Abstract
Ig and T-cell receptor (TCR) variable-region gene exons are assembled from component variable (V), diversity (D) and joining (J) gene segments during early B and T cell development. The RAG1/2 endonuclease initiates V(D)J recombination by introducing DNA double-strand breaks at borders of the germ-line segments. In mice, the Ig heavy-chain (IgH) locus contains, from 5′ to 3′, several hundred VH gene segments, 13 D segments, and 4 JH segments within a several megabase region. In developing B cells, IgH variable-region exon assembly is ordered with D to JH rearrangement occurring on both alleles before appendage of a VH segment. Also, IgH VH to DJH rearrangement does not occur in T cells, even though DJH rearrangements occur at low levels. In these contexts, V(D)J recombination is controlled by modulating substrate gene segment accessibility to RAG1/2 activity. To elucidate control elements, we deleted the 100-kb intergenic region that separates the VH and D clusters (generating ΔVH-D alleles). In both B and T cells, ΔVH-D alleles initiated high-level antisense and, at lower levels, sense transcription from within the downstream D cluster, with antisense transcripts extending into proximal VH segments. In developing T lymphocytes, activated germ-line antisense transcription was accompanied by markedly increased IgH D-to-JH rearrangement and substantial VH to DJH rearrangement of proximal IgH VH segments. Thus, the VH-D intergenic region, and likely elements within it, can influence silencing of sense and antisense germ-line transcription from the IgH D cluster and thereby influence targeting of V(D)J recombination.
Keywords: epigenetic regulation, immunoglobulin genes, lymphocyte development
Ig and T-cell receptor (TCR) variable-region exons are assembled from germ-line variable (V), diversity (D) and joining (J) gene segments during early stages of B- and T-lymphocyte development, respectively (1). Each germ-line V, D, and J gene segment is flanked by a short recombination signal (RS) sequence that consists of a palindromic heptamer and a nonamer separated by 12- or 23-bp spacer. Joining occurs only between segments flanked by RSs with 12-bp and 23-bp spacers (referred to as 12RSs and 23RSs), respectively. For example, the Ig heavy-chain (IgH) V(D)J variable region is encoded by VH, D, and JH segments with VH segments and JH segments flanked by 23RSs and D segments flanked on both sides by 12RSs. Thus, complete V(D)J assembly must be mediated via the D segment; because direct VH to JH joining is prohibited by the 12/23 rule (2). V(D)J recombination is initiated by introduction of a DNA double-strand break (DSB) between a V, D or J segment and the flanking RS. Early work indicted that a single “V(D)J recombinase” assembles all antigen receptor genes (3). The nature of the common V(D)J recombinase was clarified by discovery of RAG1 and 2, which together form the RAG endonuclease that introduces the DSBs and provides the specificity of the V(D)J recombination reaction (4). The RAG endonuclease cuts paired V, D, and J segments in the context of the 12/23 rule (5, 6). DSB joining components of the V(D)J recombinase are provided by the classical nonhomologous DNA end-joining DSB repair pathway (7).
The activity of the common V(D)J recombinase is regulated in several contexts (8, 9). First, V(D)J recombination is regulated in a lineage-specific manner. Thus, Ig variable-region exons are completely assembled in B cells and not in T cells, whereas TCR variable-region exons are assembled in T but not B cells (6). Second, within a given lineage, V(D)J recombination is highly ordered. The IgH locus contains a large cluster of VH segments, a cluster of 13 D segments, and a cluster of 4 JH segments in a several-megabase region (1). In progenitor (pro) B lymphocytes, IgH variable-region exons are assembled via a process in which D to JH rearrangements occur first and on both alleles, followed by appendage of a VH segment to the DJH complex (10). Direct joining of a VH to a D is not observed, even though permitted by the 12/23 rule (10). Although not strictly ordered, the most D-proximal VH families and, in particular, the most D-proximal VH81X gene segment, rearrange more frequently than the most distal VH gene families, such as the VHJ558 family (11). After assembly of a productive IgH variable-region exon and expression of a μ heavy chain, pro-B cells advance to the precursor (pre) stage at which Ig light-chain (IgL) variable-region assembly occurs. Similarly ordered V(D)J recombination occurs during assembly of TCR variable-region exons in developing T lineage cells (12). Finally, V(D)J recombination is regulated in the context of allelic exclusion. For example, a productive VH to DJH rearrangement that generates a μ heavy-chain signals cessation of VH to DJH rearrangement at the other IgH allele if it is in the DJH configuration and retargets RAG to IgL loci (13).
Control of V(D)J recombination in a lineage-, stage-, and allele-specific manner is mediated by modulating accessibility of the substrate gene segments to the common RAG V(D)J recombinase (3). Early studies showed that pro-B cells generate sense germ-line VH transcripts from the 5′ (distal) VH segments and that these transcripts are down-regulated at later developmental stages, establishing germ-line transcription as a strong correlate of V(D)J recombinational accessibility (14). Over the years, the precise mechanism by which V(D)J recombinational accessibility is generated has remained elusive, despite many additional correlates, including antisense, as well as sense, germ-line transcription of variable-region segments throughout the locus (15). Notably, both B and T lymphocytes have germ-line “μ0” transcripts that initiate from a promoter upstream of the most 3′ D segment (DHQ52) and run downstream through the JH segments, consistent with T lymphocytes making low levels of DJH rearrangements (16). In addition, pro-B lymphocytes express low levels of antisense transcripts from the D portion of the locus (17, 18). Additional epigenetic modifications associated with V(D)J recombinational accessibility include DNA and/or histone modifications of particular regions and chromosomal positioning of the Ig or TCR loci within the nucleus (15, 19, 20). In particular, locus contraction and looping have been implicated as potential means of bringing distant variable-region gene segments into the proximity of the DJH complex to increase their probability of rearrangement (21), and locus decontraction has been suggested to move VH gene segments away to prevent further rearrangements in the context of allelic exclusion (22, 23).
As outlined above, the VH to DJH step is the regulated event in IgH variable-region gene assembly in developing B cells, both in the context of ordered rearrangement and allelic exclusion. In addition, although developing T cells generate low levels of DJH rearrangements, they do not append VH segments to the DJH complexes or generate VH to D rearrangements, implicating VH to DJH rearrangement as the regulated step with respect to B versus T lineage specificity (14). In these contexts, the ≈100-kb intergenic region between the mouse VH and D clusters is a likely candidate for harboring sequences that might regulate VH to DJH rearrangement (15, 24, 25). Correspondingly, insertion of a VH segment just upstream of the most 5′ D segment deregulated its rearrangement both in terms of order and lineage specificity (26). To test for potential V(D)J recombinational regulatory properties of the VH to D intergenic region, we deleted this region in mice. Our analyses of mice harboring this deletion indicate that it has a silencing function with respect to antisense transcription and that it also can influence lineage-specific IgH VH to DJH rearrangement.
Results
The large intergenic region between the most 3′ functional VH segment (VH81X) (11) and the most 5′ D segment (DFL16.1) (27) has a number of properties suggestive of a potential regulatory function, including multiple DNase I hypersensitivity sites, differential histone modifications in VH and D regions in pro-B versus pro-T cells, and two defined CTCF binding sites located several kilobases upstream of DFL16.1 (25, 28-30). To evaluate potential regulatory properties of the VH to D intergenic region, we used the Velocigene technology (31) to create an IgH allele termed ΔVH-D that harbors a 109.8-kb deletion that encompasses the region from 6.4 kb 5′ of VH81X (VH7183.a2.3) to 847 bp 3′ of DFL16.1 (Fig. 1A and Fig. S1). We chose to include VH81X and DFL16.1 in the deletion to ensure that we captured any regulatory elements proximal to or overlapping with gene segments at each end of the intergenic region. We have left intact the downstream D segments, including DSPs, DFL16.2, and DSTs (Fig. 1A). Flow cytometric analyses of mice homozygous for the ΔVH-D mutation (ΔVH-D/ΔVH-D mice) indicated that this deletion had no readily discernable effects on T or B cell development (Fig. S2). More detailed analyses of potential outcomes of the ΔVH-D mutation on IgH gene rearrangement in B cells are ongoing. In this study, we concentrated on effects of the deletion in T cells, with pro-B cells or pro-B lines being used to analyze certain findings in greater depth.
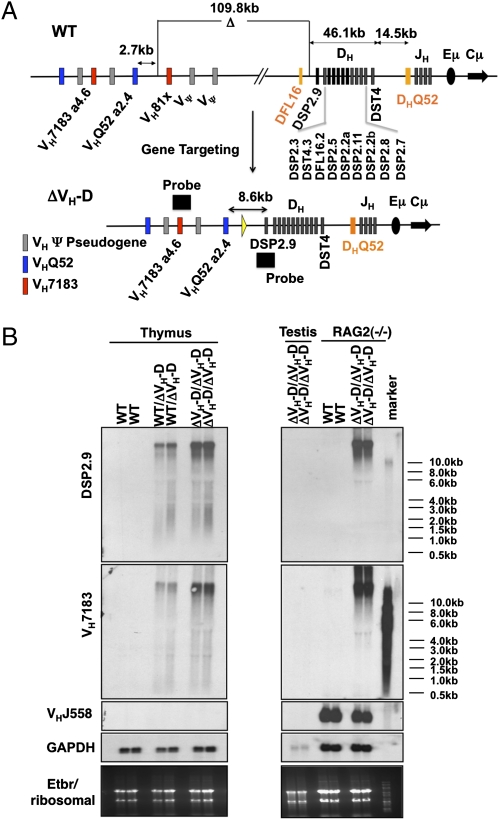
Fig. 1.
Deletion of VH to DH intergenic region leads to generation of long VH- and D-containing transcripts. (A) Schematic diagram of proximal WT murine 129sVe IgH locus before (Upper) and after gene targeting to delete the 109-kb intergenic region (Lower). The order of the D segments is indicated. (B) Northern analyses of total RNA from WT (+/+), ΔVH-D/+, and ΔVH-D/ΔVH-D thymocytes (Left) as well as total RNA from RAG2−/−(+/+) and RAG2−/−(ΔVH-D/ΔVH-D) A-MuLV-transformed pro-B lines after probing with (from top to bottom): a 1-kb fragment encompassing DSP2.9 (see A); a 511-bp fragment derived from the germ-line VH7183.a4.6 segment (see A); a 631-bp probe derived from the germ line corresponding to the VHJ558.29 segment; and a GAPDH cDNA. Ribosomal RNA loading is shown at the bottom as additional loading control.
To assess potential effects of the ΔVH-D on germ-line IgH transcription, we conducted RT-PCR assays for germ-line IgH DSP and VH7183 transcripts in purified CD4−/CD8− (double negative or DN) and CD4+CD8+ (double positive or DP) T cells, as well as in purified pro-B and pre-B cells from WT and ΔVH-D /ΔVH-D mice. We observed low levels of DSP germ-line transcripts in the absence of detectable VH7183 transcripts in WT DN and DP T cells but found increased levels of both types of transcripts in ΔVH-D /ΔVH-D DN and DP T cells (Fig. S3). Both types of transcripts were also detectable in WT pro-B and pre-B cells, but their levels were greatly increased in pro-B and pre-B cells of ΔVH-D /ΔVH-D mice (Fig. S3). The size of germ-line transcripts in ΔVH-D /ΔVH-D cells was consistent with unprocessed transcripts, suggesting the possibility, confirmed below, that they were antisense (Fig. S3).
To further characterize the apparent germ-line DSP and VH7183 transcriptional activation by the ΔVH-D mutation, we performed Northern blotting with total RNA from ΔVH-D/+ or ΔVH-D/ΔVH-D thymocytes. Northern blotting with a DSP2.9 probe revealed high-level expression of very long (>10 kb) steady-state transcripts in both ΔVH-D/+ and ΔVH-D/ΔVH-D thymocytes that were undetectable in WT thymocyte or sperm RNA (Fig. 1B). Likewise, we detected extremely high levels of large DSP2.9-hybridizing transcripts in total RNA from a RAG2−/− ΔVH-D/ΔVH-D A-MuLV-transformed pro-B line but not a RAG2−/− WT A-MuLV-transformed pro-B line (Fig. 1B); because Rag-2−/− cells do not undergo V(D)J recombination, these transcripts must come from unrearranged IgH alleles. Assays of the same RNA preparations revealed that the ΔVH-D allele led to similarly high levels of large VH7183-specific transcripts that were not detectable in WT thymocytes, WT A-MuLV-transformed pro-B cells, or sperm (Fig. 1B). Additional Northern blot analyses with a VHJ558 probe revealed expected germ-line VHJ558 transcripts in RAG-2−/−A-MuLV-transformed pro-B lines that appeared unchanged in RAG2−/−, ΔVH-D/ΔVH-D A-MuLV-transformed pro-B lines (Fig. 1B). Germ-line VHJ558 transcripts are not observed in WT or ΔVH-D/ΔVH-D thymocytes (Fig. 1B). We conclude that the ΔVH-D mutation activates the expression in both developing B and T cells of long germ-line transcripts that potentially appear to be antisense transcripts running from the IgH D region to the juxtaposed proximal VH region.
The high levels of steady-state transcripts from the DSP and VH7183 loci of ΔVHD alleles might result either from increased transcriptional initiation or from stabilization of transcripts that also are generated in WT cells. To distinguish these possibilities, we did run-on transcription assays with nuclei from WT and ΔVH-D/ΔVH-D A-MuLV-transformed RAG-2-deficient pro-B lines. For the nuclear run-on experiments we used probes from various regions within the D and VH portions of the IgH locus (Fig. 2 A and B). These assays demonstrated robust transcription of the VHJ558 and DHQ52 regions, but not of tested intervening regions of IgH (e.g., DSP or VH7183 sequences), in RAG2-deficient pro-B lines. These results are consistent with substantial steady-state levels of germ-line VHJ558 transcripts and μ0 transcripts but lack readily detectable DSP or VH7183 transcripts in these cells. In contrast, the run-on transcription assays of ΔVH-D/ΔVH-D A-MuLV-transformed RAG-2-deficient pro-B lines revealed nascent transcription throughout the DSP cluster, through the “intergenic probe” region, and through VH7183 segments as well as through the DHQ52 and VHJ558 portions of IgH (Fig. 2 C and D). Although we could not do run-on experiments with RAG2-deficient thymocytes because of lack of sufficient material, we obtained similar run-on results in experiments done with nuclei from total WT and ΔVH-D/ΔVH-D thymocytes (Fig. S4).
Fig. 2.
Deletion of VH to DH intergenic region leads increased germ-line transcription. Nuclear run-ons were conducted with nuclei from RAG2−/− (+/+) and RAG2−/−(ΔVH-D/ΔVH-D) A-MuLV-transformed pro-B lines. 32P-UTP–labeled nascent transcripts were hybridized to a series of double-stranded restriction endonuclease-generated DNA fragments (named on x axes) that were separated by electrophoresis and immobilized on Southern membranes. (A) The location of nuclear run-on probes (A through S) within the WT (Upper) and ΔVH-D (Lower) IgH alleles is shown. (B) Schematic diagram of location of probe fragments in gels shown in C and D. Open squares depict backbone vector sequences, and filled squares depict IgH fragments/probes. (C and D) (Upper) Ethidium bromide–stained gel of Southern membrane. (Lower) Same membrane exposed after probing with labeled transcripts from RAG2−/− (+/+) (Left) and RAG2−/−(ΔVH-D/ΔVH-D) (Right) A-MuLV-transformed pro-B lines. Results are representative of three independent experiments with different lines of the indicated genotype. See SI Materials and Methods for details.
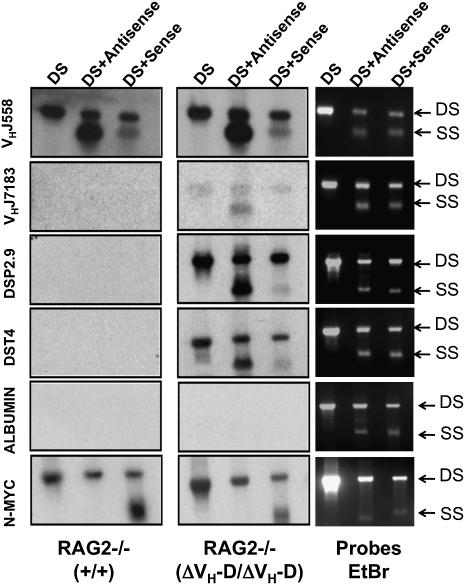
To further characterize the germ-line transcription from ΔVH-D alleles, we performed run-on experiments with nuclei from WT and ΔVH-D/ΔVH-D A-MuLV-transformed, RAG-2-deficient pro-B lines, using single strand sense and antisense DNA probes for VHJ558, VH7183, and DSP sequences, as well as for a positive (N-myc) and negative (albumin) control probes. As expected (32), sense N-myc transcription was found in both cell types. Notably, we found a consistent (n = 3) 10- to 20-fold excess of antisense versus sense transcription across VHJ558 gene segments in both cell types (Fig. 3). Although we failed to detect transcription through the DSP and VH7183 sequences in RAG-2-deficient pro-B lines, we found readily detectable nascent antisense, but not sense, transcription of VH7183 sequences, as well as substantial antisense and, to a lesser extent, sense transcription through DSP sequences in the ΔVH-D/ΔVH-D, RAG-2-deficient pro-B lines. Given that there are many more 129sVe VHJ558 segments than VH7183 segments, we cannot directly compare their signal strength. Finally, although we cannot exclude a very low level of VH7183 sense transcription in either WT or ΔVH-D/ΔVH-D RAG-2-deficient pro-B lines, we note that RT-PCR failed to show spliced sense germ-line VH7183 transcripts in these cells (Fig. S3). We conclude that there is a strong bias toward nascent antisense versus sense transcription in both the D and VH7183 regions of the ΔVH-D IgH loci.
Fig. 3.
Deletion of VH to DH intergenic region leads to antisense transcription of adjacent regions. Nuclear run-ons were conducted with nuclei from RAG2−/− (+/+) and RAG2−/−(ΔVH-D/ΔVH-D) A-MuLV-transformed pro-B lines. 32P-UTP–labeled nascent transcripts were hybridized to indicated double strand (DS), antisense, and sense DNA fragments from the IgH and control loci as indicated (see text and SI Materials and Methods for further details). (Left and Center) Run-on results. (Right) Loading as revealed by ethidium bromide staining. SS, single strand.
We took advantage of the high level of DSP region transcription in ΔVH-D/ΔVH-D thymocytes to localize potential start sites via 5′ RACE of antisense transcripts. However, we were unable to detect RACE products from the DSP region in WT thymocyte RNA, consistent with very low steady-state transcript levels from this region. In contrast, PCR amplification, with two independent sets of nested primers, of 5′ RACE products from ΔVH-D/ΔVH-D thymocyte RNA revealed a heterogeneous array of products (Fig. S5), potentially representing heterogeneous transcription initiation sites such as those observed in the TCRα locus (33). However, we cannot rule out potential “strong stops” attributable to secondary RNA structures as an origin of some products. In this regard, nuclear run-on analyses suggested transcription initiated in a region centered on DST4, given that a probe −958 bp 5′ of DST4 (5′DST4) had a positive run-on signal, whereas a probe −365 bp 5′ of DST4 (3′DST4) did not (Fig. 2). Together, these data are consistent with the possibility that antisense transcription through the DSP region may initiate heterogeneously but that there may be a downstream boundary near the DST4 region.
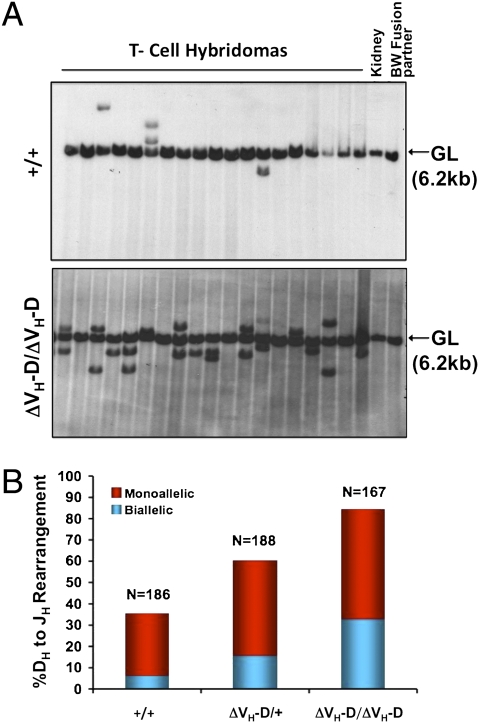
The normal B cell development in ΔVH-D/ΔVH-D mice (Fig. S2) suggests that the increase in antisense transcription in the D region does not markedly interfere with IgH locus V(D)J recombination. Therefore, we asked whether high levels of antisense transcription coupled with low-level sense transcription on the ΔVH-D allele might enhance IgH D to JH recombination in T cells. For this purpose, we generated splenic T cell hybridomas from WT, ΔVH-D/+, or ΔVH-D/ΔVH-D mice. We then analyzed rearrangement status of the IgH locus in StuI-digested DNA from each hybridoma by Southern blotting with a probe that detects JH rearrangements (Fig. 4A). In these assays, the fusion partner contributes a germ-line JH band; thus, we score rearrangements distinct non-germ-line–sized bands with two additional non-germ-line bands representing “biallelic” rearrangement. Consistent with earlier reports (34), ∼30% of WT T cells had JH rearrangements and the majority (>90%) were monoallelic (Fig. 4B). Strikingly, however, >60% of ΔVH-D/+ T cells had JH rearrangements with ∼20% having biallelic rearrangements, and nearly 90% of ΔVH-D/ΔVH-D T cells had JH rearrangements, over a third of which were biallelic (Fig. 4B).
Fig. 4.
VH to DH intergenic deletion up-regulates DH to JH recombination in thymocytes. (A) Two sets of independent splenic T-cell hybridomas were obtained from WT (n = 186), ΔVH-D/+ (n = 188), and ΔVH-D/ΔVH-D (n = 167) mice. DNA was digested with StuI, and Southern blot analysis was performed with an EcoRI/HindIII fragment probe downstream of JH4. The germ-line JH locus yields a 6.2-kb band (GL), and DH to JH rearrangements are scored as rearranged bands with a size distinct from the germ-line band. DNA from the fusion partner BW5147 and kidney DNA is shown as controls. A representative set of analyses is shown. (B) Percentage of cells containing monoallelic (red) and biallelic (blue) JH rearrangements as determined by either one or two non-germ-line bands in analyses of A. The average of two independent experiments is shown.
To test whether deletion of the intergenic VH-D region altered VH to DJH rearrangement in developing T cells, we first used a PCR approach to assay rearrangements of proximal and distal VH families in thymocytes from WT, ΔVH-D/+, or ΔVH-D/ΔVH-D mice, with ΔVH-D/ΔVH-D B cells and ES cells serving as positive and negative controls. Consistent with prior findings (12), normal T cells did not generate IgH locus VH to DJH rearrangements of any VH gene family tested. Strikingly, however, both ΔVH-D/+ and ΔVH-D/ΔVH-D DP thymocytes showed rearrangements of the two most proximal VH gene families (VH7183 and VHQ52) but not of more distal family members (VHJ558 and VHGAM3.8) (Fig. 5A). Indeed, cloning and sequencing of VH7183 rearrangements and VH7183 germ-line transcripts indicated that, within the VH7183 gene family, mainly D proximal VH segments were germ-line transcribed and rearranged (Fig. S6). To quantify VHDJH rearrangements, we prepared large panels of splenic T cell hybridomas from WT and mutant mice and assayed for VH(D)JH versus DJH rearrangement by a Southern blotting and PCR approach (35, 36). These analyses confirmed lack of VHDJH rearrangements in WT T cells and revealed that >5% of splenic ΔVH-D/ΔVH-D T cells contained VHDJH rearrangements (Fig. 5B).
Fig. 5.
Deletion of the VH to DH intergenic region leads to VH to DJH recombination in thymocytes. (A) PCR assay for VH to DJH for indicated gene families (Right) in DNA from sorted DP-T cells. Size of rearrangements to various J segments is indicated on Left. Analysis of Igκ rearrangement (VJκ) controlled for potential B cell contamination. (B) The level of VH to DJH rearrangements in the same panel of hybridomas shown in Fig. 4 was determined by a combination of hybridization to a JH probe, a probe between VH and DH segments, plus PCR for proximal VH to DJH rearrangements in each hybridoma (see SI Materials and Methods for details).
Discussion
The intergenic region between the most 3′ VH segment and the most 5′ D segment has been speculated to harbor elements that control VH to DJH rearrangement. Correspondingly, we find that deletion of the VH-D intergenic region in T cells dramatically increases germ-line transcription and rearrangement of VH segments just upstream of the deletion as well as that of the D segments downstream of the deletion. In developing T and B cells, absence of this region leads to high-level antisense transcription through both regions with the accumulation of high steady-state levels of germ-line antisense transcripts that contain both VH and D sequences. Various lines of evidence suggest that these antisense transcripts initiate in the D cluster and proceed upstream through proximal VH segments. This antisense transcription strongly correlates with increased rearrangement potential of the transcribed germ-line VH and D segments, at least in developing T lineage cells. The direct correlation between antisense transcription through and V(D)J recombination of particular germ-line IgH V and D segments indicates that, in this situation, antisense transcription has a positive role in mediating V(D)J recombinational accessibility. Our findings implicate a regulatory element or elements in the VH-D intergenic region that may modulate germ-line transcription, V(D)J recombination, or both.
Currently, we do not know the nature of the regulatory elements in the VH-D intergenic region or their function in normal development. Although there are several potential scenarios, this region clearly exerts a silencing effect on transcription from downstream antisense and, to a lesser extent, sense promoters within the D cluster. In this context, there may be insulators or other elements within the VH-D intergenic region that silence downstream D promoters by modulating their interaction with positive regulatory elements in the IgH locus (e.g., the intronic or 3′ IgH enhancers) or elsewhere via looping or related mechanisms (21, 37). One candidate would be the CTCF binding sites within the VH-D intergenic region that are postulated to serve as boundary elements between the VH versus D compartments (25, 28). Alternatively, the ΔVH-D deletion might activate D region promoters by bringing them into proximity to a putative positive transcriptional regulatory element in the VH region.
The normal role of the antisense transcriptional silencing function of the VH-D intergenic region remains to be determined. In its absence, the generation of long antisense transcripts that appear to activate proximal VH gene segments for rearrangement in T cells provides a striking example of its ability to maintain lineage-specific VH to DJH rearrangement. Whether this region serves such a role in normal cells, where it remains intact following D to JH rearrangement, is not clear. Conceivably, deregulated T-cell rearrangement of proximal VH segments on the ΔVH-D allele, reminiscent of the Pax-5 overexpression phenotype (38, 39), reflects a physiological suppressive function of the intergenic region in T lineage cells. In so, this suppressive function must normally be inactivated in pro-B cells. Alternatively, the region might function to regulate downstream D segment transcription, with deregulated VH to DJH rearrangement in ΔVH-D T cells reflecting up-regulation of downstream antisense transcription combined with artificial juxtaposition of the VH segment to the transcription origin. In this context, prior studies concluded that low-level antisense transcription in the DH cluster suppresses D segment rearrangement (18). The apparent discrepancy between those findings and our current findings might have several explanations, including relative transcription rates, steady-state transcript levels, or transcript structure differences. Another possibility is that activation of downstream transcription on the ΔVH-D allele might reflect a function normally activated by deletion of this region via VH to DJH recombination, in which case the role may be in transcriptional activation of the completely assembled V(D)J exon. Finally, additional studies will be important to test whether the intergenic region, as postulated (26), contains elements important for regulating allelic exclusion or ordered rearrangement of VH, D, and JH segments in B cells.
We describe a gain of function mutation that dramatically up-regulates nascent noncoding RNA transcription. This finding suggests potential existence of other silencing regions/sequences within the mammalian genome that may dictate levels of antisense intergenic noncoding RNAs through suppression of transcription. Thus, further characterization of mechanisms by which the VH-D intergenic region suppresses antisense noncoding RNA transcription could have more broad implications. In this regard, antisense transcription is often considered to be synonymous with transcriptional suppression stemming from paradigms such as imprinting (40–42). However, we now demonstrate that antisense transcription, even in the presence of sense transcription, does not necessarily lead to negative regulation. Finally, we find that antisense transcription of VH558 gene segments is surprisingly more robust than their sense transcription. However, steady-state antisense transcripts from VHJ558 segments are not more abundant than sense transcripts (14, 43), suggesting VHJ558 antisense transcripts are preferentially processed or degraded or that VHJ558 sense transcripts are stabilized (44).
Materials and Methods
Gene Targeting.
To make the ΔVH-D mutant IgH locus, Velocigene technology was used (31). Briefly, a targeting vector made from 129sVe DNA (Fig. S1A) was constructed via recombineering. The vector upstream and downstream arms are sequences flanking the intergenic VH to DH region. A neomycin resistance gene flanked by loxP sites allows removal of the selection cassette via Cre recombinase. F1 ES cells with IgMa (129sVe) and IgMb (C57BL/6) haplotypes were electroporated with the targeting construct. To screen for targeted clones, quantitative PCR was used to measure the copy number of the unmodified gene. Targeting was detected by a copy-number reduction from two to one per cell by a quantitative TaqMan PCR and confirmed by Southern analysis. See Fig. S1A and SI Materials and Methods for further details.
Nuclear Run-On Assays.
Nuclear run-on analyses were performed as described (32, 45). Further details are in SI Materials and Methods and Table S1.
Northern Blot Analysis.
Isolation of RNA and Northern analyses were done as described (14). Particular dsDNA probes used for indicated Northern blots were generated with Klenow and random hexamers (see Table S2 for further details).
V(D)J Rearrangement Assays.
PCR assays for D to JH or VH to DJH rearrangements were performed as described (46). See SI Materials and Methods for further details and Table S2 for primers.
T-Cell Hybridoma Analyses.
Splenic T cell hybridomas and analysis of IgH and Igκ rearrangements were done as described (36, 47). See the legend to Fig. 5 and SI Materials and Methods for more details.
RT-PCR Analyses.
RT-PCR assays of germ-line transcripts of IgH gene segments were performed as described (16). See Table S2 for primers.
Supplementary Material
Acknowledgments
We thank Drs. Kees Murre, David Schatz, Barry Sleckman, and Hidde Ploegh for critical review of this manuscript. This work was supported by National Institutes of Health Grant AI20047 (to F.W.A.) and K08 Grant AI070839 (to C.C.G.). C.G. is an Irvington Fellow of the Cancer Research Institute, and F.W.A. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015954107/-/DCSupplemental.
References
- 1.Schatz DG. V(D)J recombination. Immunol Rev. 2004;200:5–11. doi: 10.1111/j.0105-2896.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- 2.Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- 3.Yancopoulos GD, Blackwell TK, Suh H, Hood L, Alt FW. Introduced T cell receptor variable region gene segments recombine in pre-B cells: Evidence that B and T cells use a common recombinase. Cell. 1986;44:251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- 4.Schatz DG, Baltimore D. Uncovering the V(D)J recombinase. Cell. 2004;116:S103–S106. doi: 10.1016/s0092-8674(04)00042-x. [DOI] [PubMed] [Google Scholar]
- 5.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: Complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 6.Jung D, Alt FW. Unraveling V(D)J recombination; insights into gene regulation. Cell. 2004;116:299–311. doi: 10.1016/s0092-8674(04)00039-x. [DOI] [PubMed] [Google Scholar]
- 7.Rooney S, Chaudhuri J, Alt FW. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol Rev. 2004;200:115–131. doi: 10.1111/j.0105-2896.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 8.Yancopoulos GD, Alt FW. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- 9.Krangel MS. Gene segment selection in V(D)J recombination: Accessibility and beyond. Nat Immunol. 2003;4:624–630. doi: 10.1038/ni0703-624. [DOI] [PubMed] [Google Scholar]
- 10.Alt FW, et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancopoulos GD, et al. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984;311:727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- 12.von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 13.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–544. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Yancopoulos GD, Alt FW. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. [PubMed] [Google Scholar]
- 15.Corcoran AE. The epigenetic role of non-coding RNA transcription and nuclear organization in immunoglobulin repertoire generation. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.08.001. 10.1016/j.smim.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Subrahmanyam R, Sen R. RAGs’ eye view of the immunoglobulin heavy chain gene locus. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.08.003. 10.1016/j.smim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Bolland DJ, et al. Antisense intergenic transcription precedes Igh D-to-J recombination and is controlled by the intronic enhancer Emu. Mol Cell Biol. 2007;27:5523–5533. doi: 10.1128/MCB.02407-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty T, et al. Repeat organization and epigenetic regulation of the DH-Cmu domain of the immunoglobulin heavy-chain gene locus. Mol Cell. 2007;27:842–850. doi: 10.1016/j.molcel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Feeney A. Epigenetic regulation of V(D)J recombination. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.09.002. 10.1016/j.smim.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, et al. The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell. 2010;141:419–431. doi: 10.1016/j.cell.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jhunjhunwala S, et al. The 3D structure of the immunoglobulin heavy-chain locus: Implications for long-range genomic interactions. Cell. 2008;133:265–279. doi: 10.1016/j.cell.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewitt SL, et al. Association between the Igk and Igh immunoglobulin loci mediated by the 3′ Igk enhancer induces ‘decontraction’ of the Igh locus in pre-B cells. Nat Immunol. 2008;9:396–404. doi: 10.1038/ni1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roldán E, et al. Locus ‘decontraction’ and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat Immunol. 2005;6:31–41. doi: 10.1038/ni1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degner-Leisso SC, Feeney AJ. Epigenetic and 3-dimensional regulation of V(D)J rearrangement of immunoglobulin genes. Semin Immunol. 2010 doi: 10.1016/j.smim.2010.08.002. 10.1016/j.smim.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Featherstone K, Wood AL, Bowen AJ, Corcoran AE. The mouse immunoglobulin heavy chain V-D intergenic sequence contains insulators that may regulate ordered V(D)J recombination. J Biol Chem. 2010;285:9327–9338. doi: 10.1074/jbc.M109.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bates JG, Cado D, Nolla H, Schlissel MS. Chromosomal position of a VH gene segment determines its activation and inactivation as a substrate for V(D)J recombination. J Exp Med. 2007;204:3247–3256. doi: 10.1084/jem.20071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 28.Degner SC, Wong TP, Jankevicius G, Feeney AJ. Cutting edge: Developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J Immunol. 2009;182:44–48. doi: 10.4049/jimmunol.182.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury M, et al. Analysis of intergenic transcription and histone modification across the human immunoglobulin heavy-chain locus. Proc Natl Acad Sci USA. 2008;105:15872–15877. doi: 10.1073/pnas.0808462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morshead KB, Ciccone DN, Taverna SD, Allis CD, Oettinger MA. Antigen receptor loci poised for V(D)J rearrangement are broadly associated with BRG1 and flanked by peaks of histone H3 dimethylated at lysine 4. Proc Natl Acad Sci USA. 2003;100:11577–11582. doi: 10.1073/pnas.1932643100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valenzuela DM, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 32.Morrow MA, Lee G, Gillis S, Yancopoulos GD, Alt FW. Interleukin-7 induces N-myc and c-myc expression in normal precursor B lymphocytes. Genes Dev. 1992;6:61–70. doi: 10.1101/gad.6.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Abarrategui I, Krangel MS. Noncoding transcription controls downstream promoters to regulate T-cell receptor α recombination. EMBO J. 2007;26:4380–4390. doi: 10.1038/sj.emboj.7601866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Born W, White J, Kappler J, Marrack P. Rearrangement of IgH genes in normal thymocyte development. J Immunol. 1988;140:3228–3232. [PubMed] [Google Scholar]
- 35.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR α enhancer in αβ and γδ T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 36.Alt F, Rosenberg N, Lewis S, Thomas E, Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: Rearrangement of heavy but not light chain genes. Cell. 1981;27:381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- 37.Wuerffel R, et al. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souabni A, Jochum W, Busslinger M. Oncogenic role of Pax5 in the T-lymphoid lineage upon ectopic expression from the immunoglobulin heavy-chain locus. Blood. 2007;109:281–289. doi: 10.1182/blood-2006-03-009670. [DOI] [PubMed] [Google Scholar]
- 39.Fuxa M, et al. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latos PA, Barlow DP. Regulation of imprinted expression by macro non-coding RNAs. RNA Biol. 2009;6:100–106. doi: 10.4161/rna.6.2.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malecová B, Morris KV. Transcriptional gene silencing through epigenetic changes mediated by non-coding RNAs. Curr Opin Mol Ther. 2010;12:214–222. [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammad F, Mondal T, Kanduri C. Epigenetics of imprinted long noncoding RNAs. Epigenetics. 2009;4:277–286. [PubMed] [Google Scholar]
- 43.Bolland DJ, et al. Antisense intergenic transcription in V(D)J recombination. Nat Immunol. 2004;5:630–637. doi: 10.1038/ni1068. [DOI] [PubMed] [Google Scholar]
- 44.Preker P, et al. RNA exosome depletion reveals transcription upstream of active human promoters. Science. 2008;322:1851–1854. doi: 10.1126/science.1164096. [DOI] [PubMed] [Google Scholar]
- 45.Smith RK, Zimmerman K, Yancopoulos GD, Ma A, Alt FW. Transcriptional down-regulation of N-myc expression during B-cell development. Mol Cell Biol. 1992;12:1578–1584. doi: 10.1128/mcb.12.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perlot T, Li G, Alt FW. Antisense transcripts from immunoglobulin heavy-chain locus V(D)J and switch regions. Proc Natl Acad Sci USA. 2008;105:3843–3848. doi: 10.1073/pnas.0712291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakai E, Bottaro A, Davidson L, Sleckman BP, Alt FW. Recombination and transcription of the endogenous Ig heavy chain locus is effected by the Ig heavy chain intronic enhancer core region in the absence of the matrix attachment regions. Proc Natl Acad Sci USA. 1999;96:1526–1531. doi: 10.1073/pnas.96.4.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.