Abstract
Mammals expend energy in many ways, including basic cellular maintenance and repair, digestion, thermoregulation, locomotion, growth and reproduction. These processes can vary tremendously among species and individuals, potentially leading to large variation in daily energy expenditure (DEE). Locomotor energy costs can be substantial for large-bodied species and those with high-activity lifestyles. For humans in industrialized societies, locomotion necessary for daily activities is often relatively low, so it has been presumed that activity energy expenditure and DEE are lower than in our ancestors. Whether this is true and has contributed to a rise in obesity is controversial. In humans, much attention has centered on spontaneous physical activity (SPA) or non-exercise activity thermogenesis (NEAT), the latter sometimes defined so broadly as to include all energy expended due to activity, exclusive of volitional exercise. Given that most people in Western societies engage in little voluntary exercise, increasing NEAT may be an effective way to maintain DEE and combat overweight and obesity. One way to promote NEAT is to decrease the amount of time spent on sedentary behaviours (e.g. watching television). The effects of voluntary exercise on other components of physical activity are highly variable in humans, partly as a function of age, and have rarely been studied in rodents. However, most rodent studies indicate that food consumption increases in the presence of wheels; therefore, other aspects of physical activity are not reduced enough to compensate for the energetic cost of wheel running. Most rodent studies also show negative effects of wheel access on body fat, especially in males. Sedentary behaviours per se have not been studied in rodents in relation to obesity. Several lines of evidence demonstrate the important role of dopamine, in addition to other neural signaling networks (e.g. the endocannabinoid system), in the control of voluntary exercise. A largely separate literature points to a key role for orexins in SPA and NEAT. Brain reward centers are involved in both types of physical activities and eating behaviours, likely leading to complex interactions. Moreover, voluntary exercise and, possibly, eating can be addictive. A growing body of research considers the relationships between personality traits and physical activity, appetite, obesity and other aspects of physical and mental health. Future studies should explore the neurobiology, endocrinology and genetics of physical activity and sedentary behaviour by examining key brain areas, neurotransmitters and hormones involved in motivation, reward and/or the regulation of energy balance.
Keywords: dopamine, endocannabinoid system, energy budget, leptin, non-exercise activity thermogenesis, orexin, personality, wheel running
Introduction
The goal of this review is to provide an overview of the biological control of voluntary exercise and spontaneous physical activity (SPA) in relation to total daily energy expenditure (DEE), food consumption and obesity in humans and laboratory rodents. In so doing, we bring together areas of the literature that rarely overlap, even though are clearly relevant to one another.
By biological control we mean that, in a given environment, two individuals will exhibit an innately different behavioural or physiological state, or will respond differently to a change in that environment. In this context, environment refers to all external and internal factors that influence (and alter) development and functioning within an individual from fertilization (formation of the zygote), or even before. Such an idealized definition of biological control is essentially impossible to make operational because we cannot ensure that the environment is 100% identical for multiple individuals. Nonetheless, careful experimental and/or statistical control can allow us to probe the extent of biological control in various behavioural or physiological phenotypes. Considerable evidence, reviewed below, demonstrates that both voluntary exercise and SPA are under substantial biological control in both humans and rodents (Rowland, 1998; Thorburn and Proietto, 2000; Eisenmann and Wickel, 2009; Swallow et al., 2009; Feder et al., 2010). However, few studies have examined the relationship between voluntary exercise and SPA, especially in rodents, in spite of the fact that compensatory interactions between voluntary and spontaneous activity could have major implications for overall energy expenditure. For many phenotypes, the effects of genetic variation among individuals play a major role in biological control, so we also briefly review the evidence for genetic effects on physical activity. We begin with some definitions.
Defining voluntary exercise and SPA
Locomotion is a defining characteristic of animal life, and in most mammalian species it constitutes a key element of daily life as individuals search for food, shelter and mates, interact with competitors and avoid predators. This sort of physical activity can be termed obligatory (e.g. FAO, 2004). One can also think of discretionary activity or voluntary exercise, which we define as locomotor activity that is not directly required for survival or homeostasis and not directly motivated by any external factor. Human voluntary exercise occurs in a seemingly endless variety of ways (e.g. sports) and varies greatly in both intensity and duration, both of which affect its energetic cost and may modulate its other physiological consequences.
In humans, the motivation for voluntary exercise can be multifactorial, exceedingly complex (Dishman, 2008) and related to major personality traits (Rhodes and Smith, 2006) (see below). It can also be rewarding (i.e. psychologically and/or physically) and apparently even addictive [Aidman and Woollard, and others (Aidman and Woollard, 2003; Brene et al., 2007; MacLaren and Best, 2010) and references therein]. In domesticated rodents under laboratory conditions, one presumes that the motivation for voluntary exercise – usually measured by wheel running – is simpler (e.g. societal effects are absent), but it can nonetheless be related to personality traits (Jonas et al., 2010b) (see below). Wheel running is clearly rewarding for rodents, and comparative psychologists have considered it to represent the classic self-motivated behaviour. Sherwin has reviewed evidence indicating that various species are often highly motivated to run on wheels, even in the absence of any external reward (Sherwin, 1998). Importantly, wheel running is not a behaviour exhibited only by laboratory strains of rodents (e.g. Dewsbury et al., 1980), and some species of wild rodents run more on wheels than even those that have been bred specifically for high wheel running (Garland, 2003). Evidence is accumulating that wheel running can be addictive in rodents [Werme et al. and others (Werme et al., 2000; Brene et al., 2007; Kanarek et al., 2009; De Chiara et al., 2010) and references therein], and it is clear that removing wheel access from animals that have had it for some period of time can lead to changes in behaviour (e.g. Malisch et al., 2009) and in the brain [e.g. on neuronal activity in specific regions (Rhodes et al., 2003a)].
Humans and rodents engage in much physical activity that does not qualify as voluntary exercise [e.g. fidgeting, non-specific ambulatory behaviour (pacing)], and this is often termed SPA (e.g. Ravussin et al., 1986; Kotz et al., 2008). Again, such activity can vary greatly in both intensity and duration. Recently, Levine et al. emphasized the importance of NEAT, which they describe as follows: ‘Physical activity thermogenesis can be subdivided into volitional exercise (sports and fitness-related activities) thermogenesis and what we characterize as nonexercise activity thermogenesis (NEAT).... NEAT is the thermogenesis that accompanies physical activities other than volitional exercise, such as the activities of daily living, fidgeting, spontaneous muscle contraction, and maintaining posture when not recumbent’ [(Levine et al., 1999) p. 924]. In the present review, we are not generally concerned with thermogenesis, temperature regulation or heat balance per se, but the same general distinctions apply when we consider physical activity (i.e. some aspects are obligatory and others discretionary or voluntary).
In subsequent papers, the definition of NEAT in humans has been made even more inclusive, e.g. “NEAT is... akin to the energy expenditure of spontaneous physical activity. For a human it would be... performing all of our daily tasks such as walking, talking, yard work, and fidgeting” [(Levine et al., 2003) p. 169 (see also Donahoo et al., 2004)]. Under this sort of very broad definition, lots of NEAT can be voluntary and even encompass what is commonly viewed as exercise (Kotz et al., 2008) (see also Johanssen and Ravussin, 2008; Owen et al., 2010). We believe that NEAT should be more narrowly defined in order to facilitate study of logically and operationally separable components of energy expenditure.
With respect to animals, Novak and Levine [(Novak and Levine, 2007) p. 924 (see also Levine et al., 2003)] argued that: “Because animal [sic] do not have volitional exercise per se, we define animal NEAT as encompassing the energy expenditure of all activity, including spontaneous, locomotor, and more stationary or repetitive activities such as grooming. All animal physical activities require energy expenditure and are subject to biological regulation and thus are a part of NEAT and equivalent to human NEAT.” Noting that wheel running and SPA are not equivalent (Sherwin, 1998), they express concern that “a running wheel introduces confounding effects on the amount of activity and energy expenditure that are independent of energy balance regulation” [(Novak and Levine 2007) p. 924]. Of course, the same may occur in human exercise, e.g. in exercise addiction or anorexia nervosa, the latter of which may sometimes be related to dysregulation of physical activity (activity-based anorexia) (Hillebrand et al., 2008; Kas et al., 2009). Moreover, following Eikelboom (Eikelboom, 1999), we have argued elsewhere that voluntary wheel running in rodents may be a reasonable model of human volitional exercise (Rezende et al., 2009; Kelly et al., 2010). For example, the day-to-day variability in wheel running by individual mice from lines selectively bred for high voluntary wheel running (High Runner lines) and from a non-selected control line was found to be similar to that observed for activity levels of free-living human children, adolescents and young adults, which has been interpreted as evidence that biological mechanisms influence daily levels of physical activity (Eisenmann et al., 2009).
Irrespective of how broadly one defines NEAT, it is clear that we have a large ‘gray area’ between voluntary (or planned) (e.g. Johanssen and Ravussin, 2008) exercise and SPA, both in humans and other animals (see also Stubbe and de Geus, 2009; Owen et al., 2010). For example, in humans, how should we classify mowing your lawn when you have sufficient financial resources to hire someone else to do it? What about walking to buy lunch at a restaurant even though you had brought a sack lunch for the day? What about play behaviour, which occurs commonly in the young, and sometimes in the adults, of most mammals (Byers and Walker, 1995), including laboratory house mice (Walker and Byers, 1991); hyperactivity in the attention-deficit hyperactivity disorder (ADHD) child; and exercise addiction or activity-based anorexia?
Our conclusion from such considerations is that further scientific progress will be facilitated by the development of operational definitions for the components of overall physical activity (see also Tou and Wade, 2002). In other words, we need definitions that are tied, more or less directly, to measurement protocols (Table 1). We also need to pay close attention to whether activity is measured as duration, intensity or a combination, as these can have differential effects on, for example, energetic costs (e.g. Koteja et al., 1999b; Rezende et al., 2009), be differentially affected by pharmacological interventions (e.g. Keeney et al., 2008) and can have different genetic bases (e.g. Nehrenberg et al., 2009b; Kelly et al., 2010; Leamy et al., 2010). Moreover, if animal models are to be used to elucidate the human condition, then we need to make claims – and eventually support them with data on underlying mechanisms – regarding how behavioural phenotypes equate across species (see also Dishman, 2008). Of course, one can also view the situation in reverse, e.g. by noting that studies of human voluntary exercise may serve as good models for understanding the basis of wheel running in other species (see also Eikelboom, 1999).
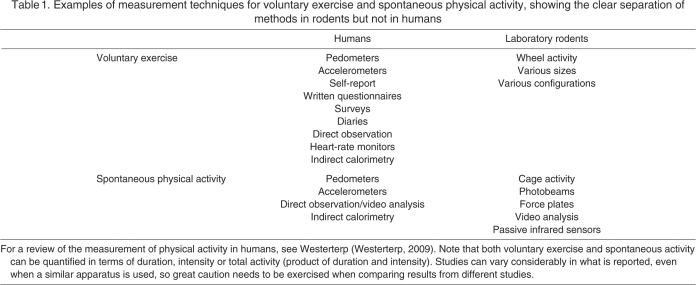
Table 1.
Examples of measurement techniques for voluntary exercise and spontaneous physical activity, showing the clear separation of methods in rodents but not in humans
As noted by Stubbe and de Geus, “Operational definitions of exercise behaviour have differed strongly across studies” [(Stubbe and de Geus, 2009) p. 343]. In humans, voluntary exercise is quantified in various ways, including questionnaires, surveys, diaries, direct observation, motion sensors (pedometers and accelerometers), heart-rate monitors and indirect calorimetry (Table 1) (Westerterp, 2009). Each method has advantages and disadvantages. Issues of reliability, validity and feasibility are important considerations. In general, there is an inverse relationship between validity and feasibility. For instance, a feasible method in large population studies, such as a questionnaire, is probably the least valid measure (e.g. Shephard, 2003) (see also Ebstein, 2006); however, the most valid measures of energy expenditure (as opposed to voluntary exercise per se), such as indirect calorimetry (e.g. Joosen et al., 2005), are neither practical nor feasible in large studies. Given the various methodologies and limitations of measuring physical activity, it is difficult to compare studies. Moreover, studies that impose relatively greater experimental control on their subjects with respect to certain factors (e.g. instructing subjects to refrain from voluntary exercise and/or confining them to metabolic chambers or hospital wards) may sacrifice face validity with respect to the phenomenon they wish to study (e.g. behavior and physiology of free-living subjects).
In captive rodents, voluntary exercise (as we define it) has been measured almost exclusively using running wheels, which may come in a variety of sizes, shapes, surface textures and configurations (Sherwin, 1998; De Bono et al., 2006). Depending on the counting device employed, and with due methodological cautions (Eikelboom, 2001; Koteja and Garland, 2001), wheel running over some period of time (e.g. 24 h) can be quantified as total revolutions, the amount of time activity and/or the average intensity of activity (e.g. Girard et al., 2007; Dlugosz et al., 2009; Gomes et al., 2009; Rezende et al., 2009). With video analysis, details of individual running bouts and the degree of intermittent locomotion can be quantified (Girard et al., 2001) (see also Waters et al., 2008).
The assessment of SPA in human subjects has also been accomplished using pedometers, accelerometers and direct observation/video analysis (Table 1). As noted above, much recent work concerns the variously defined NEAT, which has been estimated in a variety of ways, corresponding to different definitions (e.g. Levine et al., 1999; Levine et al., 2003; Levine and Kotz, 2005; Levine et al., 2005). In most of these studies, accelerometry or multi-sensor units are used to capture NEAT.
In captive rodents, SPA in cages has been measured using photobeams in various configurations, force plates (e.g. Malisch et al., 2009), video analysis and passive infrared detectors (e.g. Vaanholt et al., 2008; Gebczynski and Konarzewski, 2009a; Gebczynski and Konarzewski, 2009b). Here, as elsewhere, one needs to be extremely careful when reading the literature to note what a particular study actually means by ‘locomotor activity’, as it is often used to describe either activity in home cages after a period of acclimation or habituation (of main interest in the present review) (see also Kotz et al., 2008), locomotion in acute tests in novel arenas (e.g. open-field tests) (see also Viggiano, 2008; Hesse et al., 2010) or even wheel running. Variation exists even within studies focusing specifically on home-cage activity, ranging from variation in measurement devices and cages or arenas to the software used to process and analyse the data. These different aspects of locomotor behaviour generally do not show strong positive relations with each other (e.g. Sherwin, 1998; Bronikowski et al., 2001) (http://phenome.jax.org/). Additionally, locomotion most often refers to ambulation, but SPA refers to all activities, including non-ambulatory events, such as grooming and rearing behavior.
Some activities of daily living are commonly termed sedentary behaviours in humans, including reading books, playing cards, watching television or videos, using a computer (all of which typically occur while sitting) or sitting in automobiles (e.g. Speakman and Selman, 2003; Ford et al., 2005; Prentice and Jebb, 2006; Chaput and Tremblay, 2009; Jackson et al., 2009; Owen et al., 2010). It is apparent that these sorts of behaviours are not necessarily the opposites of active behaviours, such as jogging, in either a psychological or a physiological sense (e.g. see Owen et al., 2010). In other words, voluntary exercise, SPA and sedentary behaviours do not necessarily lie along a single continuum or axis of variation, either phenotypically or genetically. Many sedentary behaviours expressed by humans, especially those involving man-made objects or devices, cannot have direct counterparts in rodents. However, rodents will spend time (and energy) interacting with (e.g. playing with) objects placed in their cages (similar to young children playing and interacting with objects), and a substantial research effort in the area of enrichment has relevance in this context (Young, 2003). Environmental enrichment can have substantial effects on brain neurochemistry, even blunting the addictive effects of certain drugs in mice given enrichment beginning at a young age (Solinas et al., 2009). Some of the molecules affected by environmental enrichment (e.g. brain-derived neurotrophic factor) are also affected by exercise and diet (Johnson et al., 2003; Pietropaolo et al., 2008; van Praag, 2009; Fahnestock et al., 2010), but interactions among enrichment, diet and propensity for exercise or SPA have not yet been studied to our knowledge. Nonetheless, one can imagine that such interactive effects exist. As reviewed by Chaput and Tremblay, both sleep (“the most sedentary of human activities”) and cognitive or knowledge-based work have relatively low rates of energy expenditure, but the former is associated with a hormonal profile that facilitates appetite control, whereas the latter enhances food intake (as does television viewing) (Chaput and Tremblay, 2009). Apparently, no study has yet attempted to simultaneously examine voluntary exercise, SPA and analogs of human sedentary behaviours in a laboratory rodent. Nor are we aware of any studies that have attempted to quantify these simultaneously in free-living non-human mammals.
DEE and its components
The first law of thermodynamics states that energy can be transformed, but it can be neither created nor destroyed. Applied to a living animal, this means that the energy entering via food consumed (Fig. 1) will be balanced by the sum of energy expended (e.g. in physical activity), stored as fat or glycogen, and used for maintenance, growth and reproduction. The so-called energy balance equation (e.g. Schoeller, 2009) is simplified for adult mammals that are not reproducing and not storing energy, in which case energy intake is balanced by energy expended on basal metabolic rate (BMR), digestion and subsequent processing of food [thermic effect of food (TEF)], thermoregulation and activity energy expenditure (AEE). The BMR accounts for a substantial proportion of DEE, whereas the TEF is thought to contribute ∼5–10% of DEE (FAO, 2004; Schoeller, 2009). The most malleable component of DEE is AEE, the main focus of this review. As noted above, AEE can be further divided into the energy expended in voluntary exercise and SPA, also termed habitual physical activities of daily living, which generate NEAT.
Fig. 1.
Partitioning of consumed food energy. Note that energy going to the thermic effect of food is not available for ATP production or biosynthesis, but it can be used for thermoregulation. In addition, a considerable portion of the chemical potential energy in absorbed food molecules is lost as heat during the production of ATP (but again, some of this heat may be used for thermoregulation).
Locomotor costs can be a substantial portion of DEE for mammals, especially for large-bodied species and those with a highly active lifestyle (Garland, 1983; Gorman et al., 1998; Girard, 2001; Carbone et al., 2005). As illustrated in Figs 2 and 3, the energy expended on voluntary exercise can range from negligible to one-third of DEE in a mouse and two-thirds of DEE in a human in the Tour de France cycling race (Saris et al., 1989; Swallow et al., 2001; Vaanholt et al., 2007a; Vaanholt et al., 2007b; Rezende et al., 2009). In all cases, except perhaps during extreme human endurance activities, the energy expended in SPA is also appreciable. It is also worth noting that the amount of energy expended while sitting (a sedentary behavior) can be substantial. For example, Widdowson et al. estimated that young male military cadets, with an average DEE equivalent to men engaged in ‘moderate work’, spent almost 40% of their time and almost one-third of their energy while sitting (Widdowson et al., 1954).
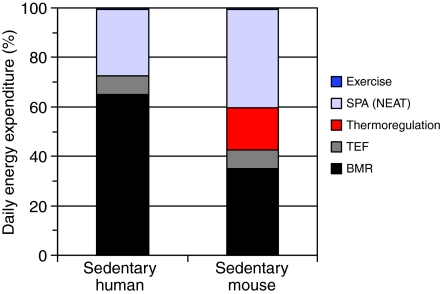
Fig. 2.
(A,B) Partitioning of daily energy expenditure in a sedentary human and a sedentary laboratory mouse. The human engages in negligible voluntary exercise, and the mouse is housed without a wheel. At room temperature (∼21°C), people wear appropriate clothing and so do not have any extra energy expenditure to maintain body temperature. For mice, however, 21°C is below their thermoneutral zone, so they have a substantial cost of thermoregulation (e.g. see Hart, 1971; Hudson and Scott, 1979; Lacy and Lynch, 1979). The values depicted are approximations, based on the synthesis of a number of sources (e.g. http://www.fao.org/docrep/007/y5686e/y5686e04.htm) [Garland and others (Garland, 1983; Saris et al., 1989; Hammond and Diamond, 1997; Gorman et al., 1998; Girard, 2001; Swallow et al., 2001; Donahoo et al., 2004; Westerterp, 2004; Carbone et al., 2005; Vaanholt et al., 2007a; Vaanholt et al., 2007b; Johanssen and Ravussin, 2008; Rezende et al., 2009; Secor, 2009) and references therein]. BMR, basal metabolic rate; NEAT, non-exercise activity thermogenesis; SPA, spontaneous physical activity; TEF, thermic effect of food.
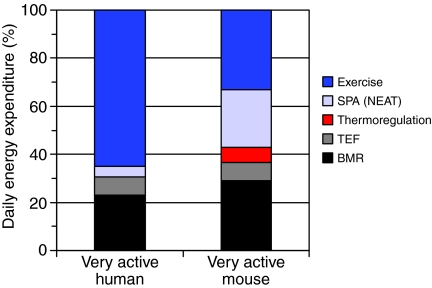
Fig. 3.
(A,B) Partitioning of daily energy expenditure (DEE) when the amount of voluntary exercise is extraordinarily high. For humans, this occurs during the Tour de France cycling race (Saris et al., 1989) and for mice it represents the High Runner lines housed with wheel access (Swallow et al., 2001; Vaanholt et al., 2007a; Vaanholt et al., 2007b; Rezende et al., 2009). For the mice, some of the heat produced during wheel running is used for thermoregulation, thus reducing costs of thermoregulation per se. SPA is still a substantial part of the energy budget for mice (Rezende et al., 2009). SPA may also appreciable for these human ultra-racers, but it has not been directly measured to our knowledge. In general, larger-bodied mammals are predicted to expend a larger fraction of their DEE on costs of locomotion, based on previous allometric analyses (Garland, 1983; Goszczynski, 1986). For additional literature sources, see Fig. 2 legend.
The techniques for estimating or measuring DEE in free-living human subjects include doubly labeled water (DLW), heart-rate monitoring, accelerometry and multi-sensor devices, and questionnaires that include physical activity and/or food consumption (Nydon and Thomas, 1989). Questionnaires yield estimates of the amount of time spent in various activities, and these can be translated into energy via time–energy budgets, given estimates of the costs of various activities (Ainsworth et al., 2000). Although direct and indirect calorimetry are seen as the most reliable and accurate methods for assessing energy expenditure (e.g. Ravussin et al., 1986; Joosen et al., 2005; McDonald et al., 2009), these techniques are not feasible for individuals moving about in their normal environment. Hence, DLW is considered the gold standard for capturing DEE in free-living populations (Speakman, 1997; Nagy, 2001; Nagy, 2005; Westerterp and Speakman, 2008). However, a limitation of DLW for studies of physical activity is that, although AEE can be calculated, e.g. as the difference between DEE and resting metabolism, no information on the nature (spontaneous or voluntary) or intensity (sedentary, low, moderate, vigorous) can be ascertained without some sort of additional information, e.g. from accelerometry. In cold environments, there may also be thermoregulatory expenditures (shivering) that further confound DLW-derived estimates of activity. The method of heart-rate monitoring requires measurement of the relationship between heart rate and oxygen consumption in an individual across a range of activities (sedentary to vigorous activity); similarly, accelerometers or multi-sensor devices depend on calibration of output (counts, etc.) and energy expenditure as determined by independent methods (e.g. Freedson et al., 1998; Franks et al., 2003; Welk et al., 2007; Westerterp, 2009). The least-valid manner of determining DEE is through subjective self-reported surveys and diaries. Diet records that allow the calculation of total daily energy intake (kJ day–1) can also be used if no weight gain occurs and energy balance is maintained.
Methods for determining energy use in free-living rodents or other animals parallel those used for humans (and have similar advantages and limitations). DLW is the de facto standard for determining energy use over long periods (e.g. days). This method yields accurate estimates of average DEE [often called field metabolic rate (FMR) for free-living animals], but does not provide a detailed breakdown of the costs of various activities. Some studies have combined DLW with time-budget analysis and, if sample size is sufficiently large and there is substantial variation in both FMR and activity, regression techniques can give insight into energy costs of particular activities (e.g. Vehrencamp et al., 1989; Chappell et al., 1993; Girard, 2001). The DLW method has been used to determine FMR in a wide range of species and its relationship to animal body size, thermal environment, ecology and phylogeny have been analysed in considerable detail (e.g. Nagy et al., 1999; Nagy, 2005). In addition to DLW, investigators have employed heart-rate monitors to estimate activity energy costs in numerous species (e.g. Bevan et al., 1994; Bevan et al., 1995), and accelerometry in various forms has been used for a limited range of subjects (e.g. Shepard et al., 2008). Finally, time-budget studies combined with laboratory measurements of different activity costs (and, where appropriate, biophysical modeling of thermoregulatory costs) have also been used to estimate DEE (e.g. Goldstein, 1988). Comparisons with DLW suggest that carefully done time-budget studies can be quite accurate (e.g. Weathers et al., 1984).
In summary, both voluntary exercise and SPA can be important components of DEE. Apparently, the latter can still be important even when exercise is high. Over the long term, relatively small alterations in either component of physical activity can lead to weight gain (or loss), as can small changes in energy intake (Hill et al., 2003). However, many human studies have shown that, beyond approximately 1 year, the amount of weight loss predicted to occur following a sustained increase in energy expenditure or a decrease in energy intake is often not realized, implying that other changes must be occurring, e.g. in hunger, satiety or dietary adherence (Schoeller, 2009). Moreover, alterations in specific dietary components can have effects that go far beyond their effects on energy intake [Jebb (Jebb, 2007) and references therein] (Meek et al., 2010). Hence, prospective use of the energy balance equation can be a risky proposition with respect to bringing about reductions in human body fat (Schoeller, 2009).
Voluntary exercise, SPA and energy expenditure in human beings
In studies of human subjects confined within metabolic chambers for 24 h, Ravussin et al. found that SPA varied widely and was a rather strong positive predictor of DEE (Ravussin et al., 1986). They commented (p. 1577) that: “Because the subjects were not allowed to carry out physical exercise such as isometric exercises or calisthenics, it is possible that such activity represents an unconscious need to be active.” The implication is that forced reduction in voluntary exercise may lead to an increase in other types of physical activity. Of course, it is also possible that an increase in voluntary exercise would be accompanied by a decrease in SPA.
As AEE is a major component of DEE (Figs 2 and 3), it is often assumed that if one initiates (or increases) structured exercise training or voluntary exercise in free-living humans, then DEE will necessarily increase. Of course, SPA (or NEAT) may decrease during the remaining portion of the day (e.g. a compensatory behaviour or mechanism that homeostatically controls DEE) following voluntary exercise, thus resulting in no change in overall AEE or DEE. This has not gone unnoticed in the literature (reviewed in Westerterp and Plasqui, 2004; Westerterp, 2008; Schoeller, 2009). Other human studies have addressed the question more from the perspective of appetite and resting metabolic rate (e.g. Speakman and Selman, 2003; King et al., 2007a; King et al., 2007b), and related studies have examined rodents forced to work for food (Vaanholt et al., 2007b). During free-living daily life, the amount of energy expended during human locomotion and SPA (i.e. AEE) can also be greatly impacted by cultural and/or societal factors and environmental conditions (Dishman, 2008), including the so-called ‘built environment’ (Sallis and Glanz, 2006). Rowland suggested that habitual physical activity (and DEE) is subject to substantial biological control (Rowland, 1998). He coined the term ‘activity stat’ to refer to a hypothetical control center, maintaining energy expenditure at a particular set point via regulatory changes in SPA, resting energy expenditure and/or AEE via voluntary exercise. Others have noted that activity levels may sometimes be a consequence of, rather than a contributor to, body weight (e.g. Tou and Wade, 2002). Long-term voluntary exercise does not consistently affect resting metabolic rate in humans (Speakman and Selman, 2003).
Studying the relationships between voluntary exercise, SPA and DEE in humans can be accomplished either by experimental exercise-training studies or by observational studies of habitual physical activity. In the former, pre- and post-exercise measures of DEE (and its components) are assessed, whereas in the latter DEE and its components can be monitored over a period of time [e.g. what happens to DEE and NEAT during a particular day when moderate-to-vigorous physical activity (MVPA) increases?].
Most studies examining this question have measured DEE in free-living subjects using DLW (Speakman, 1997). If DEE does not change with the addition of exercise training, then (barring changes in thermoregulatory costs) that implies that other activities of low-to-moderate intensities (i.e. SPA) must be reduced during the day, although there is no specific measure of these activities available from a DLW study in and of itself. In addition, DLW cannot document the energy expenditure or MVPA from the exercise-training portion of the day. This methodological issue can be resolved by using an accelerometer/multi-sensor device (e.g. ActiGraph accelerometer, Pensacola, FL, USA).
Summary of experimental studies with humans
In general, the effect of exercise training on non-training physical activity seems to be age dependent, with no change in DEE in older adults and an increase in DEE in younger subjects (Westerterp, 2008) (see also Schoeller, 2009). The matter of the contribution of the exercise vs non-training activity to the total AEE and DEE was addressed by Westerterp's research group using an additional physical activity measure with DLW (Meijer et al., 1999; Meijer et al., 1991). They used the Tracmor tri-axial accelerometer, a device the size of a small pager that is worn on the waist. Their results provide evidence that older individuals compensate for increased exercise training by lowering their non-training activity during the remainder of the day, whereas no such compensation occurs in younger subjects, who show an increase in both accelerometer output and DEE. More specifically, the average accelerometer output of 16 women and 16 men (age 28–41) increased after 8 and 20 weeks (Meijer et al., 1991), and this increase was attributed to the exercise-training program because non-exercise activity remained fairly stable. By contrast, total physical activity did not change in 55- to 68-year-old subjects (19 women and 18 men) who decreased their non-exercise activity (Meijer et al., 1999). However, a recent report provides conflicting results. Hollowell et al. reported an increase in total AEE (DEE was not measured) in sedentary, overweight, older subjects (mean age=53 years) randomly assigned to groups of inactive control, low-amount/moderate-intensity, low-amount/vigorous-intensity or high-amount/vigorous-intensity aerobic exercise (Hollowell et al., 2009). The intervention lasted 8 months, substantially longer than previous studies (e.g. 7–14 weeks).
Studies of free-living humans
The previous section considered the effects of structured exercise training on DEE and on non-training activity, but of equal interest is the normal activity of ‘ordinary’ people not engaged in a specified exercise-training regimen (free-living, unmanipulated humans). In general, DEE is relatively stable from day to day, despite considerable variation in the amount of energy expended in MVPA. In a meta-analysis of 21 DLW studies, the mean within-individual coefficient of variation for DEE was 12%, ranging from 6.5–22.6% (Black and Cole, 2000). Hence, on days when MVPA levels are high, the amount of low-intensity activity (e.g. fidgeting, SPA, NEAT) is reduced; conversely, on days when MVPA levels are low, the amount of low-intensity activity is increased.
These results have been replicated in young adults using a 3-day diary reporting method. Results showed a large coefficient of variation for energy expended during MVPA (84%), but a small coefficient of variation for DEE (12%) (Wickel and Eisenmann, 2006). Therefore, when energy expended during MVPA was higher, the amount of energy expended during inactivity was lower, such that some degree of compensation occurred for DEE. Fig. 4 shows the negative relationship between the amount of energy expended during inactivity and during MVPA. Importantly, the slope is shallower than –1 (dashed line), indicating that compensation for increased energy expenditure during days with high MVPA is not complete.
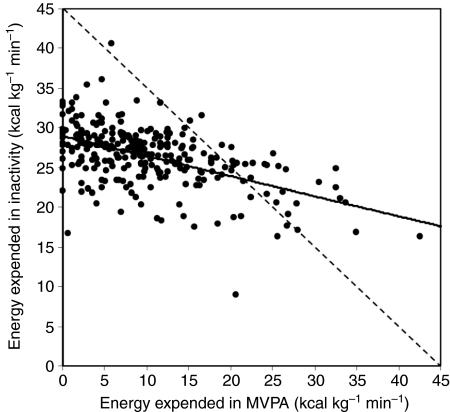
Fig. 4.
Relationship between estimated energy expenditure during inactivity and during moderate-to-vigorous physical activity (MVPA) in 125 human males and 152 females aged 18–24 years [data from Wickel and Eisenmann (Wickel and Eisenmann, 2006)]. On days when MVPA was relatively high, DEE was also relatively high [see fig. 3 in Wickel and Eisenmann (Wickel and Eisenmann, 2006)], but, as shown here, some compensation occurred in that the energy expended during inactive behaviours was reduced. However, the slope (–0.25) is much shallower than –1 (dashed line), indicating that compensation was incomplete.
Voluntary exercise, SPA and food consumption in laboratory rodents
Access to wheels has been shown by many studies to increase food consumption in rodents (e.g. Tokuyama et al., 1981; Bell et al., 1997), including in the High Runner (HR) lines of mice that have been selectively bred for high daily wheel running, as well as their non-selected control lines (Koteja et al., 1999b; Swallow et al., 2001). However, in female DBA/2J mice, which ran only 1–2 km day–1, food consumption was not significantly increased. This anomalous finding may be attributable to the level of activity being too low to stimulate food intake (Jung et al., 2010) (see also Jung and Luthin, 2010) (on C57Bl/6 mice). Even with the concomitant increase in food consumption, access to a running wheel, particularly in males and especially if access begins at a young age, tends to reduce growth, body mass and body fat in both the HR and control lines of mice [Tokuyama et al. and others (Tokuyama et al., 1981; Bell et al., 1997; Koteja et al., 1999b; Swallow et al., 1999; Swallow et al., 2001; Kelly et al., 2006) and references therein]. However, this effect is not always observed in rodents. In a recent report, wheel access had no significant effect on % body fat in young male C57Bl/6 mice [Jung and Luthin (Jung and Luthin, 2010) and references therein]. The cause of such discrepant results deserves systematic investigation. As previously noted for HR and control mice measured for wheel running, body mass and apparent food consumption weekly from ages 4 to 84 weeks, the mean ontogenetic trajectories for food consumption are more complex than those for either wheel running or body mass (Morgan et al., 2003). In any case, if wheel access causes a significant increase in food consumption, then we take that as prima facie evidence that the energy cost of wheel running is not fully compensated by reduced cage activity (see also Kumar et al., 2010). In this context it is important to note that long-term voluntary exercise does not consistently affect resting metabolic rate in rodents (Speakman and Selman, 2003; Kane et al., 2008).
Forced treadmill exercise can affect SPA in rats [Kotz (Kotz, 2008) and references therein], but because very few studies report simultaneous measurements of SPA and wheel running, the extent to which reductions in SPA may partially counteract various effects of voluntary exercise on wheels is currently unclear. Lachmansingh and Rollo found a negative, non-significant correlation between time spent in wheel running and in non-wheel running locomotion in transgenic mice with high growth rates (r=–0.01) and in their normal siblings (r=–0.15), but when transgenic and normal mice were pooled, the correlation was significantly positive (r=0.45) (Lachmansingh and Rollo, 1994). Koteja et al. found that, after 6 weeks of acclimation, access to a free vs locked wheel reduced cage locomotion in both HR and control lines of mice, especially in males, based on focal-animal scan observations (Koteja et al., 1999a). Harri et al. reported that adult male C57BL/6J mice ran for an average of 114 min in a 24-h period, while spending an additional 22 min feeding on the cage floor, compared with mice without wheel access (Harri et al., 1999). The extra time spent running and feeding was deducted from time spent resting, climbing/feeding on the cage lid, locomoting on the cage floor and grooming. Weight gain and food consumption were similar for wheel-access and control groups, indicating that wheel running can substitute for other forms of energy-consuming behaviours and vice versa. However, these mice were running only 2 km day–1, and higher amounts of wheel running, typical of some other inbred strains, may lead to different results (see also Festing, 1977; Lightfoot et al., 2004; Jung et al., 2010; Jung and Luthin, 2010). de Visser et al. found that, during a 6-day observation period, female C57BL/6J mice decreased home-cage activity when allowed simultaneous access to a wheel, but increased the total amount of time in activity (cage plus wheel) (de Visser et al., 2005).
We emphasize that laboratory strains of house mice have been evolving for many generations in relatively small cages without a wheel. Such conditions have been in force since their ancestors were first brought into laboratory culture in the early 1900s, or even earlier. Thus, laboratory mice have been evolving in the absence of any opportunity for voluntary exercise. We estimate the number of generations in captivity as approximately 400 (100 years × 4 generations per year).
Many aspects of the phenotype are known to evolve during domestication and/or adaptation to laboratory culture [Garland et al. (Garland et al., 1995) and references therein]. Given how rapidly this evolutionary change can occur (Hare et al., 2005; Albert et al., 2009; Simoes et al., 2009), laboratory strains of mice may now be rather well adapted to this low-activity environment, in which they do not need to move long distances to secure food, other resources or mates, and they do not have to avoid or escape from predators. Many (perhaps most) populations of modern Homo sapiens are living in similar conditions. We estimate the number of reduced-activity generations as perhaps 10–25 (200–500 years divided by 20 years per generation) (see also Booth et al., 2002b), far fewer than for laboratory mice. It is possible that some evolutionary adaptations may have occurred in response to the altered selective regime even over this relatively small number of generations (Byars et al., 2010). Nevertheless, it is hard to imagine that the modern human genome is optimally adapted to a low-activity lifestyle, and it is thus not surprising that low levels of physical activity are associated with a broad range of human diseases and disorders (Booth and Lees, 2007). As Booth and colleagues have argued, physical activity is probably a prerequisite for normal physiological gene expression (Booth et al., 2002a; Booth et al., 2002b). The foregoing leads to the disturbing perspective that the normal state for laboratory house mice is the absence of voluntary exercise, whereas that for humans is the presence of exercise. If valid, this perspective would call into question numerous studies of mice that have tried to draw inferences about human physiology and behaviour, and vice versa.
Energy intake, physical activity and obesity
The current human obesity epidemic in industrialized countries is stunning in its magnitude and probable effects. For example, healthy-weight adults are now in the minority in the US (Schoeller, 2009). The cost of this increase in obesity and related conditions and diseases has been estimated to approach 100 billion dollars in the US alone (Finkelstein et al., 2003). Moreover, it has been speculated that the current generation is the first that will, on average, have a lifespan shorter than that of their parents (Olshansky et al., 2005).
Given the energy balance equation (see DEE and its components), it seems intuitive that obesity is caused by an increased energy intake and/or reduced DEE and, more specifically, a reduced level of AEE. Indeed, cross-sectional, longitudinal and experimental studies have all demonstrated the role of energy intake and energy expenditure in human weight gain and/or loss (Zurlo et al., 1992; Miller et al., 1997; Prentice and Jebb, 2004; Tremblay et al., 2004; Jebb, 2007; Bray, 2008). An additional factor that contributes to increased energy intake in many modern human populations is the availability of highly palatable, energy-dense foods, and Prentice and Jebb argue for the importance of “interactions between energy-dense diets and low-levels of physical activity... due to an asymmetry between the hunger and satiety arms of human appetite control”, which will result in a disruption of an individual's energy balance [(Prentice and Jebb, 2004) p. S98]. Needless to say, eating can also be strongly influenced by hedonic responses (Blundell, 2006) and social facilitation (Drewett, 2007), and obesity can spread through large social networks (Christakis and Fowler, 2007). More recently, the possible stimulatory effect of knowledge-based work on appetite has been emphasized (Chaput and Tremblay, 2009).
In spite of the received wisdom that industrialized humans have reduced physical activity levels [e.g. see references in Booth et al. (Booth et al., 2002a; Booth et al., 2002b)], Westerterp and Speakman provide evidence that physical AEE has not declined over the same period that obesity rates have increased, and argue that it is unlikely that decreased DEE has been a major contributor to the human obesity epidemic (Westerterp and Speakman, 2008). Furthermore, recent reviews of the temporal trend in human obesity and the ‘big two’ (i.e. physical activity and diet) are challenging this traditional view and offering alternative (or additional) putative contributors, including microorganisms, epigenetics, increasing maternal age, greater fecundity among people with higher adiposity, assortative mating, sleep debt, endocrine disruptors, pharmaceutical iatrogenesis, reduction in variability of ambient temperatures, and intrauterine and intergenerational effects (Bray and Champagne, 2005; Eisenmann, 2006; Pomp and Mohlke, 2008; McAllister et al., 2009). Previously, Sclafani classified nine different origins of obesity in animals: neural, endocrine, pharmacological, nutritional, environmental, seasonal, genetic, idiopathic and viral (Sclafani, 1984). Just as there are several etiological factors acting independently or together in the development of obesity at any point in time, it is possible that the increase in obesity was due to the temporal change in a single or combination of etiological factors in each individual case. One may argue that the trend can be attributed to changes in several environmental factors acting and interacting with each other during prenatal and postnatal growth, puberty and into adulthood. Another factor adding complexity is that genotype–environment interactions are likely to be common (see also Dishman, 2008). For example, the relationships between physical activity, energy intake and body composition can differ dramatically between the sexes, among individual humans and among strains of mice, and the effects of exercise training are also known to vary among individual humans in relation to genotype (Swallow et al., 2001; Melzer et al., 2005; Girard et al., 2007; Vaanholt et al., 2008; Nehrenberg et al., 2009a; Jung and Luthin, 2010; Jung et al., 2010; Meek et al., 2010; Timmons et al., 2010). As noted in the previous section, wheel access usually, but not always, reduces body fat in rodents, especially in males.
This leads us to the concept of the obesogenic environment, which can be defined as “a set of circumstances that encourages people to eat and drink more calories than they expend and to become obese” (NHS, 2008). For humans, components of an obesogenic environment may include ready availability of highly palatable and energy-dense foods, lack of easy access to the opportunity for exercise (e.g. no safe parks nearby), use of motorized transportation for essential activities, social facilitation of eating, social facilitation of sedentary activities and a lack of education for making good decisions with respect to diet and exercise. For laboratory rodents, an obesogenic environment may be considered as one with ad libitum availability of palatable foods (including standard chow and, even more so, ‘Western’ diets) (e.g. Meek et al., 2010), housing in relatively small cages with no need to move to find food or other resources and the absence of a wheel. Lab rodents are routinely housed in same-sex groups of a few individuals, and it is possible that some social facilitation of eating or of sedentary behaviours occurs, although we are not aware of any studies that specifically address this issue. As discussed above, it is reasonable to view both modern humans and laboratory rodents as living in ‘unnatural’ environments relative to their not-so-distant ancestors (see also Booth et al., 2002a; Booth et al., 2002b; Booth and Lees, 2007). Instead, an environment that requires, promotes or at least allows higher levels of physical activity is more attuned to their genetic complements, perhaps especially for humans. In addition, the process of domestication and selection of rodents for good breeding success, large litters, successful parental care, etc., has led to genetic changes that probably interact with the laboratory environment. In any case, as noted by de Krom et al., when using animal models to elucidate aspects of human obesity, it is important to consider whether and how to model components of obesogenic environments (de Krom et al., 2009).
In rodents, diets high in fat are routinely used to induce obesity, although effects can vary substantially among strains (e.g. Brownlow et al., 1996; Funkat et al., 2004; Svensson et al., 2007; Bjursell et al., 2008; Novak et al., 2010) (http://jaxmice.jax.org/diomice/diets.html). The obesogenic effect of high-fat diets is related to their higher caloric density; although rodents typically decrease the total amount (mass) of food consumed when given a high-fat diet, they do not decrease food intake enough to avoid gaining body fat. Recently, Meek et al. showed that a Western diet (42% of kJ from fat, plus added sucrose) was obesogenic in males of both HR and control lines of mice, beyond its effects on caloric intake (Meek et al., 2010). They converted food consumption in grams to an equivalent caloric intake, and found that even with caloric intake and amount of daily wheel running as covariates, statistical analysis indicated that Western diet caused increased body mass and retroperitoneal fat pad mass in both HR and control mice. This result emphasizes the importance of dietary components for weight gain (Jebb, 2007).
In human studies, both under- and overeating have been shown to influence DEE and its components. It is fairly clear that both DEE and AEE will decrease during energy-restricted diets (Westerterp, 2008). This reduction in energy expenditure is partly due to a reduction in body weight with a negative energy balance (∼40%), but the majority (∼60%) is a consequence of lower activity levels. This was perhaps best demonstrated in the semi-starvation studies at the University of Minnesota following World War II (Keys et al., 1950).
Although it has long been known that humans differ in the susceptibility to weight gain in response to overfeeding, few studies have quantified the changes in AEE in response to overfeeding. As one example, Leibel et al. chronically over- or underfed human subjects to produce 10% changes from initial body mass, and did not restrict exercise (Leibel et al., 1995). Individuals compensated for this gain or loss both through resting metabolic rate and AEE. No differences were observed in compensation between non-obese and obese subjects, and the extent of compensation was greater than the predicted change in energy expenditure due to adjustments in body mass. In another experiment, Levine and colleagues overfed 16 sedentary, lean adults (both sexes) an extra 1000 kcal day–1 for 8 weeks, while restricting voluntary exercise. NEAT was estimated by subtracting of basal and postprandial energy expenditure from DEE, which was measured by DLW. Subjects displayed changes in NEAT that ranged from –98 to +692 kcal day–1, and 59% of the variation in fat gain could be statistically explained by the variation in NEAT (Levine et al., 1999).
With such a wide array of causes to weight gain, future studies of human susceptibility/resistance to obesity need to be more all encompassing. For example, they need to determine simultaneously the effects of voluntary exercise, NEAT/SPA and sedentary behaviours (including breaks in sedentary behaviour) (e.g. Healy et al., 2008) on weight gain/loss and body composition, while simultaneously measuring DEE, food consumption and specific dietary components (at least macronutrients) with robust methods (e.g. not based solely on self-reporting). In addition, biomarkers of appetite and energy regulation (see below) should also be considered. Effects of age and sex need to be considered with sample sizes adequate to detect interactive effects with reasonable statistical power. And, eventually, comparisons among socioeconomic, cultural and racial groupings need to be performed (e.g. Simonen et al., 2003a; Stubbe and de Geus, 2009). We recognize that such studies will be neither easy nor inexpensive, but they are warranted to better understand the complex nature of energy homeostasis and body-weight regulation in human populations. Similar studies need to be performed with rodents as putative models of the human condition, and similarities/differences elucidated.
Neurobiology of voluntary exercise and SPA
Relative to other areas of exercise physiology, very little is known about the neurobiology of voluntary exercise, SPA or sedentary behaviours in healthy animals. Similarly, we are far from understanding the neurobiology of human ADHD (van der Kooij and Glennon, 2007). At present, it is widely accepted that the mammalian brain directs complex behaviours, and that there can be vast individual variation in these behaviours. However, we lack a comprehensive understanding of the neurobiological regulation of motivated behaviours, including voluntary exercise (and eating). Various neural correlates of generalized locomotor activity have long been the subject of considerable research, but central themes are difficult to extract, often because operational definitions of locomotor activity vary widely in the literature, as has been noted elsewhere in this paper. For example, studies that purport to address SPA often do so from the construct of activity in an open-field and/or in a novel environment, rather than in a standard housing cage with habituated animals. Although all behaviours within the spectrum of locomotor activity do involve locomotor activation, it is abundantly clear that not all are created equal (e.g. Dishman, 1997; Dishman, 2008; Sherwin, 1998; Bronikowski et al., 2001; Ang and Gomez-Pinilla, 2007; Kotz et al., 2008; Leasure and Jones, 2008; Pietropaolo et al., 2008). The following sections will discuss neurobiological underpinnings of both voluntary exercise and SPA, but we include some studies that involve other measures of locomotion. We also include a consideration of how aspects of personality may be related to locomotor behaviour of various types. The endocrine bases of variation in voluntary exercise and SPA are also of great importance, but that literature is beyond the scope of the present review (e.g. see Girard et al., 2007; Levine et al., 2003; Castaneda et al., 2005; Malisch et al., 2007; Vaanholt et al., 2007c; Vaanholt et al., 2008; Lightfoot, 2008a).
Neurobiology of voluntary exercise
In general, locomotor activity in both humans and rodents is controlled by a complex cascade of neurochemical interactions that coordinate central neural impulses with motor outputs. At this point, it not known to what extent voluntary behaviour, like eating and sex, can be considered a classical motivated behaviour. However, because it can be agreeable to be physically active, a litany of other neural systems beyond those required for basic motor control become relevant, including those involved in aversion, conditioning, learning and the desire for and perception of both exogenous and endogenous rewards.
During earlier stages of human evolution, locomotion, including endurance running, was closely linked to caloric acquisition (Dudley, 2001; Bramble and Lieberman, 2004). The reward derived from exercise could be at least part of the proverbial ‘bringing home the bacon’. For contemporary humans in Western societies, locomotion is rarely required for food acquisition, but exercise activities, including endurance running, are often described as both rewarding and pleasurable. Moreover, endurance training has been reported to induce an array of pleasant psychophysical effects, including stress reduction (Long, 1983), increased anxiolysis and mood improvement (Ledwidge, 1980). Many athletes claim a pleasurable state of euphoria following or during sustained endurance-type exercise, commonly referred to as a ‘runner's high’ (Morgan, 1985; Dietrich and McDaniel, 2004). Thus, it has been suggested that evolution can ‘use’ neurobiological rewards (e.g. euphoria, anxiety reduction, reduced pain sensation or exercise-induced analgesia) (e.g. Li et al., 2004) to motivate animals to engage in activities, such as endurance running, that otherwise may be painful, stressful, energetically costly, time-consuming or risky (Ekkekakis et al., 2005). This hypothesis can be partially tested using selective breeding experiments (Keeney et al., 2008) (for reviews, see Rhodes and Kawecki, 2009; Swallow et al., 2009; Feder et al., 2010) and through comparisons of species that differ in their propensity for exercise under natural conditions (Raichlen et al., 2010).
As previously stated, voluntary wheel running can be a classic self-rewarding behaviour in laboratory rats and mice (Premack, 1964; Timberlake and Wozny, 1979; Belke and Heyman, 1994; Belke, 1996; Sherwin and Nicol, 1996; Sherwin, 1998; Ekkekakis et al., 2005; Brené et al., 2007). Many studies have shown that both wild rodents and laboratory strains are highly motivated to run on wheels and will voluntarily run long distances (e.g. Dewsbury et al., 1980; Rodnick et al., 1989; Lambert et al., 1996; Sherwin, 1998; Allen et al., 2001; Burghardt et al., 2004; Naylor et al., 2005; Leasure and Jones, 2008; Rhodes et al., 2005). Similarly, rats show conditioned place-preference for environments associated with bouts of wheel running (Lett et al., 2000), as well as the arm of a T-maze allowing access to a running wheel (Hill, 1961). In addition, rats and mice can be entrained to perform a variety of tasks to receive a reward of access to wheel running, including crossing an aversive water barrier (Sherwin and Nicol, 1996) or lever-pressing (Kagan and Berkum, 1954; Collier and Hirsch, 1971; Iversen, 1993; Belke and Garland, 2007).
In extreme cases, exercise can be enjoyable to the point where it becomes habit-forming, sometimes so much so that it justifies comparison with traditional addictive behaviours [Werme et al. and others (Werme et al., 2000; Aidman and Woollard, 2003; Brené et al., 2007; Kanarek et al., 2009; De Chiara et al., 2010; MacLaren and Best, 2010) and references therein]. As synthesized by Scheurink et al., some regular distance runners have reported a progression of feelings towards exercise similar to those experienced during the course of addiction to a drug of abuse (Scheurink et al., 2010). The runners reported sensations of euphoria after strenuous bouts of exercise, often coincident with a perceived need to increase the duration or intensity of exercise to achieve similar feelings of well-being (i.e. they appeared to develop tolerance), followed by increasing encroachment of the addiction into everyday life (e.g. difficulties in job performance and social interactions) and, lastly, symptoms of withdrawal, including depression, irritability and anxiety, when prohibited from running (Aidman and Woollard, 2003; Allegre et al., 2006). It is worth noting that, like exercise, some types of overeating may represent a form of addictive behaviour (Barry et al., 2009; DiLeone, 2009; Johnson and Kenny, 2010; Liu et al., 2010).
It has traditionally been hypothesized that dopamine is the crucial transmitter for the mediation of reinforcement phenomena (Salamone and Correa, 2002). Although the literature has yet to reach a consensus on the behavioural significance of dopamine (Salamone and Correa, 2002), several lines of evidence highlight its role in reward (Berridge and Robinson, 1998; Schultz, 2001; Schultz, 2002), learning (Wise, 2004; Owesson-White et al., 2008), motivation (Salamone and Correa, 2002; Wise, 2004), emotion (Sevy et al., 2006), addiction (Hyman and Malenka, 2001; Volknow et al., 2004) and the control of complex motor movement (Beninger, 1983; Salamone, 1992).
At present, a relationship between dopaminergic signaling and the performance of voluntary exercise seems fairly well established. Disruption of dopaminergic transmission in certain parts of the brain can strongly affect a wide range of locomotor behaviours. For example, administration of dopamine antagonists systemically or into the nucleus accumbens, as well as depletions of dopamine within the nucleus accumbens, reduces exploratory (Ahlenius et al., 1987), open-field (Correa et al., 2002; Correa et al., 2004), spontaneous, conditioned and drug-induced locomotor activity in rats (Jones and Robbins, 1992). When considering voluntary exercise, however, there remains significant debate about whether an individual's dopaminergic profile is a result of or an effecter of activity levels (Knab and Lightfoot, 2010). For instance, it has been suggested that differences in activity between inbred strains of mice result from expression differences of dopamine 1 (D1)-like receptors and tyrosine hydroxylase (Knab et al., 2009). In a related fashion, obesity-prone rats have decreased receptor expression, accompanied by decreased extracellular dopamine levels in the nucleus accumbens (Geiger et al., 2008; Geiger et al., 2009; Rada et al., 2010). Obese humans have also been shown to have decreased D2 receptor expression, based on a series of six single nucleotide polymorphisms related to D2 receptor expression (Davis et al., 2008; Davis et al., 2009). However, studies in rats have shown that acute bouts of exercise increase central dopamine concentrations (Meeusen et al., 1997), and that these effects are mediated by locomotor speed, direction and posture (Freed and Yamamoto, 1985).
Work from the Garland laboratory suggests that the HR lines of mice have an altered reward threshold for wheel running compared with their non-selected control lines (Belke and Garland, 2007), and that this difference is related to overall dopaminergic tone (Mathes et al., 2010). This ‘dysregulation’ could affect one or many of the factors that influence the sensitivity or reactivity of the neural network that regulates the anticipation of rewards. These factors can include the amount of dopamine present, the density and localization of dopamine receptors, and the rapidity of its transport back into the cell (Davis et al., 2008). Several studies confirm that HR mice have diverged with respect to control mice in dopamine receptor expression, as well as response to drugs that affect dopaminergic signaling. For example, HR mice had a 20% increase in mRNA for D2 and D4 receptors in the hippocampus compared with control mice (Bronikowski et al. 2004). Pharmacological studies with dopamine transporter blockers found differential effects on wheel running in HR and control mice, attributed to altered functionality of the D1 receptor system (but not the D2 receptor, serotonergic or opioidergic systems) (Rhodes et al., 2001; Rhodes and Garland, 2003; Rhodes et al., 2005; Li et al., 2004). In addition, HR and control mice also have different wheel-running responses to D1-like antagonists (Rhodes and Garland, 2003).
The behavioural significance of these observed differences in dopamine functioning between HR and control mice may be tantamount to an addiction to wheel running in HR mice (Rhodes et al., 2005). Consistent with this idea, Fos immunohistochemistry studies show that, when wheel access is blocked, HR mice have a greater proportional increase in activity in several brain regions implicated in reward and motivation, suggestive of a classic state of withdrawal (Rhodes et al., 2003a). If HR mice are addicted to wheel running, then it is possible that they run at higher speeds (and thus achieve greater distances) to achieve the same neurobiological reward from wheel running that control mice receive during or after lower-speed running. By this logic, one could hypothesize that HR mice have decreased dopaminergic functioning, and thus differentially seek rewards to increase dopamine tone. This is consistent with studies of humans, in which it has been suggested that individuals with relatively low dopaminergic function may seek rewarding substances (e.g. such natural rewards as food or sex, or drugs of abuse) to increase endogenous dopamine levels and thus ameliorate mood (Blum et al., 2000; Davis et al., 2008). Of course, it is also possible that HR mice have increased dopamine functioning relative to controls. As suggested by Mathes et al., HR mice could run more because they are hypersensitive to rewards, perhaps as a result of increased dopaminergic functioning (Mathes et al., 2010). The authors suggest that this combination may enhance the reinforcement value of a given reward and thus motivate individuals to further pursue its acquisition.
Consistent with the idea that HR mice may have acquired reward-related neurobiological alterations over the course of selective breeding, we have also observed sex-specific differences in the wheel-running response of HR mice to pharmacological manipulation of the endocannabinoid system (ECS) (Keeney et al., 2008). The ECS is a complex endogenous signaling system made up of transmembrane cannabinoid receptors (CB receptors), their ligands (endocannabinoids) and proteins involved in synthesis and modification of endocannabinoids (De Petrocellis et al., 2004; Cota and Woods, 2005; Demuth and Molleman, 2006). There are two primary cannabinoid receptors, CB1 and CB2. The CB1 receptor is the most abundant G protein-coupled receptor expressed in the brain (Pagotto et al., 2006), with particularly dense expression in the hypothalamus, pituitary, cerebellum and mesolimbic dopaminergic reward pathways (Herkenham et al., 1990; Matsuda et al., 1990; Demuth and Molleman, 2006). Endocannabinoid signaling via the CB1 receptor is thought to play an important role in incentive stimuli, in part because of the close involvement of endocannabinoid signaling with the dopaminergic system (Lupica and Riegel, 2005; Laviolette and Grace, 2006; Maldonado et al., 2006; Pillolla et al., 2007). Studies have shown that, in some cases, both the ECS and the dopaminergic system mutually influence the performance of locomotor behaviours (Giuffrida et al., 1999; Beltramo et al., 2000; Gorriti et al., 2005).
Ample evidence supports a relationship between the ECS and the regulation of physical activity (for review, see Fuss and Gass, 2010). In particular, it is thought that the CB1 receptor may control the expression of wheel running in rodents (Dubreucq et al., 2010), as well as exercise in humans (Dietrich and McDaniel, 2004). Hill et al. have recently shown that voluntary exercise (wheel running) increases CB1 receptor binding and the tissue content of anandamide in the hippocampus of rats (Hill et al., 2010). Sparling et al. showed that a single bout of exercise in trained humans increases blood levels of anandamide (Sparling et al., 2003). Although these and other studies demonstrate a clear link between ECS signaling and exercise behavior, much still remains to be known, particularly in reference to the directionality of the behaviour–neuropeptide relationship, as well as the extent of any sex differences.
Neurobiology of SPA and NEAT
Understanding of the neurobiological underpinnings of SPA and NEAT is in its infancy, but it is clear that the brain plays the critical regulatory role in determining their levels. Brain lesion and stimulation studies demonstrate changes in SPA, which translate into differences in NEAT. Although many brain areas contribute to SPA, none can be considered in isolation as the brain operates in a network, such that firing activity in one area – which is positively or negatively affected by environmental cues or physiology – ultimately influences firing patterns in other brain areas. Fig. 5 incorporates some of these ideas by showing some of the central and peripheral mediators that interact to produce SPA and the resultant NEAT. Behavioural studies can determine the output of specific brain activity, which will aid in understanding the brain areas and neurotransmitter systems that play dominant roles. For instance, in rats, direct electrical stimulation of the ventral tegmental area and substantia nigra, which have dopaminergic projections to the nucleus accumbens and striatum, respectively, elevates SPA and probably NEAT (Kotz et al., 2008). Many neurotransmitters have been shown to influence SPA, although most studies that report locomotor activity do not study SPA as a primary endpoint. Rather, locomotor activity, measured in a beam-break chamber, is often used as an assessment of non-specific drug effects, e.g. in studies of drugs of abuse. Similarly, low locomotor activity is used as a diagnostic criterion for depression or illness in rats and mice (e.g. Malisch et al., 2009). Thus, the data are not always easy to find, and there is probably much missing. Further, the energetic consequence of locomotor activity is often not considered, and thus the relative impact on body weight cannot be assessed.
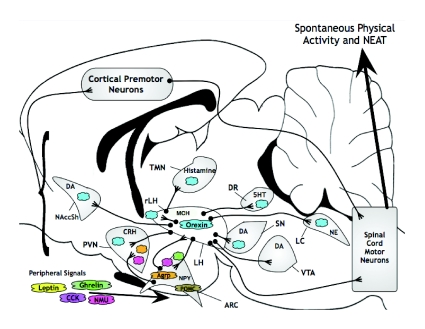
Fig. 5.
SPA (and resulting NEAT) regulatory brain areas and associated neuropeptides/transmitters [updated from fig. 1 in Kotz (Kotz, 2008)]. Colors correspond to specific neuropeptides/hormones as follows: blue, orexin; purple, CCK; pink, NMU; orange, Agrp; brown, POMC; green, ghrelin; yellow, leptin. Areas with these colors indicate site of synthesis (e.g. AgRP, POMC and ARC; orexin, LH), peripheral source (NMU, ghrelin, leptin and CCK), areas in which the neuropeptide/hormone has been injected and effects on SPA reported, or proposed site(s) of action (see text). Signals from all of these areas have the potential to influence cortical premotor neurons. Brain areas are not to scale, and connections and neuropeptides/transmitters indicated are not all-inclusive. Outline of rat brain was modified from Paxinos and Watson (Paxinos and Watson, 1990). For an alternative depiction, see fig. 2 in Castaneda et al. (Castaneda et al., 2005). 5HT, serotonin; Agrp, Agouti-related protein; ARC, hypothalamic arcuate nucleus; CCK, cholecystokinin; CRH, corticotrophin releasing hormone; DA, dopamine; LC, locus coeruleus; LH, lateral hypothalamus; MCH, melanin concentrating hormone; NAccSH, shell of nucleus accumbens; NE, norepinephrine; NMU, neuromedin U; NPY, neuropeptide Y; POMC, proopiomelanocortin; PVN, hypothalamic paraventricular nucleus; VTA, ventral tegmental area; rLH, rostral LH; SN, substantia nigra; TMN, tuberomammillary nucleus.
Like the complex regulatory network for feeding behaviour, a network of mediators regulates SPA. Very recent reviews (Kotz et al., 2008; Teske et al., 2008) have covered the specific data linking the action of several neuropeptides to SPA and NEAT, and hence that information will not be repeated here; rather, we will put some of the information into the context of physiological mechanisms contributing to obesity and obesity resistance. The studies discussed in those papers included only literature in which the endpoint was obtained from animals that had been habituated (usually for >24 h) to a measurement chamber large enough to encourage SPA (i.e. unlike most home-cage environments). The habituation period to these chambers reduces the potential novelty-induced anxiety behavior that is often observed in classic open-field testing paradigms (Castaneda et al., 2005).
Although not comprehensive, the major neuropeptide systems (peptide and receptor) that have been studied relative to SPA include cholecystokinin, corticotropin-releasing hormone, neuromedin U, neuropeptide Y, leptin, agouti-related protein, orexins and ghrelin. As mentioned above, dopamine may be the final common signaling mechanism for the action of all of these neuropeptides. Importantly, all have established roles in feeding behaviour, and thus the reported effects on SPA must be considered in the context of concurrent changes in other behaviours. Relevant to obesity is the proportional effect of stimulation and/or inhibition of relevant brain pathways on energy balance. If the energetic consequences of one behaviour (e.g. SPA) cancel out those of another behaviour (e.g. feeding), then the sum effect on energy balance will be nil. More likely, there is a disproportionate effect, with one elicited behaviour impacting energy balance more so than the other. Thus, in considering any of these neuropeptides as obesity targets, one must consider the full effect of the neuropeptide action.
Orexin A (hypocretin) is one such peptide that has been studied in detail. Orexin is synthesized in the lateral hypothalamic area, with projections throughout the hypothalamus and the rest of the brain (de Lecea et al., 1998; Sakurai et al., 1998). There are two orexin receptors, also distributed throughout the brain (Sakurai et al., 1998; Trivedi et al., 1998). Orexin B is derived from the same preprohormone as orexin A, but its effects are less studied, in part because its effects are less robust, probably because of its reduced binding affinity to both orexin receptors relative to orexin A (Sakurai et al., 1998; Trivedi et al., 1998). The large orexin network is suggestive of an integrative function for these neurons and the neuroanatomical origin of a particular orexin neuron population may define its functions. With respect to feeding behaviour and SPA, orexin neurons projecting from more caudal medial lateral hypothalamus regions may be more important to SPA as a result of projections to the midbrain ventral tegmental area, which contain dopamine-producing neurons important to locomotor activity.
Orexin A injected into specific brain areas markedly stimulates SPA (Kotz et al., 2006; Kotz et al., 2008) and NEAT in rats. This effect is much larger than that for orexin B. Mice lacking orexin have less SPA and weigh significantly more than wild-type littermates, despite reduced feeding behaviour (Hara et al., 2001), suggesting that changes in SPA may have a more profound influence on body weight than energy intake in these orexin-depleted animals, and implicating orexin in the regulation of energy balance. SPA and NEAT induced by orexin A are blocked by pre-administration of the orexin 1 receptor antagonist SB334867 (Kiwaki et al., 2004; Kotz et al., 2002; Kotz et al., 2006; Novak et al., 2006; Teske et al., 2006). Although this suggests that the orexin 1 receptor governs orexin A-induced effects on SPA and NEAT, the role of orexin 2 receptors cannot be discounted as orexin A binds to both receptors, and other literature suggests that the orexin 2 receptor may be important in the effect of orexin on physical activity (Kotz, 2006); the lack of availability of a specific orexin 2 receptor antagonist has hampered this line of investigation.
Of note is that behavioural effects of orexin depend upon the site of action. In the Kotz laboratory, orexin A injected in almost all brain areas increased SPA, whereas feeding behaviour was only influenced after injection into some of these same sites (Kotz et al., 2008). The time course of action is different for the feeding and activity effects of orexin A (Thorpe et al., 2003), indicting that the presence of one behaviour (feeding or SPA) does not depend upon the other. Thus, the question of displacement – whether one action of SPA displaces another – is still relevant, but may be less so owing to the temporal response. Likewise, whether orexin A-induced SPA is a derivative of enhanced wakefulness is uncertain, but this may be too simplistic an explanation. One must be awake to exhibit SPA, and so a sequence of waking, prior to physical activity, would be advantageous in producing elevated SPA. Narcoleptic humans lacking orexin are equally as awake as non-narcoleptics, yet are significantly heavier (Dahmen et al., 2001; Kotagal et al., 2004; Schuld et al., 2000). This argues against the idea that amount of time spent awake is directly related to the amount of time in SPA. In support of this concept, obesity-resistant (OR) rats with elevated SPA are awake the same amount of time as obesity prone (OP) animals with reduced SPA (Mavanji et al., 2010).
The standard laboratory rat model – the Sprague Dawley rat – is subject to variability in weight gain, regardless of diet exposure. Understanding the difference between rats that remain lean vs those who become obese (with aging) is the subject of intense investigation (e.g. Rada et al., 2010). Differences in absolute food intake clearly contribute to differences in body weight gain, but caloric intake is not the sole determinant. Studies in the Kotz laboratory and others suggest that energy expenditure differences, due to SPA, may underlie phenotypic differences in body weight gain (Levin et al., 1997; Ricci and Levin, 2003; Teske et al., 2006).
Indirect calorimetry studies show that, on a daily basis, OR and OP rats expend approximately the same number of absolute kilojoules (Kotz et al., 2008), in spite of reduced body fat, reduced energy intake and smaller body size in OR rats. This is important to note because several factors influence energy expenditure measurements, including body circumference (heat loss is a function of total area of the body), amount of body fat and lean mass (adipose tissue is approximately 20% as metabolically active as lean tissue) and energy intake differences (energy consumed results in energy expended, if body mass remains constant). The value of correcting estimates of energy expenditure for these factors is hotly debated, and there is not an agreed-upon standard method (Arch et al., 2006; Kaiyala et al., 2010). Nonetheless, the existing data suggest that NEAT in OR rats may be an important factor in preventing these rats from developing obesity (Teske et al., 2006).
Evidence from the Kotz laboratory suggests that OR rats are more sensitive to the SPA-promoting effects of orexin A, relative to both Sprague Dawley (Teske et al., 2006) and OP rats (Novak et al., 2006; Teske et al., 2006), implicating neurobiological differences in orexin signaling. Potential orexin signaling differences in OP and OR rats are now being explored, but the existing data show increased brain gene expression (Teske et al., 2006) and peptide content of orexin receptors (T. A. Butterick, personal communication) in OR rats. Together these data suggest that sensitivity to orexin A SPA promotion is inherent to OR rats and may be critically important to their obesity resistance. This is supported primarily by: (1) data showing OR rats remain lean despite enhanced food intake (on a per body weight basis relative to OP rats) (Teske et al., 2006), (2) observations in humans that loss of brain orexin (due to autoimmunity to orexin in narcolepsy) results in increased body mass index (Kotagal et al., 2004; Schuld et al., 2000), and (3) in rodents, loss of brain orexin (e.g. through pre- and post-natal genetic engineering approaches) results in reduced SPA and increased body weight, despite reduced feeding behaviour (Hara et al., 2001).
In conclusion, both human and animal studies indicate that SPA, and the resultant energy expenditure (NEAT) are inherent, biologically regulated and may protect against obesity. Multiple neuromodulators exist that interact and respond to environmental and physiological stimuli to define the level of SPA (and thus NEAT). These brain networks, which are important to SPA (and thus NEAT; Fig. 5), are beginning to be defined and may serve as new therapeutic targets of obesity research.
Personality correlates of physical activity
As discussed by Rhodes and Smith, the study of human personality “has a long and tumultuous history” with “numerous definitions, but most encompass the concepts that traits are enduring and consistent individual-level differences in tendencies to show consistent patterns of thoughts, feelings and actions” [(Rhodes and Smith, 2006) p. 958]. Their meta-analysis showed that the amount of physical activity in humans was positively related to the major personality traits of extraversion and conscientiousness, and negatively to neuroticism (Rhodes and Smith, 2006). Importantly, this meta-analysis did not consider sedentary behaviours as a separate variable, nor did it consider obesity or other indicators of physical health. However, previous studies (see also Dishman et al., 2008) have shown that human personality is related to health-related behaviours (e.g. exercise and maintaining a good diet) (Booth-Kewley and Vickers, 1994), obesity (Lykouras, 2008; Barry et al., 2009), and the metabolic syndrome (Sutin et al., 2010). Donahoo et al. (Donahoo et al., 2004) and Johanssen and Ravussin (Johanssen and Ravussin, 2008) speculated that individuals with inherently higher SPA may subconsciously tend to choose jobs that are more physically demanding. Ebstein reviewed empirical studies of the molecular genetic architecture of human personality (Ebstein, 2006).
In other species, concepts similar to personality are often termed coping styles, temperaments, behavioural tendencies, strategies, syndromes, axes or constructs (Gosling, 2001; Sih et al., 2004a; Koolhaas et al., 2007; Stamps and Groothuis, 2010). For example, Réale et al. stated that: “Temperament describes the idea that individual behavioural differences are repeatable over time and across situations” [(Réale et al., 2007) p. 291]. Of course, studies of human and non-human personality necessarily entail some major conceptual and empirical differences. First, human personality is usually measured using questionnaires that attempt to sample major, independent axes of behavioural variation in various situations of modern life. Obviously, such questionnaires cannot be administered to other animals. Nevertheless, the basic idea remains the same – a few major traits (or axes of variation in correlated traits) underlie (or at least predict) an individual's behaviour in many situations. Second, human personality traits are generally conceptualized and studied independent of each other, whereas animal personality traits are often assumed to be correlated strongly enough that they form behavioural syndromes. A third major difference is that, in non-human personality studies, consistently high vs low voluntary activity in a familiar environment is viewed as a temperament trait in and of itself, i.e. one of five major temperament trait categories: shyness–boldness, exploration–avoidance, activity, sociability and aggressiveness (Réale et al., 2007). In a risky environment, high activity becomes boldness. In a non-risky but novel environment, activity is interpreted as exploration (Réale et al., 2007). Some caution is due when reading the literature on personality of non-human animals, as this term is sometimes used merely to increase the appeal of a rather ordinary study of behaviour (e.g. concerning the repeatability of behaviour or correlations between different aspects of behaviour). Nonetheless, it has been established that, in rodents, birds and fish, some individuals are consistently more risk-taking and exploratory than others, and these aspects consistently reflect other behaviours, such as foraging style and invasion of novel territories (see below).
Irrespective of the extent to which personality traits correlate with the habitual level of physical activity due to some common underlying mechanisms, physical activity can have strong effects on cognitive and affective functions (Dey, 1994; Dietrich and McDaniel, 2004; Ekkekakis et al., 2005; Hillman et al., 2008; Tomporowski et al., 2008). Daily exercise in humans has been shown to attenuate anxiety and depression (Kligman and Pepin, 1992; Antunes et al., 2005) and to stimulate learning capability (Hillman et al., 2008), and similar effects of voluntary wheel running on emotionality, stress coping and learning have been demonstrated in rodents (Greenwood et al., 2007; Pietropaolo et al., 2008; van Praag et al., 1999; Rhodes et al., 2003b). Although this is certainly interesting in light of the idea that obese individuals are more frequently depressed than lean ones (McElroy et al., 2004), it does not necessarily mean that individuals display a high level of voluntary activity to ‘subconsciously medicate’ themselves. A perhaps more probable explanation is that the display of voluntary activity is a trait characteristic of one's personality.
In non-human studies, it is recognized that a fundamental factor structuring personality is the degree to which individual behaviour is guided by environmental stimuli (Sih et al., 2004; Groothuis and Carere, 2005; Korte et al., 2005). Individuals can differ along an axis that ranges from paying close attention to environmental stimuli and quickly adapting their behaviour to the prevailing conditions (i.e. flexible, reactive individuals) to those that show more rigid, routine-like behaviours, regardless of conditions (i.e. proactive individuals). Relative to reactive animals, proactive animals are often aggressive to male conspecific rivals, are more impulsive, take higher risks in the face of potential dangers and are impulsive in decision-making (Steimer et al., 1999; Steimer et al., 2003; Groothuis and Carere, 2005). Specific behaviours like these have been referred to as coping style, and they have been observed in birds (van Oers et al., 2004; Verbeek et al., 2008), mammals (Koolhaas et al., 1999) and primates including humans (Aron and Aron, 1997). To our knowledge, no distinction has been made on the basis of physical activity (or sedentary behaviours as such), and it remains to be investigated whether the display of voluntary exercise and/or SPA aligns with the above-mentioned behavioural categories. It can nevertheless be hypothesized that proactive individuals tend to be more agile and physically active (to oppose environmental challenges) than reactive animals. This idea needs empirical testing. Recently, rats from lines that vary in coping style were shown also to vary in responses to a high-fat diet, leading to the suggestion that “a proactive personality might be protected against the development of diet-induced insulin resistance” [(Boersma et al., 2010) p. 401].
Because the expression of proactive-like behaviours (activity, aggressiveness, boldness) is supposedly energy demanding, Careau et al. recently suggested the existence of fundamental associations between personality types and energy expenditure (Careau et al., 2008). Empirical support for associations between personality and energetics has just begun to accumulate in fishes (Huntingford et al., 2010), dogs (Careau et al., 2010) and rodents (Careau et al., 2009) (but see Jonas et al., 2010b), but how these associations may relate to SPA and voluntary exercise is unclear.
Because the expression of personality can be strongly contingent on the environmental context, Careau and colleagues argued that psychological stress (e.g. as a response to conspecific rivalry, caused by assessment of locomotor behaviour or energy expenditure) may lead to more freezing behaviour in passively coping animals, which would be scored by locomotion detectors as resting (sedentary) behaviour (Careau et al., 2008). However, whether animals would freeze for periods long enough to be a problem in practice has not been established through empirical studies. The converse may also be true – that some animals with a proactive coping style are trying to escape from confinement; i.e. they would have a relative overestimation of energy devoted to SPA, as this activity may have a large volitional component (see above). However, as noted by Careau et al. (Careau et al., 2008), many investigators carefully exclude individuals that do not appear to rest sufficiently – which may lead to a bias. These possibilities pose an intrinsic difficulty for assessment of several aspects of DEE under certain conditions, because animal personality may affect the amount of energy expended under resting conditions, SPA and volitional activity (Careau et al., 2008). For this reason, it is useful to investigate in more detail the behavioural syndrome or personality of animals that are known to differ in SPA under normal, habituated conditions.
We have previously investigated whether and how certain personality traits are associated with the level of SPA in mice from HR lines and their controls (Swallow et al., 1998). If some personality traits are indeed related to voluntary physical activity, then one would hypothesize that the expression of homologous behavioural traits will be altered in HR mice relative to control lines. Extraversion has homology with exploratory behaviour (Garcia-Sevilla, 1984), which can be assessed in mice by placing them in a open field, typically a circular or square arena of approximately 1 m diameter. The tendency of the animal to explore the open inner surface is positively related to the level of exploratory behaviour (Archer, 1973). At generation 22, Bronikowski et al. did not observe any statistically significant differences between HR and control mice for distance traveled, defecation, time spent in the interior or average distance from the center of the arena during a 3-min trial (Bronikowski et al., 2001). However, HR mice of both sexes turned less in their travel paths, and HR females had slower travel times (longer latencies) to reach the wall on trial day 1. Recently, we re-evaluated this outcome in two of the HR lines and one control line from a later generation (45) (Jonas et al., 2010b). One of the two HR lines (line 8) showed more locomotion and visit frequency towards the middle surface than the controls, and also showed more exploration in the center when the novel object was placed in the center of the open field. Another HR line (line 7), however, did not increase locomotion towards the middle ring, but instead showed almost a doubling of rearing or upright behaviour exploring the wall compared with control and line 8 mice. If scored with automated locomotion detection, this may have lead to tremendously different interpretations. Furthermore, when a novel object was placed in the center of the open field, only HR line 8 mice showed increased approach and (presumed) risk-assessment behaviour (Augustsson et al., 2004). The response in this particular risk-taking line of mice may be secondary to the horizontal expression of exploratory behaviour, which is more likely to be followed by approach behaviour to the novel object than the vertical expression of exploratory behaviour displayed by line 7 mice. Thus, although expressed differently, both HR lines clearly showed increased information-gathering behaviour in the empty open field. The willingness of mice to explore an open field bears an emotional stress component. In other words, if the anxiety level is high the tendency of an animal is to stay away from the open inner compartment of the open field. One way to test anxiety more directly in mice is to place them on an elevated plus maze, with two opposing arms fenced off by high walls, and the other two opposing arms left open (Lister, 1987). The extent to which animals expose themselves on the open arms vs the closed arms is correlated with the level of anxiety (with low open exposure linked to a high level of anxiety). To our surprise, HR mice spent, on average, less time in the open arms than the control mice, suggesting that the former have higher anxiety levels. This seems incongruent with the aforementioned findings in the surveyable open-field tests, where HR mice showed more exploratory and risk-taking behaviour (in line 8). Moreover, it seems incongruent with the overarching hypothesis that high and (relatively) low voluntarily physically active mice would fall into the same categories as the proactive and reactive coping styles.
One interesting aspect of behavioural changes in the HR mice is increased predatory aggression when non-fasted mice are offered live crickets, possibly related to alterations in dopamine signaling (Gammie et al., 2003). This difference does not generalize to other types of aggression, as HR and control lines show no statistical differences in intermale or maternal aggression. The positive genetic correlation between predatory tendencies and the propensity and/or ability to run long distances on a daily basis could, if it occurs in wild populations of rodents, facilitate the adaptive evolution of an active, widely foraging lifestyle (Feder et al., 2010).
An important factor not previously mentioned in this context is the nutritional status of the animal, which could alter behavioural responses to environmental stimuli (and perhaps including those in the elevated plus maze), as well as affect activity levels directly (see Meek et al., 2010). In rodents, food restriction has been reported both to alleviate (Inoue et al., 2004; Yamamoto et al., 2009) and to increase anxiety (Jahng et al., 2007). However, a field study of great tits found that food availability in the environment was a major determinant in the differential survival of fast- and slow-exploring birds (Dingemanse et al., 2004). After a sudden drop in food availability, fast-exploring juvenile tits more rapidly invaded new territories than slow-exploring individuals (van Overveld et al., 2010). Although this needs a much more elaborate experimental approach using carefully characterized animals, it does suggests that fast-exploring and thus potentially physically active individuals range over larger territories compared with slow-exploring and thus potentially inactive ones. As mentioned earlier and suggested by Careau et al. (Careau et al., 2008), inactive animals may have an advantage over active animals under high predation pressure if their frugal energy budget allows them to be less noticeable to predators (e.g. Clobert et al., 2000). It can be hypothesized that these alternative strategies belong to different suites of correlated behaviours (or personalities by some definitions), which have been shaped by the trade-off between retaining energy (i.e. a thrifty phenotype) and spending energy anticipating future energy return (i.e. an exploratory phenotype). It would be of interest to test the relevance of any of these interactions and potential trade-offs in mice living in natural habitats (Benus et al., 1991). Recently, a theoretical basis was provided for the idea that energy trade-offs can, in fact, rapidly shape different personalities, and can lead to dimorphic populations (Wolf et al., 2007; Wolf et al., 2008).
A major challenge is to understand correlated behaviours in a neurobiological context. An attempt can be made based on the transient hypofrontality hypothesis of Dietrich and colleagues (Dietrich, 2003; Dietrich and Sparling, 2004). They suggested that there is a temporary inhibition of neural activity in the prefrontal lobe during monotonous, automated movements (Dietrich and McDaniel, 2004). At the same time, when neuronal activity in the prefrontal lobes becomes reduced, the basal ganglia – which control routine behaviour – are being activated (Dietrich, 2003; Dietrich and McDaniel, 2004; Graybiel, 2008). It is possible that mice from the HR lines have a higher capacity of re-allocating neuronal activity from prefrontal cortical regions to the basal ganglia during exercise, which may help them to sustain this behaviour. This could provide a neurobiological explanation for the observation that HR mice, after extensive maze learning, visited a former habituated exit in the complex maze when a new exit is somewhere else (Jonas et al., 2010b). In a novel environment, however, where the HR mice are faced with uncertainty (such as in the open field), this switch of neuronal activity from prefrontal cortex to basal ganglia probably does not occur. If anything, the HR mice show increased curiosity and exploration in a novel environment, which reduces uncertainty and vigilance (Dember and Earl, 1957; Berlyne, 1966). Thus, in nature, where resources sometimes become temporarily reduced and/or predation increases, the behavioural characteristics of increased locomotor activity associated with – or perhaps causal to – curiosity and exploration may be favored by selection. This hypothesis could be partially tested by housing of individuals in semi-natural enclosures (e.g. see Potts et al., 1991; Carroll et al., 2004).
Synthesis; physical activity, neurobiology and personality
Given that both eating and voluntary exercise can be rewarding and invoke hedonic responses, can be addictive and are affected by some of the same neurotransmitter systems (e.g. dopamine) and brain regions (e.g. nucleus accumbens), it would be expected that factors affecting one may also affect the other (see also Dishman, 2008; De Chiara et al., 2010). Hence, we may expect genetic variants that mainly affect, say, appetite or responses to (preferences for) specific dietary components also to have pleiotropic effects on the propensity for voluntary exercise. Accordingly, Mathes et al. compared one of the HR lines of mice, a hyperphagic line that had been bred for increased body mass and body fat (M16), with a non-selected Institute of Cancer Research (ICR) line representing the base population from which both HR and M16 were selected (Mathes et al., 2010). HPLC analysis showed significant strain effects on brain neurotransmitter concentrations and microarray analysis showed significant differences in brain gene expression between HR and M16 compared with ICR mice in both dorsal striatum and nucleus accumbens. Results demonstrate that alterations within central reward pathways can contribute to both obesity and excessive exercise, and suggest that similar modifications within the dopamine system may contribute to the expression of opposite phenotypes in mice.
Of course, several other hormones, neuropeptides and neurotransmitters may be involved in the exerciser phenotype. For example, leptin affects appetite, energy balance, motivation, reward and locomotor activity via effects on the central nervous system (e.g. Girard et al., 2007; Hillebrand et al., 2008; DiLeone, 2009; Gluckman and Hanson, 2009; Zheng et al., 2009; Davis, 2010; Davis et al., 2010) and hence could lead to pleiotropic genetic effects. A recent study indicates that restoration of leptin receptors exclusively in POMC neurons in hypoactive, leptin-receptor deficient mice (db/db) enhances locomotor activity, suggesting that POMC mediates locomotor activity (Huo et al., 2009). Young adult female HR mice show reduced circulating leptin levels, even after adjusting statistically for their reduced body fat (Girard et al., 2007). Both sexes of HR mice show elevated circulating adiponectin (Vaanholt et al., 2007c; Vaanholt et al., 2008) and corticosterone (Malisch et al., 2007; Malisch et al., 2008) levels. In children (Metcalf et al., 2009), physical activity measured by accelerometers (over 7 days and averaged for readings taken at ages of 5, 6, 7 and 8 years) showed a weak negative correlation with circulating adiponectin level (partial r controlling for sex=–0.18, P=0.02, N=213), a result that is at odds with findings from the HR lines of mice. In the same study, activity was not correlated with circulating leptin level (r=0.04), which again seems at odds with the HR mice and with some studies of adult humans (e.g. Franks et al., 2003). The causality of such relationships is presently unclear, e.g. because the HR mice show elevated SPA when housed without wheels (Malisch et al., 2009). One possibility worth exploring is whether genetically elevated levels of adiponectin not only stimulate fat oxidation (e.g. in active muscles that allow sustainable increases in physical activity), but also augment SPA or voluntary exercise by actions in the brain. Although a number of rodent studies have investigated effects of genetic (e.g. by overexpression) or pharmacological manipulation of adiponectin on aspects of energy balance (see reviews below), they apparently have not addressed specific locomotor effects of these treatments.
Some rodent studies show that high-fat diet can affect (typically lower) SPA and/or wheel running, although others have not found an effect [Hesse et al. (Hesse et al., 2010) and references therein]. Brownlow et al. reported no statistical effect of high-fat diets administered for 10 weeks on SPA of male C57BL/6J or A/J mice measured by photobeams for 1 hour during the dark phase (Brownlow et al., 1996). Cheng et al. reported that male Long–Evans rats fed high-fat diet for up to 6 weeks did not show any statistical effect on voluntary wheel-running distance compared with rats fed a standard diet (Cheng et al., 1997). Funkat et al. administered a high-fat diet to males from three strains of mice for 6 weeks, then measured SPA and wheel running in separate groups of individuals on days 3–7 of a 7-day test (Funkat et al., 2004). High-fat diet significantly decreased SPA (in horizontal, vertical and ambulatory measures) in one of three strains (C57BL/6) whereas it decreased the vertical component in another strain (129T2). By contrast, wheel running was significantly decreased in both C57BL/6 and DBA/2 mice. Bjursell et al. also reported that high-fat diet reduced SPA in male C57Bl/6J mice, both acutely (within 24 h) and over a 21-day period (Bjursell et al., 2008). When housed without wheel access, both the high capacity runner (HCR) and low capacity runner (LCR) lines of endurance-selected rats showed a similar decrease in daily activity levels in response to high-fat feeding for 1 month (Novak et al., 2010). In a study of mice bred for low or high percent body fat, low-body-fat mice did not change their total wheel-running distance on being fed a high-fat diet (Simoncic et al., 2008). The high-body-fat mice fed a high-fat diet increased wheel running compared with high-body-fat mice fed regular chow, but running distances did not exceed values of low-body-fat animals on either diet (see fig. 3 in their paper). A high-fat diet did not appear to affect habituated SPA of control lines of mice in cages over a 48-hour measurement period, but tended to increase it in HR lines, although the effects were not statistically significant (Vaanholt et al., 2008). Remarkably, a Western diet greatly stimulated wheel running in HR mice whereas it had little or no effect in control mice (Fig. 6) (Meek et al., 2010); unfortunately, home-cage activity was not measured. So far as we are aware, this stimulation of voluntary exercise by Western diet has not been previously reported in any rodent or human study [but see Simoncic et al. (Simoncic et al., 2008) and discussion of that paper (Meek et al., 2010)]. In future studies, it will be important to determine the neural and/or metabolic mechanisms underlying this unique effect, as well as possible effects on NEAT/SPA and sedentary behaviours.
Fig. 6.
Western diet increased voluntary wheel running of mice from High Runner (HR) lines by ∼52% (+36% duration of running, +18% average speed of running) during days 17–30 of wheel access, whereas it had no effect on control lines [standard diet (Std)] [data from Meek et al. (Meek et al., 2010)]. This stimulation of voluntary exercise is remarkable in particular because the lines have been at an apparent selection limit for ∼35 generations (see Kolb et al., 2010). Data are adjusted means + s.e.m.
We are not aware of any human studies that have specifically investigated the effects of dietary intervention (e.g. Western diet) on the amount of voluntary exercise (Jebb, 2007). Most studies of dietary intervention either restrict diet or reduce a macro- or micronutrient (e.g. low fat, low sodium) and focus on weight loss or change in a risk factor for cardiovascular disease (e.g. blood pressure, cholesterol level), and perhaps measure physical activity secondarily. However, in an observational study of young adult men, habitual high-fat consumers had somewhat lower levels of physical activity and significantly more periods of sedentary behaviour than habitual low-fat consumers (Blundell and Cooling, 2000). Thus, dietary preferences and activity levels may be related.
Genetic basis of variation in physical activity and related phenotypes
Simonen et al. noted that: “The genetic dissection of complex traits represents a daunting challenge” [(Simonen et al., 2003b) p. 1358]. As one example, the genetics of obesity per se is currently the subject of intense research in both human populations and rodent models, and the more we learn about this topic, the more complicated it seems (Rankinen et al., 2005; O'Rahilly and Farooqi, 2006; O'Rahilly and Farooqi, 2008; Pomp et al., 2008; Speakman et al., 2008; de Krom et al., 2009; Fawcett et al., 2010; Rankinen et al., 2010; Speakman, 2010). Results to date make it clear that the effects of physical activity on the human body mass index (BMI) vary with genotype (Rankinen et al., 2010), and similar results have been observed for different strains of mice (Nehrenberg et al., 2009a; Meek et al., 2010). More generally, an individual's adult obesity phenotype may be affected by epigenetic effects passed on from mothers, their in utero and early-life experiences (including diet and opportunity for exercise), developmental plasticity and their bacterial complement (Ozanne et al., 2004; Kotz, 2008; Pomp and Mohlke, 2008; Gluckman and Hanson, 2009; Metges, 2009). We also have strong evidence for genetic effects on aspects of eating behaviour in both humans and rodents (Koteja et al., 2003; de Castro, 2004; Rankinen and Bouchard, 2006; de Krom et al., 2009), and emerging evidence that in rodents and humans the propensity for physical activity and aspects of eating behaviour can be genetically related and possibly genetically correlated (Cai et al., 2006; Jonas et al., 2010a; Kumar et al., 2010). Here, we provide a brief summary of the genetics of physical activity and related phenotypes, many of which are related to obesity and/or energy intake. Our focus here is not on the genetics of personality [e.g. see references in Stamps and Groothuis (Stamps and Groothuis, 2010)], but, as noted in the previous section, evidence suggests that personality may be an important component of weight gain and obesity (e.g. Booth-Kewley and Vickers, 1994; Lykouras, 2008; Barry et al., 2009; Sutin et al., 2010). Readers should note that the molecular-genetic analysis of behavioral traits, especially with mice, is undergoing rapid technical and conceptual progress (Flint and Mott, 2008).
Before delving into specifics, it is worth emphasizing some general points about the genetic architecture of quantitative traits, such as body size, metabolic rate and activity level. A recent review of humans, mice and Drosophila concluded that most quantitative traits in all of these species are affected by a large number of genetic loci, most harboring alleles with small effects on the total phenotypic variance in a population (Flint and Mackay, 2009). In addition, pleiotropic genetic effects may be pervasive, and many important genetic variants have been found to occur outside of genes, likely in regulatory regions, where they may act by altering gene expression.
A wide range of studies in both humans and rodents has demonstrated some genetic basis for individual variation in both exercise propensity and ability (see also Dishman, 2008). In laboratory mice, strain differences in both voluntary wheel running and treadmill endurance capacity have been documented, thus demonstrating significant broad-sense heritability (Ebihara and Tsuji, 1976; Lightfoot et al., 2001; Lightfoot et al., 2004). Breeding designs have demonstrated narrow-sense heritability of maximal oxygen consumption in laboratory strains of house mice (Dohm et al., 2001; Wone et al., 2009). In quantitative genetics, a response to selective breeding is generally considered to represent the gold standard for demonstrating that a trait in a given population has significant narrow-sense heritability. Studies of rodents have demonstrated that selective breeding can alter various types of voluntary physical activity, ranging from behaviour in a novel open-field arena over 3 min to wheel running over 2 days, as well as performance abilities in tests of forced exercise (for reviews, see Rhodes and Kawecki, 2009; Swallow et al., 2009; Feder et al., 2010; Zombeck et al., 2011). In addition, lines of mice bred for heat loss per se also show differences in locomotor activity that contribute to differences in food intake (Mousel et al., 2001).
The level of voluntary exercise is viewed as a function of both ability and motivation, with their relative importance likely to vary among species, individuals, and activities. Further studies of activity-selected lines of mice (the HR lines) and of rats bred for treadmill endurance have revealed alterations in brain function that seem to indicate changes in motivation or propensity to exercise on wheels (Rhodes et al., 2005; Foley et al., 2006; Morishima et al., 2006; Belke and Garland, 2007; Keeney et al., 2008; Waters et al., 2008) (R. P. Waters, R. B. Pringle, G. L. Forster, K. J. Renner, J. L. Malisch, T.G. Jr and J. G. Swallow, unpublished results). Many other studies of these same lines demonstrate alterations in exercise abilities (e.g. Howlett et al., 2009; Meek et al., 2009; Kolb et al., 2010), including altered plasticity for some traits in the HR lines of mice (Rhodes et al., 2003b; Swallow et al., 2005; Garland and Kelly, 2006; Gomes et al., 2009). Therefore, genetic factors are known to be involved in both motivation and ability to engage in voluntary wheel running in rodents. Further, the increase in endurance by lines of mice bred for high voluntary wheel running (Meek et al., 2009) and the divergence in wheel running of two rat lines bred for high or low endurance capacity indicate that exercise propensity and ability are positively genetically correlated in these rodents. [Based on mouse selection experiments, Nehrenberg et al. have suggested that physical activity and body composition are negatively genetically correlated (Nehrenberg et al., 2009a).] Finally, these same lines show parallel differences in SPA, body size, percent body fat and circulating leptin levels when housed without wheels (Girard et al., 2007; Malisch et al., 2009; Meek et al., 2010; Novak et al., 2009; Novak et al., 2010) (T.G., H.S., M.A.C., T.H.M., L.E.C., W.A. and R.C.M., unpublished results), with the HR lines of mice having very low body fat relative to other mouse strains (Nehrenberg et al., 2009a) (see also Svenson et al., 2007).
Studies of human populations have demonstrated significant broad-sense heritabilities for both exercise propensity and ability, as well as trainability (Cai et al., 2006; Seabra et al., 2008; Teran-Garcia et al., 2008; Stubbe and de Geus, 2009). The significant heritability of exercise propensity is consistent with one of Turkheimer's laws of behavior genetics, which states “all human behavioral traits are heritable” (Turkheimer, 2000). The estimated heritability of physical activity varies considerably (range 18–69%) because of differences in measurement and expression of physical activity (e.g. retrospective questionnaire vs accelerometer, sports participation vs MVPA) and composition of the study population (e.g. family structure, race) (Stubbe and de Geus, 2009).
Recent genome wide-association studies (GWAS) are beginning to identify individual genes, or at least chromosomal regions, that contribute to variation in exercise propensity or ability (e.g. Ways et al., 2007). Studies in rodents (see also Kumar et al., 2010) have identified a number of quantitative trait loci (QTL) that account for a statistically significant fraction of the variation in experimentally created mapping populations (e.g. Lightfoot et al., 2008), including populations that originated by crossing an HR line with an inbred strain (Hartmann et al., 2008; Nehrenberg et al., 2009b; Kelly et al., 2010). Good et al. have proposed that the helix-loop-helix transcription factor Nhlh7 affects body weight through control of physical activity levels via effects on either the motivation or the ability to exercise (Good et al., 2008).
One interesting finding from Kelly et al. is that some QTL were associated with the trajectory of running across 6 days of wheel access (Kelly et al., 2010). Moreover, the QTL observed for the regression slope of the wheel-running traits across days often did not coincide with locations of the QTL for individual days. This result suggests that the trajectory of voluntary exercise over multiple days is partly controlled by different genomic regions than those controlling the behaviour on individual days, which could have important implications regarding individual variation in human adherence to exercise regimens or sports participation (see also Lightfoot, 2008b). Since then, Leamy et al. analysed QTL for wheel running over a 21-day period in an F2 population created by crossing two inbred strains (Leamy et al., 2010). They reported that some QTL affected activity early in the period whereas others affected activity later in the period, and concluded that: (1) the genetic architecture of physical activity is more complicated than previously appreciated and (2) it may change with age, as has been observed for other complex traits, such as body weight (see also Morgan et al., 2003). Also, sex-specific, wheel-running QTL have been reported by both Nehrenberg et al. (Nehrenberg et al., 2009) and Leamy et al. (Leamy et al., 2010), reinforcing the complexity of the underlying genetic architecture. Mechanisms that may underlie sex differences in the genetic basis of voluntary activity (see also Garland et al., 2010) include the endocannabinoid system and the sex hormones (Keeney et al., 2008; Lightfoot, 2008a).
Viggiano (Viggiano, 2008) provides a meta-analysis based on a database of genetic modifications, brain lesions and pharmacological interventions that increase locomotor activity in a novel environment in rodents, i.e. not the same as habituated SPA or voluntary exercise, which are the foci of the present paper. Nonetheless, the results are of interest because they may prove to be instructive for SPA or voluntary exercise. Viggiano estimated that 1.56% of the genes in the genome are associated with hyperactivity whereas 0.75% result in hypoactivity when altered (Viggiano, 2008); thus, hundreds of genes are involved. Emphasizing the neurobiological complexity of hyperactivity, he found that alterations in most neurotransmitter systems can give rise to a hyperactive phenotype whereas, perhaps surprisingly, fewer changes are associated with decreases in locomotor activity. More generally, Viggiano concluded that there is a net imbalance in the number of altered genes, brain lesions and/or toxins that can induce hyperactivity vs hypoactivity (Viggiano, 2008). Importantly, he hypothesized the existence of a control system that continuously inhibits a basally hyperactive locomotor tone and that this control system is highly vulnerable to genetic or environmental effects that occur during prepubertal stages.
Among adult humans, a genome-wide scan based on 432 markers typed in 767 subjects from 207 Quebec families found ‘promising’ evidence of linkage (P<0.0023) for a measure of physical inactivity on chromosome 2 (Simonen et al., 2003b). We find it noteworthy that this measure of what may be termed sedentary behaviour (see above) showed stronger linkage that any of the three measures of physical activity (total physical activity, moderate to strenuous physical activity and time spent in physical activity). There is also evidence that polymorphisms in the dopamine D2 receptor gene (Simonen et al., 2003a) and melanocortin-4 receptor gene (Loos et al., 2005) are associated with physical activity in adults. A QTL on chromosome 18q contributes to the variation in both physical activity and dietary intake in children from Hispanic families at high risk for obesity (Cai et al., 2006). De Moor et al. provide the first genome-wide association study of physical activity levels, derived from questionnaires and categorizing individuals simply as exercisers or nonexercisers, with a prevalence of approximately 50% of exercisers in both American and Dutch study populations (De Moor et al., 2009). Although none of the 1.6 million single nucleotide polymorphisms (SNPs) reached genome-wide significance, SNPs in three genomic regions showed suggestive significance levels (see Rankinen et al., 2010). Studies of genetic polymorphisms associated with performance-related phenotypes (as opposed to measures of how much they engage in physical activity) are more numerous and reviewed elsewhere (Teran-Garcia et al., 2008; Rankinen et al., 2010).
Conclusions and future directions
Both voluntary exercise and SPA can be measured in humans and rodents (Table 1), but not all activity fits cleanly into these categories. Moreover, the comparability of various measures across species is unclear. Both types of physical activity can have important effects on energy balance and, hence, on growth rates or body composition (and reproduction). Over longer time scales, small alterations in physical activity or energy intake (e.g. ±100 kcal day–1) can ‘tip the scales’, leading to human obesity. Physical activity can aid in resistance against overweight and obesity (Bi et al., 2005; Bell et al., 1995; Donahoo et al., 2004; Brock et al., 2005; Hayes et al., 2008; Levin et al., 2004; Patterson and Levin, 2008), and obese humans score lower for SPA compared with lean individuals (Levine, 2007). Aside from this, physical activity has numerous beneficial effects on various aspects of both mental and physical health (Booth et al., 2002a,b; Dishman et al., 2006; Booth and Lees, 2007; Cotman et al., 2007; Hillman et al., 2008; Pietropaolo et al., 2008; Blair and Morris, 2009; Fair and Montgomery, 2009; van Praag, 2009), including cardiovascular (aerobic) fitness, which may have independent beneficial effects on health (Hillman et al., 2008; Church, 2009). Thus, from multiple clinical standpoints, physical activity should be promoted, particularly for those who live sedentary lives (Church and Blair, 2009).
Separate from effects of physical activity, a growing number of human studies indicate that too much or prolonged sitting (sedentary behaviours) can adversely affect body weight, biomarkers of metabolic health and other chronic health problems (Owen et al., 2009; Owen et al., 2010). Given that people in industrialized societies typically spend the majority of their waking hours engaged in sedentary behaviours, it has been argued that “reducing sitting time may have at least as important a role as promoting physical activity” [(Owen et al., 2009) p. 82]. We are not aware of rodent studies that have directly investigated sedentary behaviours separate from measures of voluntary exercise and SPA (Table 1) in these contexts, so this is an important area for future research.
The effects of voluntary exercise on SPA may depend on duration and intensity, measurement technique, species, sex and age. Clear evidence that complete compensation often does not occur is apparent in numerous rodent studies that have shown exercise-training effects when wheels are provided, e.g. studies on the HR and control lines of mice (see Dumke et al., 2001; Swallow et al., 2001; Bronikowski et al., 2002; Rhodes et al., 2003b; Swallow et al., 2005; Garland and Kelly, 2006; Gomes et al., 2009).
Although wheel access generally increases food consumption in laboratory rodents, a variety of studies in both rodents and humans indicate that energy expenditure is not necessarily tightly coupled to energy intake during relatively short-term exposure. This mismatch occurs, in part, because eating entails hedonic responses that can lead to non-homeostatic feeding (Davis et al., 2010). In addition, certain diets or dietary components are obesogenic beyond the effect that would be predicted from an elevated digestibility or caloric content (e.g. Meek et al., 2010). Further, eating can be affected by societal and cultural factors that often make it much more than simply a matter of getting enough energy.
As complicated as the control of eating may be, the control of physical activity is probably at least as complex – and is probably intertwined mechanistically with the control of eating. In mice, it has even been shown that certain diets can increase maximal sprint speed (C. Turbill and T. Ruf, personal communication) or stimulate voluntary exercise (Meek et al., 2010) and possibly SPA (Vaanholt et al., 2008) in a genotype-dependent fashion (Simoncic et al., 2008). Eating and physical activity are related not only through various biochemical and physiological pathways, but also via brain reward pathways and, it would seem, they may respond to environmental stimuli or evolve in non-intuitive ways. For example, it is well known that pharmaceuticals used to treat ADHD or eating disorders have a variety of side effects involving, respectively, appetite or activity. Therefore, it seems likely that effective countermeasures to encompass the full range of variation in obesity and its causes will need to target feeding behaviour, SPA, voluntary exercise, sedentary behaviours and perhaps other aspects of personality – or some combination thereof – depending on the individual (e.g. King et al., 2007a) and with due recognition of likely sex differences. If one could predict the extent to which compensation occurs (e.g. the extent to which prescribed exercise leads to reduced SPA or increased sedentary and eating behaviour), then one could tailor treatment of obesity and metabolic derangements more effectively. Thus, a major challenge lies ahead of us to discover potentially suitable prospective markers in personality, endocrinology or genetic make-up.
Acknowledgments
T.G. thanks T. J. Lightfoot, S. A. Kelly and D. Pomp for many helpful discussions over the years. We thank V. Careau and two anonymous reviewers for comments on the manuscript.
List of abbreviations
- ADHD
attention deficit hyperactivity disorder
- AEE
activity energy expenditure
- BMR
basal metabolic rate
- DEE
daily energy expenditure
- DLW
doubly labeled water
- ECS
endocannabinoid system; a complex endogenous signaling system made up of transmembrane cannabinoid receptors, their ligands (endocannabinoids) and proteins involved in synthesis and modification of endocannabinoids
- FMR
field metabolic rate
- GWAS
genome wide-association study
- HR
High Runner; four replicate lines of mice that have been bred for high voluntary wheel running on days 5 and 6 of a 6-day period of wheel access while they are young adults (Swallow et al., 1998)
- MVPA
moderate-to-vigorous physical activity, as used in studies of humans
- NEAT
non-exercise activity thermogenesis
- QTL
quantitative trait locus
- SNP
single nucleotide polymorphism
- SPA
spontaneous physical activity
- TEF
thermic effect of food
Footnotes
Preparation of this manuscript was supported by US NSF grants IOB-0543429 (to T.G.) and BCS-0925793 (to L.E.C.) and by NIH/NIDDK R01 DK078985 (to C.M.K.). Deposited in PMC for release after 12 months.
References
- Ahlenius S., Hillegaart V., Thorell G., Magnusson O., Fowler C. J. (1987). Suppression of exploratory locomotor activity and increase in dopamine turnover following the local application of cis-flupenthixol into limbic projection areas of the rat striatum. Brain Res. 402, 131-138 [DOI] [PubMed] [Google Scholar]
- Aidman E. V., Woollard S. (2003). The influence of self-reported exercise addiction on acute emotional and physiological responses to brief exercise deprivation. Psychol. Sport Exerc. 4, 225-226 [Google Scholar]
- Ainsworth B. E., Haskell W. L., Whitt M. C., Irwin M. L., Swartz A. M., Strath S. J., O’Brien W. L., Bassett D. R., Jr, Schmitz K. H., Emplaincourt P. O., et al. (2000). Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 32, S498-S504 [DOI] [PubMed] [Google Scholar]
- Albert F. W., Carlborg O., Plyusnina I., Besnier F., Hedwig D., Lautenschlager S., Lorenz D., McIntosh J., Neumann C., Richter H., et al. (2009). Genetic architecture of tameness in a rat model of animal domestication. Genetics 182, 541-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegre B., Souville M., Therma P., Griffiths M. (2006). Definitions and measures of exercise dependance. Addict. Res. Theory. 14, 631-646 [Google Scholar]
- Allen D. L., Harrison B. C., Maass A., Bell M. L., Byrnes W. C., Leinwand L. A. (2001). Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 90, 1900-1908 [DOI] [PubMed] [Google Scholar]
- Ang E. T., Gomez-Pinilla F. (2007). Potential therapeutic effects of exercise to the brain. Curr. Med. Chem. 14, 2564-2571 [DOI] [PubMed] [Google Scholar]
- Antunes H. K., Stella S. G., Santos R. F., Bueno O. F., de Mello M. T. (2005). Depression, anxiety and quality of life scores in seniors after an endurance exercise program. Rev. Bras. Psiquiatr. 27, 266-271 [DOI] [PubMed] [Google Scholar]
- Arch J. R., Hislop D., Wang S. J., Speakman J. R. (2006). Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obes. (Lond.) 30, 1322-1331 [DOI] [PubMed] [Google Scholar]
- Archer J. (1973). Tests for emotionality in rats and mice: a review. Anim. Behav. 21, 205-235 [DOI] [PubMed] [Google Scholar]
- Aron E. N., Aron A. (1997). Sensory-processing sensitivity and its relation to introversion and emotionality. J. Pers. Soc. Psychol. 73, 345-368 [DOI] [PubMed] [Google Scholar]
- Augustsson H., Meyerson B. J. (2004). Exploration and risk assessment: a comparative study of male house mice (Mus musculus musculus) and two laboratory strains. Physiol. Behav. 81, 685-698 [DOI] [PubMed] [Google Scholar]
- Barry D., Clarke M., Petry N. M. (2009). Obesity and its relationship to addictions: is overeating a form of addictive behavior? Am. J. Addict. 18, 439-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke T. W. (1996). Investigating the reinforcing properties of running: or, running is its own reward. In Activity Anorexia: Theory, Research, and Treatment (ed. Epling W. F., Pierce W. D.), pp. 45-55 Mahwah, NJ: Lawrence Erlbaum Associates; [Google Scholar]
- Belke T. W., Garland T., Jr (2007). A brief opportunity to run does not function as a reinforcer for mice selected for high daily wheel-running rates. J. Exp. Anal. Behav. 88, 199-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke T. W., Heyman G. M. (1994). A matching law analysis of the reinforcing efficacy of wheel running in rats. Anim. Learn. Behav. 22, 267-274 [Google Scholar]
- Bell R. R., Spencer M. J., Sherriff J. L. (1995). Diet-induced obesity in mice can be treated without energy restriction using exercise and/or a low fat diet. J. Nutr. 125, 2356-2363 [DOI] [PubMed] [Google Scholar]
- Bell R. R., Spencer M. J., Sherriff J. L. (1997). Voluntary exercise and monounsaturated canola oil reduce fat gain in mice fed diets high in fat. J. Nutr. 127, 2006-2010 [DOI] [PubMed] [Google Scholar]
- Beltramo M., de Fonseca F. R., Navarro M., Calignano A., Gorriti M. A., Grammatikopoulos G., Sadile A. G., Giuffrida A., Piomelli D. (2000). Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J. Neurosci. 20, 3401-3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger R. J. (1983). The role of dopamine in locomotor activity and learning. Brain Res. 287, 173-196 [DOI] [PubMed] [Google Scholar]
- Benus R. F., Bohus B., Koolhaas J. M., van Oortmerssen G. A. (1991). Heritable variation for aggression as a reflection of individual coping strategies. Experientia 47, 1008-1019 [DOI] [PubMed] [Google Scholar]
- Berlyne D. E. (1966). Curiosity and exploration. Science 153, 25-33 [DOI] [PubMed] [Google Scholar]
- Berridge K. C., Robinson T. E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 28, 309-369 [DOI] [PubMed] [Google Scholar]
- Bevan R., Woakes A., Butler P., Boyd I. (1994). The use of heart rate to estimate oxygen consumption of free-ranging black-browed albatrosses (Diomedea melanophrys). J. Exp. Biol. 193, 119-137 [DOI] [PubMed] [Google Scholar]
- Bevan R. M., Speakman J. R., Butler P. J. (1995). Daily energy expenditure of tufted ducks: a comparison between indirect calorimetry, doubly labelled water, and heart rate. Funct. Ecol. 9, 40-47 [Google Scholar]
- Bi S., Scott K. A., Hyun J., Ladenheim E. E., Moran T. H. (2005). Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology 146, 1676-1685 [DOI] [PubMed] [Google Scholar]
- Bjursell M., Gerdin A. K., Lelliott C. J., Egecioglu E., Elmgren A., Tornell J., Oscarsson J., Bohlooly Y. M. (2008). Acutely reduced locomotor activity is a major contributor to Western diet-induced obesity in mice. Am. J. Physiol. Endocrinol. Metab. 294, E251-E260 [DOI] [PubMed] [Google Scholar]
- Black A. E., Cole T. J. (2000). Within- and between-subject variation in energy expenditure measured by the doubly-labelled water technique: implications for validating reported dietary energy intake. Eur. J. Clin. Nutr. 54, 386-394 [DOI] [PubMed] [Google Scholar]
- Blair S. N., Morris J. N. (2009). Healthy hearts-and the universal benefits of being physically active: physical activity and health. Ann. Epidemiol. 19, 253-256 [DOI] [PubMed] [Google Scholar]
- Blum K., Braverman E. R., Holder J. M., Lubar J. F., Monastra V. J., Miller D., Lubar J. O., Chen T. J., Comings D. E. (2000). Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoactive Drugs 32, 1-112 [DOI] [PubMed] [Google Scholar]
- Blundell J. E. (2006). Perspective on the central control of appetite. Obesity 14, S160-S163 [DOI] [PubMed] [Google Scholar]
- Blundell J. E., Cooling J. (2000). Routes to obesity: phenotypes, food choices and activity. Br. J. Nutr. 83, S33-S38 [DOI] [PubMed] [Google Scholar]
- Boersma G. J., Benthem L., van Dijk G., Steimer T. J., Scheurink A. J. (2010). Coping style predicts the (in)sensitivity for developing hyperinsulinemia on a high fat diet in rats. Physiol. Behav. 100, 401-407 [DOI] [PubMed] [Google Scholar]
- Booth F. W., Lees S. J. (2007). Fundamental questions about genes, inactivity, and chronic diseases. Physiol. Genomics 28, 146-157 [DOI] [PubMed] [Google Scholar]
- Booth F. W., Chakravathy M. V., Spangenburg E. E. (2002a). Exercise and gene expression: physiological regulation of the human genome through physical activity. J. Physiol. (Lond.) 543, 399-411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth F. W., Chakravathy M. U., Gordon S. E., Spangenburg E. E. (2002b). Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J. Appl. Physiol. 93, 3-30 [DOI] [PubMed] [Google Scholar]
- Booth-Kewley S., Vickers R. R. (1994). Associations between major domains of personality and health behavior. J. Pers. 62, 281-298 [DOI] [PubMed] [Google Scholar]
- Bramble D. M., Lieberman D. E. (2004). Endurance running and the evolution of Homo. Nature 432, 345-352 [DOI] [PubMed] [Google Scholar]
- Bray G. A. (2008). Lifestyle and pharmacological approaches to weight loss: efficacy and safety. J. Clin. Endocrinol. Metab. 93, S81-S88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. A., Champagne C. M. (2005). Beyond energy balance: there is more to obesity than kilocalories. J. Am. Diet Assoc. 105, S17-S23 [DOI] [PubMed] [Google Scholar]
- Brené S., Bjørnebekk A., Åberg E., Mathé A. A., Olson L., Werme M. (2007). Running is rewarding and antidepressive. Physiol. Behav. 92, 136-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock C. M., King D. S., Wofford M. R., Harrell T. K. (2005). Exercise, insulin resistance, and hypertension: a complex relationship. Metab. Syndr. Relat. Disord. 3, 60-65 [DOI] [PubMed] [Google Scholar]
- Bronikowski A. M., Carter P. A., Swallow J. G., Girard I. A., Rhodes J. S., Garland T., Jr (2001). Open-field behavior of house mice selectively bred for high voluntary wheel running. Behav. Genet. 31, 309-316 [DOI] [PubMed] [Google Scholar]
- Bronikowski A. M., Morgan T. J., Garland T., Jr, Carter P. A. (2002). Antioxidant gene expression in active and sedentary house mice (Mus domesticus) selected for high voluntary wheel-running behavior. Genetics 161, 1763-1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronikowski A. M., Rhodes J. S., Garland T., Jr, Prolla T. A., Awad T., Gammie S. C. (2004). The evolution of gene expression in the hippocampus in response to selective breeding for increased locomotor activity. Evolution 5, 2079-2086 [DOI] [PubMed] [Google Scholar]
- Brownlow B. S., Petro A., Feinglos M. N., Surwit R. S. (1996). The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol. Behav. 60, 37-41 [DOI] [PubMed] [Google Scholar]
- Burghardt P. R., Fulk L. J., Hand G. A., Wilson M. A. (2004). The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 1019, 84-96 [DOI] [PubMed] [Google Scholar]
- Byars S. G., Ewbank D., Govindaraju D. R., Stearns S. C. (2010). Colloquium paper: Natural selection in a contemporary human population. Proc. Natl. Acad. Sci. USA 107, 1787-1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers J. A., Walker C. (1995). Refining the motor training hypothesis for the evolution of play. Am. Nat. 146, 25-40 [Google Scholar]
- Cai G., Cole S. A., Butte N., Bacino C., Diego V., Tan K., Goring H. H., O’Rahilly S., Farooqi I. S., Comuzzie A. G. (2006). A quantitative trait locus on chromosome 18q for physical activity and dietary intake in Hispanic children. Obesity 14, 1596-1604 [DOI] [PubMed] [Google Scholar]
- Carbone C., Cowlishaw G., Isaac N. J. B., Rowcliffe J. M. (2005). How far do animals go? Determinants of day range in mammals. Am. Nat. 165, 290-297 [DOI] [PubMed] [Google Scholar]
- Careau V., Thomas D., Humphries M. M., Reale D. (2008). Energy metabolism and animal personality. Oikos 117, 641-653 [Google Scholar]
- Careau V., Bininda-Emonds O. R. P., Thomas D. W., Réale D., Humphries M. M. (2009). Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct. Ecol. 23, 150-156 [Google Scholar]
- Careau V., Reale D., Humphries M. M., Thomas D. W. (2010). The pace of life under artificial selection: personality, energy expenditure, and longevity are correlated in domestic dogs. Am. Nat. 175, 753-758 [DOI] [PubMed] [Google Scholar]
- Carroll L. S., Meagher S., Morrison L., Penn D. J., Potts W. K. (2004). Fitness effects of a selfish gene (the Mus t complex) are revealed in an ecological context. Evolution 58, 1318-1328 [DOI] [PubMed] [Google Scholar]
- Castaneda T. R., Jürgens H., Wiedmer P., Pfluger P., Diano S., Horvath T. L., Tang-Christensen M., Tschöp M. H. (2005). Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J. Nutr. 135, 1314-1319 [DOI] [PubMed] [Google Scholar]
- Chappell M. A., Shoemaker V. H., Janes D. N., Maloney S. K., Bucher T. L. (1993). Energetics of foraging in breeding Adélie Penguins. Ecology 74, 2450-2461 [Google Scholar]
- Chaput J. P., Tremblay A. (2009). Obesity and physical inactivity: the relevance of reconsidering the notion of sedentariness. Obesity Facts 2, 249-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Karamizrak O., Noakes T. D., Dennis S. C., Lambert E. V. (1997). Time course of the effects of a high-fat diet and voluntary exercise on muscle enzyme activity in Long-Evans rats. Physiol. Behav. 61, 701-705 [DOI] [PubMed] [Google Scholar]
- Christakis N. A., Fowler J. H. (2007). The spread of obesity in a large social network over 32 years. N. Engl. J. Med. 357, 370-379 [DOI] [PubMed] [Google Scholar]
- Church T. S. (2009). The low-fitness phenotype as a risk factor: more than just being sedentary? Obesity 17, S39-S42 [DOI] [PubMed] [Google Scholar]
- Church T. S., Blair S. N. (2009). When will we treat physical activity as a legitimate medical therapy...even though it does not come in a pill? Br. J. Sports. Med. 43, 80-81 [DOI] [PubMed] [Google Scholar]
- Clobert J., Oppliger A., Sorci G., Ernande B., Swallow J. G., Garland T., Jr (2000). Trade-offs in phenotypic traits: endurance at birth, growth, survival, predation, and susceptibility to parasitism in a lizard, Lacerta vivipara. Funct. Ecol. 4, 675-684 [Google Scholar]
- Collier G., Hirsch E. (1971). Reinforcing properties of spontaneous activity in the rat. J. Comp. Physiol. Psychol. 77, 155-160 [DOI] [PubMed] [Google Scholar]
- Correa M., Carlson B. B., Wisniecki A., Salamone J. D. (2002). Nucleus accumbens dopamine and work requirements on interval schedules. Behav. Brain Res. 137, 179-187 [DOI] [PubMed] [Google Scholar]
- Correa M., Wisniecki A., Betz A., Dobson D. R., O’Neill M. F., O’Neill M. J., Salamone J. D. (2004). The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav. Brain Res. 148, 47-54 [DOI] [PubMed] [Google Scholar]
- Cota D., Woods S. C. (2005). The role of the endocannabinoid system in the regulation of energy homeostasis. Curr. Opin. Endocrinol. Diabetes 12, 338-351 [Google Scholar]
- Cotman C. W., Berchtold N. C., Christie L. A. (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464-472 [DOI] [PubMed] [Google Scholar]
- Dahmen N., Bierbrauer J., Kasten M. (2001). Increased prevalence of obesity in narcoleptic patients and relatives. Eur. Arch. Psychiatry Clin. Neurosci. 251, 85-89 [DOI] [PubMed] [Google Scholar]
- Davis C., Levitan R. D., Kaplan A. S., Carter J., Reid C., Curtis C., Patte K., Hwang R., Kennedy J. L. (2008). Reward sensitivity and the D2 dopamine receptor gene: a case-control study of binge eating disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 620-628 [DOI] [PubMed] [Google Scholar]
- Davis C. A., Levitan R. D., Reid C., Carter J. C., Kaplan A. S., Patte K. A., King N., Curtis C., Kennedy J. L. (2009). Dopamine for “Wanting” and opioids for “Liking”: a comparison of obese adults with and without binge eating. Obesity 17, 1220-1225 [DOI] [PubMed] [Google Scholar]
- Davis J. F. (2010). Adipostatic regulation of motivation and emotion. Discov. Med. 9, 462-467 [PubMed] [Google Scholar]
- Davis J. F., Choi D. L., Benoit S. C. (2010). Insulin, leptin and reward. Trends Endocrinol. Metab. 21, 68-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono J. P., Adlam D., Paterson D. J., Channon K. M. (2006). Novel quantitative phenotypes of exercise training in mouse models. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R926-R934 [DOI] [PubMed] [Google Scholar]
- De Castro J. M. (2004). Genes, the environment and the control of food intake. Br. J. Nutr. 92, S59-S62 [DOI] [PubMed] [Google Scholar]
- De Chiara V., Errico F., Musella A., Rossi S., Mataluni G., Sacchetti L., Siracusano A., Castelli M., Cavasinni F., Bernardi G., et al. (2010). Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology 35, 374-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Krom M., Bauer F., Collier D., Adan R. A. H., la Fleur S. E. (2009). Genetic variation and effects on human eating behavior. Annu. Rev. Nutr. 29, 283-304 [DOI] [PubMed] [Google Scholar]
- De Lecea L., Kilduff T. S., Peyron C., Gao X., Foye P. E., Danielson P. E., Fukuhara C., Battenberg E. L., Gautvik V. T., Bartlett F. S., et al. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Nat. Acad. Sci. USA 95, 322-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dember W. N., Earl R. W. (1957). Analysis of exploratory, manipulatory, and curiosity behaviors. Psychol. Rev. 64, 91-96 [DOI] [PubMed] [Google Scholar]
- De Moor M. H., Liu Y. J., Boomsma D. I., Li J., Hamilton J. J., Hottenga J. J., Levy S., Liu X. G., Pei Y. F., Posthuma D., et al. (2009). Genome-wide association study of exercise behavior in Dutch and American adults. Med. Sci. Sports Exerc. 41, 1887-1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. G., Molleman A. (2006). Cannabinoid signalling. Life Sci. 78, 549-563 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L., Cascio M. G., Di Marzo V. (2004). The endocannabinoid system: a general view and latest additions. Br. J. Pharmacol. 141, 765-774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Visser L., van den Bos R., Spruijt B. M. (2005). Automated home cage observations as a tool to measure the effects of wheel running on cage floor locomotion. Behav. Brain Res. 160, 382-388 [DOI] [PubMed] [Google Scholar]
- Dewsbury D. A. (1980). Wheel-running behavior in 12 species of muroid rodents. Behav. Process. 5, 271-280 [DOI] [PubMed] [Google Scholar]
- Dey S. (1994). Physical exercise as a novel antidepressant agent: possible role of serotonin receptor subtypes. Physiol. Behav. 55, 323-329 [DOI] [PubMed] [Google Scholar]
- Dietrich A. (2003). Functional neuroanatomy of altered states of consciousness: the transient hypofrontality hypothesis. Conscious. Cogn. 12, 231-256 [DOI] [PubMed] [Google Scholar]
- Dietrich A., McDaniel W. F. (2004). Endocannabinoids and exercise. Br. J. Sports Med. 38, 536-541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A., Sparling P. B. (2004). Endurance exercise selectively impairs prefrontal-dependent cognition. Brain Cogn. 55, 516-524 [DOI] [PubMed] [Google Scholar]
- DiLeone R. J. (2009). The influence of leptin on the dopamine system and implications for ingestive behavior. Int. J. Obes. 33, S25-S29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M. (2004). Fitness consequences of avian personalities in a fluctuating environment. Proc. Biol. Sci. 271, 847-852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman R. K. (1997). Brain monoamines, exercise, and behavioral stress: animal models. Med. Sci. Sports Exerc. 29, 63-74 [DOI] [PubMed] [Google Scholar]
- Dishman R. K. (2008). Gene-physical activity interactions in the etiology of obesity: behavioral considerations. Obesity 16, S60-S65 [DOI] [PubMed] [Google Scholar]
- Dishman R. K., Berthoud H.-R., Booth F. W., Cotman C. W., Edgerton V. R., Fleshner M. R., Gandevia S. C., Gomez-Pinilla F., Greenwood B. N., Hillman C. H., et al. (2006). Neurobiology of exercise. Obesity 14, 345-356 [DOI] [PubMed] [Google Scholar]
- Dlugosz E. M., Chappell M. A., McGillivray D. G., Syme D. A., Garland T., Jr (2009). Locomotor trade-offs in mice selectively bred for high voluntary wheel running. J. Exp. Biol. 212, 2612-2618 [DOI] [PubMed] [Google Scholar]
- Dohm M. R., Hayes J. P., Garland T., Jr (2001). The quantitative genetics of maximal and basal rates of oxygen consumption in mice. Genetics 159, 267-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahoo W. T., Levine J. A., Melanson E. L. (2004). Variability in energy expenditure and its components. Curr. Opin. Clin. Nutr. Metab. Care 7, 599-605 [DOI] [PubMed] [Google Scholar]
- Drewett R. F. (2007). The social facilitation of food intake. Arch. Dis. Child. 92, 377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S., Koehl M., Abrous D. N., Marsicano G., Chaouloff F. (2010). CB1 receptor deficiency decreases wheel-running activity: Consequences on emotional behaviours and hippocampal neurogenesis. Exp. Neurol. 224, 106-113 [DOI] [PubMed] [Google Scholar]
- Dudley R. (2001). Limits to human locomotor performance: Phylogenetic origins and comparative perspectives. J. Exp. Biol. 204, 3235-3240 [DOI] [PubMed] [Google Scholar]
- Dumke C. L., Rhodes J. S., Garland T., Jr, Maslowski E., Swallow J. G., Wetter A. C., Cartee G. D. (2001). Genetic selection of mice for high voluntary wheel running: effect on skeletal muscle glucose uptake. J. Appl. Physiol. 9, 1289-1297 [DOI] [PubMed] [Google Scholar]
- Ebihara S., Tsuji K. (1976). Strain differences in the mouse’s wheel-running behavior. Jpn. Psychol. Res. 18, 20-29 [Google Scholar]
- Ebstein R. P. (2006). The molecular genetic architecture of human personality: beyond self-report questionnaires. Mol. Psychiatry 11, 427-445 [DOI] [PubMed] [Google Scholar]
- Eikelboom R. (1999). Human parallel to voluntary wheel running: exercise. Anim. Behav. 57, F11-F12 [DOI] [PubMed] [Google Scholar]
- Eikelboom R. (2001). Bins, bouts and wheel-running speed. Anim. Behav. 61, 679-681 [Google Scholar]
- Eisenmann J. C. (2006). Insight into the causes of the recent secular trend in pediatric obesity: common sense does not always prevail for complex, multi-factorial phenotypes. Prev. Med. 42, 329-335 [DOI] [PubMed] [Google Scholar]
- Eisenmann J. C., Wickel E. E. (2009). The biological basis of physical activity in children: revisited. Ped. Exer. Sci. 21, 257-272 [DOI] [PubMed] [Google Scholar]
- Eisenmann J. C., Wickel E. E., Kelly S. A., Middleton K. M., Garland T., Jr (2009). Day-to-day variability in voluntary wheel running among genetically differentiated lines of mice that vary in activity level. Eur. J. Appl. Physiol. 106, 613-619 [DOI] [PubMed] [Google Scholar]
- Ekkekakis P., Hall E. E., Petruzzello S. J. (2005). Variation and homogeneity in affective responses to physical activity of varying intensities: an alternative perspective on dose-response based on evolutionary considerations. J. Sports Sci. 23, 477-500 [DOI] [PubMed] [Google Scholar]
- Fahnestock M., Marchese M., Head E., Pop V., Michalski B., Milgram W. N., Cotman C. W. (2010). BDNF increases with behavioral enrichment and an antioxidant diet in the aged dog. Neurobiol. Aging (in press) doi:10.1016/j.neurobiolaging.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair A. M., Montgomery K. (2009). Energy balance, physical activity, and cancer risk. Methods Mol. Biol. 472, 57-88 [DOI] [PubMed] [Google Scholar]
- FAO (2004). Human energy requirements. report of a joint FAO/WHO/UNU expert consultation. Food Nutr. Bull. 26, 166 [PubMed] [Google Scholar]
- Fawcett G. L., Jarvis J. P., Roseman C. C., Wang B., Wolf J. B., Cheverud J. M. (2010). Fine-mapping of obesity-related quantitative trait loci in an F9/10 advanced intercross line. Obesity 18, 1383-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder M. E., Garland T., Jr, Marden J. H., Zera A. J. (2010). Locomotion in response to shifting climate zones: not so fast. Annu. Rev. Physiol. 72, 167-190 [DOI] [PubMed] [Google Scholar]
- Festing M. F. W. (1977). Wheel activity in 26 strains of mouse. Lab. Anim. 11, 257-258 [DOI] [PubMed] [Google Scholar]
- Finkelstein E. A., Fiebelkorn I. C., Wang G. J. (2003). National medical spending attributable to overweight and obesity: how much, and who’s paying? Health Aff. 22, 219-226 [DOI] [PubMed] [Google Scholar]
- Flint J., Mackay T. F. C. (2009). Genetic architecture of quantitative traits in mice, flies, and humans. Genome Res. 19, 723-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J., Mott R. (2008). Applying mouse complex-trait resources to behavioural genetics. Nature 456, 724-727 [DOI] [PubMed] [Google Scholar]
- Foley T. E., Greenwood B. N., Day H. E. W., Koch L. G., Britton S. L., Fleshner M. (2006). Elevated central monoamine receptor mRNA in rats bred for high endurance capacity: implications for central fatigue. Behav. Brain Res. 174, 132-142 [DOI] [PubMed] [Google Scholar]
- Ford E. S., Kohl H. W., III, Mokdad A. H., Ajani U. A. (2005). Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes. Res. 13, 608-614 [DOI] [PubMed] [Google Scholar]
- Franks P. W., Farooqi I. S., Luan J., Wong M. Y., Halsall I., O’Rahilly S., Wareham N. J. (2003). Does physical activity energy expenditure explain the between-individual variation in plasma leptin concentrations after adjusting for differences in body composition? J. Clin. Endocrinol. Metab. 88, 3258-3263 [DOI] [PubMed] [Google Scholar]
- Freed C. R., Yamamoto K. (1985). Regional brain dopamine metabolism: a marker for the speed, direction, and posture of moving animals. Science 229, 62-65 [DOI] [PubMed] [Google Scholar]
- Freedson P. S., Melanson E., Sirard J. (1998). Calibration of the computer science and applications, inc. accelerometer. Med. Sci. Sports Exerc. 30, 777-781 [DOI] [PubMed] [Google Scholar]
- Funkat A., Massa C. M., Jovanovska V., Proietto J., Andrikopoulos S. (2004). Metabolic adaptations of three inbred strains of mice (C57BL6, DBA/2, and 129T2) in response to a high-fat diet. J. Nutr. 134, 3264-3269 [DOI] [PubMed] [Google Scholar]
- Fuss J., Gass P. (2010). Endocannabinoids and voluntary activity in mice: Runner’s high and long-term consequences in emotional behaviors. Exp. Neuro. 224, 103-105 [DOI] [PubMed] [Google Scholar]
- Gammie S. C., Hasen N. S., Rhodes J. S., Girard I., Garland T., Jr (2003). Predatory aggression, but not maternal or intermale aggression, is associated with high voluntary wheel-running behavior in mice. Horm. Behav. 44, 209-221 [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla L. (1984). Extraversion and neuroticism in rats. Pers. Individ. Dif. 5, 511-532 [Google Scholar]
- Garland T., Jr (1983). Scaling the ecological cost of transport to body mass in terrestrial mammals. Am. Nat. 121, 571-587 [Google Scholar]
- Garland T., Jr (2003). Selection experiments: an under-utilized tool in biomechanics and organismal biology. In Vertebrate Biomechanics and Evolution (ed. Bels V. L., Gasc J. P., Casinos A.), pp. 23-56 Oxford: BIOS Scientific Publishers; [Google Scholar]
- Garland T., Jr, Kelly S. A. (2006). Phenotypic plasticity and experimental evolution. J. Exp. Biol. 209, 2234-2261 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Gleeson T. T., Aronovitz B. A., Richardson C. S., Dohm M. R. (1995). Maximal sprint speeds and muscle fiber composition of wild and laboratory house mice. Physiol. Behav. 58, 869-876 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr, Kelly S. A., Malisch J. L., Kolb E. M., Hannon R. M., Keeney B. K., Van Cleave S. L., Middleton K. M. (2010). How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc. R. Soc. Lond. B Biol. Sci. (in press) doi:10.1098/rspb.2010.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebczynski A. K., Konarzewski M. (2009a). Locomotor activity of mice divergently selected for basal metabolic rate: a test of hypotheses on the evolution of endothermy. J. Evol. Biol. 22, 1212-1220 [DOI] [PubMed] [Google Scholar]
- Gebczynski A. K., Konarzewski M. (2009b). Metabolic correlates of selection on aerobic capacity in laboratory mice: a test of the model of the evolution of endothermy. J. Exp. Biol. 212, 2872-2878 [DOI] [PubMed] [Google Scholar]
- Geiger B. M., Behr G. G., Frank L. E., Caldera-Siu A. D., Beinfeld M. C., Kokkotou E. G., Pothos E. N. (2008). Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 22, 2740-2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. M., Haburcak M., Avena N. M., Moyer M. C., Hoebel B. G., Pothos E. N. (2009). Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 159, 1193-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard I. (2001). Field cost of activity in the kit fox, Vulpes macrotis. Physiol. Biochem. Zool. 74, 191-202 [DOI] [PubMed] [Google Scholar]
- Girard I., McAleer M. W., Rhodes J. S., Garland T., Jr (2001). Selection for high voluntary wheel running increases intermittency in house mice (Mus domesticus). J. Exp. Biol. 204, 4311-4320 [DOI] [PubMed] [Google Scholar]
- Girard I., Rezende E. L., Garland T., Jr (2007). Leptin levels and body composition of mice selectively bred for high voluntary activity. Physiol. Biochem. Zool. 80, 568-579 [DOI] [PubMed] [Google Scholar]
- Giuffrida A., Parsons L. H., Kerr T. M., Rodriguez de Fonseca F., Navarro M., Piomelli D. (1999). Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 2, 358-363 [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Hanson M. A. (2009). Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int. J. Obes. 32, S62-S71 [DOI] [PubMed] [Google Scholar]
- Goldstein D. L. (1988). Estimates of daily energy expenditure in birds: the time-energy budget as an integrator of laboratory and field studies. Am. Zool. 28, 829-844 [Google Scholar]
- Gomes F. R., Rezende E. L., Malisch J. L., Lee S. K., Rivas D. A., Kelly S. A., Lytle C., Yaspelkis B. B., III, Garland T., Jr (2009). Glycogen storage and muscle glucose transporters (GLUT-4) of mice selectively bred for high voluntary wheel running. J. Exp. Biol. 212, 238-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. J., Coyle C. A., Fox D. L. (2008). Nhlh2: a basic helix-loop-helix transcription factor controlling physical activity. Exerc. Sport Sci. Rev. 36, 187-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman M. L., Mills M. G., Raath J. P., Speakman J. R. (1998). High hunting costs make African wild dogs vulnerable to kleptoparasitism by hyaenas. Nature 391, 479-481 [Google Scholar]
- Gorriti M. A., Ferrer B., del Arco I., Bermúdez-Silva F. J., de Diego Y., Fernandez-Espejo E., Navarro M., Rodríguez de Fonseca F. (2005). Acute Δ9-tetrahydrocannabinol exposure facilitates quinpirole-induced hyperlocomotion. Pharmacol. Biochem. Behav. 81, 71-77 [DOI] [PubMed] [Google Scholar]
- Gosling S. D. (2001). From mice to men: what can we learn about personality from animal research. Psychol. Bull. 127, 45-86 [DOI] [PubMed] [Google Scholar]
- Goszczynski J. (1986). Locomotor activity of terrestrial predators and its consequences. Acta Theriol. 31, 79-95 [Google Scholar]
- Graybiel A. M. (2008). Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 31, 359-387 [DOI] [PubMed] [Google Scholar]
- Greenwood B. N., Strong P. V., Dorey A. A., Fleshner M. (2007). Therapeutic effects of exercise: wheel running reverses stress-induced interference with shuttle box escape. Behav. Neurosci. 121, 992-1000 [DOI] [PubMed] [Google Scholar]
- Groothuis T. G., Carere C. (2005). Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 29, 137-150 [DOI] [PubMed] [Google Scholar]
- Hammond K., Diamond J. (1997). Maximal sustained energy budgets in humans and animals. Nature 386, 457-462 [DOI] [PubMed] [Google Scholar]
- Hara J., Beuckmann C. T., Nambu T., Willie J. T., Chemelli R. M., Sinton C. M., Sugiyama F., Yagami K., Goto K., Yanagisawa M., et al. (2001). Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30, 345-354 [DOI] [PubMed] [Google Scholar]
- Hare B., Plyusnina I., Ignacio N., Schepina O., Stepika A., Wrangham R., Trut L. (2005). Social cognitive evolution in captive foxes is a correlated by-product of experimental domestication. Curr. Biol. 15, 226-230 [DOI] [PubMed] [Google Scholar]
- Harri M., Lindblom J., Malinen H., Hyttinen M., Lapveteläinen T., Eskola S., Helminen H. J. (1999). Effect of access to a running wheel on behavior of C57BL/6J mice. Lab. Anim. Sci. 49, 401-405 [PubMed] [Google Scholar]
- Hart J. S. (1971). Rodents. In Comparative Physiology of Thermoregulation. Vol. II. Mammals (ed. Whittow G. C.), pp. 1-149 New York: Academic Press; [Google Scholar]
- Hartmann J., Garland T., Jr, Hannon R. M., Kelly S. A., Muñoz G., Pomp D. (2008). Fine mapping of “Mini-Muscle”, a recessive mutation causing reduced hind-limb muscle mass in mice. J. Hered. 99, 679-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes C., Kriska A. (2008). Role of physical activity in diabetes management and prevention. J. Am. Diet. Assoc. 108, S19-S23 [DOI] [PubMed] [Google Scholar]
- Healy G. N., Dunstan D. W., Salmon J., Cerin E., Shaw J. E., Zimmet P. Z., Owen N. (2008). Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care 31, 661-666 [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., Little M. D., Johnson M. R., Melvin L. S., de Costa B. R., Rice K. C. (1990). Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA 87, 1932-1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse D., Dunn M., Heldmaier G., Klingenspor M., Rozman J. (2010). Behavioural mechanisms affecting energy regulation in mice prone or resistant to diet-induced obesity. Physiol. Behav. 99, 370-380 [DOI] [PubMed] [Google Scholar]
- Hill J. O., Wyatt H. R., Reed G. W., Peters J. C. (2003). Obesity and the environment: where do we go from here? Science 29, 853-855 [DOI] [PubMed] [Google Scholar]
- Hill M. N., Titterness A. K., Morrish A. C., Carrier E. J., Lee T. T., Gil-Mohapel J., Gorzalka B. B., Hillard C. J., Christie B. R. (2010). Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 20, 513-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. F. (1961). Effects of activity deprivation on choice of an activity incentive. J. Comp. Physiol. Psychol. 54, 78-82 [DOI] [PubMed] [Google Scholar]
- Hillebrand J. J., Kas M. J., van Elburg A. A., Hoek H. W., Adan R. A. (2008). Leptin’s effect on hyperactivity: potential downstream effector mechanisms. Physiol. Behav. 94, 689-695 [DOI] [PubMed] [Google Scholar]
- Hillman C. H., Erickson K. I., Kramer A. F. (2008). Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci. 9, 58-65 [DOI] [PubMed] [Google Scholar]
- Hollowell R. P., Willis L. H., Slentz C. A., Topping J. D., Bhakpar M., Kraus W. E. (2009). Effects of exercise training amount on physical activity energy expenditure. Med. Sci. Sports Exerc. 41, 1640-1644 [DOI] [PubMed] [Google Scholar]
- Howlett R. A., Kirkton S. D., Gonzalez N. C., Wagner H. E., Britton S. L., Koch L. G., Wagner P. D. (2009). Peripheral oxygen transport and utilization in rats following continued selective breeding for endurance running capacity. J. Appl. Physiol. 106, 1819-1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. W., Scott I. M. (1979). Daily torpor in the laboratory mouse, Mus musculus var albino. Physiol. Zool. 52, 205-218 [Google Scholar]
- Huntingford F. A., Andrew G., Mackenzie S., Morera D., Coyle S. M., Pilarczyk M., Kadri S. (2010). Coping strategies in a strongly schooling fish, the common carp Cyprinus carpio. J. Fish Biol. 76, 1576-1591 [DOI] [PubMed] [Google Scholar]
- Huo L., Gamber K., Greeley S., Silva J., Huntoon N., Leng X. H., Bjørbaek C. (2009). Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab. 9, 537-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S. E., Malenka R. C. (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2, 695-703 [DOI] [PubMed] [Google Scholar]
- Inoue K., Zorrilla E. P., Tabarin A., Valdez G. R., Iwasaki S., Kiriike N., Koob G. F. (2004). Reduction of anxiety after restricted feeding in the rat: implication for eating disorders. Biol. Psychiatry 55, 1075-1081 [DOI] [PubMed] [Google Scholar]
- Iversen I. H. (1993). Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Behav. 60, 219-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. M., Djafarian K., Stewart J., Speakman J. R. (2009). Increased TV viewing is associated with elevated body fatness but not lower energy expenditure in pre-school children. Am. J. Clin. Nutr. 89, 1-6 [DOI] [PubMed] [Google Scholar]
- Jahng J. W., Kim J. G., Kim H. J., Kim B. T., Kang D. W., Lee J. H. (2007). Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 1150, 100-107 [DOI] [PubMed] [Google Scholar]
- Jebb S. A. (2007). Dietary determinants of obesity. Obes. Rev. 8, 93-97 [DOI] [PubMed] [Google Scholar]
- Johannsen D. L., Ravussin E. (2008). Spontaneous physical activity: relationship between fidgeting and body weight control. Curr. Opin. Endocrinol. Diabetes Obes. 15, 409-415 [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Kenny P. J. (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neurosci. 13, 635-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Rhodes J. S., Jeffrey S. L., Garland T., Jr, Mitchell G. S. (2003). Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience 121, 1-7 [DOI] [PubMed] [Google Scholar]
- Jonas I., Vaanholt L., Doornbos M., Garland T., Jr, Scheurink A. J. W., Nyakas C., van Dijk G. (2010a). Effects of selective breeding for increased wheel-running behavior on circadian timing of substrate oxidation and ingestive behavior. Physiol. Behav. 99, 549-554 [DOI] [PubMed] [Google Scholar]
- Jonas I., Schubert K. A., Reijne A. C., Scholte J., Garland T., Jr, Gerkema M. P., Scheurink A. J. W., Nyakas C., van Dijk G. (2010b). Behavioral traits are affected by selective breeding for increased wheel-running behavior in mice. Behav. Genet. 40, 542-550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. H., Robbins T. W. (1992). Differential effects of mesocortical, mesolimbic, and mesostriatal dopamine depletion on spontaneous, conditioned and drug-induced locomotor activity. Pharmacol. Biochem. Behav. 43, 887-889 [DOI] [PubMed] [Google Scholar]
- Joosen A. M., Gielen M., Vlietinck R., Westerterp K. R. (2005). Genetic analysis of physical activity in twins. Am. J. Clin. Nutr. 82, 1253-1259 [DOI] [PubMed] [Google Scholar]
- Jung A. P., Luthin D. R. (2010). Wheel access does not attenuate weight gain in mice fed high-fat or high-CHO diets. Med. Sci. Sports Exerc. 42, 355-360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A. P., Curtis T. S., Turner M. J., Lightfoot J. T. (2010). Physical activity and food consumption in high- and low-active inbred mouse strains. Med. Sci. Sports Exerc. 42, 1826-1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J., Berkum M. (1954). The reward value of running activity. J. Comp. Physiol. Psychol. 47, 108 [DOI] [PubMed] [Google Scholar]
- Kaiyala K. J., Morton G. J., Leroux B. G., Ogimoto K., Wisse B., Schwartz M. W. (2010). Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59, 1657-1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanarek R. B., D’Anci K. E., Jurdak N., Mathes W. F. (2009). Running and addiction: precipitated withdrawal in a rat model of activity-based anorexia. Behav. Neurosci. 123, 905-912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. L., Garland T., Jr, Carter P. A. (2008). Basal metabolic rate of aged mice is affected by random genetic drift but not by selective breeding for high early-age locomotor activity or chronic wheel access. Physiol. Biochem. Zool. 81, 288-300 [DOI] [PubMed] [Google Scholar]
- Kas M. J., Kaye W. H., Mathes W. F., Bulik C. M. (2009). Interspecies genetics of eating disorder traits. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B, 318-327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney B. K., Raichlen D. A., Meek T. H., Wijeratne R. S., Middleton K. M., Gerdeman G. L., Garland T., Jr. (2008). Differential response to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behavior. Behav. Pharmacol. 19, 812-820 [DOI] [PubMed] [Google Scholar]
- Kelly S. A., Czech P. P., Wight J. T., Blank K. M., Garland T., Jr (2006). Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J. Morphol. 267, 360-374 [DOI] [PubMed] [Google Scholar]
- Kelly S. A., Nehrenberg D. L., Peirce J. L., Hua K., Steffy B. M., Wiltshire T., Pardo Manuel de Villena F., Garland T., Jr, Pomp D. (2010). Genetic architecture of voluntary exercise in an advanced intercross line of mice. Physiol. Genomics 42, 190-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A., Brozek J., Henschel A., Mickelson O., Anderson B. L. (1950). The Biology of Human Starvation. Minneapolis, Minnesota: University of Minnesota Press; [Google Scholar]
- King N. A., Hopkins M., Caudwell P., Stubbs R. J., Blundell J. E. (2007a). Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int. J. Obes. 32, 177-184 [DOI] [PubMed] [Google Scholar]
- King N. A., Caudwell P., Hopkins M., Byrne N. M., Colley R., Hills A. P., Stubbs J. R., Blundell J. E. (2007b). Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity 15, 1373-1383 [DOI] [PubMed] [Google Scholar]
- Kiwaki K., Kotz C. M., Wang C., Lanningham-Foster L., Levine J. A. (2004). Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am. J. Physiol. Endocrinol. Metab. 286, E551-E559 [DOI] [PubMed] [Google Scholar]
- Kligman E. W., Pepin E. (1992). Prescribing physical-activity for older patients. Geriatrics 47, 33-47 [PubMed] [Google Scholar]
- Knab A. M., Lightfoot J. T. (2010). Does the difference between physically active and couch potato lie in the dopamine system? Int. J. Biol. Sci. 6, 133-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab A. M., Bowen R. S., Hamilton A. T., Gulledge A. A., Lightfoot J. T. (2009). Altered dopaminergic profiles: implications for the regulation of voluntary physical activity. Behav. Brain Res. 204, 147-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Kolb E. M., Kelly S. A., Middleton K. M., Sermsakdi L. S., Chappell M. A., Garland T., Jr (2010). Erythropoietin elevates
 but not voluntary wheel running in mice. J. Exp. Biol. 213, 510-519
[DOI] [PubMed] [Google Scholar]
but not voluntary wheel running in mice. J. Exp. Biol. 213, 510-519
[DOI] [PubMed] [Google Scholar] - Koolhaas J. M., Korte S. M., De Boer S. F., van der Vegt B. J., Van Reenen C. G., Hopster H., De Jon I. C., Ruis M. A. W., Blokhuis H. J. (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925-935 [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M., De Boer S. F., Buwalda B., Van Reenen K. (2007). Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 70, 218-226 [DOI] [PubMed] [Google Scholar]
- Korte S. M., Koolhaas J. M., Wingfield J. C., McEwen B. S. (2005). The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 29, 3-38 [DOI] [PubMed] [Google Scholar]
- Kotagal S., Krahn L. E., Slocumb N. (2004). A putative link between childhood narcolepsy and obesity. Sleep Med. 5, 147-150 [DOI] [PubMed] [Google Scholar]
- Koteja P., Garland T., Jr (2001). Forum: response to R. Eikelboom. Anim. Behav. 61, F25-F26 [Google Scholar]
- Koteja P., Garland T., Jr, Sax J. K., Swallow J. G., Carter P. A. (1999a). Behaviour of house mice artificially selected for high levels of voluntary wheel running. Anim. Behav. 58, 1307-1318 [DOI] [PubMed] [Google Scholar]
- Koteja P., Swallow J. G., Carter P. A., Garland T., Jr (1999b). Energy cost of wheel running in house mice: implications for coadaptation of locomotion and energy budgets. Physiol. Biochem. Zool. 72, 238-249 [DOI] [PubMed] [Google Scholar]
- Koteja P., Carter P. A., Swallow J. G., Garland T., Jr (2003). Food wasting in house mice: variation among individuals, families, and genetic lines. Physiol. Behav. 80, 375-383 [DOI] [PubMed] [Google Scholar]
- Kotz C. M. (2006). Integration of feeding and spontaneous physical activity: role for orexin. Physiol. Behav. 88, 294-301 [DOI] [PubMed] [Google Scholar]
- Kotz C. M. (2008). Rewired to be thin? When exercise hits the brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R288-R289 [DOI] [PubMed] [Google Scholar]
- Kotz C. M., Teske J. A., Levine J. A., Wang C. (2002). Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul. Pept. 104, 27-32 [DOI] [PubMed] [Google Scholar]
- Kotz C. M., Wang C., Teske J. A., Thorpe A. J., Novak C. M., Kiwaki K., Levine J. A. (2006). Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience 142, 29-36 [DOI] [PubMed] [Google Scholar]
- Kotz C. M., Teske J. A., Billington C. J. (2008). Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R699-R710 [DOI] [PubMed] [Google Scholar]
- Kumar K. G., DiCarlo L. M., Volaufova J., Zuberi A. R., Smith Richards B. K. (2010). Increased physical activity cosegregates with higher intake of carbohydrate and total calories in a subcongenic mouse strain. Mamm. Genome 21, 52-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmansingh E., Rollo C. D. (1994). Evidence for a trade-off between growth and behavioural activity in giant “Supermice” genetically engineered with extra growth hormone genes. Can. J. Zool. 72, 2158-2168 [Google Scholar]
- Lacy R. C., Lynch C. B. (1979). Quantitative genetic analysis of temperature regulation in Mus musculus. I. Partitioning of variance. Genetics 91, 743-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M. I., van Zyl C., Jaunky R., Lambert E. V., Noakes T. D. (1996). Tests of running performance do not predict subsequent spontaneous running in rats. Physiol. Behav. 60, 171-176 [DOI] [PubMed] [Google Scholar]
- Laviolette S. R., Grace A. A. (2006). The roles of cannabinoid and dopamine receptor systems in emotional learning circuits: implications for schizophrenia and addiction. Cell. Mol. Life Sci. 63, 1597-1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamy L. J., Pomp D., Lightfoot J. T. (2010). A search for quantitative trait loci controlling within-individual variation of physical activity traits in mice. BMC Genet. 11, 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure J. L., Jones M. (2008). Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156, 456-465 [DOI] [PubMed] [Google Scholar]
- Ledwidge B. (1980). Run for your mind: Aerobic exercise as a means of alleviating anxiety and depression. Can. J. Behav. Sci./Rev. Can. Sci. Comp. 12, 127 [Google Scholar]
- Leibel R. L., Rosenbaum M., Hirsch J. (1995). Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621-628 [DOI] [PubMed] [Google Scholar]
- Lett B. T., Grant V. L., Byrne M. J., Koh M. T. (2000). Pairings of a distinctive chamber with the after effect of wheel running produce conditioned place preference. Appetite 34, 87-94 [DOI] [PubMed] [Google Scholar]
- Levin B. E., Dunn-Meynell A. A. (2004). Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R771-R778 [DOI] [PubMed] [Google Scholar]
- Levin B. E., Dunn-Meynell A. A., Balkan B., Keesey R. E. (1997). Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 273, R725-R730 [DOI] [PubMed] [Google Scholar]
- Levine J. A. (2007). Nonexercise activity thermogenesis-liberating the life-force. J. Intern. Med. 262, 273-287 [DOI] [PubMed] [Google Scholar]
- Levine J. A., Kotz C. M. (2005). NEAT - non-exercise activity thermogenesis – egocentric and geocentric environmental factors vs. biological regulation. Acta Physiol. Scand. 184, 309-318 [DOI] [PubMed] [Google Scholar]
- Levine J. A., Eberhardt N. L., Jensen M. D. (1999). Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283, 212-214 [DOI] [PubMed] [Google Scholar]
- Levine J. A., Nygren J., Short K. R., Nair K. S. (2003). Effect of hyperthyroidism on spontaneous physical activity and energy expenditure in rats. J. Appl. Physiol. 94, 165-170 [DOI] [PubMed] [Google Scholar]
- Levine J. A., Lanningham-Foster L. M., McCrady S. K., Krizan A. C., Olson L. R., Kane P. H., Jensen M. D., Clark M. M. (2005). Interindividual variation in posture allocation: possible role in human obesity. Science 307, 584-586 [DOI] [PubMed] [Google Scholar]
- Li G., Rhodes J. S., Girard I., Gammie S. C., Garland T., Jr (2004). Opioid-mediated pain sensitivity in mice bred for high voluntary wheel running. Physiol. Behav. 83, 515-524 [DOI] [PubMed] [Google Scholar]
- Lightfoot J. T. (2008a). Sex hormones’ regulation of rodent physical activity: a review. Int. J. Biol. Sci. 4, 126-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot J. T. (2008b). Commentary on Viewpoint: Perspective on the future use of genomics in exercise. J. Appl. Physiol. 104, 1249 [DOI] [PubMed] [Google Scholar]
- Lightfoot J. T., Turner M. J., DeBate K. A., Kleeberger S. R. (2001). Interstrain variation in murine aerobic capacity. Med. Sci. Sports Exerc. 33, 2053-2057 [DOI] [PubMed] [Google Scholar]
- Lightfoot J. T., Turner M. J., Daves M., Vordermark A., Kleeberger S. R. (2004). Genetic influence on daily wheel running activity level. Physiol. Genomics 19, 270-276 [DOI] [PubMed] [Google Scholar]
- Lightfoot J. T., Turner M. J., Pomp D., Kleeberger S. R., Leamy L. J. (2008). Quantitative trait loci for physical activity traits in mice. Physiol. Genomics 32, 401-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R. G. (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl.) 92, 180-185 [DOI] [PubMed] [Google Scholar]
- Liu Y., von Deneen K. M., Kobeissy F. H., Gold M. S. (2010). Food addiction and obesity: evidence from bench to bedside. J. Psychoactive Drugs 42, 133-145 [DOI] [PubMed] [Google Scholar]
- Long B. C. (1983). Aerobic conditioning and stress reduction: participation or conditioning? Hum. Mov. Sci. 2, 171-186 [Google Scholar]
- Loos R. J. F., Rankinen T., Tremblay A., Perusse L., Chagnon Y., Bouchard C. (2005). Melanocortin-4 receptor gene and physical activity in the Quebec Family Study. Int. J. Obes. 29, 420-428 [DOI] [PubMed] [Google Scholar]
- Lupica C. R., Riegel A. C. (2005). Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology 48, 1105-1116 [DOI] [PubMed] [Google Scholar]
- Lykouras L. (2008). Psychological profile of obese patients. Dig. Dis. 26, 36-39 [DOI] [PubMed] [Google Scholar]
- MacLaren V. V., Best L. A. (2010). Multiple addictive behaviors in young adults: student norms for the Shorter PROMIS Questionnaire. Addict. Behav. 35, 252-255 [DOI] [PubMed] [Google Scholar]
- Maldonado R., Valverde O., Berrendero F. (2006). Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 29, 225-232 [DOI] [PubMed] [Google Scholar]
- Malisch J. L., Saltzman W., Gomes F. R., Rezende E. L., Jeske D. R., Garland T., Jr (2007). Baseline and stress-induced plasma corticosterone concentrations of mice selectively bred for high voluntary wheel running. Physiol. Biochem. Zool. 80, 146-156 [DOI] [PubMed] [Google Scholar]
- Malisch J. L., Breuner C. W., Gomes F. R., Chappell M. A., Garland T., Jr (2008). Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen. Comp. Endocrinol. 156, 210-217 [DOI] [PubMed] [Google Scholar]
- Malisch J. L., Breuner C. W., Kolb E. M., Wada H., Hannon R. M., Chappell M. A., Middleton K. M., Garland T., Jr (2009). Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav. Genet. 39, 192-201 [DOI] [PubMed] [Google Scholar]
- Mathes W. F., Nehrenberg D. L., Gordon R., Hua K., Garland K., Jr, Pomp D. (2010). Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav. Brain Res. 210, 155-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda L. A., Lolait S. J., Brownstein B. J., Young A. C., Bonner T. I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 356, 561-564 [DOI] [PubMed] [Google Scholar]
- Mavanji V., Teske J. A., Billington C. J., Kotz C. M. (2010). Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int. J. Obes. (Lond.) 34, 1576-1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister E. J., Dhurandhar N. V., Keith S. W., Aronne L. J., Barger J., Baskin M., Benca R. M., Biggio J., Boggiano M. M., Eisenmann J. C., et al. (2009). Ten putative contributors to the obesity epidemic. Crit. Rev. Food Sci. Nutr. 49, 868-913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. M., Ramsey J. J., Miner J. L., Nielsen M. K. (2009). Differences in mitochondrial efficiency between lines of mice divergently selected for heat loss. J. Anim. Sci. 87, 3105-3113 [DOI] [PubMed] [Google Scholar]
- McElroy S. L., Kotwal R., Malhotra S., Nelson E. B., Keck P. E., Nemeroff C. B. (2004). Are mood disorders and obesity related? A review for the mental health professional. J. Clin. Psychiatry 65, 634-651 [DOI] [PubMed] [Google Scholar]
- Meek T. H., Lonquich B. P., Hannon R. M., Garland T., Jr (2009). Endurance capacity of mice selectively bred for high voluntary wheel running. J. Exp. Biol. 212, 2908-2917 [DOI] [PubMed] [Google Scholar]
- Meek T. H., Eisenmann J. C., Garland T., Jr (2010). Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int. J. Obes. 34, 960-969 [DOI] [PubMed] [Google Scholar]
- Meeusen R., Smolders I., Sarre S., de Meirleir K., Keizer H., Serneels M., Ebinger G., Michotte Y. (1997). Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta Physiol. Scand. 159, 335-341 [DOI] [PubMed] [Google Scholar]
- Meijer E. P., Westerterp K. R., Verstappen F. T. (1999). Effect of exercise training on total daily physical activity in elderly humans. Eur. J. Appl. Physiol. Occup. Physiol. 80, 16-21 [DOI] [PubMed] [Google Scholar]
- Meijer G. A., Janssen G. M., Westerterp K. R., Verhoeven F., Saris W. H., ten Hoor F. (1991). The effect of a 5-month endurance-training programme on physical activity: evidence for a sex-difference in the metabolic response to exercise. Eur. J. Appl. Physiol. Occup. Physiol. 62, 11-17 [DOI] [PubMed] [Google Scholar]
- Melzer K., Kayser B., Saris W. H., Pichard C. (2005). Effects of physical activity on food intake. Clin. Nutr. 24, 885-895 [DOI] [PubMed] [Google Scholar]
- Metcalf B. S., Jeffery A. N., Hosking J., Voss L. D., Sattar N., Wilkin T. J. (2009). Objectively measured physical activity and its association with adiponectin and other novel metabolic markers: a longitudinal study in children (EarlyBird 38). Diabetes Care 32, 468-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metges C. C. (2009). Early nutrition and later obesity: animal models provide insights into mechanisms. In Early Nutrition Programming and Health Outcomes in Later Life: Obesity and Beyond (ed. Koletzko B., Decsi T., Molnár D., De la Hunty A.), pp. 105-112 Springer Science+Business Media B.V. [DOI] [PubMed] [Google Scholar]
- Miller W. C., Koceja D. M., Hamilton E. J. (1997). A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int. J. Obes. Relat. Metab. Disord. 21, 941-947 [DOI] [PubMed] [Google Scholar]
- Morgan W. P. (1985). Affective beneficience of vigorous physical activity. Med. Sci. Sports Exerc. 17, 94-105 [PubMed] [Google Scholar]
- Morgan T. J., Garland T., Jr, Carter P. A. (2003). Ontogenies in mice selected for high voluntary wheel-running activity. I. Mean ontogenies. Evolution 57, 646-657 [DOI] [PubMed] [Google Scholar]
- Morishima M., Harada N., Hara S., Sano A., Seno H., Takahashi A., Morita Y., Nakaya Y. (2006). Monoamine oxidase A activity and norepinephrine level in hippocampus determine hyperwheel running in SPORTS rats. Neuropsychopharmacology 31, 2627-2638 [DOI] [PubMed] [Google Scholar]
- Mousel M. R., Stroup W. W., Nielsen M. K. (2001). Locomotor activity, core body temperature, and circadian rhythms in mice selected for high or low heat loss. J. Anim. Sci. 79, 861-868 [DOI] [PubMed] [Google Scholar]
- Nagy K. A. (2001). Food requirements of wild animals: predictive equations for free-living mammals, reptiles, and birds. Nutr. Abstr. Rev. B 71, 21R-31R [Google Scholar]
- Nagy K. A. (2005). Field metabolic rate and body size. J. Exp. Biol. 208, 1621-1625 [DOI] [PubMed] [Google Scholar]
- Nagy K. A., Girard I. A., Brown T. K. (1999). Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 19, 247-277 [DOI] [PubMed] [Google Scholar]
- Naylor A. S., Persson A. I., Eriksson P. S., Jonsdottir I. H., Thorlin T. (2005). Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J. Neurophysiol. 93, 2406-2414 [DOI] [PubMed] [Google Scholar]
- Nehrenberg D. L., Hua K., Estrada-Smith D., Garland T., Jr, Pomp D. (2009a). Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity 17, 1402-1409 [DOI] [PubMed] [Google Scholar]
- Nehrenberg D. L., Wang S., Hannon R. M., Garland T., Jr, Pomp D. (2009b). QTL underlying voluntary exercise in mice: interactions with the “mini muscle” locus and sex. J. Hered. 101, 42-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS (2008). A call to debate: a call to action. A report on the health of the population of NHS Greater Glasgow and Clyde 2007–2008, 93 pp. Glasgow: NHS Greater Glasgow and Clyde; http://www.nhsggc.org.uk/content/default.asp?page=s1009_3_10 [Google Scholar]
- Novak C. M., Levine J. A. (2007). Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J. Neuroendocrinol. 19, 923-940 [DOI] [PubMed] [Google Scholar]
- Novak C. M., Kotz C. M., Levine J. A. (2006). Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am. J. Physiol. Endocrinol. Metab. 290, E396-E403 [DOI] [PubMed] [Google Scholar]
- Novak C. M., Escande C., Gerber S. M., Chini E. N., Zhang M., Britton S. L., Koch L. G., Levine J. A. (2009). Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS ONE 4, e5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak C. M., Escande C., Burghardt P. R., Zhang M., Barbosa M. T., Chini E. N., Britton S. L., Koch L. G., Akil H., Levine J. A. (2010). Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm. Behav. 58, 355-367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nydon J., Thomas R. B. (1989). Methodological procedures for analysing energy expenditure. In Research Methods in Nutritional Anthropology (ed. Pelto G. H., Pelto P. J., Messer E.), 217 pp. Tokyo, Japan: United Nations University Press; [Google Scholar]
- Olshansky S. J., Passaro D. J., Hershow R. C., Layden J., Carnes B. A., Brody J., Hayflick L., Butler R. N., Allison D. B., Ludwig D. S. (2005). A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 352, 1138-1145 [DOI] [PubMed] [Google Scholar]
- O’Rahilly S., Farooqi I. S. (2006). Genetics of obesity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1095-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly S., Farooqi I. S. (2008). Human obesity as a heritable disorder of the central control of energy balance. Int. J. Obes. 32, S55-S61 [DOI] [PubMed] [Google Scholar]
- Owen N., Bauman A., Brown W. (2009). Too much sitting: a novel and important predictor of chronic disease risk? Br. J. Sports Med. 43, 81-84 [DOI] [PubMed] [Google Scholar]
- Owen N., Healy G. N., Matthews C. E., Dunstan D. W. (2010). Too much sitting: the population health science of sedentary behavior. Exerc. Sport Sci. Rev. 38, 105-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White C. A., Cheer J. F., Beyene M., Carelli R. M., Wightman R. M. (2008). Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc. Natl. Acad. Sci. USA 105, 11957-11962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne S. E., Lewis R., Jennings B. J., Hales C. N. (2004). Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin. Sci. 106, 141-145 [DOI] [PubMed] [Google Scholar]
- Pagotto U., Cervino C., Vicennati V., Marsicano G., Lutz B., Pasquali R. (2006). How many sites of action for endocannabinoids to control energy metabolism? Int. J. Obesity 30, S39-S43 [DOI] [PubMed] [Google Scholar]
- Patterson C. M., Levin B. E. (2008). Role of exercise in the central regulation of energy homeostasis and in the prevention of obesity. Neuroendocrinology 87, 65-70 [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (1990). The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; [Google Scholar]
- Pietropaolo S., Sun Y., Li R., Brana C., Feldon J., Yee B. K. (2008). The impact of voluntary exercise on mental health in rodents: a neuroplasticity perspective. Behav. Brain Res. 192, 42-60 [DOI] [PubMed] [Google Scholar]
- Pillolla G., Melis M., Perra S., Muntoni A. L., Gessa G. L., Pistis M. (2007). Medial forebrain bundle stimulation evokes endocannabinoid-mediated modulation of ventral tegmental area dopamine neuron firing in vivo. Psychopharmacology 191, 843-853 [DOI] [PubMed] [Google Scholar]
- Pomp D., Mohlke K. L. (2008). Obesity genes: so close and yet so far... J. Biol. 7, 36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomp D., Nehrenberg D., Estrada-Smith D. (2008). Complex genetics of obesity in mouse models. Annu. Rev. Nutr. 28, 331-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts W. K., Manning C. J., Wakeland E. K. (1991). Mating patterns in seminatural populations of mice influenced by MHC genotype. Nature 352, 619-621 [DOI] [PubMed] [Google Scholar]
- Premack D., Schaeffer R. W., Hundt A. (1964). Reinforcement of drinking by running: effect of fixed ratio and reinforcement time. J. Exp. Anal. Behav. 7, 91-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A., Jebb S. (2004). Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr. Rev. 62, S98-S104 [DOI] [PubMed] [Google Scholar]
- Prentice A., Jebb S. (2006). TV and inactivity are separate contributors to metabolic risk factors in children. PLoS Med. 3 e481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P., Bocarsly M. E., Barson J. R., Hoebel B. G., Leibowitz S. F. (2010). Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol. Behav. 101, 394-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichlen D. A., Giuffrida A., Gerdeman G. L., Foster A. D. (2010). Neurobiological rewards and the evolution of the runner’s high in humans and cursorial mammals. AAPA annual meeting, April, 2010 Abstract in Am. J. Phys. Anthropol. 50, 238 [Google Scholar]
- Rankinen T., Bouchard C. (2006). Genetics of food intake and eating behavior phenotypes in humans. Annu. Rev. Nutr. 26, 413-434 [DOI] [PubMed] [Google Scholar]
- Rankinen T., Zuberi A., Chagnon Y. C., Weisnagel S. J., Argyropoulos G., Walts B., Perusse L., Bouchard C. (2005). The human obesity gene map: the 2005 update. Obesity 14, 529-644 [DOI] [PubMed] [Google Scholar]
- Rankinen T., Roth S. M., Bray M. S., Loos R., Perusse L., Wolfarth B., Hagberg J. M., Bouchard C. (2010). Advances in exercise, fitness, and performance genomics. Med. Sci. Sports Exerc. 42, 835-846 [DOI] [PubMed] [Google Scholar]
- Ravussin E., Lillioja S., Anderson T. E., Christin L., Bogardus C. (1986). Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J. Clin. Invest. 78, 1568-1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291-318 [DOI] [PubMed] [Google Scholar]
- Rezende E. L., Gomes F. R., Chappell M. A., Garland T., Jr (2009). Running behavior and its energy cost in mice selectively bred for high voluntary locomotor activity. Physiol. Biochem. Zool. 82, 662-679 [DOI] [PubMed] [Google Scholar]
- Rhodes J. S., Garland T., Jr (2003). Differential sensitivity to acute administration of Ritalin, apomorphine, SCH 23390, and raclopride in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology 167, 242-250 [DOI] [PubMed] [Google Scholar]
- Rhodes J. S., Kawecki T. J. (2009). Behavior and neurobiology. In Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments (ed. Garland T., Jr, Rose M. R.), pp. 263-300 Berkeley, CA: University of California Press; [Google Scholar]
- Rhodes J. S., Hosack G. R., Girard I., Kelley A. E., Mitchell G. S., Garland T., Jr (2001). Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacol. 158, 120-131 [DOI] [PubMed] [Google Scholar]
- Rhodes J. S., Garland T., Jr, Gammie S. C. (2003a). Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav. Neurosci. 117, 1243-1256 [DOI] [PubMed] [Google Scholar]
- Rhodes J. S., Van Praag H., Jeffrey S., Girard I., Mitchell G. S., Garland T., Jr, Gage F. H. (2003b). Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav. Neurosci. 117, 1006-1016 [DOI] [PubMed] [Google Scholar]
- Rhodes J. S., Gammie S. C., Garland T., Jr (2005). Neurobiology of mice selected for high voluntary wheel-running activity. Integr. Comp. Biol. 45, 438-455 [DOI] [PubMed] [Google Scholar]
- Rhodes R. E., Smith N. E. (2006). Personality correlates of physical activity: a review and meta-analysis. Br. J. Sports Med. 40, 958-965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci M. R., Levin B. E. (2003). Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R610-R618 [DOI] [PubMed] [Google Scholar]
- Rodnick K. J., Reaven G. M., Haskell W. L., Sims C. R., Mondon C. E. (1989). Variations in running activity and enzymatic adaptations in voluntary running rats. J. Appl. Physiol. 66, 1250-1257 [DOI] [PubMed] [Google Scholar]
- Rowland T. W. (1998). The biological basis of physical activity. Med. Sci. Sports Exerc. 30, 392-399 [DOI] [PubMed] [Google Scholar]
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., Williams S. C., Richardson J. A., Kozlowski G. P., Wilson S., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573-585 [DOI] [PubMed] [Google Scholar]
- Salamone J. D. (1992). Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology (Berl.) 107, 160-174 [DOI] [PubMed] [Google Scholar]
- Salamone J. D., Correa M. (2002). Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav. Brain Res. 137, 3-25 [DOI] [PubMed] [Google Scholar]
- Sallis J. F., Glanz K. (2006). The role of built environments in physical activity, eating, and obesity in childhood. Future Child. 16, 89-108 [DOI] [PubMed] [Google Scholar]
- Saris W. H., van Erp-Baart M. A., Brouns F., Westerterp K. R., ten Hoor F. (1989). Study on food intake and energy expenditure during extreme sustained exercise: the Tour de France. Int. J. Sports Med. 10, S26-S31 [DOI] [PubMed] [Google Scholar]
- Scheurink A. J. W., Boersma G. J., Nergårdh R., Södersten P. (2010). Neurobiology of hyperactivity and reward: agreeable restlessness in Anorexia Nervosa. Physiol. Behav. 100, 490-495 [DOI] [PubMed] [Google Scholar]
- Schoeller D. A. (2009). The energy balance equation: looking back and looking forward are two very different views. Nutr. Rev. 67, 249-254 [DOI] [PubMed] [Google Scholar]
- Schuld A., Hebebrand J., Geller F., Pollmacher T. (2000). Increased body-mass index in patients with narcolepsy. Lancet 355, 1274-1275 [DOI] [PubMed] [Google Scholar]
- Schultz W. (2001). Reward signaling by dopamine neurons. Neuroscientist 7, 293-302 [DOI] [PubMed] [Google Scholar]
- Schultz W. (2002). Getting formal with dopamine and reward. Neuron 36, 241-263 [DOI] [PubMed] [Google Scholar]
- Sclafani A. (1984). Animal models of obesity: classification and characterization. Int. J. Obes. 8, 491-508 [PubMed] [Google Scholar]
- Seabra A. F., Mendonc D. M., Goring H. H., Thomis M. A., Maia J. A. (2008). Genetic and environmental factors in familial clustering in physical activity. Eur. J. Epidemiol. 23, 205-211 [DOI] [PubMed] [Google Scholar]
- Secor S. M. (2009). Specific dynamic action: a review of the postprandial metabolic response. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 179, 1-56 [DOI] [PubMed] [Google Scholar]
- Sevy S., Hassoun Y., Bechara A., Yechiam E., Napolitano B., Burdick K., Delman H., Malhotra A. (2006). Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology (Berl.) 188, 228-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard E. L. C., Wilson R. P., Quintana F., Laich A. G., Liebsch N., Albareda D. A., Halsey L. G., Gleiss A., Morgan D. T., Myers A. E., et al. (2008). Identification of animal movement patterns using tri-axial accelerometry. Endanger. Species Res. 10, 47-60 [Google Scholar]
- Shephard R. (2003). Limits to the measurement of habitual physical activity by questionnaires. Br. J. Sports Med. 37, 197-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin C. M. (1998). Voluntary wheel running: a review and novel interpretation. Anim. Behav. 56, 11-27 [DOI] [PubMed] [Google Scholar]
- Sherwin C. M., Nicol C. J. (1996). Reorganization of behaviour in laboratory mice, Mus musculus, with varying cost of access to resources. Anim. Behav. 51, 1087-1093 [Google Scholar]
- Sih A., Bell A., Johnson J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372-378 [DOI] [PubMed] [Google Scholar]
- Simoes P., Santos J., Matos M. (2009). Experimental evolutionary domestication. In Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments (ed. Garland T., Jr, Rose M. R.), pp. 89-110 Berkeley, CA: University of California Press; [Google Scholar]
- Simoncic M., Horvat S., Stevenson P. L., Bunger L., Holmes M. C., Kenyon C. J., Speakman J. R., Morton N. M. (2008). Divergent physical activity and novel alternative responses to high fat feeding in polygenic fat and lean mice. Behav. Genet. 38, 292-300 [DOI] [PubMed] [Google Scholar]
- Simonen R. L., Rankinen T., Perusse L., Leon A. S., Skinner J. S., Wilmore J. H., Rao D. C., Bouchard C. (2003a). A dopamine D2 receptor gene polymorphism and physical activity in two family studies. Physiol. Behav. 78, 751-757 [DOI] [PubMed] [Google Scholar]
- Simonen R., Rankinen T., Perusse L., Rice T., Rao D., Chagnon Y., Bouchard C. (2003b). Genome-wide linkage scan for physical activity levels in the Quebec Family study. Med. Sci. Sports Exerc. 35, 1355-1359 [DOI] [PubMed] [Google Scholar]
- Solinas M., Thiriet N., El Rawas R., Lardeux V., Jaber M. (2009). Environmental enrichment during early stages of life reduces the behavioral, neurochemical, and molecular effects of cocaine. Neuropsychopharmacol. 34, 1102-1111 [DOI] [PubMed] [Google Scholar]
- Sparling P. B., Giuffrida A., Piomelli D., Rosskopf L., Dietrich A. (2003). Exercise activates the endocannabinoid system. Neuroreport 14, 2209-2211 [DOI] [PubMed] [Google Scholar]
- Speakman J. R. (1997). Doubly Labeled Water: Theory and Practice, p. 399 London and New York: Chapman and Hall; [Google Scholar]
- Speakman J. R. (2010). FTO effect on energy demand versus food intake. Nature. 464, E1 [DOI] [PubMed] [Google Scholar]
- Speakman J. R., Selman C. (2003). Physical activity and resting metabolic rate. Proc. Nutr. Soc. 62, 621-634 [DOI] [PubMed] [Google Scholar]
- Speakman J. R., Rance K. A., Johnstone A. M. (2008). Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity 16, 1961-1965 [DOI] [PubMed] [Google Scholar]
- Stamps J., Groothuis T. G. G. (2010). The development of animal personality: relevance, concepts and perspectives. Biol. Rev. 85, 301-325 [DOI] [PubMed] [Google Scholar]
- Steimer T., Driscoll P. (2003). Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress 6, 87-100 [DOI] [PubMed] [Google Scholar]
- Steimer T., Python A., Driscoll P., de Saint H. Z. (1999). Psychogenetically selected (Roman high- and low-avoidance) rats differ in 24-hour sleep organization. J. Biol. Rhythms 14, 221-226 [DOI] [PubMed] [Google Scholar]
- Stubbe J. H., de Geus E. J. C. (2009). Genetics of exercise behavior. In Handbook of Behavior Genetics (ed. Kim Y.-K.), pp. 343-358 New York: Springer Science and Business Media; [Google Scholar]
- Sutin A. R., Costa P. T., Jr, Uda M., Ferrucci L., Schlessinger D., Terracciano A. (2010). Personality and metabolic syndrome. Age 32, 513-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson K. L., Smith R. V., Magnani P. A., Suetin H. R., Paigen B., Naggert J. K., Li R., Churchill G. A., Peters L. L. (2007). Multiple trait measurements in 43 inbred mouse strains capture the phenotypic diversity characteristic of human populations. J. Appl. Physiol. 102, 2369-2378 [DOI] [PubMed] [Google Scholar]
- Swallow J. G., Carter P. A., Garland T., Jr (1998). Artificial selection for increased wheel-running behavior in house mice. Behav. Genet. 28, 227-237 [DOI] [PubMed] [Google Scholar]
- Swallow J. G., Koteja P., Carter P. A., Garland T., Jr (1999). Artificial selection for increased wheel-running activity in house mice results in decreased body mass at maturity. J. Exp. Biol. 202, 2513-2520 [DOI] [PubMed] [Google Scholar]
- Swallow J. G., Koteja P., Carter P. A., Garland T., Jr (2001). Food consumption and body composition in mice selected for high wheel-running activity. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 171, 651-659 [DOI] [PubMed] [Google Scholar]
- Swallow J. G., Rhodes J. S., Garland T., Jr (2005). Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integr. Comp. Biol. 45, 426-437 [DOI] [PubMed] [Google Scholar]
- Swallow J. G., Hayes J. P., Koteja P., Garland T., Jr (2009). Selection experiments and experimental evolution of performance and physiology. In Experimental Evolution: Concepts, Methods, and Applications of Selection Experiments (ed. Garland T., Jr, Rose M. R.), pp. 301-351 Berkeley, CA: University of California Press; [Google Scholar]
- Teran-Garcia M., Rankinen T., Bouchard C. (2008). Genes, exercise, growth, and the sedentary, obese child. J. Appl. Physiol. 105, 988-1001 [DOI] [PubMed] [Google Scholar]
- Teske J. A., Levine A. S., Kuskowski M., Levine J. A., Kotz C. M. (2006). Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R889-R899 [DOI] [PubMed] [Google Scholar]
- Teske J. A., Billington C. J., Kotz C. M. (2008). Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology 87, 71-90 [DOI] [PubMed] [Google Scholar]
- Thorburn A. W., Proietto J. (2000). Biological determinants of spontaneous physical activity. Obes. Rev. 1, 87-94 [DOI] [PubMed] [Google Scholar]
- Thorpe A. J., Mullett M. A., Wang C., Kotz C. M. (2003). Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1409-R1417 [DOI] [PubMed] [Google Scholar]
- Timberlake W., Wozny M. (1979). Reversibility of reinforcement between eating and running by schedule changes – comparison of hypotheses and models. Anim. Learn. Behav. 7, 461-469 [Google Scholar]
- Timmons J. A., Knudsen S., Rankinen T., Koch L. G., Sarzynski M., Jensen T., Keller P., Scheele C., Vollaard N. B. J., Nielsen S., et al. (2010). Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J. Appl. Physiol. 108, 1487-1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama K., Saito M., Okuda H. (1982). Effects of wheel running on food intake and weight gain of male and female rats. Physiol. Behav. 28, 899-903 [DOI] [PubMed] [Google Scholar]
- Tomporowski P. D., Davis C. L., Miller P. H., Naglieri J. A. (2008). Exercise and children’s intelligence, cognition, and academic achievement. Educ. Psychol. Rev. 20, 111-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou J. C. L., Wade C. E. (2002). Determinants affecting physical activity levels in animal models. Exp. Biol. Med. Vol. 227, 587-600 [DOI] [PubMed] [Google Scholar]
- Tremblay A., Pérusse L., Bouchard C. (2004). Energy balance and body-weight stability: impact of gene-environment interactions. Br. J. Nutr. 92, S63-S66 [DOI] [PubMed] [Google Scholar]
- Trivedi P., Yu H., MacNeil D. J., van der Ploeg L. H. T., Guan X. M. (1998). Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438, 71-75 [DOI] [PubMed] [Google Scholar]
- Turkheimer E. (2000). Three laws of behavior genetics and what they mean. Curr. Dir. Psychol. Sci. 9, 160-164 [Google Scholar]
- Vaanholt L. M., Garland T., Jr, Daan S., Visser G. H. (2007a). Wheel-running activity and energy metabolism in relation to ambient temperature in mice selected for high wheel-running activity. J. Comp. Physiol. B 177, 109-118 [DOI] [PubMed] [Google Scholar]
- Vaanholt L. M., De Jong B., Garland T., Jr, Daan S., Visser G. H. (2007b). Behavioural and physiological responses to increased foraging effort in male mice. J. Exp. Biol. 210, 2013-2024 [DOI] [PubMed] [Google Scholar]
- Vaanholt L. M., Meerlo P., Garland T., Jr, Visser G. H., van Dijk G. (2007c). Plasma adiponectin is increased in mice selectively bred for high wheel-running activity, but not by wheel running per sé. Horm. Metab. Res. 39, 377-383 [DOI] [PubMed] [Google Scholar]
- Vaanholt L. M., Jonas I., Doornbos M., Schubert K. A., Nyakas C., Garland T., Jr, Visser G. H., van Dijk G. (2008). Metabolic and behavioral responses to high-fat feeding in mice selectively bred for high wheel-running activity. Int. J. Obesity. 32, 1566-1575 [DOI] [PubMed] [Google Scholar]
- van der Kooij M. A., Glennon J. C. (2007). Animal models concerning the role of dopamine in attention-deficit hyperactivity disorder. Neurosci. Biobehav. Rev. 31, 597-618 [DOI] [PubMed] [Google Scholar]
- van Oers K., de Jong G., Drent P. J., van Noordwijk A. J. (2004). A genetic analysis of avian personality traits: correlated, response to artificial selection. Behav. Genet. 34, 611-619 [DOI] [PubMed] [Google Scholar]
- van Overveld T., Matthysen E. (2010). Personality predicts spatial responses to food manipulations in free-ranging great tits (Parus major). Biol. Lett. 6, 187-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. (2009). Exercise and the brain: something to chew on. Trends Neurosci. 32, 283-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Christie B. R., Sejnowski T. J., Gage F. H. (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 96, 13427-13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehrencamp S. L., Bradbury J. W., Gibson R. M. (1989). The energetic cost of display in male sage grouse. Anim. Behav. 38, 885-896 [Google Scholar]
- Verbeek P., Iwamoto T., Murakami N. (2008). Variable stress-responsiveness in wild type and domesticated fighting fish. Physiol. Behav. 93, 83-88 [DOI] [PubMed] [Google Scholar]
- Viggiano D. (2008). The hyperactive syndrome: Metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav. Brain Res. 194, 1-14 [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Fowler J. S., Wang G. J., Swanson J. M. (2004). Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatr. 9, 557-569 [DOI] [PubMed] [Google Scholar]
- Walker C., Byers J. A. (1991). Heritability of locomotor play in house mice, Mus domesticus. Anim. Behav. 42, 891-898 [Google Scholar]
- Waters R. P., Renner K. J., Pringle R. B., Summers C. H., Britton S. L., Koch L. G., Swallow J. G. (2008). Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiol. Behav. 93, 1044-1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways J., Smith B., Barbato J., Ramdath R., Pettee K., DeRaedt S., Allison D., Koch L., Lee S., Cicila G. (2007). Congenic strains confirm aerobic running capacity quantitative trait loci on rat chromosome 16 and identify possible intermediate phenotypes. Physiol. Genomics 29, 91-97 [DOI] [PubMed] [Google Scholar]
- Weathers W. W., Buttemer W. A., Hayworth A. M., Nagy K. A. (1984). An evaluation of time-budget estimates of daily energy expenditure in birds. Auk 101, 459-472 [Google Scholar]
- Welk G. J., McClain J. J., Eisenmann J. C., Wickel E. E. (2007). Field validation of the MTI Actigraph and BodyMedia armband monitor using the IDEEA monitor. Obesity 15, 918-928 [DOI] [PubMed] [Google Scholar]
- Werme M., Thoren P., Olson L., Brene S. (2000). Running and cocaine both upregulate dynorphin mRNA in medial caudate putamen. Eur. J. Neurosci. 12, 2967-2974 [DOI] [PubMed] [Google Scholar]
- Westerterp K. R. (2008). Physical activity as determinant of daily energy expenditure. Physiol. Behav. 93, 1039-1043 [DOI] [PubMed] [Google Scholar]
- Westerterp K. R. (2009). Assessment of physical activity: a critical appraisal. Eur. J. Appl. Physiol. 105, 823-828 [DOI] [PubMed] [Google Scholar]
- Westerterp K. R., Plasqui G. (2004). Physical activity and human energy expenditure. Curr. Opin. Clin. Nutr. Metab. Care. 7, 607-613 [DOI] [PubMed] [Google Scholar]
- Westerterp K. R., Speakman J. R. (2008). Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int. J. Obesity 32, 1256-1263 [DOI] [PubMed] [Google Scholar]
- Wickel E. E., Eisenmann J. C. (2006). Within- and between-individual variability in estimated energy expenditure and habitual physical activity among young adults. Eur. J. Clin. Nutr. 60, 538-544 [DOI] [PubMed] [Google Scholar]
- Widdowson E. M., Edholm O. G., McCance R. A. (1954). The food intake and energy expenditure of cadets in training. Br. J. Nutr. 8, 147-155 [DOI] [PubMed] [Google Scholar]
- Wise R. A. (2004). Dopamine, learning and motivation. Nat. Rev. Neurosci. 5, 483-494 [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Leimar O., Weissing F. J. (2007). Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581-584 [DOI] [PubMed] [Google Scholar]
- Wolf M., van Doorn G. S., Weissing F. J. (2008). Evolutionary emergence of responsive and unresponsive personalities. Proc. Natl. Acad. Sci. USA 105, 15825-15830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wone B., Sears M. W., Labocha M. K., Donovan E. R., Hayes J. P. (2009). Genetic variances and covariances of aerobic metabolic rates in laboratory mice. Proc. R. Soc. Lond. B Biol. Sci. 276, 3695-3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Tanahashi T., Kawai T., Chikahisa S., Katsuura S., Nishida K., Teshima-Kondo S., Sei H., Rokutan K. (2009). Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol. Genomics 39, 227-235 [DOI] [PubMed] [Google Scholar]
- Young R. J. (2003). Environmental Enrichment for Captive Animals (UFAW Animal Welfare), p. 228 Oxford, UK: Blackwell Science, Limited; [Google Scholar]
- Zheng H., Lenard N. R., Shin A. C., Berthoud H. R. (2009). Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int. J. Obes. 33, S8-S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck J. A., DeYoung E., Brzezinska W. J., Rhodes J. S. (2011). Selective breeding for increased home cage physical activity in Collaborative Cross and Hsd:ICR mice. Behav. Genet. In press [DOI] [PubMed] [Google Scholar]
- Zurlo F., Ferraro R. T., Fontvielle A. M., Rising R., Bogardus C., Ravussin E. (1992). Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am. J. Physiol. Endocrinol. Metab. 263, E296-E300 [DOI] [PubMed] [Google Scholar]