Abstract
OBJECTIVE:
The goal was to test the effects of high-efficiency, particulate-arresting (HEPA) air cleaners on unscheduled asthma visits and symptoms among children with asthma exposed to secondhand smoke.
METHODS:
We enrolled 225 eligible children who were 6 to 12 years of age, had physician-diagnosed asthma, and were exposed to ≥5 cigarettes per day. We conducted a double-blind, randomized trial. Children were assigned randomly to receive 2 active or inactive HEPA air cleaners.
RESULTS:
Of 225 enrolled children, 110 (49%) were assigned to the intervention group and 115 (51%) to the control group; 215 (95%) completed the trial. During the trial, there were 42 fewer unscheduled asthma visits among children in the intervention group (18.5% [95% confidence interval: 1.25%–82.75%]; P = .043), compared with those in the control group, after adjustment for baseline differences. There was a significant difference in the reductions of levels of particles of >0.3 μm according to group assignment; there was a 25% reduction in particle levels in the intervention group, compared with a 5% reduction in the control group (P = .026). There were no significant differences in parent-reported asthma symptoms, exhaled nitric-oxide levels, air nicotine levels, or cotinine levels according to group assignment.
CONCLUSIONS:
These results hold promise for using HEPA air cleaners as part of a multifaceted strategy to reduce asthma morbidity, but further research is necessary before they can be recommended routinely for the medical management of asthma.
Keywords: asthma, children, secondhand smoke, air cleaner, randomized controlled trial, unscheduled asthma visits, exacerbations
WHAT'S KNOWN ON THIS SUBJECT:
Exposure to secondhand smoke (SHS) is associated with asthma exacerbations in children. Anticipatory guidance has failed to reduce SHS in controlled trials. It is not known whether high-efficiency, particle-arresting (HEPA) air cleaners can reduce SHS or improve asthma symptoms in children.
WHAT THIS STUDY ADDS:
HEPA air cleaners led to reductions in unscheduled asthma visits and fine airborne particle levels but not asthma symptoms or cotinine levels. HEPA air cleaners may be useful as part of a multifaceted strategy to reduce asthma morbidity among children.
Exposure to secondhand smoke (SHS) is associated with wheezing and asthma exacerbations in children.1–7 Children who are exposed to SHS are ∼1.5 times more likely to have physician-diagnosed asthma or wheezing than are unexposed children, which accounts for 130 000 excess cases of asthma among US children.3,7 The mechanism for asthma exacerbations resulting from SHS is not clear, but cigarette smoke is the major source of airborne particles in households with smokers.8 Indeed, concentrations of airborne particles of <2.5 μm are 2 to 3 times higher in households with smokers than in households without smokers.8–10 More than 20% of US children are exposed to household SHS.11
Numerous studies have tested the efficacy of anticipatory guidance to reduce SHS exposure for children, but the majority failed to show significant reductions in children's SHS exposure by using measurements of cotinine, a metabolite of nicotine, or improvements in asthma symptoms.12–23 This problem might be overcome if there was a technology to reduce SHS exposure passively, such as high-efficiency, particulate-arresting (HEPA) air cleaners.
There are limited data on the efficacy of HEPA air cleaners in reducing asthma symptoms. More than 10 trials have tested the effects of HEPA air cleaners, but the largest involved 45 subjects; only 2 studied children exclusively.24,25 None of those trials tested HEPA air cleaners among SHS-exposed children with asthma, but HEPA air cleaners can reduce levels of airborne particles and nicotine.26,27 Reisman et al26 reported a 73% reduction in the level of airborne particles of ≥0.3 μm with HEPA air cleaners. Bascom et al27 reported 50% reductions in levels of airborne particles and nicotine. Other trials incorporated HEPA air cleaners to reduce environmental triggers among children with asthma, but it was not possible to quantify the specific contribution of the air cleaners because the studies involved multiple environmental interventions.28,29 We speculated that children with asthma who were exposed to SHS would particularly benefit by using air cleaners. The purpose of this double-blind, randomized trial was to test the efficacy of HEPA air cleaners in reducing unscheduled asthma visits, asthma symptoms, and SHS exposure among children with asthma.
METHODS
Study Design
The Cincinnati Asthma Prevention Study was a randomized, double-blind trial to test the effects of HEPA air cleaners, in the homes of 225 SHS-exposed children with physician-diagnosed asthma, on unscheduled asthma visits and symptoms. Families assigned to the intervention group received 2 active HEPA air cleaners (Austin Healthmate [Buffalo, NY]), that is, HEPA air cleaners surrounded by a carbon-potassium permanganate-zeolite insert, whereas families in the control group received 2 inactive (placebo) air cleaners. For both groups, 1 air cleaner was installed in the main activity room and the other was installed in the child's bedroom at the baseline home visit. The air cleaners were equipped with monitors to measure the number of hours they operated. There were no attempts to reduce tobacco use or other asthma triggers.
HEPA air cleaners are certified to remove >99% of airborne particles of >0.3 μm in a 1500-ft2 room. The carbon-potassium permanganate-zeolite filter insert was designed to absorb odors and gases. The inactive air cleaners, which were indistinguishable from the active HEPA air cleaners, contained 3 layers of prefilter cloth (a blend of cotton and polyester fibers), which also were present in the active air cleaners. At the end of the trial, we offered to install HEPA filters in the air cleaners for families assigned to receive inactive air cleaners during the trial.
The Cincinnati Children's Hospital Medical Center institutional review board approved this study. Parents or legal guardians provided written consent before enrollment.
Study Participants
We screened children and their families for eligibility from May 31, 2001, through March 27, 2003, by using a sampling frame of children who had received treatment for asthma at a clinic, emergency department, or hospital affiliated with Cincinnati Children's Hospital Medical Center or Group Health Associates. Initially, we mailed letters to families. Potential participants were able to decline any further involvement or telephone contact by returning a prepaid postcard or by telephoning our research coordinators. If the family did not decline participation within 10 days, then a research coordinator contacted the family to describe the study, to determine eligibility, and, if the family was eligible, to invite the members to participate in the trial.
Children were eligible if they were 6 to 12 years of age at enrollment, had physician-diagnosed asthma in the previous 12 months, according to International Classification of Diseases, Ninth Revision, billing codes (from hospital records, emergency department visit records, or primary care physician records), experienced ≥1 exacerbation requiring an unscheduled visit in the past year, were exposed to the smoke of ≥5 cigarettes per day in and around the house, and lived within a 9-county area surrounding Cincinnati. Children were excluded if they were already using an air cleaner, if their home lacked electricity, if they had coexisting medical problems (eg, mental retardation, congenital heart disease, or cystic fibrosis), or if their family planned to move in the next year.
Random Assignment
Children were assigned randomly to receive active HEPA air cleaners or inactive air cleaners. Allocation concealment was performed by using opaque envelopes, which were opened by a research assistant immediately before the baseline home visit was conducted. With the exception of the serial numbers, the active and inactive HEPA air cleaners were indistinguishable. All of the investigators, research staff members, and participants were masked to group assignment until the end of the trial, except for the biostatistician (Dr Hornung), who was responsible for random permutation of the air cleaners by using serial numbers.
Outcome Measures
We conducted extensive surveys at baseline to characterize the children, their families, and the home environment. Research assistants surveyed the children's parent or guardian at baseline and at 3-month intervals about unscheduled asthma visits, asthma symptoms, therapy, and tobacco exposure. Our primary outcome measure was asthma exacerbation, defined as any unscheduled visit to a health care provider (clinic visit, emergency visit, or hospitalization). We selected unscheduled asthma visits as our primary outcome measure a priori because it was the most-objective measure of asthma exacerbation. We estimated that, to detect a 20% reduction in unscheduled visits attributable to asthma exacerbations with ≥80% power, we would require a sample size of 110 children per group, or a total of 220 children (α = .05, 2-tailed test). This calculation assumed that we would have a rate of attrition of <10%. Secondary outcome measures included asthma symptoms assessed with the Child Health Asthma Survey (an instrument shown to be reliable, internally consistent, and able to distinguish levels of asthma severity30,31), tobacco smoke exposure, indoor airborne particle levels, and exhaled nitric-oxide levels.
Air nicotine levels, which provide an objective measure of ambient tobacco smoke exposure, were measured in each subject's home by using nicotine dosimeters.32–34 For standardization, the dosimeters were housed in a metal compartment on the air cleaner that was located in the main activity room. Dosimeters were placed at the baseline and 6-month visits and were retrieved at the 6- and 12-month visits, respectively. The dosimeters, which have a limit of detection of 0.01 μg per filter, were analyzed by using a standardized protocol.32–34
To test the efficacy of the HEPA air cleaners in reducing children's SHS exposure, we measured cotinine levels in children's serum and hair at baseline and at 6 and 12 months.35 Serum samples were analyzed at the National Center for Environmental Health, Centers for Disease Control and Prevention (Atlanta, GA), by using high-performance liquid chromatography linked to atmospheric pressure chemical ionization/tandem mass spectrometry.36 The detection limit for cotinine in serum was 0.05 ng/mL. We collected 20 strands of hair from the occipital region of the scalp. The hair samples were analyzed for cotinine at the Hospital for Sick Children (Toronto, Ontario, Canada), by using a radioimmunoassay.37 The detection limit for cotinine in hair was 0.005 ng/mg.37
To test the effect of the HEPA air cleaners on airborne particles, we used a GT-321 particle counter (Met One Instruments, Grant Pass, OR) to measure concentrations of indoor particles (particles per cubic foot) at the baseline and 6- and 12-month visits. We took measurements of the numbers of airborne particles of >0.3 μm and >5 μm; each reading was the average of ten 6-second measurements taken over 1 minute in 3 different locations in each housing unit. The data used in the analysis are the mean of 3 readings from the main activity room, the child's bedroom, and the kitchen, taken at the beginning of each home visit.
We assessed nitric-oxide levels in exhaled air at the baseline and 6- and 12-month visits. Exhaled air was collected in Mylar balloons from each participant by using a validated offline technique to assess the effect of HEPA air cleaners on airway inflammation.38,39 A model 280i nitric-oxide analyzer (Sievers Instruments, Boulder, CO) was used for nitric-oxide analysis. As described previously, exhaled nitric-oxide concentrations were imputed when the exhaled nitric-oxide analysis did not occur within 24 hours after collection.40
Serum samples were obtained at the baseline visit by a trained pediatric phlebotomist, for determination of allergen-specific immunoglobulin E by using the ImmunoCap test (Pharmacia Diagnostics, Portage, MI). Children with class I or higher immunoglobulin E levels (≥0.35 kU/L) for dust mite, dog, cat, or cockroach allergen were considered to have atopy.
Statistical Analyses
We calculated descriptive statistics according to group assignment. For continuous variables, we calculated means, SDs, and ranges. For categorical variables, we calculated frequencies and proportions. All analyses were performed according to the intention to treat. For continuous variables, we used multivariate regression analyses for either the untransformed or logarithmically transformed outcome, as appropriate. For repeated-measures analyses over the 12 months of the study, we used mixed-effects linear models with an autoregressive covariance structure. The interaction of group assignment and time was tested as the primary hypothesis of an intervention effect. When the outcome was a rate or count (eg, unscheduled asthma visits), the generalized estimating equation version of Poisson regression was used for repeated measures, in a manner similar to the mixed-effects linear model. In secondary a priori analyses, we tested the efficacy of the HEPA air cleaners for families who used either HEPA air cleaner for >6130 hours (ie, ≥70% of the entire trial).
RESULTS
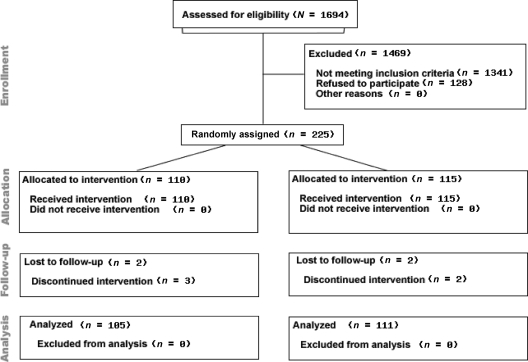
Of the 2240 children in the sampling frame who were 6 to 12 years of age, had physician-diagnosed asthma according to International Classification of Diseases, Ninth Revision, billing codes, and resided in a 9-county area surrounding Cincinnati, we screened 1694 (76%) for eligibility between May 31, 2001, and March 27, 2003. Of these children, 353 (21%) were eligible to participate (Fig 1). Of the 353 eligible children and their families, 225 (64%) agreed to participate in the trial. There were no significant differences in children's age, gender, asthma severity, or race, parents' education, marital status, household income, or health insurance, or number of cigarettes smoked in or around the house per day for families that chose to participate versus those that chose not to participate.
FIGURE 1.
Enrollment, random assignment, and retention of Cincinnati Asthma Prevention study participants.
Of the 225 families that agreed to participate, 110 (49%) were assigned randomly to the intervention group and 115 (51%) to the control group. At baseline, the mean age of the children was 8.6 years (range: 5.3–11.7 years); 62% of the children were male. Children were exposed to a geometric mean of 13 cigarettes per day. Numbers of unscheduled asthma visits and airborne particle levels were significantly greater in the control group. Some other measures of asthma severity tended to be greater in the control group, but these differences were not statistically significant (Tables 1 and 2). Of the 225 children enrolled in the trial, 215 (95%) completed the study (Fig 1).
TABLE 1.
Baseline Characteristics of Study Population According to Group Assignment
| Characteristics | Intervention Group (N = 110) | Control Group (N = 115) | P |
|---|---|---|---|
| Age, mean ± SD, y | 8.8 ± 1.8 | 8.4 ± 1.8 | .160 |
| Male, n (%) | 74 (67) | 65 (57) | .097 |
| Race, n (%) | .565 | ||
| Black | 65 (59) | 60 (52) | — |
| White | 43 (39) | 52 (45) | — |
| Other | 2 (2) | 3 (3) | — |
| Annual household income, mean ± SD, $ | 30 524 ± 24 416 | 29 595 ± 21 649 | .767 |
| No. of unscheduled asthma visits in previous 3 mo, mean ± SD | 0.6 ± 1.0 | 1.0 ± 1.4 | .013 |
| Measures of asthma severity, n (%) | |||
| Allergy severity (moderate to very severe) | 47 (43) | 55 (48) | .443 |
| Shortness of breath (moderate to very severe) | 46 (42) | 61 (53) | .104 |
| Tightness in chest (moderate to very severe) | 29 (27) | 37 (33) | .317 |
| Wheeze (moderate to very severe) | 44 (40) | 46 (40) | .955 |
| Difficulty sleeping (moderate to very severe) | 42 (38) | 53 (46) | .208 |
| Steroid therapy in previous 3 mo | 30 (27) | 37 (34) | .305 |
| Long-acting steroid therapy | 58 (53) | 60 (52) | .877 |
| Duration of asthma, mean ± SD, d | 9.8 ± 18.7 | 10.2 ± 11.7 | .841 |
| Serum immunoglobulin E levels of ≥0.35 kU/L for common allergens, n (%) | 74 (67) | 70 (61) | .310 |
| Exhaled nitric-oxide level, mean ± SD, ppb | 14.7 ± 10.0 | 16.3 ± 12.3 | .302 |
TABLE 2.
Baseline Exposure to Environmental Tobacco Smoke and Indoor Airborne Particles According to Group Assignment
| Characteristics | Intervention Group (N = 110) | Control Group (N = 115) | P |
|---|---|---|---|
| Tobacco exposure | |||
| No. of cigarettes smoked around house per d, mean ± SD | 12.9 ± 1.8 | 13.3 ± 2.2 | .716 |
| Serum cotinine level, mean ± SD, ng/mL | 1.18 ± 3.9 | 1.23 ± 3.3 | .622 |
| Hair cotinine level, mean ± SD, ng/mg | 0.14 ± 3.8 | 0.14 ± 3.5 | .970 |
| No. of airborne particles, mean ± SD, 106/ft3 | |||
| Particles of >0.3 μm | 4.0 ± 2.56 | 4.7 ± 2.58 | .003 |
| Particles of >5 μm | 0.003 ± 2.31 | 0.004 ± 2.14 | .154 |
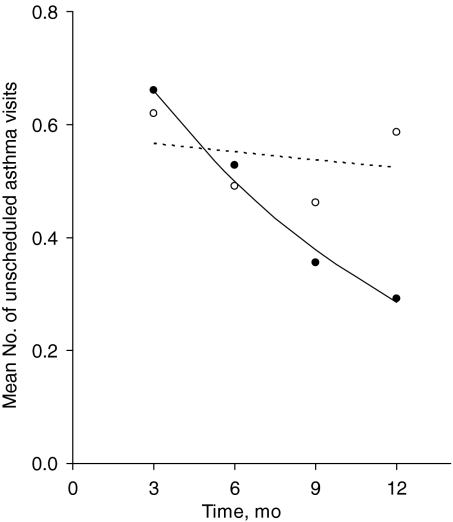
In unadjusted analyses, there were fewer unscheduled asthma visits at follow-up assessment in the intervention group (189 vs 274 visits; P = .046). This difference persisted after adjustment for baseline differences in the multivariate analyses. With adjustment for baseline differences, there was a significant difference in the rate of decrease in the number of unscheduled asthma visits among children in the intervention group, compared with the control group (Fig 2). The adjusted mean number of unscheduled visits for the intervention group decreased by 8.9% per month, compared with a decrease of 0.9% per month in the control group. With adjustment for baseline differences, there were 185 unscheduled asthma visits among children in the intervention group, compared with 227 unscheduled asthma visits among children in the control group, a reduction of 42 visits (95% confidence interval: 1.25–82.75 visits; P = .043). This is equivalent to a reduction of 18.5% (95% confidence interval: 1%–36%) in the number of unscheduled asthma visits during the study (Table 3).
FIGURE 2.
Mean numbers of unscheduled asthma visits in the previous 3 months, reported at quarterly intervals during the trial, according to group assignment, with adjustment for differences in the numbers of unscheduled asthma visits at baseline. The solid line and closed circles indicate the intervention group; dashed line and open circles, control group.
TABLE 3.
Numbers of Unscheduled Asthma Visits, Asthma Symptoms, Reported Exposure to Tobacco Smoke, and Exhaled Nitric-Oxide Levels According to Group Assignment
| Characteristics | 3 mo | 6 mo | 9 mo | 12 mo | P |
|---|---|---|---|---|---|
| No. of unscheduled asthma visits in previous 3 mo, mean | .043a | ||||
| Intervention group (n = 110) | 0.7 | 0.5 | 0.4 | 0.3 | |
| Control group (n = 115) | 0.6 | 0.5 | 0.9 | 0.6 | |
| Moderate/severe allergy in previous 3 mo, % | .618 | ||||
| Intervention group (n = 110) | 35 | 32 | 40 | 33 | |
| Control group (n = 115) | 38 | 40 | 34 | 34 | |
| In previous 2 wk, how much of time has <child's name> had | |||||
| Shortness of breath (some, most, or all of time), % | .415 | ||||
| Intervention group (n = 110) | 34 | 28 | 29 | 19 | |
| Control group (n = 115) | 36 | 27 | 24 | 28 | |
| Tightness in chest (some, most, or all of time), % | .612 | ||||
| Intervention group (n = 110) | 22 | 16 | 18 | 14 | |
| Control group (n = 115) | 22 | 10 | 16 | 17 | |
| Wheeze (some, most, or all of time), % | .168 | ||||
| Intervention group (n = 110) | 32 | 15 | 22 | 13 | |
| Control group (n = 115) | 25 | 20 | 15 | 19 | |
| Difficulty sleeping (some, most, or all of time), % | .299 | ||||
| Intervention group (n = 110) | 26 | 21 | 27 | 21 | |
| Control group (n = 115) | 27 | 18 | 16 | 18 | |
| Prescription for steroid therapy in previous 3 mo, % | .811 | ||||
| Intervention group (n = 110) | 19 | 13 | 16 | 19 | |
| Control group (n = 115) | 27 | 25 | 18 | 27 | |
| Prescription for long-acting steroid therapy in previous 3 mo, % | .135 | ||||
| Intervention group (n = 110) | 36 | 43 | 42 | 39 | |
| Control group (n = 115) | 46 | 39 | 30 | 37 | |
| No. of episodes of asthma, mean | .294 | ||||
| Intervention group (n = 110) | 8.9 | 4.3 | 6.5 | 4.5 | |
| Control group (n = 115) | 9.3 | 6.0 | 6.3 | 6.8 | |
| Exhaled nitric-oxide level, mean, ppb | .313 | ||||
| Intervention group (n = 110) | — | 16.4 | — | 16.1 | |
| Control group (n = 115) | — | 15.0 | — | 16.8 | |
| No. of cigarettes smoked around house per d, mean | .977 | ||||
| Intervention group (n = 110) | 7.5 | 11.2 | 7.5 | 9.5 | |
| Control group (n = 115) | 7.5 | 10.6 | 7.1 | 9.5 |
Test of group difference in trends over time (group-time interaction), adjusted for differences in the number of baseline visits.
In a secondary analysis, developed a priori, we examined the effect of HEPA air cleaners among 141 (68%) of 207 families with functioning monitors who used 1 or both air cleaners >70% of the time. With adjustment for baseline differences in unscheduled asthma visits, the rate of reduction in the number of unscheduled asthma visits over time was significantly greater in the intervention group, compared with the control group (P = .006). The number of unscheduled asthma visits for the intervention group decreased by 12.7% per month, compared with an increase of 0.7% per month in the control group.
There was a significant difference in the reductions in the numbers of airborne particles of >0.3 μm according to group assignment (P = .026) (Table 4). The absolute mean reduction in levels of airborne particles of >0.3 μm during the trial was 1.1 × 106 particles per ft3 for the intervention group, which was equivalent to a 25% reduction in airborne particle levels. In contrast, there was a reduction of only 0.3 × 106 particles per ft3 (5%) for the control group. There was no significant difference in levels of airborne particles of >5 μm according to group assignment (Table 4).
TABLE 4.
Exposures to Tobacco and Indoor Particles, According to Group Assignment, During Study
| Characteristic | Baseline | 6 mo | 12 mo | P |
|---|---|---|---|---|
| Serum cotinine level, mean, ng/mL | .738 | |||
| Intervention group (n = 110) | 1.1 | 1.1 | 1.1 | |
| Control group (n = 115) | 1.2 | 1.1 | 1.0 | |
| Hair cotinine level, mean, ng/mg | .984 | |||
| Intervention group (n = 110) | 0.1 | 0.12 | 0.15 | |
| Control group (n = 115) | 0.1 | 0.11 | 0.15 | |
| Air nicotine level, μg | .191 | |||
| Intervention group (n = 110) | — | 3.1 | 2.5 | |
| Control group (n = 115) | — | 2.5 | 2.7 | |
| No. of particles of >0.3 μm, mean ± SD, 106/ft3 | .026a | |||
| Intervention group (n = 110) | 4.0 | 2.5 | 3.0 | |
| Control group (n = 115) | 4.7 | 4.6 | 4.4 | |
| No. of particles of >5 μm, mean ± SD, 106/ft3 | .219a | |||
| Intervention group (n = 110) | 0.0033 | 0.0029 | 0.0027 | |
| Control group (n = 115) | 0.0037 | 0.0028 | 0.0024 |
Test of differences in group means, including baseline particle levels.
There were no significant differences between groups with respect to severity of asthma symptoms, medication use, exhaled nitric-oxide levels, air nicotine levels, serum cotinine levels, or hair cotinine levels during the trial (Table 3). There were no significant group-season (P = .328) or group-allergy severity (P = .583) interactions for unscheduled asthma visits.
To validate parents' reports of unscheduled visits, we examined the rate of agreement of parent-reported unscheduled asthma visits by using medical billing records from the Cincinnati Children's Hospital Medical Center. We found that 87% of parent-reported unscheduled asthma visits (714 of 824 visits) were in agreement with billing records for each 3-month interval.
DISCUSSION
We found, in a randomized, double-blind trial, that HEPA air cleaners led to an 18.5% reduction in unscheduled asthma visits. The differences in unscheduled asthma visits were apparent only at 9 and 12 months. We also found a significant difference in the reduction of levels of airborne particles of >0.3 μm in the intervention group, compared with the control group. In contrast, there were not significant reductions in levels of the gaseous phase of SHS exposure, asthma symptoms, or exhaled nitric oxide. These data suggested that HEPA air cleaners reduced unscheduled asthma visits for children by reducing exposure to airborne particles of >0.3 μm but without a corresponding reduction in the gaseous phase of tobacco smoke.
Although these results are somewhat paradoxical, our results are consistent with other controlled trials of environmental interventions. In a multifaceted trial to reduce indoor pollutants that included portable HEPA air cleaners, Morgan et al28 reported a 13.6% reduction in the number of unscheduled visits attributable to asthma and a significant reduction in asthma symptoms. In a trial of multiple environmental interventions that included HEPA air cleaners, Eggleston et al29 reported a significant improvement in asthma symptoms but no difference in unscheduled asthma visits according to group assignment. In a trial to reduce exposures to indoor asthma triggers among inner-city children that did not include portable air cleaners, Krieger et al41 reported a 15% reduction in unscheduled asthma visits but no significant improvement in asthma symptoms. It is not clear whether the differences across these studies are attributable to the type or intensity of the environmental intervention, sample size, or eligibility criteria. Collectively, however, these trials provide evidence that environmental interventions can reduce asthma morbidity in children.42
Results of studies that have examined the contribution of SHS exposure to asthma exacerbations in children >3 years of age have been inconsistent. Several studies found that SHS was associated with asthma exacerbations or bronchial hyperactivity in older children, whereas others did not.3,6,7,43,44 The vast majority of studies relied on parents' reports of smoking behavior to quantify the risk of asthma, wheezing, or diminished pulmonary functions associated with SHS exposure; fewer studies used objective biomarkers such as cotinine levels.7,43,44 Although we found a significant reduction in the number of unscheduled asthma visits among children, there was not a corresponding reduction in the gaseous phase of tobacco smoke, as assessed with objective measures of air nicotine levels and biomarkers of internal doses. In contrast, there was a reduction in the levels of fine indoor particles, the predominant size generated by tobacco smoke. Therefore, this study provides some evidence that airborne particles from SHS or other pollutants represent a risk factor for asthma exacerbations in children.
More than 10 trials have tested the efficacy of anticipatory guidance regarding children's exposure to tobacco smoke, by using cotinine as an objective biomarker of exposure.12–23 Only from 1 of those studies was a sustained reduction in exposure to environmental tobacco smoke, measured as cotinine levels, reported, and from another study a significant reduction in the number of unscheduled asthma visits was reported.12,13 Collectively, these trials raise serious questions about whether it is prudent to continue to rely on anticipatory guidance to reduce children's exposure to tobacco smoke. Our failure to show a reduction in the gaseous phase of children's exposure to SHS by using a passive environmental intervention provides additional support for regulations to ban smoking in public places and residential settings to reduce children's exposure to tobacco smoke.
There are some limitations of this study. First, our primary outcome measure, that is, numbers of unscheduled asthma visits, and airborne particle levels were not equally distributed in our 2 treatment arms at baseline; although the decreases in the number of unscheduled asthma visits and levels of airborne particles of >0.3 μm remained statistically significant after adjustment for baseline differences, this suggests that the 2 groups were not equivalent. Second, we examined many relevant end points and our primary result, that the air cleaners led to a significant reduction in the number of unscheduled asthma visits, might have been spurious. This is unlikely, however, because we observed a larger effect among the families who used the air cleaners consistently. Third, use of a different HEPA air cleaner might have led to different results. Fourth, we used only 2 HEPA air cleaners in each housing unit. A portable HEPA air cleaner is certified to clean 1 average-sized room and not an entire house. Fifth, the air nicotine dosimeters, which were placed on the HEPA air cleaners in the main activity rooms, might not have provided representative measurements of household exposure. Finally, by focusing on older children, who are less vulnerable to SHS exposure,7,43,44 we might have decreased our chances of showing an effect of HEPA air cleaners.
CONCLUSIONS
Our ultimate goal should be to eliminate tobacco use and SHS exposure for children. Despite efforts to reduce exposure, >20% of US children are exposed to SHS in their homes.11 Moreover, air quality in housing often is inadequate.10 Therefore, it is critical to identify ways to reduce exposure to SHS and other pollutants, especially for children with asthma. We found that HEPA air cleaners led to significant reductions in numbers of unscheduled asthma visits and levels of fine airborne pollutants but not in parent-reported asthma symptoms, cotinine levels, or exhaled nitric-oxide levels. These results hold promise for using HEPA air cleaners as part of a multifaceted strategy to reduce asthma morbidity, but they also emphasize the importance of finding ways to reduce the sources of exposure further.
ACKNOWLEDGMENTS
This work was funded by National Institutes of Health grant RO1-65731. Austin Air provided the HEPA air cleaners at a reduced cost, but the company was not involved in study design, data interpretation, or manuscript preparation.
We acknowledge the contributions of Katharine Hammond, the Cincinnati Asthma Prevention Study research staff members, and the pediatricians at Cincinnati Children's Hospital Medical Center and Group Health Associates.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00006565).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
- HEPA
- high-efficiency, particle-arresting
- SHS
- secondhand smoke
MISTAKES IN THE HOSPITAL: Patients are taken to the hospital to get better. Unfortunately, hospitalization carries with it a significant risk for adverse events. As reported on NewYorkTimes.com (November 16, 2010:1–3), one of every seven hospitalized Medicare beneficiaries is harmed as a result of the medical care received. The report on patient safety from the Inspector General for the Department of Health and Human Services is based on an exhaustive review of a representative sample of 780 patient files. The report estimates that in October 2008 alone, approximately 134,000 hospitalized Medicare beneficiaries experienced at least one adverse event. Events ranged from mild and temporary to severe and permanent including death. Almost 44% of these events were likely preventable. Medication errors leading to such problems as excessive bleeding were the most frequent adverse event. Poor patient management, e.g. leading to fluid overload was also common. Hospital acquired infections continue to be a major issue. Extremely infrequent are problems that should never happen under any condition such as performing surgery on the wrong patient. While physicians and hospitals have designed systems to help prevent errors, errors continue to happen at a distressing rate. The report recommends not only to broaden the definitions of error and harm in the hospital but to give hospitals financial incentives to reduce errors. While pediatricians infrequently take care of Medicare patients, the report emphasizes that we all need to be mindful of a cardinal rule in medicine: first, do no harm.
Noted by WVR, MD
REFERENCES
- 1. Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328(23):1665–1669 [DOI] [PubMed] [Google Scholar]
- 2. Cunningham J, O'Connor GT, Dockery DW, Speizer FE. Environmental tobacco smoke, wheezing, and asthma in children in 24 communities. Am J Respir Crit Care Med. 1996;153(1):218–224 [DOI] [PubMed] [Google Scholar]
- 3. Strachan DP, Cook DG. Health effects of passive smoking: parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53(3):204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mannino DM, Moorman JE, Kingsley B, Rose D, Repace J. Health effects related to environmental tobacco smoke exposure in children in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Pediatr Adolesc Med. 2001;155(1):36–41 [DOI] [PubMed] [Google Scholar]
- 5. DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113(4 suppl):1007–1015 [PubMed] [Google Scholar]
- 6. Martinez FD, Antognoni G, Macri F, et al. Parental smoking enhances bronchial responsiveness in nine-year-old children. Am Rev Respir Dis. 1988;138(3):518–523 [DOI] [PubMed] [Google Scholar]
- 7. Lanphear BP, Aligne C, Auinger P, Byrd R, Weitzman M. Residential exposures associated with asthma in U.S. children. Pediatrics. 2001;107(3):505–511 [DOI] [PubMed] [Google Scholar]
- 8. Wallace L, Mitchell H, O'Connor GT, et al. Indoor particle concentrations in inner-city homes of children with asthma: the effect of smoking, cooking, and outdoor pollution. Environ Health Perspect. 2003;111(9):1265–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dockery DW, Spengler JD. Indoor-outdoor relationships of respirable sulfates and particles. Atmos Environ. 1981;15(3):335–343 [Google Scholar]
- 10. Spengler JD, Dockery DW, Turner WA, Wolfson JM, Ferris BG., Jr Long-term measurements of respirable sulfates and particles inside and outside homes. Atmos Environ. 1981;15(1):23–30 [Google Scholar]
- 11. Marano C, Schober SE, Brody DJ, Zhang C. Secondhand tobacco smoke exposure among children and adolescents: United States, 2003–2006. Pediatrics. 2009;124(5):1299–1305 [DOI] [PubMed] [Google Scholar]
- 12. Hovell MF, Zakarian JM, Matt GE, Hofstetter R, Bernert JT, Pirkle J. Effect of counseling mothers on their children's exposure to environmental tobacco smoke: randomised controlled trial. BMJ. 2000;321(7257):337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilson SR, Yamada EG, Sudhaker R, et al. A controlled trial of an environmental tobacco smoke reduction intervention in low-income children with asthma. Chest. 2001;120(5):1709–1722 [DOI] [PubMed] [Google Scholar]
- 14. Irvine L, Crombie IK, Clark RA, et al. Advising parents of asthmatic children on passive smoking: randomised controlled trial. BMJ. 1999;318(7196):1456–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chilmonczyk B, Palomaki G, Knight G, Williams J, Haddow J. An unsuccessful cotinine-assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. Am J Dis Child. 1992;146(3):357–360 [DOI] [PubMed] [Google Scholar]
- 16. Meltzer SB, Hovell MF, Meltzer EO, Atkins CJ, de Peyster A. Reduction of secondary smoke exposure in asthmatic children: parent counseling. J Asthma. 1993;30(5):391–400 [DOI] [PubMed] [Google Scholar]
- 17. Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–446 [DOI] [PubMed] [Google Scholar]
- 18. Greenberg RA, Strecher VJ, Bauman KE, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. J Behav Med. 1994;17(3):273–290 [DOI] [PubMed] [Google Scholar]
- 19. McIntosh NA, Clark NM, Howatt WF. Reducing tobacco smoke in the environment of the child with asthma: a cotinine-assisted, minimal-contact intervention. J Asthma. 1994;31(6):453–462 [DOI] [PubMed] [Google Scholar]
- 20. Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children: a 2-year follow-up. Chest. 1997;111(1):81–88 [DOI] [PubMed] [Google Scholar]
- 21. Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(1):18–24 [DOI] [PubMed] [Google Scholar]
- 22. Kallio K, Jokinen E, Hämäläinen M, et al. Impact of repeated lifestyle counseling in an atherosclerosis prevention trial on parental smoking and children's exposure to tobacco smoke. Acta Paediatr. 2006;95(3):283–290 [DOI] [PubMed] [Google Scholar]
- 23. Wakefield M, Banham D, McCaul K, et al. Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low-income parents of children with asthma: a controlled trial. Prev Med. 2002;34(1):58–65 [DOI] [PubMed] [Google Scholar]
- 24. McDonald E, Cook D, Newman T, Griffith L, Cox G, Guyatt G. Effect of air filtration systems on asthma: a systematic review of randomized trials. Chest. 2002;122(5):1535–1542 [DOI] [PubMed] [Google Scholar]
- 25. Sulser C, Schulz G, Wagner P, et al. Can the use of HEPA cleaners in homes of asthmatic children and adolescents sensitized to cat and dog allergens decrease bronchial hyperresponsiveness and allergen contents in solid dust? Int Arch Allergy Immunol. 2009;148(1):23–30 [DOI] [PubMed] [Google Scholar]
- 26. Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990;85(6):1050–1057 [DOI] [PubMed] [Google Scholar]
- 27. Bascom R, Fitzgerald TK, Kesavanathan J, Swift DL. A portable air cleaner partially reduces the upper respiratory response to sidestream tobacco smoke. Appl Occup Environ Hyg. 1996;11(6):553–559 [Google Scholar]
- 28. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–1080 [DOI] [PubMed] [Google Scholar]
- 29. Eggleston PA, Butz A, Rand C, et al. Home environmental intervention in inner city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol. 2005;95(6):518–524 [DOI] [PubMed] [Google Scholar]
- 30. Asmussen L, Olson LM, Grant EN, Landgraf JM, Fagan J, Weiss KB. Use of the child health questionnaire in a sample of moderate and low-income inner-city children with asthma. Am J Respir Crit Care Med. 2000;162(4):1215–1221 [DOI] [PubMed] [Google Scholar]
- 31. Asmussen L, Olson LM, Grant EN, Landgraf JM, Fagan J, Weiss KB. Reliability and validity of the Children's Health Survey for Asthma. Pediatrics. 1999;104(6). Available at: www.pediatrics.org/cgi/content/full/104/6/e71 [DOI] [PubMed] [Google Scholar]
- 32. Hammond SK, Sorensen G, Youngstrom R, Ockene JK. Occupational exposure to environmental tobacco smoke. JAMA. 1995;274(12):956–960 [PubMed] [Google Scholar]
- 33. Marbury MC, Hammond SK, Haley NJ. Measuring exposure to environmental tobacco smoke in studies of acute health effects. Am J Epidemiol. 1993;137(10):1089–1097 [DOI] [PubMed] [Google Scholar]
- 34. Eisner MD, Katz PP, Yelin EH, Hammond SK, Blanc PD. Measurement of environmental tobacco smoke exposure among adults with asthma. Environ Health Perspect. 2001;109(8):809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204 [DOI] [PubMed] [Google Scholar]
- 36. Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem. 1997;43(12):2281–2291 [PubMed] [Google Scholar]
- 37. Klein J, Koren G. Hair analysis: a biological marker for passive smoking in pregnancy and childhood. Hum Exp Toxicol. 1999;18(4):279–282 [DOI] [PubMed] [Google Scholar]
- 38. Baraldi E, Azzolin NM, Cracco A, Zacchello F. Reference values of exhaled nitric oxide for healthy children 6–15 years old. Pediatr Pulmonol. 1999;27(1):54–58 [DOI] [PubMed] [Google Scholar]
- 39. American Thoracic Society Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal oxide in adults and children: 1999. Am J Respir Crit Care Med. 1999;160(6):2104–2117 [DOI] [PubMed] [Google Scholar]
- 40. Spanier AJ, Hornung RW, Kahn RS, Lierl MB, Lanphear BP. Seasonal variation and environmental predictors of exhaled nitric oxide in children with asthma. Pediatr Pulmonol. 2008;43(6):576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu F, Takaro TK. Childhood asthma and environmental interventions. Environ Health Perspect. 2007;115(6):971–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101(2). Available at: www.pediatrics.org/cgi/content/full/101/2/e8 [DOI] [PubMed] [Google Scholar]
- 44. Lanphear BP, Kahn RS, Berger O, Auinger P, Bortnick SM, Nahhas RW. Contribution of residential exposures to asthma in US children and adolescents. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e98 [DOI] [PubMed] [Google Scholar]