Abstract
Control of blood-borne infections is dependent on antigen-specific effector and memory T cells and high-affinity IgG responses. In chronic infections characterized by a high antigen load, it has been shown that antigen-specific T and B cells are vulnerable to downregulation and apoptosis. Anaplasma marginale is a persistent infection of cattle characterized by acute and chronic high-load bacteremia. We previously showed that CD4+ T cells primed by immunization with an A. marginale outer membrane protein were rapidly deleted following infection. Furthermore, peripheral blood T cell responses to bacteria were not observed after acute infection was controlled, suggesting dysfunctional T cell priming to other A. marginale antigens. The current study more closely investigated the kinetics of A. marginale-specific CD4+ T cell responses primed during infection. Frequent sampling of peripheral blood and spleens revealed that antigen-specific CD4+ T cell responses were first detected at 5 to 7 weeks, but the responses were sporadic and transient thereafter. A similar pattern was observed in animals sampled weekly for nearly 1 year. Paradoxically, by 2 weeks of infection, cattle had developed high titers of A. marginale-specific IgG, which remained high throughout persistent infection. This dysfunctional CD4+ T cell response to infection is consistent with continual downregulation or deletion of newly primed effector T cells, similar to what was observed for immunization-induced T cells following A. marginale infection. The failure to establish a strong memory T cell response during A. marginale infection likely contributes to bacterial persistence.
Many pathogens have evolved strategies to evade innate and antigen-specific host responses, which may result in persistent infection (14, 23, 40, 53). Chronic infections caused by blood-borne pathogens that achieve and maintain high antigen loads can result in progressive dysfunction and eventual apoptosis of antigen-specific effector and memory T or B cells (17, 28, 38, 50, 54, 55). The immune response to infections that persist with a high antigen load is unlike that following immunization or infection with a pathogen that is rapidly cleared (28). In the latter situation, the lymphocyte response contracts gradually, yielding a population of memory cells that are rapidly recalled upon subsequent encounter with antigen (24, 31). Anaplasma marginale is a persistent bacterial pathogen of cattle that is characterized by high levels of bacteremia, attaining levels of 108 to 109 organisms/ml of blood during acute infection. Control of acute infection generally begins at 4 to 5 weeks, but infection is not eliminated, and recurring peaks of 104 to 107 organisms/ml of blood occur throughout lifelong persistent infection (15, 19, 42). Thus, even during persistent infection, a continuous high antigen load is a prominent feature of this infection.
The mechanisms of immune control of A. marginale have not been completely elucidated, but in cattle protectively immunized against infection using purified outer membranes (OMs), protection was associated with OM protein (OMP)-specific CD4+ T cell responses, including gamma interferon (IFN-γ) production and proliferation and IgG2 production (10, 11). Nonimmunized infected cattle consistently produce high levels of OMP-specific IgG1 and IgG2 (43, 44, 46, 57), which are thought to be CD4+ T cell dependent (42). A. marginale continually undergoes antigenic variation in major surface protein 2 (MSP2) and MSP3 during infection (5, 37, 40), and variant-specific IgG2 is produced in response to each emerging variant (18), suggesting that IgG2 responses control the newly emerging variants but fail to eliminate the pathogen because new variants continually escape the immune response. Thus, the current paradigm of the role of CD4+ T cells in immunity to this infection is that antigen-specific CD4+ T lymphocyte priming and activation result in T cell expansion and IFN-γ secretion. IFN-γ, which in cattle induces isotype switching to IgG2 (16), is proposed to promote opsonization of bacteria or infected erythrocytes and to activate macrophages for enhanced phagocytosis and cytokine and nitric oxide production, which help eliminate intracellular bacteria (42).
Two previous studies have shown that cattle immunized with either A. marginale MSP2 or A. marginale MSP1a developed robust antigen-specific memory CD4+ T lymphocyte proliferation and IFN-γ secretion and then experienced a rapid loss of antigen-specific CD4+ T cell responses following infection (1, 22). The loss of the MSP1a-specific response was associated with physical loss of antigen-specific CD4+ T cells from the peripheral blood, monitored with major histocompatibility complex (MHC) class II tetramers (22). In addition, the response to MSP2 as well as to A. marginale homogenate remained undetectable for up to 1 year during persistent infection, but sampling was infrequent (1). This suggested that high-level bacteremia not only downregulated preexisting immunization-induced CD4+ T cell responses to a specific OMP but also impaired responses to these and additional bacterial antigens that should prime T cells during infection. We hypothesize that a continual high antigen load during acute and persistent anaplasmosis prevents the establishment of long-lived functional antigen-specific memory T cells.
In the present work, we investigated the kinetics of antigen-specific CD4+ T cell responses, primed by A. marginale infection, over the course of both acute and persistent infection in the peripheral blood and spleens of naïve cattle infected with A. marginale South Idaho or Florida strain organisms. Antigen-specific T cell proliferation and IFN-γ-secreting cells were monitored throughout infection. Our results are consistent with a functional dysregulation of A. marginale-specific CD4+ T lymphocytes primed during infection and failure to establish long-term memory. Paradoxically, high titers of A. marginale-specific IgG were maintained throughout infection, in spite of the impaired CD4+ T cell response. The failure to establish long-term memory T cell responses may be an important mechanism by which this bacterial pathogen modulates the host immune response.
MATERIALS AND METHODS
Cattle.
Four 6-month-old Holstein steers (animals C1028b1, C1030b1, 33875, and 33901) were serologically negative for A. marginale and received killed Clostridium sp. vaccine (Vision 7; Intervet) prior to onset of the study. Additionally, animals 33875 and 33901 underwent a surgery to marsupialize the body and tail of the spleen as previously described (49). All animals expressed one MHC class II DRB3*1101 allele and one different DRB3 allele, as determined by restriction fragment length polymorphism of exon II and sequencing (39, 45, 48). Spleen aspirates were obtained as described previously (21). Peripheral blood mononuclear cells (PBMCs) and splenocytes were washed and purified as described previously (2, 21, 22). Lymphocytes were immediately used in assays or cryopreserved in fetal bovine serum containing 10% dimethyl sulfoxide and stored in liquid nitrogen. All animal studies were conducted using an approved Institutional Animal Care and Use Center (Washington State University, Pullman, WA) protocol.
Infection with A. marginale.
Animals C1028b1 and C1030b1 were infected with A. marginale via transmission feeding of adult male Dermacentor andersoni ticks. Ticks were applied beneath a dermal patch and allowed to transmission feed for 7 days. Animals 33875 and 33901 were infected by intravenous inoculation of 1.0 × 106 fresh erythrocytes infected with the A. marginale Florida strain obtained from an acutely infected donor calf and diluted in Hanks' balanced salt solution (HBSS). All cattle were monitored daily by determination of rectal temperature, packed cell volumes (PCVs), and percent infected erythrocytes, as determined by light microscopy of Giemsa-stained blood films.
A. marginale-specific antibody quantitation.
Sera were collected approximately weekly during infection, and antibody specific to A. marginale MSP5 was measured. Sera were stored at −20°C until the assays were performed simultaneously on all samples. MSP5-specific antibody was detected using a competitive enzyme-linked immunosorbent assay (C-ELISA) commercial kit (VMRD) as previously described (29) and following the manufacturer's instructions. Immunoblotting was used to determine the titers of IgG antibody specific for A. marginale using sera diluted 1:100, 1:1,000, 1:3,000, 1:30,000, 1:100,000, and 1:1,000,000 in blocking buffer (I-block; Applied Biosystems) containing 5% bovine serum albumin (BSA) and 0.5% Tween 20 (New England Biolabs) essentially as described previously (10) but with the following modifications. A. marginale homogenate (200 μg) was boiled in sample buffer and electrophoresed on 10% Tris-HCl polyacrylamide Criterion gels (Bio-Rad) under denaturing conditions for 1 h at 100 V, and protein was transferred to 0.45-μm-pore-size nitrocellulose membranes at 100 V for 30 min. Membranes were cut into individual strips, dried overnight, and washed repeatedly with blocking buffer. Each strip was incubated with individual dilutions of serum for 1.5 h with rocking at room temperature. Membranes were washed and blocked with blocking buffer and incubated with mouse anti-bovine IgG1 and mouse anti-bovine IgG2 (both from Serotec) at a 1:100 dilution in blocking buffer for 1.5 h. Tertiary antibody was horseradish peroxidase-conjugated goat anti-mouse IgG (Kirkegaard and Perry Laboratories) diluted 1:10,000 in blocking buffer. Blots were washed and developed with enhanced chemiluminescence Western blotting substrate (Thermo Scientific Pierce). Preimmune sera and uninfected erythrocyte (URBC) membranes were used as negative controls, and serum from an A. marginale South Idaho strain-infected calf (C894b1) with known A. marginale-specific IgG2 and IgG2 titers was used as a positive control to standardize the immunoblots.
Quantitative PCR for detection and quantitation of bacteria.
To quantify A. marginale in blood during infection, a previously described quantitative TaqMan assay (Applied Biosystems) based on the copy number of the A. marginale msp5 gene was performed (20, 47). Peripheral blood collected weekly during infection was washed three times in phosphate-buffered saline (PBS; pH 7.2) with centrifugation to remove leukocytes. Washed erythrocytes were diluted 1:1 in PBS and frozen at −20°C until samples were processed. DNA was extracted from 300 μl of washed erythrocytes using a genPURE kit (Qiagen). Reverse transcription-PCRs (RT-PCRs) were performed in triplicate using 100 ng of template DNA per reaction mixture in a total volume of 50 μl including 25 μl TaqMan Universal buffer, 12.5 μg msp5 forward primer, 12.5 μg msp5 reverse primer, 1.25 μg of msp5 probe, and 17.4 μl of nuclease-free water. Once the bacterial copy number was determined for the 100 ng of template DNA, the number of organisms per ml of whole blood was calculated.
Lymphocyte proliferation assays.
Lymphocytes from fresh or cryopreserved PBMCs and spleen biopsy specimens were assayed in triplicate using 2.5 × 105 viable cells/well in complete RPMI 1640 medium (Gibco) as previously described (1, 2, 10, 22). Cells were stimulated in 96-well round-bottomed plates in a volume of 100 μl/well with 15.0 μg/ml of antigen using A. marginale South Idaho or Florida strain homogenate (matching infection strain) and negative-control URBC or Babesia bovis membranes. T cell growth factor diluted 1:10 and Clostridium sp. vaccine antigen diluted 1:100 in complete RPMI 1640 medium were positive controls (22). Cells were cultured for 6 days at 37°C in 5% CO2, labeled with 0.25 μCi [3H]thymidine for 8 h, harvested, and counted in a beta counter. Short-term cell lines were established by culturing 4.0 × 106 cryopreserved PBMCs in 1.5 ml complete RPMI 1640 medium with 5 μg/ml of A. marginale homogenate in a 24-well plate for 6 days. Proliferation assays with these cells were performed as described above using 2.0 × 104 lymphocytes with 2.5 × 105 fresh autologous irradiated PBMCs/well, which were cultured for 4 days before they were harvested and counted. Results are presented as the mean cpm to A. marginale minus the mean cpm to negative-control antigen.
In some experiments, cryopreserved PBMCs or cell lines were depleted of CD4+, CD8+, and/or γδ T cells and cultured in 6- or 4-day proliferation assays. Depletion was accomplished by incubating cells with monoclonal antibodies (MAb)s, obtained from the Washington State Monoclonal Antibody Center, specific for CD4+ (ILA-11), CD8+ (7C2B), and γδ (GB21A) T cells at 15 μg MAb per 107 cells rotating at 4°C for 30 min. Cells were repeatedly washed with compete RPMI 1640 medium and MACS bead buffer (Miltenyi) with 5% BSA. MAb-labeled cells were incubated on ice for 20 min with 80 μl of goat anti-mouse IgG magnetic microbeads (Miltenyi) per 107 cells. MAb- and bead-labeled cells were depleted by filtration through an LS magnetic column (Miltenyi) under a strong magnetic field and washed with 3 ml of MACS buffer with BSA. Depletion and enrichment of cell subsets were verified by flow cytometry, and cells were assayed for proliferation to antigen as described above for cell lines and PBMCs. Statistical significance was determined for each proliferation assay by comparing the mean counts per minute of cells cultured with A. marginale antigen versus the mean counts per minute of cells cultured with negative-control antigen at the same concentration, using a Student's one-tailed t test with a Welch correction, where significant results had P values of <0.05. The results of proliferation assays with significant A. marginale-specific responses were then compared over the course of infection in reference to preinfection values using a one-way analysis of variance with a Bonferroni correction for multiple comparisons, and results were considered significant where P values were <0.05.
Bovine IFN-γ ELISPOT assays.
Bovine IFN-γ enzyme-linked immunospot (ELISPOT) assays were performed in triplicate using 1.0 × 106 cryopreserved PBMCs and splenocytes/well and cultured with 15.0 μg/ml A. marginale South Idaho or Florida strain homogenate or medium as described previously (1, 2). A mixture of 1.0 μg/ml phytohemagglutinin (Sigma-Aldrich) plus 0.01 ng/ml recombinant human interleukin-12 (IL-12) (Genetics Institute) was used as the positive control. Antigen-specific responses were determined by comparing the mean number of spot-forming cells (SFCs) cultured with A. marginale homogenate to the mean number of SFCs cultured with medium, and statistical significance was determined using a Student's one-tailed t test, where a response with a P value of <0.05 was considered significant.
RESULTS
Infection with A. marginale South Idaho strain.
To observe the dynamics of infection-primed antigen-specific CD4+ T cell and antibody responses during acute and persistent infection, two calves were infected by transmission feeding D. andersoni ticks infected with the South Idaho strain of A. marginale. Animals C1028b1 and C1030b1 developed fever, malaise, and clinical anemia that were concurrent with peak blood levels of bacteria of 4.95 × 108 and 4.51 × 107 organisms/ml of blood, respectively, on day 31 postinfection (Fig. 1A and B). Both animals resolved the anemia by day 55 postinfection. Maximal decreases in PCVs were 34.5% (C1028b1) and 43.8% (C1030b1). Persistent-phase anaplasmosis was characterized by cyclical waves of bacteremia that peaked as high as 9.6 × 106 organisms/ml of blood until day 336 postinfection, the termination of the study (Fig. 1A and B).
FIG. 1.
Antigen-specific antibody and lymphocyte proliferative responses during the course of infection with A. marginale South Idaho strain. Animals C1028b1 and C1030b1 were infected via transmission feeding of A. marginale South Idaho strain-infected D. andersoni ticks. (A and B) A. marginale-specific antibody was measured weekly throughout infection with the MSP5 C-ELISA, and results are presented as percent inhibition, where values of >30% inhibition were considered significant; (C and D) A. marginale-specific lymphocyte proliferation was determined using fresh PBMCs; (E and F) proliferation specific for an unrelated vaccine antigen, a Clostridium sp. vaccine antigen, was determined using cryopreserved PBMCs. Asterisks denote significant responses compared with those on URBC or B. bovis membranes, where P is <0.05. All graphs are superimposed over levels of bacteremia, presented as log10 number of organisms/ml of blood. (G and H) Cryopreserved lymphocytes from selected time points with significant A. marginale-specific proliferation were cultured for 1 week and depleted of CD4+, CD8+, or γδ T cells. Depleted cells were used in proliferation assays with A. marginale South Idaho strain antigen. Significant decreases in proliferation compared to that for the nondepleted cell line are denoted with asterisks, where P is <0.05.
Humoral responses to A. marginale measured by MSP5 C-ELISA showed that animals developed significant levels of A. marginale-specific antibody (>30% inhibition) by day 28 postinfection, and the levels remained elevated (>50% inhibition) throughout persistent infection (Fig. 1A and B). Serum IgG1 and IgG2 titers (Table 1), determined by Western blotting using A. marginale South Idaho strain antigen, showed that A. marginale-specific IgG1 and IgG2 were detectable by day 9 postinfection with titers of 1,000 and 30,000, respectively, for animal C1028b1 and 30,000 and 10,000, respectively, for animal C1030b1. Titers remained high over the course of infection, reaching 100,000 late in infection, consistent with C-ELISA data.
TABLE 1.
A. marginale-specific serum IgG1 and IgG2 titers from South Idaho strain-infected cattlea
| Day p.i. | Titer |
|||
|---|---|---|---|---|
| IgG1 |
IgG2 |
|||
| C1028b1 | C1030b1 | C1028b1 | C1030b1 | |
| 9 | 1,000 | 10,000 | 30,000 | 10,000 |
| 29 | 30,000 | 30,000 | 10,000 | 10,000 |
| 94 | 100,000 | 10,000 | 100,000 | 100,000 |
| 189 | 30,000 | 100,000 | 10,000 | 1,000 |
| 274 | 100,000 | 100,000 | 100,000 | 100,000 |
Serum was collected on the specified days postinfection (p.i.) and frozen at −20°C until Western blots were performed simultaneously. Blots for animals C1028b1 and C1030b1 were prepared with frozen stabilate using approximately 2 × 108 red blood cells infected with A. marginale South Idaho strain. All responses were interpreted as a response to a protein of ∼35 kDa, corresponding to MSP2. Serum was diluted 1:100 to 1:1,000,000 with I-block.
T cell proliferation assays were performed with fresh PBMCs stimulated with A. marginale South Idaho strain homogenate (Fig. 1C and D). Animals failed to developed significant antigen-specific proliferative responses until days 106 (C1028b1) and 112 (C1030b1) postinfection, and responses were transient and not significant 1 to 2 weeks later. Proliferation was not consistently associated with the level or fluctuation in the level of bacteremia, as in animal C1028b1 the response seemed to parallel bacteremia, whereas in animal C1039b1 peaks of proliferation were observed when levels of bacteremia dropped. However, in both animals initial proliferative responses occurred after bacteremia dropped below 106 organisms/ml of blood. Between days 106 and 336 postinfection, significant antigen-specific lymphocyte proliferation was observed eight and seven separate times in animals C1028b1 and C1030b1, respectively, and the responses were similarly transient. These results were repeated using cryopreserved cells (data not shown). In contrast, T lymphocytes responded to positive-control Clostridium sp. vaccine antigen, and responses were statistically significant throughout the course of the infection, although they did fluctuate (Fig. 1E and F). These results indicate that the poor A. marginale-specific T cell responses were not due to generalized or nonspecific immune suppression.
To characterize the lymphocytes responding to A. marginale at time points where significant responses were observed, proliferation was repeated using short-term cell lines derived from cryopreserved PBMCs from responding time points. Cell lines were depleted of CD4+ cells, CD8+ cells, or γδ T cells using magnetic beads coated with lymphocyte-specific MAb (Fig. 1G and H). A. marginale-specific proliferation was maintained when cell lines were enriched for CD4+ T lymphocytes by depleting either CD8+ cells or γδ T cells, but responses were lost when cell lines were depleted of CD4+ T lymphocytes. In one experiment with C1030b1 cells, depleting γδ T cells also resulted in significantly diminished proliferation (Fig. 1H). These results demonstrate that A. marginale-specific lymphocyte proliferation was predominantly due to antigen-specific CD4+ T lymphocytes, although in some experiments γδ T cells may contribute to this response, as shown for certain γδ T cell clones (30).
To enumerate antigen-specific cells and to characterize IFN-γ production over the course of infection, IFN-γ ELISPOT assays were performed using cryopreserved PBMCs stimulated with A. marginale. PBMCs from time points that had antigen-specific CD4+ T lymphocyte proliferation did not show statistically significant numbers of A. marginale-specific IFN-γ-secreting cells compared to the background numbers of antigen-specific IFN-γ-producing cells (cultured without antigen), although for the majority of time points sampled there were fewer SFCs in antigen-stimulated cells. Significant numbers of antigen-specific SFCs were noted only one time in each calf on days not associated with significant proliferation (Table 2).
TABLE 2.
Frequency of IFN-γ-secreting cells in PBMCs over the course of infection in A. marginale South Idaho-infected cattlea
| C1028b1 |
C1030b1 |
||||
|---|---|---|---|---|---|
| Day p.i. | Mean no. of SFCs per 106 PBMCs in response to: |
Day p.i. | Mean no. of SFCs per 106 PBMCs in response to: |
||
| Medium | A. marginale | Medium | A. marginale | ||
| 21 | 146 | 80 | 21 | 480 | 212 |
| 28 | 27 | 112 | 49 | 688 | 354 |
| 49 | 20 | 21 | 56 | 292 | 142 |
| 63 | 286 | 354 | 126 | 676 | 497 |
| 84 | 57 | 122 | 133 | 476 | 349 |
| 119 | 854 | 274 | 168 | 637 | 264 |
| 126 | 272 | 123 | 175 | 431 | 132 |
| 140 | 84 | 52 | 196 | 925 | 765 |
| 175 | 217 | 269 | 231 | 663 | 403 |
| 224 | 103 | 72 | 238 | 445 | 448 |
| 231 | 138 | 39 | 252 | 374 | 140 |
| 238 | 206 | 74 | 259 | 596 | 415 |
| 273 | 130 | 89 | 273 | 384 | 348 |
| 280 | 174 | 206 | 280 | 200 | 103 |
| 294 | 222 | 28 | 294 | 325 | 492 |
| 302 | 164 | 109 | 301 | 345 | 264 |
| 309 | 265 | 103 | |||
| 315 | 48 | 28 | |||
Cryopreserved PBMCs were thawed and used simultaneously in the same assay to reduce assay-to-assay variation. IFN-γ ELISPOT assays were performed in triplicate with 1 × 106 cells per well with either medium or 15 μg/ml A. marginale South Idaho strain homogenate for 48 h at 37°C. Results are presented as the mean number of SFCs, with boldface values showing the number of SFCs in response to antigen that are significantly greater than the number of SFCs in response to medium (P < 0.05). p.i., postinfection.
Infection of spleen-marsupialized cattle with A. marginale Florida strain.
The lack of antigen-specific CD4+ T lymphocyte responses during acute infection is difficult to reconcile with early production of antigen-specific IgG and development of high and persistent IgG titers, which is typically dependent on CD4+ T lymphocyte help. This led us to determine whether antigen-specific CD4+ T lymphocytes were present in the spleen, where infected erythrocytes are presumably removed (36), and whether responses occurred more transiently than we may have detected by sampling animals weekly. Also, it was possible that exposure to ticks induced an early immune suppression to A. marginale (51). To address these possibilities and to determine whether similar poor T cell priming occurred in response to a different A. marginale strain, naïve animals with spleens surgically marsupialized to permit frequent sampling by needle biopsy were infected intravenously with the non-tick-transmissible Florida strain, and the sampling frequency was increased. Florida strain-infected animals showed typical signs of acute infection, including fever, malaise, and bacteremia, peaking at 4.78 × 109 (calf 33875) and 6.58 × 109 (calf 33901) organisms/ml of blood on days 31 and 26 postinfection, respectively (Fig. 2 A and B). Anemia was quite severe and was associated with decreases in PCVs of 61.8% (animal 33875) and 62.3% (animal 33901). Both cattle resolved anemia by day 69 postinfection and remained persistently infected, with levels of bacteremia ranging from 104 to 108 organisms/ml of blood. PBMCs and splenocytes were collected every 2 to 3 days during acute infection and then approximately weekly over the remainder of the study to monitor lymphocyte responses.
FIG. 2.
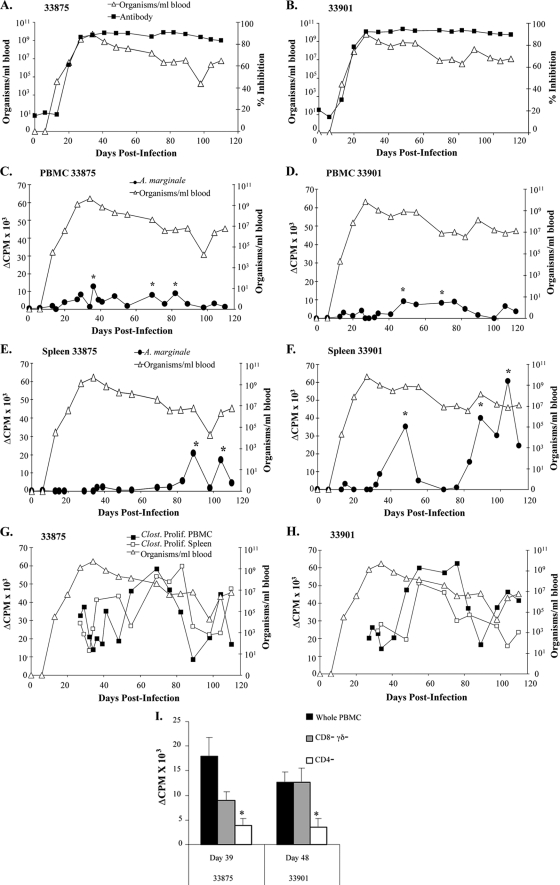
A. marginale-specific antibody and proliferative responses of PBMCs and splenocytes over the course of infection with A. marginale Florida strain. (A and B) Animals 33875 and 33901 were infected intravenously with A. marginale Florida strain-infected erythrocytes, and A. marginale-specific antibody was measured weekly with C-ELISA; (C to F) A. marginale-specific lymphocyte proliferation was performed with fresh PBMCs (C and D) or splenocytes (E and F). Cells were collected every 3 to 7 days during acute infection and in general weekly thereafter. Asterisks denote significant responses compared with those on the URBC membrane, where P is <0.05. (G and H) Proliferation specific for an unrelated vaccine antigen, Clostridium sp. vaccine antigen, was determined using fresh PBMCs and splenocytes, and responses were significant at all time points (although they are not indicated by asterisks). All graphs are superimposed over levels of bacteremia, presented as log10 number of organisms/ml of blood, for 110 days following infection. (I) Cryopreserved lymphocytes from selected time points with significant A. marginale-specific proliferation were depleted of either CD4+ cells or CD8+cells and γδ T cells, and proliferation assays were performed. Significant decreases in proliferation compared to that of the nondepleted cell line are denoted with asterisks, where P is <0.05.
Antigen-specific antibody and lymphoproliferative responses in A. marginale Florida-infected animals.
Florida strain-infected animals produced MSP5-specific antibody by day 20 postinfection (Fig. 2A and B). Antibody levels increased sharply and by day 27 postinfection maintained levels of 83.49 and 94.56% inhibition in the two animals, respectively, for the duration of the study. IgG1 and IgG2 titers determined by Western blotting using A. marginale Florida strain homogenate (Table 3) were 30,000 and 3,000 for the two animals, respectively, on the first day of sampling (day 13 postinfection). Titers fluctuated between 3,000 and 30,000 thereafter. These data and those in Table 1 show that both IgG1 and IgG2 were produced in response to infection.
TABLE 3.
A. marginale-specific serum IgG1 and IgG2 titers from Florida strain-infected calvesa
| Day p.i. | Titer |
|||
|---|---|---|---|---|
| IgG1 |
IgG2 |
|||
| 33875 | 33901 | 33875 | 33901 | |
| 0 | <100 | <100 | <100 | <100 |
| 13 | 30,000 | 3,000 | 30,000 | 3,000 |
| 20 | 30,000 | 3,000 | 3,000 | 30,000 |
| 76 | 3,000 | 3,000 | 3,000 | 3,000 |
Serum was collected on specified days postinfection (p.i.) and frozen at −20°C until Western blots were performed simultaneously. Blots for calves 33875 and 33901 were prepared with 20 μg of Florida strain initial body lysate. All responses were interpreted as a response to a protein of ∼35 kDa, corresponding to MSP2. Serum was diluted 1:100 to 1:1,000,000 with I-block. A value of <100 denotes a nondetectable antigen-specific response.
Antigen-specific T lymphocyte responses in PBMCs were detected earlier in Florida strain-infected animals than in South Idaho-infected animals on days 36 (animal 33875) and 48 (animal 33901) postinfection, concurrent with declining levels of bacteremia (Fig. 2C and D). Earlier detection of antigen-specific lymphocyte proliferation in the blood may be due to more frequent sampling. However, similar to South Idaho-infected animals, in Florida strain-infected animals antigen-specific proliferation was transient, lost within 1 to 2 weeks following initial detection, and recurred sporadically on days 69 and 82 (calf 33875) and on day 69 (calf 33901) postinfection. Cryopreserved cells from all responsive time points were retested in lymphocyte proliferation assays, and similar proliferation results were obtained at least twice (data not shown). Overall, our results with PBMCs indicate that antigen-specific T cell responses do occur in the peripheral blood during acute infection, although responses are transient and detectable for no more than two consecutive weeks. Additionally, responses prior to the peak of infection, in the face of ascending and high levels of bacteremia, could not be detected, despite increasing the sampling frequency to every 2 to 3 days.
Significant antigen-specific proliferation in the spleen was initially detected in animal 33901 on day 48, which coincided with significant proliferation in the peripheral blood, and in animal 33875 on day 89 postinfection. These responses were transient, as in the peripheral blood, and were absent the following week, recurring sporadically on day 104 (animal 33875) and days 89 and 104 (animal 33901) postinfection (Fig. 2E and F). The responses were also repeatable using cryopreserved cells (data not shown). These data suggest that poor PBMC responses to A. marginale were not explained by sequestration of responding cells in the spleen during acute or persistent infection.
Lymphocytes were cultured over the course of infection with the Clostridium sp. vaccine antigen as a positive control (1, 22). PBMCs and splenocytes maintained significant proliferative responses to this antigen at all time points in both animals, although the levels varied, again demonstrating that poor A. marginale-specific T cell responses were not due to generalized immune suppression (Fig. 2G and H).
Antigen-specific lymphocytes in PBMCs were further characterized by depleting CD4+ T cells or CD8+ and γδ T cells and repeating the proliferation assays (Fig. 2I). PBMCs maintained significant A. marginale-specific proliferation when the cells were enriched for CD4+ T lymphocytes following depletion of CD8+ and γδ T cells but had insignificant proliferation following depletion of CD4+ T cells. This again indicates that antigen-specific lymphocyte proliferation was predominantly mediated by CD4+ T lymphocytes.
To enumerate responding CD4+ T cells and to characterize IFN-γ production during infection, responding and nonresponding cryopreserved PBMCs and splenocytes cultured with A. marginale Florida strain homogenate were used in IFN-γ ELISPOT assays. In these animals, background numbers of SFCs increased following infection, although significant numbers of A. marginale-specific IFN-γ-secreting cells were not detected in PBMCs or the spleen at any time point where it was possible to perform the statistical analysis (Table 4). As observed in Table 2, for the majority of time points sampled, SFC numbers were lower following antigen stimulation of PBMCs, although this was not the case with spleen cells.
TABLE 4.
Frequency of IFN-γ-secreting cells in PBMCs and spleen lymphocytes over the course of infection in A. marginale Florida-infected cattlea
| Animal, cell or organ, and day p.i. | Mean no. of SFCs per 106 lymphocytes in response to: |
|
|---|---|---|
| Medium | A. marginale | |
| 33875, PBMC | ||
| 0 | 19 | 79 |
| 18 | 226 | 180 |
| 27 | 82 | 52 |
| 76 | 110 | 33 |
| 104 | 144 | 110 |
| 33901, PBMC | ||
| 0 | 4 | 6 |
| 6 | 5 | 12 |
| 18 | 40 | 15 |
| 27 | 52 | 37 |
| 32 | 49 | 22 |
| 76 | 102 | 65 |
| 104 | 118 | 91 |
| 33875, spleen | ||
| 0 | 28 | 86 |
| 36 | 600 | 600 |
| 55 | 121 | 600 |
| 76 | 369 | 600 |
| 98 | 254 | 89 |
| 33901, spleen | ||
| 0 | 20 | 12 |
| 25 | 7 | 9 |
| 48 | 65 | 55 |
| 55 | 94 | 427 |
| 76 | 48 | 96 |
| 98 | 91 | 95 |
| 104 | 8 | 28 |
Cryopreserved PBMCs were thawed and used simultaneously in the same assay to reduce assay-to-assay variation. IFN-γ ELISPOT assays were performed in triplicate with 1 × 106 cells per well with either medium or 15 μg/ml A. marginale Florida strain homogenate for 48 h at 37°C. Results are presented as the mean numbers of SFCs. p.i., postinfection. For values of ≥600 SFCs (too numerous to count), a statistical comparison was not performed.
DISCUSSION
Cattle that survive infection with the intracellular bacterium A. marginale are incapable of completely eliminating the organism and remain persistently infected for life, although they are asymptomatic and otherwise immunocompetent (41). Our work has focused on understanding how A. marginale escapes and modulates the immune response to facilitate long-term persistence in this natural disease model. In previous studies we found that cattle immunized with A. marginale outer membranes were completely protected from infection, and protection correlated with OMP-specific CD4+ T cell responses, including IFN-γ and IgG2 production (10, 11). Conversely, when cattle were immunized with native MSP2 or a recombinant partial MSP1a, which did not elicit protection against infection, there was a rapid loss of immunization-induced antigen-specific T cell responses, in one case documented as a loss in specific CD4+ T cells, concurrent with peak levels of bacteremia during acute infection (1, 22). Furthermore, the animals failed to develop new A. marginale-specific peripheral blood T cell responses during infection. This suggested that infection with A. marginale may impair priming of additional CD4+ T cells in response to infection. The current study was designed to systematically monitor Anaplasma-specific T cell responses during acute and long-term persistent infection and to determine if specific T cells were sequestered in the spleen. We also examined responses to Clostridium vaccine antigen, which were significant throughout A. marginale infection in both spleen and peripheral blood. The results support the generation of an abnormal memory CD4+ T cell response during A. marginale infection and not sequestration of cells to the spleen or a generalized immune suppression.
Our data show that the predominant lymphocytes that do proliferate in response to A. marginale during infection are CD4+ T cells, based on the findings of depletion experiments. This is logical, as A. marginale infects erythrocytes, which do not express MHC molecules. Thus, exogenous antigen must be taken up and presented by professional antigen-presenting cells, which favors CD4+ T cell priming. However, in one experiment depletion of γδ T cells also reduced the proliferative response. We have previously isolated γδ T cell clones from cattle immunized with MSP2 that responded to A. marginale and MSP2 (30), so it is possible that at some time points during infection γδ T cells do respond to A. marginale. It is not likely that γδ T cells are more important early in infection, as depletion of these cells from PBMCs harvested on days 39 and 48 (Fig. 2I) did not significantly reduce the proliferative response.
The mechanisms resulting in impaired antigen-specific T cell responses during A. marginale infection are not known. It does not appear that exposure to ticks can explain the dysfunctional response, as this was observed in cattle inoculated intravenously with infected blood as well. One possibility is that transient T cell responses result from periodic escape from a regulatory environment, such as that imposed by regulatory T cells, a mechanism that has been well described in many other persistent bacterial and viral infections (3). Another explanation is that impaired T cell responses result from continual deletion of newly primed antigen-specific CD4+ T cells in response to high antigen load. Mechanistically, this may occur through activation-induced cell death from chronic antigen stimulation of the T cell receptor, resulting in deletion of antigen-specific T cell clones (28). It is likely that naïve T cells are continually primed to new antigenic variants of immunodominant MSP2 and MSP3 that arise during the course of infection (6, 37), as we have shown that both conserved and hypervariable regions of MSP2 are immunogenic (1, 2, 7-10, 18). There may also be continuous priming and expansion of CD4+ T cells to subdominant OMP epitopes (33, 34) over the course of persistent infection, as has been described in chronic viral and mycobacterial infections (27, 52).
Dysfunction of antigen-specific CD4+ T cells has been described in other persistent blood-borne infections characterized by a chronic high antigen load. Examples include human immunodeficiency virus (HIV) (56) and mouse models of malaria and Brugia pahangi microfilaremia (25, 55). During infection with HIV, CD4+ T cells undergo progressive dysfunction characterized by loss of IL-2 production ability but a retained ability to produce IFN-γ. This resulted in a short-lived effector phenotype of cells incapable of proliferation and therefore undetectable in antigen-specific proliferation assays. Such cells have been successfully rescued in vitro by culturing with IL-2 (56). In the mouse malaria model, adoptively transferred antigen-specific CD4+ T cells were rapidly deleted from blood and tissues in an IFN-γ-dependent manner following infection (55). During infection with Brugia pahangi microfilariae, CD4+ T cells had defective proliferation but did produce IFN-γ. The T cells underwent apoptosis ex vivo in response to antigen in an IFN-γ-dependent manner (25). In other models of infection-mediated T cell apoptosis, T cell-produced IFN-γ was shown to be mechanistically involved in the dysfunctional response by driving intrinsic and extrinsic pathways of apoptosis (13, 32). We rarely detected antigen-specific IFN-γ-secreting T cells following A. marginale infection (Table 2), and culturing PBMCs from nonresponding time points with antigen and several concentrations of IL-2 from suboptimal to optimal failed to elicit an antigen-specific proliferative response greater than that of IL-2 alone (data not shown). These results suggest that the lack of proliferation at many time points sampled throughout infection is not simply due to a lack of IL-2 production or inhibitory effects of IFN-γ.
Paradoxically, in spite of the inability to detect T cell responses early in infection, all cattle produced high titers of antigen-specific IgG1 and IgG2 as early as 9 days postinfection and maintained high titers for up to 1 year. Several possibilities may explain the isotype switching and high titers of IgG1 and IgG2 in the absence of detectable antigen-specific CD4+ T cell responses. One is that antigen-specific T cells were present in other lymphoid organs not sampled, such as lymph nodes, lungs, liver, bone marrow, or gut. Interestingly, T cells specific for Plasmodium yoelii sporozoite antigens were primed in mouse lymph nodes draining the site of mosquito bites (12). In tick-transmitted A. marginale South Idaho strain-infected cattle, T cell priming could similarly occur in draining lymph nodes, but the cells should then traffic to the spleen, where infected erythrocytes are removed. In cattle infected intravenously with the Florida strain, one would expect T cell priming and expansion to occur in the spleen. Despite sampling repeatedly early in infection, we failed to detect antigen-specific proliferative and IFN-γ-productive responses in the spleen. It is also possible, but less likely, that IgG is produced in a CD4+ T cell-independent (TI) manner (4, 26, 35), although TI antibody responses are generally characterized by higher and shorter-lived antigen-specific IgM responses and seldom account for prolonged high-affinity IgG antibody responses.
In summary, we provide evidence that A. marginale-specific CD4+ T cells primed during infection develop a poor memory response. Downregulation of the T cell response may prevent prolonged and likely deleterious systemic inflammation in the infected host in response to continual high levels of bacteria. A. marginale-mediated immune regulation would be beneficial for the pathogen as well as the host, which acts as a reservoir to ensure pathogen survival at high enough concentrations for efficient tick transmission of A. marginale to other naïve animals within areas of endemicity.
Acknowledgments
We thank Xiaoya Cheng, Emma Karel, Beverley Hunter, and Shelley Whidbee for excellent technical assistance and Will Goff, Waithaka Mwangi, and Viveka Vadyvaloo for help with procedures.
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases, grants R01-AI44005, R01-AI053692, and T32-AI07025-30 and United States Department of Agriculture, National Institute of Food and Agriculture, grant 2010-65119-20456.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Abbott, J. R., G. H. Palmer, K. A. Kegerreis, P. F. Hetrick, C. J. Howard, J. C. Hope, and W. C. Brown. 2005. Rapid and long-term disappearance of CD4+ T lymphocyte responses specific for Anaplasma marginale major surface protein-2 (MSP2) in MSP2 vaccinates following challenge with live A. marginale. J. Immunol. 174:6702-6715. [DOI] [PubMed] [Google Scholar]
- 2.Abbott, J. R., G. H. Palmer, C. J. Howard, J. C. Hope, and W. C. Brown. 2004. Anaplasma marginale major surface protein 2 CD4+ T cell epitopes are evenly distributed in conserved and hypervariable regions (HVR), whereas linear B-cell epitopes are predominantly located in the HVR. Infect. Immun. 72:7360-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belkaid, Y. 2007. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7:875-888. [DOI] [PubMed] [Google Scholar]
- 4.Bitsaktsis, C., N. Bisweswar, R. Racine, K. C. MacNamara, and G. M. Winslow. 2007. T-cell-independent humoral immunity is sufficient for protection against fatal intracellular Ehrlichia infection. Infect. Immun. 75:4933-4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brayton, K. A., P. F. Meeus, A. F. Barbet, and G. H. Palmer. 2003. Simultaneous variation of the immunodominant outer membrane proteins, MSP2 and MSP3, during Anaplasma marginale persistence in vivo. Infect. Immun. 71:6627-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brayton, K. A., D. P. Knowles, T. C. McGuire, and G. H. Palmer. 2001. Efficient use of a small genome to generate antigenic diversity in tick-borne ehrlichial pathogens. Proc. Natl. Acad. Sci. U. S. A. 98:4130-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, W. C., K. A. Brayton, C. M. Styer, and G. H. Palmer. 2003. The hypervariable region of Anaplasma marginale major surface protein 2 (MSP2) contains multiple immunodominant CD4+ T lymphocyte epitopes that elicit variant-specific proliferative and IFN-γ responses in MSP2 vaccinates. J. Immunol. 170:3790-3798. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4+ T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:1114-1124. [DOI] [PubMed] [Google Scholar]
- 9.Brown, W. C., G. H. Palmer, H. A. Lewin, and T. C. McGuire. 2001. CD4+ T lymphocytes from calves immunized with Anaplasma marginale major surface protein 1 (MSP1), a heteromeric complex of MSP1a and MSP1b, preferentially recognize the MSP1a carboxyl terminus that is conserved among strains. Infect. Immun. 69:6853-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, W. C., D. Zhu, V. Shkap, T. C. McGuire, E. F. Blouin, K. M. Kocan, and G. H. Palmer. 1998. The repertoire of Anaplasma marginale antigens recognized by CD4+ T-lymphocyte clones from protectively immunized cattle is diverse and includes major surface protein 2 (MSP-2) and MSP-3. Infect. Immun. 66:5414-5422. [DOI] [PMC free article] [PubMed]
- 12.Chakravarty, S., I. A. Cockburn, S. Kuk, M. G. Overstreet, J. B. Sacci, and F. Zavala. 2007. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat. Med. 9:1035-1041. [DOI] [PubMed] [Google Scholar]
- 13.Dalton, D. K., L. Haynes, C. Q. Chu, S. L. Swain, and S. Wittmer. 2000. Interferon-γ eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietsch, K. W., S. A. Lukehart, and J. R. Stringer. 2009. Common strategies for antigenic variation by bacterial, fungal, and protozoan pathogens. Nat. Rev. Microbiol. 7:493-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eid, G., D. M. French, A. M. Lundgren, A. F. Barbet, T. F. McElwain, and G. H. Palmer. 1996. Expression of major surface protein 2 antigenic variants during acute Anaplasma marginale rickettsemia. Infect. Immun. 64:836-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes, D. M., N. M. Closser, and G. K. Allen. 1994. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-mu and mitogen. Cell. Immunol. 154:287-295. [DOI] [PubMed] [Google Scholar]
- 17.Freeman, G. J., E. J. Wherry, R. Ahmed, and A. H. Sharpe. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 203:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French, D. M., W. C. Brown, and G. H. Palmer. 1999. Emergence of Anaplasma marginale antigenic variants during persistent rickettsemia. Infect. Immun. 67:5834-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Futse, J. E., K. A. Brayton, S. D. Nydam, and G. H. Palmer. 2009. Generation of antigenic variants via gene conversion: evidence for recombination fitness selection at the locus level in Anaplasma marginale. Infect. Immun. 77:3181-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Futse, J. E., M. W. Ueti, D. P. Knowles, and G. H. Palmer. 2003. Transmission of Anaplasma marginale by Boophilus microplus: retention of vector competence in the absence of vector-pathogen interaction. J. Clin. Microbiol. 41:3829-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff, W. L., W. C. Johnson, C. R. Wyatt, and C. W. Cluff. 1996. Assessment of bovine mononuclear phagocytes and neutrophils for induced l-arginine-dependent nitric oxide production. Vet. Immunol. Immunopathol. 55:45-62. [DOI] [PubMed] [Google Scholar]
- 22.Han, S., J. Norimine, G. H. Palmer, W. Mwangi, K. K. Lahmers, and W. C. Brown. 2008. Rapid deletion of antigen-specific CD4+ T cells following infection represents a strategy of immune evasion and persistence for Anaplasma marginale. J. Immunol. 181:7759-7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horneff, M. W., M. J. Wick, M. Rhen, and S. Normark. 2002. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat. Immunol. 3:1033-1040. [DOI] [PubMed] [Google Scholar]
- 24.Jameson, S. C., and D. Masopust. 2009. Diversity of T cell memory: an embarrassment of riches. Immunity 31:859-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenson, J. S., R. O'Connor, J. Osborne, and E. Devaney. 2002. Infection with Brugia microfilariae induces apoptosis of CD4+ T lymphocytes: a mechanism of immune unresponsiveness in filariasis. Eur. J. Immunol. 32:858-867. [DOI] [PubMed] [Google Scholar]
- 26.Juleff, N., M. Windsor, E. A. Lefevre, S. Gubbins, P. Hamblin, E. Reid, K. McLaughlin, P. C. L. Beverley, I. W. Morrison, and B. Charleston. 2009. Foot-and-mouth disease virus can induce a specific and rapid CD4+ T-cell-independent neutralizing and isotype class-switched antibody response in naïve cattle. J. Virol. 83:3626-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemball, C. C., E. D. Han Lee, V. Vezys, T. C. Pearson, C. P. Larsen, and A. E. Lukacher. 2005. Late priming and variability of epitope-specific CD8+ T cell responses during persistent virus infection. J. Immunol. 174:7950-7960. [DOI] [PubMed] [Google Scholar]
- 28.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Rev. Immunol. 6:873-879. [DOI] [PubMed] [Google Scholar]
- 29.Knowles, D. P., S. Torioni de Echaide, G. H. Palmer, T. C. McGuire, D. Stiller, and T. F. McElwain. 1996. Antibody against an Anaplasma marginale MSP5 epitope common to tick and erythrocyte stages identifies persistently infected cattle. J. Clin. Microbiol. 34:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahmers, K. K., J. Norimine, M. S. Abrahamsen, G. H. Palmer, and W. C. Brown. 2005. The CD4+ T-cell immunodominant Anaplasma marginale major surface protein 2 stimulates γδ T-cell clones that express unique T-cell receptors. J. Leukoc. Biol. 77:199-208. [DOI] [PubMed] [Google Scholar]
- 31.Lanzavecchia, A., and F. Sallusto. 2009. Human B cell memory. Curr. Opin. Immunol. 21:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, X., K. K. McKinstry, S. L. Swain, and D. K. Dalton. 2007. IFN-γ acts directly on activated CD4+ T cells during mycobacterial infection to promote apoptosis by inducing components of the intracellular apoptosis machinery and by inducing extracellular proapoptosis signals. J. Immunol. 179:939-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez, J. E., P. A. Beare, R. A. Heinzen, J. Norimine, K. K. Lahmers, G. H. Palmer, and W. C. Brown. 2008. High-throughput identification of T-lymphocyte antigens from Anaplasma marginale expressed using in vitro transcription and translation. J. Immunol. Methods 332:129-144. [DOI] [PubMed] [Google Scholar]
- 34.Lopez, J. E., W. F. Siems, K. A. Brayton, G. H. Palmer, T. C. McGuire, and W. C. Brown. 2005. Identification of novel antigenic proteins in a complex Anaplasma marginale outer membrane immunogen by mass spectrometry and genomic mapping. Infect. Immun. 73:8109-8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 36.Mebius, R. E., and G. Kraal. 2005. Structure and function of the spleen. Nat. Rev. Immunol. 5:606-616. [DOI] [PubMed] [Google Scholar]
- 37.Meeus, P. F., M. A. Brayton, G. H. Palmer, and A. F. Barbet. 2003. Conservation of gene conversion mechanism in two distantly related paralogues of Anaplasma marginale. Mol. Microbiol. 47:633-643. [DOI] [PubMed] [Google Scholar]
- 38.Morrison, L. J., L. Marcello, and R. McCulloch. 2009. Antigenic variation in the African trypanosome: molecular mechanisms and phenotypic complexity. Cell. Microbiol. 11:1724-1734. [DOI] [PubMed] [Google Scholar]
- 39.Norimine, J., and W. C. Brown. 2005. Intrahaplotype and interhaplotype pairing of bovine leukocyte antigen DQA and DQB molecules generate functional DQ molecules important for priming CD4+ T-lymphocyte responses. Immunogenetics 57:750-762. [DOI] [PubMed] [Google Scholar]
- 40.Palmer, G. H., and K. A. Brayton. 2007. Gene conversion is a convergent strategy for pathogen antigenic variation. Trends Parasitol. 23:408-413. [DOI] [PubMed] [Google Scholar]
- 41.Palmer, G. H., W. C. Brown, and F. R. Rurangirwa. 2000. Antigenic variation in the persistence and transmission of the ehrlichia Anaplasma marginale. Microbes Infect. 2:1-10. [DOI] [PubMed] [Google Scholar]
- 42.Palmer, G. H., F. R. Rurangirwa, K. M. Kocan, and W. C. Brown. 1999. Molecular basis for vaccine development against the ehrlichial pathogen Anaplasma marginale. Parasitol. Today 15:281-286. [DOI] [PubMed] [Google Scholar]
- 43.Palmer, G. H., and T. F. McElwain. 1995. Molecular basis of vaccine development against anaplasmosis and babesiosis. Vet. Parasitol. 57:233-253. [DOI] [PubMed] [Google Scholar]
- 44.Palmer, G. H., and T. C. McGuire. 1984. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J. Immunol. 133:1010-1015. [PubMed] [Google Scholar]
- 45.Park, Y. H., Y. S. Joo, J. Y. Park, J. S. Moon, S. H. Kim, N. H. Kwon, J. S. Ahn, W. C. Davis, and C. J. Davies. 2004. Characterization of lymphocyte subpopulations and major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J. Vet. Sci. 5:29-39. [PubMed] [Google Scholar]
- 46.Tebele, N., T. C. McGuire, and G. H. Palmer. 1991. Induction of protective immunity using Anaplasma marginale initial body membranes. Infect. Immun. 59:3199-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueti, M. W., J. O. Reagan, D. P. Knowles, and G. A. Scoles. 2007. Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect. Immun. 75:2959-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Eijk, M. J., J. A. Stewart-Haynes, and H. A. Lewin. 1992. Extensive polymorphism of the BoLA-DRB3 gene distinguished by PCR-RFLP. Anim. Genet. 23:483-496. [DOI] [PubMed] [Google Scholar]
- 49.Varma, S., and A. M. Shatry. 1980. A technique for partial marsupialization of the spleen in calves. Vet. Rec. 106:127-128. [DOI] [PubMed] [Google Scholar]
- 50.Wherry, E. J., D. L. Barber, S. M. Kaech, J. N. Blattman, and R. Ahmed. 2004. Antigen independent memory CD8+ T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 45:16004-16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]
- 52.Winslow, G. M., A. D. Roberts, M. A. Blackman, and D. L. Woodland. 2003. Persistence and turnover of antigen-specific CD4+ T cells during chronic tuberculosis infection in the mouse. J. Immunol. 170:2046-2052. [DOI] [PubMed] [Google Scholar]
- 53.Woolard, M. D., and J. A. Frelinger. 2008. Outsmarting the host: bacteria modulating the immune response. Immunol. Res. 41:188-202. [DOI] [PubMed] [Google Scholar]
- 54.Wykes, M. N., Y. H. Zhou, X. Q. Liu, and M. F. Good. 2005. Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J. Immunol. 175:2510-2516. [DOI] [PubMed] [Google Scholar]
- 55.Xu, H., J. Wipsa, H. Yan, M. Zeng, M. O. Makobongo, F. D. Finkelman, A. Kelso, and M. F. Good. 2002. The mechanism and significance of deletion of parasite-specific CD4+ T cells in malaria infection. J. Exp. Med. 195:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhuang, Y., J. E. Futse, W. C. Brown, K. A. Brayton, and G. H. Palmer. 2007. Maintenance of antibody to pathogen epitopes generated by segmental gene conversion is highly dynamic during long-term persistent infection. Infect. Immun. 75:5185-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]