Abstract
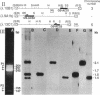
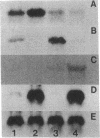
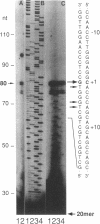
The plant hormone ethylene is believed to be responsible for the ability of rice to grow in the deepwater regions of Southeast Asia. Ethylene production is induced by hypoxia, which is caused by flooding, because of enhanced activity of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, the key enzyme in the ethylene biosynthetic pathway. We have cloned three divergent members, (OS-ACS1, OS-ACS2, and OS-ACS3), of a multigene family encoding ACC synthase in rice. OS-ACS1 resides on chromosome 3 and OS-ACS3 on chromosome 5 in the rice genome. The OS-ACS1 and OS-ACS3 genes are induced by anaerobiosis and indoleacetic acid (IAA) + benzyladenine (BA) + LiCl treatment. The anaerobic induction is differential and tissue specific; OS-ACS1 is induced in the shoots, whereas OS-ACS3 is induced in the roots. These inductions are insensitive to protein synthesis inhibitors, suggesting that they are primary responses to the inducers. All three genes are actually induced when protein synthesis is inhibited, indicating that they may be under negative control or that their mRNAs are unstable. The OS-ACS1 gene was structurally characterized, and the function of its encoded protein (M(r) = 53 112 Da, pI 8.2) was confirmed by expression experiments in Escherichia coli. The protein contains all eleven invariant amino acid residues that are conserved between aminotransferases and ACC synthases cloned from various dicotyledonous plants. The amino acid sequence shares significant identity to other ACC synthases (69-34%) and is more similar to sequences in other plant species (69% with the tomato LE-ACS3) than to other rice ACC synthases (50-44%). The data suggest that the extraordinary degree of divergence among ACC synthase isoenzymes within each species arose early in plant evolution and before the divergence of monocotyledonous and dicotyledonous plants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Bailey B. A., Avni A., Li N., Mattoo A. K., Anderson J. D. Nucleotide Sequence of the Nicotiana tabacum cv Xanthi Gene Encoding 1-Aminocyclopropane-1-Carboxylate Synthase. Plant Physiol. 1992 Nov;100(3):1615–1616. doi: 10.1104/pp.100.3.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Boorstein W. R., Craig E. A. Primer extension analysis of RNA. Methods Enzymol. 1989;180:347–369. doi: 10.1016/0076-6879(89)80111-9. [DOI] [PubMed] [Google Scholar]
- Botella J. R., Arteca J. M., Schlagnhaufer C. D., Arteca R. N., Phillips A. T. Identification and characterization of a full-length cDNA encoding for an auxin-induced 1-aminocyclopropane-1-carboxylate synthase from etiolated mung bean hypocotyl segments and expression of its mRNA in response to indole-3-acetic acid. Plant Mol Biol. 1992 Nov;20(3):425–436. doi: 10.1007/BF00040602. [DOI] [PubMed] [Google Scholar]
- Botella J. R., Schlagnhaufer C. D., Arteca J. M., Arteca R. N., Phillips A. T. Identification of two new members of the 1-aminocyclopropane-1-carboxylate synthase-encoding multigene family in mung bean. Gene. 1993 Jan 30;123(2):249–253. doi: 10.1016/0378-1119(93)90132-m. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Cohen E., Kende H. In vivo 1-aminocyclopropane-1-carboxylate synthase activity in internodes of deepwater rice : enhancement by submergence and low oxygen levels. Plant Physiol. 1987 Jun;84(2):282–286. doi: 10.1104/pp.84.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh K., Tepperman J., Christensen A. H., Quail P. H. phyB is evolutionarily conserved and constitutively expressed in rice seedling shoots. Mol Gen Genet. 1991 Feb;225(2):305–313. doi: 10.1007/BF00269863. [DOI] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Walker J. C., Lavin M., Peacock W. J. Anaerobically regulated aldolase gene of maize. A chimaeric origin? J Mol Biol. 1988 Aug 20;202(4):759–767. doi: 10.1016/0022-2836(88)90556-6. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F. Nearest neighbor procedure for relating progressively aligned amino acid sequences. Methods Enzymol. 1990;183:659–669. doi: 10.1016/0076-6879(90)83043-9. [DOI] [PubMed] [Google Scholar]
- Franco A. R., Gee M. A., Guilfoyle T. J. Induction and superinduction of auxin-responsive mRNAs with auxin and protein synthesis inhibitors. J Biol Chem. 1990 Sep 15;265(26):15845–15849. [PubMed] [Google Scholar]
- Freeling M., Bennett D. C. Maize Adh1. Annu Rev Genet. 1985;19:297–323. doi: 10.1146/annurev.ge.19.120185.001501. [DOI] [PubMed] [Google Scholar]
- Freeling M. Simultaneous induction by anaerobiosis or 2,4-D of multiple enzymes specificed by two unlinked genes: differential Adh1-Adh2 expression in maize. Mol Gen Genet. 1973 Dec 31;127(3):215–227. doi: 10.1007/BF00333761. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P. L., Parks J. E., Rottmann W. H., Theologis A. Two genes encoding 1-aminocyclopropane-1-carboxylate synthase in zucchini (Cucurbita pepo) are clustered and similar but differentially regulated. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7021–7025. doi: 10.1073/pnas.88.16.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. H., Hu Y. Y., Wu C. H., Holcenberg J. A simple method for direct cloning cDNA sequence that flanks a region of known sequence from total RNA by applying the inverse polymerase chain reaction. Nucleic Acids Res. 1990 Apr 11;18(7):1922–1922. doi: 10.1093/nar/18.7.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. A., Rumpho M. E., Fox T. C. Anaerobic metabolism in plants. Plant Physiol. 1992 Sep;100(1):1–6. doi: 10.1104/pp.100.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. T., Silverstone A., Yip W. K., Dong J. G., Yang S. F. Induction of 1-aminocyclopropane-1-carboxylate synthase mRNA by auxin in mung bean hypocotyls and cultured apple shoots. Plant Physiol. 1992 Feb;98(2):465–471. doi: 10.1104/pp.98.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Abel S., Keller J. A., Shen N. F., Theologis A. The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11046–11050. doi: 10.1073/pnas.89.22.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Olson D. C., White J. A., Edelman L., Harkins R. N., Kende H. Differential expression of two genes for 1-aminocyclopropane-1-carboxylate synthase in tomato fruits. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. Y., Drory A., Woodson W. R. Molecular cloning of an 1-aminocyclopropane-1-carboxylate synthase from senescing carnation flower petals. Plant Mol Biol. 1992 Jan;18(2):377–386. doi: 10.1007/BF00034964. [DOI] [PubMed] [Google Scholar]
- Raskin I., Kende H. Mechanism of aeration in rice. Science. 1985 Apr 19;228(4697):327–329. doi: 10.1126/science.228.4697.327. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Glucocorticoid regulation of mouse mammary tumor virus gene expression. Biochim Biophys Acta. 1979 Dec 19;560(4):487–508. doi: 10.1016/0304-419x(79)90014-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Jardetzky O., Walbot V., Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann W. H., Peter G. F., Oeller P. W., Keller J. A., Shen N. F., Nagy B. P., Taylor L. P., Campbell A. D., Theologis A. 1-aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991 Dec 20;222(4):937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satler S. O., Kende H. Ethylene and the growth of rice seedlings. Plant Physiol. 1985 Sep;79(1):194–198. doi: 10.1104/pp.79.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Oeller P. W., Theologis A. The 1-aminocyclopropane-1-carboxylate synthase of Cucurbita. Purification, properties, expression in Escherichia coli, and primary structure determination by DNA sequence analysis. J Biol Chem. 1991 Feb 25;266(6):3752–3759. [PubMed] [Google Scholar]
- Sato T., Theologis A. Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6621–6625. doi: 10.1073/pnas.86.17.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Takaiwa F., Oono K., Sugiura M. The complete nucleotide sequence of a rice 17S rRNA gene. Nucleic Acids Res. 1984 Jul 11;12(13):5441–5448. doi: 10.1093/nar/12.13.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Theologis A. One rotten apple spoils the whole bushel: the role of ethylene in fruit ripening. Cell. 1992 Jul 24;70(2):181–184. doi: 10.1016/0092-8674(92)90093-r. [DOI] [PubMed] [Google Scholar]
- Van der Straeten D., Rodrigues-Pousada R. A., Villarroel R., Hanley S., Goodman H. M., Van Montagu M. Cloning, genetic mapping, and expression analysis of an Arabidopsis thaliana gene that encodes 1-aminocyclopropane-1-carboxylate synthase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9969–9973. doi: 10.1073/pnas.89.20.9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Straeten D., Van Wiemeersch L., Goodman H. M., Van Montagu M. Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-carboxylate synthase in tomato. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4859–4863. doi: 10.1073/pnas.87.12.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wu R. Rice alcohol dehydrogenase genes: anaerobic induction, organ specific expression and characterization of cDNA clones. Plant Mol Biol. 1989 Jul;13(1):53–68. doi: 10.1007/BF00027335. [DOI] [PubMed] [Google Scholar]
- Yip W. K., Dong J. G., Kenny J. W., Thompson G. A., Yang S. F. Characterization and sequencing of the active site of 1-aminocyclopropane-1-carboxylate synthase. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7930–7934. doi: 10.1073/pnas.87.20.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip W. K., Moore T., Yang S. F. Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2475–2479. doi: 10.1073/pnas.89.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]