Abstract
Cytotoxic T lymphocyte-associated antigen (CTLA-4), also known as CD152, is a co-inhibitory molecule that functions to regulate T cell activation. Antibodies that block the interaction of CTLA-4 with its ligands B7.1 and B7.2 can enhance immune responses, including anti-tumor immunity. Two CTLA-4 blocking antibodies are presently under clinical investigation: ipilimumab and tremelimumab. CTLA-4 blockade has shown promise in treatment of patients with metastatic melanoma, with a recently completed randomized, double-blind Phase III trial demonstrating a benefit in overall survival (OS) in the treated population. However, this approach appears to benefit only a subset of patients. Understanding the mechanism(s) of action of CTLA-4 blockade and identifying prognostic immunologic correlates of clinical endpoints to monitor are presently areas of intense investigation. Several immunologic endpoints have been proposed to correlate with clinical activity. This review will focus on the endpoints of immune monitoring described in studies to date and discuss future areas of additional work needed.
Introduction
The interaction between cancer and the immune system is complex and multifaceted. While evidence of an anti-tumor immune response is detectable in many cancer patients, cancers develop multiple strategies to evade immune detection and destruction.1 Immunotherapies aim to generate or augment anti-tumor immunity to gain clinical benefit. Advances in defining the mechanisms and molecules that regulate immune responses have provided new targets for therapeutic intervention. The identification of CTLA-4, a co-inhibitory molecular expressed on T cells, led to the clinical development of CTLA-4 blocking antibodies that are capable of stimulating potent anti-tumor immunity.2 Two CTLA-4 blocking antibodies are presently under clinical investigation, ipilimumab and tremelimumab.3, 4 These antibodies have been most extensively tested in patients with melanoma but studies have now broadened to include prostate, ovarian, breast, and renal cell cancer. Clinical responses to ipilimumab and tremelimumab have been notable for their durability; however, a minority of patients treated (~10–15%) achieve objective radiographic responses at conventional timepoints while others may benefit months later, even after clinical progression.5 The side effect profile for CTLA-4 blockade includes the development of tissue specific inflammatory symptoms such as colitis, dermatitis and hypophysitis, designated immune-related adverse events (irAEs).6
Monitoring parameters of immune activation and anti-tumor immunity during the clinical testing of ipilimumab and tremelimumab has begun to shed light on the putative mechanisms of clinical activity for these agents. Immune monitoring is an approach that is presently being used to: (1) identify endpoints that correlate with, or predict, clinical benefit, (2) identify endpoints that correlate with, or predict, irAEs, (3) observe anti-tumor immune responses in real-time to better characterize the steps involved in successful (or unsuccessful) anti-tumor immunity. In this review, we aim to survey the endpoints of immune monitoring that have been identified as the most promising targets for future study.
Background
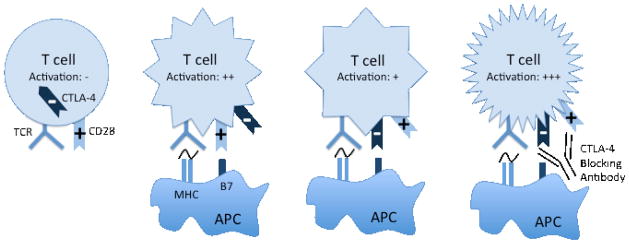
Two signals are required for full T cell activation.7 The first signal is provided by engagement of the T cell receptor (TCR) with a cognate peptide bound major histocompatibility complex (MHC). A second, co-stimulatory, signal is provided by engagement of a co-receptor. The canonical co-receptor, CD28, binds to members of the B7 family present on antigen presenting cells (APC). CTLA-4 was initially described as a new member of the immunoglobulin gene family notably upregulated in activated T cells.8 Both CD28 and CTLA-4 are members of the immunoglobulin gene family, which also includes PD-1, ICOS, and BTLA. Later studies showed that, like CD28, CTLA-4 binds to B7, but with markedly higher affinity.9 In contrast to CD28, CTLA-4 functions to inhibit T cell activation. The development of agonist and antagonist antibodies to CTLA-4 permitted the first characterizations of CTLA-4 function in vitro and in vivo. The blockade of CTLA-4 interaction with B7 enhances T cell activation in vitro; whereas antibodies that engage CTLA-4 signaling attenuate T cell activation.10, 11 CTLA-4 blocking antibodies were subsequently tested in vivo, where they again enhanced immune responses in several mouse models.12 Lastly, characterization of CTLA-4 −/− mice strongly supported the proposed immune regulatory role for CTLA-4.13–15 These mice develop a profound, autoreactive, hyperproliferative lymphocyte expansion, which is lethal within 3 weeks of age.
Preclinical Activity of CTLA-4 Blockade
Based on the evidence that CTLA-4 functions to regulate immune responses in vitro and in vivo, it was proposed that blockade of CTLA-4 could enhance immune responses against tumors by inhibiting this “checkpoint” in the immune response.16 CTLA-4 blockade as a monotherapy demonstrated efficacy in mouse models of transplantable tumors, including colon carcinoma, prostate carcinoma, fibrosarcoma, ovarian carcinoma, and lymphoma.17–21 In several of these studies, CTLA-4 mediated rejection of tumors afforded protection against subsequent tumor challenge, suggesting the generation of durable anti-tumor immunity. In some tumor models, such as the B16 melanoma and the SM1 mammary tumor, CTLA-4 monotherapy was not effective, but combination with GM-CSF-expressing tumor cells, peptide, or DNA vaccines was synergistic.22–24 Side-effects of CTLA-4 blockade, such as depigmentation and prostatitis, seen in mouse models of melanoma and prostate cancer respectively, prefigured some of the immune related side effects of CTLA-4 blockade later seen in humans.22, 25, 26 The autoimmune potentiating effect of CTLA-4 blockade was also seen in several mouse models of autoimmunity.27–30
CTLA-4 Mechanism(s) of Action
CTLA-4 is inducibly expressed on activated effector CD4+ and CD8+ T cells. In addition, CTLA-4 is constitutively expressed on a subset of regulatory T cells (Treg). Understanding the precise mechanism of CTLA-4 activity in vivo, and by extension, the mechanism of anti-tumor immune activity mediated by CTLA-4 blockade, is an area of active investigation. Two distinct, but not mutually exclusive, hypotheses to explain CTLA-4 activity have gained experimental support: a cell intrinsic and cell extrinsic mechanism. In the cell intrinsic model, CTLA-4 acts in cis on activated T cells to oppose the co-stimulatory signal provided by CD28 and CD7 interaction (Figure 1). In support of this model, conventional CD4+ and CD8+ cells that do not express CTLA-4 have a higher proliferative capacity in vitro and in vivo.15, 31–35 The cell extrinsic activity of CTLA-4 on immune response, focusing on a role for Tregs, has been more challenging to define. Early experiments demonstrating that CTLA-4 −/− T cells transferred into RAG ½ −/− hosts could be inhibited by concomitant transfer of WT T cells suggested trans-regulation by CTLA-4 sufficient WT cells36, 37, later defined as a CD4+CD25+ Tregs.38 Further support for a role of Tregs was provided by the generation of a murine conditional knock out where the mice lack CTLA-4 in the Treg compartment.39 These mice develop a lethal lymphoproliferative disorder, albeit in a delayed fashion compared with the CTLA-4 −/− mice, suggesting that both conventional T cells and Tregs contribute to this phenotype. The relative contribution of conventional T cells versus Tregs to the effects of CTLA-4 blockade appears to depend upon the disease model under investigation. Several pre-clinical models of anti-tumor immunity appear support a more important contribution of the effector T cell compartment in mediating the effects of CTLA-4 blockade.40, 41 Utilizing a transgenic mouse expressing human CTLA-4, Peggs et al. were able to independently assess the relative contributions of CTLA-4 expression on effector T cells versus Tregs. These studies demonstrate an absolute requirement for effector T cells, but not Tregs. However, concomitant blockade of both effector T cells and Tregs is required for maximal anti-tumor effects of CTLA-4 blockade.41
Figure 1. CTLA-4 is a negative regulator of T cell activation.
Conventional T cells are activated by engagement of MHC (signal 1) and B7 (signal 2). Upon activation, T cells express CTLA-4 on the cell surface. CTLA-4 engagement with B7 inhibits T cell activation. Antibody blockade of CTLA-4 interaction with B7 prevents this inhibitory signal.
CTLA-4 Blocking Antibodies for Humans: Ipilimumab and Tremelimumab
CTLA-4 blocking antibodies for use in humans were developed based on the pre-clinical activity seen in mouse models of anti-tumor immunity. Both ipilimumab (MDX-010) and tremelimumab (CP-675,206) are fully human antibodies against CTLA-4.42–44 Ipilimumab (Bristol-Myers Squibb, Princeton, NJ) is an IgG1 with a plasma half-life of 12–14 days. Tremelimumab (Pfizer, New York, NY) is an IgG2 with a plasma half-life of approximately 22 days. Both of these agents have been most widely tested in patients with metastatic melanoma, where durable clinical responses have been well documented. In metastatic melanoma, response rates to ipilimumab at the optimal dose of 10 mg/kg have ranged from 5.8 to 15.8%.45–47 A recently completed randomized, double-blind, Phase III study examining 676 patients treated with ipilimumab at a dose of 3 mg/kg compared with patients treated with peptide vaccine alone or peptide vaccine plus ipilimumab demonstrated a best overall response rate (BORR) of 10.9% among patients treated with ipilimumab alone and a benefit in OS (10.0 months for ipilimumab plus peptide vaccine vs. 10.1 months for ipilimumab alone vs. 6.4 months for peptide vaccine alone) favoring ipilimumab treatment.48 In this trial, survival rates for ipilimumab treated patients were 45.6% at 1 year and 23.5% at the 2-year mark. A second randomized, placebo-controlled, Phase III clinical trial comparing ipilimumab at a dose of 10 mg/kg plus dacarbazine to dacarbazine combined with a placebo has completed accrual.49 A randomized, open-label Phase III trial comparing tremelimumab with dacarbazine or temozolomide was halted after an interim analysis failed to demonstrate a benefit (OS 10.7 vs. 11.7 months).
From the clinical trials completed to date, several general features of anti-CTLA-4 therapy have been defined including: kinetics of a typical response, duration of response, and side effect profile. CTLA-4 blockade functions as a general activator of immune responses and thus its pattern of clinical activity is distinct from the cytotoxic agents that are the mainstay of cancer therapy. Specifically, the kinetics of radiographic responses to CTLA-4 blockade may be delayed when compared with typical responses to cytotoxic agents. Clinical responses to cytotoxic therapy are expected within the first weeks of treatment. In contrast, some patients treated with ipilimumab who have stable, or even progressive disease, in the first months of treatment, go on to experience objective responses or durable stable disease. Metastatic melanoma patients treated with ipilimumab can achieve durable responses measured in years. Durable responses to dacarbazine, the only FDA approved chemotherapy for metastatic melanoma, are exceedingly rare, even among clinical responders. Response criteria for solid tumors, including the WHO and RECIST guidelines were developed to monitor patients treated with cytotoxic chemotherapies. The unique features of responses to CTLA-4 blockade prompted the development of new guidelines for the evaluation of immune therapy activity in solid tumors.50 These immune-related response criteria (irRC) were developed to better identify clinical activity in patients treated with immunotherapies that might be neglected by conventional response criteria.
Immune-Related Adverse Events
For some patients, the potent ability of CTLA-4 blockade to activate the immune system results in inflammatory manifestations characterized as immune-related adverse events (irAEs). The most clinically significant irAE is enterocolitis which can range in severity; grade III/IV enterocolitis is seen in ~15% of patients treated with ipilimumab at 10 mg/kg. 46 With vigilance and early intervention with corticosteroids and/or anti-TNF therapy, colitis symptoms are readily treatable and rarely result in life-threatening complications. Notably, colitis treatments do not appear to compromise the anti-tumor activity or duration of response to ipilimumab.51 Additional irAEs include rash/pruritus (>50%), hepatitis (5–10%), hypophysitis (5%), uveitis (<2%), pancreatitis (<2%), and leucopenia (<2%).6, 52–55
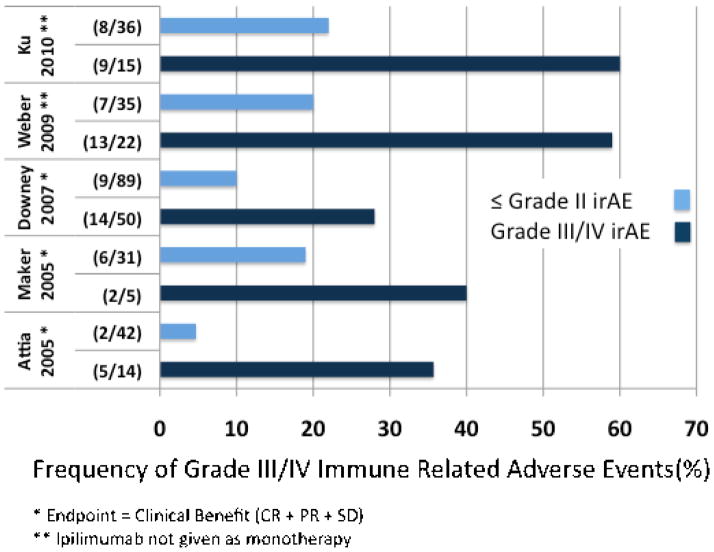
Overlap between patients who develop irAEs and those who derive clinical benefit from CTLA-4 blockade was noticed in early studies. For example, in a Phase I study of 14 patients with metastatic melanoma, Phan et al. reported that 3/3 responding patients (1 CR, 2 PR) had grade III/IV toxicities, whereas only 3/11 (27%) of non-responders had similar toxicities.56 The correlation between grade III/IV irAEs and clinical response has since been substantiated in several larger analyses.44, 51, 52, 54, 57, 58 The presence of grade III/IV irAEs correlates with higher rates of clinical response (Figure 2). Conversely, among clinical responders, irAEs are more frequent. It should be emphasized, however, that high-grade irAEs are not required for clinical response; nor does a high-grade irAE guarantee a clinical response. The factors that determine the focus (anti-tumor, autoimmune, or both) of immune responses activated by CTLA-4 are an area of active investigation.
Figure 2. Grade III/IV irAEs correlated with clinical response or clinical benefit.
The five studies evaluated each independently demonstrate a correlation between irAEs and either clinical response (CR+PR) (Attia 2005, Maker 2005, Downey 2007) or clinical benefit (CR+PR+SD) (Weber 2009 and Ku 2010). Several of the studies combined ipilimumab treatment with a second therapy (Attia 2005, Maker 2005, Downey 2007), limiting direct comparison. In Weber 2009, patients were treated with ipilimumab combined with a placebo or with budesonide; only the patients treated with ipilimumab monotherapy are analyzed here.
Immunological Monitoring
Immunological monitoring has been an integral part of the completed and ongoing clinical trials of ipilimumab and tremelimumab. Approaches to immunological monitoring have included: (1) monitoring the frequency of specific populations of cells in peripheral blood or tumor, (2) monitoring changes in expression levels of specific markers on immune cells (3) quantifying antigen specific immune responses including antibody and CD4+ or CD8+ T cell responses, (4) monitoring changes in peripheral cytokine levels of cytokines produced by specific immune cell populations, among others. A major focus has been the identification of pertinent endpoints of immune monitoring for patients treated with CTLA-4 blockade, or for immunotherapy in general. To date, immune monitoring has identified several endpoints that may correlate with a variety of clinical parameters (Table 1). Most of these biomarkers have been identified in small, retrospective analyses; larger, prospective studies are needed.
Table 1.
Immunological Biomarkers that Correlate with Clinical Endpoints in Patients Treated with CTLA-4 Blockade for Advanced or Metastatic Melanoma
| Study Population | Biomarker | Clinical Endpoint | Reference |
|---|---|---|---|
| Patients with MM pooled from 4 clinical trials receiving ipilimumab at a dose of 0.3, 3, or 10 mg/kg | Berman et al., 2009. | ||
| - 379 patients pooled from 3 clinical trials (retrospectively analyzed) | Mean rate of ALC change | CB P= 0.0013 | |
| - 64 patients (prospectively analyzed) | Mean rate of ALC change | CB P=0.00042 | |
| 51 patients with MM treated with ipilimumab at a dose of 10 mg/kgA | ALC ≥ 1000 wk 7 | CB wk 24 P<0.01 | Ku et al., 2010 |
| OS P<0.0001 | |||
| 35 patients with MM treated in 4 trials with ipilimumab at a dose of 10 mg/kg | Mean rate of ALC change | CB P<0.0001 | Yang et al., 2010 |
| Mean rate of CD8 change | CB P=0.0294 | ||
| 10 patients with MM treated with tremelimumab at a dose of 15 mg/kg | Resistance of CD4+ T cells to Treg mediated inhibition in vitro | PFS P=0.001 | Menard et al., 2008 |
| OS P=0.035 | |||
| 75 patients with stage IIIC/IV melanoma treated with MART-1/gp100/tyrosinase peptides plus ipilimumab at a dose of 3 or 10 mg/kg | Frequency of IL-17 secreting CD4+ T cells | FFR P=0.049 | Weber et al., 2009 |
| 6 patients with MM treated with GVAX plus ipilimumab at a dose of 3 mg/kg | Intra-tumoral CD8+ T cells/FoxP3+ Tregs | Tumor necrosis P<0.0001 | Hodi et al., 2008 |
| 1/intra-tumoral FoxP3+ Tregs | Tumor necrosis P<0.0001 | ||
| 35 patients with MM treated with ipilimumab at a dose of 3 or 10 mg/kgB | Intra-tumoral FoxP3 expression | CB P= 0.014 | Hamid et al., 2009 |
| Intratumoral IDO expression | CB P=0.012 | ||
The study analyzed 51 patients, only 41 were evaluable for ALC at wk 7 and CB wk 24.
33 patients were evaluable for FoxP3 expression.
CB= clinical benefit (CR + PR + SD); CR= complete response; PR= partial response; SD= stable disease; OS= overall survival; PFS= progression free survival; NS= not significant; FFR= freedom from response; MM= metastatic melanoma; NS= not significant; ALC= absolute lymphocyte count; PBMC= peripheral blood mononuclear cells; wk= week; mo= month; GVAX=irradiated, autologous tumor cells engineered to secrete GM-CSF
Absolute Lymphocyte Count
The absolute lymphocyte count (ALC) is routinely measured to exclude lymphopenias associated with some therapies.
In the largest evaluation of biomarkers in patients treated with ipilimumab reported to date, the rate of rise in ALC was identified to correlate with clinical benefit.59 This study was a pooled analysis of patients treated on 4 different clinical trials receiving ipilimumab at 0.3, 3, or 10 mg/kg. Patients were classified into 2 groups: those with clinical activity (CR+PR+SD>24 weeks) or without clinical activity. The analysis was performed in 2 parts. In the first part, pooled data from 3 studies (379 patients) was analyzed retrospectively. For the 55 patients (15.8%) with evidence of clinical activity there was a positive correlation with mean rate of ALC change (P= 0.0013). The mean rate of ALC change is the slope of the ALC over time prior to and during ipilimumab treatment. A positive slope represents a rise in ALC with treatment. This correlation was then tested and confirmed in a prospective fashion in 64 additional patients (P= 0.00042). Furthermore, accounting for the variable doses of ipilimumab given, the rate of change of ALC was dose dependent, favoring the highest ipilimumab dose (10 mg/kg).
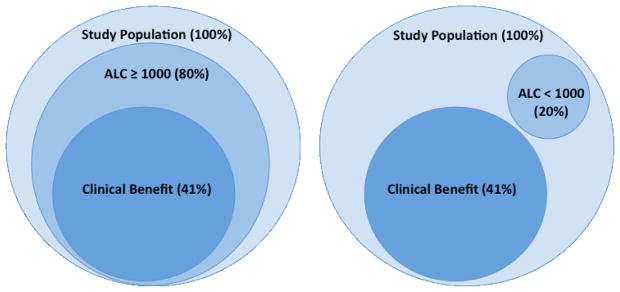
The significance of ALC was highlighted in a second, independent study.58 This analysis included 51 patients with advanced, refractory melanoma treated with ipilimumab at a dose of 10 mg/kg. In this population, there was a 9% ORR by RECIST criteria and a 12% ORR by irRC. Grade III and IV irAEs were reported in 29% of the population. Clinical benefit, defined as the sum of CR+PR+SD at 24 weeks, was reported for 33% of the population. Patients were stratified into 2 groups using an ALC < 1000/mL (low ALC) as a cut-off. ALC was evaluated pre-treatment and after the first (week 4) and second (week 7) dose of ipilimumab. Patients with an ALC ≥ 1000 after the second dose of ipilimumab had a higher clinical benefit rate at week 24 (52% vs 0%; p < 0.01). Likewise, the high ALC group had higher rates of 6-month (75% vs 0%) and 12 month (47% vs 0%) OS. ALC at earlier time points, including the pre-treatment time point, showed a non-significant trend toward clinical benefit for ALC ≥ 1000. This trend suggests that baseline characteristics (i.e. prior therapy, tumor burden, etc.) that contribute to ALC may impact response to future therapy. While low ALC may identify a poor-risk group, the correlation between ALC and clinical benefit persists after adjusting for LDH, an established marker of disease burden and predictor of survival 60, 61. For those that fall into the low ALC category after 2 doses, the likelihood of clinical benefit appears very low; 8 patients in this study met these criteria and none of them experienced clinical benefit at 24 weeks (Figure 3).
Figure 3. ALC≥1000 correlates with clinical benefit.
In a study by Ku et al.58, all of the patients with clinical benefit (CR+PR+SD) at 24 weeks had an ALC ≥1000 at week 7 of the study. Conversely, none of the patients with an ALC<1000 at week 7 had clinical benefit. The entire study includes a total of 51 patients, 41 patients were evaluable for ALC at week 7 and clinical benefit at week 24.
Further characterization of ALC as a biomarker points to CD8+ T cells as the pertinent lymphocyte subset, as seen in a recent pooled analysis of 35 patients treated with ipilimumab at a dose of 10 mg/kg.62 In this study, PBMCs were collected pre-treatment (week 1) and after dose 2 (week 7). In a retrospective analysis, increased rate of ALC change correlated with clinical benefit, as previously observed (P<0.0001). In addition to assessing ALC, peripheral lymphocyte subsets were monitored using multiparametric flow cytometry to detect CD8+, CD4+, and CD25+ populations. Clinical benefit was found to correlate with statistically significant increases in absolute numbers of CD8+ T cells between weeks 1 and 7 (P=0.0294). This correlation was not seen with CD4+ T cells (P= 0.2237) or CD4+CD25+ T cells (P=0.2912).
Inducible Costimulator (ICOS)
Inducible costimulator (ICOS), a member of the immunoglobulin gene family, is structurally related to CD28 and CTLA-4. CD4+ and CD8+ T cells express ICOS on their cell surface following activation.63 ICOS functions as a co-stimulatory molecule on activated T cells and has been associated with increased effector T cell survival.64
The correlation between CTLA-4 blockade and ICOS was first described in an analysis of 6 bladder cancer patients receiving ipilimumab in the pre-operative setting.65 The design of this study permitted analysis of both peripheral blood cells and tumor tissue. Patients were treated with ipilimumab at a dose of 3 mg/kg for 2 doses prior to radical cystoprostatectomy. Peripheral blood samples were monitored 3 weeks and 7 weeks post-treatment. Analysis of multiple parameters identified a positive correlation between ipilimumab treatment and frequency of CD4+ cells expressing high levels of ICOS in both peripheral blood and tumor samples. Furthermore, after ipilimumab treatment, the subset of CD4+ICOShigh cells collected from peripheral blood produced higher levels of IFN-γ than the same population pre-treatment or in healthy donors. Additionally, in 3 patients with tumors that express NY-ESO-1, a cancer-testis antigen, IFN-γ production by CD4+ICOShigh cells stimulated with NY-ESO-1 peptides could be detected post-treatment. Subsequently, in the same patient population, prostate tissue removed during radical cystoprostatectomy was analyzed.66 Out of 7 patients, 3 patients had incidental low-grade prostate adenocarcinoma. In both normal prostate tissue and prostate adenocarcinoma, the same pattern of increased frequency of CD4+ICOShigh cells after ipilimumab treatment was observed. This association was also reported in a recent Phase I study of tremelimumab given in combination with exemestane in hormone responsive metastatic breast cancer.67 A total of 26 patients were treated with tremelimumab, and 9 of these were analyzed for changes in lymphocyte subsets. A significant increase in percentage of CD4+ICOShigh cells was observed in PBMCs after tremelimumab treatment. There was also an association with increased ICOS expression on CD8+ cells, although the magnitude of change was less pronounced.
A retrospective analysis of melanoma patients treated with ipilimumab identified increased frequency of CD4+ICOShigh T cells, sustained over a period of 12 weeks, as a correlate of OS.68 This study analyzed 14 patients with metastatic melanoma treated with ipilimumab at a dose of 10 mg/kg. PBMCs from study subjects were analyzed at weeks 7, 12, and 24. Again, an increase in the frequency of CD4+ICOShigh cells was seen in patients treated with ipilimumab, but not in untreated melanoma patients or healthy volunteers. Of the 14 treated patients, 8 patients were noted to have a persistent increase in CD4+ICOShigh levels, defined by a greater than two-fold increase in percentage of CD4+ICOShigh cells over baseline at week 7 or 12, which was sustained at week 12. For these 8 patients, 7 had evidence of clinical benefit at week 24, defined as either SD for 6 months, PR, or CR by irRC, meeting statistical significance (P=0.004). Likewise, this group had a higher rate OS (P=0.03).
HLA-DR and CD45RO
HLA-DR, the human MHC II molecule, is expressed on T cells and upregulated at late time points after activation. Increased levels of HLA-DR on CD4+ and CD8+ T cells from PBMCs after treatment with ipilimumab has been reported in several early clinical trials.42, 57, 69, 70 CD45RO is an established marker for memory T cells. An increased level of CD45RO on PBMC CD4+, and in some studies CD8+ T cells, was seen after ipilimumab treatment as well.42, 57, 69, 70 A similar association between CTLA-4 blockade and increased surface expression of these markers in patients treated with tremelimumab. However, these early studies did not detect any correlation between increased expression of HLA-DR or CD45RO and clinical outcome.
One study of 12 patients treated with tremelimumab has offered some support of a correlation between HLA-DR and CD45RO with clinical response.71 In this study, 2 different analytic approaches were utilized. First, after converting data to a normalized scale, a hierarchical unsupervised clustering analysis was performed. When this analysis was performed focusing on HLA-DR and CD45RO, the 3 clinical responders clustered together along with 4 patients who experienced irAEs in a statistically significant fashion (P=0.05). Focusing on CD45RO and HLA-DR in the 3 clinical responders, 2 data points reached statistical significance: increased CD45RO on CD4+ cells after 3 doses of tremelimumab and increase in HLA-DR on CD8+ cells after 2 doses (P=0.03).
Antigen Specific Immune Responses
Characterization of antigen specific immune responses during CTLA-4 blockade has been performed for several cancer-related antigens including NY-ESO-1, MAGE, Melan-A, MART-1, gp-100, tyrosinase, PSA, PAP, and PSMA. Antigen specific immune responses to NY-ESO-1 have been the most extensively characterized and may be correlated with clinical activity. NY-ESO-1 is a prototypical cancer-testis antigen expressed in approximately 30–40% of melanomas and also expressed in some non-melanoma cancers including ovarian, breast, bladder, prostate and hepatocellular carcinoma. NY-ESO-1 is a well-characterized antigen with defined antibody and CD4+/CD8+ T cells responses. Spontaneously developed serological and T cell responses to NY-ESO-1 may be detected in untreated cancer patients. Serologic and T cell responses may be quantified ex vivo using tetramer analysis, T cell stimulation assays, and ELISA. The largest study thus far to specifically characterize NY-ESO-1 responses in the setting of CTLA-4 blockade examined 15 patients with metastatic melanoma treated with ipilimumab72. Within this group, 5/8 (62.5%) clinical responders demonstrated antibody, CD4+ or CD8+ responses to NY-ESO-1. By comparison, only 1/7 (14.3%) non-responders developed a CD4+ response. Among patients who had antibody responses, NY-ESO-1 specific antibody titer increased with ipilimumab treatment. Similarly, patients who developed NY-ESO-1 specific T cell responses after CTLA-4 blockade demonstrated a more robust, polyfunctional T cell response after treatment. These findings implicate the development of polyfunctional NY-ESO-1 specific T cells as a surrogate of a broader anti-tumor immune compartment and/or as direct mediators of anti-tumor immunity. The case of NY-ESO-1-specific antibodies deserves special comment. Positive serologies for NY-ESO-1 tightly correlate with T cell responses against the same antigen. In a dedicated serologic analysis of 46 melanoma patients treated with ipilimumab, 9/46 had detectable NY-ESO-1 specific antibodies.73 Among these 9 patients with positive serology, 6 (66%) experienced an objective clinical response by RECIST criteria; whereas a minority of seronegative patients achieved clinical response. In a Phase I study of 24 patients with metastatic prostate cancer treated with ipilimumab in combination with GM-CSF, 5 patients had positive NY-ESO-1 serologies with 2 patients demonstrating seroconversion after treatment.74
Similar studies investigating antigen-specific responses to additional melanoma associated antigens, Melan-A, MART-1, gp-100, and tyrosinase suggest epitope-specific or tumor-specific patterns, which will require larger studies to generalize. For example, in a case report of a patient with melanoma who experienced a CR after treatment with ipilimumab, Melan-A specific CD8+T cells were detected after treatment in the peripheral blood, the regressing tumor, and in the skin at the site of an immune related skin rash.75 However, in a study of 12 patients with MART-1 positive melanoma treated with tremelimumab, no significant changes in peripheral blood T cells specific for MART-1, gp-100, or tyrosinase were observed. 71 Highlighting the potential for discordance between T cell populations in the peripheral blood and tumor microenvironment, 1 patient who experienced a PR had an increase in gp-100 specific CD8+ T cells detected when pre-treatment and post-treatment tumor biopsy samples were compared.
Regulatory T Cells
Regulatory T cells (Treg) characterized by expression of CD4, CD25 and FoxP3 have been associated with poor outcomes in patients with cancer.76–79 Approaches to targeting Tregs for therapeutic benefit are under development.80–83 Preclinical studies suggest that CTLA-4 blockade may act, in part, by influencing Treg function.41
FoxP3 is a member of the family of forkhead box transcription factors, which is associated with the CD4+CD25+ Treg population. The frequency of FoxP3+ cells in patients treated with CTLA-4 blockade has been assessed in several studies. The studies to date have not reported a consistent pattern of change in frequency of FoxP3+ cells in the peripheral blood. Kavanagh et al. reported a significant expansion of FoxP3+ cells in the peripheral blood of 24 patients with metastatic prostate cancer treated with ipilimumab (0.5–3 mg/kg) in combination with GM-CSF in a Phase I dose escalation study.84 This trend was also observed in a small study of 10 patients with melanoma treated with tremelimumab.85 However, several studies including patients with melanoma, bladder cancer, and prostate cancer, treated with ipilimumab or tremelimumab have failed to identify any clear pattern of treatment related change in peripheral FoxP3+ cells.65, 71, 86
The FoxP3+ population at the tumor site may be a more relevant or reliable marker. In a study of 6 patients with metastatic melanoma treated with a combination of irradiated, autologous tumor cells engineered to secrete GM-CSF (GVAX) and ipilimumab at a dose of 3 mg/kg, the frequency of tumor infiltrating FoxP3+ cells inversely correlated with the extent of tumor necrosis in tumor biopsy samples (P<0.0001).87 In a second, independent study, 35 patients treated with ipilimumab at 3 mg/kg or 10 mg/kg were evaluated with tumor biopsies pre-treatment and during treatment.88 In this sample, FoxP3 expression in the tumor biopsy at baseline correlated with clinical activity (CR + PR + SD ≥ 24 weeks). Among patients who achieved clinical activity, 6/8 (75%) had elevated intratumoral FoxP3 levels at baseline (P=0.014), whereas only 9/25 (36%) of patients without clinical activity had increased FoxP3 expression. In the same study, 35 patients were evaluated for intratumoral levels of the immunoregulatory enzyme, indoleamine 2,3-dioxygenase (IDO). IDO expression has been associated with Treg activity. Intratumoral levels of IDO similarly correlated with clinical activity among patients treated with ipilimumab (P=0.012). Both of these findings suggest the hypothesis that a patient with an immune system which has tried unsuccessfully to combat the tumor may be more likely to respond than a patient with no prior response at all.
In an alternative approach to assessing the effect of CTLA-4 blockade on Treg function, Menard et al. tested purified CD4+CD45RO+ memory cells from tremelimumab treated patients for their susceptibility to inhibition by Tregs from healthy volunteers 85. In assessing the CD4+ memory cells from 10 patients with advanced melanoma, they observed that for 7 patients, the CD4+ memory cells developed a resistance to regulation by normal Tregs. These 7 patients were deemed biological responders, and were compared to the 3 patients (biological non-responders) whose CD4+ memory cells remained sensitive to Treg inhibition. For the biological responders, there was a positive association with PFS (90 days vs. 30 days in non-responders, P= 0.001) and a positive association with OS (10.6 vs. 5.2 months, P=0.035).
T Helper 17 Cells
T helper 17 (Th17) cells represent a distinct lineage of CD4+ T cells characterized by production of the specific cytokines, IL-17, and IL-22 and are implicated in the development of autoimmunity.89 A positive role for Th17 cells in anti-tumor immunity has been suggested in preliminary studies.90, 91 Moreover, a recent report offers experimental evidence that CTLA-4 blockade may potentiate Th17 differentiation in vitro and in vivo, offering a potential mechanism to link CTLA-4 blockade, Th17 cells, and anti-tumor immunity.92 Observations from clinical studies offer tentative support for this connection. In a study of 27 patients with metastatic melanoma treated with tremelimumab at a dose of either 10 or 15 mg/kg either alone or in combination with peptide pulsed dendritic cells, an increase in Th17 cells was detected post treatment in collected PBMCs stimulated in vitro.93 In a study of 18 patients with metastatic prostate cancer treated with GVAX in combination with ipilimumab at doses of 0.3–5 mg/kg, increase in the frequency of Th17 cells after in vitro stimulation was noted in 5/18 patients. Among these 5 patients, there was suggestion of increased likelihood of clinical response with 3/5 PRs and 2/5 SDs and increased frequency of irAEs with 3/5 patients developing hypophysitis or adrenal insufficiency.93 Lastly, Weber at al. reported on a Phase II study including 75 patients treated with ipilimumab (3 or 10 mg/kg) alone or in combination with MART-1, gp-100, and tyrosinase peptides.94 In this study, increased frequency of Th17 cells was associated positively with freedom from relapse (P=0.049).
Future Directions
The immune monitoring that has accompanied the clinical development of anti-CTLA-4 antibodies poses opportunities and challenges. At present, immune monitoring is still in a phase of exploration and several novel avenues of investigation have been opened by this approach. The identification of immunological markers, such as ALC, ICOS, and Th17 cells, that correlate with clinical benefit suggest this approach is likely to be an important tool in guiding the further clinical development and application of anti-CTLA-4 therapy as well as novel immunotherapies to come. However, there are notable limitations in the studies performed to date. Most potential biomarkers have been identified in retrospective analysis of Phase I and Phase II clinical studies. Validation of these markers in a prospective fashion and in randomized studies will be an important next step.
As immunological markers are validated, expanded and connected mechanistically, immune monitoring may help address important clinical questions : Who responds to CTLA-4 blockade and why? Who develops irAEs and why? Can anti-tumor immunity be separated from irAEs? Can dosing be optimized for individual patients? How can CTLA-4 blockade be rationally combined with additional therapies? Moreover, lessons learned from immune monitoring during CTLA-4 blockade are likely to form a template against which subsequent generations of immunotherapies may be measured.95
Acknowledgments
The authors would like to thank Sumit Subudhi for his critical review of the manuscript.
Abbreviations
- ALC
absolute lymphocyte count
- BORR
best overall response rate
- CB
clinical benefit (CR + PR + SD)
- CR
complete response
- CTLA-4
cytotoxic T lymphocyte-associated antigen 4
- GVAX
irradiated, autologous tumor cells engineered to secrete GM-CSF
- IDO
indoleamine 2,3-dioxygenase
- irAE
immune-related adverse events
- irRC
immune-related response criteria
- OS
overall survival
- PBMC
peripheral blood mononuclear cell
- PFS
progression free survival
- PR
partial response
- SD
stable disease
- Treg
regulatory T cells
- WT
wild type
Footnotes
Financial Disclosure: Drs. Allison and Wolchok act as consultants for Bristol-Myers Squibb. The intellectual property rights to Dr. Allison’s invention of anti-CTLA-4 are held by the University of California and licensed to Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribas A. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with tremelimumab (CP-675,206) Oncologist. 2008;13 (Suppl 4):10–5. doi: 10.1634/theoncologist.13-S4-10. [DOI] [PubMed] [Google Scholar]
- 4.Weber J. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with ipilimumab (MDX-010) Oncologist. 2008;13 (Suppl 4):16–25. doi: 10.1634/theoncologist.13-S4-16. [DOI] [PubMed] [Google Scholar]
- 5.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15:169–73. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 6.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–30. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 9.Collins AV, Brodie DW, Gilbert RJ, et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–10. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 10.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 11.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearney ER, Walunas TL, Karr RW, et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–6. [PubMed] [Google Scholar]
- 13.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 14.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 15.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–95. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 16.Allison JP, Hurwitz AA, Leach DR. Manipulation of costimulatory signals to enhance antitumor T-cell responses. Curr Opin Immunol. 1995;7:682–6. doi: 10.1016/0952-7915(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 17.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 18.Kwon ED, Hurwitz AA, Foster BA, et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc Natl Acad Sci U S A. 1997;94:8099–103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YF, Zou JP, Mu J, et al. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–41. [PubMed] [Google Scholar]
- 20.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–93. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 21.Sotomayor EM, Borrello I, Tubb E, Allison JP, Levitsky HI. In vivo blockade of CTLA-4 enhances the priming of responsive T cells but fails to prevent the induction of tumor antigen-specific tolerance. Proc Natl Acad Sci U S A. 1999;96:11476–81. doi: 10.1073/pnas.96.20.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davila E, Kennedy R, Celis E. Generation of antitumor immunity by cytotoxic T lymphocyte epitope peptide vaccination, CpG-oligodeoxynucleotide adjuvant, and CTLA-4 blockade. Cancer Res. 2003;63:3281–8. [PubMed] [Google Scholar]
- 24.Gregor PD, Wolchok JD, Ferrone CR, et al. CTLA-4 blockade in combination with xenogeneic DNA vaccines enhances T-cell responses, tumor immunity and autoimmunity to self antigens in animal and cellular model systems. Vaccine. 2004;22:1700–8. doi: 10.1016/j.vaccine.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 25.van Elsas A, Sutmuller RP, Hurwitz AA, et al. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: comparison of prophylaxis and therapy. J Exp Med. 2001;194:481–9. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–8. [PubMed] [Google Scholar]
- 27.Hurwitz AA, Sullivan TJ, Krummel MF, Sobel RA, Allison JP. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an activelyinduced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 28.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–6. [PubMed] [Google Scholar]
- 29.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyteassociated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–32. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur J Immunol. 1998;28:3137–43. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Chambers CA, Kuhns MS, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4(+) T cell responses. Proc Natl Acad Sci U S A. 1999;96:8603–8. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–55. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 34.Greenwald RJ, Oosterwegel MA, van der Woude D, et al. CTLA-4 regulates cell cycle progression during a primary immune response. Eur J Immunol. 2002;32:366–73. doi: 10.1002/1521-4141(200202)32:2<366::AID-IMMU366>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 35.McCoy KD, Hermans IF, Fraser JH, Le Gros G, Ronchese F. Cytotoxic T lymphocyteassociated antigen 4 (CTLA-4) can regulate dendritic cell-induced activation and cytotoxicity of CD8(+) T cells independently of CD4(+) T cell help. J Exp Med. 1999;189:1157–62. doi: 10.1084/jem.189.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–31. [PubMed] [Google Scholar]
- 37.Tivol EA, Gorski J. Re-establishing peripheral tolerance in the absence of CTLA-4: complementation by wild-type T cells points to an indirect role for CTLA-4. J Immunol. 2002;169:1852–8. doi: 10.4049/jimmunol.169.4.1852. [DOI] [PubMed] [Google Scholar]
- 38.Friedline RH, Brown DS, Nguyen H, et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–34. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 40.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116:1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–77. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 44.Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–6. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 45.Weber J, Thompson JA, Hamid O, et al. A randomized, double-blind, placebocontrolled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 46.Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, doseranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 47.O’Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 48.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.http:/clinicaltrials.govct2showNCT00324155term=ipilimumab&recr=Closed&phase=2&rank=3.
- 50.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 51.Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–819. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 54.Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–8. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 56.Phan GQ, Touloukian CE, Yang JC, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–56. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berman D, Wolchok JD, Weber J, Hamid O, O’Day S, Chasalow S. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab [abstract] J Clin Oncol. 2009;27:3020. [Google Scholar]
- 60.Bedikian AY, Johnson MM, Warneke CL, et al. Prognostic factors that determine the long-term survival of patients with unresectable metastatic melanoma. Cancer Invest. 2008;26:624–33. doi: 10.1080/07357900802027073. [DOI] [PubMed] [Google Scholar]
- 61.Minor DR, Moore D, Kim C, et al. Prognostic factors in metastatic melanoma patients treated with biochemotherapy and maintenance immunotherapy. Oncologist. 2009;14:995–1002. doi: 10.1634/theoncologist.2009-0083. [DOI] [PubMed] [Google Scholar]
- 62.Yang A, Kendle R, Ginsberg B, et al. CTLA-4 blockade with ipilimumab increases peripheral CD8+ T cells: Correlation with clinical outcomes. [abstract] J Clin Oncol. 2010;28:2555. [Google Scholar]
- 63.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 64.Burmeister Y, Lischke T, Dahler AC, et al. ICOS controls the pool size of effectormemory and regulatory T cells. J Immunol. 2008;180:774–82. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 65.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–92. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Liakou CI, Kamat A, et al. Anti-CTLA-4 therapy results in higher CD4+ICOShi T cell frequency and IFN-gamma levels in both nonmalignant and malignant prostate tissues. Proc Natl Acad Sci U S A. 2009;106:2729–34. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–94. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 68.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16:2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–16. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29:455–63. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Comin-Anduix B, Lee Y, Jalil J, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. doi: 10.1186/1479-5876-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–5. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gnjatic S, Yuan J, Ritter E, et al. Serum antibodies as predictive markers of clinical response to anti-CTLA-4 (Ipimilumab) treatment in advanced melanoma patients. ASCO 2008 Annual Meeting. [Google Scholar]
- 74.Fong L, Kwek SS, O’Brien S, et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–15. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 75.Klein O, Ebert LM, Nicholaou T, et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15:2507–13. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 76.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 77.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 78.Deng L, Zhang H, Luan Y, et al. Accumulation of Foxp3+ T Regulatory Cells in Draining Lymph Nodes Correlates with Disease Progression and Immune Suppression in Colorectal Cancer Patients. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-1073. [DOI] [PubMed] [Google Scholar]
- 79.Teng MW, Ritchie DS, Neeson P, Smyth MJ. Biology and Clinical Observations of Regulatory T Cells in Cancer Immunology. Curr Top Microbiol Immunol. 2010 doi: 10.1007/82_2010_50. [DOI] [PubMed] [Google Scholar]
- 80.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 81.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48:1671–5. [PubMed] [Google Scholar]
- 82.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kavanagh B, O’Brien S, Lee D, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–83. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Menard C, Ghiringhelli F, Roux S, et al. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14:5242–9. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 86.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15:390–9. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 88.Hamid O, Chasalow SD, Tsuchihashi Z, Alaparthy S, Galbraith S, Berman D. Association of baseline and on-study tumor biopsy markers with clinical activity in patients (pts) with advanced melanoma treated with ipilimumab. [abstract] J Clin Oncol. 2009:27. [Google Scholar]
- 89.Hirota K, Martin B, Veldhoen M. Development, regulation and functional capacities of Th17 cells. Semin Immunopathol. 2010;32:3–16. doi: 10.1007/s00281-009-0187-y. [DOI] [PubMed] [Google Scholar]
- 90.Canderan G, Dellabona P. T helper 17 T cells do good for cancer immunotherapy. Immunotherapy. 2010;2:21–4. doi: 10.2217/imt.09.83. [DOI] [PubMed] [Google Scholar]
- 91.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–56. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ying H, Yang L, Qiao G, et al. Cutting edge: CTLA-4-B7 interaction suppresses Th17 cell differentiation. J Immunol. 2010;185:1375–8. doi: 10.4049/jimmunol.0903369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.von Euw E, Chodon T, Attar N, et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weber JS, Sarnaik A, Targan S, et al. Phase II trial of extended dose anti-CTLA-4 antibody ipilimumab (formerly MDX-010) with a multipeptide vaccine for resected stages IIIC and IV melanoma. J Clin Oncol (abstract) 2009:27. [Google Scholar]
- 95.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent antiprogrammed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]