Abstract
Small hibernating mammals show regular oscillations in their heart rate and body temperature throughout the winter. Long periods of torpor are abruptly interrupted by arousals with heart rates that rapidly increase from 5 beats/min to over 400 beats/min and body temperatures that increase by ∼30°C only to drop back into the hypothermic torpid state within hours. Surgically implanted transmitters were used to obtain high-resolution electrocardiogram and body temperature data from hibernating thirteen-lined ground squirrels (Spermophilus tridecemlineatus). These data were used to construct a model of the circulatory system to gain greater understanding of these rapid and extreme changes in physiology. Our model provides estimates of metabolic rates during the torpor-arousal cycles in different model compartments that would be difficult to measure directly. In the compartment that models the more metabolically active tissues and organs (heart, brain, liver, and brown adipose tissue) the peak metabolic rate occurs at a core body temperature of 19°C approximately midway through an arousal. The peak metabolic rate of the active tissues is nine times the normothermic rate after the arousal is complete. For the overall metabolic rate in all tissues, the peak-to-resting ratio is five. This value is high for a rodent, which provides evidence for the hypothesis that the arousal from torpor is limited by the capabilities of the cardiovascular system.
Keywords: mathematical model, heart rate, electrocardiogram, ground squirrel
mammalian hibernation is characterized by profound reductions in metabolism, oxygen consumption, and heart rate as an animal enters a state of physical inactivity known as torpor. Torpor allows small mammals to survive the environmental extremes of near-freezing temperatures and insufficient food availability beginning in late fall and extending through winter (19, 20). Besides the inherent interest in understanding the extreme physiological events that occur during hibernation, the mechanisms of this natural phenomenon may be applied to improve various pathophysiological conditions (6, 33, 55). For example, circulatory failure is the primary cause of death from hypothermia in nonhibernating mammals (4, 22). Hibernators are resistant to such failure, even from artificially induced hypothermia during seasons that usually contain only normothermic activity (12, 49).
For a small mammal such as the thirteen-lined ground squirrel (Spermophilus tridecemlineatus), physiological extremes reached during torpor include core body temperatures of 4–6°C, oxygen consumption that holds at 2 to 3% of the aroused condition, and heart rates of 3 to 10 beats/min, compared with 200 to 400 beats/min when the animal is active (30). Even more extreme versions of this phenotype have been observed in the Arctic ground squirrel (S. parryii), where core body temperatures have been measured at −2.9°C (2).
The torpid state is not continually maintained throughout the hibernation season, but is interrupted by a series of interbout arousals (IBAs) during which the animal rapidly rewarms to the aroused state. A thorough review of the physiological characteristics of hibernating mammals can be found in Lyman et al. (30) and Carey et al. (7), and a review of the molecular biology of hibernation can be found in Andrews (1). In the present study, physiological parameters including electrocardiogram, body temperature, and motor activity of thirteen-lined ground squirrels were measured using implanted transmitters. These data were compared with historic physiological data (9, 10, 35) and used to develop our model.
Mathematically modeling the circulatory system of hibernators provides a virtual laboratory to examine the details of how they cope with physiological extremes. Quantitative models can make predictions for many types of data that are very difficult to measure directly. Such detailed understanding is crucial for guiding further experimentation and the development of novel medical interventions. For example, a circulatory compartment model, such as the one presented here, could be used for pharmacokinetic modeling, either by extending it directly or to develop appropriate allometric regression models (21, 26, 42).
The extraordinary cycles of change observed during hibernation can be modeled from the ground up with molecular and genetic mechanistic models, or from the top down by considering large-scale physiology of organs and tissues. In previous work, we presented a first attempt at the former strategy (17). In this paper, we have constructed a physiological model of the circulatory system of a hibernating ground squirrel.
In Fishman and Lyman (13) the authors comment
“The whole animal seems geared to warm from its low body-temperature in the least possible time, and the heart, beating at a rapid rate against a high head of pressure, may be an inefficient pump, but must contribute significantly as a heat source.”
The primary aim of this work is to quantify and test the essentials of the first part of that statement that the arousal phase of an IBA occurs in the least possible time. An important issue in quantifying the thermodynamics of hibernation is the profound differences in relative blood flow in different tissues during cycles of torpor and arousal (29, 30). Essential organs such as the brain, heart, and liver rewarm much faster and are more perfused during arousals than others such as the white adipose tissue, peripheral skeletal muscle, and digestive organs (29, 44). By combining the more active organs and tissues into one compartment and the less active organs and tissues into another we obtain estimates of the metabolic rates that are necessary to account for the heart rate and temperature changes seen during arousals. An advantage of this modeling approach is that it would be difficult to obtain direct measurements of these tissue-specific metabolic rates.
MATERIALS AND METHODS
Animal care.
All animal use was approved by the University of Minnesota Institutional Animal Care and Use Committee. Thirteen-lined ground squirrels (S. tridecemlineatus) were wild-caught in central Minnesota in July and August and transferred to the Research Animal Resources at the University of Minnesota Duluth for housing under controlled conditions. Squirrels were kept at 23°C, 12:12-h light-dark cycle, fed with Laboratory Rodent Diet 5001 (PMI Nutrition International, Henderson, CO) supplemented with black oil sunflower seeds, and water ad libitum.
Physiological data acquisition and analysis.
Squirrels were implanted with CTA-F40 transmitters (Data Sciences International, St. Paul, MN) to monitor body temperature, ECG, and movement (changes in location). Squirrels were anesthetized using 3% isoflurane administered through a nose cone throughout the procedure. The transmitter was secured to the abdominal wall ventral to the intestine. The two ECG leads extending from the transmitter were tunneled subcutaneously, with the negative lead secured in the right pectoral muscle, and the positive lead secured directly left of the xyphoid space. This placement of the ECG leads created a diagonal line crossing the heart to monitor changes in cardiac polarity. Squirrels were given liquid ibuprofen (15 mg per kg body wt; McNeil, Skillman, NJ) and allowed to recover from surgery for at least 7 days. In mid-November, eight squirrels were placed in an environmental chamber set to 5°C, 24 h of dark, with no food, and water ad libitum. These conditions allow deep torpor to occur during the hibernation season. The transmitters were activated, and squirrel cages were placed over receivers that detected the transmitter signals and relayed body temperatures, ECG, and physical activity information into a computer, to be monitored and stored. The animals were monitored using this telemetry for 2 mo.
All data collected by the transmitters were analyzed using Dataquest Analysis ART 4.1 and a modified version of ECG Analysis 404 (Data Sciences International); modifications were required because of the large range of heart rates relative to other laboratory animals. ECG data were recorded at 1-ms intervals. Body temperature and motor activity data were recorded at 30-s intervals. ECG data were converted to heart rate in 30-s increments, and all heart rate data were converted to a 1-h moving average for comparison.
For consistent comparison of changes in body temperature, heart rate, and activity, torpor bout reference points were defined. Torpor occurs when physiological activity is lower than normal. Thirteen-lined ground squirrels experience three phases of torpor: 1) entrance, consisting of decreasing physiological activity; 2) torpor maintenance, consisting of steady, low physiological activity; and 3) arousal, consisting of physiological activity increasing to normal active levels. The beginning of the entrance phase of torpor was defined as the beginning of the decline in body temperature. This occurred when the body temperature dropped at least 0.1°C in 10 min in a decline to steady, low levels typical of a torpid thirteen-lined ground squirrel. Since there is a great level of variability in the heart rates of active animals, the beginning of the decrease in heart rate upon entrance into torpor is not immediately apparent. It was determined that the initial decrease in heart rate at the onset of torpor occurred when the heart rate decreased nearest to the beginning of the drop in body temperature. The entrance phase ended, and the torpor maintenance phase began when body temperature and heart rate both stabilized. Stabilization of the body temperature occurred when the body temperature drop was < 0.1°C in 10 min. The heart rate stabilized when the average heart rate declined < 1 beats/min in 10 min. At the end of the maintenance phase of a torpor bout, body temperature increase occurred when body temperature increased at least 0.1°C in 10 min, and average heart rate increased by at least 1 beats/min in 10 min. During the arousal phase, each of these parameters subsequently increased until they reached active levels. The lengths of early- and mid-season torpor bouts were compared using Student's t-test with α = 0.05.
Arrhythmia analysis.
To quantify arrhythmic heartbeats, the interbeat interval (IBI) coefficient of variation (COV) was used. IBI values from eight ground squirrels were collected from 5-min ECG segments beginning 24 h before the first seasonal torpor bout at an ambient temperature of 5°C and continuing until arousal from torpor. The COV percentage was calculated by dividing the SD by the mean IBI and multiplying by 100. For each animal replicate, a mean of 10 repeated measures was taken. The mean from each hibernation phase (i.e., active, entrance, torpor maintenance, and arousal) was compared using an ANOVA (α = 0.05) followed by Tukey's multiple comparison test.
Hematocrit.
Squirrels (n = 33) were anesthetized using 3% isoflurane administered through a nose cone, and blood samples were taken by cardiac puncture. All blood samples were collected with heparinized capillary tubes and centrifuged for 5 min at 12,700 g. The percentage of packed red blood cells was determined for six samples per animal and a mean was taken. An ANOVA was used to determine statistical significance of the difference between groups using α = 0.05.
Modeling.
Our model implementation is done within the Sage platform (54) using Cython (http://www.cython.org; Python-like code compiled to C) and Euler's (forward) method with a uniform time step of one-tenth of a millisecond (11). Euler's method is mainly used because of its simplicity and the complications introduced by the heart valve backflow; it would be possible to adjust the implementation so higher-order methods could be used, but for our purposes Euler's method was sufficient. The accuracy of the model at this resolution was checked by the smaller time steps Δt = 10−6 s and Δt = 10−7 s. Computations were done at double precision (i.e., 53 bits of binary precision).
In addition, some critical data for our modeling was obtained from previous physiological work. The most important was that from Popovic (48), which provided cardiac output, oxygen consumption, arterial and venous oxygen content, body weight, and temperature for S. tridecemlineatus, with eight winter animals in torpor and five normothermic summer animals. The summary data from Hochachka and Guppy (19) was also used to account for differences in fuel utililization and oxygen consumption. Data on blood pressure in hibernating S. tridecemlineatus from Lyman and O'Brien (29) was used to calibrate the compartment resistances in our model (described in more detail in Model development in results). They found that during IBA, animals had a mean arterial blood pressure of 120 mmHg (16,000 Pa) with a systolic-to-diastolic ratio of 135:90 mmHg (18,000:12,000 Pa) and a heart rate of 280 beats/min. For a torpid animal the systolic-to-diastolic ratio was 80:40 mmHg (10,666:5,333 Pa) at a body temperature of 7.7°C and heart rate of 5 beats/min (29). It should be noted that these numbers are subject to large individual and temporal variation. The lowest diastolic pressure observed in torpor was 7 mmHg (933 Pa) (29).
Most of our parameters that correspond to the model in Ottesen et al. (45) were derived from that reference using exponential scaling laws of the form py = px(my/mx){↑ep} where (my/mx) is the ratio of masses of the two organisms x and y, py and px are the parameters, and ep is the scaling exponent for the parameter (27, 52, 60). Other parameters, and the overall model output, were checked by using the data in Lyman and O'Brien (29), Hochachka and Guppy (19), and Popovic (48). The determination of the parameters kambA and kambB, which determine the rate at which heat is lost to the environment, was done using our own temperature data using a least-squares fit to the Newtonian model of cooling. The remaining parameters described in the Model development were either determined from previously published data or in a least-squares fit to the cardiac output data of Popovic (48) and the blood pressure data from Lyman and O'Brien (29) described above.
RESULTS
Heart rate variation.
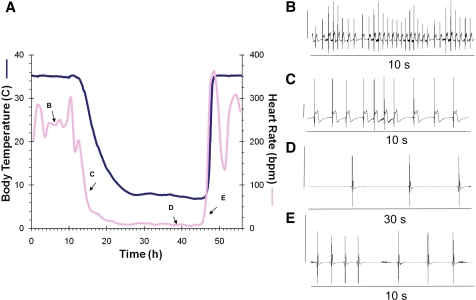
To develop a mathematical model of the physiological changes that occur during hibernation, we collected body temperature, heart rate, and movement data. An example of the changes in heart rate and body temperature during the initial seasonal entrance into torpor at 5°C ambient temperature is shown in Fig. 1A. ECG samples from squirrels that were active, entering torpor, maintaining torpor, and arousing from torpor all exemplify typical cardiac arrhythmias (Fig. 1A, insets B, C, D, and E, respectively). Note that inset D shows a longer 30-s interval, which is required to display multiple peaks during torpor maintenance.
Fig. 1.
A: simultaneous tracing of heart rate (pink line; 1-h moving average) and core body temperature (blue line) collected with an implanted transmitter in a thirteen-lined ground squirrel during the initial seasonal torpor bout (November) at an ambient temperature of 5°C. ECG readings from the indicated points on the heart rate tracing show 10-s interval where the heart rate was ∼240 beats/min (inset B); entry into torpor over 10 s at a heart rate of ∼80 beats/min (inset C); 30 s during torpor to show multiple beats at a heart rate of 8 beats/min (inset D); arousal ECG where the heart rate is 15 beats/min (inset E). In B–E the vertical line on the left side of the ECG equals 1 mV to indicate the changes in height of the R peak.
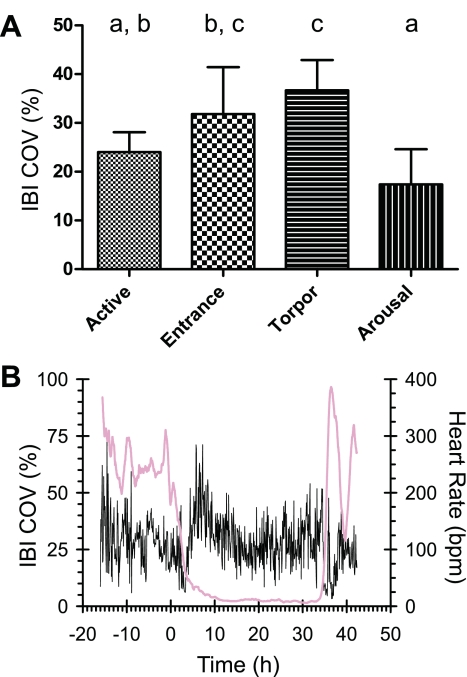
Arrhythmias typical of the entrance phase of torpor were observed. For a review of the rhythmic patterns of heart rate during hibernation see Milsom et al. (35). We hypothesized that the increase in arrhythmias prior to torpor can be used to predict the occurrence of torpor bouts. To measure the level of arrhythmic heartbeats in large amounts of ECG data, the COV of the IBI was computed. The COV of the IBI provides a numerical value of the variation of the time between heartbeats relative to the mean time between heartbeats. As shown in Fig. 2, the amount of arrhythmia was higher during torpor maintenance (36.7%; SD 6.2) than during active (24.0%; SD 4.1) and arousal (17.4%; SD 7.2) states. During entrance (31.8%; SD 9.6) there were more occurrences of arrhythmia than during arousal, which had the lowest frequency of arrhythmias.
Fig. 2.
Occurrence of arrhythmias during a torpor bout in thirteen-lined ground squirrels. The level of arrhythmias was measured by calculating the interbeat interval (IBI) coefficient of variation (COV) for 8 squirrels during the first seasonal torpor bout at 5°C (A). Arrhythmic patterns occurred more frequently as squirrels entered and maintained a torpor bout and less frequently during the arousal phase. Groups shown to be statistically different by an ANOVA followed by a Tukey's test do not share the same letter above the bar. B: example of the changes in IBI COV (black) relative to the changes in heart rate (pink). For comparison, the same animal is used as an example as in Fig. 1.
Changes in physiological parameters.
During a 24-h active period just prior to the initial seasonal (November) entrance into torpor at an ambient temperature 5°C, body temperatures ranged from 33.7 to 35.4°C with a mean active body temperature of 35.0°C. During the same period of activity, the mean minimum heart rate was 200 beats/min. The mean maximum heart rate was 350 beats/min, but heart rates as high as 450 beats/min were recorded. The average 24-h active heart rate was 250 beats/min.
The time spans of changes in body temperature and heart rate are given in Table 1. Entrance and arousal time spans in November were the same as during mid-season (January and February) torpor bouts, with P = 0.83 and P = 0.23, respectively. In all squirrels, the heart rate decreased before the body temperature. The movement of squirrels stopped between 3.7 h before to 3.5 h after the initial decline in body temperature. The time that movement ceases may be affected by whether the animal was asleep or awake when entrance into torpor began, and this may have contributed to this large range. After temperature and heart rate decreased, these parameters would stabilize, and the average minimum body temperature was 6.1°C and heart rate was 5 beats/min. During this first torpor bout at an ambient temperature of 5°C, the hearts of the eight animals maintained a minimal rate for an average of 54 h (SD 42) with a range of 10 to 140 h. Low body temperature was maintained for an average of 49 h (SD 40) with a range of 6 to 132 h during the maintenance period. Mid-season torpor bouts were longer, with a mean of 181.8 h (SD 28.4), (P = 1.6 × 10−6). Upon completion of the maintenance phase of torpor, the increase in heart rate always occurred before the increase in body temperature. The body temperature increase in arousal was faster than the decrease in torpor entrance (see Table 1). The squirrels began movement within 2 h after the time the body temperature was at active levels and no longer increased.
Table 1.
Length of transitions (in hours) during initial fall torpor bout at 5°C for 8 thirteen-lined ground squirrels
| Entrance |
Arousal |
|||
|---|---|---|---|---|
| Means | Range | Means | Range | |
| HR | 7.0 (1.7) | 4.6 to 10.3 | 3.2 (0.8) | 1.4 to 4.1 |
| Tb | 11.9 (3.0) | 9.1 to 18.6 | 2.8 (0.6) | 1.8 to 3.5 |
| Tb start - HRstart | 1.4 (1.1) | 0.1 to 3.0 | 0.9 (0.2) | 0.7 to 1.2 |
| Tbend - HRend | 6.2 (3.4) | 2.2 to 13.2 | 0.4 (0.2) | 0.1 to 0.7 |
Means and SD (in parentheses). The time for body temperature (Tb) and heart rate (HR) transitions to occur during entrance and arousal phases of torpor was calculated using the definitions of entrance, torpor maintenance, and arousal start times from materials and methods. The start of entrance occurred at the initial decline, and the end of entrance occurred at the beginning of the maintenance phase. The start of arousal occurred at the initial rise, and the end occurred at the beginning of the interbout arousal.
Hematocrit.
To determine whether blood viscosity was affected by changes in plasma volume (50, 59), hematocrit was measured for torpid, IBA, and spring-active squirrels. Rectal body temperatures were taken prior to blood sampling, and 21 torpid squirrels had body temperatures ranging from 4.4 to 7.7°C with a mean of 6.1°C, 10 squirrels in IBA had body temperatures ranging from 19.5 to 34.4°C with a mean of 28.1°C, and two spring-active squirrels had temperatures of 36.6 and 37.6°C. The difference between values for the three groups was not statistically significant using an ANOVA (P = 0.46). Overall hematocrit was 51.0 (SD 4.7).
Model development.
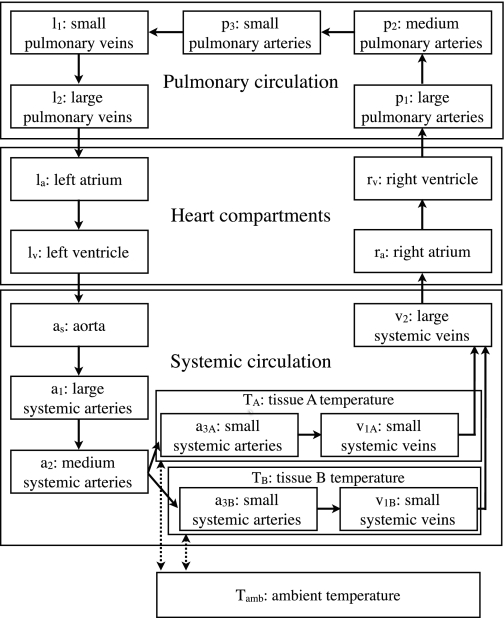
The model presented here is based on a compartment model of the human circulatory system described in Chapter 6 of Ottesen et al. (45). Although this model is fairly simple, it can reproduce many features of observed circulation (45). For our purposes it might have been possible to use even simpler nonpulsatile models, but our approach allows greater flexibility for future elaborations. The large and rapid variations in heart rate would complicate averaging to a nonpulsatile model. Also, our ECG data is on such a fine time scale (milliseconds) that a pulsatile model is appropriate. To facilitate comparisons between our model and that in Ottesen et al. (45) we have tried to be consistent with their notation. Figure 3 shows an overview of the compartments in our model.
Fig. 3.
Circulation compartments of the hibernator model.
Modeling circulation for hibernators requires a variety of modifications and choices in comparison with previous work on human or canine modeling. During an IBA, some organs and tissues such as the heart, brain, liver, and brown adipose tissue rewarm much faster than the extremities and posterior of the body, probably due to differential vasoconstriction (29, 30, 47). To include this in the model, the small arteries and vein compartments were split into two compartments (indexed as A and B). Figure 3 shows their relationship to the other compartments. The additional vasoconstriction is modeled by a parameter Rf that decreases the unstressed volume of the small arteries by a factor (Rf−1) and multiplies the resistance by Rf4 in accordance with Poiseuille's law; for an active animal Rf = 1 and it has no effect. In addition, two compartments, TA and TB, representing the tissue perfused by the two small artery and vein compartments were included in the model to account for the heat capacity of tissues separately from the blood. These compartments are necessary since the blood of a mammal is ∼10% of its mass (52), so other tissues account for most of the heat capacity. TA represents the tissues that rewarm relatively quickly: the heart, brain, liver, and brown adipose tissue, as well some anterior skeletal muscle and bone. The compartment TB accounts for the remainder, mostly posterior or peripheral tissues including posterior skeletal muscle and bone, the forelimbs, skin, white adipose tissue, and intestines.
To scale human models to small hibernators we used empirical exponential scaling laws as much as possible. Most of these are well known (27, 52, 60), but fitting them to our lumped compartment models required some adjustments. For example, it is expected that the overall resistance of the systemic circulation scales proportionally to M−0.75 where M is the mass of the organism. However, this scaling does not apply to individual compartments in our model. This is clear from considering Poiseuille's law in which resistance is proportional to the length traversed and inversely proportional to the fourth power of the radius. If the length and radii of blood vessels modeled by a compartment are proportional to M−1/3, then the resistance that the vessel contributes to the compartment resistance scales as M−1. This is most applicable to the large-vessel compartments. At the other extreme, the diameter of capillaries and their density in tissue is approximately constant among mammals regardless of their size (60). This suggests that the resistance for the smallest vessel compartments could be proportional to M−2/3. We therefore constrained three exponents of the scaling of resistance of the compartments to be between these extremes, fitting them using data on blood pressure (29) and cardiac output (48). Blood pressures in thirteen-lined ground squirrels are somewhat higher than in humans (29); to achieve this result in our model we also increased the blood volume and decreased the unstressed volume slightly from the scaled human values. The error between our model and experimental data on cardiac output was minimized by choosing the scaling exponent of the resistance of the largest compartments to be −0.94, that of the medium compartments to be −0.89, and that of the smallest compartments to be −0.84.
The viscosity of the blood increases with decreased temperature. Our model uses viscosity data from Maclean (31) and Halikas and Bowers (16), which are consistent with similar data from hibernating hedgehogs (24). For simplicity, we use a single linear function of temperature to account for this effect of temperature on viscosity [v(Tj) for compartment j]:
| (1) |
Although we did not explicitly include the dependence of viscosity on the rate of flow, this effect is indirectly modeled by the various resistances in each compartment.
The vasculature (not including the heart) is modeled by 13 compartments, five for the pulmonary circulation and eight for the systemic circulation (Fig. 3). For a given compartment (j) with upstream compartment (j − 1) and downstream compartment (j + 1), the pressure (Pj), volume (Vj), temperature (Tj), oxygen concentration (Oj) and flow (Qj) are computed from the equations:
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
where Vun,j is the unstressed volume of compartment j [i.e., the volume at zero pressure (41)], Cj is the compliance, Lj is the inductance (i.e., an inertial term), Rj is the resistance, and t is time. The Oin,j term is only non-zero for the small pulmonary vein compartment l1 and represents oxygen input from the respiratory system. The inductance terms (Lj) are zero, except for the largest arteries and veins where inertial effects may become important. Note that when Lj = 0, the relationship between pressure and flow becomes algebraic, and we can simply update those flows at each time step by
| (7) |
In addition to the 13 vasculature compartments there are two tissue compartments and four heart compartments. The compartments and their labels are given in Fig. 3.
Two compartments are treated differently: the two small artery compartments (a3A and a3B) are thermally coupled to the two tissue compartments (A and B with temperature, TA and TB). Of course, other compartments are coupled in reality but so weakly that including those interactions is unlikely to improve the model's accuracy, and, given our available data, it would be difficult to fit the additional parameters reproducibly. The temperatures in the tissue compartments A and B are modeled by Newton's law of heat exchange (39) with source terms from metabolic heat. For tissue A this is:
| (8) |
where MA is the mass of tissue A, and Tamb is the ambient temperature. The metabolic rate of the tissue in compartment A is rA. The parameters kambA and kaA determine the rate of heat exchange between compartments amb (the ambient environment) and A, and a3A and A, respectively. The parameter kmrt is a conversion factor between the metabolic rate rA and the resulting rise in temperature of the tissue. The equation for TB is of the same form. These compartments are coupled to the systemic small artery compartments as follows:
| (9) |
The parameter kAa determines the strength of the thermal coupling between the small arterial compartment a3A and its surrounding tissue A.
The relative fraction of the small arterial and venous blood in compartments A and B was chosen to be 0.70 and 0.30, respectively, while the tissue compartments are split 0.50 and 0.50. One can interpret the vascular compartment a3A as the blood in the small arteries of the brain, myocardium, liver, and anterior skeletal muscle and anterior white and brown adipose tissue. This is consistent with the results of Osborne, et al. (44) on the regulation of blood flow in hibernating hamsters, which is probably quite similar to that in the ground squirrels.
Over the course of our experiments, two animals died while being monitored in the environmental chamber; although the precise time of death could not be determined, their temperature measurements provide the best data we have for determining the thermodynamic parameters kambA and kambB, which determine how fast heat is lost to the ambient environment in our model (see Eq. 8). We assumed that the heat capacity of the tissue was ∼3.35 J·g −1·°C−1, as used in Nicol and Andersen (40); the heat capacity for whole blood has been measured as 3.6 J·g −1·°C−1 (5), but fat has a lower heat capacity. The amount of heat generated by a milliliter of oxygen consumed depends on the fuel source used. For simplicity, and because we are most interested in the physiology during torpor and arousal, we assume a constant relationship between oxygen volume and heat production based on lipid-derived catabolism of 19.9 J/ml (28). These assumptions in the model let us translate the heat production into oxygen consumption.
The small artery compartments a3A and a3B are assumed to undergo increased vasoconstriction during torpor and arousal (29), which is modeled by an increase in resistance and change in unstressed volume. In each compartment these effects are both derived from factors RfA and RfB. Since the vasoconstriction is greater in compartment a3B, RfB > RfA. For i = A or i = B these effects are
| (10) |
| (11) |
and the Rfi are determined by the temperature in compartment a3A by
| (12) |
| (13) |
These values were chosen to obtain consistent results with the available data from Lyman and O'Brien (29) on differences between heart and rectal temperatures. The temperature of compartment a3A is chosen since we assume that this vasoconstriction is determined by the central nervous system rather than locally.
The heart is modeled by four compartments corresponding to the right and left atria and ventricles. The atrial compartments are similar to the vasculature except for the mitral and tricuspid valves that close when the ventricular pressure exceeds the atrial pressure. The ventricular contractions are represented by time- and temperature-dependent elastances, Elv and Erv; for the left ventricle we have
| (14) |
where Plv is the pressure in the left ventricle, Vlv is the volume of the left ventricle, and Vun,lv is the unstressed volume of the left ventricle.
The length of time over which the heart contracts and relaxes (tce) depends upon both the period of heart contractions (th) and the temperature of the heart (e.g., for the left ventricle, Tlv). We modeled tce as a weighted average of effects from the period and temperature. The effect of temperature is sigmoidal, of the form
| (15) |
with a1 = 15.0, a2 = 0.05, a3 = 0.25, and a4 = 2.08 [a Goldbeter-Koshland function (15)].
For simplicity, we used a linear function to model the effect of the period of heart contractions,
| (16) |
These are combined as a weighted average tce = sT (T)w(T, 362 th)+[1−w(T, th)]sp(th), where w(T, th) is given by
| (17) |
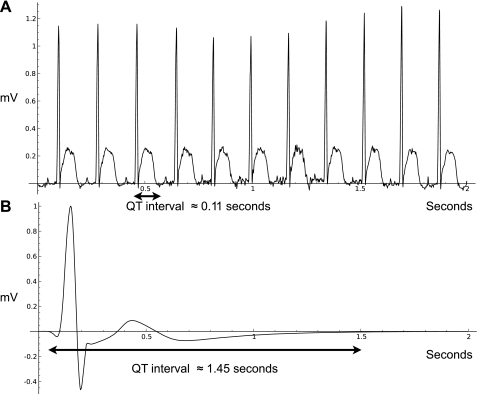
These phenomenological functions were chosen to fit data from the arousals and may not be accurate during the entrance into torpor. The QT intervals of the ECG were used to estimate the contraction time. During torpor, the QT interval could be as long as 2 s, compared with 0.1 s at normothermia. Figure 4 shows representative examples of ECGs from a normothermic squirrel in an IBA and a squirrel in torpor, with QT intervals indicated.
Fig. 4.
ECGs from 2 squirrels over a time period of 2 s. A: data are from a squirrel at 35.1°C and a heart rate of 333 beats/min, during the middle of an IBA. B: data are from a squirrel in torpor with a temperature of 5.2°C and a heart rate of 5 beats/min.
To determine the start of each contraction, the model records the last time that a contraction began (tf). A new contraction begins only if t − tf > th − tce, and then the values of th and tce at the start of the contraction are used to compute Elv from:
| (18) |
| (19) |
| (20) |
The factor Etf accounts for some loss of elastance at low temperatures.
The flows and volume changes are given by
| (21) |
| (22) |
The valves begin to shut if the pressure past the valve exceeds the pressure behind the valve. We allow a fixed volume of blood to flow back through the valves until they are completely shut (this volume is denoted as Vmax,lvb for the left ventricle; other heart compartments follow this notation). The right ventricle is modeled in the same way, with different parameter values. See the supplementary data for these and all other parameter values. (Supplemental data for this article are available online at the American Journal of Physiology–Regulatory, Integrative and Comparative Physiology website.)
Data-driven model simulations.
After determining the parameters in our model as described above, we used the measured heart rates and body temperatures of live thirteen-lined ground squirrels to obtain approximate reconstructions of other quantities, such as blood pressures and metabolic rates that are difficult to measure directly. Examples of these reconstructions are shown in Figs. 5 and 6.
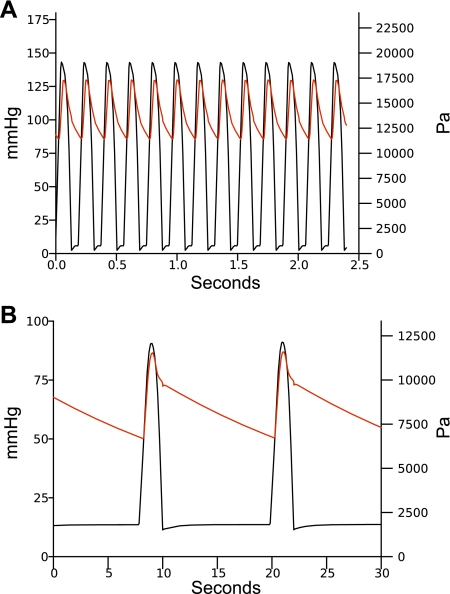
Fig. 5.
Model blood pressures. In both plots, the black line is the left ventricular pressure and the red line is the pressure in the large systemic arteries compartment (a1). A: pressures for a heart rate of 320 beats/min with a fixed blood temperature of 35.0°C. B: pressures for a heart rate of 5 beats/min with a fixed blood temperature of 7.7°C.
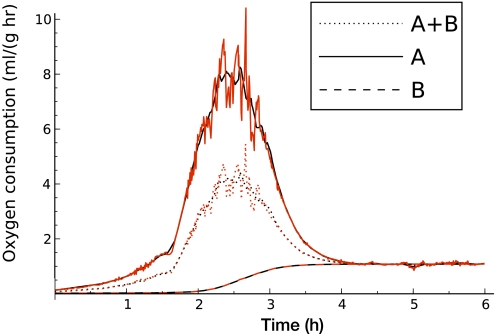
Fig. 6.
Oxygen consumption during arousal from torpor (model output) driven by averaged (black) and raw data (red) from a single animal. Solid lines are from compartment A, dashed lines are from compartment B, and dotted lines are A+B. Plot begins at the start of an arousal.
Figure 5 shows the model blood pressures in the left ventricular and large systemic arterial compartments. In Fig. 5A the model is driven with a constant body temperature of 35°C and a heart rate of 320 beats/min. In Fig. 5B, the model is driven with a constant body temperature of 7.7°C and a heart rate of 5 beats/min. These pressures and temperatures are consistent with the data in Lyman and O'Brien (29).
Figure 6 is driven by data from a first IBA after initially entering torpor at 5°C ambient temperature in November from a single animal (other bouts may have occurred undetected at higher ambient temperature). This data is a representative example, although details of the changes in body temperature on a short time scale vary with individual animals. Two simulations were run, one with the heart and temperature data Gaussian-smoothed over a 10-min window, the other with the measured data. The start of the arousal is defined in Physiological data acquisition and analysis in materials and methods.
The metabolic rate of compartment A (the more active tissue compartment) is found by solving Eq. 8 for rA at each time point with TA set to the measured body temperature. During an arousal, for compartment B (the less active tissue compartment) we assumed that the metabolic rate merely increases to match a basal level for the corresponding temperature of the perfusing blood; that is, we set
| (23) |
Another way to characterize this relationship is that we set rB to match the amount of heat needed to maintain compartment B at temperature Ta3B (the temperature of the perfusing blood). This is a somewhat higher metabolic rate than if we used the tissue compartment temperature TB, but it is a relatively passive response compared with compartment A.
Sensitivity analysis.
Our model is not particularly sensitive to any of its parameter values. This is not surprising since the circulatory system itself is robust to perturbations. We studied the sensitivity by computing the finite difference ratios of relative changes of various model outputs to relative changes in parameters. Since the metabolic rates rA and rB are the highlight of our model, we computed the sensitivity of these variables after 10 min of simulation time for the model driven by observed body temperatures. Besides the parameter kambB, for which the sensitivity can be computed analytically (equal to 1 because of the linear relationship), we computed the sensitivity σri,x of each rate ri to a parameter x by:
| (24) |
with Δri/ri = 0.01. All such sensitivities were < 1, usually much less.
For example, the sensitivity of rA to a combined change in kambA and kambB during the middle of an arousal is only 0.1, so a 10% error in estimating these parameters would result in a change of only 1% in our prediction of the metabolic rate in compartment A. Even considering 10% errors in all of our parameters our predicted metabolic rate changes by < 10%, unless the errors are very highly correlated.
DISCUSSION
Blood pressure and cardiac output.
A mathematical model of the circulatory system capable of predicting blood pressure, cardiac output, and metabolic rate was developed from recorded thirteen-lined ground squirrel body temperature, ECG, and hematocrit data. The model predicted blood pressures and cardiac outputs during phases of hibernation. These predicted values agree with previously measured blood pressures (29) and cardiac outputs (48).
During an IBA, the systolic/diastolic blood pressures shown in Lyman and O'Brien (29) range from 175/120 mmHg (23,330/16,000 Pa) to 125/70 mmHg (16,666/9,333 Pa). We used 135/90 mmHg (18,000/12,000 Pa) as the most representative value to help fit the model resistance parameters. At a constant temperature of 35°C and a constant heart rate of 320 beats/min, the blood pressures in the large arterial compartment a1 of our model are 130/85 mmHg (17,332/11,332 Pa). The cardiac output of the model under these conditions is 72 ml/min, which is within the variation found in Popovic (48) of 5.5 ml/min (SD 69). The data in Popovic (48) does not include the heart rate, but the cardiac output does not change dramatically with heart rate at 35°C in our model.
Immediately before arousal from torpor, the blood pressures shown in Lyman and O'Brien (29) are ∼75/40 mmHg (10,000/5,333 Pa), although there is significant variability with blood pressures falling as low as 60/30 mmHg (8,000/4,000 Pa). At a constant temperature of 7°C and a constant heart rate of 3 beats/min the blood pressures in the large arterial compartment a1 of our model are 72/36 mmHg (9,600/4,800 Pa). Cardiac output of the model under these conditions is 1 ml/min, in good agreement with the value of 1.04 ml/min (SD 0.1) measured by Popovic (48).
Metabolic rate.
The model we developed predicted that extremely high metabolic rates occur during arousal relative to normothermic animals. This result strongly supports the hypothesis that arousing ground squirrels rewarm as quickly as possible. Our results for the overall metabolic rate are higher than those found in data for echidnas (40), marmots (18, 43), and arctic ground squirrels (23). When the larger masses of these species are accounted for with the M−0.75 dependence of basal metabolic rate, our results are comparable, although still higher. There is substantial evidence that maximal metabolic rates scale with a somewhat higher exponent (M−0.87), which makes our predicted rates in the thirteen-lined ground squirrel even more impressive (8, 57, 58). That the smaller mass and higher basal metabolic rate of the thirteen-lined ground squirrel compared with those species means that heat loss during rewarming and euthermia is much greater. Comparisons to experimental data are complicated by the fact that metabolic rates during arousal can change very quickly, with transient spikes of much higher values than average. Table 2 gives comparative data from the above references for the maximal metabolic rate during arousal in the alpine marmot (Marmota marmota), short-beaked echidna (Tachyglossus aculeatus), and arctic ground squirrel (S. parryii). None of the studies used in Table 2 were focused specifically on measuring maximal metabolic rate during arousal and should be considered lower bounds since their data is averaged over longer time periods than in our model. Table 2 also includes values for the basal metabolic rates at normothermia and metabolic rates during torpor for comparison.
Table 2.
Comparative thermodynamics of arousal rates in 4 hibernating mammalian species
| Species | Mass, g | Maximum, ml O2·g−1·hr−1 | Basal, ml O2·g−1·hr−1 | Torpor, ml O2·g−1·hr−1 |
|---|---|---|---|---|
| Tachyglossus aculeatus (40) | 3,900 | 1.4 | 0.15 | 0.02 |
| Marmota marmota (18, 43) | 3,200 | 1.5 | 0.19 | 0.014 |
| Spermophilus parryii (23) | 800 | 2.65 | 0.5 | 0.014 |
| Spermophilus tridecemlineatus (48, 51) | 240 | 4.7 | 0.96 | 0.02 |
Data for species is taken from reference number in parentheses. The maximum metabolic rate for S. tridecemlineatus is derived from the authors' mathematical model.
The model does not explicitly take into account changes in the rate of ventilation. In the middle of an arousal, when the metabolic rate is very high, the rate of ventilation must be correspondingly high. One effect of this is that some additional heat loss would occur. Since this is not taken into account, our model might underestimate metabolic rate during arousal.
The peak metabolic rate in the active tissue (i.e., our compartment A, which includes the heart, brain, liver, and brown adipose tissue) during arousal can reach 10 ml O2·g−1·h−1 for short bursts. This metabolic rate, which occurs during the middle of the arousal, is 10 times the rate needed for the entire animal to maintain a constant temperature (which at that point is 19°C in the active compartment's small arteries, although it is probable that some tissues within our active compartment, such as the heart and brown adipose tissue, are at a significantly higher temperature than the compartment average). Averaged over both tissue compartments, the metabolic rate in our model peaks at 4.7 ml O2·g−1·h−1. That is six times the metabolic rate predicted by our model for a normothermic animal at rest at an ambient temperature of 5°C, five times that of the basal rate for a thirteen-lined ground squirrel measured by Popovic (48) during summer months at an ambient temperature of 23°C, and 235 times the rate measured by Popovic during torpor. It is important to note that the basal rate during the summer is probably higher than during torpor; in the alpine marmot, the basal metabolic rate is twice as high in the summer (43), and, in the closely related species S. mexicanus, reported basal metabolic rates (38) are only 70% of the value reported by Popovic (48).
The high metabolic rates achieved during arousals in our model are remarkable. It is difficult to directly compare these rates to larger mammals since the most common measurement is of maximal metabolic rate, which can only be maintained for short time periods: a few minutes as opposed to the hours spent during the arousal period in the thirteen-lined ground squirrel. Maximal metabolic rate scales allometrically as aM0.87 (57) (where a is a constant) as opposed to the basal metabolic scaling of M0.75 (61). This means that a given multiple of metabolic rate compared with the basal rate should be considered more extreme as the mass of the species considered decreases. Given the time span of arousal from torpor we could compare it to an endurance run, such as a marathon. A trained marathon runner can maintain a metabolic rate ∼80% of their maximal metabolic rate (3). For a rat (Rattus norvegicus), a mammal of comparable weight to our ground squirrels, the maximal metabolic rate given by Weibel et al. (57) is 11.7 ml O2·g−1·h−1. Eighty percent of that maximal metabolic rate is 9.4 ml O2·g−1·h−1; although that is twice the value we find for the ground squirrel during arousal, it is also from a normothermic animal instead of one at 19°C. This exemplifies that a peak metabolic rate of 4.7 ml O2·g−1·h−1 at 19°C is remarkably high for a small rodent and the transport of oxygen by the cardiovascular system may be the limiting factor in the speed of arousal from torpor (46).
Heart rate variation.
The occurrence of arrhythmias in ground squirrel ECGs was quantified by calculating the IBI COV. Occurrence of arrhythmias increased as an active squirrel proceeded through entrance and into maintenance of torpor. The increase in occurrence of arrhythmias upon entrance into torpor agrees with previously published data [see Milsom et al. (35) for review]. Arrhythmias occurred more frequently relative to the total number of heartbeats during maintenance of torpor compared with active-state ECGs. This may be due to temporary increases in heart rate associated with respirations during torpor maintenance (9). During the rapid increase in heart rate from 5 to 250 beats/min in only 3 h, the occurrence of arrhythmias decreased compared with the other phases of torpor. According to Eagles et al. (10), the occurrence of arrhythmias during arousal is limited to the body temperature window of 11 to 20°C, and the heart rate is rhythmic during the rest of an arousal. Mertens et al. (34) have shown a similar increase in IBI variability during the entrance phase of daily torpor and subsequent decrease in variability during the arousal phase in the Djungarian hamster.
We hypothesized that the pattern of heart rate variation could be used to predict the onset of a bout of torpor. The extreme decrease in heart rate was predicted to be preceded by an increase in arrhythmias, which would detect a bout of torpor earlier than using a drop in body temperature as an indicator. However, the regular occurrence of arrhythmias in active animals that are not about to enter torpor prevents the IBI COV from being used to reliably detect an entrance into torpor.
Some of the arrhythmia in hibernators appears to be related to variations in respiration rates (25, 36), but we did not obtain direct measurements of respiration in this study. Combining such measurements with heart rate and temperature data would allow a more complete cardiovascular model that could provide estimates of new quantities such as O2 partial pressures during apnea in torpor.
Heart rate vs. temperature.
It has been well established that heart rate declines prior to body temperature decline during entrance into torpor due to parasympathetic activation, and that heart rate increases first during arousal from torpor through sympathetic activation [reviewed in Milsom et al. (35)]. Our recording of heart rate and body temperature changes in the thirteen-lined ground squirrel also shows this pattern. The length of entrance and arousal phases of torpor in early season (November) were representative of torpor bouts even during mid-season (January and February) when the maintenance phase of torpor is longer.
Hematocrit.
In Popovic (48), hematocrit values for S. tridecemlineatus of 57% (SD 2) for summer samples and 40% (SD 2) for samples from hibernating winter animals are presented. A similar difference has been observed in S. lateralis (32). This led us to wonder whether the volume of plasma changed significantly between torpor and an IBA. Such an effect is plausible given data on rapid changes in blood volume and hematocrit in thoroughbred horses (56), greyhounds (37), and human athletes (53). Other data on hematocrit levels in hibernators is inconsistent with those large changes (14), so we began our own hematocrit measurements in torpid animals and during IBAs. We found the difference between the hematocrits of animals in these two states was not statistically significant. This indicates that hematocrit does not fall during entrance to torpor to alleviate the increase in blood viscosity. Our hematocrit values are consistent with the 49.7% (SD 1.11) obtained in Maclean (31) from normothermic S. tridecemlineatus blood taken during January through March.
Perspectives and Significance
By using implanted transmitters, accurate physiological data were collected from thirteen-lined ground squirrels without the anesthesia and restraint complications associated with older, traditional methods. The data collected using transmitters agreed with historical data, and the sensitive, long-term collection of ECG data confirmed the changes in arrhythmicity observed during torpor previously indicated with short-term ECG collection. The collection of high-resolution and accurate physiological data allowed the production of a pulsatile circulatory model of a hibernator for the first time. This physiological model used heart rate and temperature data to produce predicted blood pressures during the extremes in the physiological states experienced by the hibernators. The predicted blood pressures during those extremes corresponded to historical data.
The mathematical model presented in this paper is capable of predicting the metabolic rate in specified tissue compartments, which is very difficult to do in practice. Modeling the physiological system provides an excellent method for understanding the metabolic changes during hibernation without the difficulties involved with tissue-specific measurements in a whole animal during torpor and arousals. The metabolic rate of an arousing thirteen-lined ground squirrel at 19°C was predicted to be six times the predicted rate for a normothermic (35°C) animal at rest at the same ambient temperature of 5°C, and five times the metabolic rate measured by Popovic (48) for normothermic animals at an ambient temperature of 23°C. This supports the hypothesis that the torpid hibernator warms from its low body temperature in the least possible time, and that this warming is limited by oxygen delivery to the most active tissues. Thus this physiological model provides a more detailed understanding of the metabolic changes during hibernation without the complications posed by direct measurement. Our model also provides a basis that can be extended for understanding other physiological phenomena in mammalian hibernators. In addition to its intrinsic scientific interest, we believe that uncovering the mechanisms behind the extreme and rapid physiological changes in hibernators will eventually lead to profound applications in human health.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute grant 1RC2-HL-101625-01 (to M. T. Andrews). Some of our simulations were run on one of the Sage Foundation's 24-core Sun X4450s, supported by National Science Foundation Grant DMS-0821725.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Vicki Caskey, Sarah Diekman, Heather Moline, Eli Narveson, and Steve Skolasinski for help in data collection and analysis and Lois Heller, Richard Melvin, and David Mohrman for helpful discussion. We also thank all of the reviewers of the manuscript for their questions and suggestions.
Glossary of Compartments
All of the following compartments (j) have an associated temperature (Tj). All of them except tisA, tisB, and amb also have associated pressure (Pj), flow rate (Qj), oxygen (Oj), and volume (Vj). The aorta compartment does not have a volume that appears in the model, as its purpose is to incorporate purely inertial effects.
- as,
aorta
- a1,
large systemic arteries
- a2,
medium systemic arteries
- a3A,
small systemic arteries, core/anterior organs
- a3B,
small systemic arteries, peripheral/posterior organs
- l1,
small pulmonary veins
- l2,
large pulmonary veins
- p1,
large pulmonary arteries
- p2,
medium pulmonary arteries
- p3,
small pulmonary arteries
- la,
left atrium
- lv,
left ventricle
- ra,
right atrium
- rv,
right ventricle
- v1A,
small systemic veins, core/anterior organs
- v1B,
small systemic veins, peripheral/posterior organs
- v2,
large systemic veins
- tisA,
core/anterior tissue compartment (temperature only)
- tisB,
peripheral/posterior tissue component (temperature only)
- amb,
ambient environment (temperature only, independent of all other components)
REFERENCES
- 1.Andrews MT. Advances in molecular biology of hibernation in mammals. Bioessays 29: 431–607 440, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Barnes BM. Freeze avoidance in a mammal: body temperatures below 0 degree C in an arctic hibernator. Science 244: 1593–1595, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Bassett DJ, Howley E. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 32: 70–84, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia: its possible role in cardiac surgery. Ann Surg 132: 849–866, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake AS, Petley GW, Deakin CD. Effects of changes in packed cell volume on the specific heat capacity of blood: implications for studies measuring heat exchange in extracorporeal circuits. Br J Anaesth 84: 28–32, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bradbury J. How hibernators might one day solve medical problems. Lancet 358: 1164, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Darveau CA, Suarez RK, Andrews RD, Hochachka PW. Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Dawe AR, Morrison PR. Characteristics of the hibernating heart. Am Heart J 49: 367–384, 1955 [DOI] [PubMed] [Google Scholar]
- 10.Eagles DA, Jacques LB, Taboada J, Wagner CW, Diakun TA. Cardiac arrhythmias during arousal from hibernation in three species of rodents. Am J Physiol Regul Integr Comp Physiol 254: R102–R108, 1988 [DOI] [PubMed] [Google Scholar]
- 11.Euler L. Institutionum Calculi Integralis. St. Petersburg, Russia: The Imperial Academy of Arts and Sciences, 1768 [Google Scholar]
- 12.Fedorov VV, Glukhov AV, Sudharshan S, Egorov Y, Rosenshtraukh LV, Efimov IR. Electrophysiological mechanisms of antiarrhythmic protection during hypothermia in winter hibernating versus nonhibernating mammals. Heart Rhythm 5: 1587–1596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman AP, Lyman LP. Hibernation in mammals. Circulation 24: 434–445, 1961 [DOI] [PubMed] [Google Scholar]
- 14.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to cerebral ischemia. J Cereb Blood Flow Metab 14: 193–205, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Goldbeter A, Koshland DE. An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci USA 78: 6840–6844, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halikas G, Bowers K. Seasonal variation in blood viscosity of the hibernating arctic ground squirrel (Spermophilus undulatus plesius). Comp Biochem Physiol 44A: 677–681, 1973 [DOI] [PubMed] [Google Scholar]
- 17.Hampton M, Andrews MT. A simple molecular mathematical model of mammalian hibernation. J Theor Biol 247: 297–302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141: 317–329, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hochachka PW, Guppy M. Metabolic Arrest and the Control of Biological Time. Cambridge, MA: Harvard University Press, 1987 [Google Scholar]
- 20.Humphries MM, Thomas DW, Kramer DL. The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool 76: 165–179, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Ings RM. Interspecies scaling and comparisons in drug development and toxicokinetics. Xenobiotica 20: 1201–1231, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Johansson BW. The hibernator heart–nature's model of resistance to ventricular fibrillation. Cardiovasc Res 31: 826–832, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Karpovich SA, Tøien Ø, Buck CL, Barnes BN. Energetics of arousal episodes in hibernating ground squirrels. J Comp Physiol B 179: 691–700, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Kirkebo A. Cardiovascular investigations on hedgehogs during arousal from the hibernating state. Acta Physiol Scand 73: 394–406, 1968 [DOI] [PubMed] [Google Scholar]
- 25.Landua BR, Dowe AR. Sensitivity to low temperature in hibernating rodents. Am J Physiol 194: 75–82, 1958 [DOI] [PubMed] [Google Scholar]
- 26.Lavé T, Coassolo P, Reigner B. Prediction of hepatic metabolic clearance based on interspecies allometric scaling techniques and in vitro-in vivo correlations. Clin Pharmacokinet 36: 211–231, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Li JKJ. Comparative Cardiovascular Dynamics of Mammals. Boca Raton, FL: CRC, 1996 [Google Scholar]
- 28.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr 47: 608–628, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Lyman CP, O'Brien RC. Circulatory changes in the 13-lined ground squirrel during the hibernating cycle. Bull Mus Comp Zool 124: 353–372, 1960 [PubMed] [Google Scholar]
- 30.Lyman CP, Willis JS, Malan A, Wang LCH. Hibernation and Torpor in Mammals and Birds. New York: Academic, 1982 [Google Scholar]
- 31.Maclean GS. Blood viscosity of two mammalian hibernators: Spermophilus tridecemlineatus and Tamias striatus. Physiol Zool 54: 122–131, 1981 [Google Scholar]
- 32.Maginniss LA, Milsom WK. Effects of hibernation on blood oxygen transport in the golden-mantled ground squirrel. Respir Physiol 95: 195–208, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Martin SL. Mammalian hibernation: a naturally reversible model for insulin resistance in man? Diab Vasc Dis Res 5: 76–81, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Mertens A, Stiedl O, Steinlechner S, Meyer M. Cardiac dynamics during daily torpor in the Djungarian hamster (Phodopus sungorus). Am J Physiol Regul Integr Comp Physiol 294: R639–R650, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Milsom WK, Zimmer MB, Harris MB. Regulation of cardiac rhythm in hibernating mammals. Comp Biochem Physiol A Mol Integr Physiol 124: 383–391, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Milsom WK, Zimmer MB, Harris MB. Vagal control of cardiorespiratory function in hibernation. Exp Physiol 86: 791–796, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Neuhaus D, Fedde MR, Gaehtgens P. Changes in haemorheology in the racing greyhound as related to oxygen delivery. Eur J Appl Physiol Occup Physiol 65: 278–285, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Neumann RL, Cade TJ. Torpidity in the Mexican ground squirrel. Can J Zool 43: 133–140, 1965 [DOI] [PubMed] [Google Scholar]
- 39.Newton I. Scala graduum caloris. Philos Transact 22: 824–829, 1701 [Google Scholar]
- 40.Nicol SC, Andersen NA. Rewarming rates and thermogenesis in hibernating echidnas. Comp Biochem Physiol A Mol Integr Physiol 150: 189–195, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Noordergraaf A. Circulatory System Dynamics. San Diego, CA: Academic, 1978 [Google Scholar]
- 42.Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther 283: 46–58, 1997 [PubMed] [Google Scholar]
- 43.Ortmann S, Heldmaier G. Regulation of body temperature and energy requirements of hibernating alpine marmots (Marmota marmota). Am J Physiol Regul Integr Comp Physiol 278: R698–R704, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Osborne PG, Sato J, Shuke N, Hashimoto M. Sympathetic α-adrenergic regulation of blood flow and volume in hamsters arousing from hibernation. Am J Physiol Regul Integr Comp Physiol 289: R554–R562, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Ottesen JT, Olufsen MS, Larsen JK. Applied Mathematical Models in Human Physiology. Philadelphia, PA: SIAM, 2004 [Google Scholar]
- 46.Painter P. Allometric scaling of the maximum metabolic rate of mammals: oxygen transport from the lungs to the heart is a limiting step (Abstract). Theor Biol Med Model 2: 31, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips PK, Heath JE. Comparison of surface temperature in 13-lined ground squirrel (Spermophilus tridecimlineatus) and yellow-bellied marmot (Marmota aviventris) during arousal from hibernation. Comp Biochem Physiol A Mol Integr Physiol 138: 451–457, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Popovic V. Cardiac output in hibernating ground squirrels. Am J Physiol 207: 1345–1348, 1964 [DOI] [PubMed] [Google Scholar]
- 49.Popovic V, Kent KM. Cardiovascular responses in prolonged hypothermia. Am J Physiol 209: 1069–1074, 1965 [DOI] [PubMed] [Google Scholar]
- 50.Pries AR, Neuhaus D, Gaehtgens P. Blood viscosity in tube flow: dependence on diameter and hematocrit. Am J Physiol Heart Circ Physiol 263: H1770–H1778, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Savage VM, Gillooly JF, Woodruff WH, West GB, Allen AP, Enquist BJ, Brown JH. The predominance of quarter-power scaling in biology. Funct Ecol 18: 257–282, 2004 [Google Scholar]
- 52.Schmidt-Nielsen K. Scaling. New York: Cambridge University Press, 1984 [Google Scholar]
- 53.Schumacher YO, Pottgiesser T, Ahlgrim C, Ruthardt S, Dickhuth HH, Roecker K. Haemoglobin mass in cyclists during stage racing. Int J Sports Med 29: 372–378, 7232008 [DOI] [PubMed] [Google Scholar]
- 54.Stein W. Sage mathematics software (version 3.4.2). The Sage Development Team, 2009. http://www.sagemath.org [2010]. [Google Scholar]
- 55.Trivedi B. Suspended animation: putting life on hold. New Scientist 189: 28–32, 2006 [Google Scholar]
- 56.Weber JM, Dobson GP, Parkhouse WS, Wheeldon D, Harman JC, Snow DH, Hochachka PW. Cardiac output and oxygen consumption in exercising thoroughbred horses. Am J Physiol Regul Integr Comp Physiol 253: R890–R895, 1987 [DOI] [PubMed] [Google Scholar]
- 57.Weibel ER, Bacigalupe LD, Schmitt B, Hoppeler H. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir Physiol Neurobiol 140: 115–132, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Weibel ER, Hoppeler H. Exercise-induced maximal metabolic rate scales with muscle aerobic capacity. J Exp Biol 208: 1635–1644, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Wells RE, Merrill EW. Influence of flow properties of blood upon viscosity hematocrit relationships. J Clin Invest 41: 1591–1598, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science 276: 122–126, 1997 [DOI] [PubMed] [Google Scholar]
- 61.White C, Seymour R. Allometric scaling of mammalian metabolism. J Exp Biol 208: 1611–1619, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.