Abstract
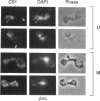
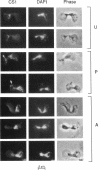
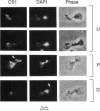
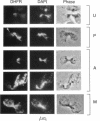
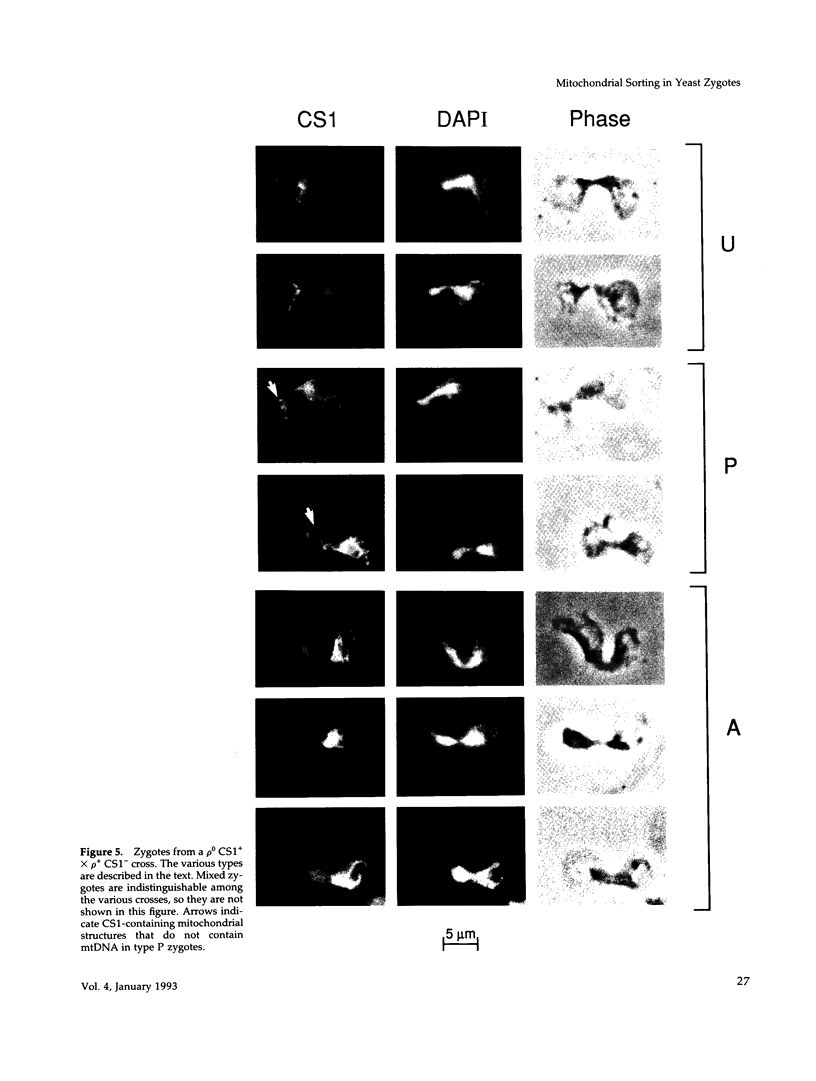
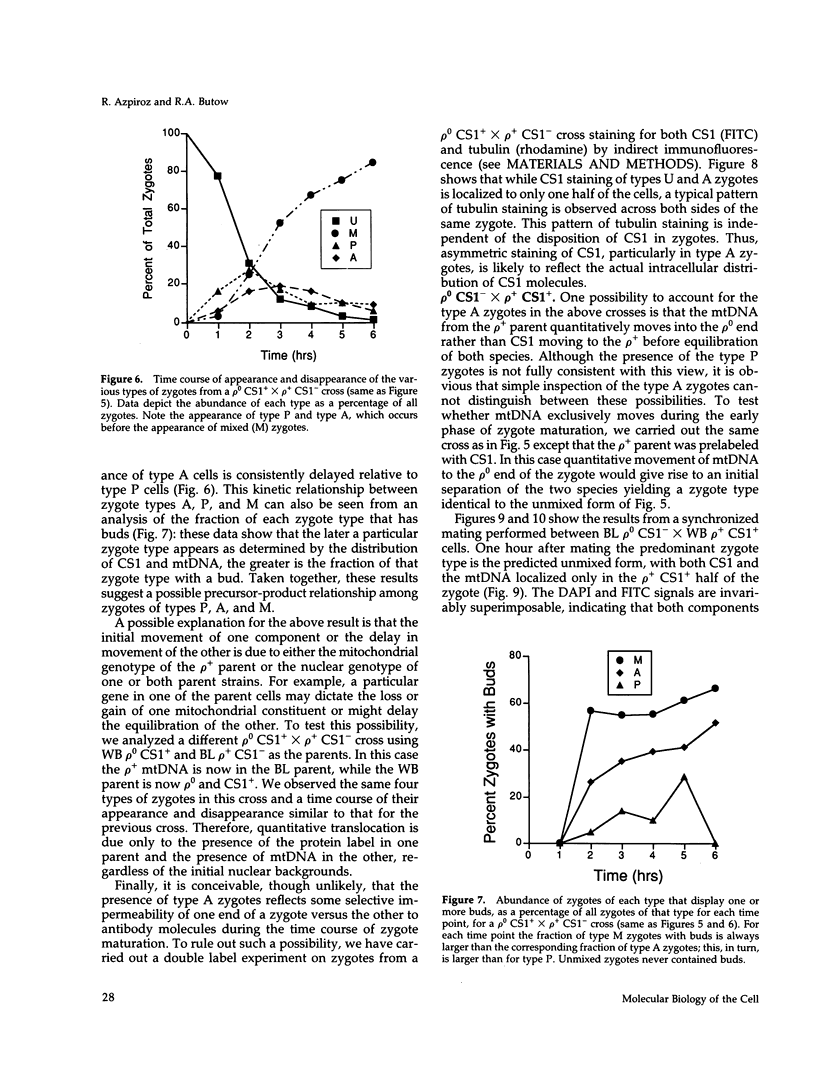
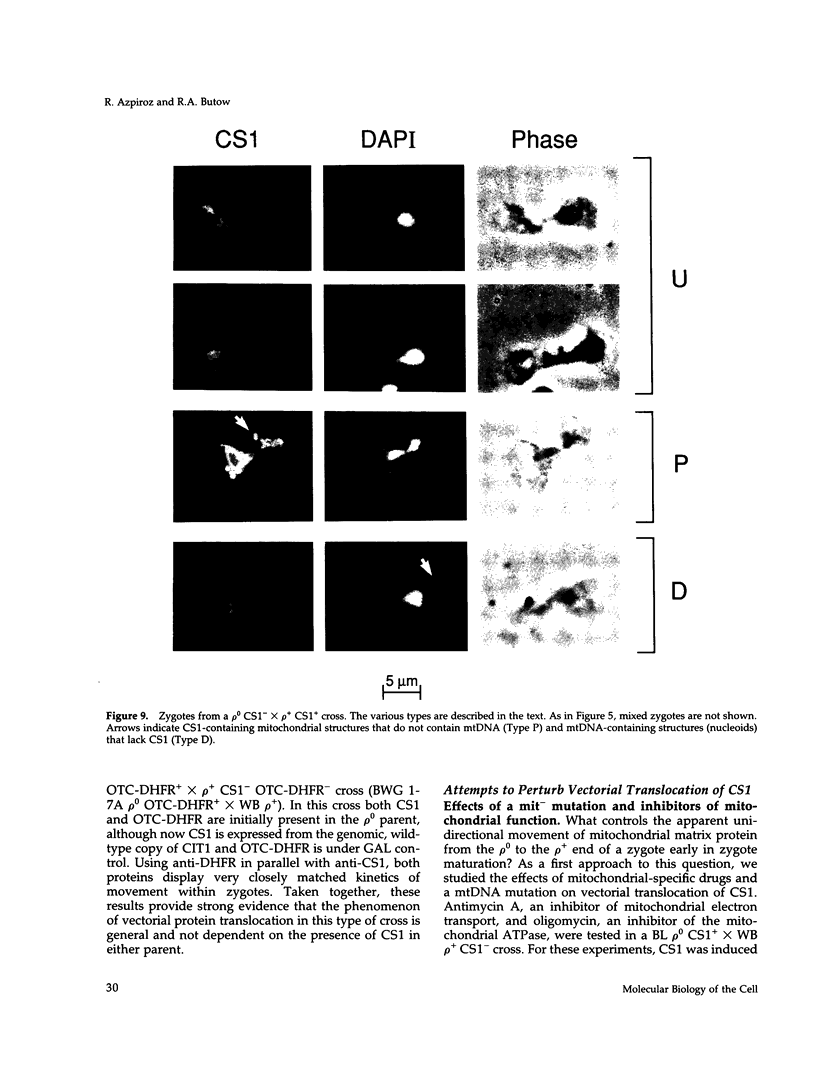
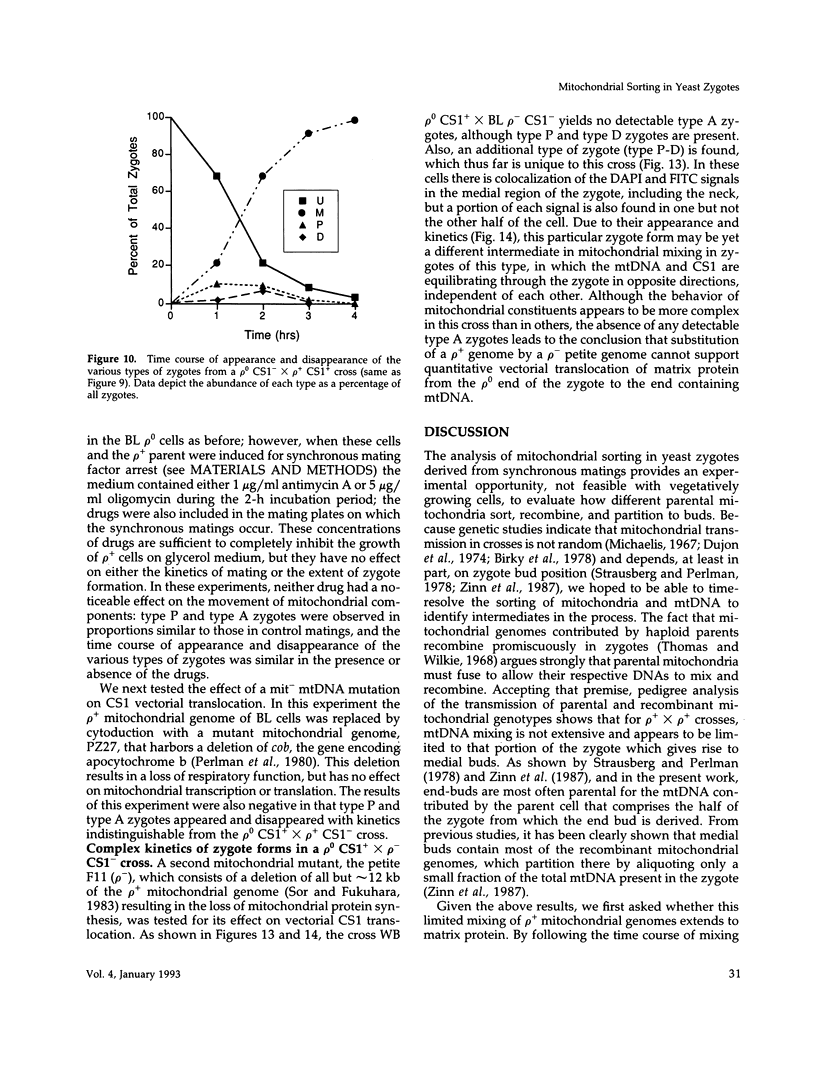
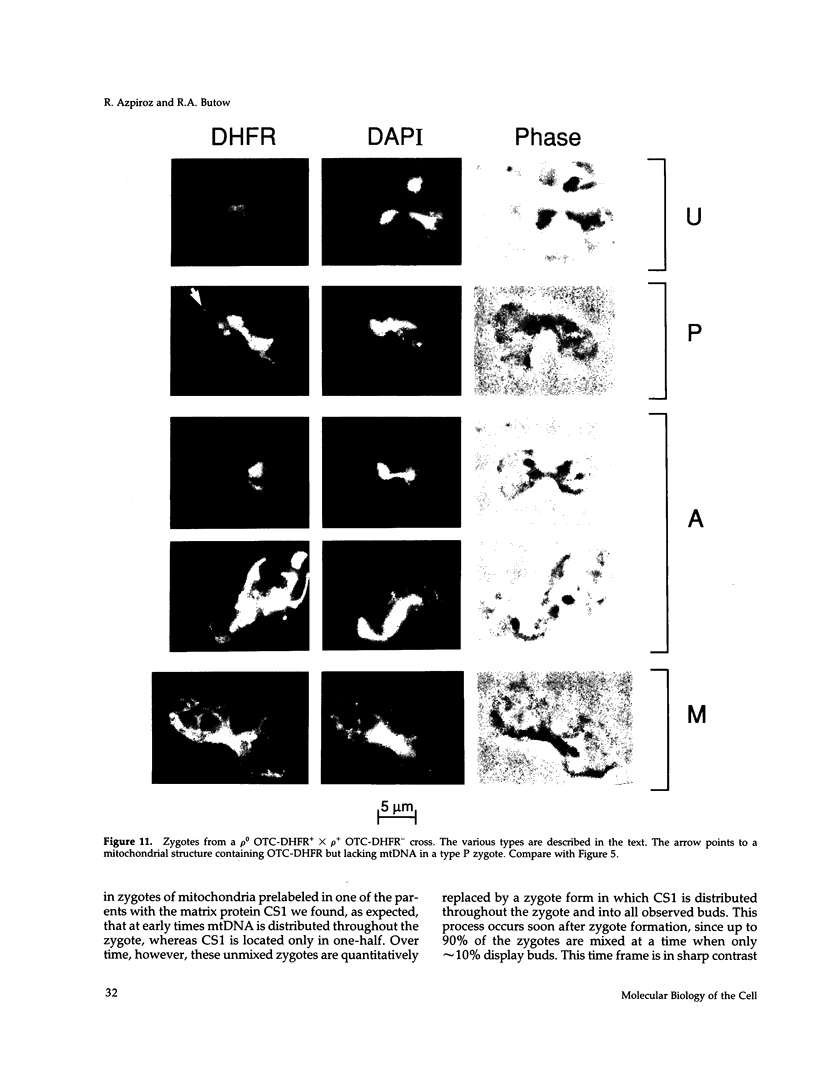
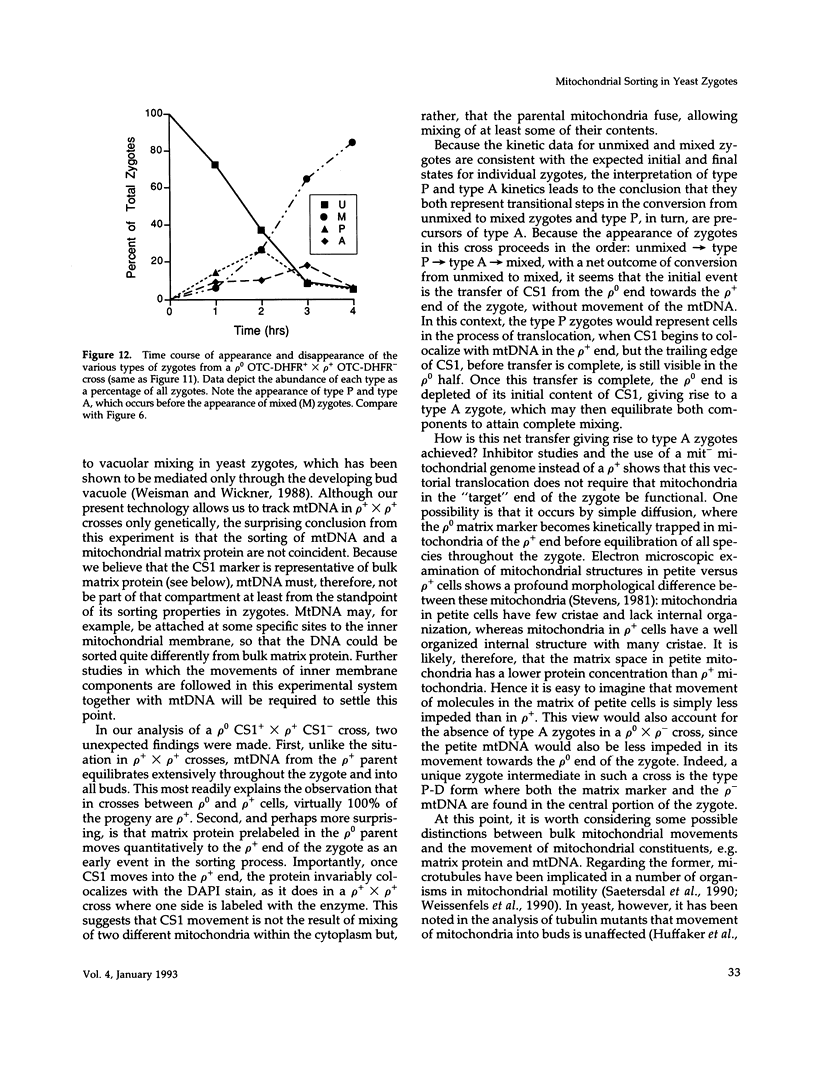
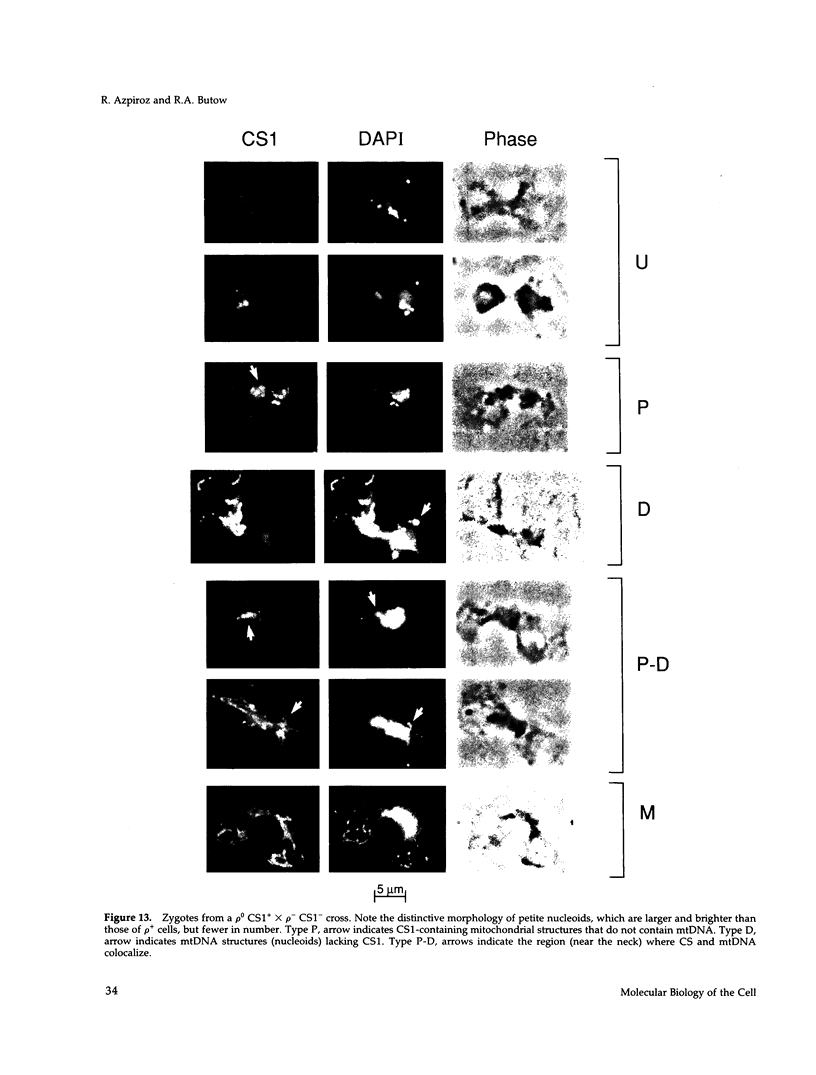
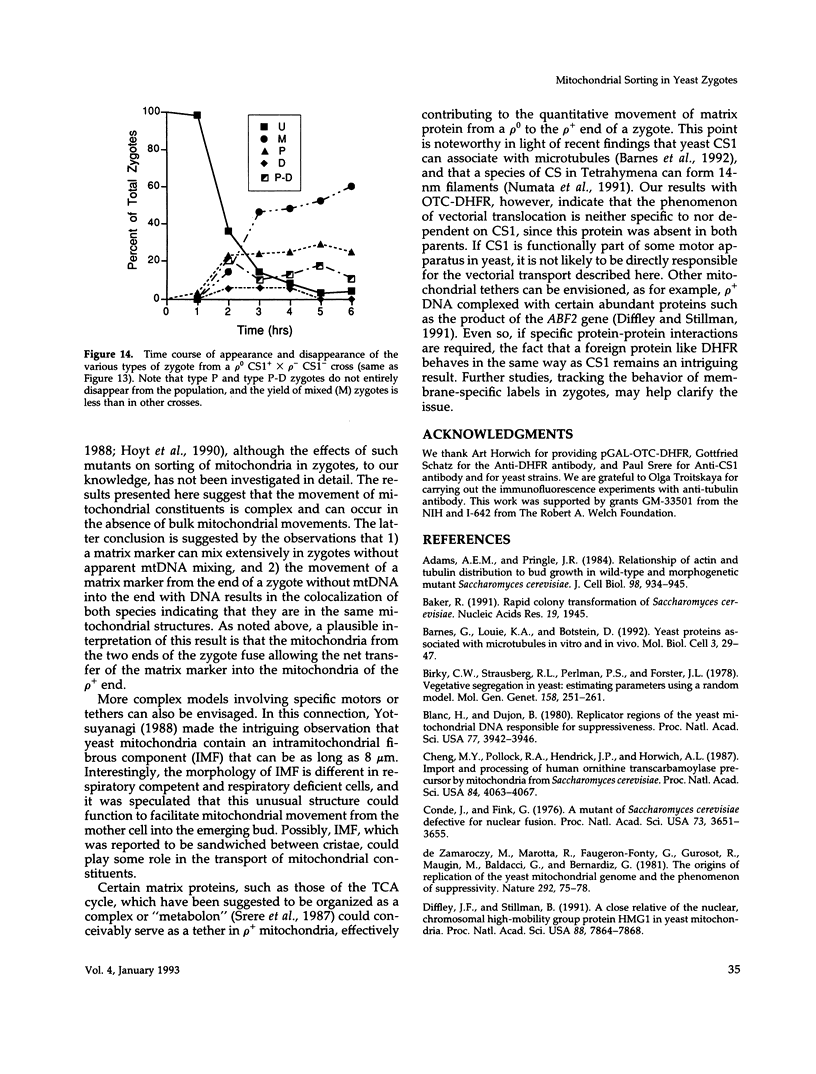
Inheritance of mitochondrial DNA (mtDNA) in Saccharomyces cerevisiae is usually biparental. Pedigree studies of zygotic first buds indicate limited mixing of wild-type (p+) parental mtDNAs: end buds are frequently homoplasmic for one parental mtDNA, while heteroplasmic and recombinant progeny usually arise from medial buds. In crosses involving certain petites, however, mitochondrial inheritance can be uniparental. In this study we show that mitochondrial sorting can be influenced by the parental mtDNAs and have identified intermediates in the process. In crosses where mtDNA mixing is limited and one parent is prelabeled with the matrix enzyme citrate synthase 1 (CS1), the protein freely equilibrates throughout the zygote before the first bud has matured. Furthermore, if one parent is p0 (lacking mtDNA), mtDNA from the p+ parent can also equilibrate; intracellular movement of mtDNA is unhindered in this case. Surprisingly, in zygotes from a p0 CS1+ x p+ CS1- cross, CS1 is quantitatively translocated to the p+ end of the zygote before mtDNA movement; subsequently, both components equilibrate throughout the cell. This initial vectorial transfer does not require respiratory function in the p+ parent, although it does not occur if that parent is p-. Mouse dihydrofolate reductase (DHFR) present in the mitochondrial matrix can also be vectorially translocated, indicating that the process is general. Our data suggest that in zygotes mtDNA movement may be separately controlled from the movement of bulk matrix constituents.
Full text
PDF


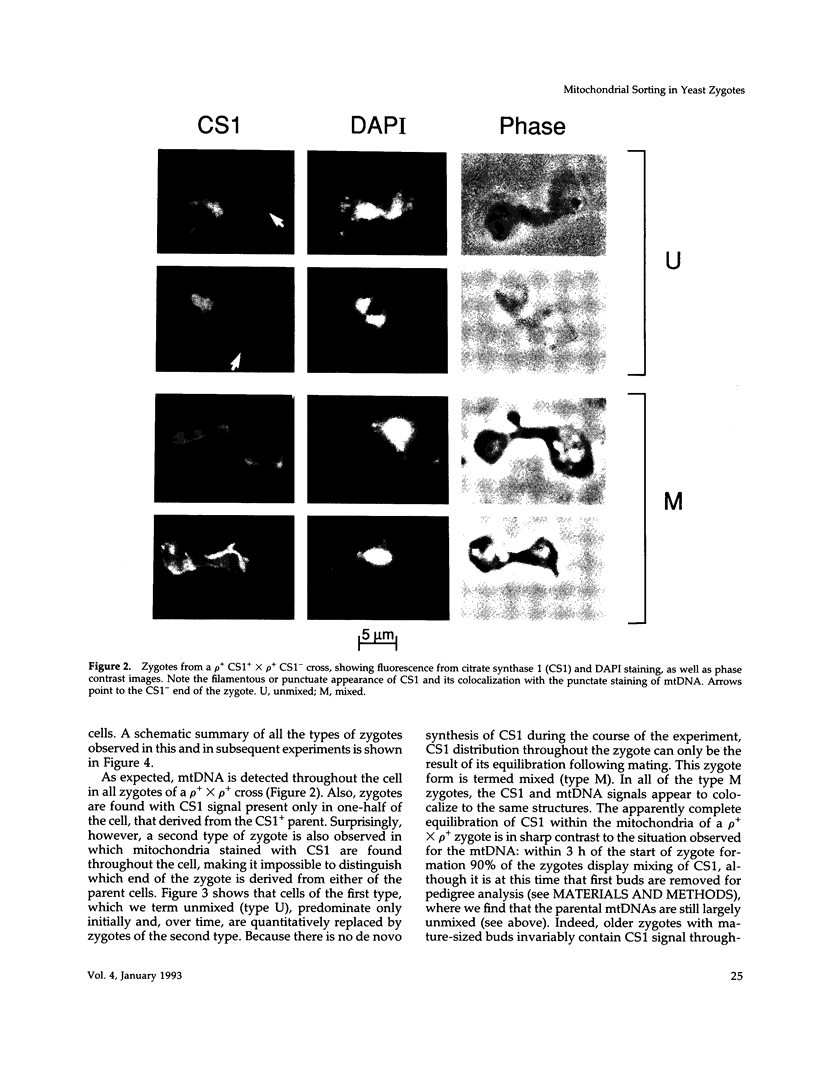
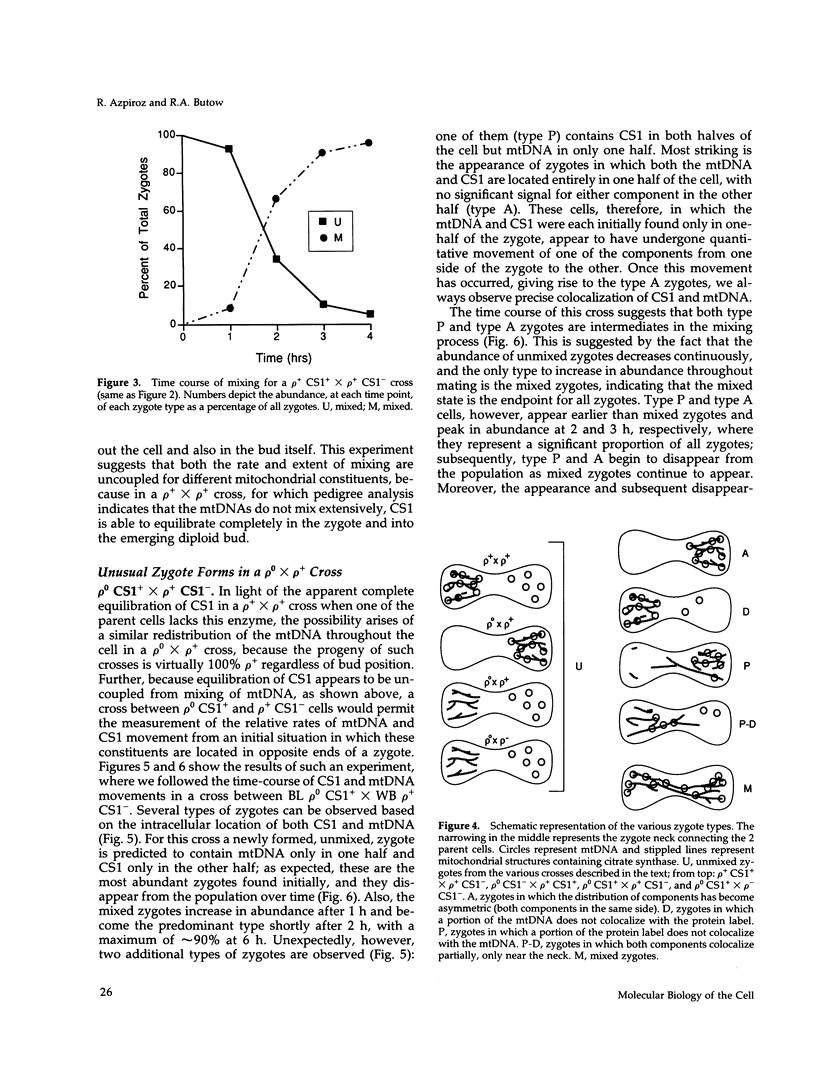


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. Rapid colony transformation of Saccharomyces cerevisiae. Nucleic Acids Res. 1991 Apr 25;19(8):1945–1945. doi: 10.1093/nar/19.8.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G., Louie K. A., Botstein D. Yeast proteins associated with microtubules in vitro and in vivo. Mol Biol Cell. 1992 Jan;3(1):29–47. doi: 10.1091/mbc.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3942–3946. doi: 10.1073/pnas.77.7.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. Y., Pollock R. A., Hendrick J. P., Horwich A. L. Import and processing of human ornithine transcarbamoylase precursor by mitochondria from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4063–4067. doi: 10.1073/pnas.84.12.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7864–7868. doi: 10.1073/pnas.88.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B., Slonimski P. P., Weill L. Mitochondrial genetics IX: A model for recombination and segregation of mitochondrial genomes in saccharomyces cerevisiae. Genetics. 1974 Sep;78(1):415–437. doi: 10.1093/genetics/78.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury F. Cloning and sequencing of the nuclear gene MIP1 encoding the catalytic subunit of the yeast mitochondrial DNA polymerase. J Biol Chem. 1989 Dec 5;264(34):20552–20560. [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Stearns T., Botstein D. Chromosome instability mutants of Saccharomyces cerevisiae that are defective in microtubule-mediated processes. Mol Cell Biol. 1990 Jan;10(1):223–234. doi: 10.1128/mcb.10.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. A., Fangman W. L. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 1992 Mar;6(3):380–389. doi: 10.1101/gad.6.3.380. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Adams A. E. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984 Mar;98(3):922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Rosenkrantz M. S., Guarente L. Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol Cell Biol. 1986 Jun;6(6):1936–1942. doi: 10.1128/mcb.6.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowsky T., Michaelis G. A nuclear gene essential for mitochondrial replication suppresses a defect of mitochondrial transcription in Saccharomyces cerevisiae. Mol Gen Genet. 1988 Oct;214(2):218–223. doi: 10.1007/BF00337714. [DOI] [PubMed] [Google Scholar]
- Lundin M., Baltscheffsky H., Ronne H. Yeast PPA2 gene encodes a mitochondrial inorganic pyrophosphatase that is essential for mitochondrial function. J Biol Chem. 1991 Jul 5;266(19):12168–12172. [PubMed] [Google Scholar]
- McConnell S. J., Stewart L. C., Talin A., Yaffe M. P. Temperature-sensitive yeast mutants defective in mitochondrial inheritance. J Cell Biol. 1990 Sep;111(3):967–976. doi: 10.1083/jcb.111.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata O., Takemasa T., Takagi I., Hirono M., Hirano H., Chiba J., Watanabe Y. Tetrahymena 14-nm filament-forming protein has citrate synthase activity. Biochem Biophys Res Commun. 1991 Jan 31;174(2):1028–1034. doi: 10.1016/0006-291x(91)91522-e. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B. Analysis of cytoskeletal structures using blot-purified monospecific antibodies. Methods Enzymol. 1986;134:467–472. doi: 10.1016/0076-6879(86)34112-0. [DOI] [PubMed] [Google Scholar]
- Rogers D., Bussey H. Fidelity of conjugation in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Jun 14;162(2):173–182. doi: 10.1007/BF00267874. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz M., Alam T., Kim K. S., Clark B. J., Srere P. A., Guarente L. P. Mitochondrial and nonmitochondrial citrate synthases in Saccharomyces cerevisiae are encoded by distinct homologous genes. Mol Cell Biol. 1986 Dec;6(12):4509–4515. doi: 10.1128/mcb.6.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetersdal T., Greve G., Dalen H. Associations between beta-tubulin and mitochondria in adult isolated heart myocytes as shown by immunofluorescence and immunoelectron microscopy. Histochemistry. 1990;95(1):1–10. doi: 10.1007/BF00737221. [DOI] [PubMed] [Google Scholar]
- Slonimski P. P., Perrodin G., Croft J. H. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal "petites". Biochem Biophys Res Commun. 1968 Feb 15;30(3):232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Complete DNA sequence coding for the large ribosomal RNA of yeast mitochondria. Nucleic Acids Res. 1983 Jan 25;11(2):339–348. doi: 10.1093/nar/11.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere P. A., Sumegi B., Sherry A. D. Organizational aspects of the citric acid cycle. Biochem Soc Symp. 1987;54:173–178. [PubMed] [Google Scholar]
- Stewart L. C., Yaffe M. P. A role for unsaturated fatty acids in mitochondrial movement and inheritance. J Cell Biol. 1991 Dec;115(5):1249–1257. doi: 10.1083/jcb.115.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
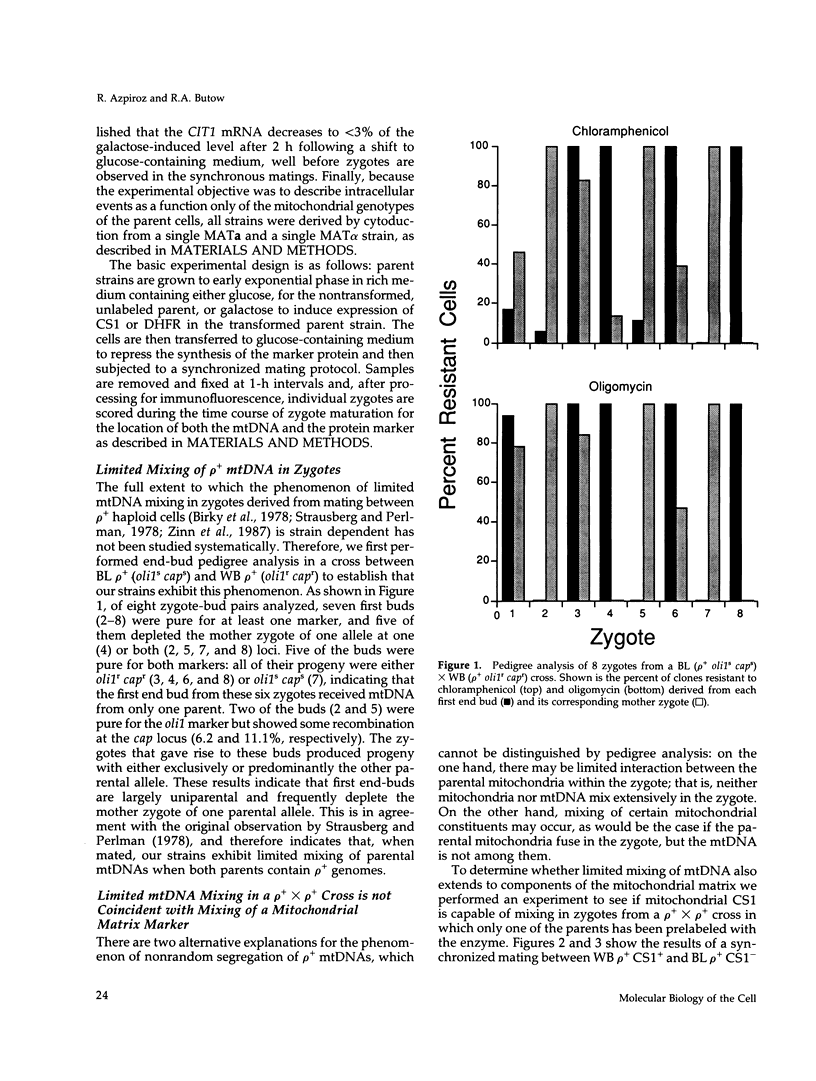
- Strausberg R. L., Perlman P. S. The effect of zygotic bud position on the transmission of mitochondrial genes in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Jul 11;163(2):131–144. doi: 10.1007/BF00267404. [DOI] [PubMed] [Google Scholar]
- Suissa M., Suda K., Schatz G. Isolation of the nuclear yeast genes for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J. 1984 Aug;3(8):1773–1781. doi: 10.1002/j.1460-2075.1984.tb02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. Y., Wilkie D. Recombination of mitochondrial drug-resistance factors in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1968 Feb 26;30(4):368–372. doi: 10.1016/0006-291x(68)90753-5. [DOI] [PubMed] [Google Scholar]
- Weislogel P. O., Butow R. A. Low temperature and chloramphenicol induction of respiratory deficiency in a cold-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1970 Sep;67(1):52–58. doi: 10.1073/pnas.67.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman L. S., Wickner W. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988 Jul 29;241(4865):589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Weissenfels N., Wachtmann D., Stockem W. The role of microtubules for the movement of mitochondria in pinacocytes of fresh-water sponges (Spongillidae, Porifera). Eur J Cell Biol. 1990 Aug;52(2):310–314. [PubMed] [Google Scholar]
- West R. W., Jr, Yocum R. R., Ptashne M. Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol Cell Biol. 1984 Nov;4(11):2467–2478. doi: 10.1128/mcb.4.11.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuyanagi Y. Fibrous component of yeast mitochondria. J Ultrastruct Mol Struct Res. 1988 Mar;98(3):254–266. doi: 10.1016/s0889-1605(88)80918-2. [DOI] [PubMed] [Google Scholar]
- Zinn A. R., Pohlman J. K., Perlman P. S., Butow R. A. Kinetic and segregational analysis of mitochondrial DNA recombination in yeast. Plasmid. 1987 May;17(3):248–256. doi: 10.1016/0147-619x(87)90033-3. [DOI] [PubMed] [Google Scholar]
- Zweifel S. G., Fangman W. L. A nuclear mutation reversing a biased transmission of yeast mitochondrial DNA. Genetics. 1991 Jun;128(2):241–249. doi: 10.1093/genetics/128.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]