Diffusion-weighted MR imaging with subsequent apparent diffusion coefficient measurement was an efficient technique for detecting and monitoring histopathologic changes during the progression of renal fibrosis in a murine model of unilateral ureteral obstruction.
Abstract
Purpose:
To test, in a murine model of unilateral ureteral obstruction (UUO), whether the magnetic resonance (MR) imaging–derived apparent diffusion coefficient (ADC) changes during the progression of renal fibrosis and correlates with the histopathologic changes observed in renal fibrogenesis.
Materials and Methods:
This study was approved by the institutional animal care and use committee. A UUO was created in each of 14 mice. In five mice, longitudinal diffusion-weighted (DW) imaging was performed before the UUO (day 0) and on days 3 and 7 after the UUO and was followed by histopathologic analysis. The nine remaining mice were examined with cross-sectional studies on days 0 (n = 4) and 3 (n = 5). ADCs were measured with a spin-echo echo-planar sequence at five b values ranging from 350 to 1200 sec/mm2. Differences in ADC among the time points and between the sides were assessed by using Tukey-Kramer and Student t tests, respectively. ADC was correlated with cell density and α–smooth muscle actin (α-SMA, a marker of myofibroblasts) expression at linear regression analysis.
Results:
Histopathologic examination revealed typical renal fibrosis on the side with UUO. The ADC decreased over time on the UUO side, from (1.02 ± 0.06 [standard deviation]) × 10−3 mm2/sec on day 0 to (0.70 ± 0.08) × 10−3 mm2/sec on day 3 (P < .001) and (0.57 ± 0.10) × 10−3 mm2/sec on day 7 (P < .001). The percentage change in ADC was greater on the UUO side than on the contralateral side on days 3 (29% ± 9, P = .05) and 7 (44% ± 11, P < .01). ADC correlated with both increased cell density and increased α-SMA expression (P < .001 for both correlations).
Conclusion:
An ADC decrease in renal fibrosis is associated with an increased number of cells, including fibroblasts. ADC has the potential to serve as a sensitive noninvasive biomarker of renal fibrosis.
© RSNA, 2010
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.10091735/-/DC1
Introduction
Chronic kidney disease (CKD) is defined as a state of progressive decline in renal function over a time span of years (1). More than 26 million Americans—or 13%—have CKD, which can result in renal failure and is increasingly recognized as a major public health problem in the aging society (2). CKD may be caused by various renal diseases, including diabetic nephropathy, hypertensive nephropathy, and chronic glomerulonephritis. Regardless of the underlying renal disease, the final common abnormality of CKD is renal fibrosis (3). Hence, assessment of the presence and extent of renal fibrosis is important and may enable prediction of the long-term outcome of renal function in CKD (4–7). Currently, renal biopsy is the only method that renders a definitive diagnosis of renal fibrosis. However, it is invasive and subject to sampling error; therefore, it is difficult to monitor the progression of fibrosis in the clinical setting. It would be beneficial to establish a noninvasive technique to detect and monitor the progression of renal fibrosis.
Renal fibrosis has been investigated in a large number of histopathologic studies by using a well-established animal model of unilateral ureteral obstruction (UUO). UUO is a simple surgical procedure in which one of the two ureters is ligated to obstruct the urinary flow from the kidney (8,9). Ureteral obstruction increases hydrostatic pressure in the lumen of the upstream urinary tracts, including the pelvis, collecting ducts, and renal tubules, eventually resulting in renal fibrosis within approximately 1 week after the UUO (10). The histologic changes observed in the kidney with UUO include infiltration of inflammatory cells and proliferation of myofibroblasts in the interstitial space. Myofibroblasts are α–smooth muscle actin (α-SMA)-positive mesenchymal cells with migratory and proliferative phenotypes of tubular epithelial cells (9,11). Consequently, the number of cells in the interstitial space increases throughout the fibrogenic process, as observed in humans (12).
The magnetic resonance (MR) imaging measurement of the apparent diffusion coefficient (ADC) reflects water molecule movement (ie, Brownian motion), which is restricted in biologic conditions. The ADC has demonstrated sensitivity to cellularity: Dense cells provide more restriction of movement, and, thus, the ADC decreases (13). Accordingly, ADC measurement has been implemented for the assessment of brain and extracranial tumors in clinical settings (14–18). We hypothesized that ADC decreases during the progression of renal fibrosis, reflecting the accumulation of cells. In our study, we sought to determine, in a murine model of UUO, whether the ADC changes during the progression of renal fibrosis and correlates with the histopathologic changes observed during renal fibrogenesis.
Materials and Methods
Animal Protocol
The animal protocols were approved by the institutional animal care and use committee of UT Southwestern Medical Center, and the experiments were performed in accordance with the National Institutes of Health guidelines on the use of laboratory animals. Fourteen 8-week-old C57BL/6 J mice (Jackson Laboratory, Bar Harbor, Me) with a mean body weight of 22.1 g ± 1.3 (standard deviation) were used. UUO was induced as described previously (19). Briefly, after a midline incision was made, the right ureter was exposed and permanently ligated twice with 4-0 nylon suture. The study design is shown in Figure 1. Five mice (group 1) were examined with MR imaging before the UUO was induced (day 0) and on days 3 and 7 after the UUO. For histopathologic correlation at each time point, DW imaging was performed in the nine remaining mice on days 0 (group 2, four mice) and 3 (group 3, five mice). All animals were sacrificed, and both kidneys were harvested after the final MR examination.
Figure 1:
Diagram illustrates study design and assignment of mice to study groups. Five mice in group 1 were examined with longitudinal diffusion-weighted (DW) imaging (solid arrows) before UUO (day 0) and on days 3 and 7 after UUO. For histopathologic correlation at each diffusion-weighted imaging time point (dotted arrows), an additional group of mice were examined on days 0 (group 2, four mice) and 3 (group 3, five mice).
MR Imaging
MR imaging was conducted by using a 7-T small-animal MR system (Varian, Palo Alto, Calif) with a 40-mm inner diameter Horizontal Millipede coil (Varian). All animals were anesthetized with 1%–2% isoflurane (Aerrane; Baxter Health Care, Deerfield, Ill) mixed in 100% oxygen. They were placed head first in the supine position in the system with a respirator sensor and with the abdomen centered with respect to the center of the radiofrequency coil. First, low-spatial-resolution multisection imaging of the abdominal region was performed to confirm the location and orientation of the kidneys. For volume measurements, axial T2-weighted multisection images encompassing the ipsilateral and contralateral kidneys were obtained with a fast spin-echo sequence (2500/40 [repetition time msec/echo time msec], 30 × 30-mm field of view, 128 × 128 matrix, 1-mm section thickness, no intersection gap, four signals acquired, fat suppression). DW imaging was performed three times by using a multishot spin-echo echo-planar imaging sequence, with a single 1-mm coronal slab delineating both kidneys. Motion-sensitive gradients were applied (diffusion time, 20 msec; gradient pulse duration, 5 msec) with five b values—350, 600, 800, 1000 and 1200 sec/mm2—in each orthogonal direction (section, readout, and phase encode). Other parameters were 3000/38, a field of view of 30 × 30 mm, a matrix of 64 × 64, a section thickness of 1 mm, no intersection gap, two signals acquired, an echo-planar imaging factor of 16, and fat suppression. To minimize motion artifacts, respiratory gating was performed with an MR imaging–compatible small-animal respiratory gating device (SA Instruments, Stony Brook, NY). The total image acquisition time for each animal was approximately 30 minutes.
MR Data Analysis
Volume measurement.—Volume measurements were performed in the group 1 mice by using axial T2-weighted MR images. The renal parenchyma (cortex and medulla) and pelvis on both the UUO side and the contralateral side were manually segmented, and the volumes were measured by image-processing software (ImageJ, version 1.40g; National Institutes of Health, Bethesda, Md).
ADC measurement.—For all animals (mice in groups 1–3), ADC maps were generated pixel by pixel on the basis of the slope of the logarithm of the five signal intensities versus the b values by using the image-processing software. Subsequently, three ADC maps in each gradient direction were averaged for each animal. The ADC was measured in the renal cortex of each kidney delineated on the averaged ADC map in a coronal slab at five regions of interest. The five regions of interest were placed every 45° from the superior (0°) to the posterior (180°) poles, clockwise in the left kidney (contralateral side) and counterclockwise in the right kidney (UUO side). Each region of interest had an area of 2.5 mm2 and was placed at the center of the cortex. The average value for three ADCs after the maximal and minimal values of five measurements were excluded was representative of the kidney.
Histopathologic Examinations
At completion of the MR examinations, the kidneys were resected, fixed in buffered 10% formalin, embedded in paraffin, and sectioned into 5-μm-thick coronal slices by using a standard procedure. A coronal slice at the middle level on the anterior-posterior axis, which corresponded to the DW imaging slab, was selected. The sections were stained with hematoxylin-eosin for general histologic analysis and cell density measurements in the kidney. Immunohistochemical staining for expression of α-SMA and type 1 collagen was performed according to previously reported procedures (10).
Five fields on the coronal section were selected for the calculation of cell density. We determined the locations of these fields in the same way that we selected the five regions of interest for the ADC measurements to match the locations as closely as possible. In each selected location, the cells were counted automatically in a high power field (original magnification, ×400) by using the ImageJ software. First, a digitized high power field was converted into an 8-bit gray-scale image. A binary image was then derived by using a threshold value estimated from the histogram of the original image so that cell nuclei could be extracted. By using the “analyze particle” function in the software, the cells in a unit of area in a high power field (0.137 mm2) could be automatically counted to calculate the cell density. A value averaged from three measurements (excluding the maximal and minimal values of five measurements) was considered to be the representative cell density of the kidney.
Western Blot Analysis of α-SMA
For detection of α-SMA, one-third of the kidney was homogenized on ice in 0.85 mL of homogenizing buffer (20 mM HEPES, 100 mM NaCl, 0.5 mM edetic acid; pH 7.4) that contained a protease inhibitor cocktail (Sigma-Aldrich, St Louis, Mo). After the addition of nonionic surfactant (Triton X; Sigma-Aldrich) (final concentration, 1.5%), the homogenate was incubated for 30 minutes at 4°C and then centrifuged at 13500g for 12 minutes to remove tissue debris. The protein content of the cell lysate was determined by performing the bicinchoninic acid protein assay (Pierce, Rockford, Ill).
Lysates (10 μg per lane) were subjected to sodium dedecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After blocking with 5% nonfat milk powder and 2% fetal bovine serum in tris(hydroxymethyl) aminomethane–buffered saline containing 0.1% Tween 20 detergent (Sigma-Aldrich), the blots were incubated for 1 hour at room temperature with anti–α-SMA antibody (Sigma-Aldrich) at a 1:1000 dilution. After being washed with tris(hydroxymethyl) aminomethane–buffered saline containing 0.1% Tween 20, the membrane was incubated for 1 hour at room temperature with a horseradish peroxidase conjugated sheep antimouse immunoglobulin G antibody (GE Healthcare, Little Chalfont Buckinghamshire, England) at a 1:5000 dilution. A monoclonal anti–α-tubulin antibody (Sigma-Aldrich) was used as an internal control. The signals were detected by using the Super Signal West Dura system (Pierce). The intensity of each signal was quantified by using image software (Scion, Frederick, Md).
Statistical Analysis
All values were expressed as means ± standard deviations. Comparisons of absolute volumes and ADCs measured in the kidneys at three time points were made by using one-way analysis of variance followed by post hoc multiple comparisons with the Tukey-Kramer test. In the longitudinal assessment (group 1), percentage changes in the volume or ADC relative to the value on day 0 were calculated on days 3 and 7 and tested by using the Student t test to compare the UUO side with the contralateral side at each time point. To examine the relationships between ADC and either cell density or α-SMA expression in all analyzed kidneys, a simple linear regression analysis was performed. All statistical analyses were performed by using commercially available software (Prism 5.0; GraphPad Software, San Diego, Calif), and P ≤ .05 was considered to indicate a statistically significant difference.
Results
Volume Measurements
On T2-weighted MR images, dilatation of the renal pelvis on the UUO side was observed on day 3 after induced UUO and was exacerbated on day 7, while there was no marked change on the contralateral side (Fig E1 [online]). The mean volume of the renal pelvis on the UUO side rapidly increased after the UUO, from 5.2 mm3 ± 0.9 on day 0 to 35.7 mm3 ± 6.0 mm3 on day 3 and 84.8 mm3 ± 11.4 on day 7, with significant differences in values between days 0 and 3 and between day 7 and days 0 and 3 (P < .001 for all comparisons). There was no marked change over time on the contralateral side, with mean volumes of 5.0 mm3 ± 1.7 on day 0, 4.7 mm3 ± 1.0 on day 3, and 5.0 mm3 ± 0.8 on day 7. The volume of the parenchyma on the UUO side tended to decrease by day 7, with mean volumes of 170.0 mm3 ± 16.1 on day 0, 170.3 mm3 ± 16.6 on day 3, and 149.6 mm3 ± 16.6 on day 7. In contrast, the parenchymal volume on the contralateral side was increased by day 7, with mean volumes of 150.7 mm3 ± 5.2 on day 0, 157.9 mm3 ± 16.2 on day 3, and 172.2 mm3 ± 8.8 on day 7 (P < .05), implying adaptive changes to compensate for the renal dysfunction on the UUO side. As a result, there were significant differences in the percentage change in volume relative to the baseline value between the UUO and contralateral sides on days 3 (P < .001) and 7 (P < .001) in the pelvis and on day 7 (P < .01) in the parenchyma (Fig E1 [online]).
ADC Measurements
Absolute renal cortex ADCs measured in all kidneys (mouse groups 1–3) are summarized in the Table. The ADC was found to decrease over time on the UUO side, whereas it was consistent over time on the contralateral side. In the group 1 mice (Fig 2), the UUO side had mean reductions in ADC of 29% ± 9 on day 3 (to [0.73 ± 0.09] × 10−3 mm2/sec) and 44% ± 11 on day 7 (to [0.57 ± 0.10] × 10−3 mm2/sec) compared with the baseline ADC ([1.04 ± 0.04] × 10−3 mm2/sec), whereas there was no marked change on the contralateral side. Significant differences in percentage change in ADC between the two sides were observed on days 3 (P = .05) and 7 (P < .01).
Renal Cortex ADCs before and after UUO
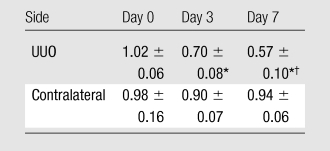
Note.—Data are mean ADCs (in ×10−3 mm2/sec) ± standard deviations. MR-derived ADC measurements were obtained in nine mice on day 0, in 10 mice on day 3, and in five mice on day 7.
P < .001 for comparison with day 0 value (Tukey-Kramer multiple comparison test).
P < .05 for comparison with day 3 value (Tukey-Kramer multiple comparison test).
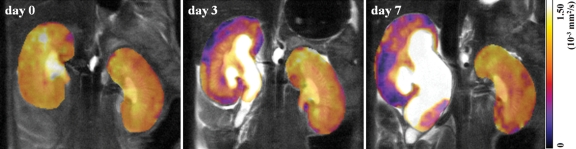
Figure 2a:
(a) Typical coronal ADC maps of the kidneys in a mouse in group 1 before UUO (day 0) and on days 3 and 7 after UUO. ADCs were averaged over three orthogonal directions to eliminate possible anisotropic effect. (b) Graph illustrates changes in renal cortex ADC, which decreased in a time-dependent manner on the UUO side (five mice in group 1). All data are expressed as percentage changes, in means ± standard deviations, relative to baseline value, with P = .05 (*)and P < .01 (**) for comparisons of UUO and contralateral sides at unpaired two-tailed Student t testing.
Figure 2b:
(a) Typical coronal ADC maps of the kidneys in a mouse in group 1 before UUO (day 0) and on days 3 and 7 after UUO. ADCs were averaged over three orthogonal directions to eliminate possible anisotropic effect. (b) Graph illustrates changes in renal cortex ADC, which decreased in a time-dependent manner on the UUO side (five mice in group 1). All data are expressed as percentage changes, in means ± standard deviations, relative to baseline value, with P = .05 (*) and P < .01 (**) for comparisons of UUO and contralateral sides at unpaired two-tailed Student t testing.
Histopathologic Analysis
Hematoxylin-eosin staining revealed a progressive increase in cells in the interstitial space on the UUO side (Fig 3). Abnormal changes such as tubular atrophy, tubular dilatation, and expanded interstitial space filled with massive cells were evident on day 7. Immunohistochemical staining revealed an increase in α-SMA–positive myofibroblasts (Fig 4) and the deposition of extracellular matrix (type 1 collagen) (Fig E2 [online]). No abnormal changes were observed in the pre-UUO or contralateral kidneys.
Figure 3a:
(a) Micrographs of renal cortex before UUO (day 0) and on days 3 and 7 after UUO show evolution of massive cells. (Hematoxylin-eosin stain; original magnification, ×400.) (b) A field in the day 7 sample (magnification, ×400) demonstrates morphologic changes manifested by tubular atrophy (arrows), tubular dilatation (*), and expanded interstitial space filled with numerous cells (+). Bars = 100 μm.
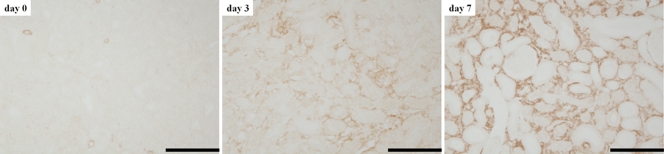
Figure 4:
Renal cortex specimens immunohistochemically stained for α-SMA expression (original magnification, ×400) before UUO (day 0) and on days 3 and 7 after UUO. Stained area and cell density gradually increase over time, indicating proliferation of α-SMA–positive myofibroblasts. Bars = 100 μm.
Figure 3b:
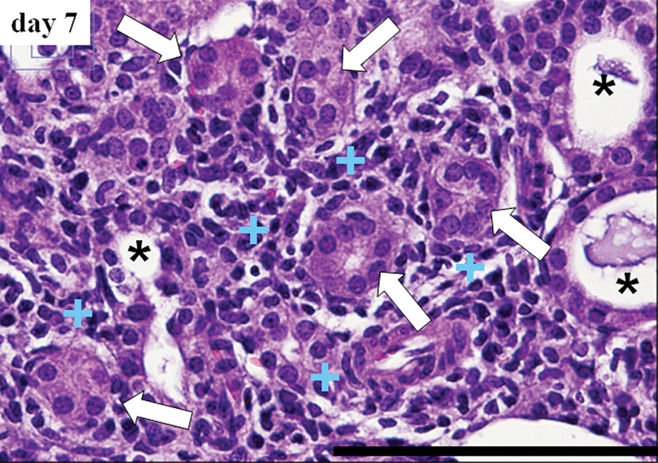
(a) Micrographs of renal cortex before UUO (day 0) and on days 3 and 7 after UUO show evolution of massive cells. (Hematoxylin-eosin stain; original magnification, ×400.) (b) A field in the day 7 sample (magnification, ×400) demonstrates morphologic changes manifested by tubular atrophy (arrows), tubular dilatation (*), and expanded interstitial space filled with numerous cells (+). Bars = 100 μm.
Cell Density and Relationship with ADC
The mean cell density was markedly increased over time on the UUO side, whereas there was no obvious change on the contralateral side (Fig 5). Cell density on the UUO side was greater than that on the contralateral side on days 3 and 7 (P < .001 for both comparisons). The kidneys without UUO (on day 0 and on contralateral side) and the kidneys with UUO were clearly dissociated in terms of ADC ([0.81–1.08] × 10−3 mm2/sec vs [0.47–0.76] × 10−3 mm2/sec) and cell density ([6.3–7.9] × 103 cells/mm2 vs [9.0–13.0] × 103 cells/mm2 [Fig 5]). There was a strong correlation between ADC and cell density (P < .001, R2 = 0.74) (Fig 5) .
Figure 5a:
(a) Bar graph illustrates mean cell density in renal cortex before UUO (day 0, eight kidneys) and on days 3 (five kidneys for each side) and 7 (five kidneys for each side) after UUO. On day 0, data were obtained in both kidneys as they were intact (eight kidneys), with P ≤ .001 (***) for comparison of UUO and contralateral (contra)sides at unpaired two-tailed Student t testing. (b) Graph illustrates relationship between ADC and cell density, which were measured in corresponding locations and had a strong correlation (P < .001, R2 = 0.74; simple regression analysis).
Figure 5b:
(a) Bar graph illustrates mean cell density in renal cortex before UUO (day 0, eight kidneys) and on days 3 (five kidneys for each side) and 7 (five kidneys for each side) after UUO. On day 0, data were obtained in both kidneys as they were intact (eight kidneys), with P ≤ .001 (***) for comparison of UUO and contralateral (contra)sides at unpaired two-tailed Student t testing. (b) Graph illustrates relationship between ADC and cell density, which were measured in corresponding locations and had a strong correlation (P < .001, R2 = 0.74; simple regression analysis).
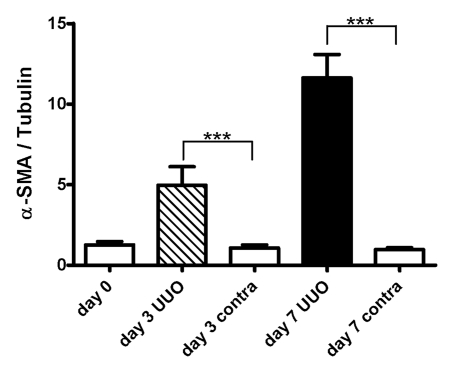
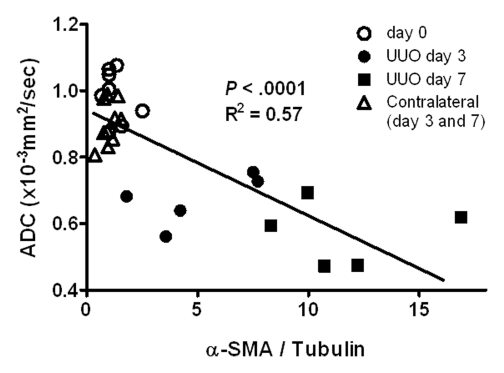
α-SMA Measurements and Relationship with ADC
The mean α-SMA expression on the UUO side increased progressively during the experimental period, whereas no change was observed on the contralateral side (Fig 6). α-SMA expression on the UUO side was greater than that on the contralateral side on days 3 and 7 (P < .001 for both comparisons). Simple regression analysis revealed a significant correlation between ADC and α-SMA expression (P < .001, R2 = 0.57) (Fig 6).
Figure 6a:
(a) Western blot analysis of α-SMA expression. Upper panels were probed with antibody that recognizes α-SMA. Lower panels were probed with antibody that recognizes tubulin, with tubulin expression used as internal control. (b) Graph illustrates mean amounts of α-SMA in kidney (α-SMA-to-tubulin expression ratios) before UUO (day 0, eight kidneys) and on days 3 (five kidneys for each side) and 7 (five kidneys for each side) after UUO. On day 0, data were obtained in both kidneys as they were intact (eight kidneys), with P < .001 (***) for comparisons of UUO and contralateral sides at unpaired two-tailed Student t testing. (c) Graph illustrates relationship between ADC and total amount of α-SMA; data indicate a strong correlation (P < .001, R2 = 0.57; simple regression analysis).
Figure 6b:
(a) Western blot analysis of α-SMA expression. Upper panels were probed with antibody that recognizes α-SMA. Lower panels were probed with antibody that recognizes tubulin, with tubulin expression used as internal control. (b) Graph illustrates mean amounts of α-SMA in kidney (α-SMA-to-tubulin expression ratios) before UUO (day 0, eight kidneys) and on days 3 (five kidneys for each side) and 7 (five kidneys for each side) after UUO. On day 0, data were obtained in both kidneys as they were intact (eight kidneys), with P < .001 (***) for comparisons of UUO and contralateral sides at unpaired two-tailed Student t testing. (c) Graph illustrates relationship between ADC and total amount of α-SMA; data indicate a strong correlation (P < .001, R2 = 0.57; simple regression analysis).
Figure 6c:
(a) Western blot analysis of α-SMA expression. Upper panels were probed with antibody that recognizes α-SMA. Lower panels were probed with antibody that recognizes tubulin, with tubulin expression used as internal control. (b) Graph illustrates mean amounts of α-SMA in kidney (α-SMA-to-tubulin expression ratios) before UUO (day 0, eight kidneys) and on days 3 (five kidneys for each side) and 7 (five kidneys for each side) after UUO. On day 0, data were obtained in both kidneys as they were intact (eight kidneys), with P < .001 (***) for comparisons of UUO and contralateral sides at unpaired two-tailed Student t testing. (c) Graph illustrates relationship between ADC and total amount of α-SMA; data indicate a strong correlation (P < .001, R2 = 0.57; simple regression analysis).
Discussion
In our study, we performed longitudinal and cross-sectional ADC measurements and rigorous histopathologic correlation in the kidney in mice during fibrogenic progression that was induced by UUO. Local ADCs in the renal parenchyma rapidly decreased after UUO to approximately 70% of their initial value at day 3, and they kept decreasing until they reached 55% of their initial value on day 7. In the longitudinal study in group 1, mean ADCs on days 0, 3, and 7 were (1.04 ± 0.04) × 10−3 mm2/sec, (0.73 ± 0.09) × 10−3 mm2/sec, and (0.57 ± 0.10) × 10−3 mm2/sec, respectively. ADCs on the contralateral side remained constant. Histologic examination revealed progressive accumulation of α-SMA–positive myofibroblasts, collagen deposition, and tubular atrophy, which are the typical findings of renal fibrogenesis observed both in UUO murine models (8,9) and in patients with CKD (20). Renal cortex ADC correlated highly with local cell density and total amount of α-SMA protein expression. These results provide strong evidence that in this application, the ADC reflects diffusion changes related to the process of renal fibrogenesis.
The strong correlations between ADC and both cell density and α-SMA expression indicate that an increase in cell density leads to a decrease in ADC in the renal parenchyma during fibrogenesis. It is widely accepted that increased cell density causes reduced ADC (13). In fact, ADC measurements have been used to assess cellularity in the brain and extracranial tumors in clinical settings (14–18). High cellularity causes an increase in the density of cell membranes, which is the sole mechanism that restricts water molecule movement (21,22). In addition, high cell density causes decreases in the extracellular water fraction and reciprocally increases the intracellular water fraction, which in turn exhibits a lower ADC (23). Therefore, we believe that the predominant cause of the lower ADC in the fibrotic kidneys was the increase in density of cell membranes and associated increase in intracellular water fraction. One could also argue that collagen deposition may contribute to reduced ADC; however, the issue of whether collagen deposition can cause reduced MR-derived ADCs is still controversial (24–26).
Several investigators have reported the use of diffusion-weighted MR imaging in normal human kidneys (27–31). The ADCs in these studies, however, had large variations and often were higher ([2.0–5.0] × 10−3 mm2/sec) than the ADCs of nonrestricted water diffusion, (2.0–2.5) × 10−3 mm2/sec (32). Yamada et al (33) stated that the ADC measured by using low b values (0–300 sec/mm2) was contaminated by the effect of local blood and urinary flow in the kidney and reported that the mean ADC for the normal kidneys in humans was (1.38 ± 0.29) × 10−3 mm2/sec when it was measured with b values greater than 300 sec/mm2. According to our measurements, the mean ADC for the kidneys in normal mice (day 0 in Table) seems somewhat lower than the reported values measured with high b values in humans (33,34). In our study, we used five b values ranging from 350 to 1200 sec/mm2 to calculate the ADC as the slope (R2 > 0.9) of the logarithm of the signal intensities; our values did not appear to be affected by the flow effect. Furthermore, we averaged three ADCs calculated in each orthogonal direction to eliminate any possible anisotropic effect that has been shown to be associated with renal ADC (34). We attribute this difference to the difference in species (human vs mice) and/or the minimization of motion artifacts achieved by using respiratory gating in our ADC measurements. It has been shown that at a lower signal-to-noise ratio (< 20) and with use of log-linear fitting, ADCs tend to be underestimated, especially when they are measured at larger b values (35). Therefore, besides the difference in species, noise bias could have caused the lower ADCs.
There are, to our knowledge, only five prior studies of the correlation between kidney ADC and renal function in patients with CKDs (30,34,36–38). Although in four of these five studies, the investigators measured the ADCs by using low b values (0–300 sec/mm2), all of these studies revealed a substantial reduction in ADC, which correlated with the renal dysfunction severity determined according to serum creatinine level and/or glomerular filtration rate. Although these studies did not include histologic examination, we postulate that renal fibrogenesis could have been a cause of the reduced ADC in the patients with CKDs despite the variety of underlying etiologies.
In previously reported studies, the size or volume of the kidney in patients was measured to evaluate the presence or severity of CKD (39,40), where the kidney atrophy paralleled the deterioration of renal function. In our study, the volume of the renal parenchyma on the UUO side did not markedly change by day 7. This might indicate that the ADC in the kidney changes during the earlier stages of renal fibrogenesis and can be a sensitive biomarker before an atrophic reduction in kidney tissue is observed.
There currently is no clinically available treatment that is effective in halting the progressive loss of renal function in patients with CKD. However, reports suggest that candidate drugs for therapeutic use are under development (41,42). By establishing this UUO animal model of renal fibrosis, we may, by using ADC measurements, be able to screen drugs that are potentially efficacious in preventing the progression of renal disorders.
One of the limitations of this study was that no correlations between ADC measurements and standard tests of kidney function were made. Furthermore, our study lacked evidence of specificity for renal fibrosis: Other renal diseases such as acute inflammation (34), and neoplastic cells (43) in the kidney have yielded reduced ADCs due to cell density increases. However, diffusion-weighted imaging of the kidney combined with standard noninvasive tests should enable clinicians to distinguish renal fibrosis from other renal diseases.
In conclusion, diffusion-weighted MR imaging and subsequent ADC measurement can be an efficient technique for detecting and monitoring abnormal changes during the progression of renal fibrosis in a murine UUO model. Our findings suggest that the decreased ADC in renal fibrosis primarily results from the accumulation of cells, including myofibroblasts, in the kidney. ADC could serve as a noninvasive metric for examining patients with CKD.
Advances in Knowledge.
The apparent diffusion coefficient (ADC) measured with diffusion-weighted MR imaging decreased during the progression of renal fibrosis—from (1.02 ± 0.06) × 10−3 mm2/sec before unilateral ureteral obstruction was induced (day 0) to (0.70 ± 0.08) × 10−3 mm2/sec on day 3 and (0.57 ± 0.10) × 10−3 mm2/sec on day 7 after the obstruction.
The ADC decrease was closely related to the increase in cell density that resulted from the accumulation of cells, including fibroblasts in the interstitial space, which typically are observed during renal fibrogenesis.
Implication for Patient Care.
ADC may be a useful noninvasive biomarker for monitoring the progression of renal fibrosis in patients with chronic kidney disease.
Supplementary Material
Received September 16, 2009; revision requested October 20; revision received November 6; accepted November 25; final version accepted December 17.
Funding: This research was supported by the National Institutes of Health (grant RR02584-22719).
Authors stated no financial relationship to disclose.
See also Science to Practice in this issue.
Abbreviations:
- ADC
- apparent diffusion coefficient
- CKD
- chronic kidney disease
- α-SMA
- α–smooth muscle actin
- UUO
- unilateral ureteral obstruction
References
- 1.Levey AS, Stevens LA, Coresh J. Conceptual model of CKD: applications and implications. Am J Kidney Dis 2009;53(3suppl 3):S4–S16 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298(17):2038–2047 [DOI] [PubMed] [Google Scholar]
- 3.Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol 2009;296(6):F1239–F1244 [DOI] [PubMed] [Google Scholar]
- 4.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 1992;20(1):1–17 [DOI] [PubMed] [Google Scholar]
- 5.Bohle A, Wehrmann M, Bogenschütz O, Batz C, Müller CA, Müller GA. The pathogenesis of chronic renal failure in diabetic nephropathy: investigation of 488 cases of diabetic glomerulosclerosis. Pathol Res Pract 1991;187(2-3):251–259 [DOI] [PubMed] [Google Scholar]
- 6.Shiiki H, Saito T, Nishitani Y, et al. Prognosis and risk factors for idiopathic membranous nephropathy with nephrotic syndrome in Japan. Kidney Int 2004;65(4):1400–1407 [DOI] [PubMed] [Google Scholar]
- 7.Wehrmann M, Bohle A, Bogenschütz O, et al. Long-term prognosis of chronic idiopathic membranous glomerulonephritis: an analysis of 334 cases with particular regard to tubulo-interstitial changes. Clin Nephrol 1989;31(2):67–76 [PubMed] [Google Scholar]
- 8.Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int 2005;68(3):925–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 2009;75(11):1145–1152 [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Masaki T, Doi S, et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Invest 2009;89(1):47–58 [DOI] [PubMed] [Google Scholar]
- 11.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 2008;19(12):2282–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masaki T, Foti R, Hill PA, Ikezumi Y, Atkins RC, Nikolic-Paterson DJ. Activation of the ERK pathway precedes tubular proliferation in the obstructed rat kidney. Kidney Int 2003;63(4):1256–1264 [DOI] [PubMed] [Google Scholar]
- 13.Lyng H, Haraldseth O, Rofstad EK. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 2000;43(6):828–836 [DOI] [PubMed] [Google Scholar]
- 14.Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001;22(6):1081–1088 [PMC free article] [PubMed] [Google Scholar]
- 15.Zelhof B, Pickles M, Liney G, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int 2009;103(7):883–888 [DOI] [PubMed] [Google Scholar]
- 16.Humphries PD, Sebire NJ, Siegel MJ, Olsen OE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 2007;245(3):848–854 [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Tehrani YM, Wang L, Ishill NM, Schwartz LH, Hricak H. Renal masses: characterization with diffusion-weighted MR imaging—a preliminary experience. Radiology 2008;247(2):458–464 [DOI] [PubMed] [Google Scholar]
- 18.Marini C, Iacconi C, Giannelli M, Cilotti A, Moretti M, Bartolozzi C. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol 2007;17(10):2646–2655 [DOI] [PubMed] [Google Scholar]
- 19.Kuratsune M, Masaki T, Hirai T, et al. Signal transducer and activator of transcription 3 involvement in the development of renal interstitial fibrosis after unilateral ureteral obstruction. Nephrology (Carlton) 2007;12(6):565–571 [DOI] [PubMed] [Google Scholar]
- 20.De Heer E, Sijpkens YW, Verkade M, et al. Morphometry of interstitial fibrosis. Nephrol Dial Transplant 2000;15(suppl 6):72–73 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Hackney DB, Zhang G, et al. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci U S A 2002;99(25):16192–16196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szafer A, Zhong J, Gore JC. Theoretical model for water diffusion in tissues. Magn Reson Med 1995;33(5):697–712 [DOI] [PubMed] [Google Scholar]
- 23.Chin CL, Wehrli FW, Hwang SN, Takahashi M, Hackney DB. Biexponential diffusion attenuation in the rat spinal cord: computer simulations based on anatomic images of axonal architecture. Magn Reson Med 2002;47(3):455–460 [DOI] [PubMed] [Google Scholar]
- 24.Luciani A, Vignaud A, Cavet M, et al. Liver cirrhosis: intravoxel incoherent motion MR imaging—pilot study. Radiology 2008;249(3):891–899 [DOI] [PubMed] [Google Scholar]
- 25.Annet L, Peeters F, Abarca-Quinones J, Leclercq I, Moulin P, Van Beers BE. Assessment of diffusion-weighted MR imaging in liver fibrosis. J Magn Reson Imaging 2007;25(1):122–128 [DOI] [PubMed] [Google Scholar]
- 26.Taouli B, Tolia AJ, Losada M, et al. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol 2007;189(4):799–806 [DOI] [PubMed] [Google Scholar]
- 27.Fukuda Y, Ohashi I, Hanafusa K, et al. Anisotropic diffusion in kidney: apparent diffusion coefficient measurements for clinical use. J Magn Reson Imaging 2000;11(2):156–160 [DOI] [PubMed] [Google Scholar]
- 28.Müller MF, Prasad PV, Bimmler D, Kaiser A, Edelman RR. Functional imaging of the kidney by means of measurement of the apparent diffusion coefficient. Radiology 1994;193(3):711–715 [DOI] [PubMed] [Google Scholar]
- 29.Ries M, Jones RA, Basseau F, Moonen CT, Grenier N. Diffusion tensor MRI of the human kidney. J Magn Reson Imaging 2001;14(1):42–49 [DOI] [PubMed] [Google Scholar]
- 30.Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging 1999;9(6):832–837 [DOI] [PubMed] [Google Scholar]
- 31.Siegel CL, Aisen AM, Ellis JH, Londy F, Chenevert TL. Feasibility of MR diffusion studies in the kidney. J Magn Reson Imaging 1995;5(5):617–620 [DOI] [PubMed] [Google Scholar]
- 32.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168(2):497–505 [DOI] [PubMed] [Google Scholar]
- 33.Yamada I, Aung W, Himeno Y, Nakagawa T, Shibuya H. Diffusion coefficients in abdominal organs and hepatic lesions: evaluation with intravoxel incoherent motion echo-planar MR imaging. Radiology 1999;210(3):617–623 [DOI] [PubMed] [Google Scholar]
- 34.Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 2005;235(3):911–917 [DOI] [PubMed] [Google Scholar]
- 35.Kristoffersen A. Optimal estimation of the diffusion coefficient from non-averaged and averaged noisy magnitude data. J Magn Reson 2007;187(2):293–305 [DOI] [PubMed] [Google Scholar]
- 36.Carbone SF, Gaggioli E, Ricci V, Mazzei F, Mazzei MA, Volterrani L. Diffusion-weighted magnetic resonance imaging in the evaluation of renal function: a preliminary study. Radiol Med (Torino) 2007;112(8):1201–1210 [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging 2007;26(3):678–681 [DOI] [PubMed] [Google Scholar]
- 38.Toyoshima S, Noguchi K, Seto H, Shimizu M, Watanabe N. Functional evaluation of hydronephrosis by diffusion-weighted MR imaging: relationship between apparent diffusion coefficient and split glomerular filtration rate. Acta Radiol 2000;41(6):642–646 [DOI] [PubMed] [Google Scholar]
- 39.Khati NJ, Hill MC, Kimmel PL. The role of ultrasound in renal insufficiency: the essentials. Ultrasound Q 2005;21(4):227–244 [DOI] [PubMed] [Google Scholar]
- 40.Widjaja E, Oxtoby JW, Hale TL, Jones PW, Harden PN, McCall IW. Ultrasound measured renal length versus low dose CT volume in predicting single kidney glomerular filtration rate. Br J Radiol 2004;77(921):759–764 [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Liu Y. Delayed administration of hepatocyte growth factor reduces renal fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 2003;284(2):F349–F357 [DOI] [PubMed] [Google Scholar]
- 42.Sung SA, Jo SK, Cho WY, Won NH, Kim HK. Reduction of renal fibrosis as a result of liposome encapsulated clodronate induced macrophage depletion after unilateral ureteral obstruction in rats. Nephron Exp Nephrol 2007;105(1):e1–e9 [DOI] [PubMed] [Google Scholar]
- 43.Taouli B, Thakur RK, Mannelli L, et al. Renal lesions: characterization with diffusion-weighted imaging versus contrast-enhanced MR imaging. Radiology 2009;251(2):398–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.