Abstract
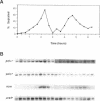
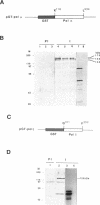
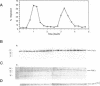
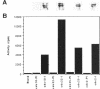
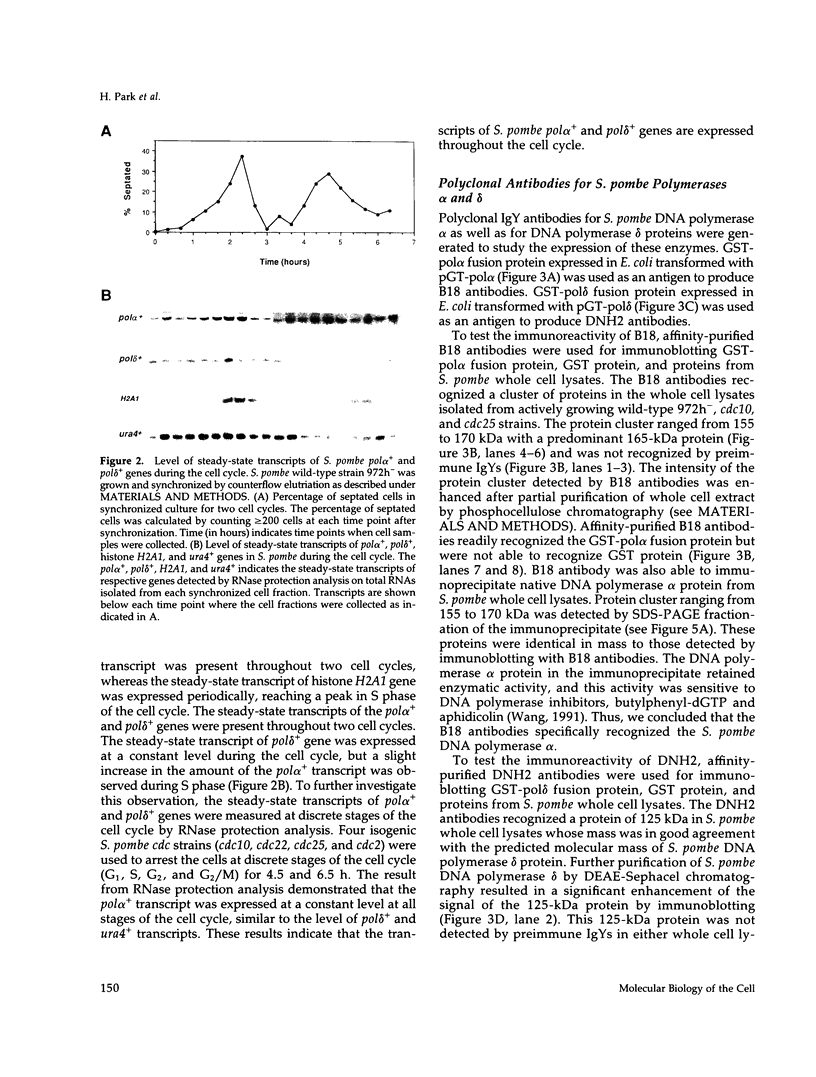
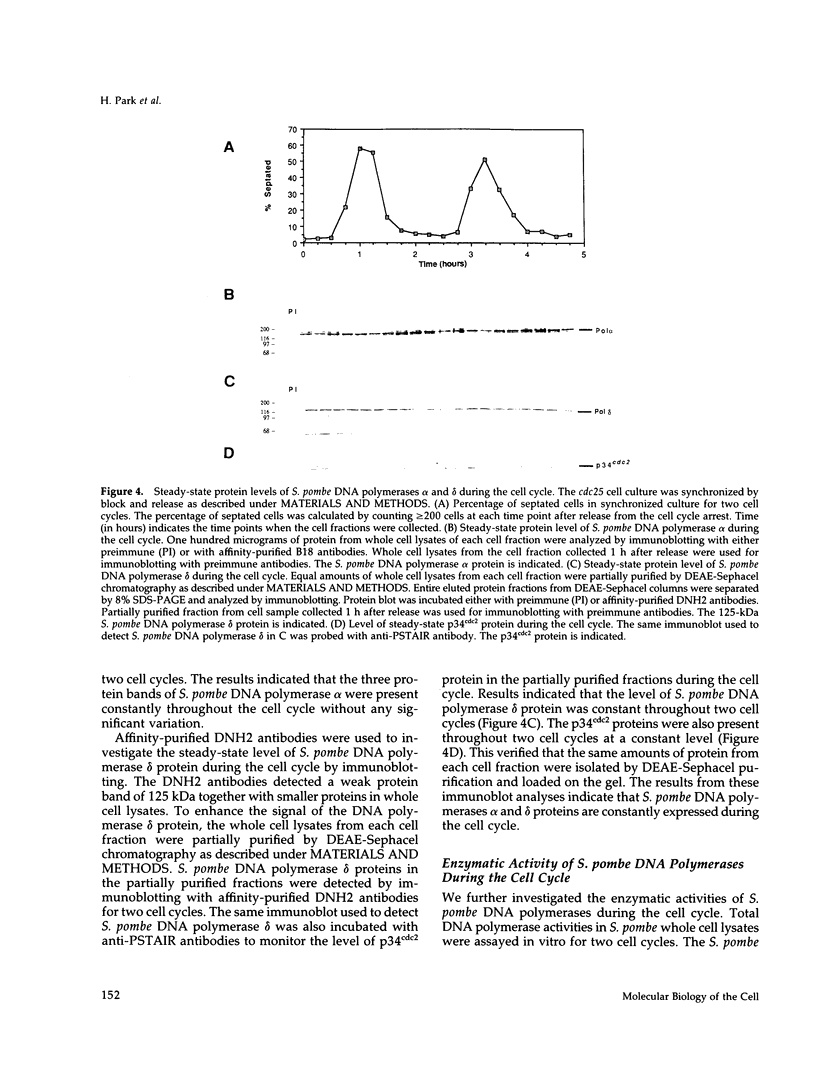
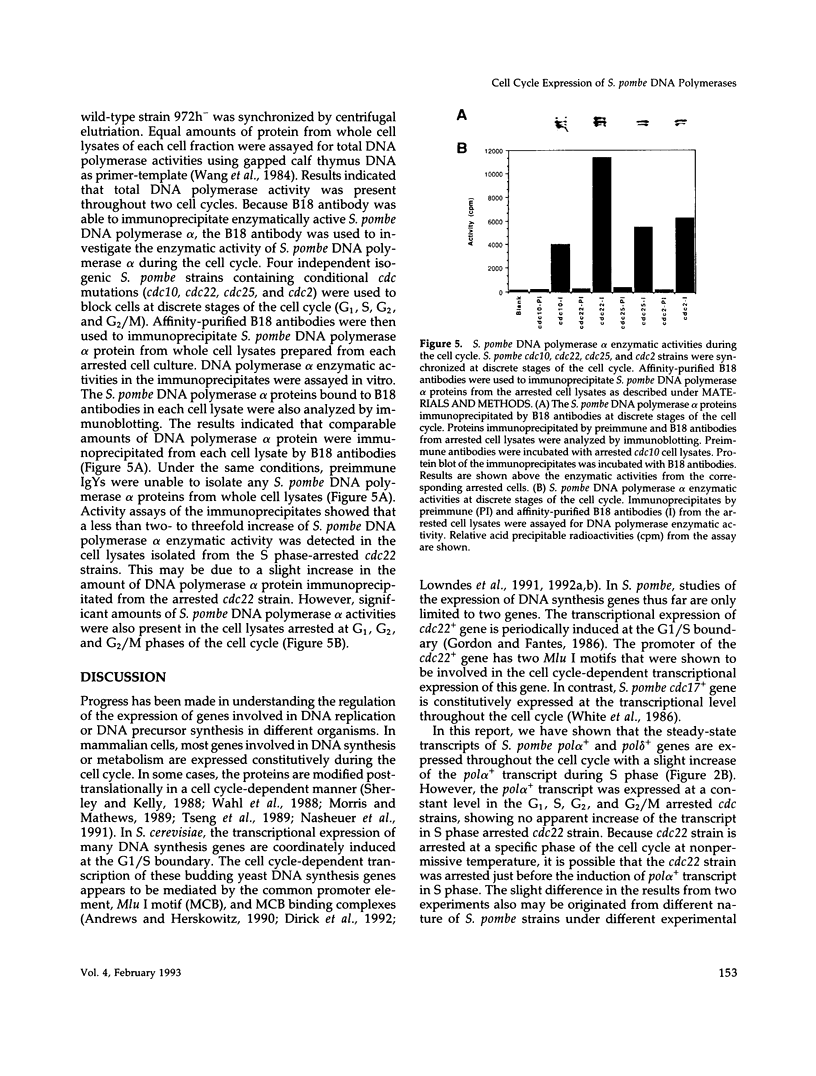
We have investigated the expression of two Schizosaccharomyces pombe replicative DNA polymerases alpha and delta during the cell cycle. The pol alpha+ and pol delta+ genes encoding DNA polymerases alpha and delta were isolated from S. pombe. Both pol alpha+ and pol delta+ genes are single copy genes in haploid cells and are essential for cell viability. In contrast to Saccharomyces cerevisiae homologs, the steady-state transcripts of both S. pombe pol alpha+ and pol delta+ genes were present throughout the cell cycle. Sequence analysis of the pol alpha+ and pol delta+ genes did not reveal the Mlu I motifs in their upstream sequences that are involved in cell cycle-dependent transcription of S. cerevisiae DNA synthesis genes as well as the S. pombe cdc22+ gene at the G1/S boundary. However, five near-match Mlu I motifs were found in the upstream region of the pol alpha+ gene. S. pombe DNA polymerases alpha and delta proteins were also expressed constantly throughout the cell cycle. In addition, the enzymatic activity of the S. pombe DNA polymerase alpha measured by in vitro assay was detected at all stages of the cell cycle. Thus, these S. pombe replicative DNA polymerases, like that of S. pombe cdc17+ gene, are expressed throughout the cell cycle at the transcriptional and protein level. These results indicate that S. pombe has at least two regulatory modes for the expression of genes involved in DNA replication and DNA precursor synthesis.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. J., Herskowitz I. Regulation of cell cycle-dependent gene expression in yeast. J Biol Chem. 1990 Aug 25;265(24):14057–14060. [PubMed] [Google Scholar]
- Araki H., Ropp P. A., Johnson A. L., Johnston L. H., Morrison A., Sugino A. DNA polymerase II, the probable homolog of mammalian DNA polymerase epsilon, replicates chromosomal DNA in the yeast Saccharomyces cerevisiae. EMBO J. 1992 Feb;11(2):733–740. doi: 10.1002/j.1460-2075.1992.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G. A., Burgers P. M. Molecular cloning, structure and expression of the yeast proliferating cell nuclear antigen gene. Nucleic Acids Res. 1990 Jan 25;18(2):261–265. doi: 10.1093/nar/18.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet A., Simon M., Faye G., Bauer G. A., Burgers P. M. Structure and function of the Saccharomyces cerevisiae CDC2 gene encoding the large subunit of DNA polymerase III. EMBO J. 1989 Jun;8(6):1849–1854. doi: 10.1002/j.1460-2075.1989.tb03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeden L., Nasmyth K. Similarity between cell-cycle genes of budding yeast and fission yeast and the Notch gene of Drosophila. Nature. 1987 Oct 15;329(6140):651–654. doi: 10.1038/329651a0. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Stillman B. Replication factor-A from Saccharomyces cerevisiae is encoded by three essential genes coordinately expressed at S phase. Genes Dev. 1991 Sep;5(9):1589–1600. doi: 10.1101/gad.5.9.1589. [DOI] [PubMed] [Google Scholar]
- Budd M. E., Wittrup K. D., Bailey J. E., Campbell J. L. DNA polymerase I is required for premeiotic DNA replication and sporulation but not for X-ray repair in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):365–376. doi: 10.1128/mcb.9.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Damagnez V., Tillit J., de Recondo A. M., Baldacci G. The POL1 gene from the fission yeast, Schizosaccharomyces pombe, shows conserved amino acid blocks specific for eukaryotic DNA polymerases alpha. Mol Gen Genet. 1991 Apr;226(1-2):182–189. doi: 10.1007/BF00273602. [DOI] [PubMed] [Google Scholar]
- Din S., Brill S. J., Fairman M. P., Stillman B. Cell-cycle-regulated phosphorylation of DNA replication factor A from human and yeast cells. Genes Dev. 1990 Jun;4(6):968–977. doi: 10.1101/gad.4.6.968. [DOI] [PubMed] [Google Scholar]
- Dirick L., Moll T., Auer H., Nasmyth K. A central role for SWI6 in modulating cell cycle Start-specific transcription in yeast. Nature. 1992 Jun 11;357(6378):508–513. doi: 10.1038/357508a0. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Davis R. W. Two genes differentially regulated in the cell cycle and by DNA-damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev. 1990 May;4(5):740–751. doi: 10.1101/gad.4.5.740. [DOI] [PubMed] [Google Scholar]
- Fantes P. Epistatic gene interactions in the control of division in fission yeast. Nature. 1979 May 31;279(5712):428–430. doi: 10.1038/279428a0. [DOI] [PubMed] [Google Scholar]
- Fantes P., Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977 Jul;107(2):377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Foiani M., Santocanale C., Plevani P., Lucchini G. A single essential gene, PRI2, encodes the large subunit of DNA primase in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Jul;9(7):3081–3087. doi: 10.1128/mcb.9.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Nurse P. Cell cycle regulation in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Annu Rev Cell Biol. 1991;7:227–256. doi: 10.1146/annurev.cb.07.110191.001303. [DOI] [PubMed] [Google Scholar]
- Gassmann M., Thömmes P., Weiser T., Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990 May;4(8):2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- Gordon C. B., Campbell J. L. A cell cycle-responsive transcriptional control element and a negative control element in the gene encoding DNA polymerase alpha in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6058–6062. doi: 10.1073/pnas.88.14.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. B., Fantes P. A. The cdc22 gene of Schizosaccharomyces pombe encodes a cell cycle-regulated transcript. EMBO J. 1986 Nov;5(11):2981–2985. doi: 10.1002/j.1460-2075.1986.tb04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi K. L., Copeland W. C., Wang T. S. Human DNA polymerase alpha catalytic polypeptide binds ConA and RCA and contains a specific labile site in the N-terminus. Nucleic Acids Res. 1990 Nov 11;18(21):6231–6237. doi: 10.1093/nar/18.21.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J., Dean F. B., Kwong A. D., Lee S. H. The in vitro replication of DNA containing the SV40 origin. J Biol Chem. 1990 Oct 25;265(30):18043–18046. [PubMed] [Google Scholar]
- Johnson L. M., Snyder M., Chang L. M., Davis R. W., Campbell J. L. Isolation of the gene encoding yeast DNA polymerase I. Cell. 1985 Nov;43(1):369–377. doi: 10.1016/0092-8674(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., Eberly S. L., Chapman J. W., Araki H., Sugino A. The product of the Saccharomyces cerevisiae cell cycle gene DBF2 has homology with protein kinases and is periodically expressed in the cell cycle. Mol Cell Biol. 1990 Apr;10(4):1358–1366. doi: 10.1128/mcb.10.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. H., White J. H., Johnson A. L., Lucchini G., Plevani P. Expression of the yeast DNA primase gene, PRI1, is regulated within the mitotic cell cycle and in meiosis. Mol Gen Genet. 1990 Mar;221(1):44–48. doi: 10.1007/BF00280366. [DOI] [PubMed] [Google Scholar]
- Johnston L. H., White J. H., Johnson A. L., Lucchini G., Plevani P. The yeast DNA polymerase I transcript is regulated in both the mitotic cell cycle and in meiosis and is also induced after DNA damage. Nucleic Acids Res. 1987 Jul 10;15(13):5017–5030. doi: 10.1093/nar/15.13.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Johnson A. L., Breeden L., Johnston L. H. SWI6 protein is required for transcription of the periodically expressed DNA synthesis genes in budding yeast. Nature. 1992 Jun 11;357(6378):505–508. doi: 10.1038/357505a0. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., Johnson A. L., Johnston L. H. Coordination of expression of DNA synthesis genes in budding yeast by a cell-cycle regulated trans factor. Nature. 1991 Mar 21;350(6315):247–250. doi: 10.1038/350247a0. [DOI] [PubMed] [Google Scholar]
- Lowndes N. F., McInerny C. J., Johnson A. L., Fantes P. A., Johnston L. H. Control of DNA synthesis genes in fission yeast by the cell-cycle gene cdc10+. Nature. 1992 Jan 30;355(6359):449–453. doi: 10.1038/355449a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Yanagida M. Histone gene organization of fission yeast: a common upstream sequence. EMBO J. 1985 Dec 16;4(13A):3531–3538. doi: 10.1002/j.1460-2075.1985.tb04113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh E. M., Atkinson T., Storms R. K., Smith M. Characterization of a short, cis-acting DNA sequence which conveys cell cycle stage-dependent transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1991 Jan;11(1):329–337. doi: 10.1128/mcb.11.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Morris G. F., Mathews M. B. Regulation of proliferating cell nuclear antigen during the cell cycle. J Biol Chem. 1989 Aug 15;264(23):13856–13864. [PubMed] [Google Scholar]
- Nasheuer H. P., Moore A., Wahl A. F., Wang T. S. Cell cycle-dependent phosphorylation of human DNA polymerase alpha. J Biol Chem. 1991 Apr 25;266(12):7893–7903. [PubMed] [Google Scholar]
- Nasmyth K., Nurse P. Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1981;182(1):119–124. doi: 10.1007/BF00422777. [DOI] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Pearson B. E., Nasheuer H. P., Wang T. S. Human DNA polymerase alpha gene: sequences controlling expression in cycling and serum-stimulated cells. Mol Cell Biol. 1991 Apr;11(4):2081–2095. doi: 10.1128/mcb.11.4.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignède G., Bouvier D., de Recondo A. M., Baldacci G. Characterization of the POL3 gene product from Schizosaccharomyces pombe indicates inter-species conservation of the catalytic subunit of DNA polymerase delta. J Mol Biol. 1991 Nov 20;222(2):209–218. doi: 10.1016/0022-2836(91)90207-m. [DOI] [PubMed] [Google Scholar]
- Pizzagalli A., Valsasnini P., Plevani P., Lucchini G. DNA polymerase I gene of Saccharomyces cerevisiae: nucleotide sequence, mapping of a temperature-sensitive mutation, and protein homology with other DNA polymerases. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3772–3776. doi: 10.1073/pnas.85.11.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle J. R., Adams A. E., Drubin D. G., Haarer B. K. Immunofluorescence methods for yeast. Methods Enzymol. 1991;194:565–602. doi: 10.1016/0076-6879(91)94043-c. [DOI] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Sitney K. C., Budd M. E., Campbell J. L. DNA polymerase III, a second essential DNA polymerase, is encoded by the S. cerevisiae CDC2 gene. Cell. 1989 Feb 24;56(4):599–605. doi: 10.1016/0092-8674(89)90582-5. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Storms R. K., Ord R. W., Greenwood M. T., Mirdamadi B., Chu F. K., Belfort M. Cell cycle-dependent expression of thymidylate synthase in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Dec;4(12):2858–2864. doi: 10.1128/mcb.4.12.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng B. Y., Prussak C. E., Almazan M. T. Primase p49 mRNA expression is serum stimulated but does not vary with the cell cycle. Mol Cell Biol. 1989 May;9(5):1940–1945. doi: 10.1128/mcb.9.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J Biol Chem. 1991 Jan 25;266(3):1950–1960. [PubMed] [Google Scholar]
- Wahl A. F., Geis A. M., Spain B. H., Wong S. W., Korn D., Wang T. S. Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol Cell Biol. 1988 Nov;8(11):5016–5025. doi: 10.1128/mcb.8.11.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S. Eukaryotic DNA polymerases. Annu Rev Biochem. 1991;60:513–552. doi: 10.1146/annurev.bi.60.070191.002501. [DOI] [PubMed] [Google Scholar]
- Wang T. S., Hu S. Z., Korn D. DNA primase from KB cells. Characterization of a primase activity tightly associated with immunoaffinity-purified DNA polymerase-alpha. J Biol Chem. 1984 Feb 10;259(3):1854–1865. [PubMed] [Google Scholar]
- White J. H., Barker D. G., Nurse P., Johnston L. H. Periodic transcription as a means of regulating gene expression during the cell cycle: contrasting modes of expression of DNA ligase genes in budding and fission yeast. EMBO J. 1986 Jul;5(7):1705–1709. doi: 10.1002/j.1460-2075.1986.tb04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H., Green S. R., Barker D. G., Dumas L. B., Johnston L. H. The CDC8 transcript is cell cycle regulated in yeast and is expressed coordinately with CDC9 and CDC21 at a point preceding histone transcription. Exp Cell Res. 1987 Jul;171(1):223–231. doi: 10.1016/0014-4827(87)90265-5. [DOI] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright A., Maundrell K., Heyer W. D., Beach D., Nurse P. Vectors for the construction of gene banks and the integration of cloned genes in Schizosaccharomyces pombe and Saccharomyces cerevisiae. Plasmid. 1986 Mar;15(2):156–158. doi: 10.1016/0147-619x(86)90051-x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chung D. W., Tan C. K., Downey K. M., Davie E. W., So A. G. Primary structure of the catalytic subunit of calf thymus DNA polymerase delta: sequence similarities with other DNA polymerases. Biochemistry. 1991 Dec 24;30(51):11742–11750. doi: 10.1021/bi00115a002. [DOI] [PubMed] [Google Scholar]