. Treatment with topiramate and taranabant result in significant weight loss in long-term studies, however both of these drugs have serious adverse effects. In the case of taranabant the psychiatric adverse events have led to the discontinuation of Phase III trials. Amongst the combination therapies both bupropion with naltrexone and bupropion with zonisamide have demonstrated effective weight loss and appear to be generally well tolerated based on published results from RCTs whereas there appears to be concerns regarding the safety of combination therapy using topiramate with phentermine.
References
- 1.Ioannides-Demos LL, Proietto J, McNeil JJ. Pharmacotherapy for obesity. Drugs. 2005;65(10):1391–1418. doi: 10.2165/00003495-200565100-00006. [DOI] [PubMed] [Google Scholar]
- 2.Abenhaim L, Moride Y, Brenot F, et al. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. The New England Journal of Medicine. 1996;335(9):609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 3.Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Safety. 2006;29(4):277–302. doi: 10.2165/00002018-200629040-00001. [DOI] [PubMed] [Google Scholar]
- 4.Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. The New England Journal of Medicine. 1998;339(11):719–724. doi: 10.1056/NEJM199809103391102. [DOI] [PubMed] [Google Scholar]
- 5.Loke YK, Derry S, Pritchard-Copley A. Appetite suppressants and valvular heart disease—a systematic review. BMC Clinical Pharmacology. 2002;2, article 6 doi: 10.1186/1472-6904-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachdev M, Miller WC, Ryan T, Jollis JG. Effect of fenfluramine-derivative diet pills on cardiac valves: a meta-analysis of observational studies. American Heart Journal. 2002;144(6):1065–1073. doi: 10.1067/mhj.2002.126733. [DOI] [PubMed] [Google Scholar]
- 7.Glazer G. Long-term pharmacotherapy of obesity 2000: a review of efficacy and safety. Archives of Internal Medicine. 2001;161(15):1814–1824. doi: 10.1001/archinte.161.15.1814. [DOI] [PubMed] [Google Scholar]
- 8.Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. The New England Journal of Medicine. 2000;343(25):1826–1832. doi: 10.1056/NEJM200012213432501. [DOI] [PubMed] [Google Scholar]
- 9.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. The Lancet. 2007;370(9600):1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency. Withdrawal of the marketing authorisation in the European Union, 2009, http://www.emea.europa.eu/humandocs/PDFs/EPAR/zimulti/3956009en.pdf.
- 11.Mitchell PB, Morris MJ. Depression and anxiety with rimonabant. The Lancet. 2007;370(9600):1671–1672. doi: 10.1016/S0140-6736(07)61705-X. [DOI] [PubMed] [Google Scholar]
- 12. Stiles, S. FDA:Excess of CV events with sibutramine in SCOUT. HeartWire News, http://www.theheart.org/article/1027617/print.do.
- 13.Caterson I, Coutinho W, Finer N, et al. Early response to sibutramine in patients not meeting current label criteria: preliminary analysis of SCOUT lead-in period. Obesity. 2010;18(5):987–994. doi: 10.1038/oby.2009.327. [DOI] [PubMed] [Google Scholar]
- 14.Van Gaal L, Caterson I, Coutinho W, et al. on behalf of the SCOUT Investigators. Weight and blood pressure response to weight management and sibutramine in diabetic and non diabetic high-risk patients: an analysis from the 6-week lead-in period of the sibutramine cardiovascular outcomes (SCOUT) trial. Diabetes. Obesity and Metabolism. 2010;12:26–34. doi: 10.1111/j.1463-1326.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- 15.Weeke P, Andersson C, Fosbøl EL, et al. The weight lowering effect of sibutramine and its impact on serum lipids in cardiovascular high risk patients with and without type 2 diabetes mellitus—an analysis from the SCOUT lead-in period. BMC Endocrine Disorders. 2010;10, article 3 doi: 10.1186/1472-6823-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams G. Withdrawal of sibutramine in Europe. British Medical Journal. 2010;340, article c824 doi: 10.1136/bmj.c824. [DOI] [PubMed] [Google Scholar]
- 17.James WPT, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. The New England Journal of Medicine. 2010;363(10):905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 18.Barbosa MDFS, Celis E. Immunogenicity of protein therapeutics and the interplay between tolerance and antibody responses. Drug Discovery Today. 2007;12(15-16):674–681. doi: 10.1016/j.drudis.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Fabricatore AN, Wadden TA, Moore RH, et al. Attrition from randomized controlled trials of pharmacological weight loss agents: a systematic review and analysis: obesity Management. Obesity Reviews. 2009;10(3):333–341. doi: 10.1111/j.1467-789X.2009.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson K, Neovius K, Desantis SM, Rössner S, Neovius M. Discontinuation due to adverse events in randomized trials of orlistat, sibutramine and rimonabant: a meta-analysis. Obesity Reviews. 2009;10(5):564–575. doi: 10.1111/j.1467-789X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 21.McGovern L, Johnson JN, Paulo R, et al. Treatment of pediatric obesity: a systematic review and meta-analysis of randomized trials. Journal of Clinical Endocrinology and Metabolism. 2008;93(12):4600–4605. doi: 10.1210/jc.2006-2409. [DOI] [PubMed] [Google Scholar]
- 22.Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. Journal of Clinical Endocrinology and Metabolism. 2009;94(12):4898–4906. doi: 10.1210/jc.2009-1350. [DOI] [PubMed] [Google Scholar]
- 23.Aronne LJ, Tonstad S, Moreno M, et al. A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study. International Journal of Obesity. 2010;34(5):919–935. doi: 10.1038/ijo.2010.21. [DOI] [PubMed] [Google Scholar]
- 24.Bermudez-Silva FJ, Viveros MP, McPartland JM, Rodriguez de Fonseca F. The endocannabinoid system, eating behavior and energy homeostasis: the end or a new beginning? Pharmacology Biochemistry and Behavior. 2010;95(4):375–382. doi: 10.1016/j.pbb.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Cheung BMY. Pharmacotherapy for obesity. British Journal of Clinical Pharmacology. 2009;68(6):804–810. doi: 10.1111/j.1365-2125.2009.03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padwal RS, Majumdar SR. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. The Lancet. 2007;369(9555):71–77. doi: 10.1016/S0140-6736(07)60033-6. [DOI] [PubMed] [Google Scholar]
- 27.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. British medical journal. 1968;1(5588):352–354. doi: 10.1136/bmj.1.5588.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weintraub M, Hasday JD, Mushlin AI, Lockwood DH. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Archives of Internal Medicine. 1984;144(6):1143–1148. [PubMed] [Google Scholar]
- 29.Griffen L, Anchors M. The “phen-pro” diet drug combination is net associated with valvular heart disease. Archives of Internal Medicine. 1998;158(11):1278–1279. doi: 10.1001/archinte.158.11.1278. [DOI] [PubMed] [Google Scholar]
- 30.Haddock CK, Poston WSC, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. International Journal of Obesity. 2002;26(2):262–273. doi: 10.1038/sj.ijo.0801889. [DOI] [PubMed] [Google Scholar]
- 31.McKay RH. Long-term use of diethylpropion in obesity. Current Medical Research and Opinion. 1973;1(8):489–493. doi: 10.1185/03007997309111712. [DOI] [PubMed] [Google Scholar]
- 32.Cercato C, Roizenblatt VA, Leança CC, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. International Journal of Obesity. 2009;33(8):857–865. doi: 10.1038/ijo.2009.124. [DOI] [PubMed] [Google Scholar]
- 33.Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. Journal of Physiology. 2009;587(1):49–60. doi: 10.1113/jphysiol.2008.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heisler LK, Cowley MA, Tecott LH, et al. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297(5581):609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 35.Heisler LK, Pronchuk N, Nonogaki K, et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. Journal of Neuroscience. 2007;27(26):6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lam DD, Przydzial MJ, Ridley SH, et al. Serotonin 5-HT2C receptor agonist promotes hypophagia via downstream activation of melanocortin 4 receptors. Endocrinology. 2008;149(3):1323–1328. doi: 10.1210/en.2007-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvajal A, Garcia del Pozo J, Martin de Diego I, Rueda de Castro AM, Velasco A. Efficacy of fenfluramine and dexfenfluramine in the treatment of obesity: a meta-analysis. Methods and Findings in Experimental and Clinical Pharmacology. 2000;22(5):285–290. doi: 10.1358/mf.2000.22.5.796647. [DOI] [PubMed] [Google Scholar]
- 38.Guy-Grand B, Appelbaum M, Crepaldi G, Gries A, Lefebvre P, Turner P. International trial of long-term dexfenfluramine in obesity. The Lancet. 1989;2(8672):1142–1145. doi: 10.1016/s0140-6736(89)91499-2. [DOI] [PubMed] [Google Scholar]
- 39.Mathus-Vliegen EMH, van de Voorde K, Kok AME, Res AMA. Dexfenfluramine in the treatment of severe obesity: a placebo-controlled investigation of the effects on weight loss, cardiovascular risk factors, food intake and eating behaviour. Journal of Internal Medicine. 1992;232(2):119–127. doi: 10.1111/j.1365-2796.1992.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 40.Weintraub M. Long-term weight control: the National Heart, Lung, and Blood Institute funded multimodal intervention study: introduction. Clinical Pharmacology and Therapeutics. 1992;51(5):581–585. doi: 10.1038/clpt.1992.68. [DOI] [PubMed] [Google Scholar]
- 41.Bowen R, Glicklich A, Khan M, et al. Cardiac valvulopathy associated with exposure fenfluramine or dexfenfluramine: US Department of Health and Human Services Interim Public Health Recommendations, November 1997. MMWR. 1997;46(45):1061–1066. [PubMed] [Google Scholar]
- 42.Curfman GD. Diet pills redux. The New England Journal of Medicine. 1997;337(9):629–630. doi: 10.1056/NEJM199708283370909. [DOI] [PubMed] [Google Scholar]
- 43.Delcroix M, Kurz X, Walckiers D, Demedts M, Naeije R. High incidence of primary pulmonary hypertension associated with appetite suppressants in Belgium. European Respiratory Journal. 1998;12(2):271–276. doi: 10.1183/09031936.98.12020271. [DOI] [PubMed] [Google Scholar]
- 44.Levine LR, Enas GG, Thompson WL, et al. Use of fluoxetine, a selective serotonin-uptake inhibitor, in the treatment of obesity: a dose-response study. International Journal of Obesity. 1989;13(5):635–645. [PubMed] [Google Scholar]
- 45.Bray GA. Use and abuse of appetite-suppressant drugs in the treatment of obesity. Annals of Internal Medicine. 1993;119(7):707–713. doi: 10.7326/0003-4819-119-7_part_2-199310011-00016. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein DJ, Rampey AH, Enas GG, et al. Fluoxetine: a randomised clinical trial in the treatment of obesity. International Journal of Obesity. 1994;18:129–135. [PubMed] [Google Scholar]
- 47.Wise SD. Clinical studies with fluoxetine in obesity. American Journal of Clinical Nutrition. 1992;55(1):181S–184S. doi: 10.1093/ajcn/55.1.181s. [DOI] [PubMed] [Google Scholar]
- 48.Darga LL, Carroll-Michals L, Botsford SJ, Lucas CP. Fluoxetine’s effect on weight loss in obese subjects. American Journal of Clinical Nutrition. 1991;54(2):321–325. doi: 10.1093/ajcn/54.2.321. [DOI] [PubMed] [Google Scholar]
- 49.Pedrinola F, Sztejnsznajd C, Lima N, Halpern A, Medeiros-Neto G. The addition of dexfenfluramine to fluoxetine in the treatment of obesity: a randomized clinical trial. Obesity Research. 1996;4(6):549–554. doi: 10.1002/j.1550-8528.1996.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 50.Anchors M. Fluoxetine is a safer alternative to fenfluramine in the medical treatment of obesity. Archives of Internal Medicine. 1997;157(11):p. 1270. [PubMed] [Google Scholar]
- 51.Whigham LD, Dhurandhar NV, Rahko PS, Atkinson RL. Comparison of combinations of drugs for treatment of obesity: body weight and echocardiographic status. International Journal of Obesity. 2007;31(5):850–857. doi: 10.1038/sj.ijo.0803498. [DOI] [PubMed] [Google Scholar]
- 52.Plodkowski RA, Nguyen Q, Sundaram U, Nguyen L, Chau DL, St Jeor S. Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity. Expert Opinion on Pharmacotherapy. 2009;10(6):1069–1081. doi: 10.1517/14656560902775750. [DOI] [PubMed] [Google Scholar]
- 53.Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obesity Research. 2002;10(7):633–641. doi: 10.1038/oby.2002.86. [DOI] [PubMed] [Google Scholar]
- 54.Croft H, Houser T, Leadbetter R, Jamerson B. Effect of bupropion SR on weight in the long-term treatment of depression. Obesity Research. 2000;8(supplement 1):p. 10. [Google Scholar]
- 55.Gadde KM, Parker CB, Maner LG, et al. Bupropion for weight loss: an investigation of efficacy and tolerability in overweight and obese women. Obesity Research. 2001;9(9):544–551. doi: 10.1038/oby.2001.71. [DOI] [PubMed] [Google Scholar]
- 56.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Annals of Internal Medicine. 2005;142(7):532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 57.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database of Systematic Reviews. 2004;4(3) doi: 10.1002/14651858.CD004094.pub2. Article ID CD004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. British Medical Journal. 2007;335(7631):1194–1199. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neovius M, Johansson K, Rössner S. Head-to-head studies evaluating efficacy of pharmaco-therapy for obesity: a systematic review and meta-analysis. Obesity Reviews. 2008;9(5):420–427. doi: 10.1111/j.1467-789X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- 60.Curioni C, André C. Rimonabant for overweight or obesity. Cochrane Database of Systematic Reviews. 2006;(4) doi: 10.1002/14651858.CD006162.pub2. Article ID CD006162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Archives of Family Medicine. 2000;9(2):160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 62.Heymsfield SB, Segal KR, Hauptman J, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Archives of Internal Medicine. 2000;160(9):1321–1326. doi: 10.1001/archinte.160.9.1321. [DOI] [PubMed] [Google Scholar]
- 63.Rössner S, Sjöström L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. Obesity Research. 2000;8(1):49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- 64.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the Prevention of Diabetes in Obese Subjects (XENDOS) Study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 65.Horvath K, Jeitler K, Siering U, et al. Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Archives of Internal Medicine. 2008;168(6):571–580. doi: 10.1001/archinte.168.6.571. [DOI] [PubMed] [Google Scholar]
- 66.Siebenhofer A, Horvath K, Jeitler K, et al. Long-term effects of weight-reducing drugs in hypertensive patients. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD007654.pub2. Article ID CD007654. [DOI] [PubMed] [Google Scholar]
- 67.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. Journal of the American Dietetic Association. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. American Journal of Clinical Nutrition. 2004;80(6):1461–1468. doi: 10.1093/ajcn/80.6.1461. [DOI] [PubMed] [Google Scholar]
- 69.O’Meara S, Riemsma R, Shirran L, Mather L, Ter Riet G. A systematic review of the clinical effectiveness of orlistat used for the management of obesity. Obesity Reviews. 2004;5(1):51–68. doi: 10.1111/j.1467-789x.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 70.Padwal R, Li SK, Lau DCW. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. International Journal of Obesity. 2003;27(12):1437–1446. doi: 10.1038/sj.ijo.0802475. [DOI] [PubMed] [Google Scholar]
- 71.Czernichow S, Lee CMY, Barzi F, et al. Efficacy of weight loss drugs on obesity and cardiovascular risk factors in obese adolescents: a meta-analysis of randomized controlled trials. Obesity Reviews. 2010;11(2):150–158. doi: 10.1111/j.1467-789X.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 72. FDA Drug Safety Communication: Completed safety review of Xenical/Alli (orlistat) and severe liver injury, 2010, http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm179166.htm.
- 73.Connoley IP, Liu Y-L, Frost I, Reckless IP, Heal DJ, Stock MJ. Thermogenic effects of sibutramine and its metabolites. British Journal of Pharmacology. 1999;126(6):1487–1495. doi: 10.1038/sj.bjp.0702446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones SP, Smith IG, Kelly F, Gray JA. Long term weight loss with sibutramine. International Journal of Obesity. 1995;19(supplement 2):p. 41. [Google Scholar]
- 75.Apfelbaum M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long-term maintenance of weight loss after a very-low-calorie diet: a randomized blinded trial of the efficacy and tolerability of sibutramine. American Journal of Medicine. 1999;106(2):179–184. doi: 10.1016/s0002-9343(98)00411-2. [DOI] [PubMed] [Google Scholar]
- 76.Bray GA, Ryan DH, Gordon D, Heidingsfelder S, Cerise F, Wilson K. A double-blind randomized placebo-controlled trial of sibutramine. Obesity Research. 1996;4(3):263–270. doi: 10.1002/j.1550-8528.1996.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 77.Smith IG, Goulder MA. Randomized placebo-controlled trial of long-term treatment with sibutramine in mild to moderate obesity. Journal of Family Practice. 2001;50(6):505–512. [PubMed] [Google Scholar]
- 78.James WPT, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. The Lancet. 2000;356:2119–2125. doi: 10.1016/s0140-6736(00)03491-7. [DOI] [PubMed] [Google Scholar]
- 79.McMahon FG, Fujioka K, Singh BN, et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension: a 1-year, double-blind, placebo-controlled, multicenter trial. Archives of Internal Medicine. 2000;160(14):2185–2191. doi: 10.1001/archinte.160.14.2185. [DOI] [PubMed] [Google Scholar]
- 80.Smith IG, Jones SP, Heath MJ, Kelly F. Categorical outcome analysis of weight loss in long-term sibutramine. International Journal of Obesity. 1996;20:p. 157. [Google Scholar]
- 81.Johansson K, Sundström J, Neovius K, Rössner S,, Neovius M. Long-term changes in blood pressure following orlistat and sibutramine treatment: a meta-analysis. Obesity Reviews. 2009;11(11):777–791. doi: 10.1111/j.1467-789X.2009.00693.x. [DOI] [PubMed] [Google Scholar]
- 82.Wadden TA, Berkowitz RI, Sarwer DB, Prus-Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Archives of Internal Medicine. 2001;161(2):218–227. doi: 10.1001/archinte.161.2.218. [DOI] [PubMed] [Google Scholar]
- 83.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Arnold ME, Steinberg CM. Effects of sibutramine plus orlistat in obese women following 1 year of treatment by sibutramine alone: a placebo-controlled trial. Obesity Research. 2000;8(6):431–437. doi: 10.1038/oby.2000.53. [DOI] [PubMed] [Google Scholar]
- 84.Sari R, Balci MK, Cakir M, Altunbas H, Karayalcin U. Comparison of efficacy of sibutramine or orlistat versus their combination in obese women. Endocrine Research. 2004;30(2):159–167. doi: 10.1081/erc-200027356. [DOI] [PubMed] [Google Scholar]
- 85.Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity. 2010;18(9):1739–1746. doi: 10.1038/oby.2009.478. [DOI] [PubMed] [Google Scholar]
- 86.James WPT. The SCOUT study: risk-benefit profile of sibutramine in overweight high-risk cardiovascular patients. European Heart Journal, Supplement. 2005;7:L44–L48. [Google Scholar]
- 87.Sharma AM, Caterson ID, Coutinho W, et al. Blood pressure changes associated with sibutramine and weight management—an analysis from the 6-week lead-in period of the sibutramine cardiovascular outcomes trial (SCOUT) Diabetes, Obesity and Metabolism. 2009;11(3):239–250. doi: 10.1111/j.1463-1326.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 88.Von Haehling S, Lainscak M, Anker SD. Sibutramine in cardiovascular disease: is SCOUT the new STORM on the horizon? European Heart Journal. 2007;28(23):2830–2831. doi: 10.1093/eurheartj/ehm493. [DOI] [PubMed] [Google Scholar]
- 89.Torp-Pedersen C, Caterson I, Coutinho W, et al. Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. European Heart Journal. 2007;28(23):2915–2923. doi: 10.1093/eurheartj/ehm217. [DOI] [PubMed] [Google Scholar]
- 90. GLG Expert Contributor. New Meridia SCOUT trial has major implications for obesity drug development, 2009, http://www.glgroup.com/News/New-Meridia-SCOUT-Trial-has-Major-Implications-for-Obesity-Drug-Development-45006.html.
- 91. FDA Drug Safety Communication: Follow-Up to the November 2009 Early Communication about an Ongoing Safety Review of Sibutramine, Marketed as Meridia, 2010, http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm198206.htm.
- 92.Singer N, Pollack A. Heart Patients Warned Against Using Meridia, an Anti-Obesity Drug. The New York Times, 2010, http://www.nytimes.com/2010/01/23/business/23diet.html.
- 93.Harrison-Woolrych M, Ashton J, Herbison P. Fatal and non-fatal cardiovascular events in a general population prescribed sibutramine in New Zealand: a prospective cohort study. Drug Safety. 2010;33(7):605–613. doi: 10.2165/11532440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 94.Astrup A. Drug management of obesity—efficacy versus safety. The New England Journal of Medicine. 2010;363(3):288–290. doi: 10.1056/NEJMe1004076. [DOI] [PubMed] [Google Scholar]
- 95.Cahill K, Ussher M. Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD005353.pub2. Article ID CD005353. [DOI] [PubMed] [Google Scholar]
- 96.Van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes care. 2008;31(supplement 2):S229–240. doi: 10.2337/dc08-s258. [DOI] [PubMed] [Google Scholar]
- 97.Fujioka K, Apovian C, Hill J. The Evolution of Obesity Therapies: New Applications for Existing Drugs. MedscapeCME Diabetes Endocrinology, 2010, http://cme.medscape.com/viewarticle/722366.
- 98.Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299(13):1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- 99.Astrup A, Madsbad S, Breum L, Jensen T, Kroustrup J, Larsen T. Effect of tesofensine on bodyweight loss body composion and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. The Lancet. 2008;372:1906–1913. doi: 10.1016/S0140-6736(08)61525-1. [DOI] [PubMed] [Google Scholar]
- 100.Chavez-Tapia NC, Tellez-Avila FI, Bedogni G, Crocè LS, Masutti F, Tiribelli C. Systematic review and meta-analysis on the adverse events of rimonabant treatment: considerations for its potential use in hepatology. BMC Gastroenterology. 2009;9, article no. 1471:p. 75. doi: 10.1186/1471-230X-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berton E, Bender R. UPDATE: Sanofi-Aventis stops all Acomplia clinical trials. Dow Jones Newswires, 2008, www.djnewswires.com.
- 102.McTigue KM, Hess R, Ziouras J. Obesity in older adults: a systematic review of the evidence for diagnosis and treatment. Obesity. 2006;14(9):1485–1497. doi: 10.1038/oby.2006.171. [DOI] [PubMed] [Google Scholar]
- 103.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD001872.pub2. Article ID CD001872. [DOI] [PubMed] [Google Scholar]
- 104.Viner RM, Hsia Y, Tomsic T, Wong ICK. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis: obesity management. Obesity Reviews. 2010;11(8):593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- 105.Gadde KM, Allison DB. Combination pharmaceutical therapies for obesity. Expert Opinion on Pharmacotherapy. 2009;10(6):921–925. doi: 10.1517/14656560902824152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valentino MA, Lin JE, Waldman SA. Central and peripheral molecular targets for antiobesity pharmacotherapy. Clinical Pharmacology and Therapeutics. 2010;87(6):652–662. doi: 10.1038/clpt.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aronne L, Fujioka K, Aroda V, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo-controlled, dose-escalation study. Journal of Clinical Endocrinology and Metabolism. 2007;92(8):2977–2983. doi: 10.1210/jc.2006-2003. [DOI] [PubMed] [Google Scholar]
- 108.Smith SR, Aronne LJ, Burns CM, Kesty NC, Halseth AE, Weyer C. Sustained weight loss following 12-month pramlintide treatment as an adjunct to lifestyle intervention in obesity. Diabetes Care. 2008;31(9):1816–1823. doi: 10.2337/dc08-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 110.Madsbad S. Liraglutide effect and action in diabetes (LEAD) trial. Expert Review of Endocrinology and Metabolism. 2009;4(2):119–129. doi: 10.1586/17446651.4.2.119. [DOI] [PubMed] [Google Scholar]
- 111.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. The Lancet. 2009;374(9701):1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 113.Meade LT. Practical use of exenatide and pramlintide for the treatment of type 2 diabetes. Journal of Pharmacy Practice. 2009;22(6):540–545. [Google Scholar]
- 114.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2006;8(4):436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 115.Addy C, Wright H, Van Laere K, et al. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metabolism. 2008;7(1):68–78. doi: 10.1016/j.cmet.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 116.Wadden TA, Fujioka K, Toubro S, et al. A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity. 2010;18(12):2301–2310. doi: 10.1038/oby.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kipnes MS, Hollander P, Fujioka K, et al. A one-year study to assess the safety and efficacy of the CB1R inverse agonist taranabant in overweight and obese patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2010;12(6):517–531. doi: 10.1111/j.1463-1326.2009.01188.x. [DOI] [PubMed] [Google Scholar]
- 118.Proietto J, Rissanen A, Harp JB, et al. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. International Journal of Obesity. 2010;34(8):1243–1254. doi: 10.1038/ijo.2010.38. [DOI] [PubMed] [Google Scholar]
- 119.Koch L. Obesity: taranabant no longer developed as an antiobesity agent. Nature Reviews Endocrinology. 2010;6(6):p. 300. doi: 10.1038/nrendo.2010.56. [DOI] [PubMed] [Google Scholar]
- 120.Smith SR, Weissman NJ, Anderson CM. Multicentre placebo-controlled trial of lorcaserin for weight management. The New England Journal of Medicine. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 121.Wadden TA, Foreyt JP, Foster GD. Weight loss with naltrexone sr/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD Trial. doi: 10.1038/oby.2010.147. Obesity, 2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rosenstock J, Hollander P, Gadde KM, Sun X, Strauss R, Leung A. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30(6):1480–1486. doi: 10.2337/dc06-2001. [DOI] [PubMed] [Google Scholar]
- 123. Vivus Inc. VIVUS Announces Positive Results From Two Phase 3 Studies: Obese Patients on Qnexa Achieve Average Weight Loss up to 14.7% and Significant Improvements in Co-Morbidities: Results of EQUIP and CONQUER Phase 3 Studies Exceed FDA Benchmarks for Obesity Treatments, Demonstrate Positive Safety Profile, http://ir.vivus.com/releasedetail.cfm?ReleaseID=407933.
- 124.Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine phentermine. The New England Journal of Medicine. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 125.Chakrabarti R. Pharmacotherapy of obesity: emerging drugs and targets. Expert Opinion on Therapeutic Targets. 2009;13(2):195–207. doi: 10.1517/14728220802637063. [DOI] [PubMed] [Google Scholar]
- 126.Idelevich E, Kirch W, Schindler C. Current pharmacotherapeutic concepts for the treatment of obesity in adults. Therapeutic Advances in Cardiovascular Disease. 2009;3(1):75–90. doi: 10.1177/1753944708098226. [DOI] [PubMed] [Google Scholar]
- 127.Anderson CM, Sanchez M, Krolikowski J, Mancini M, Shanahan WR. a selective 5-HT2C agonist is efficacious for weight loss across patient subgroups. American Diabetes Association 70th Scientific Meeting. Abstract 1845-P, 2010, http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=80762.
- 128.Fidler MC, Sanchez M, Raether BL, Anderson CM, Shanahan WR. Changes in glucose tolerance and cardiovascular risk factors after 52 weeks of treatment with lorcaserin. American Diabetes Association 70th Scientific Meeting. Abstract 1855-P, 2010, http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=80772.
- 129.Astrup A, Meier DH, Mikkelsen BO, Villumsen JS, Larsen TM. Weight loss produced by tesofensine in patients with Parkinson’s or Alzheimer’s disease. Obesity. 2008;16(6):1363–1369. doi: 10.1038/oby.2008.56. [DOI] [PubMed] [Google Scholar]
- 130.Holtzman SG. Suppression of appetitive behavior in the rat by naloxone: lack of effect of prior morphine dependence. Life Sciences. 1979;24(3):219–226. doi: 10.1016/0024-3205(79)90222-4. [DOI] [PubMed] [Google Scholar]
- 131.Atkinson RL, Berke LK, Drake CR. Effects of long-term therapy with naltrexone on body weight in obesity. Clinical Pharmacology and Therapeutics. 1985;38(4):419–422. doi: 10.1038/clpt.1985.197. [DOI] [PubMed] [Google Scholar]
- 132.Bertino M, Beauchamp GK, Engelman K. Naltrexone, an opioid blocker, alters taste perception and nutrient intake in humans. American Journal of Physiology. 1991;261(1):R59–R63. doi: 10.1152/ajpregu.1991.261.1.R59. [DOI] [PubMed] [Google Scholar]
- 133.Malcolm R, O'Neil PM, Sexauer JD. A controlled trial of naltrexone in obese humans. International Journal of Obesity. 1985;9(5):347–353. [PubMed] [Google Scholar]
- 134.Spiegel TA, Stunkard AJ, Shrager EE. Effect of Naltrexone on food intake, hunger, and satiety in obese men. Physiology and Behavior. 1987;40(2):135–141. doi: 10.1016/0031-9384(87)90198-3. [DOI] [PubMed] [Google Scholar]
- 135.Cone RD. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8(5):571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 136.Grossman HC, Hadjimarkou MM, Silva RM, et al. Interrelationships between mu opiod and melanocortin receptors in mediating food intake in rats. Brain Research. 2003;991:240–244. doi: 10.1016/s0006-8993(03)03442-5. [DOI] [PubMed] [Google Scholar]
- 137.Kusher R. Anti-obesity drugs. Expert Opinion on Pharmacotherapy. 2008;9:1339–1350. doi: 10.1517/14656566.9.8.1339. [DOI] [PubMed] [Google Scholar]
- 138.Mcelroy SL, Guerdjikova AI, Rosen A, Kim DD, Landbloom R, Dunayevich E. An open-label study evaluating the naltrexone SR/bupropion SR combination therapy in overweight or obese subjects with major depression. American Diabetes Association 70th Scientific Meeting, Abstract 1851-P, 2010, http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=80768.
- 139.Gupta AK, Hollander P, Bays H, et al. mproved glycemic control and weight loss with naltrexone SR/bupropion SR combination therapy in overweight/obese subjects with Type 2 Diabetes. American Diabetes Association 70th Scientific Meeting, Abstract 2633-PO, http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=81463.
- 140.Hollander P, Plodkowski R, Gupta AK. COR-Diabetes: naltrexone SR/Bupropion SR combination therapy led to significant and sustained weight loss and improved HbA1c in overweight/obese subjects with Type 2 Diabetes. American Diabetes Association 70th Scientific Meeting, Abstract 56-OR, http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=79004.
- 141.Smith SR, Church T, Geohas J. Naltrexone SR/Bupropion SR Combination Therapy Reduces Total and Visceral Adiposity in Overweight and Obese Subjects in the COR-I Study. American Diabetes Association 70th Scientific Meeting (2010). 70th Scientific Sessions, Abstract 1848-P, http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=80765.
- 142.Gadde KM, Yonish GM, Foust MS, Wagner HR., II Combination therapy of zonisamide and bupropion for weight reduction in obese women: a preliminary, randomized, open-label study. Journal of Clinical Psychiatry. 2007;68(8):1226–1229. doi: 10.4088/jcp.v68n0809. [DOI] [PubMed] [Google Scholar]
- 143.Greenway F, Anderson J, Atkinson R, et al. Bupropion and zonisamide for the treatment of obesity [abstract 52-OR] Obesity Research. 2006;14, A17 [Google Scholar]
- 144. Orexigen therapeutics, http://www.orexigen.com/candidates.
- 145.Gadde KM, Franciscy DM, Wagner HR, II, Krishnan KRR. Zonisamide for weight loss in obese adults: a randomized Controlled trial. JAMA. 2003;289(14):1820–1825. doi: 10.1001/jama.289.14.1820. [DOI] [PubMed] [Google Scholar]
- 146.Fujioka K, Lee MW. Pharmacologic treatment options for obesity: current and potential medications. Nutrition in Clinical Practice. 2007;22(1):50–54. doi: 10.1177/011542650702200150. [DOI] [PubMed] [Google Scholar]
- 147.Kaplan LM. Pharmacological therapies for obesity. Gastroenterology Clinics of North America. 2005;34(1):91–104. doi: 10.1016/j.gtc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 148.Pollack AFDA. Panel Votes Against Obesity Drug. The New York Times, 2010, www.nytimes.com/2010/07/16/health/16obese.html?_r=1&ref=food_and_drug_administration.
- 149.Ravussin E, Smith SR, Mitchell JA, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17(9):1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]