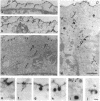
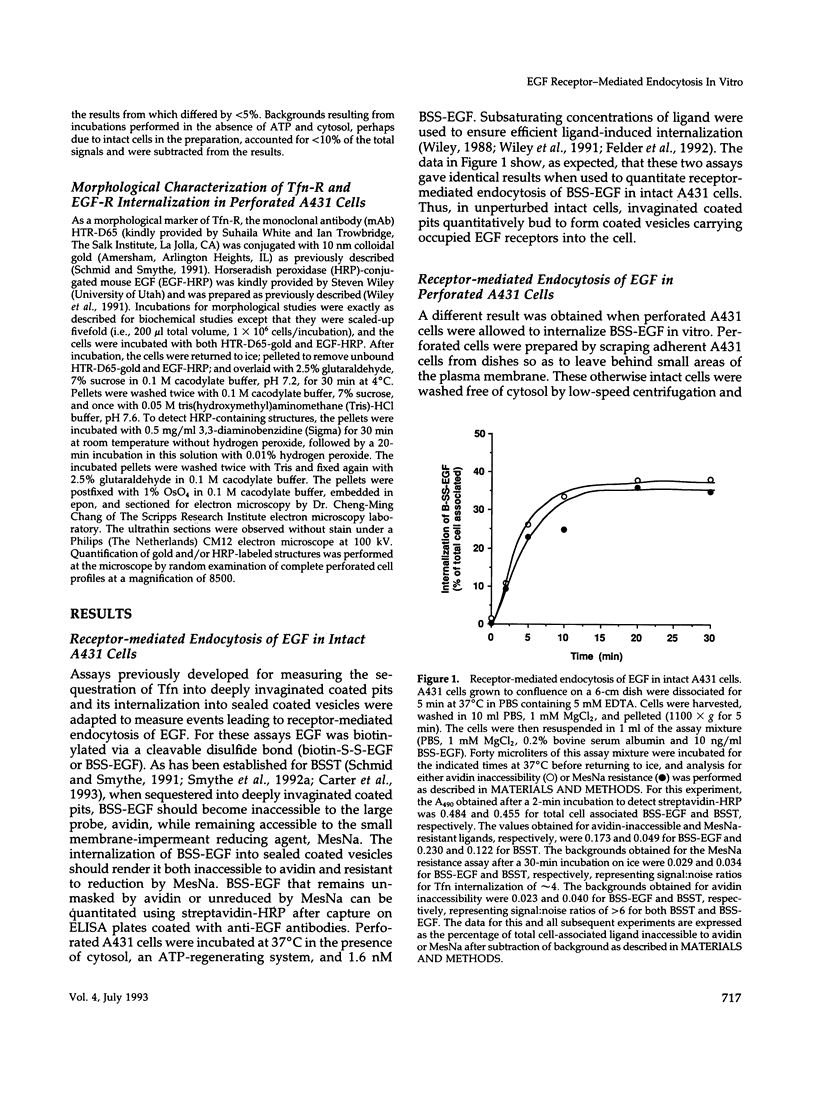
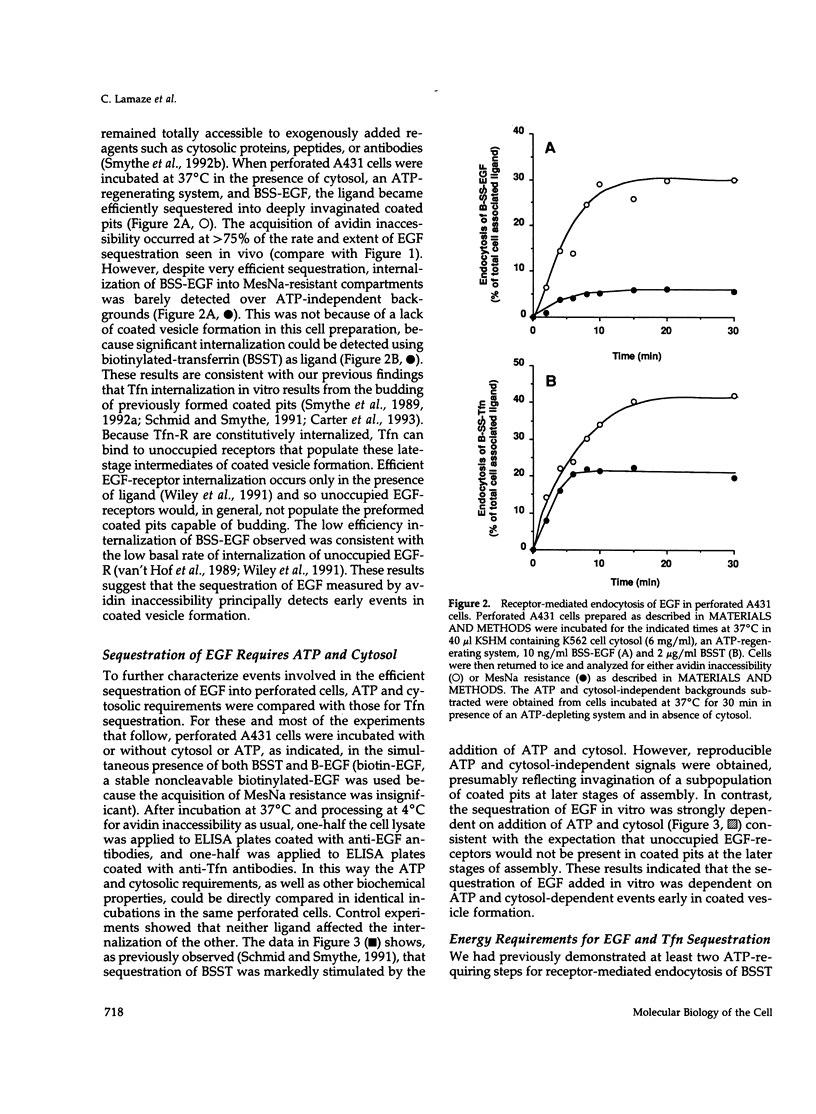
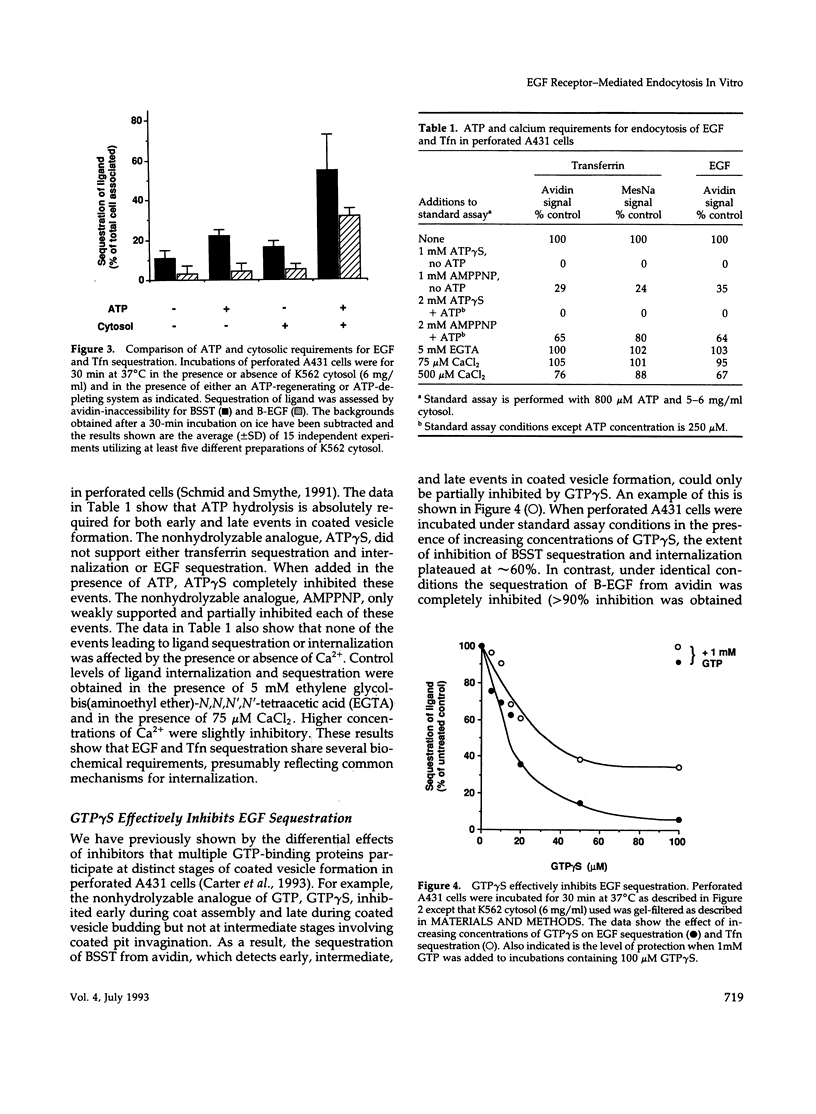
Abstract
The biochemical requirements for epidermal growth factor (EGF) and transferrin receptor-mediated endocytosis were compared using perforated human A431 cells. Morphological studies showed that horseradish peroxidase (HRP)-conjugated EGF and gold-labeled antitransferrin (Tfn) receptor antibodies were colocalized during endocytosis in vitro. The sequestration of both ligands into deeply invaginated coated pits required ATP hydrolysis and cytosolic factors and was inhibited by GTP gamma S, indicating mechanistic similarities. Importantly, several differences in the biochemical requirements for sequestration of EGF and Tfn were also detected. These included differing requirements for soluble AP (clathrin assembly protein) complexes, differing cytosolic requirements, and differing sensitivities to the tyrosine kinase inhibitor, genistein. The biochemical differences detected between EGF and Tfn sequestration most likely reflect specific requirements for the recruitment of EGF-receptors (R) into coated pits. This assay provides a novel means to identify the molecular bases for these biochemical distinctions and to elucidate the mechanisms involved in ligand-induced recruitment of EGF-R into coated pits.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Akiyama T., Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Shoelson S. E., Weiss M. A., Hua Q. X., Cheatham R. B., Haring E., Cahill D. C., White M. F. The insulin receptor juxtamembrane region contains two independent tyrosine/beta-turn internalization signals. J Cell Biol. 1992 Aug;118(4):831–839. doi: 10.1083/jcb.118.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A., Gierasch L. M. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991 Dec 20;67(6):1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. Co-localization of 125I-epidermal growth factor and ferritin-low density lipoprotein in coated pits: a quantitative electron microscopic study in normal and mutant human fibroblasts. J Cell Biol. 1982 Oct;95(1):73–77. doi: 10.1083/jcb.95.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L. L., Redelmeier T. E., Woollenweber L. A., Schmid S. L. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J Cell Biol. 1993 Jan;120(1):37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. P., Kao J. P., Lazar C. S., Walsh B. J., Wells A., Wiley H. S., Gill G. N., Rosenfeld M. G. Ligand-induced internalization and increased cell calcium are mediated via distinct structural elements in the carboxyl terminus of the epidermal growth factor receptor. J Biol Chem. 1991 Dec 5;266(34):23467–23470. [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Eberle W., Sander C., Klaus W., Schmidt B., von Figura K., Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell. 1991 Dec 20;67(6):1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- Faaland C. A., Mermelstein F. H., Hayashi J., Laskin J. D. Rapid uptake of tyrphostin into A431 human epidermoid cells is followed by delayed inhibition of epidermal growth factor (EGF)-stimulated EGF receptor tyrosine kinase activity. Mol Cell Biol. 1991 May;11(5):2697–2703. doi: 10.1128/mcb.11.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S., LaVin J., Ullrich A., Schlessinger J. Kinetics of binding, endocytosis, and recycling of EGF receptor mutants. J Cell Biol. 1992 Apr;117(1):203–212. doi: 10.1083/jcb.117.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Chen W. S., Lazar C. S., Walton G. M., Zokas L. M., Rosenfeld M. G., Gill G. N. Ligand-induced endocytosis of the EGF receptor is blocked by mutational inactivation and by microinjection of anti-phosphotyrosine antibodies. Cell. 1988 Mar 11;52(5):675–684. doi: 10.1016/0092-8674(88)90405-9. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Willingham M. C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984 Dec;39(2 Pt 1):283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R., Miller K., Beardmore J. M. Receptor-mediated endocytosis of transferrin and epidermal growth factor receptors: a comparison of constitutive and ligand-induced uptake. J Cell Sci Suppl. 1985;3:173–186. doi: 10.1242/jcs.1985.supplement_3.17. [DOI] [PubMed] [Google Scholar]
- Mahaffey D. T., Peeler J. S., Brodsky F. M., Anderson R. G. Clathrin-coated pits contain an integral membrane protein that binds the AP-2 subunit with high affinity. J Biol Chem. 1990 Sep 25;265(27):16514–16520. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Peeler J. S., Donzell W. C., Anderson R. G. The appendage domain of the AP-2 subunit is not required for assembly or invagination of clathrin-coated pits. J Cell Biol. 1993 Jan;120(1):47–54. doi: 10.1083/jcb.120.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. Adaptins. Trends Cell Biol. 1992 Oct;2(10):293–297. doi: 10.1016/0962-8924(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Carter L. L. ATP is required for receptor-mediated endocytosis in intact cells. J Cell Biol. 1990 Dec;111(6 Pt 1):2307–2318. doi: 10.1083/jcb.111.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L. Coated-vesicle formation in vitro: conflicting results using different assays. Trends Cell Biol. 1993 May;3(5):145–148. doi: 10.1016/0962-8924(93)90129-o. [DOI] [PubMed] [Google Scholar]
- Schmid S. L., Smythe E. Stage-specific assays for coated pit formation and coated vesicle budding in vitro. J Cell Biol. 1991 Sep;114(5):869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Carter L. L., Schmid S. L. Cytosol- and clathrin-dependent stimulation of endocytosis in vitro by purified adaptors. J Cell Biol. 1992 Dec;119(5):1163–1171. doi: 10.1083/jcb.119.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Pypaert M., Lucocq J., Warren G. Formation of coated vesicles from coated pits in broken A431 cells. J Cell Biol. 1989 Mar;108(3):843–853. doi: 10.1083/jcb.108.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe E., Redelmeier T. E., Schmid S. L. Receptor-mediated endocytosis in semiintact cells. Methods Enzymol. 1992;219:223–234. doi: 10.1016/0076-6879(92)19024-z. [DOI] [PubMed] [Google Scholar]
- Smythe E., Warren G. The mechanism of receptor-mediated endocytosis. Eur J Biochem. 1991 Dec 18;202(3):689–699. doi: 10.1111/j.1432-1033.1991.tb16424.x. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Westermark B., Heldin C. H., Claesson-Welsh L. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J Cell Biol. 1991 Feb;112(3):469–478. doi: 10.1083/jcb.112.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991 Aug;3(4):634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- Wiley H. S. Anomalous binding of epidermal growth factor to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J Cell Biol. 1988 Aug;107(2):801–810. doi: 10.1083/jcb.107.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley H. S., Herbst J. J., Walsh B. J., Lauffenburger D. A., Rosenfeld M. G., Gill G. N. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991 Jun 15;266(17):11083–11094. [PubMed] [Google Scholar]
- Willingham M. C., Haigler H. T., Fitzgerald D. J., Gallo M. G., Rutherford A. V., Pastan I. H. The morphologic pathway of binding and internalization of epidermal growth factor in cultured cells. Studies on A431, KB, and 3T3 cells, using multiple methods of labelling. Exp Cell Res. 1983 Jun;146(1):163–175. doi: 10.1016/0014-4827(83)90334-8. [DOI] [PubMed] [Google Scholar]
- van 't Hof R. J., Defize L. H., Nuijdens R., de Brabander M., Verkleij A. J., Boonstra J. Dynamics of epidermal growth factor receptor internalization studied by Nanovid light microscopy and electron microscopy in combination with immunogold labeling. Eur J Cell Biol. 1989 Feb;48(1):5–13. [PubMed] [Google Scholar]