Abstract
Protein aggregation is a hallmark of many diseases, including amyotrophic lateral sclerosis (ALS) where aggregation of copper/zinc superoxide dismutase (SOD1) is implicated in pathogenesis. We report here that fully metallated (holo) SOD1 under physiologically relevant solution conditions can undergo changes in metallation and/or dimerization over time and form aggregates that do not exhibit classical characteristics of amyloid. The relevance of the observed aggregation to disease is demonstrated by structural and tinctorial analyses, including the novel observation of binding of an anti-SOD1 antibody that specifically recognizes aggregates in ALS patients and mice models. ALS-associated SOD1 mutations can promote aggregation but are not essential. The SOD1 aggregation is characterized by a lag phase, which is diminished by self- or cross-seeding and by heterogeneous nucleation. We interpret these findings in terms of an expanded aggregation mechanism consistent with other in vitro and in vivo findings that point to multiple pathways for the formation of toxic aggregates by different forms of SOD1.
Keywords: Amyotropic Lateral Sclerosis (Lou Gehrig Disease), Neurological Diseases, Protein Assembly, Protein-Protein Interactions, Superoxide Dismutase (SOD), SEDI, Seeding Effects
Introduction
Amyotrophic lateral sclerosis (ALS)2 is a devastating, rapidly progressive, and invariably fatal neurodegenerative disease, characterized by motor neuron degeneration and paralysis (1). Approximately 10% of ALS cases are familial (fALS), the remaining cases being sporadic (sALS). The familial and sporadic diseases are clinically indistinguishable and so have been proposed to share common disease mechanisms (1). Mutations in copper/zinc superoxide dismutase (SOD1) account for ∼20% of fALS and represent a major known cause of the disease. It is generally accepted that fALS-linked SOD1 mutations result in a toxic gain of function rather than a loss of function (1). Much attention has focused on toxic protein aggregation as causing ALS, analogous to pathogenic protein aggregation in other neurodegenerative diseases, including Alzheimer, Huntington, Parkinson, and prion diseases (1–5). SOD1 is present in aggregates in motor neurons of SOD1-linked fALS patients (3, 6) and mice models (3, 7–9) and in some sALS patients (4, 5, 10).
Mature SOD1 is a homodimeric protein, with each β-barrel subunit containing one catalytic copper ion, one structural zinc ion, one intrasubunit disulfide bond, and two free cysteines (11) (see Fig. 1). More than 147 mainly missense mutations throughout the SOD1 structure have been associated with fALS. Aggregation has been reported previously only for immature (metal and/or disulfide deficient) or aberrant forms of SOD1, often under highly destabilizing conditions, which favor aggregation in general (12–19). The relevance to human disease of such aggregation is not known, nor is it known what forms of SOD1 may give rise to or be present in aggregates in patients (20). Mature (holo) SOD1 is a major form of SOD1 in cells for wild type and most mutant SOD1s (11). Although this form has been proposed to be incompatible with aggregation because of its very high stability (15, 21), several studies have reported observation of aggregation for metallated ALS-associated mutant SOD1s, which may be caused by the loss of metals and/or dimer dissociation (13, 22–25). Thus, it may be necessary to look beyond the immature forms of SOD1 to determine how SOD1 may be involved in ALS pathology.
FIGURE 1.
Structure of copper/zinc SOD1. A, ribbon representation of dimeric holo pWT (34) (Protein Data Bank code 1SOS). Copper and zinc ions are shown as orange and black spheres, respectively. The intrasubunit disulfide bond between cysteines 57 and 146 is shown in yellow space filling representation. The two active site loops, electrostatic loop VII (residues 121–142) and loop IV (residues 49–82), are colored blue and green, respectively. Loop IV is composed of three regions: the dimer interface region (residues 49–54), the disulfide bond region (residues 55–61), and the zinc-binding region (residues 62–82) (82). Sites of fALS mutations are colored red and labeled. B, the epitope (residues 143–151, red) for SEDI antibody is buried in the dimer form of SOD1 (left panel) and exposed in the monomer form (right panel). The dimer view is the same as in A, but the monomer is rotated to more clearly show the epitope. The figure was made using Molmol (98) and Pymol for A and B, respectively.
Recently, evidence for pathological protein aggregation under mild solution conditions via states that are close to native has been accumulating (26, 27). We report here that incubation of holo SOD1s under physiologically relevant conditions leads to decreases in activity, altered metal binding, and formation of aggregates that have common structural, tinctorial, as well as immunological characteristics with those of aggregates formed in fALS patients. The aggregation process exhibits lag phase, seeding, and heterogeneous nucleation characteristics, similar to aggregation of other disease-associated proteins. The findings have important implications for understanding protein aggregation mechanisms in ALS and other neurodegenerative diseases, as well as other diseases where aggregation may occur from the native state.
EXPERIMENTAL PROCEDURES
Preparation of Holo pWT and Mutant SOD1s
SOD1 proteins were expressed, purified, and filtered (0.2 μm) prior to storage (−80 °C), and full metallation and proper folding were carefully verified by measuring: specific activity using a pyrogallol activity assay (activity absolutely requires copper), differential scanning calorimetry (stability is highly dependent on metallation), and inductively coupled plasma atomic emission spectroscopy (ICP-AES) to measure metal content using established methods (19, 28, 29) (see supplementary text and supplemental Tables S1 and S2).
Holo SOD1 Aggregation
Samples for aggregation experiments were prepared by concentrating proteins by ultrafiltration (Nanosep 3K Omega; PALL Corporation) and then diluting to the desired final concentration, which was generally 10 mg/ml (317 μm) unless otherwise stated, in 20 mm HEPES, pH 7.8. Unless otherwise stated, the samples were then filtered (20 nm Anotop 10 Plus; Whatman) to remove dust and preformed aggregates. The samples were incubated without agitation at 37 °C in 4-ml glass vials (VWR) sealed with parafilm to minimize evaporation. To facilitate comparisons among mutant holo SOD1s, a consistent frequency of measurements was used for all samples. The samples were measured two times/day during the lag time, three times/day during rapid growth phase, and one time/day at the plateau phase.
Light Scattering Measurements
Time average light scattering and dynamic light scattering were measured using a 90Plus Particle Sizer (Brookhaven Instruments, Inc.) or a Zetasizer Nano-ZS (Malvern), which gave consistent results. The reported light scattering values are the averages of three measurements, each consisting of 10 consecutive 30-s data accumulations. For all SOD1 variants, at least three independent time courses were measured, unless otherwise stated.
Activity of Soluble SOD1 during Aggregation
The activity of the remaining soluble SOD1 during aggregation time courses was determined by removing a sample aliquot, diluting this 75-fold in water to a suitable concentration for activity measurement, centrifuging (14,000 × g, 8 min using a microcentrifuge) to remove aggregates, and determining the activity of SOD1 samples by inhibition of pyrogallol auto-oxidation activity assay (30). The protein concentration after centrifugation was determined by Lowry assay (31) and used to calculate specific activity. To minimize assay variability, specific activity was also determined in parallel for control nonaggregated pWT, and sample activity was determined as the ratio of specific activity of the aggregated sample relative to nonaggregated pWT.
Data Fitting for Aggregation and Nucleation Mechanism
Aggregation profiles were fit to an empirical equation for a sigmoidal curve,
 |
where Yi and Yf are the initial and average plateau light scattering intensities, respectively; x0 is the time of half completion; and dx is the time constant (32). The lag time was calculated as follows.
To gain insight into aggregation mechanism, the initial 10–20% of the light scattering aggregation time course data were fit as per Ferrone et al. (33) models for primary nucleation
and secondary nucleation
where A is the fitted amplitude, B is an effective rate, and t is time (33). All of the fits were performed in Origin 7.5. The two-tailed Student's t test was used for determining p values.
Seeding Effects
Aliquots of preformed aggregates were added to freshly prepared holo aggregation samples (10% (w/w)) and aggregation monitored by light scattering as above. The aliquots contained either “early” or “late” seeds obtained at the end of the lag phase or the plateau phase, respectively. Cross-seeding experiments were performed by adding early seeds of different isoforms of SOD1 to fresh pWT solution (3 mg/ml).
Atomic Force Microscopy (AFM)
Aliquots of aggregation samples at various time points were diluted in MilliQ water to a suitable concentration for AFM imaging (∼0.5 μg/ml) and then centrifuged (10,000 rpm, 6 min). Diluted nonaggregated (control) and aggregated (obtained from the bottom of the centrifuge tube) protein solutions were applied to freshly cleaved mica, rinsed with MilliQ water, and air-dried overnight. Imaging was performed at room temperature using a PicoScanTM atomic force microscope (Molecular Imaging) using the tapping mode. All of the images were acquired using a 225-μm silicon single-crystal cantilever (NCL type) with a typical tip radius of 10 nm and resonance frequency of ∼170 kHz. A scanner with the maximum scan size of 6 × 6 μm2 was used. The images were obtained at a resolution of 512 × 512 pixels on a scale of 1 μm × 1 μm.
Competitive ELISA
SOD1 exposed dimer interface (SEDI) polyclonal antibody from rabbit was prepared using C-terminal peptide (SOD1 residues 143–151, which are located in the dimer interface; see Fig. 1) and was kindly provided by Janice Robertson (7). SEDI was used in competitive ELISA assays on nonaggregated (control) and aggregated pWT, which were analyzed in parallel to ensure accurate comparison. C-terminal peptide was first added to the assay plate (0.05 μg/well), which was incubated for 1 h at room temperature, and then washed three times using PBS containing 0.05% (v/v) of Tween 20 (PBST) followed by two washes with PBS solution. Next, blocking solution (1% (w/v) BSA in PBS) was added to the plate, which was incubated overnight at 4 °C, and then washed two times with PBST followed by three washes with PBS. Meanwhile, SEDI antibody at a range of dilutions in blocking solution was combined with either nonaggregated SOD1 or aggregated SOD1 from the plateau phase (both at final SOD1 concentration of 180 nm) or with no added SOD1 and incubated for 2 h at room temperature with gentle shaking. The higher the level of exposed SOD1 dimer interface in the sample, the less SEDI will be able to bind to C-terminal peptide in the well. The SEDI solutions were added to the plates (50 μl/well), incubated for 35 min at room temperature with no agitation, and then washed five times with PBST followed by two washes with PBS. HRP-conjugated anti-rabbit secondary antibody (Pierce), diluted in blocking solution (1:2000), was added to the plate and incubated for 80 min at room temperature, and the plate was washed five times using PBST followed by two washes with PBS. For visualization, 100 μl/well of ABTS substrate (Pierce) was added, incubated for 30 min at room temperature, and read at 405 nm using a plate reader (SpectramaxPlus384, Molecular Devices). In a given assay, each sample was measured in triplicate, and three independent assays were performed. Solutions of SEDI antibody with no added SOD1 gave higher readings than those with added SOD1, as expected. The readings from the samples of SEDI with either nonaggregated or aggregated pWT were normalized against the reading for the sample of SEDI without SOD1.
RESULTS
Incubation of Holo SOD1s Results in Aggregate Formation
Aggregation arising from the holo form of SOD1 was followed using light scattering to measure particle formation as a function of time. The experiments were conducted at close to physiological conditions of temperature (37 °C), pH (7.8), and protein concentration (3–30 mg/ml; 95–952 μm) (18). A well established pseudo wild type (pWT) background was used in which the free cysteines at positions 6 and 111 are mutated to alanine and serine, respectively. The pWT has very similar structure, dynamics, stability, and activity to wild type SOD1, but aberrant disulfide bond formation caused by oxidation of free cysteines is eliminated (34, 35). Moreover, the role of the free cysteines in the formation of pathological aggregates in mice models is suggested to be limited (8). Thus, pWT is a useful model for characterizing the effects of mutations on SOD1 folding and aggregation. Diverse ALS-associated mutations were studied: G85R, which disrupts metal binding (36); A4V, A4T, A4S, and I149T in the dimer interface; and G93V, G93S, G93A and G93D at a mutational hot spot in a loop (Fig. 1).
Prior to the aggregation experiments, the proper metallation and folding of holo SOD1 preparations were carefully confirmed by differential scanning calorimetry and pyrogallol activity assay and in torch vaporization inductively coupled plasma atomic emission spectroscopy (ITV-ICP-AES) (see “Experimental Procedures”; also see supplemental text, Fig. S1, and Tables S1 and S2). Also, samples were filtered using a 20-nm cut-off filter and did not contain detectable initial (t = 0 h) aggregates based on light scattering measurements. These measurements are highly sensitive to the presence of larger species because of the dependence of scattering intensity on the sixth power of the radius of the scattering species (37). The initial light scattering measurements clearly show: 1) a single exponential autocorrelation function for all samples, indicating that the solutions are monodisperse, i.e. consist of a single species with hydrodynamic radius of ∼5 nm (as expected for a protein the size of the SOD1 native dimer (38)) (supplemental Fig. S2); and 2) consistently low light scattering intensities. In contrast, samples that were not filtered showed shorter lag times (supplemental Fig. S3). However, some of the (filtered) SOD1 aggregation profiles also showed quite short or no appreciable lag times (supplemental Fig. S4), suggesting that minute quantities of aggregates or seeds (not detectable by light scattering) may sometimes be present initially and facilitate further aggregation. These results show that samples initially consisted of highly homogeneous, fully metallated SOD1. The obvious changes observed upon subsequent sample incubation cannot be accounted for by the possible presence of a small fraction of mismetallated or aggregated protein and thus must arise from holo SOD1 (see below).
Upon incubating the samples, all of the holo SOD1s, including pWT, gave rise to aggregation, exhibiting sigmoidal-like increases in light scattering intensity with time (Fig. 2) that resemble those of many other disease-associated proteins (39). The observation of aggregation for pWT supports the hypothesis that SOD1 aggregation may also contribute to the pathology of sALS (4, 5) (see “Discussion”).
FIGURE 2.
Representative sigmoidal aggregation profiles for holo SOD1s monitored by light scattering. pWT, G85R, A4V, A4T, A4S, I149T, G93D, G93V, G93S, and G93A (from left to right and top to bottom). Dashed lines show the fit of the data to Equation 1, an empirical sigmoidal equation for aggregation. The R2 value of 0.986 (pWT), 0.963 (G85R), 0.847 (A4V), 0.977 (A4T), 0.844 (A4S), 0.969 (I149T), 0.990 (G93D), 0.984 (G93V), 0.971 (G93S), and 0.969 (G93A) are from the fitting. Plotted values (●) and error bars are the means and S.D. for three measurements (see “Experimental Procedures”). Some error bars are smaller than the symbols. An arrow is used to indicate the end of the lag phase for each aggregation sample.
An approximate comparison of aggregation for different SOD1s was made by fitting the data to an empirical equation for a sigmoidal curve (32) (see “Experimental Procedures”) to analyze lag times and apparent rate constants. Fitted values obtained from multiple independent aggregation time courses were averaged, excluding time courses that appeared to be “preseeded” based on lag time analysis (supplemental Fig. S4). On the whole, aggregation tended to be more pronounced in terms of higher amplitudes and/or shorter lag times, as well as faster loss of activity and increased formation of larger particles (see below) for mutants compared with pWT. No clear correlations are apparent in the aggregation time course parameters with respect to protein stability or ALS disease characteristics. This may not be surprising because no such strong correlations have been identified in other studies, although there is evidence for a correlation between disease duration and the combination of stability and calculated aggregation propensity (40). Furthermore, aggregation can depend on various protein properties, including global and local instability and charge, in complex ways that are not well understood (21, 26, 41–43). It is noteworthy that sALS has a similar disease duration to many fALS cases, typically ∼3–5 years (40). Also, different mutant SOD1-fALS tend to have characteristic disease durations; for example, the dimer interface mutant A4V has a particularly short duration of ∼1 year (40). Interestingly, the lag times for the dimer interface mutants tend to be shorter than for pWT and the other mutants (supplemental Fig. S5), suggesting that aggregation from holo SOD1 involves dimer dissociation. This is further supported by the protein concentration dependence of aggregation and antibody binding experiments (see Figs. 4 and 8).
FIGURE 4.
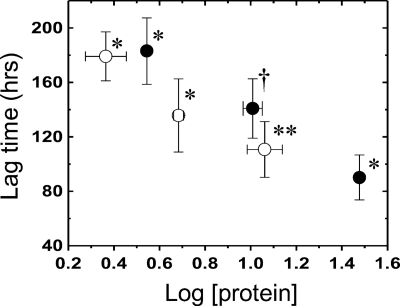
The concentration dependence of the lag phase. The concentration dependence of the lag phase for pWT (●) and G93V (○) is plotted. *, **, and † represent two, four, and five independent experiments performed, respectively. The plotted values are average lag times with the S.D. given by the error bars.
FIGURE 8.
SEDI antibody specifically recognizes aggregates formed from holo SOD1. Specific SEDI binding to aggregated (SODagg) or nonaggregated (SODunagg) pWT was measured using competition of the C-terminal peptide epitope (see “Experimental Procedures”). C represents the control with SOD1 omitted. The recognition of aggregates relative to control is significant (**, p < 0.05), but there is no difference for nonaggregated protein relative to control (*, p > 0.1). The plotted values are the means ± S.D. for three independent aggregation experiments, for which sample measurements were made at least in duplicate.
Aggregation Involves Changes in Metal Binding
The nature of the aggregation process was investigated further by measuring specific activity during aggregation as well as aggregation restart experiments. SOD1 activity absolutely requires copper and is sensitive to protein mismetallation or misfolding (11, 44). Thus, if samples initially contain some misfolded or mismetallated SOD1 with lower activity than the holo protein, aggregation of this protein should cause the specific activity of the remaining nonaggregated protein sample to increase with time. The results illustrate that the specific activity instead decreases with time by ∼20–70% (Fig. 3). Changes in metal binding and/or formation of soluble aggregates could contribute to the decreases in activity (see below). Furthermore, if aggregation originates from a pre-existing pool of mismetallated or misfolded SOD1, no further aggregation should be observed if aggregates are removed after the plateau phase of the time course is reached. Instead, another sigmoidal aggregation time course occurs after plateau phase aggregates are removed (supplemental Fig. S6). These results further confirm the homogeneity of the initial holo samples and that the holo protein changes over time to result in the aggregated species.
FIGURE 3.
SOD1 activity decreases during aggregation. Closed circles represent the normalized activity ratio (see “Experimental Procedures”) for pWT, A4V, G85R, and G93V (from left to right and top to bottom). The ratios were determined using the averages of three to five activity measurements with the S.D. shown by the error bars. Some error bars are smaller than the symbols. The arrows indicate the end of the lag phase from light scattering measurements conducted in parallel with activity measurements.
UV and FTIR absorbance analyses provided further evidence for changes in metal binding. Because of the presence of several phenylalanine residues in the vicinity of the active site, the slope of the SOD1 UV absorbance spectrum between 255 and 279 nm increases markedly upon loss of zinc and slightly upon loss of copper (45, 46). Holo pWT, G85R, and I149T exhibited clear increases in UV slope with increasing incubation time, suggesting decreased or altered metal binding (supplemental Fig. S7). FTIR-monitored thermal melting curves showed a marked change in thermal melting, which begins at ∼80 °C for nonaggregated pWT and at ∼66 °C for the aggregated pWT (supplemental Fig. S8). Based on thermal melting of various metallated forms of SOD1 measured by differential scanning calorimetry (29, 47), these results suggest a loss of some but not all bound metals upon aggregation.
Lastly, the metal content of aggregates was determined using ITV-ICP-AES. These results show that the aggregated SOD1 contains bound metals but is not fully metallated (supplemental Table S3). Taken together, the specific activity, UV, FTIR, and ITV-ICP-AES results show that holo SOD1 samples change with time and that these changes involve loss of and/or altered metal binding.
Holo SOD1 Aggregation Is Nucleation-dependent
In protein aggregation reactions, lag phases occur because the initial nucleation event is unfavorable, and this is followed by relatively favorable and rapid aggregation (48). For various disease-associated proteins, the lag phase has been found to depend on protein concentration because the nucleation step requires association of protein subunits. The concentration dependence of aggregation was determined for both pWT and the fALS-associated mutant, G93V (Fig. 4). For all samples, again, sigmoidal-like time courses were observed. The lag phase for both proteins decreases with increasing protein concentration (Fig. 4), as expected for a protein association reaction, although the concentration dependence is moderate compared with some other proteins (32, 49). One likely explanation for this is that the SOD1 aggregation involves dissociation of the native dimer to monomers. Similarly, increased formation of native dimer relative to aggregation prone monomer at increased protein concentration was found to slow aggregation of immunoglobulin light chain (50), and a similar mechanism has been proposed for SOD1 (17). Thus, although increasing protein concentration can favor protein association reactions, it may also simultaneously disfavor aggregation by favoring competing association processes, such as formation of native dimer for SOD1.
Self- and Cross-seeding Diminish the Lag Phase of Aggregation
A hallmark of nucleated aggregation reactions is that the addition of preformed aggregates as seeds decreases or abolishes the lag phase (2). Seeding has a central role in many diseases, for example, prion diseases (51). The increased lag time upon sample filtering provides evidence for the occurrence of seeding in aggregation from holo SOD1 (see above and supplemental Fig. S3). Seeding was examined further by adding aliquots of solutions that had been allowed to aggregate for different periods of time to fresh solutions of SOD1 (Fig. 5). The addition of aliquots taken early or late during the aggregation time course both decreased the lag time (p < 0.01; Fig. 5). Furthermore, SOD1 exhibits both self-seeding for a given protein (pWT, A4V, and G85R), and cross-seeding between different variants (A4T, A4S, and G93D with pWT (Fig. 5)). These findings are pertinent to understanding disease because SOD1-linked fALS is typically autosomally dominant, with patients having a single copy of the mutant gene as well as a copy of the wild type (1), and it is not yet clear what roles the two forms of the protein may have in the disease (see “Discussion”).
FIGURE 5.
The lag phase decreases upon self- and cross-seeding. The effects of self-seeding on the lag phase are shown for aggregation of pWT, A4V, and G85R (from left to right and top to bottom). The effects of cross-seeding on the lag phase are shown for aggregation of pWT upon addition of 10% (w/w) early seeds of A4T, A4S, and G93D (from left to right and top to bottom). Aggregation time courses for unseeded, 10% (w/w) early seeds, and 10% (w/w) late seeds are shown by ●, ○, and □, respectively. The plotted values are average light scattering intensity, and the vertical error bars are the S.D. from the three replicate measurements per sample. Some error bars are smaller than the symbols. The lag time of the aggregation time course for SOD1 with seeds is significantly shorter (p < 0.001 at 66.5 h in pWT and at 48 h in A4V, p < 0.01 at 20 h in G85R, and p < 0.001 at 60 h in A4T, at 140 h in A4S, and at 64 h in G93D) than for the control sample (unseeded).
Holo SOD1 Undergoes Secondary Nucleation
Two major types of nucleation mechanisms have been reported for protein aggregation: primary and secondary nucleation (52). In homogeneous nucleation, only primary nuclei are formed, generating linear, unbranched aggregates (53). In secondary nucleation, additional nucleation sites are formed; this may occur by formation of heterogeneous nucleation sites on the sides of fibrillar aggregates (33, 54, 55) or by fragmentation of fibrillar aggregates (56, 57), generating new ends for further aggregate growth. Secondary nucleation can be characterized by more rapid aggregate growth and branched aggregate structures (33, 54). The time courses of SOD1 aggregation were fit to models for primary and secondary nucleation (Fig. 6) as per Ferrone (33) (see “Experimental Procedures”). The aggregation profiles of all of the SOD1s are better fit by secondary than by primary nucleation.
FIGURE 6.
Holo SOD1 aggregation time course is indicative of secondary nucleation. Data fitting to Equations 3 and 4 (see “Experimental Procedures”) were used to distinguish primary (dashed line) and secondary (solid line) nucleation mechanisms, respectively, for pWT, A4V, A4T, G93D, G93S, and G93V (from left to right and top to bottom). The R2 values for secondary nucleation fits are 0.972 (pWT), 0.991 (A4V), 0.916 (A4T), 0.984 (G93D), 0.979 (G93S), and 0.975 (G93V), respectively, and each R2 value for secondary nucleation fits is higher than that for primary nucleation fitting.
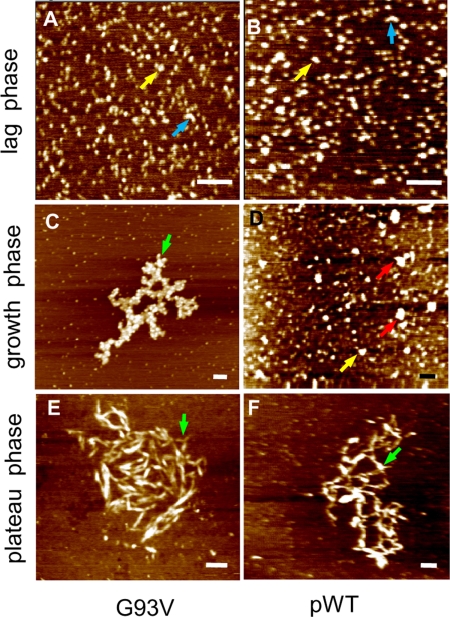
Analysis of aggregate structures by AFM provides further evidence for secondary nucleation. AFM images at different stages of aggregation are shown in Fig. 7 for pWT and G93V. The same types of species are observed for both proteins. At t = 0 h, observed particles are mostly native dimers, and there are no appreciable quantities of aggregated species (supplemental Fig. S9). Later during the lag time, globular amorphous species accumulate (Fig. 7, A and B), although the major species is still native dimer. The amorphous species resemble toxic “oligomers” formed by many other disease-associated proteins (58). There are also some elongated species that look like associated spherical species or protofibrils (2) (Fig. 7, A and B). During the rapid growth phase, native dimers are still the most abundant species, but amorphous species increase in abundance and size (Fig. 7D). Some of these immature aggregates show signs of branching (Fig. 7, A and B), which becomes more pronounced in the mature aggregates from the plateau phase (Fig. 7, C, E, and F). The branched aggregates may become smoother with time (Fig. 7, E and F). It is not clear from the available data whether the branched structures arise from the formation of heterogeneous nuclei on the sides of linear aggregates or from fragmentation; in either case, the observed branched or tangled fibrillar structures are indicative of the occurrence of secondary nucleation. Aggregates in ALS patients have been described as granules and tangled 15–20-nm granule-coated fibrils (3, 4). In addition, early aggregates in mice models are amorphous, and later aggregates have similar fibrillar morphologies to those observed here and in ALS patients (3). Thus, the structural characteristics of the holo SOD1 aggregates reported here strongly resemble those observed in ALS.
FIGURE 7.
AFM structural analyses show holo SOD1 gives rise to soluble oligomers followed by larger amorphous and tangled fibrillar species. The bars represent 100 nm. The AFM images are for G93V (A, C, and E) and pWT (B, D, and F) during the lag time (A and B), rapid growth (C and D), and plateau phases (E and F). No aggregated species are observed at t = 0 (supplemental Fig. S9). The yellow, cyan, red, and green arrows point to oligomers, protofibrils, amorphous aggregates, and branched fibrils, respectively. The cyan arrows also indicate possible branch points on the fibrils, which may arise from heterogeneous nucleation.
Aggregates Formed from Holo SOD1 Have Similar Characteristics to Those Observed in ALS
Because aggregate formation is generally highly sensitive to experimental conditions (59, 60), it is important to check that aggregates generated in vitro have the same characteristics as aggregates observed in disease. Dye and antibody binding experiments provide further evidence confirming the medical relevance of the holo SOD1 aggregates. The aggregates do not show green-gold birefringence upon binding Congo red nor strong enhancement of fluorescence upon binding ThT (supplemental Fig. S10), consistent with properties of aggregates in ALS patients (6, 61). Recently, an ALS-specific antibody was developed that recognizes the SEDI by binding to a C-terminal peptide that is buried in the native dimer (7) (Fig. 1). SEDI antibody specifically recognizes aggregated SOD1 in post mortem fALS patients and in mice models before and after the onset of disease symptoms but not native dimeric holo SOD1 (7). Competitive ELISA experiments on purified nonaggregated (control) and aggregated holo SOD1 showed that SEDI antibody binding increases upon aggregation (Fig. 8; p < 0.05). The recognition of the dimer interface in the aggregates supports the lag time and protein concentration dependence data (Figs. 2 and 4 and supplemental Fig. S5), suggesting involvement of dimer dissociation in aggregation. This striking finding is the first demonstration of specific antibody binding to aggregates formed under native conditions by purified SOD1 as in ALS.
DISCUSSION
Aggregation Arising from Holo SOD1 Is Not Different from Previous Studies
We have demonstrated here that under physiologically relevant conditions holo SOD1 slowly undergoes changes in activity and metal binding monitored by UV absorbance and forms aggregates having the same structural as well as dye and antibody binding characteristics as aggregates observed in ALS patients and mice models, by a nucleation-dependent process. Similar slow changes may also occur in vivo, because SOD1 likely has a very long lifetime in neurons (62).
It is of interest to compare the results observed here with other in vitro investigations of SOD1 aggregation. Various previous studies, mainly involving amyloid formation (see also following section), have reported a lack of observation of aggregation for holo SOD1 (12, 15, 19, 21). On the other hand, numerous studies have found that immature, destabilized forms of SOD1 aggregate more readily than the mature holo protein (12–16, 18, 62–66). Indeed, dimeric holo protein is also probably not the main aggregating species observed in the present studies; however, it appears to undergo dimer dissociation and/or metal loss and form relatively small quantities of aggregates. Our findings are similar to NMR studies of holo pWT, A4V, G37R, and S134N (a mutant with weakened metal binding), which also reported slow formation of small quantities of aggregates (13, 22, 67). Weakened metal binding (68) and loss of metal from mutant SOD1s (24) have also been reported previously. In addition, crystallographic studies of disulfide-intact metal-deficient or apo SOD1s showing fibrillar arrays formed by non-native interactions of active site loops may be related to the type of aggregation observed here (24, 25). The results obtained here are not inconsistent with numerous previous studies characterizing holo SOD1 reported by our group and others, because the majority of SOD1 in solution (>80%) is not altered over a long time scale (>300 h) in most cases. This extent of aggregation is similar to that observed for other proteins (69–71).
The differences between some of the previous studies reporting no observed aggregation for holo SOD1 (12, 15) and the results here could be due to various experimental factors. The previous studies monitored aggregation primarily using ThT binding, a probe of amyloid formation (72); the aggregates from holo SOD1 here do not bind ThT or have other characteristics of amyloid (see also following section). Instead, light scattering used here is sensitive to the formation of any larger particles, amyloid or others (37). An additional experimental difference may be the increased number and/or longer time course of measurements here, enabling observation of aggregation from the holo state of SOD1, even though this is less favorable than aggregation from other states (13–15, 19, 21). Furthermore, when we incubated identical holo SOD1 samples in parallel, with one sample being measured as usual and the other not, aggregation occurred more quickly for the regularly measured sample, suggesting that the process of making sample measurements promotes aggregation (supplemental Fig. S11). This is consistent with other studies showing that sample agitation can promote aggregation of SOD1 and other proteins (13–15, 73, 74). Taken together, the above considerations show that our results are compatible with many previous studies.
Aggregates Formed from Holo SOD1 Do Not Resemble Amyloid
Typically, aggregation reactions are complex, with competing reactions that are highly sensitive to solution conditions (59, 60) and hard to characterize because of heterogeneity in the observed aggregates (58) (this is also evident here in the mixture of structures seen by AFM, Fig. 7). As a consequence, it can be difficult to compare and ascertain the medical relevance of different in vitro and in vivo studies. A common aggregate structure formed in many neurodegenerative and other diseases is known as amyloid (2). Recently, much attention has focused on amyloid formation by SOD1. SOD1 has been shown to form amyloid under destabilizing conditions when subjected to sonication (75), low pH (16), or agitation (13–15). Furthermore, there is very extensive evidence that all proteins can form amyloid, often quite readily, under suitable, typically destabilizing, conditions (76, 77). However, there is strong evidence that ALS is not an amyloid disease. Aggregates in ALS patients do not exhibit the classic properties of amyloid, such as green-gold Congo Red birefringence or ThT binding (6, 61) or smooth unbranched fibrillar structures (3, 4). In addition, cell culture experiments have shown that aggregates formed by SOD1 are distinct from those formed by proteins associated with classical amyloid diseases (78). We consider that the aggregates studied herein are likely to be biologically relevant because they are formed under physiologically relevant conditions, and based on multiple criteria, their properties match those of aggregates formed in ALS.
SOD1 Can Aggregate by Many Pathways
The mechanism of aggregation from holo SOD1 likely involves the loss of metals and/or dimer dissociation. Based on the high stability of holo SOD1 under the solution conditions used here, the population of the unfolded state for pWT and mutants will be extremely small (28), consistent with aggregation occurring from a state that is closer to the native state. Previously, holo SOD1 has been proposed to be incapable of forming aggregates because of its high stability (21). However, aggregation could be initiated via local structural fluctuations (26), which are not necessarily linked to global stability (79). Recent experimental data suggest that the metal binding characteristics of SOD1 give rise to frustration and misfolding (80). The activity, metal analysis, UV, and FTIR thermal unfolding data (Figs. 3, supplemental Figs. S7 and S8, and supplemental Table S3) show that aggregation ultimately involves the loss of some, but not all, metal from the holo SOD1s, whereas the lag time (Figs. 2 and supplemental Fig. S5), concentration dependence (Fig. 4), and SEDI binding data (Fig. 8) suggest that dimer dissociation is occurring.
The experimental results herein support a mechanism similar to that proposed by Dokholyan and co-workers (17), modified to allow for additional partial as well as the originally proposed complete loss of metal (Fig. 9). There is considerable experimental support for this mechanism (Ref. 17 and references therein). Extensive additional data from the literature (12, 13, 15, 19, 21, 22) can be included and rationalized by additional pathways for aggregation of SOD1. Similarly, multiple pathways have been proposed for amyloid formation (2, 59). Furthermore, a key and often overlooked point is that all SOD1 mutations studied to date destabilize the holo protein (19, 28, 47), which is not consistently the case for the apo protein (42). Also, the dimer interface for holo SOD1 is only slightly stronger than for apo SOD1 (28, 81, 82); assuming that dimer dissociation is required for aggregation, this means that it is feasible for holo SOD1 to aggregate. Finally, fALS-associated mutations increase holo SOD1 dynamics, causing complex changes that propagate through the structure, as shown by NMR and x-ray studies of G93A (23), A4V (83), I113T (83), and S134N (22). Interestingly, the lag time for G85R, which has weakened metal binding (36) and dimerization (84), is shorter than for pWT and G93 variants (supplemental Fig. S5), suggesting that the loss of metal may favor dimer dissociation and consequently aggregation. This is reasonable because the zinc-binding loop leads into the dimer interface (Fig. 1) (82). Conversely, the dimer interface mutants A4V, A4T, and I113T have weakened zinc binding (68). Computational studies on many mutants have shown complex coupling between the dimer interface and metal-binding loops (79). Thus, the proposed expanded aggregation mechanism accounts for the new data herein as well as extensive previous experimental and computational findings.
FIGURE 9.
Multiple pathways for pathological SOD1 aggregation. Dholo, Mholo, DpMe/apo, MpMe/apo, Aoligo, Aamor, Afib, and Abranch represent the holo dimer, holo monomer, partially metallated or apo dimer, partially metallated or apo monomer, soluble oligomers, amorphous aggregates, fibrillar aggregates, and branched aggregates, respectively. The expanded mechanism of Dokholyan and co-workers (17) is shown with solid lines and supported by the data herein. Fully metallated SOD1s may monomerize or lose metal by local fluctuation. Either step may promote the other as the next step. The dashed lines indicate additional possible aggregation pathways (which may include multiple steps) that are not excluded by the current data and are supported by other findings from the literature. The various aggregated species included in dashed square brackets may interconvert.
The zinc-binding loop and electrostatic loops (Fig. 1) are likely regions for forming intermolecular interactions in SOD1 aggregates. Recently, as-isolated SOD1s that are partially metallated have been reported to exhibit structural and dynamic changes affecting mainly the electrostatic and zinc-binding loops (84). Although many mutant SOD1s are wild type-like and appear to be predominantly properly metallated, there is also evidence for incomplete metallation of numerous mutants in various in vivo systems (11, 44, 85–87), suggesting that mutants have weakened metal binding and more easily lose metals. A plausible mechanism for the aggregation process is formation of intermolecular interactions by active site loops, which may subsequently result in further alterations in metal binding. Common intermolecular interactions involving these loops and giving rise to branched assemblies have been identified in holo wild type and mutant SOD1 crystal structures (83). Also, many variations involving these as well as various other loops have been noted in crystal structures of partially or fully metal-depleted wild type and mutant SOD1s, often with formation of fibrillar assemblies (24, 25). Pertinent to the secondary nucleation observed here, the intermolecular interactions in the crystals provide clues regarding possible heterogeneous nucleation sites on the sides of fibers. The above considerations together with the results presented herein suggest that aggregation arising from the holo protein could play an important role in disease.
Limited Role for Intermolecular Disulfide Bond Formation in Aggregation
Many previous studies have focused on the role of intermolecular disulfide bonds in SOD1 aggregation. Recently, Borchelt and co-workers (8) have shown that intermolecular disulfide bond formation is favored only in the later stages of disease in mice, and so it is likely a secondary effect. In vitro studies of oxidative aggregation of wild type apo SOD1 have also reported evidence for noncovalent interactions (12–15). Our studies are most pertinent to understanding noncovalent association, because the free cysteines are eliminated in pWT. This may be particularly important for the formation of pathogenic aggregates early in disease and also contribute to ongoing toxic aggregation as the disease progresses.
Implications for Disease
The observation of similar aggregation for pWT and mutant holo SOD1 and cross-seeding suggests that wild type SOD1 may play significant roles in both sALS and fALS. Currently, the role(s) of pWT are controversial. Wild type SOD1 is a particularly abundant protein, placing it at high risk for aggregation (18). Furthermore, motor neurons are particularly vulnerable to the toxic effects of protein aggregation (1). However, overexpression of wild type SOD1 in mice does not result in disease, although mitochondrial alterations and subclinical defects are observed at older ages (88). Also, alterations in gene expression in the spinal cord were reported for mutant and WT SOD1 models but not in nontransgenic mice (89). Large aggregates of SOD1 are generally observed in fALS, but have only been reported for a subset of sALS cases (4, 5, 90), and are not detected by SEDI in sALS (90). However, small species of misfolded SOD1 have been proposed to be a common molecular signature in both sALS and fALS (91). The detection of SOD1 in aggregates and indeed the types of protein inclusions observed in different cases of ALS vary considerably (92, 93). SOD1 may be present in end-stage aggregates but not always be detected depending on accessibility of various SOD1 epitopes to different antibodies. It is also possible that the structures of SOD1 aggregates in ALS vary, perhaps in specific ways associated with different mutations (21, 94) and disease characteristics such as duration or onset; this is analogous to varying disease and aggregate characteristics in different prion strains (51). Consistent with this, different mutant SOD1-ALS mouse models have distinct phenotypes (92, 95). Further studies are needed to determine the characteristics of aggregates formed by different forms of SOD1 (fALS mutations and metallation states) in relation to different disease characteristics and stages.
The seeding and secondary nucleation observed here also have important implications for understanding the disease characteristics of ALS. The cross-seeding of mutant and pWT shows that the two forms can co-aggregate (Fig. 5). This is consistent with results from cell culture fALS models where wild type and mutant SOD1 co-aggregate when co-expressed (96) and with fALS mice studies that found overexpression of wild type is required to convert A4V mice to a disease phenotype (97). Combining different mutants with wild type may have different aggregation and hence disease characteristics. Mutations could enhance initiation and/or propagation of aggregation and so favor disease. Also, seeding and the very rapid growth of aggregates caused by secondary nucleation processes may be linked to the very rapid disease progression in ALS.
The aggregation observed here for holo SOD1 shares many characteristics with the aggregation of other disease-associated proteins, including occurrence of a lag phase (caused by unfavorable nucleation, which can be overcome by seeding), formation of amorphous and fibrillar species, and rapid aggregate growth caused by secondary nucleation (2). In summary, the current results provide new and potentially general insights into protein aggregation in disease and provide the basis for further studies to define aggregation mechanisms, identify inappropriate interactions, and develop urgently needed therapeutic strategies against toxic protein aggregation.
Supplementary Material
Acknowledgments
We thank Kenrick Vassall, Helen Stubbs, and Heather Primmer for insightful discussions and assistance.
This work was supported by the ALS Society of Canada, Muscular Dystrophy Canada, and the Canadian Institutes of Health Research.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text, Tables S1–S3, and Figs. S1–S11.
- ALS
- amyotrophic lateral sclerosis
- fALS
- familial ALS
- sALS
- sporadic ALS
- SOD1
- copper/zinc superoxide dismutase
- pWT
- pseudo wild type recombinant SOD1 with C6A and C111S mutations
- ITV-ICP-AES
- in torch vaporization inductively coupled plasma atomic emission spectroscopy
- SEDI
- SOD1 exposed dimer interface
- AFM
- atomic force microscopy
- ThT
- thioflavin T.
REFERENCES
- 1.Boillée S., Vande Velde C., Cleveland D. W. (2006) Neuron 52, 39–59 [DOI] [PubMed] [Google Scholar]
- 2.Chiti F., Dobson C. M. (2006) Annu. Rev. Biochem. 75, 333–366 [DOI] [PubMed] [Google Scholar]
- 3.Kato S., Nakashima K., Horiuchi S., Nagai R., Cleveland D. W., Liu J., Hirano A., Takikawa M., Kato M., Nakano I., Sakoda S., Asayama K., Ohama E. (2001) Neuropathology 21, 67–81 [DOI] [PubMed] [Google Scholar]
- 4.Shibata N., Hirano A., Kobayashi M., Sasaki S., Kato T., Matsumoto S., Shiozawa Z., Komori T., Ikemoto A., Umahara T., Asayama K. (1994) Neurosci. Lett. 179, 149–152 [DOI] [PubMed] [Google Scholar]
- 5.Shibata N., Asayama K., Hirano A., Kobayashi M. (1996) Dev. Neurosci. 18, 492–498 [DOI] [PubMed] [Google Scholar]
- 6.Okamoto K., Hirai S., Yamazaki T., Sun X. Y., Nakazato Y. (1991) Neurosci. Lett. 129, 233–236 [DOI] [PubMed] [Google Scholar]
- 7.Rakhit R., Robertson J., Vande Velde C., Horne P., Ruth D. M., Griffin J., Cleveland D. W., Cashman N. R., Chakrabartty A. (2007) Nat. Med. 13, 754–759 [DOI] [PubMed] [Google Scholar]
- 8.Karch C. M., Prudencio M., Winkler D. D., Hart P. J., Borchelt D. R. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7774–7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston J. A., Dalton M. J., Gurney M. E., Kopito R. R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 12571–12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto S., Kusaka H., Ito H., Shibata N., Asayama T., Imai T. (1996) Clin. Neuropathol. 15, 41–46 [PubMed] [Google Scholar]
- 11.Valentine J. S., Doucette P. A., Zittin Potter S. (2005) Annu. Rev. Biochem. 74, 563–593 [DOI] [PubMed] [Google Scholar]
- 12.Banci L., Bertini I., Durazo A., Girotto S., Gralla E. B., Martinelli M., Valentine J. S., Vieru M., Whitelegge J. P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11263–11267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oztug Durer Z. A., Cohlberg J. A., Dinh P., Padua S., Ehrenclou K., Downes S., Tan J. K., Nakano Y., Bowman C. J., Hoskins J. L., Kwon C., Mason A. Z., Rodriguez J. A., Doucette P. A., Shaw B. F., Selverstone Valentine J. (2009) PLoS ONE 4, e5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa Y., Kaneko K., Yamanaka K., O'Halloran T. V., Nukina N. (2008) J. Biol. Chem. 283, 24167–24176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chattopadhyay M., Durazo A., Sohn S. H., Strong C. D., Gralla E. B., Whitelegge J. P., Valentine J. S. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18663–18668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiDonato M., Craig L., Huff M. E., Thayer M. M., Cardoso R. M., Kassmann C. J., Lo T. P., Bruns C. K., Powers E. T., Kelly J. W., Getzoff E. D., Tainer J. A. (2003) J. Mol. Biol. 332, 601–615 [DOI] [PubMed] [Google Scholar]
- 17.Khare S. D., Caplow M., Dokholyan N. V. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15094–15099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakhit R., Crow J. P., Lepock J. R., Kondejewski L. H., Cashman N. R., Chakrabartty A. (2004) J. Biol. Chem. 279, 15499–15504 [DOI] [PubMed] [Google Scholar]
- 19.Stathopulos P. B., Rumfeldt J. A., Scholz G. A., Irani R. A., Frey H. E., Hallewell R. A., Lepock J. R., Meiering E. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7021–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valentine J. S. (2002) Free Radic. Biol. Med. 33, 1314–1320 [DOI] [PubMed] [Google Scholar]
- 21.Shaw B. F., Valentine J. S. (2007) Trends Biochem. Sci. 32, 78–85 [DOI] [PubMed] [Google Scholar]
- 22.Banci L., Bertini I., D'Amelio N., Gaggelli E., Libralesso E., Matecko I., Turano P., Valentine J. S. (2005) J. Biol. Chem. 280, 35815–35821 [DOI] [PubMed] [Google Scholar]
- 23.Shipp E. L., Cantini F., Bertini I., Valentine J. S., Banci L. (2003) Biochemistry 42, 1890–1899 [DOI] [PubMed] [Google Scholar]
- 24.Seetharaman S. V., Winkler D. D., Taylor A. B., Cao X., Whitson L. J., Doucette P. A., Valentine J. S., Schirf V., Demeler B., Carroll M. C., Culotta V. C., Hart P. J. (2010) Biochemistry 49, 5714–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elam J. S., Taylor A. B., Strange R., Antonyuk S., Doucette P. A., Rodriguez J. A., Hasnain S. S., Hayward L. J., Valentine J. S., Yeates T. O., Hart P. J. (2003) Nat. Struct. Biol. 10, 461–467 [DOI] [PubMed] [Google Scholar]
- 26.Chiti F., Dobson C. M. (2009) Nat. Chem. Biol. 5, 15–22 [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki M., Li W., Johnson D. J., Huntington J. A. (2008) Nature 455, 1255–1258 [DOI] [PubMed] [Google Scholar]
- 28.Rumfeldt J. A., Stathopulos P. B., Chakrabarrty A., Lepock J. R., Meiering E. M. (2006) J. Mol. Biol. 355, 106–123 [DOI] [PubMed] [Google Scholar]
- 29.Stathopulos P. B., Rumfeldt J. A., Karbassi F., Siddall C. A., Lepock J. R., Meiering E. M. (2006) J. Biol. Chem. 281, 6184–6193 [DOI] [PubMed] [Google Scholar]
- 30.Marklund S., Marklund G. (1974) Eur. J. Biochem. 47, 469–474 [DOI] [PubMed] [Google Scholar]
- 31.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 32.Nielsen L., Khurana R., Coats A., Frokjaer S., Brange J., Vyas S., Uversky V. N., Fink A. L. (2001) Biochemistry 40, 6036–6046 [DOI] [PubMed] [Google Scholar]
- 33.Ferrone F. (1999) Methods Enzymol. 309, 256–274 [DOI] [PubMed] [Google Scholar]
- 34.Parge H. E., Hallewell R. A., Tainer J. A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 6109–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lepock J. R., Frey H. E., Hallewell R. A. (1990) J. Biol. Chem. 265, 21612–21618 [PubMed] [Google Scholar]
- 36.Cao X., Antonyuk S. V., Seetharaman S. V., Whitson L. J., Taylor A. B., Holloway S. P., Strange R. W., Doucette P. A., Valentine J. S., Tiwari A., Hayward L. J., Padua S., Cohlberg J. A., Hasnain S. S., Hart P. J. (2008) J. Biol. Chem. 283, 16169–16177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lomakin A., Benedek G. B., Teplow D. B. (1999) Methods Enzymol. 309, 429–459 [DOI] [PubMed] [Google Scholar]
- 38.Wilkins D. K., Grimshaw S. B., Receveur V., Dobson C. M., Jones J. A., Smith L. J. (1999) Biochemistry 38, 16424–16431 [DOI] [PubMed] [Google Scholar]
- 39.Morris A. M., Watzky M. A., Finke R. G. (2009) Biochim. Biophys. Acta 1794, 375–397 [DOI] [PubMed] [Google Scholar]
- 40.Wang Q., Johnson J. L., Agar N. Y., Agar J. N. (2008) PLoS Biol. 6, e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiti F., Stefani M., Taddei N., Ramponi G., Dobson C. M. (2003) Nature 424, 805–808 [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez J. A., Shaw B. F., Durazo A., Sohn S. H., Doucette P. A., Nersissian A. M., Faull K. F., Eggers D. K., Tiwari A., Hayward L. J., Valentine J. S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10516–10521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandelin E., Nordlund A., Andersen P. M., Marklund S. S., Oliveberg M. (2007) J. Biol. Chem. 282, 21230–21236 [DOI] [PubMed] [Google Scholar]
- 44.Goto J. J., Zhu H., Sanchez R. J., Nersissian A., Gralla E. B., Valentine J. S., Cabelli D. E. (2000) J. Biol. Chem. 275, 1007–1014 [DOI] [PubMed] [Google Scholar]
- 45.Beem K. M., Rich W. E., Rajagopalan K. V. (1974) J. Biol. Chem. 249, 7298–7305 [PubMed] [Google Scholar]
- 46.Mailer K., Addetia R., Livesey D. L. (1989) J. Inorg. Biochem. 37, 151–161 [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez J. A., Valentine J. S., Eggers D. K., Roe J. A., Tiwari A., Brown R. H., Jr., Hayward L. J. (2002) J. Biol. Chem. 277, 15932–15937 [DOI] [PubMed] [Google Scholar]
- 48.Harper J. D., Lansbury P. T., Jr. (1997) Annu. Rev. Biochem. 66, 385–407 [DOI] [PubMed] [Google Scholar]
- 49.Ferrone F. A., Hofrichter J., Eaton W. A. (1985) J. Mol. Biol. 183, 611–631 [DOI] [PubMed] [Google Scholar]
- 50.Souillac P. O., Uversky V. N., Millett I. S., Khurana R., Doniach S., Fink A. L. (2002) J. Biol. Chem. 277, 12666–12679 [DOI] [PubMed] [Google Scholar]
- 51.Prusiner S. B. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bishop M. F., Ferrone F. A. (1984) Biophys. J. 46, 631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S., Berthelier V., Hamilton J. B., O'Nuallain B., Wetzel R. (2002) Biochemistry 41, 7391–7399 [DOI] [PubMed] [Google Scholar]
- 54.Andersen C. B., Yagi H., Manno M., Martorana V., Ban T., Christiansen G., Otzen D. E., Goto Y., Rischel C. (2009) Biophys. J. 96, 1529–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruschak A. M., Miranker A. D. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12341–12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collins S. R., Douglass A., Vale R. D., Weissman J. S. (2004) PLoS Biol. 2, e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue W. F., Homans S. W., Radford S. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8926–8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glabe C. G. (2008) J. Biol. Chem. 283, 29639–29643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahn T. R., Radford S. E. (2008) Arch. Biochem. Biophys. 469, 100–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carulla N., Zhou M., Arimon M., Gairí M., Giralt E., Robinson C. V., Dobson C. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7828–7833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kerman A., Liu H. N., Croul S., Bilbao J., Rogaeva E., Zinman L., Robertson J., Chakrabartty A. (2010) Acta Neuropathol. 119, 335–344 [DOI] [PubMed] [Google Scholar]
- 62.Rakhit R., Cunningham P., Furtos-Matei A., Dahan S., Qi X. F., Crow J. P., Cashman N. R., Kondejewski L. H., Chakrabartty A. (2002) J. Biol. Chem. 277, 47551–47556 [DOI] [PubMed] [Google Scholar]
- 63.Furukawa Y., O'Halloran T. V. (2005) J. Biol. Chem. 280, 17266–17274 [DOI] [PubMed] [Google Scholar]
- 64.Roberts B. R., Tainer J. A., Getzoff E. D., Malencik D. A., Anderson S. R., Bomben V. C., Meyers K. R., Karplus P. A., Beckman J. S. (2007) J. Mol. Biol. 373, 877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banci L., Bertini I., Boca M., Girotto S., Martinelli M., Valentine J. S., Vieru M. (2008) PLoS ONE 3, e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stathopulos P. (2005) in Chemistry, pp. 133–171, University of Waterloo, Waterloo [Google Scholar]
- 67.Banci L., Bertini I., D'Amelio N., Libralesso E., Turano P., Valentine J. S. (2007) Biochemistry 46, 9953–9962 [DOI] [PubMed] [Google Scholar]
- 68.Crow J. P., Sampson J. B., Zhuang Y., Thompson J. A., Beckman J. S. (1997) J. Neurochem. 69, 1936–1944 [DOI] [PubMed] [Google Scholar]
- 69.Piazza R., Pierno M., Iacopini S., Mangione P., Esposito G., Bellotti V. (2006) Eur. Biophys. J. 35, 439–445 [DOI] [PubMed] [Google Scholar]
- 70.Morel B., Casares S., Conejero-Lara F. (2006) J. Mol. Biol. 356, 453–468 [DOI] [PubMed] [Google Scholar]
- 71.Huang T. H., Yang D. S., Plaskos N. P., Go S., Yip C. M., Fraser P. E., Chakrabartty A. (2000) J. Mol. Biol. 297, 73–87 [DOI] [PubMed] [Google Scholar]
- 72.LeVine I. H. (1995) Amyloid 2, 1–6 [Google Scholar]
- 73.Souillac P. O., Uversky V. N., Fink A. L. (2003) Biochemistry 42, 8094–8104 [DOI] [PubMed] [Google Scholar]
- 74.Khurana R., Gillespie J. R., Talapatra A., Minert L. J., Ionescu-Zanetti C., Millett I., Fink A. L. (2001) Biochemistry 40, 3525–3535 [DOI] [PubMed] [Google Scholar]
- 75.Stathopulos P. B., Scholz G. A., Hwang Y. M., Rumfeldt J. A., Lepock J. R., Meiering E. M. (2004) Protein Sci. 13, 3017–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiti F., Webster P., Taddei N., Clark A., Stefani M., Ramponi G., Dobson C. M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3590–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefani M., Dobson C. M. (2003) J. Mol. Med. 81, 678–699 [DOI] [PubMed] [Google Scholar]
- 78.Matsumoto G., Kim S., Morimoto R. I. (2006) J. Biol. Chem. 281, 4477–4485 [DOI] [PubMed] [Google Scholar]
- 79.Khare S. D., Dokholyan N. V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3147–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nordlund A., Leinartaite L., Saraboji K., Aisenbrey C., Gröbner G., Zetterström P., Danielsson J., Logan D. T., Oliveberg M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9667–9672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vassall K. A., Stathopulos P. B., Rumfeldt J. A., Lepock J. R., Meiering E. M. (2006) Biochemistry 45, 7366–7379 [DOI] [PubMed] [Google Scholar]
- 82.Hörnberg A., Logan D. T., Marklund S. L., Oliveberg M. (2007) J. Mol. Biol. 365, 333–342 [DOI] [PubMed] [Google Scholar]
- 83.Hough M. A., Grossmann J. G., Antonyuk S. V., Strange R. W., Doucette P. A., Rodriguez J. A., Whitson L. J., Hart P. J., Hayward L. J., Valentine J. S., Hasnain S. S. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 5976–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molnar K. S., Karabacak N. M., Johnson J. L., Wang Q., Tiwari A., Hayward L. J., Coales S. J., Hamuro Y., Agar J. N. (2009) J. Biol. Chem. 284, 30965–30973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hayward L. J., Rodriguez J. A., Kim J. W., Tiwari A., Goto J. J., Cabelli D. E., Valentine J. S., Brown R. H., Jr. (2002) J. Biol. Chem. 277, 15923–15931 [DOI] [PubMed] [Google Scholar]
- 86.Seetharaman S. V., Prudencio M., Karch C., Holloway S. P., Borchelt D. R., Hart P. J. (2009) Exp. Biol. Med. 234, 1140–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Borchelt D. R., Lee M. K., Slunt H. S., Guarnieri M., Xu Z. S., Wong P. C., Brown R. H., Jr., Price D. L., Sisodia S. S., Cleveland D. W. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8292–8296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jaarsma D. (2006) Mitochondrion 6, 48–51 [DOI] [PubMed] [Google Scholar]
- 89.D'Arrigo A., Colavito D., Pena-Altamira E., Fabris M., Dam M., Contestabile A., Leon A. (2010) J. Mol. Neurosci. 41, 404–415 [DOI] [PubMed] [Google Scholar]
- 90.Liu H. N., Sanelli T., Horne P., Pioro E. P., Strong M. J., Rogaeva E., Bilbao J., Zinman L., Robertson J. (2009) Ann. Neurol. 66, 75–80 [DOI] [PubMed] [Google Scholar]
- 91.Gruzman A., Wood W. L., Alpert E., Prasad M. D., Miller R. G., Rothstein J. D., Bowser R., Hamilton R., Wood T. D., Cleveland D. W., Lingappa V. R., Liu J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12524–12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kato S. (2008) Acta Neuropathol. 115, 97–114 [DOI] [PubMed] [Google Scholar]
- 93.Deleted in proof
- 94.Furukawa Y., Kaneko K., Yamanaka K., Nukina N. (2010) J. Biol. Chem. 285, 22221–22231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turner B. J., Talbot K. (2008) Prog Neurobiol. 85, 94–134 [DOI] [PubMed] [Google Scholar]
- 96.Prudencio M., Durazo A., Whitelegge J. P., Borchelt D. R. (2009) J. Neurochem. 108, 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng H. X., Shi Y., Furukawa Y., Zhai H., Fu R., Liu E., Gorrie G. H., Khan M. S., Hung W. Y., Bigio E. H., Lukas T., Dal Canto M. C., O'Halloran T. V., Siddique T. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7142–7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koradi R., Billeter M., Wuthrich K. (1996) J. Mol. Graph. 14, 51–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.