Abstract
The aim of this study was to investigate whether endogenous restrictions in oxygen supply are limiting for storage metabolism in developing oilseed rape (Brassica napus) seeds. Siliques were studied 30 d after flowering, when rapid lipid accumulation is occurring in the seeds. (a) By using microsensors, oxygen concentrations were measured within seeds and in the silique space between seeds. At ambient external oxygen (21% [v/v]) in the light, oxygen fell to 17% (v/v) between and 0.8% (v/v) within seeds. A step-wise reduction of the external oxygen concentration led within 2 h to a further decrease of internal oxygen concentrations, and a step-wise increase of the external oxygen concentration up to 60% (v/v) resulted in an increase in internal oxygen that rose to 30% (v/v) between and 8% (v/v) within seeds. (b) The increase in oxygen levels in the seeds was accompanied by a progressive increase in the levels of ATP, UTP, and the ATP to ADP and UTP to UDP ratios over the entire range from 0% to 60% (v/v) external oxygen. (c) To investigate metabolic fluxes in planta, 14C-sucrose was injected into seeds, which remained otherwise intact within their siliques. The increase in oxygen in the seeds was accompanied by a progressive increase in the rate of lipid (including triacylglycerol), protein and cell wall synthesis, and an increase in glycolytic flux over a range from sub- to superambient oxygen concentrations. In contrast to lipid synthesis, starch synthesis was not significantly increased at superambient oxygen levels. The levels of fermentation products such as lactate and glycerol-3P increased only at very low (0%-4% [v/v]) external oxygen concentrations. (d) When 14C-acetate or 14C-acetyl-coenzyme A (CoA) was injected into seeds, label incorporation into triacylglycerol progressively increased over the whole range of external oxygen concentrations from 0% to 60% (v/v). (e) Stimulation of lipid synthesis was accompanied by an increase in sugar levels and a decrease in the levels of hexose-phosphates and acetyl-CoA, indicating sucrose unloading and the use of acetyl-CoA as possible regulatory sites. (f) Increased lipid synthesis was also accompanied by an increase in the maximal activities of invertase and diacylglycerol acyltransferase. (g) The developmental shift from starch to lipid storage between 15 and 45 d after flowering was accompanied by an increase in the seed energy state. (h) The results show that at ambient oxygen levels, the oxygen supply is strongly limiting for energy metabolism and biosynthetic fluxes in growing rape seeds, affecting lipid synthesis more strongly than starch synthesis. The underlying mechanisms and implications for strategies to increase yield and storage product composition in oilseed crops are discussed.
Plants synthesize reduced carbon compounds from inorganic matter during photosynthesis in leaves, which are then exported to reproductive organs to be used for storage and growth. In developing seeds, Suc is converted to oil, protein, and starch, which are laid down as storage reserves. The percentage of reduced carbon stored as oil (triacylglycerol [TAG]) in seeds varies between species from 1% to 60% of total dry weight (Ohlrogge and Browse, 1995). Although the understanding of the pathways involved in Suc to TAG conversion in oil-storing seeds has improved in recent years, little is known about how the flux of carbon through these pathways is controlled or how the partitioning of carbon between oil and other storage products is determined (Rawsthorne, 2002).
The metabolism of Suc to storage lipids in developing oilseed rape (Brassica napus) involves the breakdown of the incoming Suc to glycolytic intermediates (hexose-P, phosphoenolpyruvate, pyruvate, and malate), which are subsequently imported into the plastid by specific transport proteins and used to synthesize acetyl-CoA via the pyruvate dehydrogenase complex (Rawsthorne, 2002). This is followed by acetyl-CoA carboxylase (ACCase) catalyzing the ATP-dependent conversion of acetyl-CoA to malonyl-CoA, from which two-carbon units are added to the growing acyl chain by fatty acid synthetase (White et al., 2000). Acyl-CoA is then exported to the cytosol and used for the step-wise acylation of the glycerol backbone to synthesize TAG at the endoplasmatic reticulum (Kennedy, 1961; Stymne and Stobart, 1987). In the first two steps of TAG assembly, glycerol-3P is acylated by glycerol-3P acyltransferase (G3PAT) to form lyso-phosphatidic acid, which is then acylated further by lysophosphatidate acyltransferase to produce phosphatidic acid. This is followed by dephosphorylation of phosphatidic acid by phosphatidic acid phosphohydrolase to release diacylglycerol (DAG) and the final acylation of DAG by DAG acyltransferase (DAGAT).
Most of the information on the regulation of lipid biosynthesis derives from studies performed in leaves. Here, it could be shown that changes in intermediates of fatty acid synthesis during light-dark transitions are consistent with control being exerted at the level of ACCase (Post-Beittenmiller et al., 1991, 1992a). Recent studies provide evidence that this is due to posttranslational redox modification of ACCase, leading to activation of the enzyme under the more reducing conditions found within the chloroplast in the light (Sasaki et al., 1997; Kozaki and Sasaki, 1999). Under these conditions, fatty acid synthesis will be additionally supported by the increased supply of reducing power and ATP from the photosynthetic light reactions, which serve as cofactors in the reactions of ACCase and fatty acid synthase. Other reports demonstrate feedback inhibition of ACCase in response to fatty acid supply (Shintani and Ohlrogge, 1995), providing a mechanism to adapt the rate of fatty acid synthesis to changes in the demand for fatty acids by lipid biosynthesis. Although these studies document a regulatory role of ACCase in photosynthetic leaves, less is known about the importance of this enzyme in regulating flux to lipid storage in heterotrophic seeds.
In heterotrophic seeds, ATP is produced by respiration and imported into plastids via an ATP/ADP exchanger or produced by glycolysis directly within the plastid (Rawsthorne, 2002). Previous studies with transgenic potato (Solanum tuberosum) plants document that ATP supply via the plastidial adenylate transporter is colimiting for starch biosynthesis in heterotrophic tubers (Tjaden et al., 1998; Geigenberger et al., 2001). It is not known if the ATP supply is limiting for storage lipid synthesis in seeds. It is also unclear whether starch and lipid biosynthesis, which will compete for the imported ATP in oilseeds like rape or Arabidopsis, are differentially affected by changes in ATP concentrations in the plastid. Oil-seeds like rape or Arabidopsis are green and photosynthetically active. However, it has been shown that photosynthetic activity within these seeds is too low to contribute substantially the supply of energy and reduced substrates for storage lipid biosynthesis in vivo (Eastmond et al., 1996; Eastmond and Rawsthorne, 1998). Furthermore, analyses of the overall levels of adenine nucleotide pools indicate relatively low adenylate energy states in developing seeds of Arabidopsis (Gibon et al., 2002).
Previous reports document an unexpected inhibition of seed production when plants are grown at low external oxygen, with a critical oxygen pressure of around 5% (v/v) external oxygen (compared with 21% [v/v] in ambient conditions; Quebedeaux and Hardy, 1975, 1976; Sinclair et al., 1987). More detailed analyses using series of subambient oxygen tensions revealed a linear decrease in Arabidopsis seed size when external oxygen was decreased below 15% (v/v; Porterfield et al., 1999), and a progressive arrest in Arabidopsis embryo development and inhibition of protein body formation when atmospheric oxygen was decreased below 16% (Kuang et al., 1998). Short-term experiments document a progressive decrease in the energy state in Arabidopsis seeds after exposing siliques to oxygen concentrations below 12% (v/v) for 2 h in the dark, which was accompanied by a decrease in biosynthetic fluxes to lipids and other storage components (Gibon et al., 2002). Measurements of ambient oxygen tensions in the airspace of siliques of rape and Arabidopsis using glass electrodes (Porterfield et al., 1999) document low oxygen concentrations in the silique airspace of 6% (v/v) in Arabidopsis and 12% (v/v) in rape under dark conditions, which rose in the light to only 12% and 16% (v/v), respectively. These studies suggest that internal oxygen concentrations might be relatively low within the seeds and could be limiting for oil biosynthesis. However, the actual oxygen concentrations within these seeds were not determined, and direct evidence is lacking as to whether the prevailing seed oxygen concentrations limit oil metabolism or not.
The following study investigates whether internal oxygen concentration are limiting for storage metabolism in oilseed rape. Rape seeds were chosen; since being larger, they are more accessible than Arabidopsis seeds and are an economically important oil crop. (a) Optical microsensors were inserted into siliques and seeds to analyze in situ oxygen concentrations in the silique airspace and inside the seeds. This was performed at ambient external oxygen (21% [v/v]), after decreasing external oxygen to 12% and 0% (v/v) and after increasing external oxygen to 30% and 60% (v/v). (b) To analyze the influence of changes in the oxygen concentration on cellular energy state, the levels of ATP, ADP, UTP, and UDP were analyzed in seeds in parallel. (c) To analyze the influence of changes in oxygen on metabolic fluxes to TAG, starch, structural elements, and glycolysis, 14C-Suc was injected into seeds and the metabolism of radiolabel analyzed after 2 h. In addition to this, more immediate precursors of fatty acid biosynthesis (14C-acetate and 14C-acetyl-CoA) were injected, and label incorporation into TAG was investigated. (d) Metabolite levels and enzyme activities were measured in the seeds to elucidate possible regulatory sites and mechanisms. The results show that ambient oxygen concentrations are very low within growing rape seeds (approximately 0.8% [v/v]) and can be markedly increased to more than 8% (v/v) by elevating external oxygen supply. Increased oxygen is accompanied by an increase in the energy state within seeds, increased metabolic fluxes to storage TAG biosynthesis (but not starch), and an increase in DAGAT and invertase activity. We conclude that oilseed metabolism is restricted by the prevailing low oxygen concentrations, providing an adaptive mechanism that allows oxygen consumption to be decreased to prevent internal anoxia. These results have obvious implications for strategies to increase yield and storage product composition in oilseed crops.
RESULTS
Influence of Changes in the External Oxygen Concentration on Internal Oxygen Concentrations in Siliques and Seeds of Rape
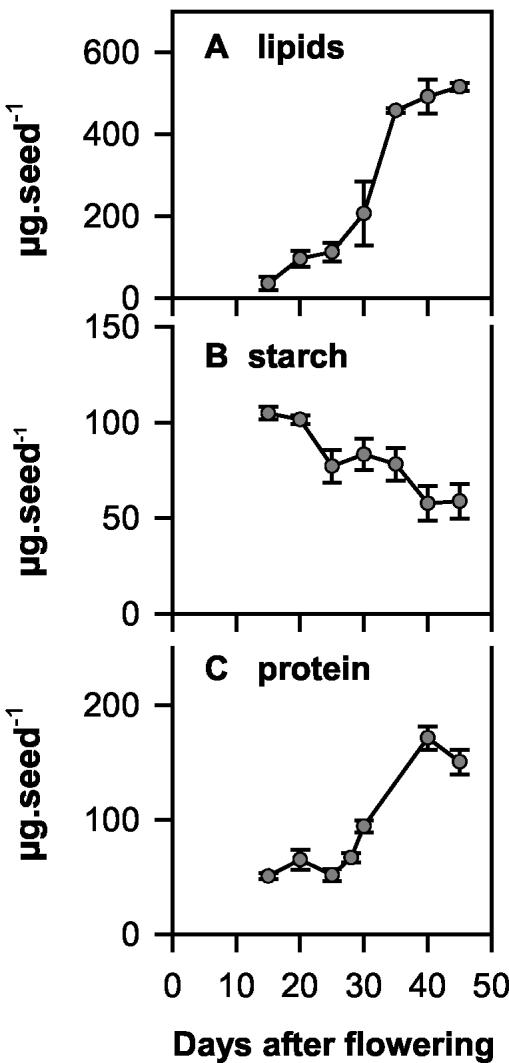
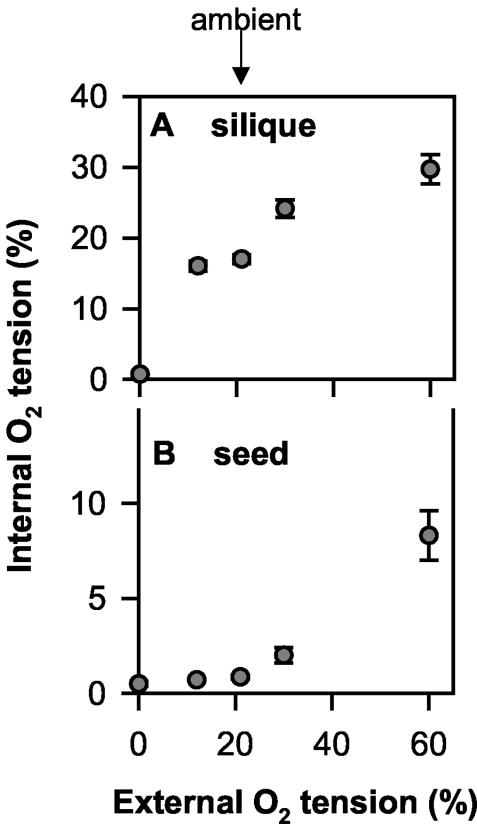
Experiments were performed with growing rape plants in the middle of the light period using siliques at 30 d after flowering (DAF). At this stage, developing seeds contained substantial amounts of lipids, starch, and protein and were characterized by high rates of lipid accumulation (Fig. 1). To analyze internal oxygen concentrations within the silique space between and inside growing seeds, an oxygen sensor (30-μm-diameter tip) was impaled into growing siliques. At ambient external oxygen (21% [v/v]) in the light, the internal oxygen concentration was 17% (v/v) in the silique airspace between seeds (Fig. 2A) and 0.8% (v/v) inside the seeds (Fig. 2B). The value for the silique airspace was similar to the value reported by Porterfield et al. (1999) for rape siliques (16% [v/v] in the light). Internal oxygen concentrations dropped to 16% and 0.7% (v/v) within the silique space and to 0.7% and 0.5% (v/v) within seeds when external oxygen around the siliques was reduced to subambient levels (12% and 0% [v/v] oxygen, respectively). When external oxygen was elevated to 30% and 60% (v/v; superambient levels), internal oxygen concentrations increased to 24% and 30% (v/v) in the silique space between seeds and to 2% and 8% (v/v) inside the seeds. These results show that internal oxygen concentrations in growing seeds are very low and can be more than proportionally increased by elevating the external oxygen concentration. When the external and internal oxygen concentrations were compared over the whole range of oxygen concentrations, the relationship was different in respect to siliques and seeds, with the levels in the airspace rising particularly markedly in the range from 0% to 12% (v/v) external oxygen, whereas the concentration in seeds did not rise strongly until superambient oxygen concentrations were supplied (compare Fig. 2, A with B).
Figure 1.
Accumulation of lipid, starch and protein in developing rape seeds. Seeds were harvested at different time points between 15 and 45 DAF to analyze their lipid (A), starch (B), and protein (C) content. Data are means ± se (n = 3 different plants).
Figure 2.
Oxygen concentrations in silique space (A) and within seeds (B) and analyzed using an optical sensor (tip diameter of approximately 30 μm). In the middle of the light period, siliques (30 DAF) were exposed to circulating air containing different oxygen concentrations. The siliques were fixed in position with adhesive tape, and the internal oxygen concentration was determined by inserting the microsensor through the silique wall, either into the center of the seed or between two seeds for measuring the oxygen tension in the silique gas space. Data are means ± se of (n = 8, 4, 27, 9, and 4 for 0%, 12%, 21%, 30%, and 60% [v/v] external oxygen, respectively). Error bars are not shown when they are smaller than the symbol.
Influence of the Oxygen Concentration on the Energy State in Seeds
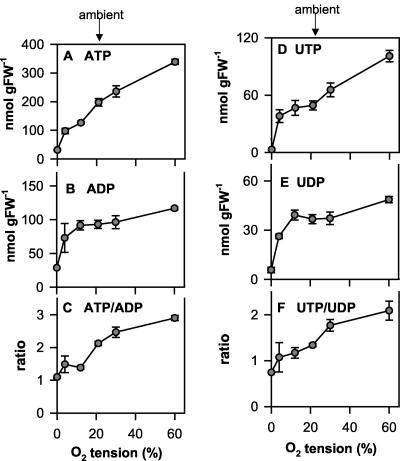
The increase in oxygen levels in the seeds was accompanied by a progressive increase in the levels of ATP (Fig. 3A), UTP (Fig. 3D), and the ratios of ATP to ADP (Fig. 3C) and UTP to UDP (Fig. 3F) over the entire range from 0% to 60% (v/v) external oxygen. These results imply that at ambient oxygen levels, the oxygen supply is strongly limiting for energy metabolism in rape seeds.
Figure 3.
Oxygen affects energy state in seeds. Siliques (30 DAF) were exposed to 0%, 4%, 12%, 21%, 30%, and 60% (v/v) O2 for 2 h, then rapidly frozen in liquid N2, and the seeds were separated from the silique wall under liquid N2 to analyze nucleotide levels: A, ATP; B, ADP; C, ATP to ADP ratio; D, UTP; E, UDP; and F, UTP to UDP ratio. Data are means ± se (n = 6 separate siliques from different plants). Error bars are not shown when they are smaller than the symbol.
Influence of the Oxygen Concentration on the Metabolism of 14C-Suc Injected into Seeds
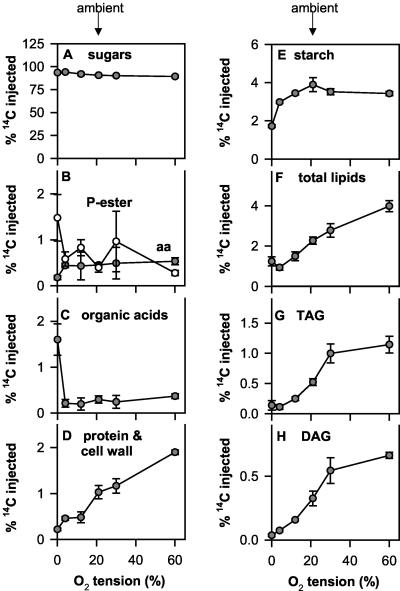
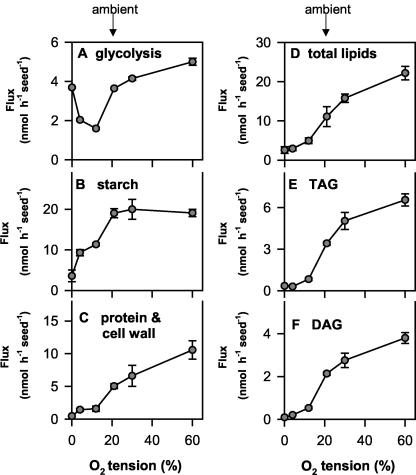
To investigate the effect of changes in external oxygen around siliques on metabolic fluxes in seeds, 14C-Suc was injected into seeds, which remained otherwise intact within their siliques. Seeds were harvested 2 h later to investigate the fate of the label (Fig. 4). This in planta labeling method provides a minimally invasive technique by which to study the metabolism of labeled precursors within developing seeds. In parallel experiments, we checked that this manipulation did not significantly alter subsequent growth and lipid accumulation of the seeds when compared with untreated controls (data not shown). During the short labeling period of 2 h, only a small portion of the labeled Suc was metabolized (Fig. 4A). The percentage label found in hexose-phosphates (Fig. 4B), amino acids (Fig. 4B), and organic acids (Fig. 4C) was low and showed no marked changes over the whole range of external oxygen concentrations, except at 0% (v/v) oxygen, where labeling of phosphoester and organic acids increased and of amino acids decreased (Fig. 4B). The increase in organic acids was especially marked (approximately 10-fold), reflecting the onset of lactic fermentation. Most of the metabolized label was converted to structural components (Fig. 4D), starch (Fig. 4E), and lipids (Fig. 4F). The proportion of the injected label metabolized to lipids (Fig. 4F), and structural components (proteins plus cell walls, Fig. 4D) progressively increased when the external oxygen was increased over the entire range from 0% to 60% (v/v). Most of the label in lipids was recovered in TAG (Fig. 4G) and to a lesser extent in DAG (Fig. 4H). In contrast to this, the percentage labeling of starch did not change substantially over the whole range of external oxygen concentrations, except at 4% and 0% (v/v) oxygen, when labeling of starch decreased (Fig. 4E). Interestingly, there was incorporation of label into starch despite overall levels of starch decreasing in seeds at this stage of development (Fig. 1B), indicating simultaneous synthesis and degradation of starch in the seeds. This is in confirmation with previous studies documenting turnover of starch in developing rape seed embryos (Eastmond and Rawsthorne, 2000).
Figure 4.
Oxygen affects the metabolism of [U-14C]Suc injected directly into seeds. To determine the influence of changes in external oxygen on metabolic fluxes, 0.5 μL of a buffered solution containing [U-14C] Suc was injected into seeds (30 DAF), which remained otherwise intact within their siliques. After 2 h of incubation in different oxygen concentrations (see Fig. 3), siliques were frozen in liquid N2, and seeds were separated from the silique wall and extracted to determine distribution of label into different fractions. A, Label remaining in sugars. B to H, Percentage of label metabolized to amino acids and phosphoesters (B; black and white symbols, respectively), organic acids (C), structural components (D; cell wall plus protein), starch (E), total lipids (F), TAG (G), and DAG (H). The results are means ± se (n = 4-5 separate siliques from different plants). Error bars are not shown when they are smaller than the symbol.
The 14C-Suc taken up into the cells will mix with internal unlabeled pools, so movement of the label will not necessarily reflect fluxes into the various pools. This is especially true in the case of Suc because Suc represents a very large internal pool in these seeds (approximately 200 μmol g fresh weight-1, see below). Label incorporated into the phosphoester fraction at the end of the 2-h incubation (see Fig. 4B) was divided by the total carbon found in phosphoesters (see below) to calculate the specific activity of the hexose-phosphate pool at the end of the labeling interval. To calculate the mean specific activity during the 2-h incubation, values were divided by a factor of 2 (for data on mean specific activities, see legend of Fig. 5; for a discussion of the assumptions involved in these calculations, see Geigenberger et al., 1997). To estimate the absolute rate of glycolytic flux, label in organic and amino acids was summed and divided by the specific activity of the phosphoester pool. The estimated rate of glycolysis increased progressively when external oxygen was increased from subambient (12% [v/v]) to suvkperambient (60% [v/v]) levels. It also increased up to 2-fold at 4% and 0% (v/v) external oxygen, reflecting the onset of fermentation at these very low oxygen concentrations (Fig. 5A). The rate of starch synthesis increased progressively when external oxygen was increased from 0% to 21% (v/v) but did not significantly change when oxygen was further increased above ambient levels (Fig. 5B). In contrast to this, there was a nearly linear increase in the rate of lipid synthesis (Fig. 5D) and in the synthesis of structural components (cell wall plus protein, Fig. 5C) over the whole range of external oxygen concentrations from 0% to 60% (v/v). The increase in flux to total lipids was reflected in the rates of TAG (Fig. 5E) and DAG (Fig. 5F) synthesis. These results imply that at ambient oxygen levels, the oxygen supply is strongly limiting for respiration, lipid biosynthesis, and the synthesis of structural components in rape seeds. The value for the rate of lipid synthesis at ambient (21% [v/v]) external oxygen (approximately 12 nmol Suc seed-1 h-1; Fig. 5D) is similar to the rate of Suc incorporation into lipids reported by Eastmond and Rawsthorne (2000) in isolated oilseed rape embryos (between 6 and 10 nmol Suc embryo-1 h-1). The flux can be increased 2-fold when more oxygen is supplied.
Figure 5.
Oxygen affects metabolic fluxes in developing seeds. The specific activity of the hexose phosphate pool (see below) and label incorporation into the relevant fractions (see Fig. 4) was used to calculate absolute rates of metabolic fluxes in seeds. The specific activity of the hexose phosphate pool was calculated by dividing the label retained in phosphoesters (Fig. 4B) by the total carbon of the phosphoester pool (see Fig. 7). The obtained values were divided by 2 to get the mean specific activities during the 2-h labeling period: 29 ± 9, 43 ± 8, 72 ± 5, 54 ± 18, 35 ± 12, and 44 ± 4 dpm nmol-1 at 0%, 4%, 12%, 21%, 30%, and 60% (v/v) O2, respectively. A, Glycolytic flux (the sum of the flux to the organic acids and amino acids); B, the rate of starch synthesis; C, flux into structural components (cell wall plus protein); D, rate of total lipid synthesis; E, rate of TAG synthesis; F, rate of DAG synthesis. The results are means ± se (n = 4-5 separate siliques from different plants). Error bars are not shown when they are smaller than the symbol.
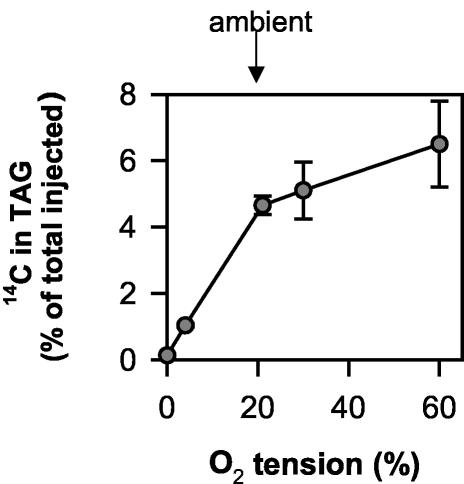
Influence of the Oxygen Concentration on the Metabolism of 14C-Acetate to TAG
The influence of the external oxygen concentration on TAG biosynthesis was also investigated by supplying 14C-acetate to the seeds, which is a more immediate precursor of fatty acid synthesis. Figure 6 shows the incorporation of label from 14C-acetate into TAG as percentage of the total label injected. Due to the short incubation time (2 h), only a small percentage of the injected label was metabolized (data not shown). Label incorporation into TAG increased over the whole range of external oxygen concentrations from 0% to 60% (Fig. 6). Similar results were obtained when 14C-acetyl-CoA was injected into seeds (data not shown). However, in this case, we cannot exclude that 14C-acetyl-CoA was converted to 14C-acetate prior to uptake into the plastid. The data indicate that at least one of the regulatory sites leading to changes in TAG biosynthesis in response to oxygen is upstream of acetyl-CoA.
Figure 6.
Oxygen affects incorporation of [U-14C]acetate into TAG. A buffered solution containing [U-14C] acetate (0.5 μL) was injected into seeds, and the experiment was performed as described in the legend to Figure 4. After 2 h, seeds were separated from the silique, and the percentage label incorporation into TAG was determined. The results are means ± se (n = 3 separate siliques from different plants). Error bars are not shown when they are smaller than the symbol.
Influence of the Oxygen Concentration on Metabolite Levels in Seeds
Metabolite levels in seeds are summarized in Figure 7. The increase in oxygen levels in seeds was accompanied by a slight but progressive increase in the level of Suc (Fig. 7A) and a strong almost linear increase in the levels of Glc (Fig. 7B) and Fru (Fig. 7C) over the whole range of external oxygen concentrations from 0% to 60% (v/v). Interestingly, sugars increased even though at the same time metabolic fluxes were increasing (see Fig. 5). This implies that Suc import has been stimulated.
Figure 7.
Oxygen affects metabolite levels in seeds. Siliques (30 DAF) were exposed to 0%, 4%, 12%, 21%, 30%, and 60% (v/v) O2 for 2 h, then rapidly frozen in liquid N2, and the seeds were separated from the silique wall under liquid N2 to analyze metabolite levels: A, Suc; B, Glc; C, Fru; D, glucose-6-phosphate; E, glucose-1-phosphate; F, acetyl-CoA; G, glycerol-3P; and H, lactate. The results are means ± se (n = 3 separate siliques from different plants). Error bars are not shown when they are smaller than the symbol.
The levels of glucose-6-phosphate (Fig. 7D), glucose-1-phosphate (Fig. 7E), and acetyl-CoA (Fig. 7F) decreased when external oxygen was increased from 12% to 30% (v/v), reflecting the stimulation of respiratory and biosynthetic fluxes (see Fig. 5). It is noteworthy that in this range of external oxygen concentrations, the stimulation of lipid synthesis was accompanied by a decrease of acetyl-CoA. This provides additional evidence that one of the sites at which flux is stimulated lies downstream of acetyl-CoA. The levels of glycerol-3P (Fig. 7G) and lactate (Fig. 7H), which are both regarded as fermentation products in plants, showed no substantial changes when external oxygen was increased from 12% to 60% (v/v) but increased progressively at 4% and 0% (v/v) external oxygen, showing that fermentation was induced at these very low external oxygen concentrations.
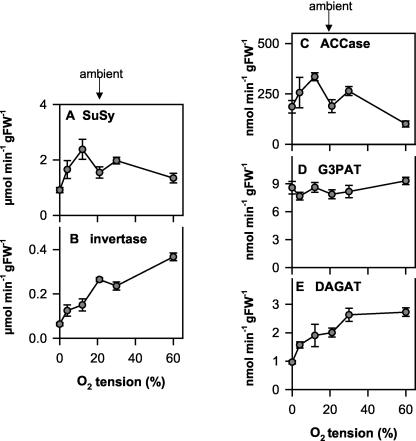
Influence of the Oxygen Concentration on Enzyme Activities in Seeds
The data so far document a progressive increase in metabolic fluxes to respiratory and biosynthetic pathways in response to increasing oxygen levels within seeds. To investigate whether this can be correlated with changes in key enzymes of Suc degradation, fatty acid synthesis, or TAG biosynthesis, we measured the maximal activities of Suc synthase (SuSy; Fig. 8A), invertase (Fig. 8B), ACCase (Fig. 8C), G3PAT (Fig. 8D), and DAGAT (Fig. 8E) in the seeds. Under ambient conditions (21% [v/v] oxygen), the activities of SuSy, ACCase, G3PAT, and DAGAT were in the same range as the values published previously for developing rape seeds (Weselake et al., 1993; Kang et al., 1994; King et al., 1997; Perry et al., 1999). The increase in oxygen levels in the seeds was accompanied by a strong increase in invertase activity over the entire range from 0% to 60% (v/v) external oxygen, consistent with the increase in the hexose to Suc ratio (compare Fig. 8B with 7, A-C). In contrast to this, SuSy activity only increased when oxygen was increased from 0% to 12% (v/v) and actually decreased at higher oxygen concentrations (Fig. 8A). Similar changes in the SuSy to invertase ratio in response to changes in external oxygen concentrations have been reported in potato tuber slices (Bologa et al., 2003) and maize (Zea mays) root tips (Zeng et al., 1999), reflecting a shift to the more energy conserving pathway of Suc degradation via SuSy when oxygen is low (Bologa et al., 2003).
Figure 8.
Oxygen affects key enzymatic activities of carbohydrate and lipid metabolism. Siliques (30 DAF) were exposed to 0%, 4%, 12%, 21%, 30%, and 60% (v/v) O2 for 2 h, then rapidly frozen in liquid N2, and seeds were separated from the silique wall under liquid N2 to analyze enzyme activities: A, SuSy; B, invertase; C, ACCase; D, G3PAT; and E, DAGAT. Results are means ± se (n = 3 separate siliques from different plants). Error bars are not shown when they are smaller than the symbol.
The activity of ACCase, which catalyzes the first step of fatty acid synthesis, increased when external oxygen was increased from 0% to 12% (v/v) but decreased at higher external oxygen concentrations (Fig. 8C). Although G3PAT, which catalyzes the first acylating step of TAG assembly and membrane lipid synthesis, showed no significant changes in activity over the whole range of external oxygen concentrations, there was a progressive increase in the activity of DAGAT especially between 0% and 30% (v/v) external oxygen. DAGAT catalyzes the final acylation of DAG to TAG and is the only step that is unique to TAG biosynthesis.
DISCUSSION
Oxygen Falls to Low Levels within Developing Rape Seeds in Planta
By using microsensors inserted into the tissue, we have shown that oxygen falls to low concentrations (0.8% [v/v]) within oil-storing rape seeds, growing at ambient external oxygen (21% [v/v]) in the light (Fig. 2B). A similar fall in internal oxygen concentrations has previously been found in other heterotrophic tissues like starch-storing pea (Pisum sativum) and broad bean (Vicia faba) seeds (Rolletschek et al., 2002), wheat (Triticum aestivum) seeds (J.T. van Dongen, P. Geigenberger, unpublished data), and potato tubers (Geigenberger et al., 2000; Bologa et al., 2003; Geigenberger, 2003). Manipulation of the external oxygen concentration around the siliques led to corresponding changes in the internal oxygen concentration within seeds, the extent being smaller when external oxygen was decreased below and much larger when external oxygen was increased above ambient level (Fig. 1B). Within the silique airspace, there was an almost linear decrease in the oxygen concentration (Fig. 1A). In contrast, the decrease in the oxygen concentration within seeds was more than proportionally diminished when external oxygen was step wise decreased from superambient to subambient levels (Fig. 1B). This provides evidence for an adaptive decrease in the rate of oxygen consumption by the seeds in response to low oxygen.
Low Internal Oxygen Restricts Respiration and Storage Metabolism within Seeds, Affecting Lipid Synthesis More Dramatically Than Starch Synthesis
The results of the present paper show that there is a decrease in metabolic activity in response to the prevailing low oxygen concentrations within seeds. When oxygen concentrations within seeds were decreased below 0.8% (v/v), the concentration found with ambient external levels, ATP to ADP and UTP to UDP ratios decreased, indicating a restriction in respiration (Fig. 3), and there was a general decrease in various biosynthetic fluxes to lipids (including TAG), starch, protein, and cell walls (Fig. 5). Conversely, when oxygen concentrations within seeds were increased above 0.8% (v/v), there was an increase in the adenylate and uridinylate energy states, an increase in glycolytic flux, and an increase in the rate of lipid, protein, and cell wall synthesis. Also, flux to TAG increased, reflecting the increase in total lipid synthesis, whereas flux to starch was not significantly changed at elevated oxygen. These results show that rapeseed metabolism is restricted by the prevailing low internal oxygen levels and that this restriction can be relieved by elevating the external oxygen supply. Crucially, these metabolic changes occurred at internal oxygen levels between 0.8% and 8% (v/v) and could clearly be separated from fermentation that occurred only at lower seed oxygen concentrations (below 0.7%), see Figures 5A and 7, G and H.
Interestingly, starch and lipid biosynthesis were differentially affected by elevated oxygen supply. Although storage lipid synthesis increased more than 2-fold when oxygen was increased above ambient levels, there was no further increase in starch synthesis above ambient oxygen concentrations (compare Fig. 5, B with D). This indicates that starch synthesis is already at its maximal rate at ambient internal oxygen levels in seeds (0.8% [v/v]), whereas the rate of lipid biosynthesis is clearly restricted under these conditions and has an optimum at superambient oxygen concentrations (8% [v/v] internal oxygen or higher). An optimum for starch synthesis at relatively low oxygen concentrations (between 1% and 12% [v/v]) has also been reported for discs of starch-storing potato tubers (Geigenberger et al., 2000; Bologa et al., 2003), where it was attributed to stimulation of ADP-Glc pyrophosphorylase by increased levels of its allosteric activator 3PGA, which increases in response to the restriction of glycolysis at low oxygen (Geigenberger et al., 2000). This contrasts with lipid and protein synthesis, which had maximal rates at 21% and 40% (v/v) oxygen in potato discs (Geigenberger, 2003).
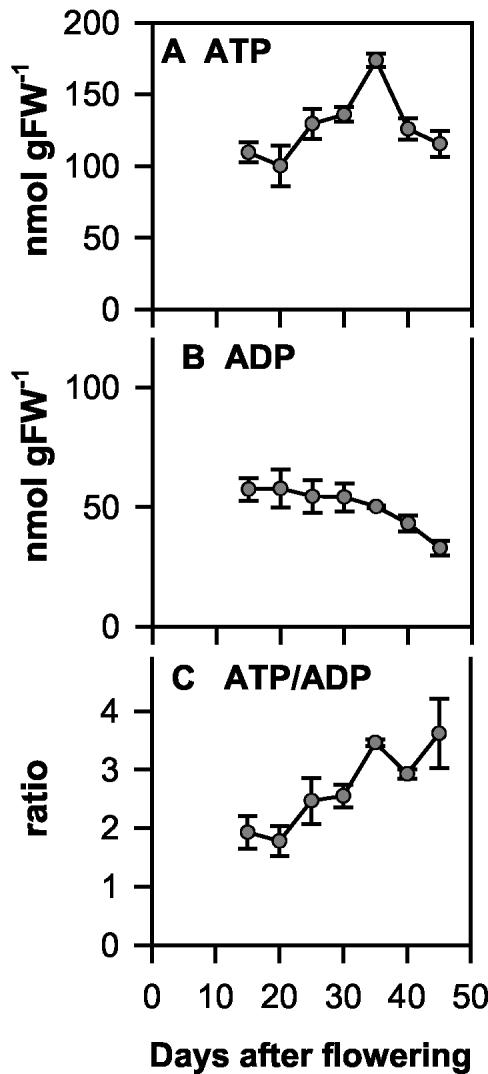
The differential effect of oxygen on starch and lipid synthesis could also be linked to the different energy requirements of the two biosynthetic processes in seeds. Based on theoretical considerations of the stoichiometry of the reaction pathways, addition of a six-carbon unit would cost one ATP in the case of starch and three ATP in the case of lipid synthesis. Experiments on isolated plastids from developing embryos of oilseed rape showed that lipid synthesis requires more ATP than starch synthesis (Neuhaus and Emes, 2000). Interestingly, the developmental shift from starch to lipid storage between 15 and 45 DAF (see Fig. 1, A and B) was accompanied by an increase in the ATP to ADP ratio in the seeds from 1.9 to 3.5 (see Fig. 9C). Intriguingly, manipulation of the internal oxygen concentration led to similar changes in the ATP to ADP ratio in seeds (compare Figs. 3C and 9C), and to a preferential stimulation of lipid synthesis (Fig. 4, E and F). This raises the question of whether the developmental shift from starch to lipid storage during seed filling (Eastmond and Rawsthorne, 2000) is related to increased oxygen availability and ATP supply. Due to technical difficulties, it was not possible to analyze oxygen concentrations in earlier or later stages of rapeseed development. Young seeds were too small to allow reliable measurements, and the seed coat of older seeds was not soft enough to allow the hole made by the sensor to be tightly sealed and oxygen diffusion from the surrounding air to be prevented. Studies in starch-storing pea seeds, however, document that oxygen concentrations are markedly lower in earlier compared with later stages of seed development (Rolletschek et al., 2002).
Figure 9.
Developmental changes in adenine nucleotide levels and energy state in growing rape seeds. At different time points between 15 and 45 DAF, siliques were rapidly frozen in liquid N2, and the seeds were separated from the silique wall under liquid N2 to analyze nucleotide levels: A, ATP; B, ADP; and C, ATP to ADP ratio. Data are means ± se (n = 3 different plants). Samples were taken from the same plants as in the experiment of Figure 1. Error bars are not shown when they are smaller than the symbol.
Changes in metabolite levels (Fig. 7) indicate that the stimulation of lipid synthesis in response to elevated oxygen occurs at two regulatory sites. The increase in the sugar levels in the face of increased metabolic fluxes provides evidence that Suc unloading has been stimulated by increased oxygen supply. This is consistent with recent studies showing an inhibition of metabolic activity paralleled by an inhibition of Suc unloading (using C-11 isotope labeling techniques) in developing wheat and rice (Oryza sativa) seeds when external oxygen was decreased from 21% to 10% (v/v; J.T. van Dongen, G. Roeb, A. Fröhlich, and P. Geigenberger, unpublished data). It is also consistent with studies documenting low oxygen concentrations within the vascular bundles of stems, restricting phloem energy metabolism and Suc transport rates (van Dongen et al., 2003).
The stimulation of lipid synthesis at elevated oxygen was also accompanied by a decrease in the levels of hexose-phosphates and acetyl-CoA (Fig. 7, D-F), indicating that one or more reactions using acetyl-CoA have been stimulated. The increased incorporation of 14C-acetate into TAG (Fig. 6) provides additional evidence that the use of acetyl-CoA for lipid synthesis has been stimulated. This could be due to increased levels of ATP (Fig. 3A), leading to a stimulation of ACCase activity in planta. However, this is not reflected by changes in the maximal ACCase activity assayed under optimal conditions in vitro (Fig. 8C), indicating the importance of metabolic fine control of ACCase for the regulation of lipid synthesis. The factors leading to a decrease in the maximal ACCase activity at elevated oxygen are not known, but it is interesting to note that changes in overall ACCase activity obviously correlate with changes in the in planta concentration of acetyl-CoA (compare Figs. 8C and 7F). It must be noted, however, that the reported metabolite levels are overall levels and may not reflect the concentrations in the plastid. Direct measurements of subcellular metabolite levels will be needed to confirm our interpretation.
The increase in overall DAGAT activity (Fig. 8E) could provide a further explanation for the stimulation of oil biosynthesis. DAGAT catalyzes the final acylating step of TAG biosynthesis. Maximal DAGAT activity increased at elevated oxygen and correlated with increased TAG biosynthesis. The reason(s) leading to increased DAGAT activity at elevated oxygen in the 2-h time frame are unclear and could involve transcriptional and/or posttranscriptional regulation. Previous studies document that increased expression of DAGAT leads to increased oil accumulation in seeds of transgenic Arabidopsis plants (Jako et al., 2001).
Lipid synthesis also requires NADPH and NADH for each C-2 addition to a growing acyl chain (Rawsthorne, 2002). Malate and pyruvate metabolism and the plastidial oxidative pentose phosphate pathway are potential sources of the reductants. It is not known how changes in oxygen will affect the redox state in seeds, but one would intuitively suggest that increased oxygen should decrease rather than increase cellular redox states. Previous studies in potato tubers and castor bean (Ricinus communis) seedlings show that changes in oxygen concentrations over a wide range are not associated with an increase in the cytosolic NADH/NAD+ ratio, unless oxygen is decreased to a very low level (Geigenberger, 2003). Further studies are needed to investigate plastidial NAD(P)H to NAD(P)+ ratios in response to changing oxygen levels.
Restriction of Oil Seed Metabolism by the Prevailing Low Oxygen Concentrations Is Part of an Adaptive Response to Decrease Oxygen Consumption and to Prevent Internal Anoxia
Plants, unlike animals, lack specialized circulation systems for efficient oxygen transport to internal tissues, and oxygen can fall to quite low concentrations within bulky, dense, or metabolically active plant tissues (Geigenberger, 2003). On the basis of work in potato tubers, it has been proposed that falling internal oxygen concentrations are sensed within plants and lead to adaptive responses to avoid internal anoxia (Geigenberger et al., 2000; Bologa et al., 2003; Geigenberger, 2003). These include a rapid restriction of metabolism to decrease oxygen consumption and a shift to pathways that conserve energy and use oxygen more efficiently. It was separated clearly from the inhibition of cytochrome oxidase and the switch to fermentation, which does not occur until much lower oxygen concentrations. The results of the present paper provide evidence for a similar adaptive response in rape seeds. First, when external oxygen levels are reduced, there is a disproportionately smaller decrease in internal oxygen levels, indicating a restriction of oxygen consumption under low oxygen. The progressive decrease in ATP level, ATP to ADP ratio, and in glycolytic flux provides additional evidence that seed respiration already starts to decline when oxygen is decreased in the superambient range. Second, there is a general decrease in biosynthetic fluxes with decreasing oxygen concentrations in seeds. This is especially so for oil synthesis over the whole range of oxygen concentrations from super- to subambient levels. The suppression of biosynthetic activities will save ATP and allows oxygen consumption to be decreased. Third, there is a decrease in the activity of invertase relative to SuSy with decreasing oxygen concentrations in the seeds, indicating a switch to an energetically less costly route of Suc degradation to conserve oxygen. More studies are needed to elucidate the signaling pathways involved in these responses and the underlying oxygen sensing system(s) in seeds.
Biotechnological Implications
The strong dependence of Suc to lipid conversion on oxygen is remarkable. Lipid biosynthesis is clearly restricted by the prevailing oxygen concentrations within seeds and can be increased by increasing oxygen supply. From radiolabeling studies, fluxes from Suc to lipids were calculated to be 12 nmol Suc seed-1 h-1 at ambient (21% [v/v]) external oxygen and 23 nmol Suc seed-1 h-1 at elevated (60% [v/v]) external oxygen (Fig. 5D). If we assume that only the 12 CH2 groups of a Suc molecule (molecular weight = 168 g mol-1) are incorporated into lipids, the rate of lipid synthesis would correspond to 48.4 and 92.7 μg lipid seed-1 d-1 at 21% and 60% (v/v) external oxygen, respectively. The value for the rate of lipid synthesis in ambient conditions (48 μg lipid seed-1 d-1) is well in the range of the maximum lipid accumulation rate derived from measurements of total lipid contents during seed development (approximately 35 μg seed-1 d-1, calculated from Fig. 1A). Similar rates of lipid accumulation have been reported in previous studies in developing rape seeds (Turnham and Northcote, 1983). Intriguingly, elevating oxygen supply leads to 2-fold higher rates of lipid biosynthesis, exceeding the rates of lipid accumulation reported earlier or in the present study under ambient oxygen. This finding has obvious implications for strategies to increase yield in oilseed crops. Molecular approaches to increase oxygen supply to seeds could involve changes in the expression of oxygen-binding proteins or changes in the gas permeability of the seed to facilitate gas diffusion or changes in the photosynthetic capacity of the seeds. The experimental conditions used in the present study are different from field conditions in that light intensities were much lower than in full sunlight. It is possible that under higher light intensities, oil synthesis may be less strongly limited by low oxygen, due to a more substantial contribution of seed photosynthesis to oxygen production and lipid synthesis under these conditions (Eastmond and Rawsthorne, 1996; King et al., 1998). More studies are needed to elucidate the long-term effects of changes in the oxygen availability on storage metabolism in seeds under different conditions.
MATERIALS AND METHODS
Plant Material
Spring rapeseed (Brassica napus cv Drakkar) plants were grown in a phytotron (25°C/day and 20°C/night) with a 16-h photoperiod at a light irradiance of 300 μmol photons m-2 s-1. Emerging flowers were tagged, and seed age was expressed in DAF. If no developmental stage is indicated in the text, then experiments were performed with seeds at the age of 30 DAF, when the seed diameter was about 3 mm, and lipid content was approximately 200 μg seed-1 (see Fig. 1). All of these experiments were done in the middle of the light period.
Chemicals
Unless stated otherwise, chemicals were obtained from Sigma (Taufkirchen, Germany) or Merck (Darmstadt, Germany).
Analysis of Total Lipid Content of Seeds
Total lipids of developing seeds between 15 and 45 DAF were extracted according to the method of Bligh and Dyer (1959), and the lipid content was measured by gas chromatography of fatty acid methyl esters, using pentadecanoic acid as internal standard (Benning and Somerville, 1992).
Determination of Protein and Starch Content of Seeds
Protein content was measured as by Eastmond and Rawsthorne (2000). The protein concentration was determined using the dye-binding assay (Bradford, 1976) with bovine serum albumin as the standard. Starch content was measured as described by Geigenberger et al. (1998).
In Planta Labeling Experiments
To determine the influence of changing oxygen concentrations on carbon flux and metabolism within the seed, a side branch bearing several siliques was enclosed in a transparent plastic bag through which air with various oxygen tensions was passed. The premixed gases (Messer Griesheim GmbH, Magdeburg, Germany) used for this treatment contained 350 ppm CO2, different oxygen concentrations as indicated in the text, and N2. Using a 5-μL Hamilton syringe (470-μm needle diameter, neolab, Heidelberg, Germany), 0.5 μL of a solution containing 115 μm [U-14C] Suc, 2.8 mm [U-14C] acetate, or 110 μm [1-14C] acetyl-CoA (Amersham-Buchler, Freiburg, Germany; specific activities 22.8 MBq μmol-1, 2.11 MBq μmol-1, or 2.11 MBq μmol-1, respectively) in 20 mm MES-buffer (pH 5.7) was injected directly into seeds. After 2 h, siliques were harvested and immediately frozen in liquid N2. During the whole experiment, seeds remained otherwise intact within their siliques.
Extraction and Fractionation of Radiolabeled Seeds
Seeds were manually separated from the silique wall under liquid N2. For each replicate, five seeds were pooled and ground to a fine powder in liquid nitrogen using a ball mill (Retsch Schwingmühle M200, Haan, Germany). Using the extraction method described by Bligh and Dyer (1959), the material was separated into a chloroform phase (lipid fraction), a water/methanol phase (water-soluble fraction), and an insoluble pellet containing starch, protein, and cell wall material. Lipids were fractionated by thin-layer chromatography as described by Stobart et al. (1997). The radioactive TAG and DAG spots were scraped from the plate, and the radioactivity was measured in a liquid scintillation counter. The methanol/water fraction was dried under an air stream at 45°C. The pellet was taken up in 1 mL of water, and neutral, anionic, and cationic compounds were separated on cationic (AG 50W-X8 Superfine, NH4+ form,) and anionic (AG 1-X8 Superfine, OH- form) columns according to the manufacturer's instructions (Bio-Rad Laboratories, Munich, Germany). The label in phosphate esters was measured according to Geigenberger et al. (1997). The insoluble fraction was separated into starch, protein, and cell wall as in Merlo et al. (1993).
Metabolite, Nucleotide, and Enzyme Analysis
To determine the influence of changing oxygen concentrations on metabolite levels, nucleotide levels, and enzyme activities in seeds, side branches containing several siliques were enclosed in a transparent plastic bag through which air with various oxygen tensions was passed. The premixed gases (Messer Griesheim GmbH) used for this treatment contained 350 ppm CO2, different oxygen concentrations as indicated in the text, and N2. After 2 h, siliques were rapidly frozen in liquid nitrogen, and seeds separated from silique walls under liquid nitrogen. Metabolite and nucleotide levels were analyzed in trichloroacetic acid extracts as described by Jelitto et al. (1992). Glc-6P, Glc-1P, glycerol-3P, acetyl-CoA, and lactate were measured as by Gibon et al. (2002). Glc, Fru, and Suc were measured as by Geigenberger et al. (1998). ADP, ATP, UTP, and UDP were quantified by high-pressure liquid chromatography using a Partisil-SAX10 anion-exchange column (Kontron Instruments, Eiching, Germany) as by Geigenberger et al. (1997). SuSy (EC 2.4.1.13) and soluble alkaline invertase (EC 3.2.1.26) were extracted according to Geigenberger and Stitt (1993) and measured as by Geigenberger et al. (1998). In 30-DAF seeds, approximately 80% of the total invertase activity is due to soluble alkaline invertase (King et al., 1997). ACCase (EC 6.4.1.2) was extracted with 0.5 m sorbitol, HEPESNaOH (pH 7.4), 10 mm KCl, 1 mm MgCl2, 1 mm EDTA, 10% (v/v) ethanediol, 5 mm dithiothreitol, and 1% (w/v) bovine serum albumin. The activity was determined by measuring the incorporation of 14C from NaH14CO3 into malonyl-CoA (Kang et al., 1994). DAGAT (EC 2.3.1.20) and G3PAT (EC 2.3.1.15) were determined according to Perry et al. (1999) and Bafor et al. (1990), respectively.
Analysis of Tissue Oxygen Concentrations
In situ oxygen tensions were measured in the light, using an oxygen microsensor with a tip diameter of approximately 30 μm, and connected to a fiber optic oxygen meter (MicroxTX2, Presens, Regensburg, Germany) as by van Dongen et al. (2003). This oxygen sensor is especially suitable for measuring very low oxygen concentrations because of its high sensitivity at low concentrations. Siliques were enclosed in a transparent plastic bag with circulating air containing different oxygen concentrations as described above. The siliques were fixed in position with adhesive tape, and the internal oxygen concentration was determined by inserting the microsensor through the plastic bag and the silique wall, either into the center of the seed or between two seeds for measuring the oxygen tension in the silique gas space. After inserting the microsensor at normal oxygen (21% [v/v]), external oxygen was changed to the respective sub- or superambient concentration for about 1 to 2 h, until the internal oxygen tension was stabilized. Seeds at 30 DAF were investigated because they were big enough (about 3 mm) to be impaled with the oxygen sensor but still soft and flexible enough that the hole made by the sensor was tightly sealed by the seed coat so that oxygen diffusion from the surrounding air was prevented.
Acknowledgments
We wish to thank Mark Stitt for his support and interest in this work, stimulating discussions, and helpful comments on the manuscript. We are grateful to Peter Dörmann for providing the gas chromatography facilities, to John E. Lunn and Alisdair R. Fernie for critical readings of the manuscript, and to Britta Hausmann and Karin Koehl for taking care of the plants.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Ge 878/1-3).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.031963.
References
- Bafor M, Jonsson L, Stobart AK, Stymne S (1990) Regulation of triacylglycerol biosynthesis in embryos and microsomal preparations from the developing seeds of Cuphea lanceolata. Biochem J 272: 31-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning C, Somerville CR (1992) Identification of an operon involved in sulfolipid biosynthesis in Rhodobacter sphaeroides. J Bacteriol 174: 6479-6487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37: 911-917 [DOI] [PubMed] [Google Scholar]
- Bologa KL, Fernie AR, Leisse A, Ehlers Loureiro M, Geigenberger P (2003) A bypass of sucrose synthase leads to low internal oxygen and impaired metabolic performance in growing potato tubers. Plant Physiol 132: 2058-2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Eastmond P, Kolacna L, Rawsthorne S (1996) Photosynthesis by developing embryos of oilseed rape (Brassica napus L). J Exp Bot 47: 1763-1769 [Google Scholar]
- Eastmond P, Rawsthorne S (1998) Comparison of the metabolic properties of plastids isolated from developing leaves or embryos of Brassica napus L. J Exp Bot 49: 1105-1111 [Google Scholar]
- Eastmond PJ, Rawsthorne S (2000) Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryos. Plant Physiol 122: 767-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P (2003) Response of plant metabolism to too little oxygen. Curr Opin Plant Biol 6: 247-256 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M (2000) Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biol Chem 381: 723-740 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Hajirezaei M, Geiger M, Deiting U, Sonnewald U, Stitt M (1998) Overexpression of pyrophosphatase leads to increased sucrose degradation and starch synthesis, increased activities of enzymes for sucrose-starch interconversions, and increased levels of nucleotides in growing potato tubers. Planta 205: 428-437 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Reimholz R, Geiger M, Merlo L, Canale V, Stitt M (1997) Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201: 502-518 [Google Scholar]
- Geigenberger P, Stamme C, Tjaden J, Schulz A, Quick PW, Betsche T, Kersting HJ, Neuhaus HE (2001) Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol 125: 1667-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geigenberger P, Stitt M (1993) Sucrose synthase catalyzes a readily reversible-reaction in vivo in developing potato-tubers and other plant-tissues. Planta 189: 329-339 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221-235 [DOI] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126: 861-874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M (1992) inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing Escherichia coli pyrophosphatase in their cytosol. Planta 188: 238-244 [DOI] [PubMed] [Google Scholar]
- Kang F, Ridout C, Morgan C, Rawsthorne S (1994) The activity of acetyl-CoA carboxylase is not correlated with the rate of lipid synthesis during development of oilseed rape (Brassica napus L.) embryos. Planta 193: 320-325 [Google Scholar]
- Kennedy EP (1961) Biosynthesis of complex lipids. Fed Proc 20: 934-935 [PubMed] [Google Scholar]
- King SP, Badger MR, Furbank RT (1998) CO2 refixation characteristics of developing canola seeds and silique wall. Aust J Plant Physiol 25: 377-386 [Google Scholar]
- King SP, Lunn JE, Furbank RT (1997) Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol 114: 153-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki A, Sasaki Y (1999) Light-dependent changes in redox status of the plastidic acetyl-CoA carboxylase and its regulatory component. Biochem J 339: 541-546 [PMC free article] [PubMed] [Google Scholar]
- Kuang A, Crispi M, Musgrave ME (1998) Control of seed development in Arabidopsis thaliana by atmospheric oxygen. Plant Cell Environ 21: 71-78 [DOI] [PubMed] [Google Scholar]
- Merlo L, Geigenberger P, Hajirezaei M, Stitt M (1993) Changes of carbohydrates, metabolites and enzyme-activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J Plant Physiol 142: 349-402 [Google Scholar]
- Neuhaus HE, Emes MJ (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51: 111-140 [DOI] [PubMed] [Google Scholar]
- Ohlrogge JB, Browse J (1995) Lipid biosynthesis. Plant Cell 7: 957-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry HJ, Bligny R, Gout E, Harwood JL (1999) Changes in Kennedy pathway intermediates associated with increased triacylglycerol synthesis in oil-seed rape. Phytochemistry 52: 799-804 [Google Scholar]
- Porterfield DM, Kuang A, Smith PJ, Crispi ML, Musgrave ME (1999) Oxygen-depleted zones inside reproductive structures of Brassicaceae: implications for oxygen control of seed development. Can J Bot 77: 1439-1446 [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworsky J, Ohlrogge JB (1991) In vivo pools of free and acylated acyl carrier proteins in spinach: evidence for sites of regulation of fatty acids synthesis. J Biol Chem 266: 1858-1865 [PubMed] [Google Scholar]
- Post-Beittenmiller D, Roughan G, Ohlrogge JB (1992a) Regulation of plant fatty acid synthesis: analysis of acyl-coenzyme A and acyl-acyl carrier protein substrate pools in spinach and pea chloroplasts. Plant Physiol 100: 923-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B, Hardy RWF (1975) Reproductive growth and dry-matter production of Glycine-max (L.) in response to oxygen concentration. Plant Physiol 55: 102-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quebedeaux B, Hardy RWF (1976) Oxygen concentration: regulation of crop growth and productivity. In RH Burris, CC Black, eds, CO2 Metabolism and Plant Productivity. University Park Press, Baltimore, pp 185-204
- Rawsthorne S (2002) Carbon flux and fatty acid synthesis in plants. Prog Lipid Res 41: 182-196 [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H (2002) Legume embryos develop in a hypoxic environment. J Exp Bot 53: 1099-1107 [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Kozaki A, Hatano M (1997) Link between light and fatty acid synthesis: thioredoxin-linked reductive activation of plastidic acetyl-CoA carboxylase. Proc Natl Acad Sci USA 94: 11096-11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani D, Ohlrogge JB (1995) Feedback inhibition of fatty acid synthesis in tobacco suspension cells. Plant J 7: 577-587 [Google Scholar]
- Sinclair T, Ward J, Randall C (1987) Soybean seed growth in response to long-term exposures to differing oxygen partial pressures. Plant Physiol 83: 467-468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart K, Mancha M, Lenmam M, Dahlqvist A, Stymne S (1997) Triacylglycerols are synthesised and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L) seeds. Planta 203: 58-66 [Google Scholar]
- Stymne S, Stobart K (1987) Triacylglycerol biosynthesis. In The Biochemistry of Plants, Vol. 9. Lipids: Structure and Function. PK Stumpf, ed, Academis Press, New York, pp 175-214
- Tjaden J, Möhlmann T, Kampfenkel K, Henrichs G, Neuhaus HE (1998) Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of starch. Plant J 16: 531-540 [Google Scholar]
- Turnham E, Northcote DH (1983) Changes in the activity of acetyl-CoA carboxylase during rape-seed formation. Biochem J 212: 223-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen JT, Schurr U, Pfister M, Geigenberger P (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol 131: 1529-1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselake R, Pomeroy M, Furukawa T, Golden J, Little D, Laroche A (1993) Developmental profile of diacylglycerol acyltransferase in maturing seeds of oilseed rape and safflower and microspore-derived cultures of oilseed rape. Plant Physiol 102: 565-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Todd J, Newman T, Focks N, Girke T, Martínez de Ilárduya O, Jaworski JG, Ohlrogge JB, Benning C (2000) A new set of Arabidopsis expressed sequence tags from developing seeds: the metabolic pathway from carbohydrates to seed oil. Plant Physiol 124: 1582-1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Wu Y, Avigne WT, Koch KE (1999) Rapid repression of maize invertases by low oxygen: invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol 121: 599-608 [DOI] [PMC free article] [PubMed] [Google Scholar]