Abstract
In pancreatic β cells, ERK1 and ERK2 participate in nutrient sensing, and their activities rise and fall as a function of glucose concentration over the physiologic range. Glucose metabolism triggers calcium influx and release of calcium from intracellular stores to activate ERK1/2. Calcium influx also activates the calcium-dependent phosphatase calcineurin, which is required for maximal ERK1/2 activation by glucose. Calcineurin controls insulin gene expression by ERK1/2-dependent and -independent mechanisms. Here, we show that, in β cells, glucose activates the ERK1/2 cascade primarily through B-Raf. Glucose activation of B-Raf, like that of ERK1/2, is calcineurin-sensitive. Calcineurin binds to B-Raf in both unstimulated and stimulated cells. We show that B-Raf is a calcineurin substrate; among calcineurin target residues on B-Raf is T401, a site of negative feedback phosphorylation by ERK1/2. Blocking calcineurin activity in β cells prevents dephosphorylation of B-Raf T401 and decreases B-Raf and ERK1/2 activities. We conclude that the major calcineurin-dependent event in glucose sensing by ERK1/2 is the activation of B-Raf.
Keywords: diabetes, dimerization, scaffold
Insulin is essential for glucose homeostasis and is synthesized in and secreted from pancreatic β cells. Glucose, together with other nutrients, stimulates acute insulin release and is a long-term regulator of β-cell proliferation and survival (1, 2); glucose activates multiple signaling pathways, including calcium, calmodulin-dependent protein kinases, and the MAPKs ERK1/2. ERK1/2 assist in maintaining β-cell function in part by mediating nutrient-induced transcription of the insulin gene. ERK1/2 are regulated by insulin secretagogues, including glucose, other nutrients, glucagon-like peptide 1, and depolarizing stimuli (3, 4). Their activation state in β cells reflects the need for insulin secretion imposed by stimuli to maintain euglycemia in the organism (5).
In most settings, the three Raf protein kinases, A-Raf, B-Raf, and C-Raf (Raf-1), are the enzymes that activate the MAP/ERK kinases MEK1/2 upstream of ERK1/2 in the kinase cascade, with C-Raf being the first identified. Raf proteins respond to extracellular signals primarily transmitted from Ras family small G proteins to regulate MEK1/2 and ERK1/2 (6–8). Disruption of B-Raf or C-Raf causes embryonic lethality in mice, whereas A-Raf KO mice have intestinal and neurological defects of variable severity (9–11). The oncogenic B-Raf mutant V600E was shown to activate the cascade through direct interaction with C-Raf (7, 12, 13). The three Raf proteins are now known to associate as homodimers or heterodimers (6, 14, 15). In one current model, B-Raf is viewed as the major pathway activator, whereas C-Raf and A-Raf fine tune the signal to impact intensity and/or duration of ERK1/2 activity (7, 16). B-Raf/C-Raf heterodimers have higher activity than B-Raf or C-Raf homodimers and may produce distinct ERK1/2 activation kinetics (15). In B-Raf/C-Raf complexes, C-Raf may phosphorylate MEK1/2 or simply act as a scaffold (7, 12). Cooperation of B-Raf and C-Raf is required for ERK1/2 activation in several systems (17, 18).
B-Raf requires the fewest activating phosphorylation events. Sites that must be phosphorylated on C-Raf are, instead, acidic residues on B-Raf (e.g., D447 and D448 in the key regulatory region). Furthermore, S445, equivalent to the essential activating site S338 in C-Raf, seems to be constitutively phosphorylated in B-Raf (19). B-Raf also has an N-terminal region preceding the Ras binding domain, which facilitates lipid-independent Ras binding and interactions with other proteins (20). Although Ras-GTP alone may be sufficient to activate B-Raf, activation of C-Raf or A-Raf may require coordinate regulation by other factors (21).
The calcium calmodulin-dependent phosphatase calcineurin has essential functions in β cells (22–24). Because of their actions as immunosuppressants, the calcineurin inhibitors FK506 and cyclosporin A are used to decrease immune rejection of transplanted tissues (25). These inhibitors reduce insulin gene transcription and may contribute to reduced function of transplanted islets (26–28). Multiple actions of calcineurin are involved in β-cell function. Nuclear factor of activated T cells (NFAT), the best known calcineurin target, is a key factor mediating glucose-induced insulin gene transcription (22, 23, 29). Calcineurin is also required for activation of ERK1/2 by glucose and other insulin secretagogues in contrast with growth factors and insulin, which activate the kinases in a largely calcineurin-independent manner (30).
To identify the calcineurin-dependent steps in the ERK1/2 cascade, we evaluated the effects of calcineurin inhibitors on the activities of upstream components. We find an essential role of calcineurin in glucose stimulation of B-Raf.
Results
Glucose Activates B-Raf in Pancreatic β Cells.
Our previous work showed that expression of inhibitory mutants of Ras or Raf inhibited ERK1/2 activation by glucose in pancreatic β cells, consistent with the idea that Ras and Raf are required for glucose-induced-ERK1/2 activation (30). To gain further evidence that glucose stimulates ERK1/2 through Ras, we assessed the amount of Ras-GTP in the mouse insulinoma cell line MIN6 in the absence or presence of 20 mM glucose using a tagged form of the N-terminal Ras binding domain of B-Raf (RBD) to selectively capture the GTP-bound form of Ras. Increased Ras-GTP was detected by 5 min of exposure to glucose (Fig. 1A). Other small G proteins were not detected, although this cannot be taken as proof that Rap1 or others are not also involved in the glucose response (31).
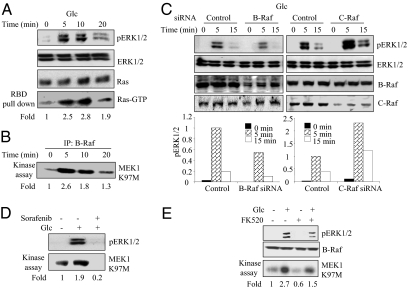
Fig. 1.
Glucose activates ERK1/2 through B-Raf in pancreatic β cells. MIN6 cells were preincubated for 2 h in KRBH containing 2 mM glucose (Glc) before stimulation with 20 mM glucose for indicated times (A–D). Quantification was with ImageJ software. (A) Ras-GTP was pulled down with GST-B-Raf-RBD and immunoblotted as were phosphorylated ERK1/2 (pERK1/2), ERK1/2, and Ras in lysates. Numbers indicate the fold change in Ras immunoblotting signal in the glucose-stimulated relative to the control sample. (B) B-Raf was immunoprecipitated, and activity was measured with MEK1 K97M as substrate. An autoradiogram is shown. (C) MIN6 cells were transiently transfected with indicated siRNA oligonucleotides 48 h before treatment. (Upper) Immunoblots of cell lysates. (Lower) pERK1/2 was quantified from blots in B. (D) Cells were treated with 10 μM Sorafenib for 20 min before a 10-min exposure to glucose. pERK1/2 was detected by immunoblotting. B-Raf kinase assays were as in B. (E) MIN6 cells were pretreated with 200 nM FK520 or DMSO (vehicle) for 20 min before addition of glucose for 10 min. pERK1/2 and B-Raf were immunoblotted in lysates. B-Raf was assayed as in B. Numbers indicate the fold change in B-Raf activity in treated samples relative to control from this experiment. An autoradiogram is shown. A, B, and E: n = 3; C and D: n = 4.
There are conflicting reports about the involvement of Raf proteins in β cells (32, 33). Using immune complex kinase assays, we found that B-Raf activity was quickly enhanced by glucose (Fig. 1B). In contrast, C-Raf activity was too low to detect in control or glucose-treated cells (SI Appendix, Fig. S1A). To show that the assay could detect C-Raf activity, we showed that C-Raf activity was stimulated by phorbol ester in HeLa cells (SI Appendix, Fig. S1B). In addition, glucose stimulation did not alter the level of C-Raf S338 phosphorylation but did cause a slight increase in phosphorylation of B-Raf S445 (SI Appendix, Fig. S1C).
To confirm these findings, we knocked down B-Raf or C-Raf expression using siRNA oligonucleotides. Reduced B-Raf expression decreased glucose-induced ERK1/2 activity after short times of glucose exposure (Fig. 1C). In contrast, suppression of C-Raf initially caused higher ERK1/2 activity but lower activity after prolonged stimulation (Fig. 1C and SI Appendix, Fig. S1D). Sorafenib, a Raf inhibitor (34), blocked B-Raf and ERK1/2 activities (Fig. 1D). Calcineurin inhibitors reduced glucose-stimulated B-Raf activity from 4.5 ± 1.3-fold over control to only 1.7 ± 0.2-fold over control (P < 0.03; n = 3) but had no effect on B-Raf activation by EGF or phorbol ester in nutrient-deprived cells (Fig. 1E and SI Appendix, Fig. S1E). Because the B-Raf and C-Raf knockdowns caused opposite phenotypes, the same siRNAs were used to knockdown B-Raf and C-Raf in a mouse fibroblast cell line (9). EGF-induced ERK1/2 activity was reduced by both C-Raf and B-Raf siRNA (SI Appendix, Fig. S1F), showing the expected effects. We conclude that B-Raf is essential for ERK1/2 activation in β cells, but the effects of C-Raf are less clear.
Dimerization of B-Raf and C-Raf Is Enhanced by Calcineurin in β Cells.
To determine if glucose affects B-Raf/C-Raf association, endogenous C-Raf and B-Raf were immunoprecipitated with isoform-selective antibodies, and coprecipitating proteins were detected by immunoblotting. Despite undetectable glucose-induced C-Raf activation, glucose did increase B-Raf/C-Raf heterodimers (Fig. 2A). We transiently expressed Myc–C-Raf and found that glucose enhanced B-Raf/Myc-C–Raf association (SI Appendix, Fig. S2A). The temporal changes suggest that B-Raf/C-Raf interaction is related to B-Raf activation.
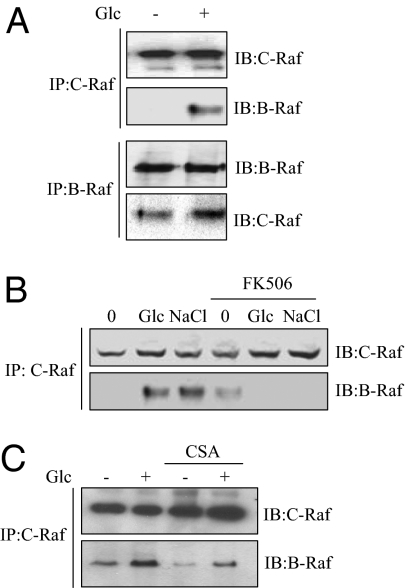
Fig. 2.
Glucose stimulates B-Raf/C-Raf dimerization. MIN6 cells were preincubated and stimulated with glucose for the indicated times as in Fig. 1. In each panel, the indicated Raf proteins were immunoprecipitated, and coprecipitating Raf proteins were detected by blotting. (A) C-Raf and B-Raf were immunoprecipitated. (B and C) Cells were pretreated with DMSO, 200 nM FK506, or 10 μM cyclosporin A (CsA) for 20 min before stimulation with glucose or 0.25 M NaCl for 10 min. NaCl is a calcinerin-sensitive ERK1/2 activator in β cells. A: n = 4; B: n = 2; C: n = 3.
B-Raf/C-Raf association triggered by glucose and NaCl was effectively suppressed by calcineurin inhibitors (Fig. 2 B and C). To explore these effects, active and inactive forms of calcineurin were expressed in 293 cells. The constitutively active form of calcineurin lacks the calmodulin binding domain and an autoinhibitory motif (SI Appendix, Fig. S2B) and increases insulin gene transcription (29, 35). B-Raf/C-Raf complexes were disrupted by inactive calcineurin but enhanced by active calcineurin in 293 cells, indicating that calcineurin can regulate B-Raf/C-Raf association (SI Appendix, Fig. S2C).
B-Raf Is Constitutively Bound to Calcineurin.
Because calcineurin contributes to B-Raf activity and B-Raf/C-Raf oligomerization, we asked if Raf proteins bind stably to calcineurin in β cells. B-Raf interacted with calcineurin, even in the absence of glucose (Fig. 3A). When expressed in 293 cells, calcineurin coprecipitated with both Raf isoforms (Fig. 3B). The inactive calcineurin mutant, which lacks the autoinhibitory and calmodulin binding regions, also binds B-Raf (SI Appendix, Fig. S3). This suggests that the interaction with B-Raf is independent of calmodulin binding and calcium signals.
Fig. 3.
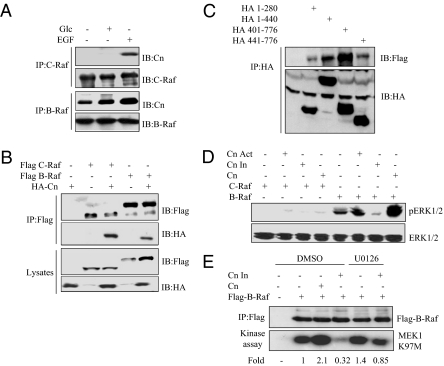
B-Raf is constitutively associated with calcineurin. (A) MIN6 cells were preincubated as in Fig. 1 and treated with 20 ng EGF for 10 min or 10 mM glucose for 2 h. Endogenous calcineurin (Cn) and Raf isoforms were detected in Raf immunoprecipitates by immunoblotting. (B) HA-calcineurin, Flag-B-Raf, or Flag-C-Raf were expressed in 293 cells. After 2 d, Raf proteins were immunoprecipitated using anti-Flag and immunoblotted. (C) Flag-calcineurin and HA-B-Raf fragments were expressed in 293 cells. Flag-calcineurin was detected by immunoblotting the HA-tagged fragment immunoprecipitates with anti-Flag. (D) WT (CN), active (1–401; Cn Act), or inactive (1–401 160A; Cn In) calcineurin mutants were expressed in 293 cells. After 2 d, ERK1/2 and pERK1/2 were detected by immunoblotting. (E) WT or inactive calcineurin was expressed in 293 cells with Flag-B-Raf. After 2 d, cells were treated with DMSO or 10 μM U0126 for 2 h. The activity of Flag-B-Raf was assayed as in Fig. 1, and numbers refer to relative B-Raf activity for this experiment. A, B, and E: n = 3; C: n = 4; D: n = 2.
To find the region of B-Raf that binds calcineurin, we examined the interactions of B-Raf truncations with calcineurin. B-Raf contains a Ras binding domain, cysteine-rich domain, kinase domain, and a middle region (280–457), which is less conserved with A- and C-Raf. This region affects Raf activity and localization through multiple phosphorylation sites and other binding proteins, such as 14–3-3 (36). We found that calcineurin bound most strongly to B-Raf residues 401–776 and 1–440 and less well to residues 441–776 and 1–280 (Fig. 3C). Thus, calcineurin preferentially interacts with sequences between B-Raf residues 280 and 440.
Calcineurin Is Required for B-Raf Activation in a Heterologous System.
We assessed the impact of calcineurin on ERK1/2 and B-Raf activity in 293 cells. Consistent with previous findings, pERK1/2 was elevated in cells transfected with B-Raf but not C-Raf (Fig. 3D). Expression of calcineurin increased B-Raf activity 2.1 ± 0.36-fold, whereas inactive calcineurin decreased B-Raf activity to about 40% of control (0.41 ± 0.21-fold; n = 3) (Fig. 3E). B-Raf activity was also regulated by ERK1/2-mediated feedback, because the MEK inhibitor U0126 enhanced B-Raf activity in the absence of calcineurin. U0126 rescued the suppression of B-Raf activity by inactive calcineurin in 293 cells, with activity returning to 1.05 ± 0.13-fold of control. This supports the contention that calcineurin promotes B-Raf activation at least in part by antagonizing ERK1/2-mediated feedback inhibition.
B-Raf pT401 Is Phosphorylated by ERK2 and Dephosphorylated by Calcineurin.
Because calcineurin binds to B-Raf, we asked if B-Raf itself is dephosphorylated by calcineurin in vitro. ERK1/2 often cause negative feedback phosphorylation upstream in the pathway. Inhibition of ERK1/2 can enhance Raf activity (37) (Fig. 3E) (compare lane 2 with 5 and lane 4 with 6). ERK1/2-mediated feedback phosphorylation on S151, T401, S750, and T753 of B-Raf effectively disrupts B-Raf/active Ras interaction and B-Raf/C-Raf heterodimerization (38). Based on these published data, we considered the possibility that ERK phosphorylation sites on B-Raf can be dephosphorylated by calcineurin.
Because the calcineurin binding sequence seems to be in the serine/threonine-rich region (280–440) of B-Raf, we focused on this region and expressed GST–B-Raf 1–440 for in vitro assays. GST–B-Raf 1–440 was phosphorylated by active ERK1 in vitro (Fig. 4A and SI Appendix, Fig. S4), and calcineurin reduced its phosphorylation. Antiphospho-specific T401 antibodies recognized B-Raf 1–440 phosphorylated by ERK1 in vitro but no longer recognized B-Raf 1–440 T401A (Fig. 4B). We reasoned that B-Raf T401A would be resistant to inhibition by inactive calcineurin and found that it had higher basal activity. The serum-stimulated activity of B-Raf T401A was also unaltered by inactive calcineurin, unlike that of WT B-Raf (Fig. 4C). Therefore, calcineurin positively regulates B-Raf activity at least in part by dephosphorylating B-Raf pT401. Because phosphorylation by ERK1/2 disrupts B-Raf/C-Raf dimerization (38), we tested and confirmed that B-Raf T401A bound better to C-Raf than did WT B-Raf, although B-Raf T401A had similar extents of S365 and S445 phosphorylation (Fig. 4B). These findings support the idea that B-Raf pT401 is linked to calcineurin-dependent effects on Raf dimerization.
Fig. 4.
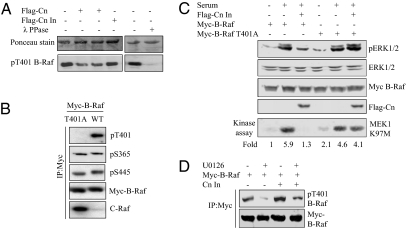
Calcineurin can dephosphorylate B-Raf pT401. (A Upper) Ponceau stain of GST B-Raf 1–440. (Lower) Anti–B-Raf pT401 immunoblot of GST-B-Raf 1–440 phosphorylated in vitro by pERK1 and dephosphorylated with immunoprecipitated WT or inactive Flag-calcineurin or with λ-phosphatase. (B) Myc-B-Raf or Myc-B-Raf T401A was immunoprecipitated from 293 cells with anti-Myc and immunoblotted with the indicated antibodies. (C) Myc-B-Raf or B-Raf T401A was expressed in 293 cells without or with inactive Flag-calcineurin. Cells were untreated or stimulated with 5% serum for 15 min. ERK1/2, pERK1/2, Myc-B-Raf, and Flag-calcineurin were immunoblotted. Myc-B-Raf kinase activity was assessed in triplicate as in Fig. 1. Activity relative to the untreated control is listed under the autoradiogram. (D) Myc-B-Raf was expressed without or with inactive calcineurin in 293 cells. Prior to harvest, cells were treated with DMSO or 10 μm U0126 for 2 h. Myc-B-Raf immunoprecipitates were immunoblotted with the indicated antibodies. A: n = 3; B–D: n = 2.
To verify that pT401 on B-Raf is one of the targets of calcineurin in cells, we examined changes in pT401 caused by inactive calcineurin. Phosphorylation of T401 was enhanced by expression of inactive calcineurin compared with the control (Fig. 4D). U0126 reduced T401 phosphorylation, but cells transfected with inactive calcineurin showed no reduction or even higher pT401. These data support the notion that ERK1/2 phosphorylates B-Raf on T401 and that calcineurin participates in pT401 dephosphorylation.
Dephosphorylation of B-Raf pT401 by Calcineurin Promotes B-Raf Activation in β Cells.
Both glucose and the calcineurin inhibitor FK506 increased phosphorylation of B-Raf T401 (Fig. 5A), consistent with the conclusion that T401 is a site of glucose-stimulated phosphorylation that is dephosphorylated by calcineurin. To suggest that phosphorylation of T401 on B-Raf inhibits its activity in β cells, we expressed B-Raf T401A in MIN6 cells and found that it had 2.1-fold higher basal activity than WT B-Raf (Fig. 5B). Furthermore, glucose-induced B-Raf T401A activity was not impaired by FK506 (4.1 ± 0.6-fold over control with FK506 compared with 4.6 ± 0.51-fold without FK506) in contrast with that of WT B-Raf.
Fig. 5.
Dephosphorylation of B-Raf T401 promotes B-Raf activation in β cells. In A and B, MIN6 cells were preincubated as in Fig. 1 and pretreated with FK506 or vehicle for 20 min before addition of 20 mM glucose for 15 min. (A) B-Raf immunoprecipitates were immunoblotted with the indicated antibodies. (B) Myc-B-Raf or Myc-B-Raf T401A was expressed in MIN6 cells for 48 h before preincubation and treatment. Myc B-Raf kinase activity was measured as in Fig. 1. (C and D) MIN6 cells were preincubated in DMEM without glucose for 6 h and then stimulated with glucose for 15 min in presence or absence of FK506 and/or U0126. (C) B-Raf was immunoprecipitated, and the precipitates were immunoblotted with the indicated antibodies. ERK1/2 and pERK1/2 were immunoblotted in lysates. (D) B-Raf was immunoprecipitated and assayed as in Fig. 1. Autoradiogram of B-Raf kinase assay. In B and D, activity relative to the untreated control is listed under the autoradiogram. A: n = 5; B: n = 2; C and D: n = 3.
We tested other Raf phosphorylation sites to determine if they are regulated by glucose and calcineurin in β cells. S365 on B-Raf is a negative regulatory site for 14–3-3 docking comparable with S259 on C-Raf (39). Glucose caused small increases in pS259 and pS365 (Fig. 5A), but FK506 had no detectable effect on either. pS338 on C-Raf and pS445 on B-Raf are prerequisites for activation of each Raf isoform. FK506 decreased pS338 on C-Raf and slightly reduced pS445 on B-Raf (SI Appendix, Fig. S5 A and B). Impaired B-Raf or C-Raf activation may also be involved in calcineurin action, although no effect of glucose on their regulation was noted. Thus, T401 seems to be a major site regulated by both calcineurin and glucose that impacts B-Raf activity.
Finally, we found that blocking ERK1/2 activation with U0126 reduced B-Raf pT401 (Fig. 5C), supporting the conclusion that ERK1/2 phosphorylates B-Raf on T401 in response to glucose. We then determined if blocking ERK1/2 activation with U0126 prevented down-regulation of B-Raf activity by FK506. As before, FK506 reduced glucose-stimulated B-Raf activity, whereas cells pretreated with both FK506 and U0126 showed 2.6 ± 0.3-fold higher B-Raf activity than cells treated with FK506 alone (Fig. 5D). These data support the conclusion that calcineurin positively regulates B-Raf activity by reversing ERK1/2-mediated negative feedback phosphorylation of T401.
Discussion
In β cells, insulin secretion is tightly coupled to nutrients, hormones, and paracrine signals that detect nutrient load. Calcium signaling, a key link, is triggered by glucose metabolism and activates both the ERK1/2 cascade and calcineurin. The use of calcineurin inhibitors as immunosuppressants is associated with a high risk of type 2 diabetes, showing the importance of calcineurin signaling in β cells (24). ERK1/2 activity rises and falls in response to changes in glucose concentration. We find that B-Raf is the primary kinase responsible for the rapid ERK1/2 response to glucose in β cells and is the major calcineurin target in the pathway. Thus, impaired B-Raf and ERK1/2 activity will result from calcineurin inhibitor-mediated immunosuppression. The phosphatase phosphoprotein phosphatase (PP)2A up-regulates Raf activity and assembles C-Raf/kinase suppressor of Ras 1 (KSR1) complexes in response to growth factors (8, 40). PP2A promotes Raf activation by dephosphorylating inhibitory sites, B-Raf pS365, or C-Raf pS259. In contrast to calcineurin, the PP2A/PP1 inhibitor okadaic acid did not inhibit glucose regulation of ERK1/2 in β cells (SI Appendix, Fig. S5C), consistent with the specificity of calcineurin action in this system.
Because loss of C-Raf enhances rather than impairs short-term increases in ERK1/2 activation, accounting for the contributions of C-Raf to normal glucose regulation is not simple. Controversies exist concerning the detailed molecular events regulating Raf isoforms (7, 12, 13, 41). B-Raf can activate C-Raf by forming B-Raf/C-Raf heterodimers. The interface on heterodimers is required for triggering Raf activation (12). Thus, activation of one Raf molecule may promote activation of the other molecule in the dimer. At the same time, differential sensitivity to activation may cause one Raf isoform to impair activation of another isoform in a dimer. Our data suggest that B-Raf/C-Raf dimerization may not always increase activation of ERK1/2, because C-Raf knockdown in β cells enhances activation of B-Raf and ERK1/2 within the first few minutes of exposure to glucose. In the short term, B-Raf seems more readily activated than C-Raf, and C-Raf may restrain activation of B-Raf in heterodimers. With longer stimulation, C-Raf may be activated to further enhance pathway activity. KSR2 is expressed in β cells and has been implicated in regulating calcium-dependent signaling to ERK1/2 (42). Initial analysis of KSR2 suggests a complex role in glucose signaling, in some respects similar to C-Raf. Knockdown experiments suggested both positive and negative effects on ERK1/2 activity that remain to be fully explained.
Raf isoforms are phosphorylated to integrate signals from other pathways. Inhibitory events may be more prevalent than activating ones. Inhibitory phosphorylations have been attributed to cAMP-dependent protein kinase, for example, as well as ERK1/2 (38, 40, 43, 44). B-Raf activity may also be affected by glycogen synthase kinase 3 (GSK3) in view of the frequent interactions between ERK1/2 and GSK3 pathways (45) and the fact that GSK3 has a related substrate specificity. Preliminary experiments suggested that inhibition of GSK3 partially reduced B-Raf T401 phosphorylation. GSK3 may contribute to the basal phosphorylation of this site in nutrient-deficient conditions, or it may have more marked effects on feedback sites identified other than T401. Some of these sites may also be targeted by calcineurin.
We conclude that calcineurin participates in the regulation of the Raf/ERK1/2 cascade through reversing negative feedback regulation of B-Raf. Calcineurin antagonizes ERK1/2-mediated feedback phosphorylation on B-Raf, thereby promoting B-Raf activation and B-Raf/C-Raf heterodimerization. Cross-talk between the Raf/ERK1/2 cascade and calcineurin facilitates maximal activation of ERK1/2 in a manner that is highly sensitive to factors that transmit the demand for insulin secretion to β cells.
Materials and Methods
Cell Culture and Reagents.
The β-cell line MIN6 was maintained as described (23). Before stimulation, β cells were incubated for 2 h in Krebs-Ringer-bicarbonate-Hepes (KRBH) buffer. HEK293T cells and C3H10T1/2 were maintained in RPMI medium 1640 with 10% FBS. Chemicals and antibodies were as described: FK506, FK520, rapamycin, and Sorafenib were from LC laboratories, cyclosporin A (CSA) was from Biomol, U0126 was from Promega, SB216763 was from Sigma-Aldrich, anti–B-Raf(F-7) and anti–C-Raf (E-10) were from Santa Cruz, anti-pS445 B-Raf (#2696) was from Cell Signaling Technology, anti-pS338 C-Raf was from Millipore, anticalcineurin (polyclonal) was from Calbiochem, anti-pT401 B-Raf and anti-p259 C-Raf were from Abgent, and antiphosphorylated ERK1/2 (pERK1/2) and anti-Flag (monoclonal) were from Sigma-Aldrich. Anti-ERK1/2 is Y691 (4).
Constructs and Transfection.
Flag- and HA-CnA (WT), HA-CnA (1–401; constitutively active), and Flag-CnA (1–401 160A; inactive) were kindly provided by Beverly Rothermel (University of Texas Southwestern, Dallas). PLNCX-Flag-B-Raf was kindly provided by Michael White (University of Texas Southwestern, Dallas). Human C-Raf and B-Raf cDNAs were amplified by PCR and subcloned into pCMV5-Myc. The RBD of human B-Raf (residues 1–280) was amplified by PCR and subcloned into BamH1 and XbaI sites of pGEX-KG. B-Raf fragments (1–280, 1–440, 401–776, and 441–776) were amplified by PCR and subcloned into pcDNA-HA or expressed as GST fusion proteins in Escherichia coli. Point mutations were introduced by site-directed mutagenesis. Transfection of MIN6 cells was with Fugene HD (Roche Molecular Biochemicals) and HEK293T cells was with Lipofectamine 2000 (Invitrogen Life Technologies) according to manufacturers' protocols.
Immunoprecipitation and Immunoblotting.
Cells (8 × 106) in 100-mm dishes were harvested at 4 °C with 0.65 mL cold lysis buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM EGTA, 1.5 mM MgCl2, 50 mM β-glycerophosphate, 10% glycerol, 0.2 mg/mL PMSF, 0.1 mM NaF, 2 mM Na3VO4, 10 μg/mL aprotinin, 5 μg/mL pepstatin A, 5 μg/mL leupeptin), homogenized through a 23-gauge needle, and centrifuged for 10 min at 12,000 × g in a microcentrifuge. Protein concentrations were measured using the BioRad Bradford reagent. Immunoprecipitations were from 0.5-mg lysates incubated with 3–4 μg of indicated antibodies overnight. Immune complexes were then incubated with 50 μL protein-A-Sepharose beads for 4 h. Beads were washed three times with lysis buffer for 5 min before adding 5× electrophoresis sample buffer. Samples were boiled and resolved on 10% acrylamide gels in SDS and transferred to membranes. Immunoblots were developed with ECL reagent.
Kinase Assays.
B-Raf and C-Raf were assayed in 30-μL reactions containing 10 mM Hepes, pH 7.5, 10 mM MgCl2, 1 mM benzamidine, 1 mM DTT, 5 μM ATP (15 cpm/fmol [γ-32P] ATP), and 2 μg GST-MEK1 K97M. A similar protocol was also used to phosphorylate B-Raf fragments with active ERK1. Reactions were incubated at 30 °C for 25–40 min, terminated with 5× sample buffer, and analyzed on gels as above. Gels were stained with Coomassie blue before autoradiography.
Ras Assays.
Ras activity was measured using a GST fusion protein containing the B-Raf RBD domain. GST-B-Raf 1–280 was expressed in E. coli and purified on glutathione-Sepharose beads. Cells in 100-mm dishes were lysed in 0.6 mL cold cell lysis buffer containing 1% Nonidet P-40. Cleared extracts were incubated with 30 μg GST-B-Raf 1–280 precoupled to GST beads at 4 °C for 4 h to capture Ras-GTP. After washing two times with cold lysis buffer, precipitates were resolved on gels as above and analyzed by immunoblotting with antipan-Ras antibodies.
siRNA Knockdown.
siRNA oligonucleotides targeting endogenous mouse B-Raf and C-Raf were purchased from Ambion. Control oligonucleotides were purchased from Dharmacon. siRNA oligonucleotides were as follows: C-Raf (siRNA ID: s99723)—sense: CACGAUUCUUCUAAGACAtt, antisense: UGUCUUAGAAGAAUCCGUGag and B-Raf (siRNA ID: s99634)—sense: CCACAGAUGCAUCACGGAAtt, antisense: UUCCGUGAUGCAUCUGUGGga. siRNA oligonucleotides were transfected into cells with Lipofectine maxi (Invitrogen).
Statistical Analyses.
Results are expressed as means ± SEM determined from three or more experiments, unless otherwise stated. Statistical significance was calculated by a one-tailed unpaired Student t test.
Dephosphorylation Assays.
After phosphorylation with ERK1, GST-B-Raf 1–440 proteins were repurified on GST beads and incubated with or without calcineurin (R&D Systems) at 37 °C for 1 h in 1 μM calmodulin, 50 mM Hepes, pH 7.5, 1 mM CaCl2, 5 mM MgCl2, 1 mM DTT, and 1 mg/mL BSA. After washing two times with cold PBS, proteins were analyzed as for kinase assays or by immunoblotting.
Supplementary Material
Acknowledgments
We thank Beverly Rothermel and Michael White (University of Texas Southwestern, Dallas), and Richard Easom (Takeda Pharmaceuticals, Indianapolis) for reagents, Michael Lawrence, Eric Wauson, Chunli Shao, Jihan Osborne, Aileen Klein, and Wen-Huang Ko for suggestions about this work, and Dionne Ware for administrative assistance. This work was supported by grants from the National Institutes of Health (DK34128 and DK55310) and the Robert A. Welch Foundation (I1243).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016630108/-/DCSupplemental.
References
- 1.Martens GA, Pipeleers D. Glucose, regulator of survival and phenotype of pancreatic beta cells. Vitam Horm. 2009;80:507–539. doi: 10.1016/S0083-6729(08)00617-1. [DOI] [PubMed] [Google Scholar]
- 2.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 3.Frödin M, et al. Glucose, other secretagogues, and nerve growth factor stimulate mitogen-activated protein kinase in the insulin-secreting β-cell line, INS-1. J Biol Chem. 1995;270:7882–7889. doi: 10.1074/jbc.270.14.7882. [DOI] [PubMed] [Google Scholar]
- 4.Khoo S, Cobb MH. Activation of MAP kinase by glucose is not required for insulin secretion. Proc Natl Acad Sci USA. 1997;94:5599–5604. doi: 10.1073/pnas.94.11.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson TB, et al. Inhibition of glucose-stimulated activation of extracellular signal-regulated protein kinases 1 and 2 by epinephrine in pancreatic β-cells. Diabetes. 2006;55:1066–1073. doi: 10.2337/diabetes.55.04.06.db05-1266. [DOI] [PubMed] [Google Scholar]
- 6.Weber CK, Slupsky JR, Kalmes HA, Rapp UR. Active Ras induces heterodimerization of cRaf and BRaf. Cancer Res. 2001;61:3595–3598. [PubMed] [Google Scholar]
- 7.Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol Cell. 2005;20:963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon AS, Meikle S, Yazici Z, Eulitz M, Kolch W. Regulation of Raf-1 activation and signalling by dephosphorylation. EMBO J. 2002;21:64–71. doi: 10.1093/emboj/21.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojnowski L, Stancato LF, Larner AC, Rapp UR, Zimmer A. Overlapping and specific functions of Braf and Craf-1 proto-oncogenes during mouse embryogenesis. Mech Dev. 2000;91:97–104. doi: 10.1016/s0925-4773(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 10.Wiese S, et al. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat Neurosci. 2001;4:137–142. doi: 10.1038/83960. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard CA, Bolin L, Slattery R, Murray R, McMahon M. Post-natal lethality and neurological and gastrointestinal defects in mice with targeted disruption of the A-Raf protein kinase gene. Curr Biol. 1996;6:614–617. doi: 10.1016/s0960-9822(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 12.Rajakulendran T, Sahmi M, Lefrançois M, Sicheri F, Therrien M. A dimerization-dependent mechanism drives RAF catalytic activation. Nature. 2009;461:542–545. doi: 10.1038/nature08314. [DOI] [PubMed] [Google Scholar]
- 13.Karreth FA, DeNicola GM, Winter SP, Tuveson DA. C-Raf inhibits MAPK activation and transformation by B-Raf(V600E) Mol Cell. 2009;36:477–486. doi: 10.1016/j.molcel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Luo Z, et al. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- 15.Rushworth LK, Hindley AD, O'Neill E, Kolch W. Regulation and role of Raf-1/B-Raf heterodimerization. Mol Cell Biol. 2006;26:2262–2272. doi: 10.1128/MCB.26.6.2262-2272.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529–545. doi: 10.1016/j.hoc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Edin ML, Juliano RL. Raf-1 serine 338 phosphorylation plays a key role in adhesion-dependent activation of extracellular signal-regulated kinase by epidermal growth factor. Mol Cell Biol. 2005;25:4466–4475. doi: 10.1128/MCB.25.11.4466-4475.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brummer T, Shaw PE, Reth M, Misawa Y. Inducible gene deletion reveals different roles for B-Raf and Raf-1 in B-cell antigen receptor signalling. EMBO J. 2002;21:5611–5622. doi: 10.1093/emboj/cdf588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran NH, Wu X, Frost JA. B-Raf and Raf-1 are regulated by distinct autoregulatory mechanisms. J Biol Chem. 2005;280:16244–16253. doi: 10.1074/jbc.M501185200. [DOI] [PubMed] [Google Scholar]
- 20.Fischer A, et al. B- and C-RAF display essential differences in their binding to Ras: The isotype-specific N terminus of B-RAF facilitates Ras binding. J Biol Chem. 2007;282:26503–26516. doi: 10.1074/jbc.M607458200. [DOI] [PubMed] [Google Scholar]
- 21.Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272:4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002;51:691–698. doi: 10.2337/diabetes.51.3.691. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence MC, McGlynn K, Park BH, Cobb MH. ERK1/2-dependent activation of transcription factors required for acute and chronic effects of glucose on the insulin gene promoter. J Biol Chem. 2005;280:26751–26759. doi: 10.1074/jbc.M503158200. [DOI] [PubMed] [Google Scholar]
- 24.Heit JJ. Calcineurin/NFAT signaling in the beta-cell: From diabetes to new therapeutics. Bioessays. 2007;29:1011–1021. doi: 10.1002/bies.20644. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Martínez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004;11:997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K, et al. Transcriptional inhibition of insulin by FK506 and possible involvement of FK506 binding protein-12 in pancreatic beta-cell. Transplantation. 1995;59:1606–1613. [PubMed] [Google Scholar]
- 27.Herold KC, Nagamatsu S, Buse JB, Kulsakdinun P, Steiner DF. Inhibition of glucose-stimulated insulin release from beta TC3 cells and rodent islets by an analog of FK506. Transplantation. 1993;55:186–192. doi: 10.1097/00007890-199301000-00035. [DOI] [PubMed] [Google Scholar]
- 28.Robertson RP. Cyclosporin-induced inhibition of insulin secretion in isolated rat islets and HIT cells. Diabetes. 1986;35:1016–1019. doi: 10.2337/diab.35.9.1016. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca(2+)-responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol. 2001;15:1758–1767. doi: 10.1210/mend.15.10.0702. [DOI] [PubMed] [Google Scholar]
- 30.Arnette D, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278:32517–32525. doi: 10.1074/jbc.M301174200. [DOI] [PubMed] [Google Scholar]
- 31.Ehses JA, Pelech SL, Pederson RA, McIntosh CH. Glucose-dependent insulinotropic polypeptide activates the Raf-Mek1/2-ERK1/2 module via a cyclic AMP/cAMP-dependent protein kinase/Rap1-mediated pathway. J Biol Chem. 2002;277:37088–37097. doi: 10.1074/jbc.M205055200. [DOI] [PubMed] [Google Scholar]
- 32.Gomez E, Pritchard C, Herbert TP. cAMP-dependent protein kinase and Ca2+ influx through L-type voltage-gated calcium channels mediate Raf-independent activation of extracellular regulated kinase in response to glucagon-like peptide-1 in pancreatic beta-cells. J Biol Chem. 2002;277:48146–48151. doi: 10.1074/jbc.M209165200. [DOI] [PubMed] [Google Scholar]
- 33.Trümper J, et al. The Rap-B-Raf signalling pathway is activated by glucose and glucagon-like peptide-1 in human islet cells. Diabetologia. 2005;48:1534–1540. doi: 10.1007/s00125-005-1820-5. [DOI] [PubMed] [Google Scholar]
- 34.Gollob JA, Wilhelm S, Carter C, Kelley SL. Role of Raf kinase in cancer: Therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin Oncol. 2006;33:392–406. doi: 10.1053/j.seminoncol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freed E, Symons M, Macdonald SG, McCormick F, Ruggieri R. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science. 1994;265:1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- 37.Slack JK, Catling AD, Eblen ST, Weber MJ, Parsons JT. c-Raf-mediated inhibition of epidermal growth factor-stimulated cell migration. J Biol Chem. 1999;274:27177–27184. doi: 10.1074/jbc.274.38.27177. [DOI] [PubMed] [Google Scholar]
- 38.Ritt DA, Monson DM, Specht SI, Morrison DK. Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol. 2010;30:806–819. doi: 10.1128/MCB.00569-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorson JA, et al. 14-3-3 proteins are required for maintenance of Raf-1 phosphorylation and kinase activity. Mol Cell Biol. 1998;18:5229–5238. doi: 10.1128/mcb.18.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol. 2003;13:1356–1364. doi: 10.1016/s0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- 41.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dougherty MK, et al. KSR2 is a calcineurin substrate that promotes ERK cascade activation in response to calcium signals. Mol Cell. 2009;34:652–662. doi: 10.1016/j.molcel.2009.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramstad C, Sundvold V, Johansen HK, Lea T. cAMP-dependent protein kinase (PKA) inhibits T cell activation by phosphorylating ser-43 of raf-1 in the MAPK/ERK pathway. Cell Signal. 2000;12:557–563. doi: 10.1016/s0898-6568(00)00097-8. [DOI] [PubMed] [Google Scholar]
- 44.Dougherty MK, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Zhou Y, Wang X, Evers BM. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene. 2006;25:43–50. doi: 10.1038/sj.onc.1209004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.