Abstract
Human natural killer (NK) cells express an abundant level of 2B4 and CD2 on their surface. Their counter-receptors, CD48 and CD58, are also expressed on the NK cell surface, raising a question about the functional consequences of potential 2B4/CD48 and CD2/CD58 interactions. Using blocking antibodies specific to each receptor, we demonstrated that both 2B4/CD48 and CD2/CD58 interactions were essential for the development of NK effector functions: cytotoxicity and cytokine secretion. However, only 2B4/CD48, but not CD2/CD58, interactions were shown to be critical for the optimal NK cell proliferation in response to interleukin (IL)-2. IL-2-activated NK cells cultured in the absence of 2B4/CD48 or CD2/CD58 interactions were severely impaired for their ability to induce intracellular calcium mobilization and subsequent ERK activation upon tumor target exposure, suggesting that the early signaling pathway of NK receptors leading to impaired cytolysis and interferon (IFN)-γ secretion was inhibited. Nevertheless, these defects did not fully account for the reduced proliferation of NK cells in the absence of 2B4/CD48 interactions, because anti-CD2 or anti-CD58 monoclonal antibody (mAb)-treated NK cells, showing defective signaling and effector functions, displayed normal proliferation upon IL-2 stimulation. These results propose the signaling divergence between pathways leading to cell proliferation and cytotoxicity/cytokine release, which can be differentially regulated by 2B4 and CD2 during IL-2-driven NK cell activation. Collectively, these results reveal the importance of homotypic NK-to-NK cell cross-talk through 2B4/CD48 and CD2/CD58 pairs and further present their differential and overlapping roles in human NK cells.
Keywords: Cell-Cell Interaction, Cellular Immune Response, Immunology, Innate Immunity, Receptors, 2B4, CD2, CD48, CD58, NK Cells
Introduction
2B4 (CD244) belongs to the CD2 family along with CD48, CD2, CD58 (LFA-3), CD84 (GR6), Ly9 (CD229), SLAM, NTB-A, and CRACC. Receptors within this family have been implicated in homophilic and heterophilic self-interactions (1). Among them, CD84, Ly9, SLAM, NTB-A, and CRACC are all self-ligands and receptors, and up-regulated in NK6 cells as well as T cells, B cells, dendritic cells, and macrophages (2). In both mouse and human, CD48 appears to be a high affinity ligand for 2B4, as well as a weak ligand for CD2 (3). Although mouse NK cells in the resting state express both 2B4 and CD2, only 2B4, but not CD2, was found to play a major role in the activation and expansion of NK cells (4, 5). The preferential role of 2B4 over CD2 in murine NK cells appears to be due to its high affinity interaction with CD48 because 2B4 binds to CD48 with 5–10-fold higher affinity than CD2 (6). Furthermore, murine 2B4 was shown to function as a co-stimulatory receptor in response to cytokine (IL-2 or IFN-α) stimulation, via triggering cell to cell cross-talk through binding to CD48 expressed on neighboring NK cells (7). These homotypic interactions via 2B4/CD48 were found to be necessary for optimal expansion, lytic potential, and cytokine secretion in murine NK cells (7). Additionally, murine 2B4 could also function as a co-stimulatory ligand for CD48, capable of generating bidirectional signals (8, 9). For example, 2B4 expressed on neighboring activated/memory T cells (10) or surrounding IL-2-activated NK cells (11) was demonstrated to promote the proliferation of TCR+ CD8+ transgenic T cells through stimulating CD48. Paradoxically, 2B4 was found to serve as a MHC class I-independent inhibitory receptor and to be involved in NK cell self-tolerance in mice (12).
Unlike murine 2B4, inhibitory function has not been found with human 2B4. Engagement of 2B4 on human NK cells by 2B4-specific monoclonal antibodies (mAbs) induced IFN-γ in vitro and triggered NK cell-mediated cytotoxicity in redirected antibody-dependent cell cytotoxicity assays (5). Furthermore, NK cytotoxicity against tumor targets was augmented when these targets expressed CD48 on their surface (13, 14). These in vitro findings were corroborated by a recent discovery that x-linked lymphoproliferative disease patients demonstrated defective clearance of Epstein-Barr virus infections due to the absence of 2B4 signaling (15). These results highlight the activating role of 2B4 in human NK cells, and further present a differential mode of 2B4 activities between human and mouse NK cells.
Similar to murine NK cells, human NK cells express abundant quantities of 2B4, CD48, and CD2 on their surface. Unlike mice, human NK cells also express CD58, a high affinity ligand for CD2, raising a question about the functional outcome of potential CD2/CD58 interactions in addition to 2B4/CD48 binding among NK cells. Therefore, this study was set up to dissect the roles of 2B4, CD48, CD2, and CD58 in NK to NK cell interactions and to provide a molecular mechanism and consequences of these interactions in the generation of effector NK cells in human. Our results present that 2B4/CD48, but not CD2/CD58, interactions among NK cells are required for the proliferation of human NK cells, whereas both 2B4/CD48 and CD2/CD58 interactions are important for the development of optimal cytolytic and secretory NK effector functions. Thus, 2B4 and CD2 differentially contribute to NK to NK cell cross-talks by providing co-stimulatory signals among NK cells themselves in human.
EXPERIMENTAL PROCEDURES
NK Cell Purification and Cell Culture
Human primary NK cells were isolated from peripheral blood mononuclear cells (PBMCs) from healthy volunteers using a RosetteSep Human NK Enrichment Mixture (Stem Cell Technologies, Vancouver, Canada) and Ficoll-Plaque (GE Healthcare) following the manufacturer's instructions. The purity of CD3−CD56+ NK cells was >95%, determined by flow cytometry (FACScalibur, BD Biosciences). Freshly purified NK cells were cultured in RPMI1640 medium (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum (FBS; Lonza, Walkersville, MD), 100 units/ml of penicillin (Lonza), 100 units/ml of streptomycin (Lonza), and 300 units/ml of human recombinant interleukin-2 (hrIL-2; Chiron, Charlotte, NC) at 37 °C in 5% CO2 for 1–2 weeks. In some experiments, 5 μg/ml of anti-2B4, anti-CD48, anti-CD2, or anti-CD58 blocking monoclonal antibodies (mAbs) were added in culture media and replenished every 5 days. Mouse immunoglobulin G1 (mIgG1) was used as a corresponding isotype control for anti-2B4, anti-CD2, and anti-CD58 and mouse immunoglobulin M (mIgM) was used for anti-CD48 mAb. The effect of mIgG1 or mIgM on cell proliferation was comparable with negative control without any antibodies, hence only the mIgG1-treated group was plotted in the figures. All procedures were approved by the Korea University Institutional Review Board and donor consent. A human myelogenous leukemia cell line, K562 (ATCC, Manassas, VA), was maintained in RPMI1640 supplemented with 10% FBS (Lonza), 100 units/ml of penicillin (Lonza), and 100 units/ml of streptomycin (Lonza), which we refer to as complete RPMI.
Antibodies
Fluorescently labeled anti-human 2B4 (clone 2–69), anti-human CD48 (YU145), purified anti-CD48 (TU145) mAbs, and Annexin V were purchased from BD Pharmingen (San Diego, CA). Fluorescence-labeled anti-CD3 (UCHT-1) and anti-CD56 (MEM-188) mAbs were purchased from DiNonA Inc. (Seoul, Korea). Purified anti-2B4 (C1.7), anti-CD2 (RPA-2.10) mAbs, mIgG1, or mIgM were purchased from Immunotec (Vaudreuil-Dorion, Canada) or eBioscience (San Diego, CA). Purified anti-CD58 mAb (IC3; BD Biosciences) was used to block CD58 binding from CD2. For intracellular staining, fluorescence-labeled anti-human perforin (δG9), granzyme B (GB11), and CD107a (eBioH4A3) mAbs were purchased from eBioscience. Anti-phospho-ERK1/2 (p44/42 MAPK) and anti-ERK1/2 (p44/42 MAPK) Abs were purchased from Cell Signaling Technology (Boston, MA). Goat anti-mouse HRP-conjugated Ab and goat anti-rabbit HRP-conjugated Ab were purchased from Amersham Biosciences.
Generation of CD48-expressing K562 Cells
To produce CD48-expressing K562 cells, human CD48 cDNA from PBMCs were subcloned into pMFG retroviral vector (16). Infectious retroviral supernatants were generated by transient transfection of a H29D packaging cell line using ExGen 500 in vitro Transfection Reagent (Fermentas Inc., Daemyung Science, Seoul, Korea). The culture supernatant was collected after 48 and 72 h post transfection. K562 cells were stably transduced with retroviral particles in the presence of 8 μg/ml of Polybrene (Sigma) and transduced cells were selected by puromycin (Sigma).
Cell Proliferation Assays
To measure DNA synthesis, 3H incorporation assay was performed. In brief, live cell numbers were counted with a hemocytometer (Improved Neubauer; Hawksley Co., Lansing, UK) by trypan blue exclusion. To assess DNA duplication, 1 μCi/ml of [3H]thymidine (Amersham Biosciences) was incorporated overnight into NK cells. The next day, cells were harvested onto filter paper with Micro96 harvester (Skatron, Norway) and disintegrations per minute were counted by a β-counter (Packard Instrument Company, Meriden, CT).
Flow Cytometry
For surface staining, the cells were stained with the indicated phycoerythrin- or FITC-conjugated mAb in 100 μl of FACS buffer (BD Biosciences). For intracellular staining, cultured NK cells were fixed and permeabilized with Cytofix/Cytoperm solution and Perm/Wash buffer (both from BD Biosciences), respectively, prior to specific staining with fluorescence-labeled perforin or granzyme B mAbs. To assess cell death, NK cells were stained with Annexin V-FITC and propidium iodide. CD107a assay was performed to determine the frequency of degranulating NK cells (17). Briefly, NK cells were mixed with K562 target cells at an E:T ratio of 1:1 and incubated for 1 h in the presence of 10 μl/ml of CD107a-FITC (BD Biosciences). GolgiStop (BD Biosciences) was added at 6 μg/ml and incubated for an additional 5 h at 37 °C. Stained cells were analyzed by a flow cytometer (FACScalibur, BD Biosciences) and CellQuest software (FACScalibur).
Cytotoxicity Assay
A standard 51Cr release assay was performed as previously described (18). In brief, K562 target cells were labeled with 51Cr (PerkinElmer Life Sciences, Boston, MA) at 50 μCi/5 × 105 cells. 5 × 103 labeled target cells were incubated for 4 h with serially diluted NK cells, cultured with or without anti-2B4, anti-CD2, anti-CD48, or anti-CD58 blocking mAbs or their isotype controls (mIgG1 or mIgGM). In some experiments, the assay was done in the presence of 5 μg/ml of anti-2B4, anti-CD2, anti-CD48, and anti-CD58 mAbs or isotype controls. The γ-scintillation of supernatant was measured by a γ-counter (PerkinElmer Life Sciences). Percent of specific lysis was calculated as follows: 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
IFN-γ Enzyme-linked Immunosorbent Assay (ELISA)
To assess IFN-γ secretion, ELISA was performed. Two hundred thousand IL-2-activated NK cells, cultured with or without anti-2B4, anti-CD2, or anti-CD48 blocking mAb or their isotype controls, were subsequently incubated with K562 (1 × 105) cells in 200 μl of complete RPMI for 18 h in the presence of the same blocking mAbs at 37 °C. The supernatant was collected and ELISA was performed using IFN-γ ELISA kits (eBioscience) following the manufacturer's instructions. Plates were read at 450 nm using a SpectraMax Microplated Reader (Molecular Devices, Sunnyvale, CA) and data were analyzed using SoftMax pro Software (Molecular Devices). All experiments were performed in duplicate and repeated at least three times.
Granzyme B Enzyme-linked Immunosorbent Spot (ELISPOT) Assay
For Granzyme B ELISPOT assay, MultiScreen-IP plates (PVDF membrane, Millipore) were coated overnight at 4 °C in 100 μl/well with anti-human Granzyme B capture antibody (eBioGrB; eBioscience). Two hundred thousand IL-2-activated NK cells in the presence of anti-2B4, anti-CD48, and anti-CD2 blocking mAbs or their isotype controls were coincubated with K562 target cells at an E:T ratio of 2:1 in 200 μl of medium. Three negative controls were applied: effector cells in the absence of target cells, target cells in the absence of effector cells, and medium alone. After incubation for 24 h, Granzyme B secretion was quantified using the Human Granzyme B ELISPOT kit (eBioscience, CA) following the manufacturer's instructions. The plates were air-dried and spots per well were counted using the Immunospot Imaging Analyzer system (Cellular Technology Ltd., Shaker Height, OH). All experiments were performed in triplicate and repeated at least two times.
Measurement of Calcium Flux
NK cells were loaded with 2 μm fura 2-AM (Molecular Probes, Invitrogen) in 2Ca+/Na buffer (140 mm NaCl, 10 mm Hepes, 2 mm CaCl2·2H2O, 1 mm MgCl2·6H2O, 10 mm glucose, and 5 mm KCl, pH 7.4) for 40 min at 37 °C. Cells were washed three times and resuspended in serum-free RPMI medium. Cells were then plated onto poly-l-lysine-coated 25-mm coverslips and incubated for 10 min. Coverslips were mounted at the bottom of a chamber placed on the stage of a Nikon Diaphot inverted epifluorescence microscope (Nikon, Garden City, NJ). Cells in a chamber were placed in 2Ca+/Na buffer (pH 7.4), and changes of the intracellular calcium concentration were measured upon addition of K562 target cells (E:T ratio = 1:1) and 4 μm ionomycin at intervals. The fluorescence ratio was monitored using a Metafluor Imaging System (Molecular Devices).
Western Blotting
K562 target cells were starved in serum-free medium overnight and fixed with 1.6% formaldehyde for 10 min. One million target cells were mixed with NK cells at 1:1 ratio and incubated at 37 °C for the designated times. Cells were harvested and lysed in ice-cold lysis buffer (20 mm Tris, pH 7.5, 100 mm NaCl, 5 mm MgCl2, 1% Nonidet P-40, 0.5% sodium deoxycholate) supplemented with protease inhibitor mixture and the phosphatase inhibitor mixture PhosSTOP (Roche Diagnostics) for 1 h at 4 °C. Equal amounts of protein was loaded on 10% SDS-PAGE gel and transferred to PVDF membrane (Gelman Science, Ann Arbor, MI) after gel electrophoresis. The resulting blot was processed using anti-phospho-ERK (Cell Signaling Technology) and developed by Immobilon Western Chemiluminescent HRP substrate (Millipore) on x-ray film. The blot was stripped and reprobed with anti-total ERK Ab (Cell Signaling Technology).
Statistics
One sample t test and analysis of variance (ANOVA) were performed to compare the mean difference between the samples (SPSS, Chicago, IL). Where p value was less than 0.05, the result was considered significant: *, p < 0.05; **, p < 0.01; ***, p < 0.001. All error bars represent S.D.
RESULTS
Ligation of 2B4 and CD48, but Not CD2 or CD58, Is Required for NK Cell Proliferation
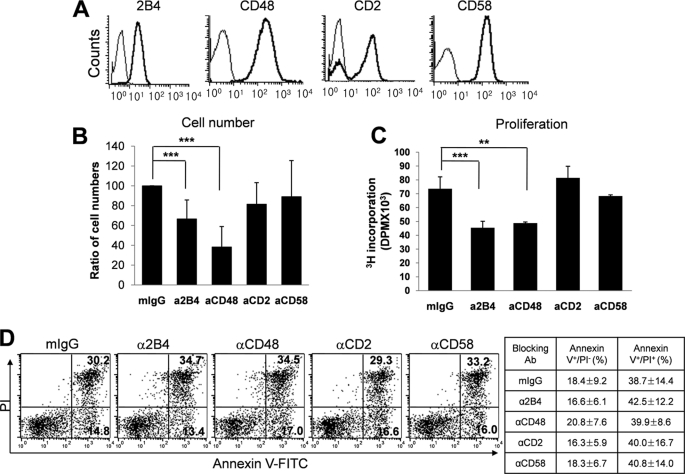
Human NK cells freshly isolated from PBMCs express 2B4, CD48, CD2, and CD58 on their surface (Fig. 1A). To dissect the role of 2B4/CD48 and CD2/CD58 interactions among NK cells and determine whether homotypic NK-NK cell interactions occur through these receptors, purified NK cells (>95%) were cultured with IL-2 in the presence or absence of blocking mAbs specific to 2B4, CD48, CD2, or CD58, or their matched isotype controls (mIgG1 or mIgM; Fig. 1 and data not shown). When the number of NK cells was assessed following 10 days in culture with IL-2, those treated with anti-2B4 or anti-CD48 mAbs, but not with anti-CD2 or anti-CD58 mAbs, demonstrated ∼45–60% reduction in total cell numbers, compared with their isotype control-treated groups (Fig. 1B).
FIGURE 1.
Human NK cell proliferation was dependent on homotypic 2B4/CD48 interactions among NK cells. A, human NK cells were isolated from PBMCs and their surface expression of 2B4, CD48, CD2, and CD58 was assessed by flow cytometry. B-D, isolated NK cells were cultured with IL-2 (300 units/ml) for 10 days in the presence of anti-2B4, anti-CD48, anti-CD2, or anti-CD58 mAb (5 μg/ml). Mouse IgG1 (mIgG1) or IgM (not shown) were used as matching isotype controls. The effect of mIgG1 or mIgM on cell proliferation was comparable with that of the untreated. B, live cell numbers were counted by trypan blue exclusion. Relative cell numbers compared with the isotype control are presented as mean (%) ± S.D. n = 9. ***, p < 0.001 (one sample t test). C, cells were incubated with 1 μCi of [3H]thymidine for the last 18 h in culture with IL-2 and incorporated [3H]thymidine into the DNA was measured. n = 4. **, p < 0.01 and ***, p < 0.001 determined by ANOVA combined with a Tukey post hoc test. D, cells were stained with FITC-conjugated Annexin V and propidium iodide and analyzed by flow cytometry. The values represent the percentage of cells in each quadrant. The results are representatives of 5 independent experiments. The mean ± S.D. from these 5 experiments are summarized in the table.
Because reduced NK cell expansion in the mAb-treated groups might have been the result of decreased cell proliferation, increased cell death, or both, the rate of cell proliferation was determined using [3H]thymidine incorporation assay and cell death was assessed using Annexin-V/PI staining. For the cells cultured with blocking anti-2B4 or anti-CD48 mAbs, the rate of [3H]thymidine incorporation into their DNAs was decreased approximately 35% compared with that of the isotype control groups, whereas cells treated with anti-CD2 or anti-CD58 mAbs remained unaffected (Fig. 1C). These results suggest that homotypic 2B4/CD48, not CD2/CD58, interactions among NK cells were critical for the expansion of human NK cells. Notably, neither blocking of 2B4/CD48 nor CD2/CD58 interactions significantly affected NK cell death during the culture, as the percentages of Annexin V+PI− early apoptotic cells and Annexin V+PI+ late apoptotic/necrotic cells in all mAb-treated groups were comparable with those of isotype controls (Fig. 1D). These data suggest that the reduced NK cell number by blocking homotypic 2B4/CD48 interactions was primarily due to the defect in cell proliferation rather than accelerating apoptotic cell death. Therefore, similar to mouse, engagement of 2B4 with CD48 on neighboring NK cells was found to be essential for the proliferation and proper expansion of NK cells in human.
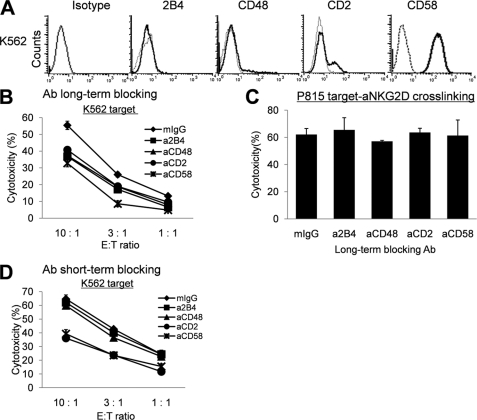
NK Cell Cross-talks through Homotypic 2B4/CD48 and CD2/CD58 Interactions Were Required for the Development of Full NK Cytotoxicity
To determine whether LAK cells generated in the absence of homotypic 2B4/CD48 or CD2/CD58 interactions could develop full cytotolytic potential, a standard 51Cr release assay was performed against the NK-sensitive leukemia K562 cells. As seen in Fig. 2A, K562 cells did not express surface 2B4 or CD48, thus the interaction between 2B4 and CD48 was localized only between NK cells, but not between NK and target cells. When human NK cells were cultured in the presence of IL-2, NK cytotoxicity increased within 48 h and remained elevated for up to 14 days (data not shown). Addition of isotype controls (mIgG1 or mIgM) during IL-2 culture did not affect elevated natural cytotoxicity. However, addition of blocking anti-2B4, anti-CD48, anti-CD2, or anti-CD58 mAbs during the culture resulted in reduction of NK cytotoxicity against K562 target cells (Fig. 2B). This reduction was apparent despite the absence of blocking mAbs during the 4-h 51Cr release assay, demonstrating that impairment of cytotoxicity was not developed instantly during the assay but rather could have occurred during the 14-day culture with IL-2 via blocking homotypic NK-NK interactions. At an E:T ratio of 10:1, cytotoxicity of the anti-2B4, anti-CD48, anti-CD2, or anti-CD58 mAb-treated NK cells was reduced by approximately 30% compared with the isotype control. Impaired cytotoxicity of anti-2B4 or anti-CD48 mAb-treated cells was not the result of poor viability or the inhibition of lytic machinery in these cells, as anti-NKG2D mAb-induced redirected antibody-dependent cell cytotoxicity of FcR+P815 targets was normal regardless of the presence of the mAbs (Fig. 2C). On the other hand, blocking 2B4/CD48 interactions by anti-2B4 or anti-CD48 mAbs only during the 4-h 51Cr release assay did not significantly alter NK cytotoxicity (Fig. 2D). These data demonstrate that prolonged interactions of 2B4 and CD48 among NK cells were required to develop maximal cytotoxicity and that the contribution of homotypic 2B4/CD48 interactions to target lysis became relatively insignificant once effector cells mounted full cytotoxic potential.
FIGURE 2.
Blocking homotypic 2B4/CD48 or CD2/CD58 interactions caused reduction of NK cytotoxicity against CD48 negative K562 targets. A, expression of 2B4, CD48, CD2, and CD58 on K562 target cells was assessed by flow cytometry. B, a standard 4-h 51Cr release assay was performed with NK cells, cultured with 300 units/ml of IL-2 in the presence of 5 μg/ml of mIgG as an isotype control (♦), anti-2B4 mAb (■), anti-CD48 mAb (▴), anti-CD2 mAb (●), or anti-CD58 mAb (×) for 14 days, as effectors and with 51Cr-labeled K562 cells as target cells (Ab long-term blocking). C, a redirected antibody-dependent cell cytotoxicity assay against 51Cr-labeled P815 cells pre-coated with anti-NKG2D mAb was performed with NK cells cultured as in B. Specific lysis observed at an E:T ratio of 10:1 was shown. D, a standard 4-h 51Cr release assay was performed using NK cells cultured with IL-2 without blocking mAbs. Mouse IgG as isotype control (♦), anti-2B4 mAb (■), anti-CD48 mAb (▴), anti-CD2 mAb (●), or anti-CD58 mAb (×) at 5 μg/ml were added only during the 4-h cytotoxicity assay (Ab short-term blocking). All the results are representatives of a minimum of 5 independent experiments. Error bars represent S.D.
Intriguingly, whereas long-term blocking of CD2/CD58 interactions during the culture with IL-2 did not affect NK cell expansion (Fig. 1), cells cultured in the presence of anti-CD2 or anti-CD58 mAbs demonstrated reduced NK cytotoxicity against K562 cells (Fig. 2B). This raised a possibility that CD2/CD58 interactions might also have provided a co-stimulatory role in the development of cytotoxic NK cells. However, because K562 target cells also expressed CD58, reduced cytotoxicity in the anti-CD2 mAb-treated cells might have resulted from the inhibition of NK-target interactions through down-regulated CD2. Indeed, addition of anti-CD2 or anti-CD58 mAbs during the 4-h 51Cr release assay caused significant reduction of cytotoxicity against K562 targets (Fig. 2D), demonstrating that the CD2 receptor was engaged in natural cytotoxicity against CD58+ targets. However, the fact that anti-CD58 mAb-treated NK cells, which expressed normal levels of CD2, also showed defects in lysing CD58+K562 cells suggests that homotypic interactions through CD2/CD58 among NK cells existed and influenced the development of full cytotoxic potentials. Therefore, unlike 2B4/CD48, CD2/CD58 interactions appeared to control both homotypic NK-NK and heterotypic NK-target interactions.
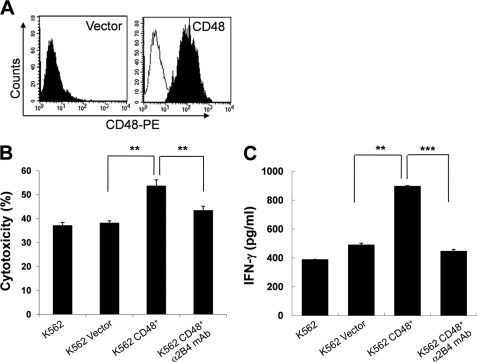
Ectopic Expression of CD48 on K562 Cells Increases Their Lysis by 2B4-expressing NK Cells
The results presented above demonstrate a co-stimulatory role of 2B4 during the IL-2-induced NK cell activation process. Because human 2B4 was also found to be an activating receptor in a variety of in vitro models (5, 19–21), the question was raised whether ectopic expression of CD48 on K562 target cells drove heterotrophic NK-target interactions and facilitated the cytoxicity of LAK cells. For this, K562 cells were transduced with a retroviral vector encoding the human CD48 gene. Selected CD48-expressing cells uniformly expressed CD48 on their surface, whereas no CD48 was detected on cells transduced with an empty vector (Fig. 3A). In accordance with previous reports (22), CD48-expressing K562 cells were more susceptible to NK lysis by LAK cells than those not transduced or empty vector-transduced (Fig. 3B). The average percent increase of NK lysis against CD48+K562 cells was calculated to be 40.9% as compared with CD48-K562 cells (K562 or K562 vector, **, p < 0.01). Increased cytotoxicity of CD48+K562 cells was mainly due to the 2B4/CD48 interactions between NK and K562 cells, as addition of blocking anti-2B4 mAb during the 4-h cytotoxicity assay abrogated this increase (Fig. 3B). Furthermore, CD48-expressing K562 cells significantly increased IFN-γ secretion in LAK cells as compared with the control untransduced or empty vector-transduced cells (Fig. 3C). This increase was also dependent on 2B4 binding of CD48 on targets, as an additive increase of IFN-γ production was hampered in the presence of blocking anti-2B4 mAbs. Together, these data demonstrate that 2B4 in human NK cells plays a role not only as a co-stimulatory receptor but also as an activating receptor leading to cytokine secretion and cytotoxicity.
FIGURE 3.
CD48-expressing K562 target cells were more susceptible to NK cytotoxicity. A, K562 cells were transduced with pMFG retroviral vector carrying human CD48 cDNA, as described under “Experimental Procedures.” CD48 expression on empty vector-transduced or CD48-transduced K562 cells was analyzed by flow cytometry. B, a standard 4-h 51Cr release assay was performed using NK cells cultured with 300 units/ml of IL-2 for 10–14 days as effector cells and parental K562 cells, empty vector-transduced K562 cells, or CD48-expressing K562 cells as targets. Where indicated, 5 μg/ml of anti-2B4 mAb was added during the 4-h cytotoxicity assay. Specific lysis (%), presented as mean ± S.D. of triplicates, at an E:T ratio of 10:1 was shown. The average NK cytotoxicity against K562, K562 vector, K562 CD48+, and K562 CD48+ with α2B4 mAb was 37.1 ± 1.2, 38.1 ± 0.9, 53.7 ± 2.5, and 43.3 ± 1.8%, respectively (**, p < 0.01). The data are representative of 5 independent experiments. C, LAK cells, cultured as described above, were incubated with parental, empty vector-transduced, or CD48-expressing K562 cells at an E:T ratio of 2:1 for 18 h. Where indicated, 5 μg/ml of anti-2B4 mAb was added during the assay. Secretion of IFN-γ into the supernatant was assessed by ELISA. The data are representatives of a minimum of 3 independent experiments. **, p < 0.01 and ***, p < 0.001, as determined by ANOVA combined with a Tukey post hoc test. Error bars represent S.D.
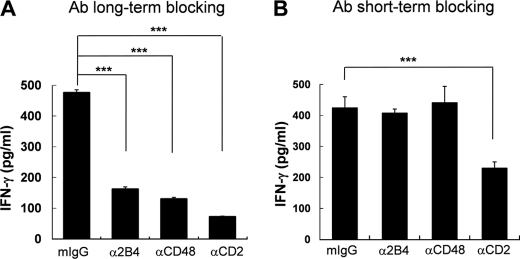
Homotypic NK Cell Interactions through 2B4/CD48 and CD2/CD58 Was Required for the Secretion of IFN-γ
To examine if reduced cytolytic functions by LAK cells generated in the absence of 2B4/CD48 or CD2/CD58 interactions was associated with impaired secretory functions, we assessed the ability of these cells to secrete IFN-γ (Fig. 4). Upon stimulation by K562 cells, NK cells secreted a substantial amount of IFN-γ (Fig. 4A). However, cells cultured in the presence of anti-2B4, anti-CD48, anti-CD2, or anti-CD58 mAbs showed dramatic impairment in secreting IFN-γ (Fig. 4A and data not shown). The effect of anti-2B4 and anti-CD2 mAbs was found to be highly comparable with anti-CD48 and anti-CD58 mAbs, respectively. When compared with cells cultured with isotype control, anti-2B4-, anti-CD48-, or anti-CD2 mAb-treated cells displayed ∼36, 29, and 16% of IFN-γ, respectively. Among the cells tested, anti-CD2 mAb caused the most profound reduction of IFN-γ, presumably due to inhibition of both homotypic NK-NK and heterotypic NK-target CD2/CD58 interactions. Indeed, addition of anti-CD2 mAbs partially inhibited IFN-γ secretion, when LAK cells were incubated with K562 cells during the cytotoxicity assay (Fig. 4B), supporting the role of CD2 as an activating receptor against CD58+K562 targets. In contrast, addition of anti-2B4 or anti-CD48 mAbs during the assay did not significantly affect the secretion of IFN-γ, demonstrating that homotypic 2B4/CD48 interactions did not contribute to the secretory functions of LAK cells once they became effector cells. These results demonstrate that the loss of NK-NK cross-talk through 2B4/CD48 or CD2/CD58 during the effector phase of IL-2 stimulation specifically impaired the ability of NK cells to secrete IFN-γ.
FIGURE 4.
LAK cells, cultured in the presence of blocking anti-2B4, anti-CD48, or anti-CD2 mAbs, produced less IFN-γ upon K562 target exposure. Human primary NK cells, cultured with 300 units/ml of IL-2 for 14 days in the presence (A) or absence (B) of 5 μg/ml of isotype control or anti-2B4, anti-CD48, or anti-CD2 mAbs, were incubated with K562 cells at an E:T ratio of 2:1 for 18 h. In B, mIgG isotype control, anti-2B4, anti-CD48, or anti-CD2 mAbs were added during the 18-h assay. Secretion of IFN-γ into the supernatant was assessed by ELISA. The data are representatives of a minimum 3 of independent experiments. ***, p < 0.001, as determined by ANOVA combined with a Tukey post hoc test. Error bars represent S.D.
Impaired Cytotoxicity in the Absence of 2B4/CD48 or CD2/CD58 Interactions Was Associated with Defective Degranulation Process
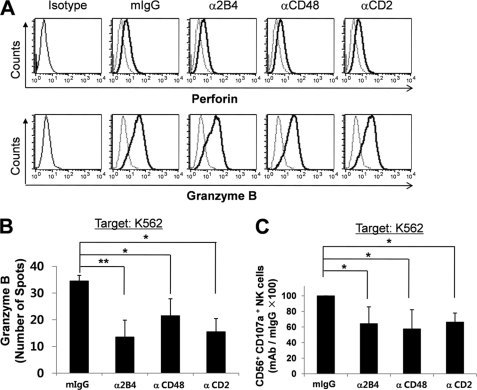
To identify the basis for the cytotoxic defects of LAK cells cultured in the absence of 2B4/CD48 or CD2/CD58 interactions, intracellular content of granzyme B and perforin was assessed by flow cytometry. As shown in Fig. 5A, LAK cells generated in the presence of blocking mAbs to 2B4, CD48, or CD2, or CD58 (data not shown) all expressed comparable levels of perforin and granzyme B, demonstrating that blocking homotypic interactions among NK cells did not affect the intrinsic capacity to produce cytotoxic molecules. However, the release of granzyme B in NK cells upon encountering K562 targets was significantly inhibited in the absence of 2B4/CD48 or CD2/CD58 binding (Fig. 5B). Degranulation events, as measured by CD107a expression, were also hampered in LAK cells cultured in the presence of blocking anti-2B4, anti-CD48, or anti-CD2 mAb (Fig. 5C). These results suggest that blocking homotypic NK cell interactions through 2B4/CD48 or CD2/CD58 specifically affected the release, but not the production, of cytotoxic granules in LAK cells and thereby caused impaired lysis against tumor targets.
FIGURE 5.
Blocking 2B4/CD48 homotypic interactions impaired degranulation events without affecting the production of granzyme B and perforin. A, isolated NK cells, cultured with 300 units/ml of IL-2 for 14 days in the presence of isotype control, anti-2B4, anti-CD48, or anti-CD2 mAbs, were incubated with K562 cells at a 1:1 ratio for 6 h. GolgiStop was added for the last 5 h. The cells were then stained with anti-granzyme B or anti-perforin mAbs and assessed by flow cytometry. The data are representatives of a minimum of 3 independent experiments. B, isolated NK cells cultured as above were co-incubated with K562 cells at an E:T ratio of 1:1 for 24 h in anti-granzyme B Ab-coated ELISPOT plates. The spot numbers per well were presented as mean ± S.D. n = 3, *, p < 0.05 and **, p < 0.01, as determined by ANOVA combined with a Tukey post hoc test. C, NK cells cultured as above were co-incubated with K562 cells at an E:T ratio of 1:1 for 6 h. GolgiStop was added for the last 5 h. The cells were stained with anti-CD56 and anti-CD107a Abs and degranulation was assessed by flow cytometry. The values represent the relative CD56+CD107a+ NK cell number as compared with the isotype control, presented as mean (%) ± S.D., n = 5 (*, p < 0.05, one sample t test).
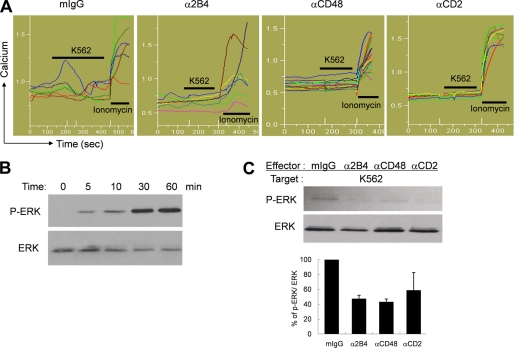
Blocking Homotypic Interactions Among NK Cells Resulted in Defective Intracellular Calcium Release and ERK Phosphorylation
Impaired cytotoxicity and cytokine secretion in anti-2B4-, anti-CD48-, or anti-CD2 mAb-treated cells suggest that the signaling pathway initiated from NK receptors might not have operated properly upon tumor target exposure. Because calcium mobilization induced by activation of PLC-γ is an initial step leading to degranulation events, we addressed whether the defective signaling in anti-2B4-, anti-CD48-, or anti-CD2 mAb-treated LAK cells emerged at the level of intracellular calcium mobilization. As seen in Fig. 6A, NK cells elevated intracellular calcium upon interacting with K562 target cells. The percentage of NK cells responded to K562 target cells varied between 50 and 70%. In contrast, LAK cells generated in the presence of anti-2B4, anti-CD48, or anti-CD2 mAbs substantially diminished intracellular calcium flux (Fig. 6A). Impaired calcium release in these mAb-treated cells was not the result of poor viability because they showed normal release of intracellular calcium by addition of ionomycin, a calcium ionophore. These results suggest that the signaling defect in the absence of homotypic 2B4/CD48 interactions might have occurred at the proximal step of PLC-γ activation. To further dissect pathways involved in homotypic NK cell interactions, ERK activation was assessed in LAK cells cultured in the presence or absence of anti-2B4, anti-CD48, or anti-CD2 mAbs. ERK is one of the known downstream molecules of 2B4 signaling, mediating polarization of the microtubule organizing center and granule exocytosis in NK cells (23–25). Upon mixing LAK cells with target cells, ERK was found to be increasingly phosphorylated in a time-dependent manner, reaching a plateau within 30 min (Fig. 6B). However, LAK cells generated in the absence of 2B4/CD48 or CD2/CD58 interactions was not able to induce phos- phorylation of ERK after encountering K562 cells (Fig. 6C). Taken together, costimulatory signals conveyed by homotypic 2B4/CD48 and CD2/CD58 interactions among NK cells were necessary for intracellular calcium mobilization and ERK phosphorylation leading to the development of maximal effector functions.
FIGURE 6.
Blocking homotypic 2B4/CD48 interactions hampered intracellular calcium release and phosphorylation of ERK. A, NK cells were cultured with 300 units/ml of IL-2 for 14 days in the presence of mIgG as isotype control, anti-2B4, anti-CD48, or anti-CD2 mAbs. Calcium flux was measured with an epifluorescence microscope. K562 cells and ionomycin were inoculated sequentially. Each line represents intracellular calcium concentration from a single cell in a given microscopic field. The data are representatives of minimum 3 independent experiments. B, Western blotting was performed to assess time-dependent changes of ERK phosphorylation in LAK cells upon stimulation with K562 cells. LAK cells were co-incubated with fixed K562 cells at 1:1 ratio and subsequently harvested at the designated time points for lysis. The upper bands represent phosphorylated ERK1/2 and the lower bands represent total ERK1/2. C, human primary NK cells were cultured with 300 units/ml of IL-2 for 14 days in the presence of mIgG as isotype control, anti-2B4, anti-CD48, or anti-CD2 mAbs. NK cells were coincubated with fixed K562 cells at an E:T ratio of 1:1 for 30 min, and Western blotting was performed as described above. The data are representatives of a minimum of 3 independent experiments. Error bars represent S.D.
DISCUSSION
Human and mouse NK cells present an analogous mode of activation mechanisms, mainly through altering the balance between activating and inhibitory signals upon stimulation. Although a number of receptors were found to be specifically expressed on human or mouse NK cells, e.g. the killer cell immunoglobulin-like receptors, CD56, CD58, and NKp44 for human and a lectin-like structure (Ly49 receptors) and NK1.1 for mouse, many receptors were found and functionally preserved in both mouse and human NK cells. 2B4 and CD2 are among those shared by mouse and human NK cells and function through binding to CD48, their common ligand. Additionally, CD2 has another high affinity ligand, CD58 in human. Although 2B4, CD2, CD48, and CD58, are all constitutively expressed on NK cells, the functional consequences of these interactions among NK cells have remained elusive until now.
Previously we reported that 2B4, but not CD2, was found to play a major role in the proliferation and activation of murine NK cells through homotypic NK-NK interactions (7). In this study, we confirm the presence of such homotypic interactions through 2B4/CD48 in human NK cells and further present the role of CD2/CD58 interactions during NK cell activation. Using blocking antibodies specific to each receptor, it was demonstrated that both 2B4/CD48 and CD2/CD58 interactions were required for the development of effector NK cells equipped with full cytolytic and secretory functions. However, 2B4/CD48, but not CD2/CD58, interactions were found to control optimal NK cell proliferation in response to IL-2. Therefore, the costimulatory function of 2B4 appeared to be preserved across the species, but that of CD2 was differentially regulated in human and mice.
These results are consistent with an earlier report by Valiante and Trinchieri (5), where 2B4 was initially found as an activating receptor on human NK cells. Inhibition of NK cell proliferation was apparent when blocking mAb against human 2B4 (clone C1.7) was added to LAK cells cultured for 7 days. In contrast, a recent report by Stark and Watzl (19) demonstrated no discernible effect on NK cell proliferation for up to 4 days when 2B4/CD48 interactions were blocked using C1.7 mAb during cultures with IL-2 and phytohemagglutinin. Those results are not entirely inconsistent from our findings because it was also noticed that the presence of C1.7 mAb for less than 7 days in culture did not significantly influence proliferation of LAK cells. Thus, NK-NK cross-talk between 2B4 and CD48 appears to become more important in the later effector phase than the earlier activation phase. However, an alternative possibility is that the culture condition containing IL-2 and phytohemagglutinin in the presence of irradiated feeder cells (K562 and 721.221, 1:1 mix), applied by Stark and Watzl (19), were highly stimulating, hence cells might become less dependent on homotypic 2B4/CD48 interactions. Notably LAK cells generated in the absence of 2B4/CD48 interactions were severely impaired for their ability to elevate intracellular calcium and subsequent ERK activation, which was required for perforin-dependent cytolysis, degranulation, and IFN-γ secretion. Therefore, NK signaling defect in the absence of 2B4/CD48 interactions appeared to have occurred at the proximal level of PLC-γ activation.
Although homotypic 2B4/CD48 interactions were shown to exist both in human and mouse NK cells, murine 2B4 could also function as an inhibitory receptor. Thus, CD48 expression on tumor targets inhibited NK lysis, whereas the absence of 2B4 induced self-fratricide in murine NK cells (26). These results provide a self-tolerance mechanism, independent of MHC class I, against surrounding autologous CD48 expressing hematopoietic cells. However, such an inhibitory function of 2B4 has not been found in mature human NK cells. Similarly, no changes of cell death rate or fratricide were detected in the human NK cell culture where 2B4/CD48 binding was blocked in our study. Furthermore, in contrast to mouse, ectopic expression of CD48 on K562 tumor targets rather facilitated than inhibited their lysis by NK cells, suggesting that 2B4 in human consistently provided co-stimulatory and activating, not inhibitory, functions. These results raised a question of the mechanism of controlling tolerance against CD48+ NK or surrounding hematopoietic cells in human. Recent reports demonstrated that the 2B4 function was tightly regulated by MHC class I (27) and by collective and simultaneous engagement of other NK receptors, e.g. LFA-1 and NKG2D, in human (28–29). Therefore, the species-specific regulatory mechanism of 2B4 function may operate in mouse and human NK cells.
Role of CD2 on NK cells was much less appreciated although it is a well known co-stimulatory receptor in T cells (30, 31). CD2 was abundantly expressed in human T cells and shown to enhance antigen-presenting cell-T cell adhesion and promote T cell activation at lower antigen concentrations (32). In addition, CD2 was also suggested to initiate intracellular signaling when cross-linked with anti-CD2 mAbs (33, 34). More recent results demonstrated that CD2 ligation with CD58-stimulated T cell signaling cascades involved Lck, TCRζ chain, and LAT through actin-dependent recruitment to the plasma membrane microdomain (35). Furthermore, CD2 interaction with CD58 on antigen-presenting cells activated PLC-γ and augmented intracellular calcium release in antigen-specific T cell clones (31). It was also shown that simulation of CD2 up-regulated IL-8 production in human intestinal intraepithelial lymphocytes upon contact with epithelial cells (36). Therefore, CD2/CD58 interactions in human T cells exhibited a potential to initiate TCR signaling independently and/or synergistically with TCR stimulation. Herein we demonstrate two distinct functions of CD2 in human NK cells: as an activating receptor toward CD58 expressed on target cells to promote their lysis, and as a co-stimulatory receptor to regulate the development of NK effector functions. This dual function of CD2 appeared to be mostly the results of binding to CD58, not CD48, in human NK cells, although CD2 could bind CD48 with relatively weak affinity. The comparable effects by anti-CD2 and anti-CD58 mAbs in blocking proliferation and cytotoxicity support this notion. Notably, LAK cells generated in the absence of CD2/CD58 interactions did not show defects in cell proliferation or production of cytotoxic granules, such as perforin and granzyme B. Nonetheless, they were much less efficient in secreting IFN-γ and tumor cytotoxicity. These impairments were likely associated with the inability of NK cells to induce intracellular calcium release and subsequent ERK activation upon tumor target exposures, similar to those observed with anti-2B4 or anti-CD48 mAb-treated cells. Our results are consistent with a previous report using a human NK cell line, NK92, wherein blockade of CD2 on NK cells reduced cytotoxicity and ERK activation against K562 targets (37).
It is not clear at present what determines the co-stimulatory versus activating interactions when 2B4 or CD2 are engaged with their cognate ligands. LAK cells generated in the absence of 2B4/CD48 or CD2/CD58 interactions both demonstrated severe intracellular singaling defects leading to impaired calcium mobilization and ERK activation, which might have accounted for defective cytotoxicity and IFN-γ secretion. However, these defects do not explain the proliferative defects in the absence of 2B4/CD48 because LAK cells cultured in the absence of CD2/CD58 interactions displayed normal proliferation. These data propose signaling divergence between cell proliferation and cytotoxicity/cytokine release, which may be controlled differentially by 2B4 or CD2 during NK activation and expansion. Studies are underway to dissect individual signaling pathways controlled by homotypic 2B4/CD48 and CD2/CD58 interactions.
The differential function of 2B4 from mouse and human may be attributed to its intracellular sequences. Although two isoforms of 2B4, 2B4L with 4 immunoreceptor tyrosine-based switch motifs and 2B4S with 1 immunoreceptor tyrosine-based switch motif, were found in mouse, only the long form of 2B4 with 4 immunoreceptor tyrosine-based switch motifs is reported so far in human. 2B4L is known to mediate inhibitory signals via SHP-1, whereas 2B4S delivered activating signals (38). In human, the third immunoreceptor tyrosine-based switch motif was shown to recruit inhibitory tyrosine phosphatases, e.g. SHP-1, SHP-2, and SHIP. The competition between adaptor molecule SAP and tyrosine phosphatases was demonstrated to drive the function of 2B4 toward activating or inhibiting (13, 39). Another adaptor molecule, 3BP2 (SH23BP2), was found to interact with human 2B4, but not with murine (24). 3BP2 acts downstream of SAP and links to the activation of PI3K, Vav, ERK, PLC-γ, and PKC (40). Thus, lack of SAP binding to 2B4, observed in x-linked lymphoproliferative disease patients, likely converts activating 2B4 into inhibitory via associated tyrosine phosphatases. Consistent with this hypothesis, SAP expression was shown to be up-regulated upon activation (41), and relative paucity of SAP failed to deliver signals to 3BP2 and subsequent PLC-γ and ERK activation (42). Unlike 2B4, the role of CD2 in control of NK effector functions was found to be specific to human, not murine, NK cells. However, we cannot rule out the possibility that the lack of CD2 function in mouse NK cells might have been due to the absence of its high affinity receptor. Therefore, it would be interesting to see whether expression of human CD58 on mouse NK cells turns CD2 into a positive regulator for homotypic NK-NK cross-talk in mouse.
In summary, these results demonstrate the existence of cell to cell cross-talks among NK cells through 2B4/CD48 and CD2/CD58 in humans. Both 2B4/CD48 and CD2/CD58 interactions are required for the development of cytotoxic and secretory effector functions, but only 2B4/CD48, not CD2/CD58, binding appears to be critical for the optimal NK cell expansion. These results suggest differential and overlapping roles of 2B4/CD48 and CD2/CD58 interactions in mouse and human NK cells, which is required to be taken into consideration to analyze in vivo immune phenomena in particular disease settings of animal models.
This work was supported in part by Korea Foundation for International Cooperation of Science and Technology Grants K20704000007-09A0500-00710 and K20601000002-09E0100-00210.
- NK
- natural killer
- PBMC
- peripheral blood mononuclear cell
- ELISPOT
- enzyme-linked immunosorbent spot
- ANOVA
- analysis of variance
- PLC
- phospholipase.
REFERENCES
- 1.McNerney M. E., Lee K. M., Kumar V. (2005) Mol. Immunol. 42, 489–494 [DOI] [PubMed] [Google Scholar]
- 2.Veillette A. (2006) Immunol. Rev. 214, 22–34 [DOI] [PubMed] [Google Scholar]
- 3.Vaidya S. V., Stepp S. E., McNerney M. E., Lee J. K., Bennett M., Lee K. M., Stewart C. L., Kumar V., Mathew P. A. (2005) J. Immunol. 174, 800–807 [DOI] [PubMed] [Google Scholar]
- 4.Mahajan S., Gollob J. A., Ritz J., Frank D. A. (2001) Exp. Hematol. 29, 209–220 [DOI] [PubMed] [Google Scholar]
- 5.Valiante N. M., Trinchieri G. (1993) J. Exp. Med. 178, 1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M. H., Boles K., van der Merwe P. A., Kumar V., Mathew P. A., Barclay A. N. (1998) J. Exp. Med. 188, 2083–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K. M., Forman J. P., McNerney M. E., Stepp S., Kuppireddi S., Guzior D., Latchman Y. E., Sayegh M. H., Yagita H., Park C. K., Oh S. B., Wülfing C., Schatzle J., Mathew P. A., Sharpe A. H., Kumar V. (2006) Blood 107, 3181–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assarsson E., Kambayashi T., Persson C. M., Ljunggren H. G., Chambers B. J. (2005) Mol. Immunol. 42, 419–423 [DOI] [PubMed] [Google Scholar]
- 9.Assarsson E., Kambayashi T., Persson C. M., Chambers B. J., Ljunggren H. G. (2005) J. Immunol. 175, 2045–2049 [DOI] [PubMed] [Google Scholar]
- 10.Kambayashi T., Assarsson E., Chambers B. J., Ljunggren H. G. (2001) J. Immunol. 167, 6706–6710 [DOI] [PubMed] [Google Scholar]
- 11.Assarsson E., Kambayashi T., Schatzle J. D., Cramer S. O., von Bonin A., Jensen P. E., Ljunggren H. G., Chambers B. J. (2004) J. Immunol. 173, 174–180 [DOI] [PubMed] [Google Scholar]
- 12.Lee K. M., McNerney M. E., Stepp S. E., Mathew P. A., Schatzle J. D., Bennett M., Kumar V. (2004) J. Exp. Med. 199, 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tangye S. G., Lazetic S., Woollatt E., Sutherland G. R., Lanier L. L., Phillips J. H. (1999) J. Immunol. 162, 6981–6985 [PubMed] [Google Scholar]
- 14.Nakajima H., Cella M., Langen H., Friedlein A., Colonna M. (1999) Eur. J. Immunol. 29, 1676–1683 [DOI] [PubMed] [Google Scholar]
- 15.Dupré L., Andolfi G., Tangye S. G., Clementi R., Locatelli F., Aricò M., Aiuti A., Roncarolo M. G. (2005) Blood 105, 4383–4389 [DOI] [PubMed] [Google Scholar]
- 16.Sadelain M., Wang C. H., Antoniou M., Grosveld F., Mulligan R. C. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 6728–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alter G., Malenfant J. M., Altfeld M. (2004) J. Immunol. Methods 294, 15–22 [DOI] [PubMed] [Google Scholar]
- 18.Yoon S. H., Yun S. O., Park J. Y., Won H. Y., Kim E. K., Sohn H. J., Cho H. I., Kim T. G. (2009) Exp. Mol. Med. 41, 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark S., Watzl C. (2006) Int. Immunol. 18, 241–247 [DOI] [PubMed] [Google Scholar]
- 20.Mathew S. O., Rao K. K., Kim J. R., Bambard N. D., Mathew P. A. (2009) Eur. J. Immunol. 39, 1632–1641 [DOI] [PubMed] [Google Scholar]
- 21.Vaidya S. V., Mathew P. A. (2006) Immunol. Lett. 105, 180–184 [DOI] [PubMed] [Google Scholar]
- 22.Messmer B., Eissmann P., Stark S., Watzl C. (2006) J. Immunol. 176, 4646–4650 [DOI] [PubMed] [Google Scholar]
- 23.Chuang S. S., Kumaresan P. R., Mathew P. A. (2001) J. Immunol. 167, 6210–6216 [DOI] [PubMed] [Google Scholar]
- 24.Saborit-Villarroya I., Del Valle J. M., Romero X., Esplugues E., Lauzurica P., Engel P., Martín M. (2005) J. Immunol. 175, 4226–4235 [DOI] [PubMed] [Google Scholar]
- 25.Chen X., Allan D. S., Krzewski K., Ge B., Kopcow H., Strominger J. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10346–10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi R. T., Guzior D., Kumar V. (2007) Blood 110, 2020–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Betser-Cohen G., Mizrahi S., Elboim M., Alsheich-Bartok O., Mandelboim O. (2010) J. Immunol. 184, 2761–2768 [DOI] [PubMed] [Google Scholar]
- 28.Fauriat C., Long E. O., Ljunggren H. G., Bryceson Y. T. (2010) Blood 115, 2167–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryceson Y. T., Ljunggren H. G., Long E. O. (2009) Blood 114, 2657–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford K., Stark A., Kitchens B., Sternheim K., Pantazopoulos V., Triantafellow E., Wang Z., Vasir B., Larsen C. E., Gabuzda D., Reinherz E., Alper C. A. (2003) Blood 102, 1745–1752 [DOI] [PubMed] [Google Scholar]
- 31.Espagnolle N., Depoil D., Zaru R., Demeur C., Champagne E., Guiraud M., Valitutti S. (2007) Int. Immunol. 19, 239–248 [DOI] [PubMed] [Google Scholar]
- 32.Bachmann M. F., Barner M., Kopf M. (1999) J. Exp. Med. 190, 1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. (1985) Nature 317, 428–430 [DOI] [PubMed] [Google Scholar]
- 34.Kanner S. B., Damle N. K., Blake J., Aruffo A., Ledbetter J. A. (1992) J. Immunol. 148, 2023–2029 [PubMed] [Google Scholar]
- 35.Kaizuka Y., Douglass A. D., Vardhana S., Dustin M. L., Vale R. D. (2009) J. Cell Biol. 185, 521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert E. C., Panja A., Praveen R. (2009) Am. J. Physiol. Gastrointest. Liver Physiol. 296, G671–677 [DOI] [PubMed] [Google Scholar]
- 37.Zheng X., Wang Y., Wei H., Sun R., Tian Z. (2009) J. Biol. Chem. 284, 21280–21287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wahle J. A., Paraiso K. H., Kendig R. D., Lawrence H. R., Chen L., Wu J., Kerr W. G. (2007) J. Immunol. 179, 8009–8015 [DOI] [PubMed] [Google Scholar]
- 39.Eissmann P., Beauchamp L., Wooters J., Tilton J. C., Long E. O., Watzl C. (2005) Blood 105, 4722–4729 [DOI] [PubMed] [Google Scholar]
- 40.Saborit-Villarroya I., Martinez-Barriocanal A., Oliver-Vila I., Engel P., Sayos J., Martin M. (2008) Mol. Immunol. 45, 3446–3453 [DOI] [PubMed] [Google Scholar]
- 41.Endt J., Eissmann P., Hoffmann S. C., Meinke S., Giese T., Watzl C. (2007) Eur. J. Immunol. 37, 193–198 [DOI] [PubMed] [Google Scholar]
- 42.Chlewicki L. K., Velikovsky C. A., Balakrishnan V., Mariuzza R. A., Kumar V. (2008) J. Immunol. 180, 8159–8167 [DOI] [PubMed] [Google Scholar]