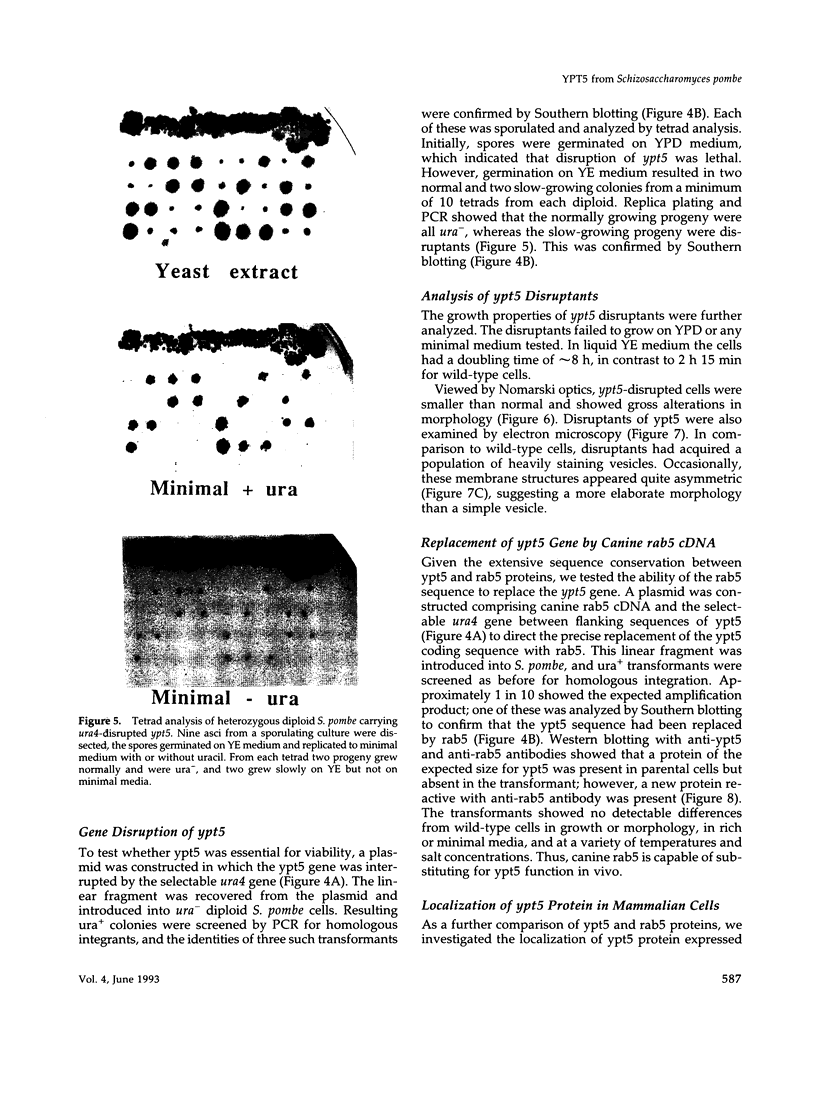
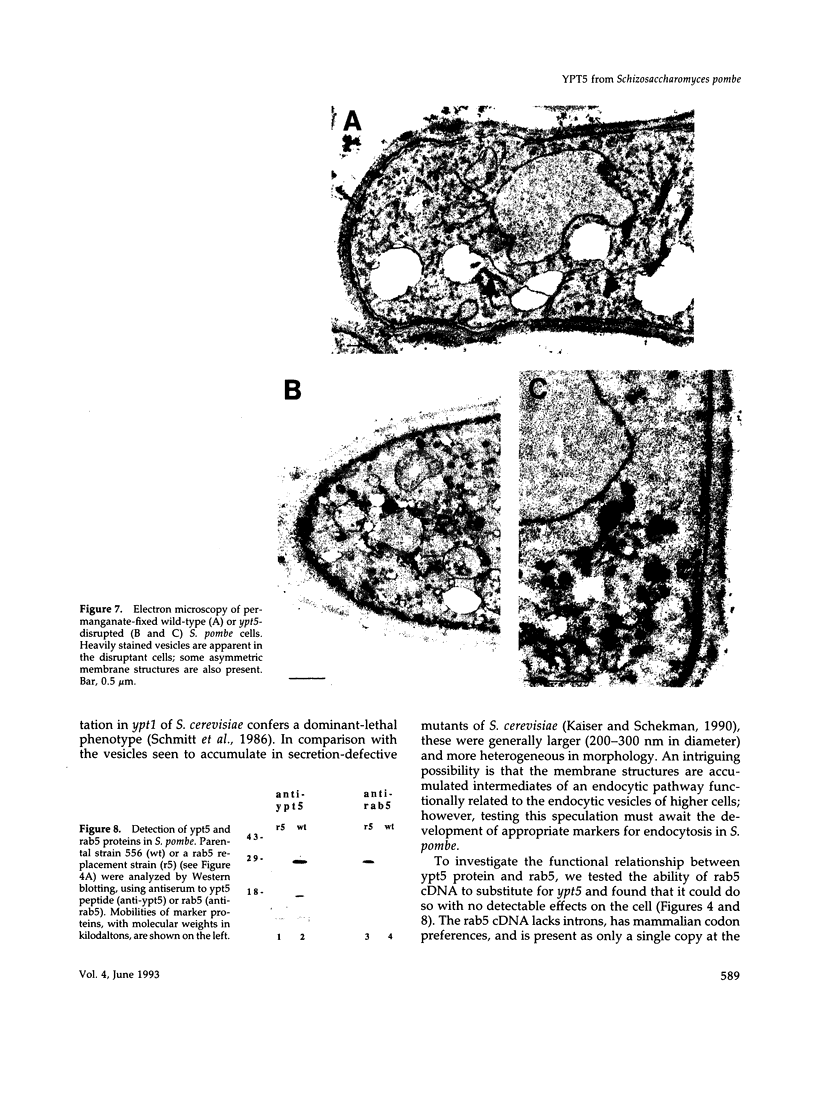
Abstract
The ypt/rab proteins are a family of small GTP-binding proteins thought to be required for different stages of membrane traffic. From the fission yeast Schizosaccharomyces pombe we have isolated and characterized ypt5, a gene encoding a homologue of rab5, a mammalian protein apparently involved in regulating fusion of early endosomes. Recombinant ypt5 protein bound GTP. The ypt5 gene was found to be essential for viability on minimal media, but ypt5-disrupted cells grew slowly on some rich media and accumulated a population of small vesicles not observed in wild-type cells. Canine rab5 cDNA could replace the ypt5 gene in S. pombe and restore normal growth and viability. Ypt5 protein expressed in mammalian cells colocalized with the transferrin receptor to early endosomes. Thus, molecular aspects of the early endocytic pathway may be conserved between mammalian cells and S. pombe and hence may be amenable to genetic analysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Patel S., Riddle P. Lysosomal sorting mutants of coronavirus E1 protein, a Golgi membrane protein. J Cell Sci. 1990 Feb;95(Pt 2):191–197. doi: 10.1242/jcs.95.2.191. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992 Sep 4;70(5):715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Warren G. A galactosyltransferase from the fission yeast Schizosaccharomyces pombe. J Cell Biol. 1989 Dec;109(6 Pt 1):2693–2702. doi: 10.1083/jcb.109.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Gorvel J. P., Stelzer E., Simons K., Gruenberg J., Zerial M. Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature. 1991 Oct 24;353(6346):769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Simons K., Zerial M. The complexity of the Rab and Rho GTP-binding protein subfamilies revealed by a PCR cloning approach. Gene. 1992 Mar 15;112(2):261–264. doi: 10.1016/0378-1119(92)90387-5. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Vingron M., Sander C., Simons K., Zerial M. Molecular cloning of YPT1/SEC4-related cDNAs from an epithelial cell line. Mol Cell Biol. 1990 Dec;10(12):6578–6585. doi: 10.1128/mcb.10.12.6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J. Mating pheromones of the fission yeast Schizosaccharomyces pombe: purification and structural characterization of M-factor and isolation and analysis of two genes encoding the pheromone. EMBO J. 1992 Mar;11(3):951–960. doi: 10.1002/j.1460-2075.1992.tb05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink L. A., Anzai K., Scheller R. H. rab15, a novel low molecular weight GTP-binding protein specifically expressed in rat brain. J Biol Chem. 1992 Mar 25;267(9):5768–5775. [PubMed] [Google Scholar]
- Farnsworth C. C., Kawata M., Yoshida Y., Takai Y., Gelb M. H., Glomset J. A. C terminus of the small GTP-binding protein smg p25A contains two geranylgeranylated cysteine residues and a methyl ester. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6196–6200. doi: 10.1073/pnas.88.14.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell E., Bowden S., Armstrong J. A homologue of the ras-related CDC42 gene from Schizosaccharomyces pombe. Gene. 1992 May 1;114(1):153–154. doi: 10.1016/0378-1119(92)90724-4. [DOI] [PubMed] [Google Scholar]
- Fawell E., Hook S., Armstrong J. Nucleotide sequence of a gene encoding a YPT1-related protein from Schizosaccharomyces pombe. Nucleic Acids Res. 1989 Jun 12;17(11):4373–4373. doi: 10.1093/nar/17.11.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell E., Hook S., Sweet D., Armstrong J. Novel YPT1-related genes from Schizosaccharomyces pombe. Nucleic Acids Res. 1990 Jul 25;18(14):4264–4264. doi: 10.1093/nar/18.14.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer von Mollard G., Mignery G. A., Baumert M., Perin M. S., Hanson T. J., Burger P. M., Jahn R., Südhof T. C. rab3 is a small GTP-binding protein exclusively localized to synaptic vesicles. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1988–1992. doi: 10.1073/pnas.87.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel J. P., Chavrier P., Zerial M., Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991 Mar 8;64(5):915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Goud B., Zahraoui A., Tavitian A., Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990 Jun 7;345(6275):553–556. doi: 10.1038/345553a0. [DOI] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Haubruck H., Disela C., Wagner P., Gallwitz D. The ras-related ypt protein is an ubiquitous eukaryotic protein: isolation and sequence analysis of mouse cDNA clones highly homologous to the yeast YPT1 gene. EMBO J. 1987 Dec 20;6(13):4049–4053. doi: 10.1002/j.1460-2075.1987.tb02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubruck H., Engelke U., Mertins P., Gallwitz D. Structural and functional analysis of ypt2, an essential ras-related gene in the fission yeast Schizosaccharomyces pombe encoding a Sec4 protein homologue. EMBO J. 1990 Jun;9(6):1957–1962. doi: 10.1002/j.1460-2075.1990.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst L., Lehmeier T., Gallwitz D. The ryh1 gene in the fission yeast Schizosaccharomyces pombe encoding a GTP-binding protein related to ras, rho and ypt: structure, expression and identification of its human homologue. EMBO J. 1990 Jun;9(6):1949–1955. doi: 10.1002/j.1460-2075.1990.tb08322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B. R. Specific binding of [alpha-32P]GTP to cytosolic and membrane-bound proteins of human platelets correlates with the activation of phospholipase C. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2261–2265. doi: 10.1073/pnas.84.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Mertins P., Gallwitz D. Nuclear pre-mRNA splicing in the fission yeast Schizosaccharomyces pombe strictly requires an intron-contained, conserved sequence element. EMBO J. 1987 Jun;6(6):1757–1763. doi: 10.1002/j.1460-2075.1987.tb02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S., Yamamoto M. Identification of ras-related, YPT family genes in Schizosaccharomyces pombe. EMBO J. 1990 May;9(5):1417–1422. doi: 10.1002/j.1460-2075.1990.tb08257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moreno S., Ruíz T., Sánchez Y., Villanueva J. R., Rodríguez L. Subcellular localization and glycoprotein nature of the invertase from the fission yeast Schizosaccharomyces pombe. Arch Microbiol. 1985 Sep;142(4):370–374. doi: 10.1007/BF00491906. [DOI] [PubMed] [Google Scholar]
- Newman C. M., Giannakouros T., Hancock J. F., Fawell E. H., Armstrong J., Magee A. I. Post-translational processing of Schizosaccharomyces pombe YPT proteins. J Biol Chem. 1992 Jun 5;267(16):11329–11336. [PubMed] [Google Scholar]
- Nilsson T., Jackson M., Peterson P. A. Short cytoplasmic sequences serve as retention signals for transmembrane proteins in the endoplasmic reticulum. Cell. 1989 Aug 25;58(4):707–718. doi: 10.1016/0092-8674(89)90105-0. [DOI] [PubMed] [Google Scholar]
- Pidoux A. L., Armstrong J. Analysis of the BiP gene and identification of an ER retention signal in Schizosaccharomyces pombe. EMBO J. 1992 Apr;11(4):1583–1591. doi: 10.1002/j.1460-2075.1992.tb05203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutner H., Cox A. D., Pind S., Khosravi-Far R., Bourne J. R., Schwaninger R., Der C. J., Balch W. E. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991 Oct;115(1):31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths S., Rohrer J., Crausaz F., Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J Cell Biol. 1993 Jan;120(1):55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985 Apr;40(4):1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. A ras-like protein is required for a post-Golgi event in yeast secretion. Cell. 1987 May 22;49(4):527–538. doi: 10.1016/0092-8674(87)90455-7. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kaibuchi K., Kabcenell A. K., Novick P. J., Takai Y. A mammalian inhibitory GDP/GTP exchange protein (GDP dissociation inhibitor) for smg p25A is active on the yeast SEC4 protein. Mol Cell Biol. 1991 May;11(5):2909–2912. doi: 10.1128/mcb.11.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H. D., Wagner P., Pfaff E., Gallwitz D. The ras-related YPT1 gene product in yeast: a GTP-binding protein that might be involved in microtubule organization. Cell. 1986 Nov 7;47(3):401–412. doi: 10.1016/0092-8674(86)90597-0. [DOI] [PubMed] [Google Scholar]
- Segev N., Mulholland J., Botstein D. The yeast GTP-binding YPT1 protein and a mammalian counterpart are associated with the secretion machinery. Cell. 1988 Mar 25;52(6):915–924. doi: 10.1016/0092-8674(88)90433-3. [DOI] [PubMed] [Google Scholar]
- Singer B., Riezman H. Detection of an intermediate compartment involved in transport of alpha-factor from the plasma membrane to the vacuole in yeast. J Cell Biol. 1990 Jun;110(6):1911–1922. doi: 10.1083/jcb.110.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith D. G., Svoboda A. Golgi apparatus in normal cells and protoplasts of Schizosaccharomyces pombe. Microbios. 1972 May-Jun;5(19):177–182. [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchot N., Chardin P., Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8210–8214. doi: 10.1073/pnas.84.23.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth N. C., Goud B., Kabcenell A. K., Novick P. J. Mutational analysis of SEC4 suggests a cyclical mechanism for the regulation of vesicular traffic. EMBO J. 1989 Jun;8(6):1685–1693. doi: 10.1002/j.1460-2075.1989.tb03560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann H., Hengst L., Gallwitz D. Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p). Cell. 1992 Dec 24;71(7):1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Pai E. F. The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci. 1991 Oct;16(10):382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- Zahraoui A., Touchot N., Chardin P., Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989 Jul 25;264(21):12394–12401. [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Webster P., Mâle P., Goud B., Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992 Sep 4;70(5):729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]