Abstract
Golgi cells (GoCs) are the primary inhibitory interneurons of the granular layer of the cerebellum. Their inhibition of granule cells is central to operate the relay of excitatory inputs to the cerebellar cortex. Parallel fibers (PFs) establish synapses to the GoCs in the molecular layer; these synapses contain AMPA, N-methyl-d-aspartate (NMDA), and mostly group II metabotropic glutamate receptors. Long-term changes in the efficacy of synaptic transmission at the PF-GoC synapse have not been described previously. We used whole cell patch-clamp recordings of GoCs in acute rat cerebellar slices to study synaptic plasticity. We report that high-frequency burst stimulation of PFs, using a current-clamp or voltage-clamp induction protocol, gave rise to long-term depression (LTD) at the PF-GoC synapse. This form of LTD was not associated with persistent changes of paired-pulse ratio, suggesting a postsynaptic origin. Furthermore, LTD induction was not dependent on activation of NMDA receptors. PF-GoC LTD does require activation of specifically group II metabotropic glutamate receptors and of protein kinase A.
INTRODUCTION
The cerebellum plays an important role in several forms of motor learning. For example, there is evidence supporting the requirement of the cerebellar cortex in associative eyelid conditioning (Koekkoek et al. 2003; McCormick and Thompson 1984). Thus activity-dependent alterations in the efficacy of cerebellar synaptic transmission (i.e., potentiation, depression) are thought to underlie learning and possibly the storage of motor patterns. From early theoretical work, it was anticipated that long-term depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses underlies motor learning (Marr-Albus-Ito models; Albus 1971; Ito 1984; Marr 1969). Such plasticity was later experimentally shown by applying in vivo conjunctive stimulation of PFs and climbing fibers (Ito and Kano 1982). An elaborate literature exists on the synaptic plasticity of the PF-PC synapse (Jörntell and Hansel 2006; for reviews, see Ito 2001) and on plasticity at other cerebellar synapses, such as the climbing fiber to PC synapse (Coesmans et al. 2004; Hansel and Linden 2000). Other synapses in the cerebellum thought to be important for motor learning are the input stage of the cerebellar cortex at the mossy fiber (MF) to granule cell (GC) synapses (D'Angelo et al. 2004) and the MF to deep cerebellar nuclei synapses, of which synaptic plasticity was also long theorized but only recently experimentally shown (Medina and Mauk 2000; Pugh and Raman 2009; Zhang and Linden 2006). Furthermore, synaptic inputs to interneurons, such as the PF to stellate cell synapses in the molecular layer (ML), have been shown to be plastic (Jörntell and Ekerot 2002; Rancillac and Crépel 2004). We chose to study whether the PF to Golgi cell synapse shows long-term plastic changes.
Golgi cells (GoCs) are the main inhibitory interneurons of the granule cell layer (GCL), gating the activity of as many as 100 billion granule cells. They typically have a large soma emitting a series of basolateral dendrites in the GCL and three to four long and thin apical dendrites, going into the ML where they branch. In the GCL, GoCs form a large ramified axonal plexus, allowing each cell to contact several thousands GCs (D'Angelo 2008). The central role of the GoC in the cerebellar cortex is exemplified by the extensive interconnections of the cell (Geurts et al. 2003; Watanabe et al. 1998). GoCs receive excitatory inputs from MFs, which form synapses on their basolateral dendrites, from PFs in the molecular layer (in the order of 5,000 inputs per cell) (Ito 2006; Pellionisz and Szentágothai 1973), and possibly also from climbing fibers (Shinoda et al. 2000). GoCs also receive inhibitory inputs from stellate and basket cells in the ML and from Lugaro cells in the GCL (Dieudonné and Dumoulin 2000; Palay and Chan-Palay 1974). The main function of the GoCs is to tonically and phasically inhibit GCs by their GABAergic output (Rossi et al. 2003). Apart from GABA, GoCs co-release glycine at their synaptic terminals (Dugué et al. 2005). In vivo, GoCs have an ongoing irregular, low-frequency firing activity and react to afferent stimulation with a burst of action potentials followed by a pause (Tahon et al. 2005; Vos et al. 1999). In vitro, GoCs behave like regular pacemakers and show phase-reset and resonant activity in the “theta” frequency band (i.e., 4–7 Hz) (Forti et al. 2006; Solinas et al. 2007).
Several studies have shown that PF to GoC synapses have functional glutamate receptors of the AMPA, N-methyl-d-aspartate (NMDA), and kainate types (Bureau et al. 2000; Dieudonné 1998; Kanichay and Silver 2008; Misra et al. 2000). Their AMPA receptors (AMPARs) are composed of GluR2 subunits, which are characterized by low Ca2+ permeability and linear I/V relationships (Menuz et al. 2008). The NMDA receptors (NMDARs) contributing to the excitatory postsynaptic currents (EPSCs) evoked by stimulating the PFs have a high conductance, arising from NR2B-containing receptors, and are present both on the synaptic and extrasynaptic membrane. Low-conductance components arising from NR2D-containing receptors are extrasynaptically localized and do not contribute to the EPSC (Misra et al. 2000). Kainate receptors (KARs) have small amplitudes and slow kinetics and summate in response to presynaptic tetanic stimulation, thus allowing the GoC to integrate excitatory inputs at different time scales (Bureau et al. 2000). Metabotropic glutamate receptors (mGluRs) are also expressed postsynaptically on PF-GoC synapses. Both group I (mGluR1 and 5) and group II (mGluR2 and 3) are expressed, but mGluR2 are the most abundant (Watanabe and Nakanishi 2003) and serve as a histological marker of GoCs (Geurts et al. 2001). Activation of mGluR2 enhances an inward rectifier potassium current, leading to a slow inhibitory postsynaptic current (IPSC) capable of silencing GoC activity after high-intensity PF input (Watanabe and Nakanishi 2003).
Long-term changes at GoC synapses have not been studied previously. A good candidate is the PF-GoC synapse because PF inputs are in general associated with activity-dependent plastic phenomena. Because of its central position in the control of information transfer to Purkinje cells, a change in the synaptic input to the GoC, even if subtle, could have a significant effect on network activity in the GCL. Here we showed that brief trains of high-frequency synaptic activation can induce long-term depression (LTD) of the parallel fiber (PF) excitatory inputs to GoCs. This PF-GoC LTD is shown using induction protocols (IPs) in voltage-clamp (V-clamp) and current-clamp (C-clamp). The PF-GoC LTD induced by a C-clamp IP is further pharmacologically analyzed.
METHODS
Slice preparation and electrophysiology
Parasagittal and coronal cerebellar slices were acutely prepared from Wistar rats (P15–P22) as previously reported (Dugué et al. 2005) and in agreement with institutional, federal, and European ethical guidelines and laws for animal experimentation. Briefly, the brain was quickly removed after decapitation, and 250 μm tissue slices were cut by a vibratome (VT 1000 S, Leica) in a ice-cold solution containing (in mM) 130 K-gluconate, 15 KCl, 0.5 EGTA, 20 HEPES, and 25 glucose, with pH adjusted to 7.4 by KOH (oxygenated with 100% O2) (Dugué et al. 2005). Slices were transferred to an incubation chamber, heated at 32°C for ≥45 min and later stored at room temperature. The artificial cerebrospinal fluid (ACSF) used for slice incubation, storage, and continuous perfusion during the electrophysiological recordings contained (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 25 glucose, 2 CaCl2, and 1 MgCl2 (compensated with 95% O2-5% CO2).
Recordings were carried out at room temperature from the soma of GoCs, visually identified by infrared differential interference contrast (DIC) videomicroscopy. The identity of GoCs was routinely assessed using previously established criteria (Dieudonné 1995) and on observation of EPSCs evoked by extracellular stimuli delivered in the molecular layer (ML) (Beierlein et al. 2007; Bureau et al. 2000). Additional morphological confirmation was obtained in a subset of cells (n = 15), filled with a low concentration (0.05%) of Lucifer yellow and imaged by epifluorescence.
Giga-seal patch-clamp recordings were obtained, under the whole cell configuration, using 2–3 MΩ borosilicate-glass pipettes. Intracellular solution contained (in mM) 135 potassium gluconate, 10 KCl, 10 HEPES, 0.2 EGTA, 4 Mg-ATP, 0.4 Na3GTP, and either 10 Na2-phosphocreatine and 10 sucrose or 14 Na2-phosphocreatine, with pH 7.25–7.3 titrated with KOH. For experiments in which BAPTA was added to strongly buffer free intracellular calcium, at increasing concentrations (i.e., 10, 20, and 40 mM), the composition of the intracellular solution was adjusted by decreasing the concentration of potassium gluconate (i.e., 135, 90, and 35 mM, respectively). Voltage- and current-clamp recordings were performed with an EPC 10 amplifier (HEKA, Lambrecht, Germany).
See Supplementary Materials and Fig. S11 for description of the voltage-clamp methods and results on parasagittal slices. All results discussed here are from recordings in coronal slices. In coronal slices, Cm was 149 ± 12 pF (range: 34–392 pF; n = 36) and Rin was 290 ± 24 MΩ (range: 79–708 MΩ; n = 36). These results are in close agreement with Forti et al. (2006).
A theta-glass pipette (1401016, Hilgenberg, Malsfeld, Germany) filled with ACSF, and accommodating two platinum-wires, was used to deliver bipolar extracellular electrical stimuli. This electrode was placed in the lower half of the ML, at lateral distances of 150–300 μm. The stimulation artifacts were small. To monitor changes in synaptic efficacy, EPSCs were systematically evoked by paired-pulses stimuli with a 50 ms interpulse interval and repeated at a frequency of 0.1 Hz. Current-controlled stimuli had amplitude and duration in the ranges 8–100 μA and 100–200 μs, respectively. The stimulation intensity was adjusted so that the equivalent excitatory postsynaptic potential (EPSP) had a peak value of 5–10 mV. GoCs are in general parasagittally organized (Barmack and Yakhnitsa 2008; Sillitoe et al. 2008). However, it was much easier to evoke and obtain EPSCs in the coronal plane where responses ≤2 nA were not unusual. No more than one or two position adjustments of the stimulating electrode in the ML were necessary.
The LTD induction protocol (IP) consisted of a high-frequency extracellular electrical stimulation (20 pulses at 100 Hz), repeated 30 times with an interval of 2 s. For the C-clamp IP recordings, the cells were held in current clamp during the IP, injecting negative current to hold the cell around −70 mV but allowing the cell to spike freely during the tetanic stimulation. For the V-clamp IP recordings, the cells were held under voltage clamp at −70 mV during the IP.
Data analysis and statistics
Currents were filtered at 2 kHz, digitized at 5 kHz, and acquired using Pulse software (Heka). Data were analyzed off-line using custom-made macros written in Igor Pro (Wavemetrics, Lake Oswego, OR). All group data are reported as means ± SE and compared statistically using the Student's t-test. A significance of P < 0.05 was indicated by an asterisk, and a significance of P < 0.01 was indicated by two asterisks.
The PPRs were calculated from the ratio of the second EPSCs over the first EPSCs of the baseline EPSCs, during the 10 min before IP and a stable recording period 25–35 min after IP (60 data points each). No corrective measure was applied to the PPR, as for example, taking the mean of second EPSCs over the mean of the first EPSCs of a series of consecutive paired EPSCs to compensate for the increase in variability of the PPR after IP in case of depression of the EPSCs (Kim and Alger 2001; Sims and Hartell 2005).
The main parameter measured to characterize the synaptic responses evoked by extracellular stimulation was the amplitude of the individual EPSCs, although other parameters like the time-to-peak, the slope of the rising-phase, and the decay-phase time constant were computed. An estimate of the charge transfer, corresponding to each EPSC, was also routinely computed by subtracting the time integral of the steady-state current from the time integral of total current during the EPSC.
Pharmacology
Inhibitory synaptic transmission was blocked by bath-applying a cocktail of 10 μM of SR95531 (gabazine) and 1 μM strychnine, a GABAA receptor and glycine-receptor antagonist, respectively. d-(−)-2-amino-5-phosphono-pentanoic acid (d-APV), (S)-α-methyl-4-carboxyphenylglycine [(S)-MCPG] and LY367385 (S)-(+)-a-amino-4-carboxy-2-methylbenzeneacetic acid were also bath-applied to block NMDA receptors, mGluR, and mGluR1, respectively. LY341495 bis-(O-aminophenoxy)-N,N,N',N'-tetraacetic acid and KT5720 (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester were prepared in DMSO as stock solutions and used to specifically block mGluR2 receptors and protein kinase A (PKA), respectively. Drug application always followed the establishment of the whole cell configuration, preceded the LTD induction protocol and persisted thereafter. All chemicals were obtained from Sigma-Aldrich, Ascent Scientific, or Tocris Cookson.
RESULTS
Characterization of parallel fiber to Golgi cell synapses
Patch-clamp recordings were established at room temperature, under the whole cell configuration, from the soma of visually identified putative GoCs in the GCL of cerebellar slices, prepared from 15 to 22 day old rats. In general, eliciting EPSCs by extracellular stimulation of the PFs further confirmed the identity of the cell recorded, complementing standard electrophysiological identification criteria (Dieudonné 1995, 1998). In addition, to isolate glutamatergic PSCs, gabazine (10 μM) and strychnine (1 μM) were bath applied for the entire duration of the experiment. A schematic representation of the position of the recording and stimulating electrodes in the coronal slice is shown in Fig. 1C.
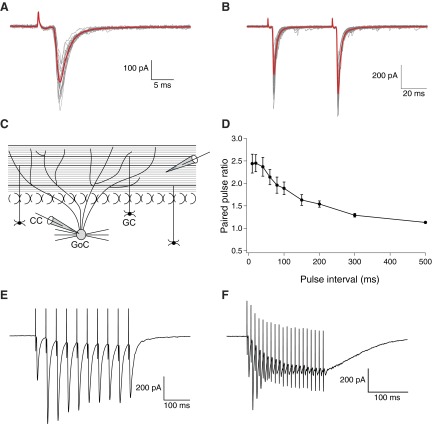
Fig. 1.
Properties of parallel fibers to Golgi cell excitatory postsynaptic currents (EPSCs). A: representative (gray) and averaged traces (red) from a series of 20 consecutive EPSCs evoked by extracellular stimulation of parallel fibers (PFs) in coronal slices. B: in all experiments, EPSCs were evoked with a paired-pulse protocol (10–100 μA, 100–200 μs) with a 50 ms interpulse interval. Representative (gray) and averaged (red) traces from 20 consecutive stimulations show paired-pulse facilitation. C: schematic diagram of representative positions of the stimulating and recording electrode in the coronal slice plane. D: on the average, paired pulse ratios (PPRs) were strongly dependent on the interpulse intervals (n = 9; 20 PPRs per data-point, with error bars indicating SE). E and F: response to tetanic stimulation, averaged over 20 responses, for stimulation at 50 (E) and 100 Hz (F); the latter was used to induce long-term depression of EPSCs.
Characteristic kinetic parameters of the evoked EPSCs are summarized in Table 1. A sample EPSC, average superposed on series of 20 consecutive EPSCs, is shown in Fig. 1A. Paired-pulse stimuli with 50 ms interpulse intervals were systematically used to evoke EPSCs; representative traces and their averages are shown in Fig. 1B. Paired stimulation of PF-GoCs with relative short time intervals led to paired-pulse facilitation (PPF), implying that the release probability of the second EPSC is increased. When systematically characterized, the paired-pulse ratio (PPRs, i.e., the ratio of the 2nd EPSC amplitude over the 1st EPSC of each pair) decreased exponentially (decay time constant 135 ± 16 ms) for increasing interpulse intervals and leveled off at an average value of 2.45 for intervals <50 ms (Fig. 1D; n = 9 cells). The variability of the responses also increased for shorter time intervals. From the traces in Fig. 1D, we estimated a PPR of 2.25 for a time interval of 50 ms between the paired EPSCs.
Table 1.
Passive cell parameters and several EPSC parameters, shown per experimental group and pre- vs. post- induction protocol
| C-clamp IP (n = 19) |
V-clamp IP (n = 7) |
NMDAR Block (n = 10) |
40 mM BAPTA (n = 9) |
Aspecific mGluR (n = 4) |
mGluRII Block (n = 7) |
mGluRI Block (n = 3) |
PKA Block (n = 6) |
|
|---|---|---|---|---|---|---|---|---|
| Rinput, Mohm | 271 ± 37 | 322 ± 52 | 286 ± 38 | 255 ± 32 | 301 ± 61 | 320 ± 50 | 573 ± 210 | 402 ± 61 |
| Cm, pF | 131 ± 13 | 142 ± 46 | 152 ± 17 | 96 ± 30 | 123 ± 25 | 141 ± 22 | 62 ± 11 | 126 ± 22 |
| Amplitude EPSCs, pA | ||||||||
| Pre-IP | 243 ± 24 | 362 ± 51 | 277 ± 35 | 263 ± 27 | 300 ± 104 | 308 ± 23 | 279 ± 16 | 372 ± 40 |
| Post-IP | 185 ± 22 | 253 ± 41 | 210 ± 36 | 210 ± 23 | 284 ± 82 | 308 ± 39 | 178 ± 6 | 361 ± 32 |
| P-value | <0.01 | <0.01 | 0.04 | <0.01 | 0.38 | 0.86 | 0.02 | 0.55 |
| PPR | ||||||||
| Pre-IP | 2.06 ± 0.12 | 2.18 ± 0.17 | 1.93 ± 0.12 | 1.96 ± 0.24 | 2.12 ± 0.40 | 1.68 ± 0.06 | 1.75 ± 0.06 | 1.71 ± 0.2 |
| Post-IP | 2.14 ± 0.15 | 2.34 ± 0.22 | 2.12 ± 0.16 | 1.88 ± 0.07 | 1.81 ± 0.26 | 1.75 ± 0.14 | 1.93 ± 0.13 | 1.70 ± 0.11 |
| P-value | 0.57 | 0.27 | 0.17 | 0.24 | 0.14 | 0.5 | 0.10 | 0.91 |
| Average slope, -pA/ms | ||||||||
| Pre-IP | 1.77 ± 0.26 | 1.77 ± 0.31 | 1.63 ± 0.38 | 1.62 ± 0.22 | 1.14 ± 0.54 | 1.63 ± 0.15 | 1.98 ± 0.04 | 2.64 ± 0.31 |
| Post-IP | 1.23 ± 0.22 | 1.11 ± 0.25 | 1.28 ± 0.36 | 1.44 ± 0.26 | 1.07 ± 0.41 | 1.68 ± 0.24 | 1.21 ± 0.1 | 2.57 ± 0.39 |
| P-value | <0.01 | <0.01 | 0.22 | 0.44 | 0.7 | 0.82 | 0.04 | 0.71 |
| Charge transfer, pC | ||||||||
| Pre-IP | 1.00 ± 0.08 | 1.69 ± 0.24 | 1.16 ± 0.10 | 1.51 ± 0.19 | 1.20 ± 0.31 | 1.24 ± 0.17 | 1.15 ± 0.08 | 1.49 ± 0.30 |
| Post-IP | 0.81 ± 0.09 | 1.28 ± 0.25 | 0.89 ± 0.09 | 1.10 ± 0.14 | 1.12 ± 0.22 | 1.28 ± 0.18 | 0.77 ± 0.02 | 1.40 ± 0.26 |
| P-value | <0.01 | <0.01 | <0.01 | <0.01 | 0.48 | 0.81 | 0.03 | 0.30 |
| Time-to-peak, ms | ||||||||
| Pre-IP | 2.01 ± 0.16 | 2.12 ± 0.21 | 2.38 ± 0.20 | 2.56 ± 0.21 | 2.63 ± 0.41 | 1.80 ± 0.10 | 2.08 ± 0.13 | 1.47 ± 0.22 |
| Post-IP | 1.78 ± 0.14 | 2.09 ± 0.14 | 2.53 ± 0.27 | 2.37 ± 0.30 | 2.68 ± 0.55 | 1.78 ± 0.08 | 1.77 ± 0.43 | 1.49 ± 0.23 |
| P-value | 0.15 | 0.90 | 0.2 | 0.54 | 0.76 | 0.1 | 0.41 | 0.92 |
| Decaytime constant, ms | ||||||||
| Pre-IP | 2.95 ± 0.21 | 2.85 ± 0.22 | 2.87 ± 0.30 | 3.26 ± 0.28 | 2.98 ± 0.74 | 2.51 ± 0.40 | 2.66 ± 0.16 | 2.55 ± 0.20 |
| Post-IP | 3.25 ± 0.21 | 3.01 ± 0.31 | 2.96 ± 0.30 | 3.82 ± 0.35 | 3.03 ± 0.59 | 2.55 ± 0.37 | 2.94 ± 0.73 | 2.43 ± 0.25 |
| P-value | 0.08 | 0.50 | 0.54 | 0.26 | 0.21 | 0.74 | 0.25 | 0.18 |
PPR, paired pulse ratio.
Data are expressed as mean ± SE. Pre-IP means the baseline activity starting 10 min before start IP. Post-IP means the activity 25–35 min after IP. P values for statistical significance have been obtained by applying paired t-tests between values of the same parameter pre- vs. post-IP.
Responses to trains of stimulations have previously been studied (Bureau et al. 2000; Kanichay and Silver 2008). A detailed study of short-term plasticity of the PF-GoC synapse was done by Beierlein et al. (2007), showing that short stimulus bursts had little overall effect on the strength of the PF-GoC synapse, GoCs do not release endocannabinoids to regulate their PF synapses, and short-term post-tetanic potentiation (PTP) is absent beyond the third pulse. When trains of stimulation pulses were delivered, both short-term facilitation and depression of PF-GoC EPSCs were apparent. Figure 1, E and F, reports sample average responses to tetani of 10 pulses at 50 Hz and of 20 pulses at 100 Hz, respectively. From Fig. 1F, the progressive depression of EPSC amplitude was particularly prominent at 100 Hz, where separable responses practically vanished by the end of the train.
LTD of the parallel fiber to Golgi cell synapse
We performed extensive preliminary work to study the parameters needed to induce synaptic plasticity at the PF-GoC synapse. In general, we used high-frequency stimulation burst pulses as the IP. This was based on having more consistent results using a high-frequency IP compared with using low-frequency IPs. More specifically, we used high-frequency, quasi-physiological IPs similar to some IPs used for LTD of the PF-PC synapse where “burst-only” IPs are used and are not necessarily combined with postsynaptic depolarization (Eilers et al. 1997; Hartell 1996). To further increase possible physiological relevancy, we performed the high-frequency IP in coronal slices under C-clamp of the postsynaptic cell, allowing the cell to spike during the IP. Control experiments were done under V-clamp conditions in coronal slices and in parasagittal slices (Supplementary Material and Fig. S1).
During the C-clamp, IP current was applied to hold the membrane potential around −70 mV, although the cell was allowed to spike during the IP stimuli. Quite a regular response developed during the IP, with evoked spiking activity occurring during the first couple of bursts, followed by progressively decreasing postsynaptic and action potentials. By the end of the IP protocol, during the last 5–10 bursts, the extracellular pulses elicited practically no effect on the postsynaptic membrane. This is a result of a complete but transient presynaptic exhaustion of the PFs and/or of postsynaptic receptor desensitization. An example of the response of a cell to the repetitive tetanus is shown in Fig. S2 (Supplementary Material).
Control experiments, where no IP was applied but EPSCs were continuously monitored at 0.1 Hz, showed a moderate decrease of the EPSC amplitude over the 50 min recording period (−9.9 ± 3.9% change at t = 25–35 min; n = 10; Fig. 2C). No significant changes were seen for the PPR controls (Fig. 2D). The C-clamp IP resulted generally in a progressive and sustained LTD of the amplitude of the evoked EPSCs (−26.5 ± 5.1% change at t = 25–35 min after IP; n = 19: P < 0.01 compared with the control group; Fig. 2, C and F). This depression was also reflected by changes in average slope and charge transfer, without any significant changes of kinetic parameters (Table 1). Fifteen of 19 experiments showed a progressive and sustained depression after IP (−34 ± 4% change at t = 25–35 min; n = 15; Fig. 2B), 3 showed no change, and 1 resulted in clear potentiation of EPSCs amplitude. Of the 15 experiments showing LTD, 8 showed a small and transient post-tetanic potentiation (PTP), 10–20 min after IP, of the order of 10–30% compared with baseline. Average EPSCs waveforms, before and after IP, are shown in Fig. 2A.
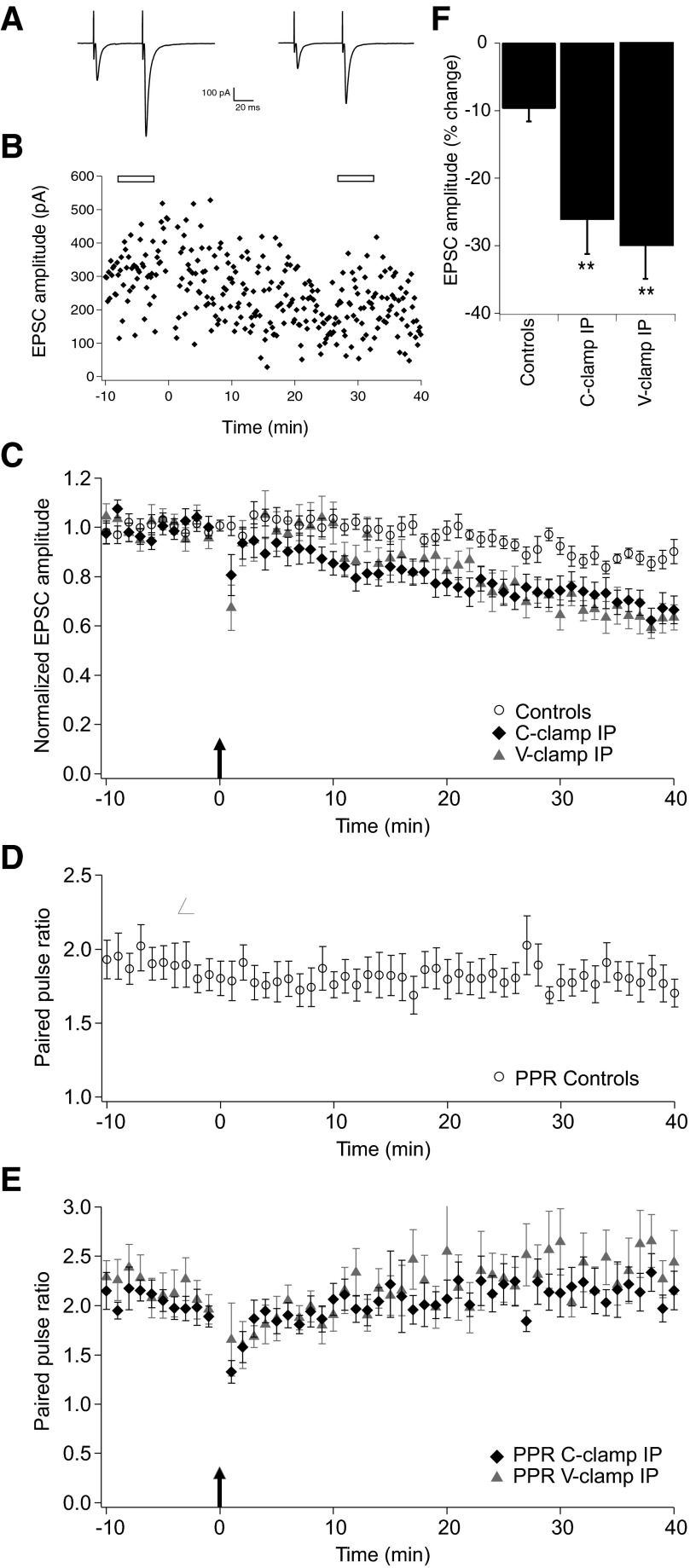
Fig. 2.
Long-term depression (LTD) of the parallel fibers to Golgi cells synaptic responses. A: representative averaged traces of evoked EPSCs waveforms 5 min before induction protocol (IP) and 30 min after IP, time points indicated by open bars in B. B: sample experiment showing LTD of EPSCs amplitude evoked by the high-frequency burst IP under current clamp (C-clamp). C: population summary showing the time course of the normalized EPSCs amplitude, comparing control conditions (n = 10) with the LTD induced by the high-frequency burst IP under C-clamp (n = 19) and voltage clamp (V-clamp) mode (n = 7). The arrow at time point 0 indicates the occurrence of the 1 min long IPs. D: population summary of the time course of the PPR for the control condition (n = 10). E: population summary of the time course of the PPR for C-clamp IP and V-clamp IP. F: box plot shows that for both C-clamp IP and V-clamp IP protocols the percentage changes of EPSC amplitude averaged over 25–35 min after IP are significantly different from controls (**P < 0.01).
Under the same experimental conditions, changes in the PPR were studied as a potential landmark of alterations in presynaptic release probability. To check whether our recording setup can detect small changes in PPR at distal dendrites and thus be a reliable test for confirming the post- versus presynaptic locus of the plasticity, control experiments were carried out in which the extracellular Ca2+ concentration was systematically lowered (see Supplementary Material and Fig. S3). These indicate that, because of the small extent of LTD, any changes in PPR at electrotonically distant sites may be difficult to detect. The C-clamp IP recordings were not associated with a significant increase of the PPR (2.06 ± 0.12 at 10 min before IP vs. 2.14 ± 0.15 at t = 25–35 min after IP; n = 19; P = 0.57), suggesting a postsynaptic origin of the LTD (Fig. 2E).
To see whether spiking behavior during the C-clamp IP was necessary to induce LTD, we applied the same high-frequency IP while voltage clamping the cell at −70 mV all through the IP. Passive cell parameters and EPSC-related parameters were comparable for the two experimental groups (Table 1). The V-clamp IP results are shown in Fig. 2, C and F (n = 7). They showed a similar amount of depression as for the C-clamp IP series (−29.6 ± 4.7% change at t = 25–35 min after IP; n = 7: P < 0.01 compared with the control group). Similar changes were seen for the slope of the rising phase and the charge transfer, whereas kinetic parameters did not show any significant changes (Table 1). A more pronounced PTP was observed in four of the seven recordings and is reflected in the summary plot and in the depression of the PPR right after the IP. No significant changes of the PPR were observed (2.18 ± 0.17 at 10 min before IP vs. 2.34 ± 0.22 at t = 25–35 min after IP; n = 7; P = 0.17; Fig. 2E), suggesting this IP also has a postsynaptic origin of the LTD.
Using similar IPs as in coronal slices, we performed a number of experiments in parasagittal slices, and these data confirmed that PF-GoC LTD can be evoked in parasagittal slices (see Supplementary Materials and Fig. S1).
Pharmacology of the LTD at the parallel fiber to Golgi cell synapse
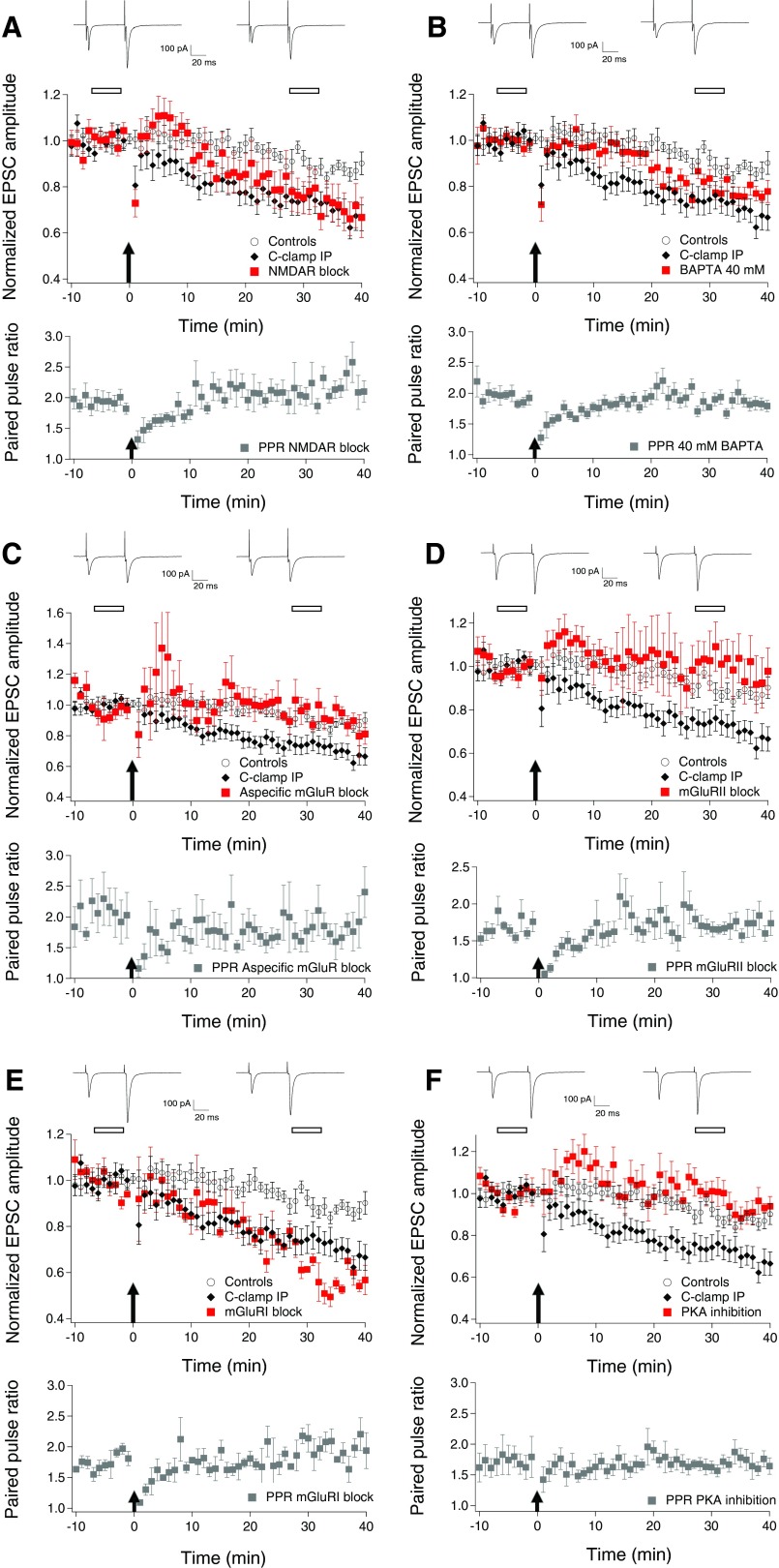
Extensive pharmacological studies were done in coronal slices using the C-clamp IP to elucidate the mechanisms involved in LTD induction. Because NMDARs are involved in many forms of synaptic plasticity and are expressed at PF-GoC synapses, we tested whether these receptors are needed for PF-GoC LTD. Bath applying 50 μM d-APV from the beginning of the experiment had no significant effect on the PF-GoC LTD (Fig. 3A and Fig. 4) compared with the results summarized in Fig. 2C (−23.0 ± 7.6% EPSCs amplitude change at t = 25–35 min; n = 10; P = 0.73). Again no significant change was present in the PPR time course (1.93 ± 0.12 at 10 min before IP vs. 2.13 ± 0.16 at t = 25–35 min after IP; n = 10; P = 0.17; Fig. 3A). Under these conditions, 6 of 10 experiments showed LTD, whereas 3 showed no change and 1 experiment showed LTP. A transient PTP was observed in 7 of 10 experiments. This was reflected in a depression of the PPRs, during the same 10 min post-IP period, possibly indicating a presynaptic origin of the PTP. Sample average EPSCs waveforms before and after induction are shown in Fig. 3A. No significant difference was observed, comparing these EPSCs with those evoked without NMDAR blockade (Table 1). When comparing the population plot of the V-clamp IP experiments (Fig. 2C) with Fig. 3A, a similar time course is observed: a transient PTP over the first 10 min after IP followed by a progression toward sustained depression at ∼20 min after IP. These results suggest that AMPAR-mediated EPSCs at PF-GoC synapses undergo NMDAR-independent LTD but that the extra depolarization caused by NMDAR activation may cause a faster induction of LTD.
Fig. 3.
Pharmacology of LTD at the parallel fibers to Golgi cells synapses. A–F: top: representative traces of paired EPSCs. Traces shown are the averages of 30 consecutive responses taken from time points indicated by an open bar before and after IP and centered respectively around −5 min before IP and 30 min after IP. Middle: time course of the normalized evoked EPSC amplitude under test conditions vs. controls (n = 10) and C-clamp IP LTD (n = 19). The data for each cell were normalized to the mean value of the EPSC amplitudes 10 min before IP. The arrow at time point 0 indicates the start of the IP lasting 1 min. Bottom: time course of the PPR under test conditions. A: LTD induced by the C-clamp IP was not blocked in the presence of 50 μM d-APV (n = 10; P = 0.73). No significant changes in PPR occurred. B: LTD induced by the C-clamp IP was not blocked in Golgi cells (GoCs) loaded with 40 mM BAPTA (n = 9; P = 0.21), although LTD is clearly slower to develop and the amplitude is smaller than usual. No significant changes in PPR occurred. C: LTD induced by the C-clamp IP was completely blocked in the presence of 500 μM (S)-MCPG (n = 4; P < 0.01). No significant changes in PPR occurred. D: LTD induced by the C-clamp IP was completely blocked in the presence of 1 μM LY341495 with DMSO as vehicle (n = 7; P < 0.05). No significant changes in PPR occurred. E: LTD induced by the C-clamp IP was not blocked in the presence of 100 μM LY367385 (n = 3; P = 0.46). No significant changes in PPR occurred. F: LTD induced by the C-clamp IP was completely blocked in the presence of 0.1 μM KT5720 with DMSO used as vehicle (n = 7; P < 0.05). No significant changes in PPR occurred.
Fig. 4.
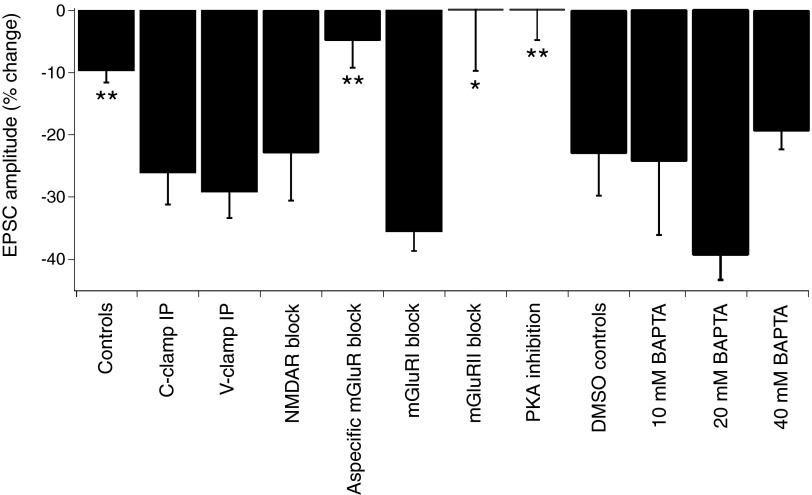
Box plot summary of all recordings showing the percentage change in EPSC amplitude. Summary showing the percentage change in EPSC amplitude averaged over time 25–35 min after IP, centered around 30 min after IP for the conditions indicated. *P < 0.05 and **P < 0.01 compared with C-clamp IP LTD.
Next, we considered the necessity of an increased intracellular free Ca2+ concentration for PF-GoC LTD. Indeed, spiking during C-clamp IP might result in postsynaptic Ca2+ transients by Ca2+ influx through voltage-gated Ca2+ channels, as well as Ca2+ release from internal stores. Entry through AMPAR and NMDAR could also be possible contributing factors. To test the Ca2+ requirement, we first used an intracellular solution containing 10 mM BAPTA (tetraacetic acid). Because this had no effect on the LTD (−25 ± 11% change at t = 25–35 min; n = 4; P = 0.94 compared with results C-clamp IP; Fig. 4), we tested a 20 mM BAPTA concentration for the intracellular solution (BAPTA tetrapotassium salt, with adjustment of the concentration of the potassium gluconate). Under these conditions, no effect on the LTD was observed (−39 ± 5% change at t = 25–35 min; n = 5; P = 0.18 compared with results C-clamp IP; Fig. 4). Because GoC apical dendrites in the ML are very thin, one might expect difficult diffusion of BAPTA to the distant spine-like protrusions that form the postsynaptic part of the PF-GoC synapse, and maybe higher concentrations of the chelating agent are necessary to see an effect on PF-GoC LTD. For this reason, we added 40 mM BAPTA to the intracellular solution and waited longer than usual after establishing the whole cell configuration, before applying the C-clamp IP (40 ± 4 min, n = 9, compared with a standard interval 24 ± 2 min, n = 19). As in the previous experiments, no significant effect of this Ca2+ chelator on LTD was observed (−19.5 ± 2.5% change at t = 25–35 min; n = 9; P = 0.23 compared with results from C-clamp IP and P = 0.01 compared with controls; Figs. 3B and 4). The parameters of the EPSCs and passive cell parameters, recorded under these conditions, are summarized in Table 1. These results do not support an essential role for postsynaptic Ca2+ transients in PF-GoC LTD induction, although a partial effect is not excluded because LTD induction was slower and less pronounced with 40 mM BAPTA.
Finally, mGluRs are known to be expressed postsynaptically at the PF-GoC synapses. Specifically, activation of mGluR2 by glutamate gives rise to slow inhibitory postsynaptic potentials through the activation of G protein-coupled inwardly rectifying K+ channels (GIRKs) (Knoflach and Kemp 1998; Watanabe and Nakanishi 2003). The mGluR2-coupling GIRK is predominantly located at GoC dendrites. Because mGluRs are, in general, important mediators for synaptic plasticity phenomena, we studied the effect of blocking these receptors on the PF-GoC LTD.
Because mGluR1 is also expressed in GoCs, although to a lesser degree than mGluR2, we first did a series of experiments whereby an aspecific mGluR blocker [i.e., (S)-MCPG, 500 μM] was bath applied. This resulted in complete blockade of PF-GoC LTD (−4.8 ± 4.4% change at t = 25–35 min; n = 4; P < 0.01 compared with results C-clamp IP; Figs. 3C and 4). No significant change was observed in the PPR, although a rather pronounced but transient decrease in the ratio was recorded after the IP (2.30 ± 0.40 at 10 min before IP vs. 1.77 ± 0.26 at t = 25–35 min after IP; n = 4; P = 0.16; Fig. 3C).
Next we studied the effect of treatment with a specific mGluR2 blocker (LY341495, 1 μM) in DMSO as vehicle. In this series of experiments, PF-GoC LTD was also completely abolished, confirming the results with the aspecific mGluR blocker and emphasizing the role of mGluR2 in PF-GoC LTD (+0.3 ± 9.5% change at t = 25–35 min; n = 7; P < 0.05 compared with results C-clamp IP; Figs. 3D and 4). In six of seven experiments, a PTP was observed, and it was reflected in a depression of the PPRs during the same 10 min post-IP period. Average EPSC waveforms before and after induction are shown in Fig. 3D. This lack of change was also reflected in the other EPSC parameters (Table 1). No significant change was observed in the PPR values (1.68 ± 0.06 at 10 min before IP vs. 1.76 ± 0.14 at t = 25–35 min after IP; n = 7; P = 0.46; Fig. 3D). Application of the vehicle alone (0.25% DMSO) had no effect on PF-GoC LTD (−23 ± 7.1% change at t = 25–35 min; n = 7; P = 0.48 compared with results C-clamp IP; Fig. 4).
These experiments do not exclude a role of mGluR1 in the induction and/or expression of LTD. We next used the selective mGluR1 antagonist LY367385 at a concentration of 100 μM in the ACSF. No effect on LTD was observed (−36.0 ± 2.6% change at t = 25–35 min; n = 3; P = 0.46 compared with results C-clamp IP; Figs. 3E and 4). No significant change was observed in the PPR (1.75 ± 0.06 at 10 min before IP vs. 1.93 ± 0.13 at t = 25–35 min after IP; n = 3; P = 0.26; Fig. 3E).
These data confirm an essential role for mGluR2 in the induction and/or expression of PF-GoC LTD. Puzzled by the fact that no clear Ca2+ dependency was shown, although an effect on the induction of LTD is not excluded, we tested whether PKA might be a downstream mediator of the induction mechanism for LTD. PKA was chosen because this kinase can be activated without a rise in intracellular Ca2+ concentration. As blocker, we used the very selective PKA inhibitor, KT5720, dissolved in DMSO, at a concentration of 0.1 μM in the ACSF. In this series of experiments, PF-GoC LTD was also completely abolished (−0.3 ± 4.3% change at t = 25–35 min; n = 6; P < 0.01 compared with results C-clamp IP; Figs. 3F and 4), suggesting a highly probable role for PKA in the biochemical cascade leading to LTD. In five of six experiments, a PTP was observed, and it was reflected in a depression of the PPRs during the same 10 min post-IP period. Average EPSC waveforms before and after induction are shown in Fig. 3F. This lack of change was also reflected in the other EPSC parameters (Table 1). No significant change was observed in the PPR values (1.71 ± 0.2 at 10 min before IP vs. 1.70 ± 0.11 at t = 25–35 min after IP; n = 6; P = 0.91; Fig. 3F).
A summary of all pharmacology experiments is shown in Fig. 4.
DISCUSSION
We reported here for the first time that the PF-GoC synapse can undergo sustained LTD after a quasi-physiological, high-frequency synaptic burst stimulation. PF-GoC LTD was shown to be NMDAR independent. A clear Ca2+ dependence could not be shown. On the other hand, PF-GoC LTD showed a strong dependence on activation of mGluR and more specifically on mGluR2, which are characteristic markers for GoCs. It was also shown that PKA acts as a mediator of PF-GoC LTD. The LTD is likely to be postsynaptically expressed because it was not associated with significant changes in the PPR and because mGluR2 is only postsynaptically expressed.
The central finding of this work is that the PF-GoC synapse can undergo LTD using quasi-physiological IPs similar to those used in PF-PC LTD experiments. This was inspired by the work of Chadderton et al. (2004), showing that sensory stimulations produce short high-frequency bursts of granule cell spikes and the current tendency to use quasi-physiological patterns of stimulation to induce synaptic plasticity in slices (Pugh and Raman 2006; Shin and Linden 2005; Zhang and Linden 2006). No important differences were observed between V-clamp IP– and C-clamp IP–based experiments.
We regularly observed a transient PTP after the IP, sometimes of quite large amplitude and lasting ≤20 min. This PTP was more pronounced for the V-clamp IP data than for the C-clamp IP data. It was not excluded that more Ca2+ enters the cell during C-clamp IPs because cells were allowed to spike. This could possibly lead to an earlier induction of LTD by itself or through the mediation of other factors necessary for the induction. We cannot exclude, however, that there could be a washout of factors necessary for LTP of the PF-GoC synapse, which is quite often described for plasticity in hippocampal interneurons (Kullmann and Lamsa 2007).
It is remarkable that protocols similar to IPs used for inducing PF-PC LTD (Coesmans et al. 2004; Sims and Hartell 2006) result in LTD of the PF-GoC synapse as well. In the same vein, recent in vivo work reported that conjunctive stimulation of peripheral afferents and climbing fibers causes depression of GoC firing activity (Xu and Edgley 2008), but the same peripheral stimulation alone does not cause plasticity of activity. The locus of these plastic changes is unclear but is supposed to lie in the inhibitory input from ML interneurons to GoCs. This represents the first experimental indication of long-term activity changes of GoCs, and it adds to recent literature that the cerebellum is highly prone to plastic changes in vivo (Jörntell 2008).
The control experiments done for the V-clamp and C-clamp IP experiments showed a rundown of their activity (about −10% of baseline activity before IP) over the course of the 50 min duration of the experiments. This depression in activity was rather small, and the differences with preIP values were not significant, both for the V-clamp and C-clamp controls. It is not excluded that the stimulation of PFs at a frequency of 0.1 Hz could lead to mild mGluR2 activation and thus be the cause of the moderate depression of activities. This hypothesis is supported by the fact that there is no rundown of the EPSCs in the mGluR blocking experiments.
Concerning the induction of PF-GoC LTD, we showed that it is NMDAR independent. This is also reflected by the lack of effect of APV on the kinetic parameters of the evoked EPSCs. This independence of the PF-GoC LTD is in a way remarkable, because it is generally expected that, if glutamergic synapses express GluR2-containing AMPAR, they are not permeable to Ca2+ and when depolarized they show strong activation of NMDAR (Kullmann and Lamsa 2007). This did not seem to be the case for Golgi cells because no clear differences were observed when comparing responses to tetanus stimulation with or without NMDAR blockers. It can of course not be excluded that the NMDAR conductance undergoes LTD with the same high-frequency IP. Cortical and hippocampal synapses, having a postsynaptic expression of mGluR2 and showing LTD, also show no dependence on NMDAR for LTD (Otani et al. 2002; Pöschel and Manahan-Vaughan 2005).
An important result of our work is the demonstration that PF-GoC LTD is mGluR dependent and more specifically mGluR2 dependent and mGluR1 independent. Postsynaptic mGluR2 endow the GoC with a capability to sense the strength of presynaptic GC inputs. Weak stimuli hardly activate mGluR2, whereas strong stimuli activate these receptors through spillover activation, causing a temporal inhibition of the GoCs. This permits the GoC to discriminate between weak and strong stimuli (Watanabe and Nakanishi 2003). This mechanism has consequences on the network level activity in the GCL because inhibition will relieve GoC-mediated feedback inhibition in a stimulus-dependent manner (Nakanishi 2009). The fact that blocking the mGluR2 in GoCs leads to relief of LTD of the PF input adds to the important role played by the mGluR2 with regard to the spatiotemporal regulation of the cerebellar circuitry.
The possible role of presynaptically expressed mGluR4 autoreceptors, which are the main group III mGluR on PFs, was not studied because of the lack of a specific blocker (Abitbol et al. 2008; Niswender and Conn 2010). LY341495 at 1 μM has no blocking effect on mGluR4 (Johnson et al. 1999; Kingston et al. 1998; Wright et al. 2000). The specific LY341495 block of PF-GoC LTD makes the contribution of a presynaptic mechanism very unlikely because mGluR2 is only postsynaptically expressed and mGluR4 only presynaptically.
We convincingly showed that PKA is involved with the biochemical pathways leading to PF-GoC LTD induction. Group II metabotropic glutamate receptors (mGluR2 and mGluR3) are known to couple to Gi/o proteins, which inhibit cAMP formation and Ca2+ channels at pre- and postsynaptic sites, and they are known to activate GIRK channels at postsynaptic sites (Bellone et al. 2008; Knoflach et al. 2001). Activation of mGluR2 can trigger a form of postsynaptically expressed LTD in different areas of the cortex and hippocampus. At Schaffer collaterals to CA1 synapses, LTD has been shown to be dependent on the simultaneous activation of mGluR2 and A1 adenosine receptors by inhibiting adenylate cyclase, leading to less cAMP and reducing PKA activity (Santschi et al. 2006). Less PKA activity promotes the dephosphorylation and endocytosis of AMPAR. This mechanism is thus a plausible biochemical mechanism for PF-GoC LTD because a postsynaptic increase in Ca2+ does not seem to be necessary.
Although in Marr's seminal theory (1969) it was suggested that only PF-PC synapses should be plastic, plasticity has now been shown in a number of cerebellar synapses (Hansel et al. 2001). Recently, there has been a growing interest in the role of inhibitory interneurons and their relation to synaptic plasticity and circuit control. This is specifically true concerning the role of the GoC in cerebellar motor learning (Dugué et al. 2009; Prsa et al. 2009; Xu and Edgley 2008).
LTD of the PF input on GoCs can have significant effects on the cortical network activity of the cerebellum. GoCs play a central role in the information processing of the cerebellar cortex (Watanabe et al. 1998) by carefully regulating the silencing and the timing of feedback inhibition of GC activity (De Schutter and Bjaalie 2001; Tahon et al. 2005; Vos et al. 1999) and by probably generating regular synchronous oscillations over large GCL fields (D'Angelo 2008; Maex and De Schutter 1998). It is assumed that this oscillatory behavior plays a central role in precise timing of sensorimotor information (Isope et al. 2002) and cognition and memory consolidation by interacting with, for example, the long-term plasticity at the input stage of the cerebellar cortex (D'Angelo et al. 2009). Recent studies show that GoCs are extensively coupled through electrical synapses and display low-frequency oscillatory synchronization, imposing rhythmic inhibition onto GCs (Dugué et al. 2009). PF-GoC LTD can be envisaged to have a pronounced effect on these oscillations and the timing of feedback inhibition.
PF synapses have a low initial probability of release of glutamate (leading to PPF) and can thus function as high-pass filters that allow the GoC easily to detect bursts in presynaptic activity (Abbott and Regehr 2004; Beierlein et al. 2007). PF-GoC LTD induced after intense presynaptic stimulation could lead to a diminishment of inhibition at the input stage and could thus allow more activation of PCs in the corresponding cortical field on a longer time scale.
We used a synchronous but dispersed stimulation IP, which is a physiologically probable input pattern. Therefore PF-GoC LTD can serve as an example of use-dependent changes evoked in a relative small number of synapses with possible consequences for the activity of an entire network of interconnected neurons.
GRANTS
This work was supported by FWO Grants G0450.03 and G.0097.04 and by the University Antwerp (GOA-BOF2004).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. P. Achard, S. Dieudonné, C. Hansel, B. Barbour, D. Gall, M. de Ruiter, and A. Artola for useful advice and help.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Abbott and Regehr, 2004. Abbott LF, Regehr WG. Synaptic computation. Nature 431: 796–803, 2004 [DOI] [PubMed] [Google Scholar]
- Abitbol et al., 2008. Abitbol K, Acher F, Daniel H. Depression of excitatory transmission at PF-PC synapse by group III metabotropic glutamate receptors is provided exclusively by mGluR4 in the rodent cerebellar cortex. J Neurochem 105: 2069–2079, 2008 [DOI] [PubMed] [Google Scholar]
- Albus, 1971. Albus JS. A theory of cerebellar function. Math Biosci 10: 25–61, 1971 [Google Scholar]
- Barmack and Yakhnitsa, 2008. Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci 28: 1140–1152, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein et al., 2007. Beierlein M, Fioravante D, Regehr WG. Differential expression of posttetanic potentiation and retrograde signaling mediate target dependent short-term synaptic plasticity. Neuron 54: 949–959, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone et al., 2008. Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci 65: 2913–2923, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau et al., 2000. Bureau I, Dieudonné S, Coussen F, Mulle C. Kainate receptor-mediated synaptic currents in cerebellar Golgi cells are not shaped by diffusion of glutamate. Proc Natl Acad Sci USA 97: 6838–6843, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton et al., 2004. Chadderton P, Margrie TW, Häusser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature 428: 856–860, 2004 [DOI] [PubMed] [Google Scholar]
- Coesmans et al., 2004. Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44: 691–700, 2004 [DOI] [PubMed] [Google Scholar]
- D'Angelo, 2008. D'Angelo E. The critical role of Golgi cells in regulating spatio-temporal integration and plasticity at the cerebellum input stage. Front Neurosci 2: 35–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo et al., 2009. D'Angelo E, Koekkoek SK, Lombardo P, Solinas S, Ros E, Garrido J, Schonewille M, De Zeeuw CI. Timing in the cerebellum: oscillations and resonance in the granular layer. Neuroscience 162: 805–815, 2009 [DOI] [PubMed] [Google Scholar]
- D'Angelo et al., 2004. D'Angelo E, Rossi P, Gall D, Prestori F, Nieus T, Maffei A, Sola E. Long-term potentiation of synaptic transmission at the mossy fiber–granule cell relay of cerebellum. Prog Brain Res 248: 71–80, 2004 [DOI] [PubMed] [Google Scholar]
- De Schutter and Bjaalie, 2001. De Schutter E, Bjaalie JG. Coding in the granular layer of the cerebellum. Prog Brain Res 130: 279–296, 2001 [DOI] [PubMed] [Google Scholar]
- Dieudonné, 1995. Dieudonné S. Glycinergic synaptic currents in Golgi cells of the rat cerebellum. Proc Natl Acad Sci USA 92: 1441–1445, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné, 1998. Dieudonné S. Submillisecond kinetics and low efficacy of parallel fibre-Golgi cell synaptic currents in the rat cerebellum. J Physiol 510: 845–866, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné and Dumoulin, 2000. Dieudonné S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci 20: 1837–1848, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuguÉ et al., 2009. DuguÉ GP, Brunel N, Hakim V, Schwartz E, Chat M, Lévesque M, Courtemanche R, Léna C, DieudonnÉ S. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron 61: 126–139, 2009 [DOI] [PubMed] [Google Scholar]
- Dugué et al., 2005. Dugué GP, Dumoulin A, Triller A, Dieudonné S. Target-dependent use of coreleased inhibitory transmitters at central synapses. J Neurosci 25: 6490–6498, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers et al., 1997. Eilers J, Takechi H, Finch EA, Augustine GJ, Konnerth A. Local dendritic Ca2+ signaling induces cerebellar long-term depression. Learn Mem 4: 159–168, 1997 [DOI] [PubMed] [Google Scholar]
- Forti et al., 2006. Forti L, Cesana E, Mapelli J, D'Angelo E. Ionic mechanisms of autorhythmic firing in rat cerebellar Golgi cells. J Physiol 574: 711–729, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts et al., 2003. Geurts FJ, De Schutter E, DieudonnÉ S. Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer. Cerebellum 2: 290–299, 2003 [DOI] [PubMed] [Google Scholar]
- Geurts et al., 2001. Geurts FJ, Timmermans J, Shigemoto R, De Schutter E. Morphological and neurochemical differentiation of large granular layer interneurons in the adult rat cerebellum. Neuroscience 104: 499–512, 2001 [DOI] [PubMed] [Google Scholar]
- Hansel and Linden, 2000. Hansel C, Linden DJ. Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron 26: 473–482, 2000 [DOI] [PubMed] [Google Scholar]
- Hansel et al., 2001. Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci 4: 467–475, 2001 [DOI] [PubMed] [Google Scholar]
- Hartell, 1996. Hartell NA. Strong activation of parallel fibers produces localized calcium transients and a form of LTD that spreads to distant synapses. Neuron 16: 601–610, 1996 [DOI] [PubMed] [Google Scholar]
- Iansek and Redman, 1973. Iansek R, Redman SJ. An analysis of the cable properties of spinal motoneurons using a brief intracellular current pulse. J Physiol 231: 613–636, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isope et al., 2002. Isope P, Dieudonné S, Barbour B. Temporal organization of activity in the cerebellar cortex: a manifesto for synchrony. Ann NY Acad Sci 978: 164–174, 2002 [DOI] [PubMed] [Google Scholar]
- Ito, 1984. Ito M. The Cerebellum and Neural Control. New York: Raven Press, 1984 [Google Scholar]
- Ito, 2001. Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81: 1143–1195, 2001 [DOI] [PubMed] [Google Scholar]
- Ito, 2006. Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78: 272–303, 2006 [DOI] [PubMed] [Google Scholar]
- Ito and Kano, 1982. Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33: 253–258, 1982 [DOI] [PubMed] [Google Scholar]
- Johnson et al., 1999. Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]-LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology 38: 1519–1529, 1999 [DOI] [PubMed] [Google Scholar]
- Jörntell, 2008. Jörntell H. Input-output plasticity of peripheral responses in cerebellar Golgi cells. in vivo. J Physiol 586: 4789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell and Ekerot, 2002. Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron 34: 797–806, 2002 [DOI] [PubMed] [Google Scholar]
- Jörntell and Hansel, 2006. Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52: 227–238, 2006 [DOI] [PubMed] [Google Scholar]
- Kanichay and Silver, 2008. Kanichay RT, Silver RA. Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J Neurosci 28: 8955–8967, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim and Alger, 2001. Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci 21: 9608–9618, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston et al., 1998. Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37: 1–12, 1998 [DOI] [PubMed] [Google Scholar]
- Knoflach and Kemp, 1998. Knoflach F, Kemp JA. Metabotropic glutamate group II receptors activate a G-protein-coupled inwardly rectifying K+ current in neurons of the rat cerebellum. J Physiol 509: 347–354, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach et al., 2001. Knoflach F, Woltering T, Adam G, Mutel V, Kemp JA. Pharmacological properties of native metabotropic glutamate receptors in freshly dissociated Golgi cells of the rat cerebellum. Neuropharmacology 40: 163–169, 2001 [DOI] [PubMed] [Google Scholar]
- Koekkoek et al., 2003. Koekkoek SKE, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJH, De Zeeuw CI. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science 301: 1736–1739, 2003 [DOI] [PubMed] [Google Scholar]
- Kullmann and Lamsa, 2007. Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci 8: 687–699, 2007 [DOI] [PubMed] [Google Scholar]
- Maex and De Schutter, 1998. Maex R, De Schutter E. Synchronization of Golgi and granule cell firing in a detailed network model of the cerebellum granule cell layer. J Neurophysiol 80: 2521–2537, 1998 [DOI] [PubMed] [Google Scholar]
- Marr, 1969. Marr D. A theory of cerebellar cortex. J Physiol 202: 437–470, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick and Thompson, 1984. McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science 223: 296–299, 1984 [DOI] [PubMed] [Google Scholar]
- Medina and Mauk, 2000. Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nat Neurosci 3: 1205–1211, 2000 [DOI] [PubMed] [Google Scholar]
- Menuz et al., 2008. Menuz K, O'Brien JL, Karmizadegan S, Bredt DS, Nicoll RA. TARP redundancy is critical for maintaining AMPA receptor function. J Neurosci 27: 8740–8746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra et al., 2000. Misra C, Brickley SG, Farrant M, Cull-Candy SG. Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol 524: 147–162, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, 2009. Nakanishi S. Genetic manipulation study of information processing in the cerebellum. Neuroscience 162: 723–731, 2009 [DOI] [PubMed] [Google Scholar]
- Niswender and Conn, 2010. Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50: 295–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani et al., 2002. Otani S, Daniel H, Takita M, Crépel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci 22: 3434–3444, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay and Chan-Palay, 1974. Palay SL, Chan-Palay V. Cerebellar Cortex. New York: Springer, 1974 [Google Scholar]
- Pellionisz and Szentágothai, 1973. Pellionisz A, Szentágothai J. Dynamic single unit stimulation of a realistic network model. Brain Res 49: 83–99, 1973 [DOI] [PubMed] [Google Scholar]
- Pöschel and Manahan-Vaughan, 2005. Pöschel B, Manahan-Vaughan D. Group II mGluR-induced long term depression in the dentate gyrus in vivo is NMDA receptor-independent and does not require protein synthesis. Neuropharmacology 49: 1–12, 2005 [DOI] [PubMed] [Google Scholar]
- Prsa et al., 2009. Prsa M, Dash S, Catz N, Dicke PW, Thier P. Characteristics of responses of Golgi cells and mossy fibers to eye saccades and saccadic adaptation recorded from the posterior vermis of the cerebellum. J Neurosci 29: 250–262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh and Raman, 2006. Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51: 113–123, 2006 [DOI] [PubMed] [Google Scholar]
- Pugh and Raman, 2009. Pugh JR, Raman IM. Nothing can be coincidence: synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci 32: 170–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancillac and Crépel, 2004. Rancillac A, Crépel F. Synapses between parallel fibers and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J Physiol 554: 707–720, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi et al., 2003. Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol 548: 97–110, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santschi et al., 2006. Santschi LA, Zhang XI, Stanton PK. Activation of receptors negatively coupled to adenylate cyclase is required for induction of long-term synaptic depression at Schaffer collateral-CA1 synapses. J Neurobiol 66: 205–219, 2006 [DOI] [PubMed] [Google Scholar]
- Shin and Linden, 2005. Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol 94: 4281–4289, 2005 [DOI] [PubMed] [Google Scholar]
- Shinoda et al., 2000. Shinoda Y, Sugihara I, Wu HS, Sugiuchi Y. The entire trajectory of single climbing fiber and mossy fibers in the cerebellar nuclei and cortex. Prog Brain Res 124: 173–186, 2000 [DOI] [PubMed] [Google Scholar]
- Sillitoe et al., 2008. Sillitoe RV, Chung SH, Fritschy JM, Hoy M, Hawkes R. Golgi cell dendrites are restricted by Purkinje cell stripe boundaries in the adult mouse cerebellar cortex. J Neurosci 28: 2820–2826, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims and Hartell, 2005. Sims RE, Hartell NA. Differences in transmission properties and susceptibility to long-term depression reveal functional specialization of ascending axon and parallel fiber synapses to Purkinje cells. J Neurosci 25: 3246–3257, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims and Hartell, 2006. Sims RE, Hartell NA. Differential susceptibility to synaptic plasticity reveals a functional specialization of ascending axon and parallel fiber synapses to cerebellar Purkinje cells. J Neurosci 26: 5153–5159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas et al., 2007. Solinas S, Forti L, Cesana E, Mapelli J, De Schutter E, D'Angelo E. Fast reset of pacemaking and theta-frequency resonance patterns in cerebellar Golgi cells: simulations of their impact in vivo. Front Neurosci 1: 1–9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahon et al., 2005. Tahon K, Volny-Luraghi A, De Schutter E. Temporal characteristics of tactile stimuli influence the response profile of cerebellar Golgi cells. Neurosci Lett 390: 156–161, 2005 [DOI] [PubMed] [Google Scholar]
- Vos et al., 1999. Vos BP, Maex R, Volny-Luraghi A, De Schutter E. Cerebellar Golgi cells in the rat: receptive fields and timing of responses to facial stimulation. Eur J Neurosci 11: 2621–2634, 1999 [DOI] [PubMed] [Google Scholar]
- Watanabe et al., 1998. Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95: 17–27, 1998 [DOI] [PubMed] [Google Scholar]
- Watanabe and Nakanishi, 2003. Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron 39: 821–829, 2003 [DOI] [PubMed] [Google Scholar]
- Wright et al., 2000. Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. Binding of [3H](2S,1′S,2′S)-2-(9-xanthylmethyl)-2-(2′-carboxycyclopropyl) glycine ([3H]LY341495) to cell membranes expressing recombinant human group III metabotropic glutamate receptor subtypes. Naunyn Schmiedebergs Arch Pharmacol 362: 546–554, 2000 [DOI] [PubMed] [Google Scholar]
- Xu and Edgley, 2008. Xu W, Edgley SA. Climbing fibre-dependent changes in Golgi cell responses to peripheral stimulation. J Physiol 586: 4951–4959, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Linden, 2006. Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci 26: 6935–6944, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.