Abstract
A role for Cu2+ ions in Alzheimer disease is often disputed, as it is believed that Cu2+ ions only promote nontoxic amorphous aggregates of amyloid-β (Aβ). In contrast with currently held opinion, we show that the presence of substoichiometric levels of Cu2+ ions in fact doubles the rate of production of amyloid fibers, accelerating both the nucleation and elongation of fiber formation. We suggest that binding of Cu2+ ions at a physiological pH causes Aβ to approach its isoelectric point, thus inducing self-association and fiber formation. We further show that Cu2+ ions bound to Aβ are consistently more toxic to neuronal cells than Aβ in the absence of Cu2+ ions, whereas Cu2+ ions in the absence of Aβ are not cytotoxic. The degree of Cu-Aβ cytotoxicity correlates with the levels of Cu2+ ions that accelerate fiber formation. We note the effect appears to be specific for Cu2+ ions as Zn2+ ions inhibit the formation of fibers. An active role for Cu2+ ions in accelerating fiber formation and promoting cell death suggests impaired copper homeostasis may be a risk factor in Alzheimer disease.
Keywords: Alzheimer Disease, Amyloid, Biophysics, Copper, Protein Folding, Protein-Metal Ion Interaction, Cu, Zn, Aggregation, Fiber Growth
Introduction
Alzheimer disease (AD)2 is characterized by extracellular amyloid plaques, composed predominantly of fibrillar amyloid-β peptide (Aβ), a 39–43-residue peptide. Genetic alterations underlying familial AD are associated with mutations or increased production of Aβ, indicating that Aβ plays a central role in the disease (1).
A notable characteristic of AD is altered metal ion concentrations in the brain and disrupted metal ion homeostasis (2). Cu2+ ions are found concentrated within senile plaques of AD patients directly bound to Aβ (3–5). Recent in vivo studies using a Drosophila model of AD have shown that impaired copper homeostasis enhances the toxic effects of Aβ (6). Furthermore, copper in a cholesterol high diet induces amyloid plaques and learning deficits in a rabbit model of AD (7). Other in vivo studies have shown that copper homeostasis can influence AD pathology. In contrast to the Drosophila model, transgenic mice have shown a reduced AD pathology with increased intracellular copper levels (8–10).
Although studies of Aβ neurotoxicity suggest that small diffusible oligomers, rather than mature amyloid fibers, are the more toxic form (11, 12), there remains strong evidence suggesting that amyloid plaques, or possibly intermediates of the fibrils, are critical in neuronal toxicity (13, 14). Aβ oligomers may be precursors of fiber formation and may also arise from fiber fragmentation. Alternatively, oligomers may be in competition with fiber formation. Both possibilities require the self-association of monomeric Aβ, and thus factors that affect fibrillization will also influence oligomer generation.
The mechanism by which Aβ is toxic is hotly debated (11, 15). It has been proposed that Aβ can form ion channels or pores or can thin the membrane, all of which will cause membrane leakage and loss of cellular Ca2+ ion homeostasis. One popular hypothesis is that the membrane integrity is compromised by lipid peroxidation from reactive oxygen species, which is a key feature of the pathogenesis of AD (16, 17). It is well established that hydrogen peroxide mediates Aβ toxicity and the antioxidant enzyme catalase protects cells from Aβ toxicity (18–20). A likely source of extracellular H2O2 is from the Fenton redox cycling of copper or iron ions (17). We and others have shown that Cu2+ bound to Aβ will readily generate hydroxyl radicals and H2O2 in the presence of a physiological reductant such as ascorbate (19, 21–23). Indeed, transfer of Cu2+ from Aβ to the redox-inactive metallothionein III removes Aβ toxic properties (24).
The three histidine residues within Aβ peptide form a tetragonal complex with Cu2+ ions (25–35; for review, see 36, 37). Recent studies point to a dynamic Cu2+ complex involving imidazole coordination in both the axial and equatorial plain (25, 27, 35). A full (1:1) stoichiometric complement will bind to both monomeric and mature Aβ fibers with identical coordination geometry and affinity (25). Affinity measurements of the Cu2+-Aβ complex have been revised, indicating a considerably tighter affinity than previously believed, setting the conditional dissociation constant, pH 7.4, at 60 × 10−12 m (25). Extracellular monomeric Aβ levels are thought to be 5 nm (38), whereas Aβ levels are higher in plaques and at the synapse. Furthermore, extracellular Cu2+ levels in the brain interstitial fluid are 100 nm. A picomolar affinity for Cu2+ allows Aβ to compete for Cu2+ ions with other extracellular Cu2+ chelators, especially at the synapse during neuronal depolarization where fluxes of Cu2+ are reported to be 20–250 μm (39).
Studies showed more than a decade ago that Zn2+ and Cu2+ ions cause marked aggregation of Aβ (40, 41). These initial studies did not make the distinction between amorphous aggregates, which are nontoxic to cells, and the formation of amyloid fibers. Further investigations using the fiber specific fluorophore thioflavin T (ThT) suggested that Zn2+ and Cu2+ only promote amorphous aggregation of Aβ and actually inhibit fiber formation and cell toxicity (42–46). We became interested in the factors that promote self-association of Aβ, the relationship between amorphous aggregation and amyloid fiber formation, and a role for Cu2+ ions in promoting fiber formation. Furthermore, we wanted to establish whether there was a link between the influence of Cu2+ ions on fiber formation and the effect of Cu2+ ions on cell toxicity of Aβ.
EXPERIMENTAL PROCEDURES
Aβ Production and Solubilization
Aβ(1–40) and Aβ(1–42) were synthesized using solid phase Fmoc (N-(9-fluorenyl)methoxycarbonyl) chemistry; the peptides were purchased commercially from ABC-London and Zinisser Analytic. HPLC indicated a single peak with the expected molecular mass. The peptides were also characterized by 1H NMR, and Met35 was confirmed to be unoxidized. Lyophilized Aβ(1–40) and Aβ(1–42) were solubilized by dissolving 0.8 mg/ml Aβ in water at pH 10 and then placed at 5 °C for 72 h. It is clear that Aβ is essentially seed-free as Aβ preparations at pH 7.4 have a lag phase of typically 100 h. The concentration of Aβ was determined using the tyrosine absorbance at 280 nm, ϵ280 = 1280 m−1 cm−1.
Fiber Growth Assay
The binding of ThT to amyloid fibers was used to monitor the kinetics of amyloid formation. ThT binding to amyloids induces the ThT to fluoresce at 487 nm. This fluorescence signal is related directly to the amount of amyloid. A BMG-Galaxy fluoro-star fluorescence 96-well plate reader was used for the ThT measurements. The central 60 wells were used, whereas wells around the edge contained buffer only, to minimize evaporation effects. Readings were taken every 30 min. The well plates were subjected to 30 s of agitation prior to each fluorescence measurement. Fluorescence excitation was at 440 nm and emission detected at 490 nm.
Fiber growth kinetics are very sensitive to a number of factors that must be carefully controlled. They include the pH, concentration, agitation, temperature, and ionic strength. Solubilization of Aβ into a seed-free form is also important. Fiber growth experiments were incubated at 30 °C in 160 mm NaCl. In addition, 50 mm HEPES buffer was used throughout; HEPES was used for its low affinity for Cu2+ and Zn2+ ions. Small adjustments were made with 10 mm NaOH or HCl to the stock Aβ solutions. The pH, a critical parameter in fiber growth rates, was measured before and after each fiber growth experiment; variations were 0.05, or fewer, pH units over the course of the experiment. Metal stocks solutions were 25 mm CuCl2 and 20 mm ZnCl2 or as a Cu(Gly)2 chelate. UHQ water (10−18 Ω−1cm−1 resistivity) was used at all times.
Growth Curve Analysis
Conversion of essentially monomeric Aβ to fibrillar Aβ follows a characteristic growth curve, typically described as the lag phase (nucleation) and a growth phase (elongation). A number of empirical parameters can be obtained from the fiber growth curve, including the time needed to reach half-maximal ThT intensity (t50) and the lag time (tlag) (see Table 1 and supplemental Table S1. The t50 is influenced by both the nucleation and elongation phases. These values can readily be extracted from the data by fitting the growth curve to the following equation (25),
where Y is the fluorescence intensity, x is the time, X0 is the time at half-height of fluorescence (t50). The apparent fiber growth rate is Kapp = 1/τ, and the lag time (tlag) is X0 − 2τ. This equation allows for a slope in the initial and final parts of the growth curve, (yi + mix), (υf + mfx), rather than forcing these to be horizontal. The fibril growth curves have also been fitted using an alternative equation to extract a rate of nucleation and elongation (47) shown in supplemental Table S1.
TABLE 1.
Kinetic data from fibril growth curves with and without Cu2+ present
Data were obtained from fitting Equation 1 (see “Experimental Procedures”) to the graphs shown in Fig. 1, with the S.E. shown in parentheses. A, B, and C indicate data obtained in separate experiments. Equation 1 determines the kinetics parameters: t(50), (Xo); t(lag), (X0 − 2dx) and; kapp, (1/dx) with the number of traces shown in n.
| Experiment | t50 (h) | tlag (h) | kapp (h−1) | n |
|---|---|---|---|---|
| A | ||||
| Apo | 72 (2) | 55 (3) | 0.120 (0.007) | 6 |
| 0.5 Cu2+ | 42.6 (0.6) | 32.2 (0.8) | 0.20 (0.01) | 6 |
| B | ||||
| Apo | 68 (2) | 53 (8) | 0.09 (0.03) | 9 |
| 0.5 Cu2+ | 20 (3) | 14 (3) | 0.20 (0.06) | 8 |
| 1.0 Cu2+ | 41 (3) | 28 (3) | 0.17 (0.03) | 9 |
| C | ||||
| Apo | 72 (1) | 49 (2) | 0.091 (0.006) | 9 |
| 1.0 Cu2+ | 36 (2) | 16 (4) | 0.12 (0.02) | 8 |
Kinetics parameters have been extracted from between six and nine raw traces. Mean values with 1 S.E. are given in Table 1 and supplemental Table S1. A two-tailed unpaired t test was used to confirm the significance of the difference between the kinetics with and without a Cu2+ ion.
Transmission Electron Microscopy (TEM)
Aβ samples were freeze-dried and resuspended to obtain a peptide concentration of 0.5 mg/ml. The samples were added to 200-mesh carbon-coated copper grids via the droplet method, and 2% uranyl acetate was used to negatively stain the samples. Images were collected with a JEOL JEM-2010 microscope operating at 200 kV.
Cell Viability
PC12 cells were used to assess the cytotoxic effect of different Aβ preparations (48). Cells were spun down at 95 × g for 5 min and resuspended in 1 ml of Opti-MEM. Opti-MEM was used due to its low protein concentration (15 μg/ml total protein concentration) to minimize the presence of potential competing copper chelators. A 10-μl aliquot of the cells was added to 10 μl of 3 mg/ml trypan blue, and the cells were counted. The cell stock was then diluted in Opti-MEM and added to the wells in a 96-well plate to give a typical concentration of 5 × 104 cells/ml, or 1 × 104 cells/well. Typically, cells were incubated with the Aβ preparations, and at various time points, between 1 and 6 days, 10% (v/v) alamarBlue was added. The wells were then incubated at 37 °C for a further 4–6 h to allow color development. The concentration of viable cells present is indicated by alamarBlue fluorescence. The fluorescence was measured in a Molecular Devices SpectraMax Gemini XPS, excitation at 530 nm and emission at 585 nm.
1H NMR
The pH-dependent protonation state of the three histidine side chains of Aβ were determined from 1H NMR chemical shifts of the ϵH and δH protons, recorded at 0.5-pH unit intervals between pH 5 and 9, as shown in supplemental Fig. S7. The singlet chemical shifts of the ϵ and δ protons were readily identified from the one-dimensional 1H NMR spectra. His CϵH and CδH assignments were confirmed using two-dimensional 1H TOCSY spectra, using standard acquisition parameters and a spin-lock of 60 ms. Assignment of the His6, His13, and His14 residues was based on previous 1H NMR studies with H6A, H13A, and H14A analogs (49). The Aβ(1–28) fragment was used rather than full-length Aβ(1–40) to improve solubility. 10 μm 4,4-dimethyl-4-silapentane-1-sulfonic acid was used as a reference, 0.1 mm Aβ(1–28) in 50 mm phosphate buffer, in 90% H2O, 10% D2O, at 25 °C. The pH-dependent shifts for ϵ and δ protons were fitted to a modified Hill equation to determine pKa for His6, His13, and His14 and are also presented in supplemental Fig. S7.
RESULTS AND DISCUSSION
Copper2+ and Fibril Growth Rates
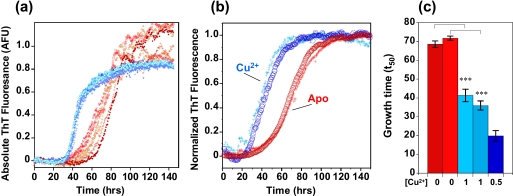
Using the well established amyloid-binding ThT fluorescence assay, we have investigated fiber formation over a range of Aβ concentrations, with and without the presence of Cu2+ ions. Aβ concentrations of >10 μm, pH 7.4, showed no detectable amyloid fibrils in the presence of 1 mol eq of Cu2+ ions (see supplemental Fig. S1), as reported previously (42, 43). However, under more dilute conditions, with Aβ between 5 and 2 μm, rapid fiber formation was detected in the presence of Cu2+ ions. Fig. 1 shows that Cu2+ ions significantly increase the rate of Aβ fiber formation at pH 7.4. In Fig. 1a, multiple ThT fluorescence traces are shown, with and without Cu2+ ions present, Fig. 1b shows normalized data from the mean of nine measurements repeated on two separate occasions (individual fluorescence traces are shown in supplemental Fig. S2). Metal-free Aβ preparations typically take more than 70 ± 2 h to reach half-maximal fluorescence (t50), whereas the same Aβ preparations with 0.5 or 1 mol eq of Cu2+ ions cause fibers to form in nearly half the time, 38 ± 2 h (Fig. 1c). A two-tailed unpaired t test confirms that Cu2+ ions significantly increase fiber growth rates with 99.9% confidence. Kinetic parameters taken from the fiber growth curves are given in Table 1 and supplemental Table S1.
FIGURE 1.
Cu2+ accelerates fiber growth. a, fiber growth curves with 0.5 mol eq Cu2+ (blue traces) and without Cu2+ (red traces) at pH 7.4. Six traces are shown for each condition, and the ThT fluorescence values are absolute. 5 μm Aβ(1–40), 50 mm HEPES buffer, 160 mm NaCl at 30 °C is shown. b, average of nine growth curves recorded on two separate occasions, apo in red, 1 mol eq of Cu2+ in blue. The fluorescence signal is normalized at maximal intensity. c, time to reach half-maximal fluorescence (t50) in two experiments with 0 (red), 1 (mid-blue), or 0.5 (dark blue) mol eq of Cu2+ ions. The presence of Cu2+ typically halves the time taken to form fibers. Error bars are for S.E. from nine traces. ***, p = 0.001.
Inspection of the growth curves indicates that both the nucleation and elongation rate are accelerated by Cu2+ ions for Aβ(1–40). However the lag time is particularly reduced by Cu2+ ions, from 49 to 16 h, for example (see Table 1). In the case of Aβ(1–42), elongation rates and total fiber content generated are significantly enhanced by the presence of Cu2+ ions (supplemental Fig. S3) whereas the lag times are less affected by Cu2+ ions. We have repeated this fiber growth experiment at a number of pH values (8.0, 8.5, and 9.0), and in each case Cu2+ increases the rate of fiber formation (supplemental Fig. S4). Studies with Cu2+ added as a Cu(glycine)2 chelate produced identical results (supplemental Fig. S5). It is notable that the total ionic strength is unaffected by the Cu2+ addition, which is constant at 160 mm NaCl.
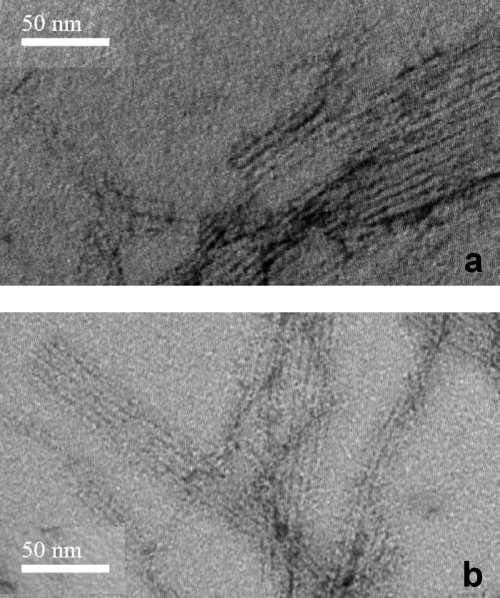
We then characterized the nature of the Cu2+-promoted amyloids. TEM images indicate the presence of fibers (Fig. 2). Based on TEM images, under these conditions, the morphology of the fibers generated appears quite similar for fibers formed with and without the presence of Cu2+ ions. We note that previous studies by other groups using TEM did not reveal fibers in the presence of Cu2+ because the high Aβ and Cu2+ concentrations used caused amorphous precipitation (42–46). Furthermore, the Cu2+-generated fibers are capable of seeding fiber formation of fresh, metal-free Aβ as indicated by a reduction in the lag time (see supplemental Fig. S6).
FIGURE 2.
TEM images of Aβ(1–42) fibrils formed in the absence (a) and presence (b) of Cu2+ ions. The morphologies of fibers formed with and without Cu2+ ions present are similar; typically long, straight, unbranched fibers are observed often stacked together. Scale bar, is 50 nm. The negatively stained fibers are generated from 3 μm Aβ(1–42) with and without 0.5 mol eq of Cu2+ ions, over 200 h with agitation every 30 min, at 30 °C in 50 mm HEPES, 160 mm NaCl. The fibrils were then freeze-dried to ∼0.5 mg/ml.
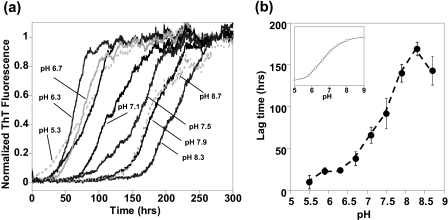
How does the presence of Cu2+ ions accelerate the rate of fiber formation? At μm concentrations of Aβ, Cu2+ does not form crossed-linked species (26, 27, 50), and the Cu2+ coordination geometry is identical in the monomer and fiber (25, 28). This rules out copper bridging to form cross-linked Aβ as a possible mechanism of accelerated fiber formation. Cu2+ coordination may trigger the Aβ misfolding that nucleates fiber assembly; however, the conformational changes in Aβ upon Cu2+ binding are small and outside of the fiber core (25, 26). It is, however, well documented that intermolecular self-association is strongly influenced by the net charge of the protein. As Aβ approaches its isoelectric point, a pI of 5.3, and an overall neutral charge, its solubility decreases (51, 52). We investigated the effect of the net charge of Aβ on the rate of fiber formation more quantitatively by varying the pH and monitoring fiber growth. The growth of Aβ fibers over a range of pH values is shown in Fig. 3a. It is clear that as the pH drops from 8.3 to 5.9 the rate at which fibers form significantly increases. Fig. 3b is a plot of lag times versus pH, lag times (tlag) reduce from 170 (±8) h at pH 8.3 to 23 (±4) h at pH 5.9.
FIGURE 3.
pH dependence of Aβ fiber formation. a, fiber growth curves (average from six traces) between pH 5.5 and 8.7, for Aβ(1–40) at 5 μm with 160 mm NaCl. The maximal ThT fluorescence signal has been normalized. b, lag times for fibril growth (tlag) versus pH. Inset, protonation state of the three histidine residues of Aβ versus pH. Data are based on 1H NMR chemical shift. The rate fibers formed increases as Aβ approaches its isoelectric point, and this is dependent on histidine protonation.
We note that pH dependence of the fiber growth rates bears a strong resemblance to the protonation state of the histidine residues and the N-terminal group within Aβ. For direct comparison we determined the protonation state of the three His residues within Aβ (His6, His13, and His14) over a range of pH values using 1H NMR chemical shift measurements (Fig. 3b inset and supplemental Fig. S7). The pKa of the His residues at 25 °C is 6.7. It appears the protonation state of the three imidazole rings and the N terminus (pKa 7.9), and consequently the net charge of Aβ, is crucial to its amyloidogenicity.
In addition to pH, the binding of metal ions will also perturb the net charge of Aβ. It is known that Cu2+ (and Zn2+) ions bind to the three histidine residues within Aβ (3, 25–28, 36, 49). At pH 7.4 Aβ histidine residues are predominantly (80%) deprotonated and neutrally charged, thus coordination of a divalent Cu2+ (or Zn2+) ion to Aβ histidines adds two positive charges. Adding two positive charges to Aβ at pH 7.4 makes the Aβ peptide complex more neutral in overall net charge, and therefore more prone to self-association, with the result that fiber growth times are almost halved.
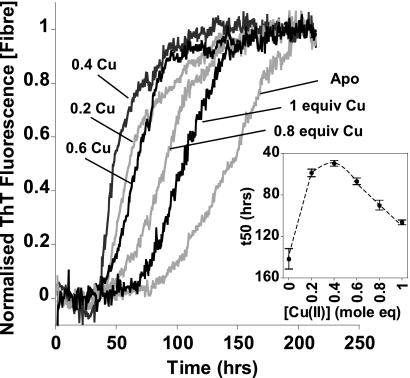
The stoichiometric effect of Cu2+ on fiber growth was investigated in more detail. All stoichiometries of Cu2+ up to 1 mol eq caused the rate of fibrillization to increase. Interestingly, substoichiometric amounts of Cu2+ between 0.2 and 0.4 mol eq display the greatest increase in fiber growth rates (Fig. 4). This supports the observation that Cu2+ accelerates nucleation (as well as elongation) as this suggests that substoichiometric amounts of Cu2+ can nucleate fiber formation. Further addition of Cu2+ beyond 1 mol eq caused precipitation of Aβ and markedly reduced the amount of fibers generated (supplemental Fig. S1), as previously noted (53, 54). The total amount of fibers generated in the presence (or absence) of Cu2+ ions can be quite variable (supplemental Fig. S8a), presumably reflecting competition between fibril formation and amorphous aggregation. Interestingly, Cu2+ ions tend to increase the total amount Aβ(1–42) fiber generated (supplemental Fig. S3). As with the effect of Cu2+ ions on Aβ(1–40), it is also notable that at lower pH values the maximal intensity of the ThT fluorescence signal is reduced (supplemental Fig. S8b). As the pH drops closer to the pI of Aβ, formation of amorphous aggregates competes with the rapid formation of ordered amyloids (55). This effect is reduced with dilution, but 2–5 μm levels of Aβ are required for reliable timely detection. This concentration-dependent process can be likened to the crystallization of proteins, in which overly precipitative conditions for self-association will cause amorphous aggregates rather than ordered crystals to form. A limitation in previous experiments that showed only amorphous aggregates was the high concentration of Aβ and Cu2+ ions used (42, 43) (typically 50 μm, 100 μm, respectively), much higher than that found in vivo.
FIGURE 4.
Effect of Cu2+ concentration on fibril growth rates. Normalized fibril growth curves with different mole equivalents of Cu2+ ions. The inset shows that fiber growth times (t50) all decrease in the presence of Cu2+ ions, from 150 h for the apo to 50 h with 0.4 mol eq of Cu2+ ions. The maximal effect is between 0.2 and 0.4 mol eq of Cu2+. With excess, 2 and 4 mol eq, of Cu2+ ions the fibril growth is completely inhibited. Growth curves represent the mean of six traces. Error bars indicate S.E. Aβ(1–40) at 5 μm in 50 mm HEPES, 160 mm NaCl, at pH 7.4, 30 °C, with intermittent agitation is shown.
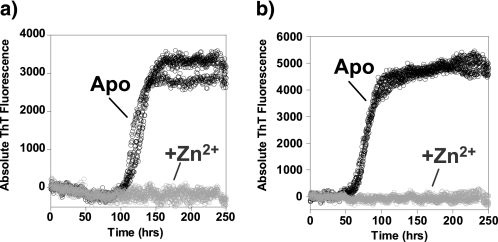
Interestingly, accelerated fibril formation appears to be quite specific for Cu2+ ions, Zn2+ ions completely inhibit fiber formation even at 3 μm Aβ(1–40) (Fig. 5). This may be due to the very different coordination geometry (at micromolar concentration) between the two metal ions. Cu2+ ions form an intramolecular complex with Aβ (25–28) whereas at micromolar levels current data suggest that Zn2+ will form an intermolecular complex, cross-linking between histidine residues on multiple Aβ molecules (34, 36, 49, 56). These cross-linked Zn2+-Aβ species will inhibit amyloids forming by interfering with the regular cross-beta assembly.
FIGURE 5.
Zn2+ inhibits fibril growth. Fibril growth curves with 1 mol eq of Zn2+ ions (gray) and metal free (black) in (a) 3 μm Aβ(1–40), pH 7.4 or (b) 4 μm Aβ(1–40), pH 7.4, is shown. The experiment was carried out in 50 mm HEPES, 160 mm NaCl, 30 °C, with agitation. Three to six traces for each experiment are shown.
Copper and Aβ Cell Toxicity
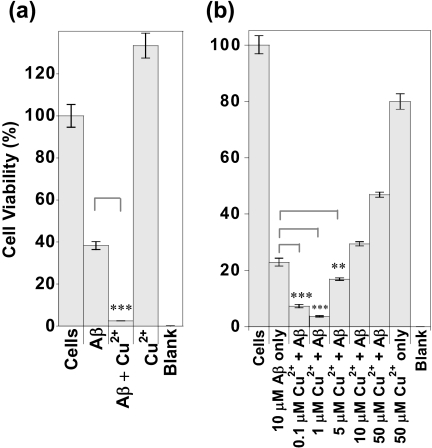
We wanted to relate the ability of Cu2+ ions to promote fiber formation of Aβ to the cell toxicity. We have added both monomeric Aβ and fibrillar Aβ to PC12 cells with and without the presence of Cu2+ ions. Cu2+ ions bound to Aβ are more cytotoxic than Aβ in the absence of Cu2+ ions, whereas the same levels of Cu2+ in the absence of Aβ are not toxic to the cells. Fig. 6a shows that Aβ(1–42) fibers incubated with PC12 cells are toxic (40% viability), whereas generation of Aβ fibers in the presence of Cu2+ ions (a half-mol eq in Cu2+ ions) makes the Aβ(1–42) fibers considerably more toxic (only 4% viability). This experiment was repeated on a number of occasions, and each time the presence of Cu2+ ions consistently enhanced Aβ toxicity to PC12 cells. Control studies show that the same levels of Cu2+ ions (2.5 μm) are not toxic to the cells. Supplemental Fig. S9a shows that free Cu2+ ions over a range of concentrations have no detectable toxic effect; Cu2+ ions were added to the cell medium as CuCl2. A further control in which Cu2+ was bound to the nonamyloidogenic Cu2+-binding fragment, Aβ(1–16), was studied. Cu2+ ions when bound to Aβ(1–16) are not toxic (supplemental Fig. S9b), showing that the toxic effects of Cu2+ are specific to their interaction with Aβ(1–42) and Aβ(1–40). The use of Aβ(1–16) is a particularly good control as Cu2+ binds to the shorter fragment of Aβ with the same affinity and coordination geometry (25–28), but Aβ(1–16) lacks the amyloidogenic region and does not form fibrils.
FIGURE 6.
Cell viability, Cu2+-Aβ is more cytotoxic than Aβ. a, Aβ(1–42) as preformed fibrils, 3 μm, with and without the presence of 1.5 μm Cu2+ was added to PC12 cells to give a concentration in the well of 2.7 μm Aβ and 1.35 μm Cu2+. Cell concentration was 5.9 × 104 cells/ml. 2.5 μm Cu2+ was added to the cells alone to test Cu2+ toxicity. Blank is buffer only. All preparations were incubated with the cells for 24 h, and then 10% (v/v) alamarBlue was added. The data shown here are after total incubation for 70 h. b, 10 μm monomeric Aβ(1–40) with a range of Cu2+ concentrations was incubated with PC12 cells for 6 days. AlamarBlue was then added and the reading taken. Cell concentration was 5.4 × 104 cells/ml. All cell experiments were conducted in Opti-MEM, pH 7.4. Error bars are S.E., n = 3. ***, p = 0.001; **, p = 0.02.
Next, we were interested in the ratios of Cu2+ to Aβ that caused toxicity. As little as 0.01 mol eq of Cu2+ (100 nm) was found to be significantly more toxic than Aβ(1–40) fibers in the absence of Cu2+ ions (Fig. 6b). With 0.1 mol eq of Cu2+, bound Aβ was even more toxic, whereas 0.5 mol eq of Cu2+ ions were also more toxic than Aβ(1–40) in the absence of Cu2+ ions. However, at the Cu2+ ratio of 1:1 mol eq, the toxic effects of Cu2+ are lost. Indeed at, 5 mol eq of Cu2+, relative to Aβ(1–40), Cu2+ is actually protective (Fig. 6b). Interestingly, these observations can be directly related to the optimum ratio of Cu2+-Aβ that generates amyloid fibers, as shown in Fig. 4. We have already shown that substoichiometric amounts of Cu2+ (0.2–0.4 mol eq) are more effective at generating amyloid fibers, whereas Cu2+ levels above 1 mol eq will actually inhibit fiber formations (supplemental Fig. S1b). Clearly, the influence of Cu-Aβ ratios on Aβ toxicity relate closely to the ability of Cu2+ to accelerate (or inhibit) fiber formation.
Both preformed fibrils (Fig. 6a) and Aβ added as a monomer (Fig. 6b) are toxic to cells. The toxic effects of fibrillar Aβ are apparent within a day of addition to the cell medium. However, addition of monomeric Aβ(1–40) shows no significant cell cytotoxicity within 2 days of incubation; it is only after this time that the toxic effects are apparent. Fiber growth in the cell medium, detected using a ThT assay, suggests that fibers will take a few days to form, supporting the hypothesis that cytotoxicity observed correlates with the rate at which Aβ fibers are generated.
Over a range of concentrations of fibrillar Aβ(1–42), Cu2+ ions increase Aβ toxic effects, shown in supplemental Fig. S10. Counterintuitively, at lower Aβ(1–42) concentrations a greater toxic effect is observed. We suggest that a larger amount of toxic species is present because more protofibrils and fewer amorphous aggregates are generated, at the more dilute concentrations of Aβ(1–42). Interestingly, Aβ(1–42) at 4.5 μm has almost no toxic effect; 89 (±14)% viability is observed. The addition of Cu2+ ions Aβ(1–42) becomes markedly toxic with 19 (±0.4)% cell viability.
There are a few studies already published investigating the relationship between copper and Aβ cell toxicity (19, 20, 24, 42). These studies appear to show conflicting results; in one study copper promoted toxicity (19), and in another it appeared to protect against toxicity (42). In these studies the nature of the Aβ preparation was not well defined (i.e. amorphous aggregate, monomer or fiber), and this may be the source of the discrepancies. Here we are able to correlate the kinetics of fibril formation in the presence of Cu2+ ions with the severity of cytotoxicity. The levels of Cu2+ used relative to Aβ can now explain contradictory observations in the literature. This effect is highlighted in Fig. 6b where substoichiometric levels of Cu2+ significantly reduce cell viability, whereas suprastoichiometric levels are actually protective. Clearly, lower substoichiometric levels of Cu2+ are the more physiologically relevant case, suggesting a role for Cu2+ ions in enhancing Aβ toxicity.
There are potentially two reasons for the enhanced toxicity of Aβ in the presence of Cu2+ ions. Diffusible oligomers of Aβ could bind Cu2+, resulting in a concentration of Cu2+ ions at the neuronal cell surface, where Cu2+ would generate toxic hydrogen peroxide and hydroxyl radicals. Indeed, Aβ oligomers are found clustered at synaptic terminals (57) and cause memory loss due to synapse failure (58). Redox-active Cu2+ ions released at synaptic terminals will cause lipid peroxidation at the cell membrane and so compromise cell integrity (19, 21, 59), leading to the neuron loss characteristic of AD. The observation that the antioxidant protein catalase and the Cu2+ chelator metallothionein III are protective strongly supports this hypothesis (18–20, 24). Alternatively, the Cu2+ ions could promote the formation of protofibrillar/fibrillar Aβ species that are toxic to the cells. The rate of production, quantity, or morphology of the Cu2+-promoted fibers and protofibrillar oligomers may cause the heightened cytotoxicity. We conclude that if it was simply a matter of reactive oxygen species generation by Cu2+ ions bound to Aβ then one might expect the more Cu2+ present, the greater the toxicity; however, in Fig. 6b we observe that small amounts of Cu2+ ions (0.1 μm) are more toxic than 50 times as much (5 μm) Cu2+ ions. Thus, the ability of Cu2+ to promote fibers (and by inference protofibrillar species) appears to be the significant factor in reactive oxygen species promoted Aβ cell toxicity.
CONCLUSIONS
Cu2+-Aβ is more toxic to PC12 cells than Aβ on its own; furthermore, cytotoxic effects are related to the ability of Cu2+ ions to promote amyloid fibers and protofibrils. We suggest that Cu2+ ions increase the rate of fiber formation, at pH 7.4, by causing Aβ to approach its isoelectric point. To put our observation in context, the increase in fiber growth rates measured here due to Cu2+ ions is comparable with that observed for (metal-free) Aβ(1–40) mutants associated with familial early onset AD (E22K/G/Q), where a halving of the growth times (t50) of fiber formation is also reported (60). Metals have also been proposed as triggers for other misfolding and assembly diseases such as dialysis-related amyloidosis (61), Parkinson disease (62), and prion diseases (63, 64), although it remains to be established whether the mechanisms by which metals induce fibrillization are shared. Our observations provide a rationale for the in vivo observations in Drosophilia and mammals which link the AD phenotype with impaired Cu2+ homeostasis (6, 7). It is known that Cu2+ levels in the brain increase with age (2); thus, our observations should refocus attention on loss of Cu2+ homeostasis as a possible risk factor in AD. Cu2+ chelators are being investigated in clinical trials as a potential therapy for AD (2, 65, 66).
Supplementary Material
Acknowledgments
We thank Harold Toms for assistance with NMR, John Nield and Zofia Luklinska for assistance with TEM, and Henry Clarke and Neveen Hosny for assistance with growth curve analysis.
This work was supported by Biotechnology and Biological Sciences Research Council (BBSRC) Project Grant BBD0050271 and a BBSRC quota studentship.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S10.
- AD
- Alzheimer disease
- Aβ
- amyloid-β-peptide
- TEM
- transmission electron microscopy
- ThT
- thioflavin T.
REFERENCES
- 1.Hardy J., Selkoe D. J. (2002) Science 297, 353–356 [DOI] [PubMed] [Google Scholar]
- 2.Barnham K. J., Bush A. I. (2008) Curr. Opin. Chem. Biol. 12, 222–228 [DOI] [PubMed] [Google Scholar]
- 3.Dong J., Atwood C. S., Anderson V. E., Siedlak S. L., Smith M. A., Perry G., Carey P. R. (2003) Biochemistry 42, 2768–2773 [DOI] [PubMed] [Google Scholar]
- 4.Lovell M. A., Robertson J. D., Teesdale W. J., Campbell J. L., Markesbery W. R. (1998) J. Neurol. Sci. 158, 47–52 [DOI] [PubMed] [Google Scholar]
- 5.Miller L. M., Wang Q., Telivala T. P., Smith R. J., Lanzirotti A., Miklossy J. (2006) J. Struct. Biol. 155, 30–37 [DOI] [PubMed] [Google Scholar]
- 6.Sanokawa-Akakura R., Cao W., Allan K., Patel K., Ganesh A., Heiman G., Burke R., Kemp F. W., Bogden J. D., Camakaris J., Birge R. B., Konsolaki M. (2010) PLoS One 5, e8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparks D. L., Schreurs B. G. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11065–11069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phinney A. L., Drisaldi B., Schmidt S. D., Lugowski S., Coronado V., Liang Y., Horne P., Yang J., Sekoulidis J., Coomaraswamy J., Chishti M. A., Cox D. W., Mathews P. M., Nixon R. A., Carlson G. A., St. George-Hyslop P., Westaway D. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14193–14198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer T. A., Schäfer S., Simons A., Kemmling A., Kamer T., Tepest R., Eckert A., Schüssel K., Eikenberg O., Sturchler-Pierrat C., Abramowski D., Staufenbiel M., Multhaup G. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14187–14192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crouch P. J., Hung L. W., Adlard P. A., Cortes M., Lal V., Filiz G., Perez K. A., Nurjono M., Caragounis A., Du T., Laughton K., Volitakis I., Bush A. I., Li Q. X., Masters C. L., Cappai R., Cherny R. A., Donnelly P. S., White A. R., Barnham K. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yankner B. A., Lu T. (2009) J. Biol. Chem. 284, 4755–4759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glabe C. G. (2008) J. Biol. Chem. 283, 29639–29643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer-Luehmann M., Spires-Jones T. L., Prada C., Garcia-Alloza M., de Calignon A., Rozkalne A., Koenigsknecht-Talboo J., Holtzman D. M., Bacskai B. J., Hyman B. T. (2008) Nature 451, 720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urbanc B., Cruz L., Le R., Sanders J., Ashe K. H., Duff K., Stanley H. E., Irizarry M. C., Hyman B. T. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 13990–13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roychaudhuri R., Yang M., Hoshi M. M., Teplow D. B. (2009) J. Biol. Chem. 284, 4749–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butterfield D. A., Reed T., Newman S. F., Sultana R. (2007) Free Radic. Biol. Med. 43, 658–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rival T., Page R. M., Chandraratna D. S., Sendall T. J., Ryder E., Liu B., Lewis H., Rosahl T., Hider R., Camargo L. M., Shearman M. S., Crowther D. C., Lomas D. A. (2009) Eur. J. Neurosci. 29, 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behl C., Davis J. B., Lesley R., Schubert D. (1994) Cell 77, 817–827 [DOI] [PubMed] [Google Scholar]
- 19.Opazo C., Huang X., Cherny R. A., Moir R. D., Roher A. E., White A. R., Cappai R., Masters C. L., Tanzi R. E., Inestrosa N. C., Bush A. I. (2002) J. Biol. Chem. 277, 40302–40308 [DOI] [PubMed] [Google Scholar]
- 20.Huang X., Atwood C. S., Hartshorn M. A., Multhaup G., Goldstein L. E., Scarpa R. C., Cuajungco M. P., Gray D. N., Lim J., Moir R. D., Tanzi R. E., Bush A. I. (1999) Biochemistry 38, 7609–7616 [DOI] [PubMed] [Google Scholar]
- 21.Nadal R. C., Rigby S. E., Viles J. H. (2008) Biochemistry 47, 11653–11664 [DOI] [PubMed] [Google Scholar]
- 22.Barnham K. J., Haeffner F., Ciccotosto G. D., Curtain C. C., Tew D., Mavros C., Beyreuther K., Carrington D., Masters C. L., Cherny R. A., Cappai R., Bush A. I. (2004) FASEB J. 18, 1427–1429 [DOI] [PubMed] [Google Scholar]
- 23.Hureau C., Faller P. (2009) Biochimie 91, 1212–1217 [DOI] [PubMed] [Google Scholar]
- 24.Meloni G., Sonois V., Delaine T., Guilloreau L., Gillet A., Teissié J., Faller P., Vasák M. (2008) Nat. Chem. Biol. 4, 366–372 [DOI] [PubMed] [Google Scholar]
- 25.Sarell C. J., Syme C. D., Rigby S. E., Viles J. H. (2009) Biochemistry 48, 4388–4402 [DOI] [PubMed] [Google Scholar]
- 26.Syme C. D., Nadal R. C., Rigby S. E., Viles J. H. (2004) J. Biol. Chem. 279, 18169–18177 [DOI] [PubMed] [Google Scholar]
- 27.Drew S. C., Noble C. J., Masters C. L., Hanson G. R., Barnham K. J. (2009) J. Am. Chem. Soc. 131, 1195–1207 [DOI] [PubMed] [Google Scholar]
- 28.Karr J. W., Szalai V. A. (2008) Biochemistry 47, 5006–5016 [DOI] [PubMed] [Google Scholar]
- 29.Shin B. K., Saxena S. (2008) Biochemistry 47, 9117–9123 [DOI] [PubMed] [Google Scholar]
- 30.Damante C. A., Osz K., Nagy Z., Pappalardo G., Grasso G., Impellizzeri G., Rizzarelli E., Sovago I. (2008) Inorg. Chem. 47, 9669–9683 [DOI] [PubMed] [Google Scholar]
- 31.Hou L., Zagorski M. G. (2006) J. Am. Chem. Soc. 128, 9260–9261 [DOI] [PubMed] [Google Scholar]
- 32.Streltsov V. A., Titmuss S. J., Epa V. C., Barnham K. J., Masters C. L., Varghese J. N. (2008) Biophys. J. 95, 3447–3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antzutkin O. N. (2004) Magn. Reson. Chem. 42, 231–246 [DOI] [PubMed] [Google Scholar]
- 34.Minicozzi V., Stellato F., Comai M., Serra M. D., Potrich C., Meyer-Klaucke W., Morante S. (2008) J. Biol. Chem. 283, 10784–10792 [DOI] [PubMed] [Google Scholar]
- 35.Dorlet P., Gambarelli S., Faller P., Hureau C. (2009) Angew Chem. Int. Ed. Engl. 48, 9273–9276 [DOI] [PubMed] [Google Scholar]
- 36.Faller P., Hureau C. (2008) Dalton Trans. 2008, 1080–1094 [DOI] [PubMed] [Google Scholar]
- 37.Faller P. (2009) ChemBioChem 10, 2837–2845 [DOI] [PubMed] [Google Scholar]
- 38.Vigo-Pelfrey C., Lee D., Keim P., Lieberburg I., Schenk D. B. (1993) J. Neurochem. 61, 1965–1968 [DOI] [PubMed] [Google Scholar]
- 39.Kardos J., Kovács I., Hajós F., Kálmán M., Simonyi M. (1989) Neurosci. Lett. 103, 139–144 [DOI] [PubMed] [Google Scholar]
- 40.Bush A. I., Pettingell W. H., Multhaup G., d Paradis M., Vonsattel J. P., Gusella J. F., Beyreuther K., Masters C. L., Tanzi R. E. (1994) Science 265, 1464–1467 [DOI] [PubMed] [Google Scholar]
- 41.Atwood C. S., Moir R. D., Huang X., Scarpa R. C., Bacarra N. M., Romano D. M., Hartshorn M. A., Tanzi R. E., Bush A. I. (1998) J. Biol. Chem. 273, 12817–12826 [DOI] [PubMed] [Google Scholar]
- 42.Yoshiike Y., Tanemura K., Murayama O., Akagi T., Murayama M., Sato S., Sun X., Tanaka N., Takashima A. (2001) J. Biol. Chem. 276, 32293–32299 [DOI] [PubMed] [Google Scholar]
- 43.Raman B., Ban T., Yamaguchi K., Sakai M., Kawai T., Naiki H., Goto Y. (2005) J. Biol. Chem. 280, 16157–16162 [DOI] [PubMed] [Google Scholar]
- 44.Ha C., Ryu J., Park C. B. (2007) Biochemistry 46, 6118–6125 [DOI] [PubMed] [Google Scholar]
- 45.Tõugu V., Karafin A., Zovo K., Chung R. S., Howells C., West A. K., Palumaa P. (2009) J. Neurochem. 110, 1784–1795 [DOI] [PubMed] [Google Scholar]
- 46.Innocenti M., Salvietti E., Guidotti M., Casini A., Bellandi S., Foresti M. L., Gabbiani C., Pozzi A., Zatta P., Messori L. (2010) J. Alzheimers Dis. 19, 1323–1329 [DOI] [PubMed] [Google Scholar]
- 47.Morris A. M., Watzky M. A., Agar J. N., Finke R. G. (2008) Biochemistry 47, 2413–2427 [DOI] [PubMed] [Google Scholar]
- 48.Simakova O., Arispe N. J. (2007) J. Neurosci. 27, 13719–13729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syme C. D., Viles J. H. (2006) Biochim. Biophys. Acta 1764, 246–256 [DOI] [PubMed] [Google Scholar]
- 50.Talmard C., Guilloreau L., Coppel Y., Mazarguil H., Faller P. (2007) ChemBioChem 8, 163–165 [DOI] [PubMed] [Google Scholar]
- 51.Hortschansky P., Schroeckh V., Christopeit T., Zandomeneghi G., Fändrich M. (2005) Protein Sci. 14, 1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo M., Gorman P. M., Rico M., Chakrabartty A., Laurents D. V. (2005) FEBS Lett. 579, 3574–3578 [DOI] [PubMed] [Google Scholar]
- 53.Smith D. P., Ciccotosto G. D., Tew D. J., Fodero-Tavoletti M. T., Johanssen T., Masters C. L., Barnham K. J., Cappai R. (2007) Biochemistry 46, 2881–2891 [DOI] [PubMed] [Google Scholar]
- 54.Jun S., Gillespie J. R., Shin B. K., Saxena S. (2009) Biochemistry 48, 10724–10732 [DOI] [PubMed] [Google Scholar]
- 55.Wood S. J., Maleeff B., Hart T., Wetzel R. (1996) J. Mol. Biol. 256, 870–877 [DOI] [PubMed] [Google Scholar]
- 56.Miller Y., Ma B., Nussinov R. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 9490–9495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacor P. N., Buniel M. C., Chang L., Fernandez S. J., Gong Y., Viola K. L., Lambert M. P., Velasco P. T., Bigio E. H., Finch C. E., Krafft G. A., Klein W. L. (2004) J. Neurosci. 24, 10191–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 59.Murray I. V., Sindoni M. E., Axelsen P. H. (2005) Biochemistry 44, 12606–12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakami K., Irie K., Morimoto A., Ohigashi H., Shindo M., Nagao M., Shimizu T., Shirasawa T. (2003) J. Biol. Chem. 278, 46179–46187 [DOI] [PubMed] [Google Scholar]
- 61.Calabrese M. F., Eakin C. M., Wang J. M., Miranker A. D. (2008) Nat. Struct. Mol. Biol. 15, 965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uversky V. N., Li J., Fink A. L. (2001) J. Biol. Chem. 276, 44284–44296 [DOI] [PubMed] [Google Scholar]
- 63.Brown D. R., Qin K., Herms J. W., Madlung A., Manson J., Strome R., Fraser P. E., Kruck T., von Bohlen A., Schulz-Schaeffer W., Giese A., Westaway D., Kretzschmar H. (1997) Nature 390, 684–687 [DOI] [PubMed] [Google Scholar]
- 64.Klewpatinond M., Davies P., Bowen S., Brown D. R., Viles J. H. (2008) J. Biol. Chem. 283, 1870–1881 [DOI] [PubMed] [Google Scholar]
- 65.Cherny R. A., Atwood C. S., Xilinas M. E., Gray D. N., Jones W. D., McLean C. A., Barnham K. J., Volitakis I., Fraser F. W., Kim Y., Huang X., Goldstein L. E., Moir R. D., Lim J. T., Beyreuther K., Zheng H., Tanzi R. E., Masters C. L., Bush A. I. (2001) Neuron 30, 665–676 [DOI] [PubMed] [Google Scholar]
- 66.Crouch P. J., White A. R., Bush A. I. (2007) FEBS J. 274, 3775–3783 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.