Abstract
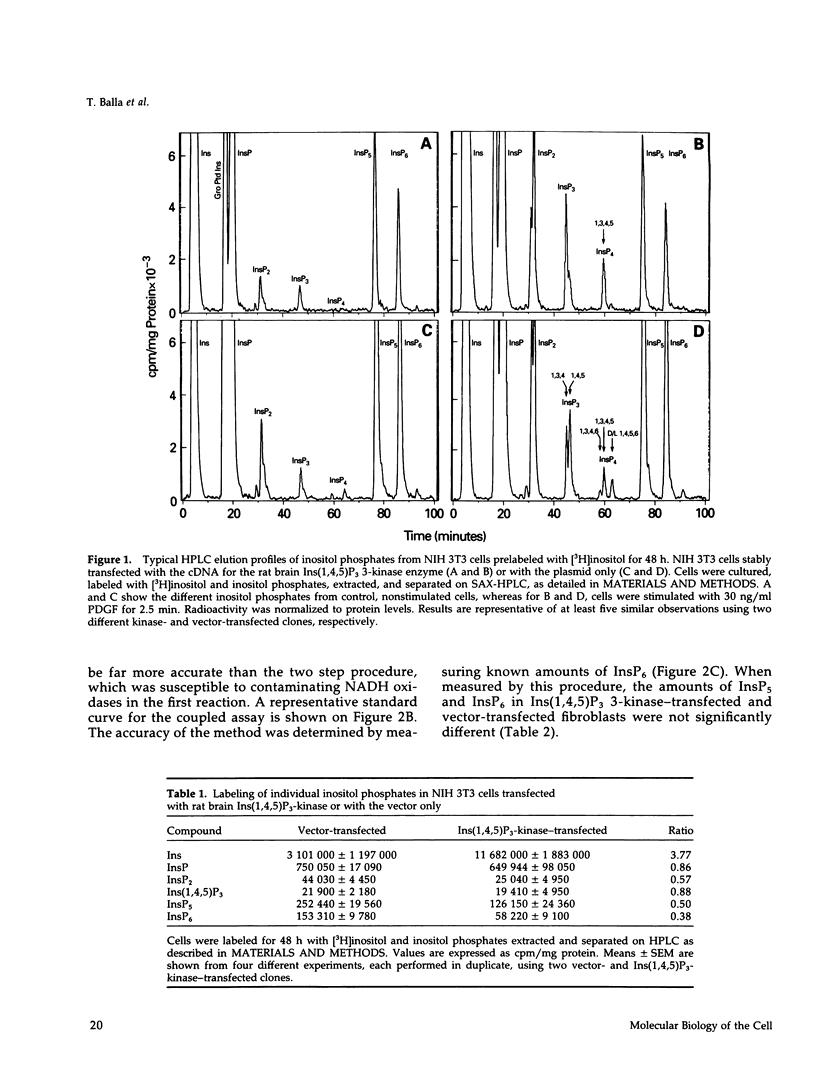
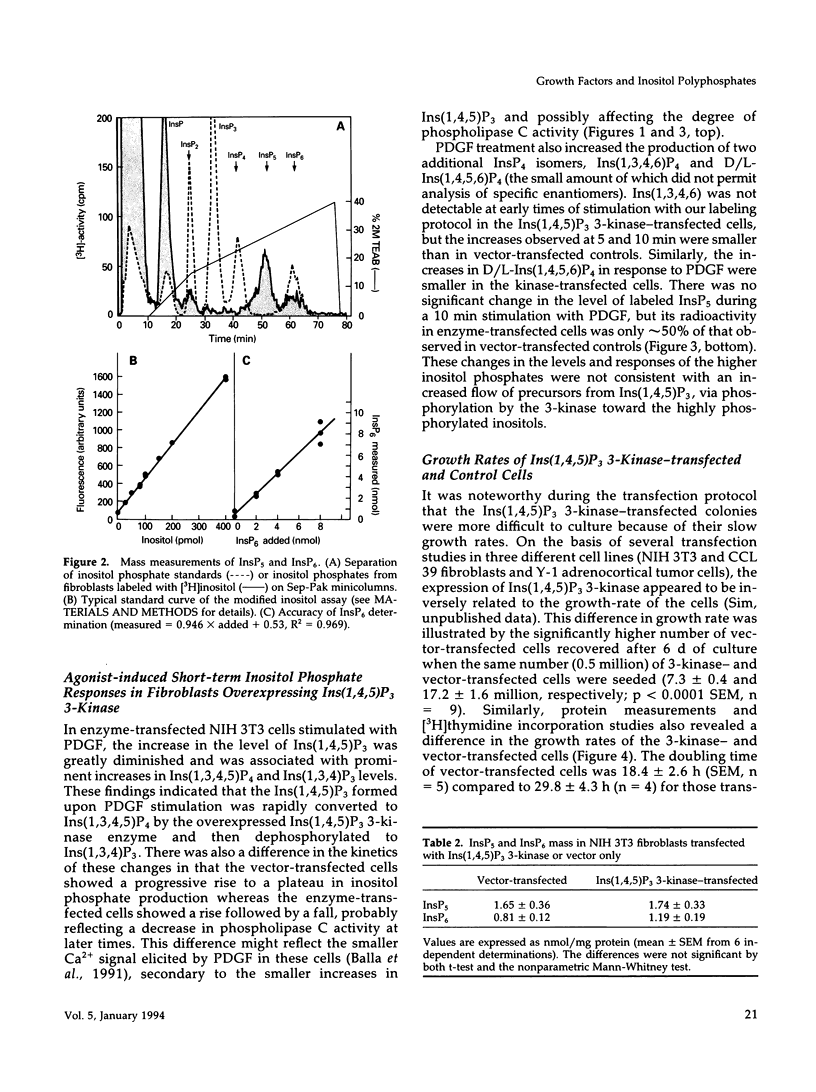
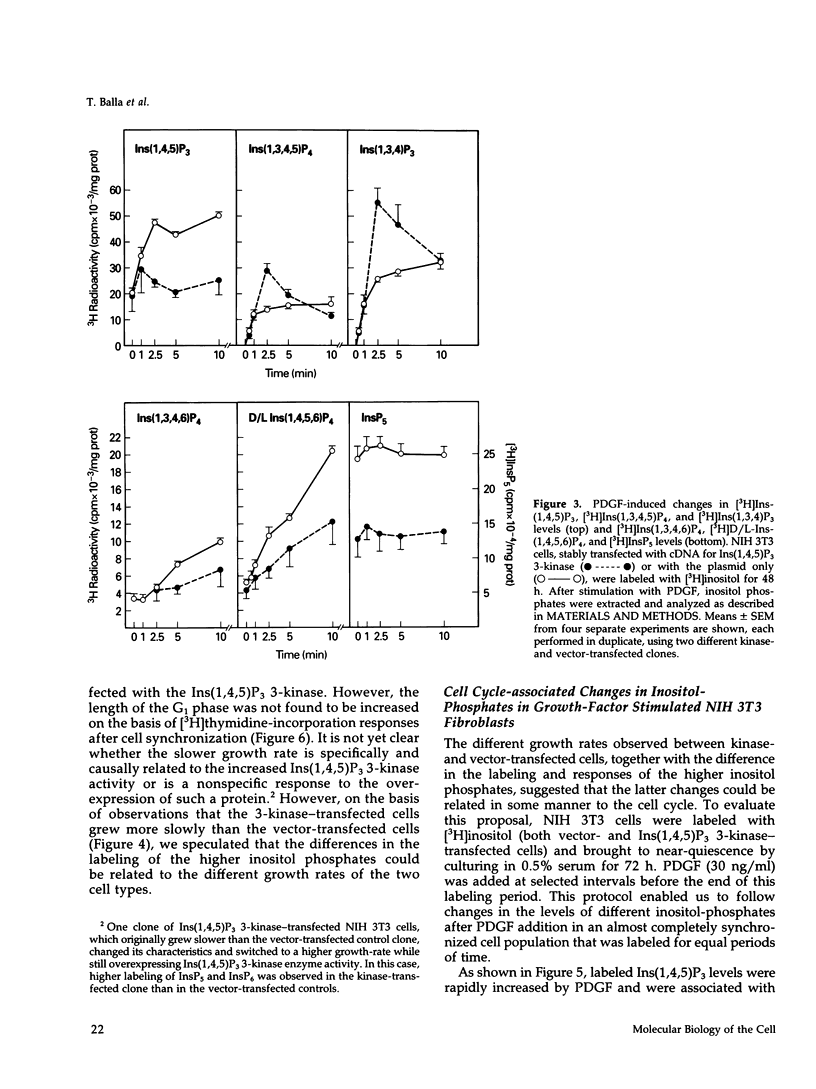
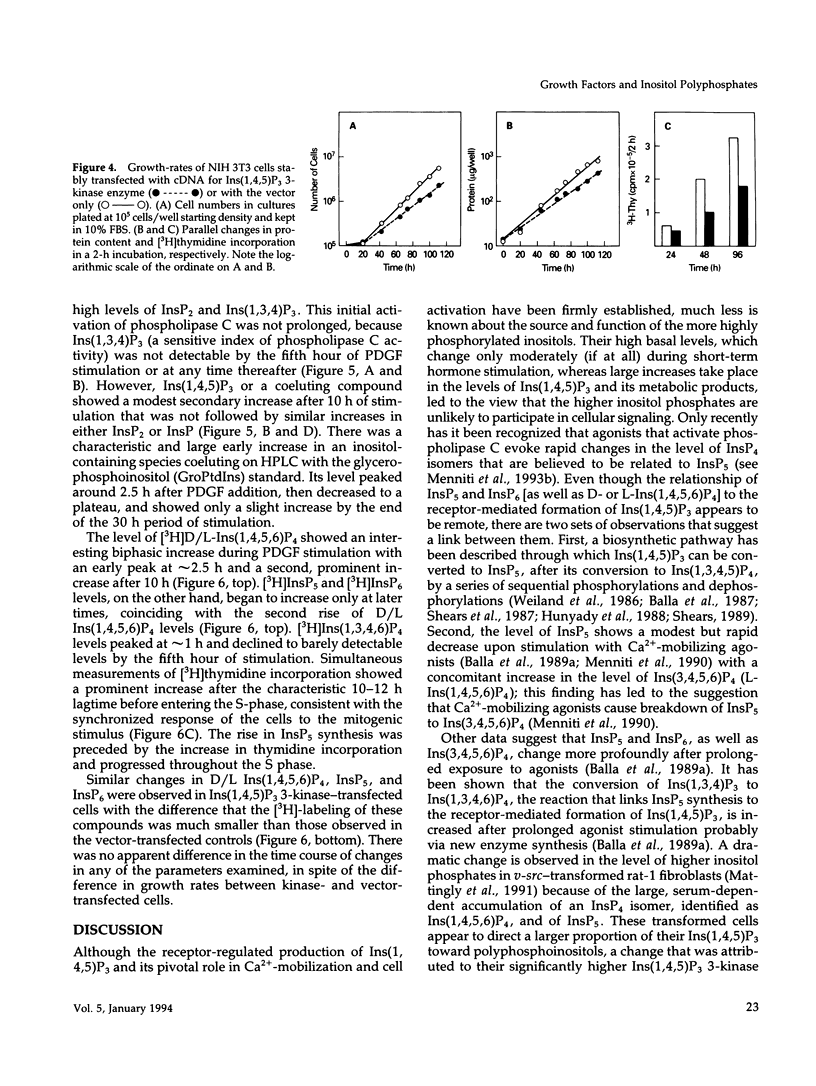
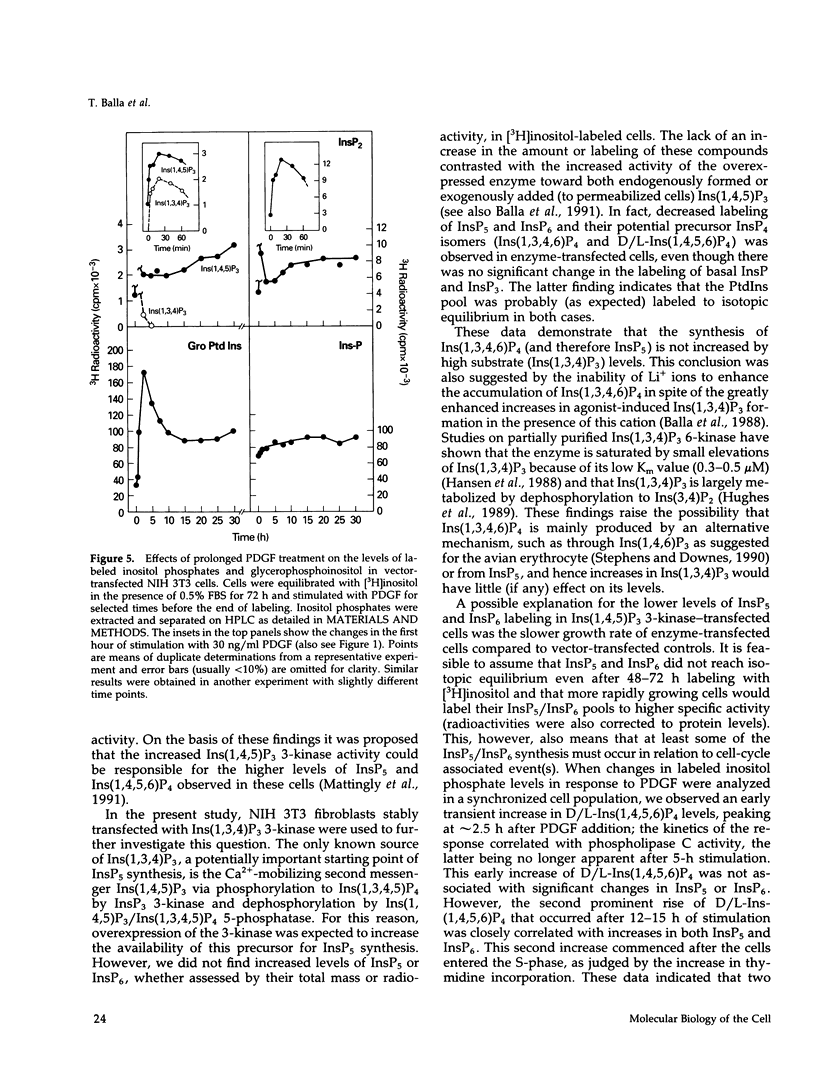
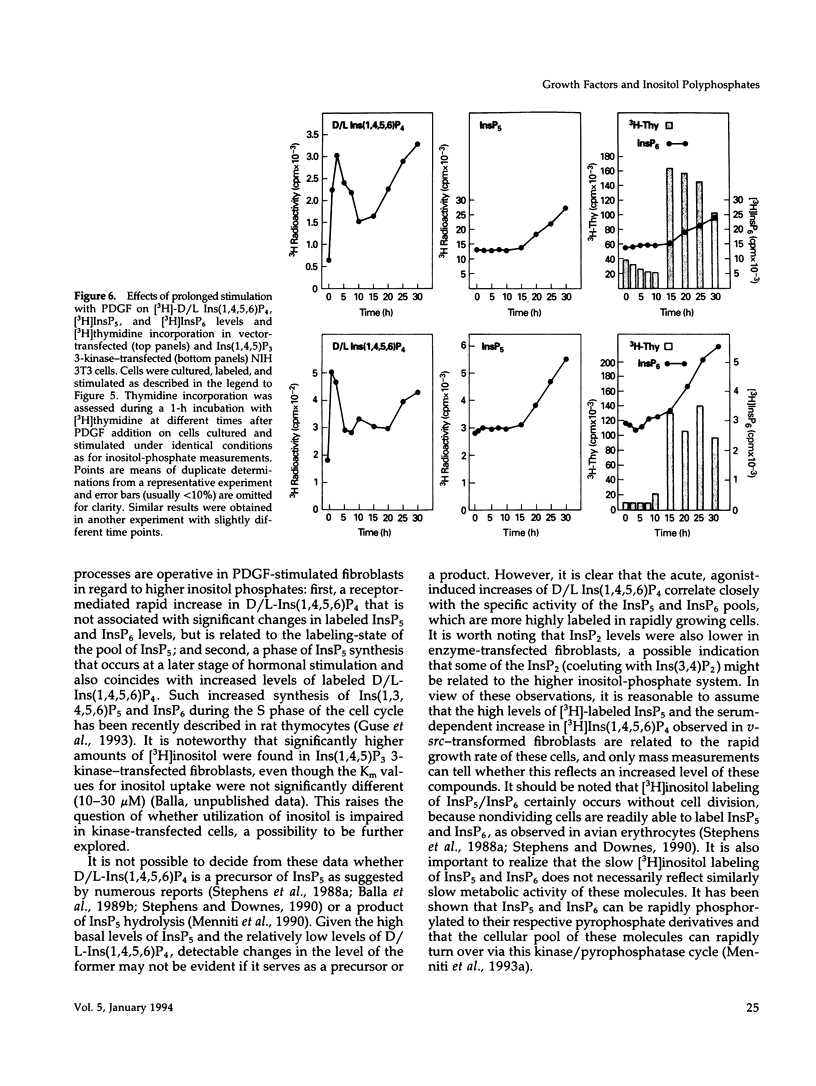
NIH 3T3 fibroblasts were stably transfected with rat brain inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) 3-kinase to explore the relationship between increased production of Ins(1,3,4,5)P4 and the formation of InsP5 and InsP6. Mass measurements of InsP5 and InsP6 revealed no significant difference between kinase- and vector-transfected fibroblasts. However, such 3-kinase-transfected cells, when labeled with [3H]inositol for 48-72 h, showed lower levels of [3H]InsP5 and [3H]InsP6, as well as [3H]Ins(1,3,4,6)P4 and D/L[3H]Ins(1,4,5,6)P4, than their vector-transfected counterparts. Because Ins(1,4,5)P3 3-kinase-transfected cells grew less rapidly than vector-transfected controls, we determined whether the synthesis of InsP5 and InsP6 was related to a specific phase of the cell cycle. When NIH 3T3 cells prelabeled with [3H]inositol were synchronized by serum deprivation followed by stimulation with platelet-derived growth factor (PDGF), the amounts of labeled InsP5 and InsP6 began to increase only after 12 h of stimulation, when cells entered the S-phase as indicated by increased [3H]thymidine incorporation. The enhanced synthesis of these inositol polyphosphates was preceded by an early increase in Ins(1,4,5)P3 and its metabolites that was no longer evident by the fifth hour of PDGF action. There was also a prominent and biphasic increase in the level of D/L-Ins(1,4,5,6)P4 with an early peak at approximately 3 h and a second rise that paralleled the increases in InsP5 and InsP6. These results indicate that the formation of highly phosphorylated inositols is not tightly coupled to the receptor-mediated formation of Ins(1,4,5)P3 and its metabolites but is mainly determined by other factors that operate at specific points of the cell cycle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Guillemette G., Catt K. J. Multiple pathways of inositol polyphosphate metabolism in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem. 1988 Mar 25;263(9):4083–4091. [PubMed] [Google Scholar]
- Balla T., Baukal A. J., Hunyady L., Catt K. J. Agonist-induced regulation of inositol tetrakisphosphate isomers and inositol pentakisphosphate in adrenal glomerulosa cells. J Biol Chem. 1989 Aug 15;264(23):13605–13611. [PubMed] [Google Scholar]
- Balla T., Guillemette G., Baukal A. J., Catt K. J. Metabolism of inositol 1,3,4-trisphosphate to a new tetrakisphosphate isomer in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem. 1987 Jul 25;262(21):9952–9955. [PubMed] [Google Scholar]
- Balla T., Hunyady L., Baukal A. J., Catt K. J. Structures and metabolism of inositol tetrakisphosphates and inositol pentakisphosphate in bovine adrenal glomerulosa cells. J Biol Chem. 1989 Jun 5;264(16):9386–9390. [PubMed] [Google Scholar]
- Balla T., Sim S. S., Iida T., Choi K. Y., Catt K. J., Rhee S. G. Agonist-induced calcium signaling is impaired in fibroblasts overproducing inositol 1,3,4,5-tetrakisphosphate. J Biol Chem. 1991 Dec 25;266(36):24719–24726. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Guse A. H., Greiner E., Emmrich F., Brand K. Mass changes of inositol 1,3,4,5,6-pentakisphosphate and inositol hexakisphosphate during cell cycle progression in rat thymocytes. J Biol Chem. 1993 Apr 5;268(10):7129–7133. [PubMed] [Google Scholar]
- Hansen C. A., vom Dahl S., Huddell B., Williamson J. R. Characterization of inositol 1,3,4-trisphosphate phosphorylation in rat liver. FEBS Lett. 1988 Aug 15;236(1):53–56. doi: 10.1016/0014-5793(88)80284-9. [DOI] [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Hughes P. J., Hughes A. R., Putney J. W., Jr, Shears S. B. The regulation of the phosphorylation of inositol 1,3,4-trisphosphate in cell-free preparations and its relevance to the formation of inositol 1,3,4,6-tetrakisphosphate in agonist-stimulated rat parotid acinar cells. J Biol Chem. 1989 Nov 25;264(33):19871–19878. [PubMed] [Google Scholar]
- Hunyady L., Baukal A. J., Guillemette G., Balla T., Catt K. J. Metabolism of inositol-1,3,4,6-tetrakisphosphate to inositol pentakisphosphate in adrenal glomerulosa cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1247–1252. doi: 10.1016/s0006-291x(88)81008-8. [DOI] [PubMed] [Google Scholar]
- Ji H., Sandberg K., Baukal A. J., Catt K. J. Metabolism of inositol pentakisphosphate to inositol hexakisphosphate in Xenopus laevis oocytes. J Biol Chem. 1989 Dec 5;264(34):20185–20188. [PubMed] [Google Scholar]
- Johnson R. M., Wasilenko W. J., Mattingly R. R., Weber M. J., Garrison J. C. Fibroblasts transformed with v-src show enhanced formation of an inositol tetrakisphosphate. Science. 1989 Oct 6;246(4926):121–124. doi: 10.1126/science.2506643. [DOI] [PubMed] [Google Scholar]
- Mattingly R. R., Stephens L. R., Irvine R. F., Garrison J. C. Effects of transformation with the v-src oncogene on inositol phosphate metabolism in rat-1 fibroblasts. D-myo-inositol 1,4,5,6-tetrakisphosphate is increased in v-src-transformed rat-1 fibroblasts and can be synthesized from D-myo-inositol 1,3,4-trisphosphate in cytosolic extracts. J Biol Chem. 1991 Aug 15;266(23):15144–15153. [PubMed] [Google Scholar]
- McConnell F. M., Stephens L. R., Shears S. B. Multiple isomers of inositol pentakisphosphate in Epstein-Barr-virus- transformed (T5-1) B-lymphocytes. Identification of inositol 1,3,4,5,6-pentakisphosphate, D-inositol 1,2,4,5,6-pentakisphosphate and L-inositol 1,2,4,5,6-pentakisphosphate. Biochem J. 1991 Dec 1;280(Pt 2):323–329. doi: 10.1042/bj2800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti F. S., Miller R. N., Putney J. W., Jr, Shears S. B. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J Biol Chem. 1993 Feb 25;268(6):3850–3856. [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Nogimori K., Obie J. F., Shears S. B., Putney J. W., Jr Origins of myo-inositol tetrakisphosphates in agonist-stimulated rat pancreatoma cells. Stimulation by bombesin of myo-inositol 1,3,4,5,6-pentakisphosphate breakdown to myo-inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 1990 Jul 5;265(19):11167–11176. [PubMed] [Google Scholar]
- Menniti F. S., Oliver K. G., Putney J. W., Jr, Shears S. B. Inositol phosphates and cell signaling: new views of InsP5 and InsP6. Trends Biochem Sci. 1993 Feb;18(2):53–56. doi: 10.1016/0968-0004(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Conroy L. A., French P. J., Bunce C. M., Lord J. M., Brown G., Baxter M. A., McConnell F., Creba J. A. Inositol lipids and phosphates in the differentiation of blood cells. Adv Second Messenger Phosphoprotein Res. 1990;24:147–151. [PubMed] [Google Scholar]
- Shears S. B., Parry J. B., Tang E. K., Irvine R. F., Michell R. H., Kirk C. J. Metabolism of D-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J. 1987 Aug 15;246(1):139–147. doi: 10.1042/bj2460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B. Regulation of the metabolism of 1,2-diacylglycerols and inositol phosphates that respond to receptor activation. Pharmacol Ther. 1991;49(1-2):79–104. doi: 10.1016/0163-7258(91)90023-f. [DOI] [PubMed] [Google Scholar]
- Shears S. B. The pathway of myo-inositol 1,3,4-trisphosphate phosphorylation in liver. Identification of myo-inositol 1,3,4-trisphosphate 6-kinase, myo-inositol 1,3,4-trisphosphate 5-kinase, and myo-inositol 1,3,4,6-tetrakisphosphate 5-kinase. J Biol Chem. 1989 Nov 25;264(33):19879–19886. [PubMed] [Google Scholar]
- Stephens L. R., Downes C. P. Product-precursor relationships amongst inositol polyphosphates. Incorporation of [32P]Pi into myo-inositol 1,3,4,6-tetrakisphosphate, myo-inositol 1,3,4,5-tetrakisphosphate, myo-inositol 3,4,5,6-tetrakisphosphate and myo-inositol 1,3,4,5,6-pentakisphosphate in intact avian erythrocytes. Biochem J. 1990 Jan 15;265(2):435–452. doi: 10.1042/bj2650435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Morris A. J., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate (3-hydroxy)kinase. Biochem J. 1988 Jan 1;249(1):283–292. doi: 10.1042/bj2490283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Stanley A. F., Moore T., Poyner D. R., Morris P. J., Hanley M. R., Kay R. R., Irvine R. F. myo-inositol pentakisphosphates. Structure, biological occurrence and phosphorylation to myo-inositol hexakisphosphate. Biochem J. 1991 Apr 15;275(Pt 2):485–499. doi: 10.1042/bj2750485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L. R., Irvine R. F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990 Aug 9;346(6284):580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M., Jackson T., Lightman S., Hanley M. R. Occurrence and extracellular actions of inositol pentakis- and hexakisphosphate in mammalian brain. Nature. 1987 Dec 17;330(6149):656–658. doi: 10.1038/330656a0. [DOI] [PubMed] [Google Scholar]
- Weiland N. G., Barraclough C. A., Catt K. J. Effects of long- and short-term gonadectomy on the hypothalamo-hypophysial (LH-releasing hormone-LH) system in oestrogen-treated male and female rats. J Endocrinol. 1986 Aug;110(2):367–373. doi: 10.1677/joe.0.1100367. [DOI] [PubMed] [Google Scholar]


