Abstract
The enzyme β-1,3-glucanase (βGlu) was found to be strongly induced by ultraviolet (UV-B; 280-320 nm) radiation in primary leaves of French bean (Phaseolus vulgaris). This was demonstrated on the level of gene transcription, protein synthesis, and enzyme activity and was due to the expression of bean class I βGlu (βGlu I). In contrast to other proteins of the family of pathogenesis-related proteins, the induction of βGlu I by UV correlated with the formation of photoreversible DNA damage, i.e. pyrimidine dimer formation. In conditions that allowed photorepair of this damage, βGlu I induction was blocked. Therefore, UV-induced DNA damage seems to constitute a primary signal in the pathway leading to the induction of the βGlu I gene(s). The induction was a local response because in partly irradiated leaves βGlu I was selectively found in leaf parts exposed to UV. Although short wavelength UV (λ < 295 nm) was most efficient in βGlu I induction, longer wavelength UV (λ > 295 nm) as present in natural radiation was still effective. In contrast to UV induction of βGlu I, the induction of flavonoids in bean leaves was optimally triggered by much more moderate fluences from the UV wavelength range no longer effective in βGlu I induction. UV induction of the flavonoid pathway shows no correlation with DNA damage and thus should be mediated via a different signal transduction pathway.
Plants require sunlight for photosynthesis and thus are constantly also exposed to potentially damaging UV radiation that is present in sunlight. UV-C (λ < 280 nm) is the band with the highest energy and most efficient in damaging DNA and proteins, but only wavelengths in the UV-B (280-320 nm) range greater than 290 nm reach the earth's surface; shorter wavelengths are completely absorbed by the stratospheric ozone layer. UV-C and UV-B cause DNA damage; this radiation induces the formation of pyrimidine dimers of which cyclobutane pyrimidine dimers (CPDs) constitute the major class (Taylor et al., 1997). Plants possess quite effective protection mechanisms against UV-induced DNA-damage. So, photolyase is an enzyme capable to repair CPDs after activation by violet light (photoreactivation; Strid et al., 1994; Taylor et al., 1997). Another important defense mechanism against UV is the production of UV-absorbing flavonoids and phenylpropanoid compounds in leaf epidermal cells in response to UV irradiation (Li et al., 1993; Beggs and Wellmann, 1994). Flavonoids are also induced by a range of other stimuli; including pathogen attack (Harborne and Williams, 2000).
Pathogenesis-related (PR) proteins are implicated in plant defense and accumulate in response to pathogen attack or to treatment with other elicitors (Leubner-Metzger and Meins, 1999). Endo-β-1,3-glucanases (βGlu; EC 3.2.1.39) are assigned to the PR proteins, where they constitute the PR-2 family. βGlu are abundant proteins, widely distributed in seed plant species. Besides plant defense, they have been implicated in several physiological and developmental processes (Doblin et al., 2001; Leubner-Metzger, 2003; Scherp et al., 2003) and are highly regulated in response to environmental factors (Leubner-Metzger and Meins, 1999). On the basis of amino acid sequence similarities, the several βGlu isoforms have been grouped into distinct classes (Simmons, 1994). Four classes are known for tobacco (Nicotiana tabacum; Leubner-Metzger and Meins, 1999), and the existence of similar classes has been postulated in the legumes soybean (Glycine max; Jin et al., 1999), alfalfa (Medicago sativa; Maher et al., 1993), and bean (Phaseolus vulgaris; Edington et al., 1991). Several PR proteins have been shown to be induced by shortwavelength UV (Brederode et al., 1991; Yalpani et al., 1994; Green and Fluhr, 1995). This induction by UV is often correlated with extensive leaf damage (Brederode et al., 1991; Yalpani et al., 1994), which itself might induce a pathogenesis-type response. For class I βGlu, it was not in all cases possible to show a UV induction. Jung et al. (1995) reported a small increase in βGlu level in response to UV for sunflower (Helianthus annuus), but for tobacco, only a marginal UV induction of βGlu (Brederode et al., 1991) or no induction at all (Thalmair et al., 1996) was found. So far, little is known about the signal pathways in UV-B mediated induction of defense genes. Brosché and Strid (2003) recently proposed a model for the molecular events following perception of UV-B by plants. According to this model, there are UV-B-specific pathways mediated by a UV-B-specific receptor as well as nonspecific pathways. Whereas low or medium UV fluences are required to induce genes via the specific pathways, high UV doses are needed for nonspecific induction of genes. PR genes have been grouped both as “medium level” genes (PR-5) or “high level” genes (like PR-1).
As a novel finding, we show in this study that there is a strong induction of class I βGlu by UV-B in bean leaves. The induction by UV-B represents a local response visible on the levels of activity, protein, and mRNA. Our results strongly suggest that DNA damage plays a key role in the signal pathway leading to induction of βGlu in response to UV-B, because photoreactivating light prevents the βGlu accumulation.
RESULTS AND DISCUSSION
In this study, we show that in contrast to other reports (Brederode et al., 1991; Jung et al., 1995; Thalmair et al., 1996), class I βGlu (βGlu I) is inducible by UV-B in primary leaves of French bean (cv Saxa) and that this induction correlates with the formation of CPDs in the DNA. The induction pattern was verified on the activity, protein, and mRNA level and compared with the UV acclimation process of flavonoid pigment formation.
UV Induces a βGlu I
To study the induction of βGlu by UV, whole plants were irradiated for 15 min with UV and then kept in red light for 8 to 48 h, before taking samples from the two primary leaves for analysis. One of the primary leaves was covered by a WG 360 filter to exclude UV-B during irradiation, whereas the other primary leaf was irradiated without filter to receive the full spectrum of the UV source (Fig. 1). The kinetics of βGlu induction was studied at the activity, the protein, and the mRNA level, and the results for UV irradiation were compared with the induction by wounding (Fig. 2, A-C). For mRNA analysis, a specific DNA probe was constructed, based on the bean βGlu I DNA sequence (Edington et al., 1991). In a northern-blot analysis on isolated RNA from UV-irradiated leaves, a single strong band was detected by this probe (Fig. 2B), whereas no band was visible in RNA preparations from the UV-free irradiated control leaves. This indicates a clear induction of βGlu I gene(s), either a single gene or few highly homologous genes encoding βGlu I isoforms, by UV. Accordingly, Edington et al. (1991) found only one βGlu I gene in bean cv Saxa after induction of a suspension cell culture with a fungal elicitor. Because no second band was detected by the cDNA probe, the further analyses of βGlu I mRNA induction were carried out using the “dot-blot” method. Immunoblot analysis was used to study the induction of βGlu on the protein level. The blots were stained with antibody specific for ethylene-inducible 33-kD class I βGlu of tobacco leaves (Fig. 2A). Class I βGlu from different organisms has similar apparent molecular masses. The apparent molecular mass of class I bean βGlu was mostly considered to be 36 kD (Vögeli et al., 1988; Mauch et al., 1992). Edington et al. (1991) determined the molecular mass from the cDNA sequence of bean βGlu to be 39 kD, which was higher than expected. According to the authors, it might therefore be a precursor sequence. Abeles et al. (1970) estimated the molecular mass of ethylene-induced βGlu from bean leaves after analytical centrifugation to be 34 kD. In our experiments, the major immunoreactive band in immunoblots probed with antitobacco class I βGlu antibody was 34 ± 0.8 kD. This is thus well in the range of molecular masses reported for bean class I βGlu. So far, only two minor βGlu from other classes (acidic isoforms) have been reported in bean leaves. These have a Mr of 28,000 and 30,000 and are therefore much smaller than the class I isoform. They were found after induction by mercuric chloride treatment and viral infection (Awade et al., 1989). On the amino acid level, tobacco class I βGlu shows 59% similarity to the published sequence of class I βGlu from bean leaves (Edington et al., 1991). The two proteins display sequence similarity throughout their peptide sequence, except at the C terminus in this region, which is cleaved to produce the mature tobacco βGlu. It has been shown that antibodies against bean βGlu I and tobacco βGlu I are both able to detect the class I βGlu in pea (Pisum sativum; Petruzelli et al., 1999; G. Leubner-Metzger, personal communication). The antibody used in this study was raised against an endo-type βGlu and, due to substrate specificities and also the activity assay performed in this study, only detects activity of endo-type βGlu, because reduced laminarin was used as substrate (Keefe et al., 1990). It has also been shown that in bean leaves the major stress-induced βGlu activities are represented by class I isozyme, which is an endo-type protein (Boller, 1983; Vögeli et al., 1988). Together, these findings strongly suggest that a bean βGlu I is detected by the tobacco βGlu I antibody in the extracts of bean leaves. The induction of the 34-kD immunoreactive band in our experiment is in agreement with the kinetics of βGlu activity and mRNA accumulation (Fig. 2, A-C). Thus the UV-induced βGlu activity found in bean primary leaves can be accounted for by the 34-kD antigen.
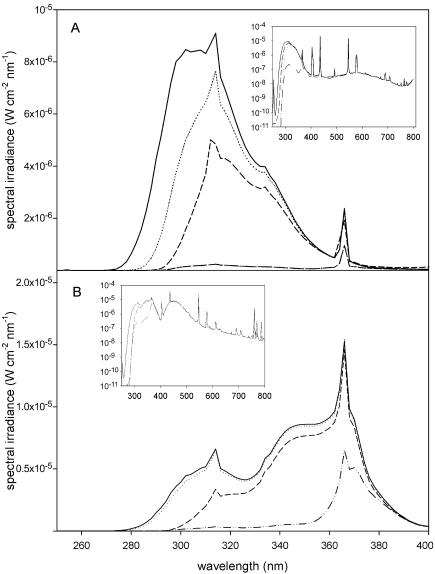
Figure 1.
A, Standard UV source used in studies for βGlu induction and UV/WL source used in experiments for flavonoid induction measured with a 250- to 800-nm double-monochromator spectroradiometer (model OL 754, Optronic, Orlando FL). A, Spectral irradiance of the standard UV source under transmission cut-off filters WG 360 (- - - -), WG 295 (· · · ·), WG 305 (—·—), and without filter (——). B, Spectral irradiance of the UV/WL source under transmission cut-off filters WG 360(—··—), WG 295 (· · · ·), WG 310 (- - - -), and under quartz (——). For better overview, the spectral irradiance in the UV range is given in linear scaling. Total spectral irradiance of the light sources (inset) is shown in logarithmic scale.
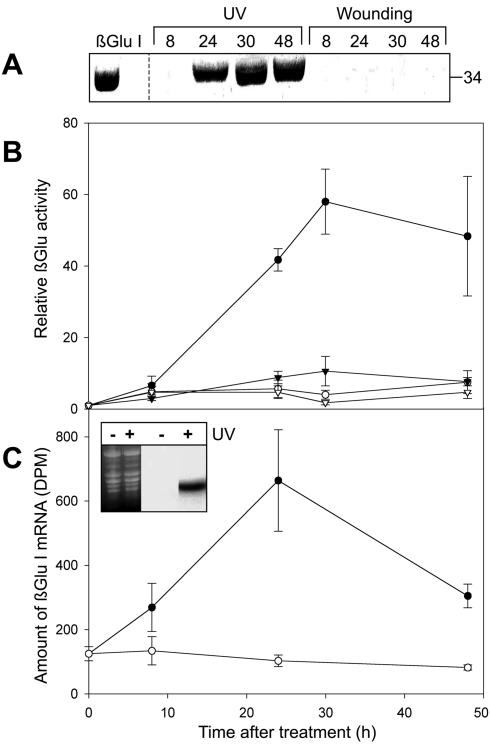
Figure 2.
Distinct effects of UV and wounding on βGlu I gene expression. Plants were irradiated for 15 min under the UV source; one of the primary leaves was covered with a WG 360 filter to exclude UV-B (control) while the opposite leaf was left uncovered. After UV irradiation, plants were kept in red light for the time indicated. For wounding response, one primary leaf was damaged using forcipes, while the opposite leaf was left untreated as a control. A, Time course of βGlu I protein accumulation. The position of βGlu I standard from tobacco is shown in the first lane. The apparent size in kilodaltons of the major immunoreactive band is indicated at the right. B, Time course of βGlu activity after UV treatment (•) or wounding (○) with controls (▾, UV cut-off filter WG 360; fl, untreated). C, Time course of βGlu I mRNA accumulation after UV treatment (•) with control kept under UV cut-off filter WG 360 (○). Inset, Northern blots (including loading control in first two lanes) 24 h after UV treatment. +UV, irradiation without filter; -UV, control irradiated under UV cut-off filter WG 360. Mean values and se from four independent experiments.
Class I βGlu was shown to be weakly induced upon wounding in tobacco leaves (Brederode et al., 1991; van den Rhee et al., 1993). In bean leaves, we found only a marginal induction of βGlu activity, hardly visible on the protein or mRNA level after wounding (Fig. 2). In contrast, after UV treatment, the activity was strongly enhanced and reached a maximum 30 h after irradiation (Fig. 2A). The accumulation of protein and mRNA showed similar patterns (Fig. 2, A-C) with mRNA accumulation preceding protein accumulation. Whereas protein and activity levels did not decline in the investigated time period, mRNA levels were found to be decreased after 48 h. Compared with the rapid induction of some enzymes such as e.g. chalcone synthase in response to UV-B (Loyall et al., 2000; Jenkins et al., 2001), the UV induction of βGlu seems to be rather slow. A much faster induction of βGlu was found in bean leaves treated with ethylene (Vögeli et al., 1988). Only 2 h after the ethylene treatment, both mRNA levels and activity were already strongly enhanced. As in our experiments, the activity stayed on a constant level after reaching a maximum, whereas the mRNA levels declined again quickly without further treatment.
βGlu I Induction in Response to UV-B Is a Local Response
In tobacco, βGlu I is induced locally as part of the hypersensitive response after infection with tobacco mosaic virus. In contrast, class II and III proteins are induced both locally and systemically in uninfected leaves of the same plant and are therefore markers for the systemic acquired resistance (Leubner-Metzger, 2003). Also in response to ozone, βGlu I is induced locally in the treated leaf (Ernst et al., 1996). Therefore, we investigated whether the UV induction of βGlu in bean leaves is a local response, too. For this purpose, part of the leaf was covered by a WG 360 filter during the irradiation, and samples were taken from both the covered and the uncovered part of the same leaf. Samples from uncovered parts of the leaves showed an activity of 0.53 ± 0.08 pkat μg-1 protein, whereas the activity measured in parts covered with WG 360 to exclude UV-B was as low as 0.08 ± 0.01 pkat μg-1 protein. This is a similar value as obtained for leaves completely covered with WG 360 (see Table I). Thus, it could be clearly shown that the UV induction of βGlu activity is a local response, because a significant βGlu activity could only be detected in uncovered parts of the leaf. Also the UV-induced accumulation of PR-1 in tobacco was a local response (Green and Fluhr, 1995); but in contrast to the flavonoid induction, it was not cell specific, and the protein could be detected in the whole cross-section of the leaf. Mauch et al. (1992) found after ethylene treatment of bean leaves a selective accumulation of βGlu in the lower epidermis and in parenchyma cells adjacent to vascular strands.
Table I.
Prevention of the UV response on ßGlu activity under conditions allowing photoreactivation
Spectral Effectiveness of βGlu I Induction
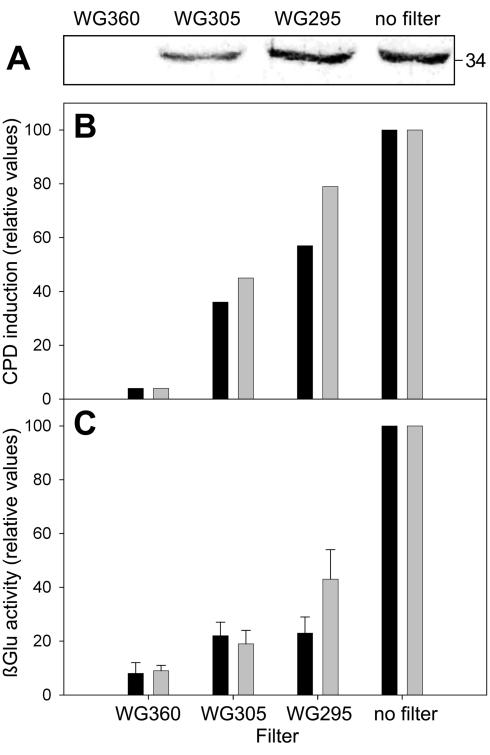
That the UV effects described in our experiments are not due exclusively to the wavelength part, which is no longer present in solar radiation, can be concluded from irradiations under UV transmission cutoff filters at wavelength ranges of λ > 295 nm (using a WG 295 filter) or λ > 305 nm (using a WG 305 filter). With both filters, the βGlu activity was still clearly enhanced compared with the activity found in leaves irradiated under WG 360 (Fig. 3C). The activity level under WG 295 reached about one-half of the level in leaves that were irradiated without filter when the irradiation time was prolonged to 30 min. The induction was also visible on the protein level after an irradiation time of 15 min (Fig. 3C). In parallel to the induction of βGlu, the accumulation of DNA damage in bean primary leaves was measured after the same irradiation times (Fig. 3B). Strikingly, the pattern of CPD formation and βGlu I induction under the different filters looked very much the same, raising the question of whether a correlation exists between CPD accumulation and UV induction of βGlu I.
Figure 3.
βGlu and CPD induction in response to varied UV spectral ranges. Plants were irradiated for 15 min (black bars) or 30 min (gray bars) under the UV source, using transmission cut-off filters and then kept in red light for 24 h for measurement of βGlu induction. For analysis of CPDs, samples were taken from leaves in dim yellow light immediately after irradiation. A, Accumulation of βGlu I protein after 30 min of UV irradiation. The apparent size of the major 34-kD immunoreactive band is indicated at the right. B, Induction of CPDs. Representative experiment that was repeated twice with similar results. C, Induction of βGlu activity. Mean values and se of two independent experiments (n = 8).
βGlu I Induction by UV Is Inhibited under Photoreactivating Light
To assess the question of whether DNA damage could be part of the signal transduction pathway leading to βGlu I induction by UV, plants were kept in white light (WL) instead of red light after UV treatment. Due to the wavelengths in the violet range present in WL, the photoreactivating enzyme photolyase is active in WL, and UV-induced CPDs should therefore be quickly repaired (Beggs and Wellmann, 1985). WL should thus prevent βGlu I accumulation, if CPDs contribute to the signaling for βGlu I induction. It could be clearly shown, that βGlu was not induced in plants kept in WL, whereas plants kept in red light after UV irradiation showed high βGlu activities 24 h after UV irradiation (Table I). In plants kept in WL, even 48 h after UV treatment, no significant increase in βGlu activity could be measured compared with control leaves irradiated under WG 360. Again, this finding is corroborated by the protein expression pattern under the same conditions (Fig. 4). No immunoreactive band could be detected in analysis of protein extracts of plants kept in WL after UV irradiation. A reduction both in βGlu I accumulation and in pyrimidine dimer formation of about 20%, to be explained as result of photoreactivation, could also be observed after keeping the plants 1 h under UV-A after UV-B irradiation (results not shown). This strongly suggests that UV-induced DNA damage is involved in signaling leading to the UV induction of βGlu I. That DNA damage can be a signal leading to the expression of several genes has mostly been demonstrated in animal systems (Kripke et al., 1992; Geyer et al., 2000; Jenkins et al., 2001). One example in plants where DNA might be involved in signaling is the accumulation of isoflavonoids like coumestrol in response to damaging UV in bean leaves (Beggs et al., 1985). As we found it for βGlu induction, this effect was also inhibited in the presence of photoreactivating light, and it was concluded that coumestrol formation was mediated via UV-induced pyrimidine dimer formation in the DNA. This kind of UV elicitor response should be clearly separated from UV-B photoreceptor responses on flavonoid pathway as described below. The UV-B photoreceptor response was found to be inhibited under UV irradiation conditions optimal for the UV elicitor response, and this inhibition could be reversed under photoreactivating conditions (Buchholz et al., 1995). In both cases, de novo synthesis of the same enzymes is affected, but there is good evidence that both responses are regulated independently (Hahlbrock and Scheel, 1989; Beggs and Wellmann, 1994).
Figure 4.
βGlu I protein accumulation under conditions allowing photoreactivation. Plants were irradiated for 15 min under UV; for control, one leaf was covered with UV transmission cut-off filter WG 360 to exclude UV-B. After irradiation plants were kept for 24 h either in red light (R) excluding the photoreactivating wavelength range or in WL including violet for photoreactivation. The apparent size in kilodaltons of 34-kD antigen is indicated.
In contrast to βGlu I, the UV induction of another PR protein, PR-1 in tobacco, seems to depend on the UV-induced accumulation of reactive oxygen species (ROS), whereas the presence of photoreactivating light did not decrease the observed response to UV (Green and Fluhr, 1995). ROS have been shown to play an important role in the induction of several PR proteins (Chen et al., 1993; Mackerness et al., 1999, 2001). We cannot exclude a role of ROS for the UV induction of βGlu in bean leaves, but the fact that photoreactivating light prevented the βGlu induction points to a key role of DNA damage.
Flavonoid Synthesis Is Induced by Moderate UV and Inhibited under Strong UV Irradiation
Our results suggest that medium or high UV fluences leading to DNA damage are needed for βGlu I formation. This pathway should be different from the pathway leading to flavonoid synthesis, the latter being mediated by moderate non-damaging fluences of UV-B. Flavonoid induction is a well-known specific UV response mediated by the postulated UV-B photoreceptor (Wellmann, 1983; Brosché and Strid, 2003). In mustard (Sinapis alba) cotyledons, it has been shown that strong UV irradiation inducing DNA damage leads to inhibition of flavonoid synthesis (Buchholz et al., 1995). Therefore, different irradiation sources were used for optimal induction of βGlu I and CPDs or flavonoids, respectively. The UV-B source containing high parts of shortwavelength UV-B and low parts of photoreactivating blue/violet light resulted in optimal effects on βGlu I and CPD formation. The UV/WL source containing higher spectral parts allowing photoreactivation, led to optimal flavonoid induction if a transmittance cutoff filter at 310 nm (WG 310) was used. The amount of CPDs formed in bean leaves in the UV/WL source under WG 310 was near the limits of detection. Compared with CPD amounts formed under quartz, only about 5% of the CPD content was detected in samples irradiated under WG 310. Therefore, irradiation under the combined UV/WL source using the cut-off filter WG 310 was considered to be non-damaging. A slight shift to shorter wavelengths, using a 305-nm cut-off filter (WG 305) resulted in reduced flavonoid formation (E. Wellmann, unpublished data) and correlated with CPD formation.
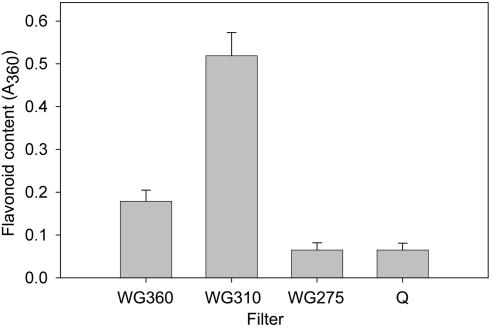
When short-wavelength UV was excluded using a WG 360 filter, only a small amount of flavonoids was present in the leaf material (Fig. 5). Upon irradiation including long-wavelength UV-B under the combined UV/WL source (WG 310; see Fig. 1B), a strong enhancement of flavonoid amounts was observed. In contrast, samples irradiated under WG 275 and quartz transparent for short-wavelength UV showed a clear inhibition of flavonoid synthesis. Thus wavelengths optimally effective in βGlu induction inhibit flavonoid induction in bean primary leaves, which corroborates the hypothesis that both effects are mediated via different pathways. The lack of correlation between flavonoid induction and DNA damage has been reported previously (Frohnmeyer et al., 1999; Kalbin et al., 2001). Moreover, photoreactivating blue light is known to stimulate UV-B photoreceptor effects on flavonoid biosynthesis (Duell-Pfaff and Wellmann, 1982; Ohl et al., 1989; Fuglevand et al., 1996).
Figure 5.
UV-induced flavonoid formation. Leaf discs were irradiated for 42 h under a combined UV/WL source in boxes covered with different UV transmission cut-off filters (WG 360, WG 310, WG 275) or quartz. Flavonoid content was quantified after PC separation by measuring the A360 as described in “Materials and Methods.”Mean values and se from nine independent experiments.
In conclusion, we showed here that UV-B is a strong inducer of βGlu I in bean leaves, visible on the levels of enzyme activity, protein, and mRNA. Most interestingly, inactivation of the DNA seems to be involved in the signal chain leading to βGlu induction following UV treatment. DNA damage in signaling has so far only been shown for isoflavonoid formation (Beggs et al., 1985) but not for proteins of the PR family. Because both isoflavonoids and βGlu have no obvious role in protecting the plant against UV-induced damage and because unnaturally high levels of UV are necessary for optimal induction, their accumulation can probably be considered as a nonspecific UV response. In contrast, the other UV response investigated here, i.e. the induction of flavonoids, is considered to be a specific acclimation response, supporting the hypothesis of the existence of different UV-signaling pathways in plants.
MATERIALS AND METHODS
Plant Material and Light Treatments
Seeds of French bean (Phaseolus vulgaris L. cv Saxa) were obtained from Samen-Vath (Freiburg, Germany). Plants were grown UV-free under PVC foil (50% transmission cut-off at 380 nm) for 10 d in a growth chamber in 12-h light/dark cycles at 25°C. For induction of βGlu or CPDs, plants with fully expanded pairs of primary leaves were irradiated for 15 or 30 min under the UV source. The spectrum of the UV source is given in Figure 1A. One leaf was covered with a 3-mm WG 360 transmission cut-off filter (Schott, Mainz, Germany; 50% transmission at the given wavelength) as UV-B-free control. The other leaf was left uncovered, except for the UV-C exclusion experiments, where it was covered with a 3-mm WG 295 or WG 305 filter. After UV treatment, plants were kept either in red light (6.5 W m-2) to allow photosynthesis but to exclude photoreactivation or in WL (335 nm cut-off; 20 W m-2) for photoreactivation. For wounding, parts of one primary leaf were squeezed using forcipes; the other leaf was left unwounded as control.
For flavonoid induction, leaf discs of 1 cm in diameter were cut before irradiation. For experimental reasons, leaf discs were used after verifying that UV-effects on flavonoid formation were similar in isolated leaf discs and intact leaves. The discs were put on wet filter paper in a plastic box covered with either 6-mm WG 305 (resulting in 50% transmission at 310 nm and referred to as WG 310) or 3-mm WG 360, WG 275, or quartz. For flavonoid induction a combined UV-WL source (13.6 W m2) was used for irradiation (Buchholz et al., 1995; Fig. 1B). Accumulation kinetics of flavonoids showed a maximum between 36 and 48 h. Therefore, flavonoids were measured after an irradiation time of 42 h.
Protein Extraction and βGlu Activity Assay
For protein extraction, two leaf discs (diameter 1 cm) were homogenized in 200 μL of H-buffer (200 mm Tris-HCl, pH 8.0, 0.25 mm EDTA, 5 mm dithiothreitol, and 0.5% [v/v] 1-Bu-OH) using a rotary bolt. This extract was centrifuged twice for 15 min at 12,500 rpm (centrifuge 2K15, Sigma-Aldrich, St. Louis). The protein content was measured using the Bio-Rad (Bradford) protein assay. For measurement, the protein solution was diluted 1:10. The procedure of the βGlu activity assay was described by Keefe et al. (1990).
Immunoblot, Northern-Blot, and Dot-Blot Analyses
Immunoblot analyses were performed as described previously (Leubner-Metzger et al., 1995) using rabbit antibodies directed against the class I βGlu from tobacco leaves. For RNA northern- and dot-blot analyses, a probe was constructed using primers for bean βGlu (http://www.ncbi.nlm.nih.gov/entrez/; accession no. X897171). The primers had the following sequences: 5′ primer, ATG AAT TCA TGA TGG GCA ACA ATC TCC C; 3′ primer, ATC TCG AGG TCA CTC TTA AGG GGA TAT G (synthesis, MWG-Biotech AG, Ebersberg, Germany). The βGlu gene from bean leaves was amplified with the help of these primers using a PCR-based method and then cloned in Escherichia coli (XL1-blue) cells. The purified βGlu was used as the probe in the northern- and dot-blot analyses.
RNA was isolated from bean leaves using the RNeasy Plant Mini kit (Qiagen USA, Valencia, CA). For northern-blot analyses, 20 μg of RNA was denatured and electrophoresed on a 1.2% (w/v) agarose gel. After electrophoresis, RNA was transferred by capillary blotting onto filter (Hybond-N, Amersham Biosciences, Uppsala). For RNA dot blots, 6 μg of RNA was applied directly to Hybond-N filter. All filters were then vacuum baked for 2 h at 80°C. Filters were prehybridized in a mixture containing 50 mm NaPi, pH 6.5, 5× SSC (20× SSC is sodium citrate, pH 7.0, and 3 m NaCl), 5× Denhard's reagent (100× Denhard's is 2% [w/v] bovine serum albumin, 2% [w/v] polyvinylpyrollidone, and 2% [w/v] ficoll 400), 0.1 mg mL-1 denatured salmon sperm DNA, and 50% (w/v) deionized formamide at 42°C for 3 h. The hybridization procedure was the same as for prehybridization, except that the mixture additionally contained the βGlu probe, labeled with 32P (50 μCi) after the random priming method of Feinberg and Vogelstein (1983). Incubation was overnight. The filters were washed at 62°C in 2× SSC containing 0.2% (w/v) SDS for 10 min, then in 1× SSC, 0.2% (w/v) SDS for 10 min, then twice 0.5× SSC, 0.2% (w/v) SDS for 10 min followed by 0.1× SSC, 0.2% (w/v) SDS for 10 min. After drying, the filters were exposed at -80°C to x-ray film (X-Omat, Eastman Kodak, Rochester, NY). For the detection of weak signals, an amplifying screen (Biomax Trans-screen-HE Intensifying, Eastman Kodak) was used in combination with a sensitive x-ray film (Biomax, Eastman Kodak). For quantification of dots in the dot-blot analysis, dots were cut from the nitrocellulose, and filter-bound radioactivity was determined by liquid scintillation counting.
DNA Extraction and Determination of CPDs
Leaf discs were frozen immediately after UV irradiation in liquid nitrogen. The DNA was extracted according to the method of Doyle and Doyle (1990) using hexadecyltrimethylammonium bromide. The CPDs were quantified by ELISA according to Mori et al. (1991) with monoclonal antibody. This antibody has been shown to recognize CPDs specifically in the decreasing order of affinity to TT-, CT-, CC-, and TC-dimers (Mori et al., 1991).
Flavonoid Extraction and Quantification
Flavonoids from four leaf discs (diameter 1 cm) were extracted in 200 μL of extraction medium (79% [v/v] ethanol and 1% [v/v] acetic acid) at 85°C for 30 min. For separation of flavonoids, a downstream paper chromatography in 15% (v/v) acetic acid was performed according to Markham (1982). Bands corresponding to flavonoids were identified by their purple color under UV-A and were cut. Elution was performed in 1 mL of 50% (v/v) ethanol at 85°C for 30 min. An absorbance spectrum of this solution from 250 to 450 nm was measured, and quantification was done at 360 nm.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.029520.
This work was supported by the European Community (project no. ICA-CT-2000-30025).
References
- Abeles FB, Bosshart RP, Forrence LE, Habig WH (1970) Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol 47: 129-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awade A, de Tapia M, Didierjean L, Burkard G (1989) Biological function of bean pathogenesis-related (PR3 and PR4) proteins. Plant Sci 63: 121-130 [Google Scholar]
- Beggs CJ, Stolzer-Jehle A, Wellmann E (1985) Isoflavonoid formation as an indicator of UV-stress in bean (Phaseolus vulgaris L.) leaves: the significance of photorepair in assessing potential damage by increased solar UV-B radiation. Plant Physiol 79: 630-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs CJ, Wellmann E (1985) Analysis of light-controlled anthocyanin formation in coleoptiles of Zea mays L.: the role of UV-B, red and far-red light. Photochem Photobiol 41: 481-486 [Google Scholar]
- Beggs CJ, Wellmann E (1994) Photocontrol of flavonoid biosynthesis. In RE Kendrick, GHM Kronenberg, eds Photomorphogenesis in Plants, Ed 2. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 733-751
- Boller T (1983) Chitinase in bean leaves: induction by ethylene, purification, properties and possible function. Planta 157: 22-31 [DOI] [PubMed] [Google Scholar]
- Brederode FT, Linthorst HJM, Bol JF (1991) Differential induction of acquired resistance and PR gene expression. Plant Mol Biol 17: 1117-1125 [DOI] [PubMed] [Google Scholar]
- Brosché M, Strid Å (2003) Molecular events following perception of ultraviolet-B radiation by plants. Physiol Plant 117: 1-10 [Google Scholar]
- Buchholz G, Ehmann B, Wellmann E (1995) Ultraviolet light inhibition of phytochrome-induced flavonoid biosynthesis and DNA photolyase formation in mustard cotyledons (Sinapis alba L.). Plant Physiol 108: 227-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Silva H, Klessig DF (1993) Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science 262: 1883-1885 [DOI] [PubMed] [Google Scholar]
- Doblin MS, DeMelis L, Newbigin E, Basic A, Read SM (2001) Pollen tubes of Nicotiana alata express two genes from different β-glucan synthase families. Plant Physiol 125: 2040-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JF, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13-15 [Google Scholar]
- Duell-Pfaff N, Wellmann E (1982) Involvement of phytochrome and a blue light photoreceptor in UV-B induced flavonoid synthesis in parsley (Petroselinum hortense Hoffm.) cell suspension cultures. Planta 136: 213-217 [DOI] [PubMed] [Google Scholar]
- Edington BV, Lamb CJ, Dixon RA (1991) cDNA cloning and characterization of a putative 1,3-β-d-glucanase transcript induced by fungal elicitor in bean cell suspension cultures. Plant Mol Biol 16: 81-94 [DOI] [PubMed] [Google Scholar]
- Ernst D, Bodemann A, Schmelzer E, Langenbartels C, Sandermann H Jr (1996) Beta-1,3-glucanase mRNA is locally, but not systemically induced in Nicotiana tabacum L. cv. BEL W3 after ozone fumigation. J Plant Physiol 148: 215-221 [Google Scholar]
- Feinberg AP, Vogelstein B (1983) A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6-13 [DOI] [PubMed] [Google Scholar]
- Frohnmeyer H, Loyall L, Blatt MR, Grabov A (1999) Millisecond UV-B irradiation evokes prolonged elevation of cytosolic-free Ca2+ and stimulates gene expression in transgenic parsley cell cultures. Plant J 20: 109-117 [DOI] [PubMed] [Google Scholar]
- Fuglevand G, Jackson JA, Jenkins G (1996) UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8: 2347-2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer RK, Nagasawa H, Little JB, Maki CG (2000) Role and regulation of p53 during an ultraviolet radiation-induced G1 cell cycle arrest. Cell Growth Differ 11: 149-156 [PubMed] [Google Scholar]
- Green R, Fluhr R (1995) UV-B-induced PR-1accumulation is mediated by active oxygen species. Plant Cell 7: 203-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol 40: 347-369 [Google Scholar]
- Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55: 481-504 [DOI] [PubMed] [Google Scholar]
- Jenkins GI, Long JC, Wade HK, Shenton MR, Bibikova TN (2001) UV and blue light signaling: pathways regulating chalcone synthase gene expression in Arabidopsis. New Phytol 151: 121-131 [DOI] [PubMed] [Google Scholar]
- Jin W, Horner HT, Palmer RG, Shoemaker RC (1999) Analysis and mapping of gene families encoding β-1,3-glucanases of soybean. Genetics 153: 445-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-L, Maurel S, Fritig B, Hahne G (1995) Different pathogenesis-related proteins are expressed in sunflower (Helianthus annuus L.) in response to physical, chemical and stress factors. J Plant Physiol 145: 153-160 [Google Scholar]
- Kalbin G, Hidema J, Brosché M, Kumagai T, Bornman JF, Strid Å (2001) UV-B-induced DNA damage and expression of defence genes under UV-B stress: tissue-specific molecular marker analysis in leaves. Plant Cell Environ 24: 983-990 [Google Scholar]
- Keefe D, Hinz U, Meins F Jr (1990) The effect of ethylene on the cell-typespecific and intracellular localization of beta-1,3-glucanase and chitinase in tobacco leaves. Planta 182: 43-51 [DOI] [PubMed] [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB (1992) Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci USA 89: 7516-7520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G (2003) Functions and regulation of β-1,3-glucanases during seed germination, dormancy release and after-ripening. Seed Sci Res 13: 17-34 [Google Scholar]
- Leubner-Metzger G, Frundt C, Vögeli-Lange R, Meins F Jr (1995) Class I β-1,3-glucanases in the endosperm of tobacco during germination. Plant Physiol 109: 751-759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G, Meins F Jr (1999) Functions and regulation of plant β-1,3-glucanases (PR-2). In SK Datta, S Muthukrishnan, eds, Pathogenesis-Related Proteins in Plants. CRC Press, Boca Raton, FL, pp 49-76
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5: 171-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyall L, Uchida K, Braun S, Furuya M, Frohnmeyer H (2000) Glutathione and a UV light-induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell 12: 1939-1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerness SAH, John CF, Jordan BR, Thomas B (2001) Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett 489: 237-242 [DOI] [PubMed] [Google Scholar]
- Mackerness SAH, Surplus SL, Blake P, John CF, Buchanan-Wollaston V, Jordan BR, Thomas B (1999) Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signaling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ 22: 1413-1423 [Google Scholar]
- Maher EA, Lamb CJ, Dixon RA (1993) Stress responses in alfalfa (Medicago sativa L.): XVII. Identification of multiple hydrolases and molecular characterization of an acidic glucanase. Physiol Mol Plant Pathol 43: 329-342 [Google Scholar]
- Markham KR (1982) Techniques of Flavonoid Identification. Academic Press, London, New York
- Mauch F, Meehl JB, Staehlin LA (1992) Ethylene-induced chitinase and β-1,3-glucanase accumulate specifically in the lower epidermis and along vascular strands of bean leaves. Planta 186: 367-375 [DOI] [PubMed] [Google Scholar]
- Mori T, Nakane M, Hattori I, Matsunaga T, Ihara M, Nikaido O (1991) Simultaneous establishment of monoclonal antibodies specific for either cyclobutane pyrimidine dimer or (6-4) photoproduct from the same mouse immunized with ultraviolet-irradiated DNA. Photochem Photobiol 54: 225-232 [DOI] [PubMed] [Google Scholar]
- Ohl S, Hahlbrock K, Schaefer E (1985) A stable blue-light-derived signal modulates UV-light-induced activation of the chalcone synthase gene in cultured parsley cells. Planta 95: 228-236 [DOI] [PubMed] [Google Scholar]
- Petruzelli L, Kunz C, Waldvogel R, Meins F Jr, Leubner-Metzger G (1999) Distinct ethylene- and tissue-specific regulation of β-1,3-glucanases and chitinases during pea seed germination. Planta 209: 195-201 [DOI] [PubMed] [Google Scholar]
- Scherp P, Grotha R, Kutschera U (2003) Occurrence and phylogenetic significance of cytokinesis-related callose in green algae, bryophytes, ferns and seed plants. Plant Cell Rep 20: 143-149 [DOI] [PubMed] [Google Scholar]
- Simmons CR (1994) The physiology and molecular biology of plant 1,3-β-d-glucanases and 1,3; 1,4-β-d-glucanases. Crit Rev Plant Sci 13: 325-387 [Google Scholar]
- Strid Å, Chow WS, Anderson JM (1994) UV-B damage and protection at the molecular level in plants. Photosynth Res 39: 475-489 [DOI] [PubMed] [Google Scholar]
- Taylor RM, Tobin AK, Bray CM (1997) DNA damage and repair in plants. In PJ Lumsden, ed, Plants and UV-B. Cambridge University Press, Cambridge, UK, pp 53-75
- Thalmair M, Bauw G, Thiel S, Döhring T, Langebartels C, Sandermann H Jr (1996) Ozone and ultraviolet B effects on the defense-related proteins β-1,3-glucanase and chitinase in tobacco. J Plant Physiol 148: 222-228 [Google Scholar]
- van den Rhee MD, Lemmers R, Bol JF (1993) Analysis of regulatory elements involved in stress-induced and organ-specific expression of tobacco acidic and basic beta-1, 3-glucanase genes. Plant Mol Biol 21: 451-461 [DOI] [PubMed] [Google Scholar]
- Vögeli U, Meins F Jr, Boller T (1988) Co-ordinated regulation of chitinase and β-1,3-glucanase in bean leaves. Planta 174: 364-372 [DOI] [PubMed] [Google Scholar]
- Wellmann E (1983) UV radiation in photomorphogenesis. In W Shropshire Jr, H Mohr, eds, Photomorphogenesis, Encyclopedia of Plant Physiology, New Series, Vol 16 B. Springer-Verlag, Berlin, pp 745-756 [Google Scholar]
- Yalpani N, Enyedi AJ, Léon J, Raskin I (1994) Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193: 372-376 [Google Scholar]