Abstract
Both connexin 50 (Cx50) and aquaporin 0 (AQP0) have important roles in lens development and homeostasis, and their mutations are associated with human congenital cataracts. We have previously shown that Cx50 directly interacts with AQP0. Here, we demonstrate the importance of the Cx50 intracellular loop (IL) domain in mediating the interaction with AQP0 in the lens in vivo. AQP0 significantly increased (~20–30%) the intercellular coupling and conductance of Cx50 gap junctions. However, this increase was not observed when the IL domain was replaced with those from other lens connexins. The Cx50–AQP0 interaction had no effect on Cx50 hemichannel function. A fusion protein containing three extracellular loop domains of AQP0 efficiently blocked the cell-to-cell adhesion of AQP0 and attenuated the stimulatory effect of AQP0 on Cx50 gap junction conductance. These data suggest that the specific interaction between Cx50 and AQP0 enhances the coupling of Cx50 gap junctions, but not hemichannels, through the cell adhesion function of AQP0. This result establishes a physiological role of AQP0 in the functional regulation of gap junction channels.
Keywords: Gap junction, Connexin, Aquaporin, Hemichannel, Lens, Cell adhesion
Introduction
As an avascular organ, the lens is formed by an anterior epithelial cell layer and highly differentiated fiber cells constituting the bulk of the lenticular mass. Mitotically active epithelial cells at the lens equator start to differentiate and form new lens fibers, which gradually lose their intracellular nuclei and organelles during lens development and turn into mature lens fibers with accumulating high concentrations aquaporin 0 (AQP0) and soluble proteins known as crystallins. The eye lens is dependent upon an extensive network of gap-junction-mediated intercellular communication, a crucial part of a micro-circulatory system, to compensate for lack of vasculature and to maintain lens transparency and homeostasis (Mathias et al., 2007).
Gap junctions are clusters of transmembrane channels connecting the cytoplasm of adjacent cells, which allow small molecules (Mr≤1 kDa), such as metabolites, ions and second messengers, to pass through. The structural units of gap junctions are a group of membrane proteins called connexins, which belong to a multigene family consisting of more than 20 members (Mese et al., 2007). All connexins have four conserved transmembrane domains and two extracellular loop (EL) domains, whereas their intracellular loop (IL) domains and C-terminal (CT) domains are the most variable regions. Three major connexins have been identified in the vertebrate lens. Connexin 43 (Cx43) is mainly localized in the anterior epithelial cells and in the lens bow region along with Cx50; whereas Cx50 and Cx46 predominantly colocalize in the lens fibers and are able to form heteromeric connexons (Jiang and Goodenough, 1996). The physiological importance of lens gap junctions has been recognized in the past decade through the identification of connexin mutations that cause lens congenital cataracts in humans and the lens phenotypes displayed in connexin-deficient mouse models (for reviews see Gerido and White, 2004; Gong et al., 2007).
AQP0, also known as lens major intrinsic protein (MIP), is the most abundant membrane protein expressed in lens fibers. Although it assembles as a tetramer in the lens fiber plasma membrane, each monomer functions independently as a water channel (Chepelinsky, 2003). Unlike other aquaporins, the water permeability of AQP0 is remarkably low, being 40-times lower than that of the AQP1 channel in lens anterior epithelial cells (Zampighi et al., 2002). Besides functioning as a water channel, AQP0 is also reported to have a crucial structural role as an adhesion molecule in mediating the formation of thin junctions between lens fibers (Engel et al., 2008; Fotiadis et al., 2000; Kumari and Varadaraj, 2009). In addition, the CT domain of AQP0 has been shown to interact with several proteins, such as calmodulin (Girsch and Peracchia, 1991), lens-specific intermediate filament proteins filensin and CP49 (Lindsey Rose et al., 2006), as well as two types of γ-crystallins (Fan et al., 2004; Fan et al., 2005). Our previous studies have shown that only Cx50, not the other two lens connexins, directly associates with AQP0 in the differentiating lens fibers (Yu and Jiang, 2004). The IL domain of Cx50 and the CT domain of AQP0 are the two domains responsible for this interaction (Yu et al., 2005). Cleavage at the IL domain of Cx50 during lens development is the possible cause of dissociation of these two proteins in the mature lens fibers. However, the molecular mechanism and functional importance of the Cx50–AQP0 interaction has been largely unexplored.
In this study, we show that the IL domain of Cx50 is important in mediating the interaction with AQP0 in the lens in vivo. Moreover, the Cx50–AQP0 interaction enhances the ability of Cx50 to form functional gap junctions on the cell surface through the cell adhesion function of AQP0, but not hemichannels. Together, these results suggest that AQP0 has a specific role in facilitating the function of gap junctions during early lens development, which is independent of hemichannel function.
Results
The IL domain of Cx50 facilitates the interaction with AQP0 in differentiating lens fibers
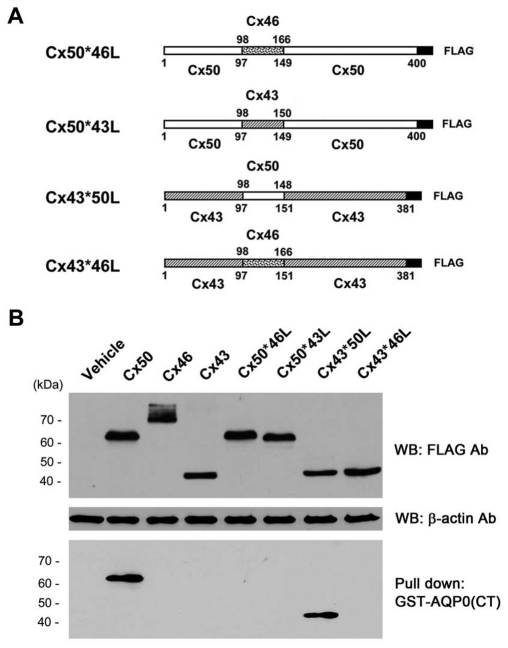
We have previously shown that the IL domain of Cx50 directly interacts with the CT domain of AQP0 in vitro (Yu et al., 2005). To address whether the IL domain of Cx50 is essential for the association of Cx50 with AQP0 in the lens fibers in situ, four chimeric retroviral constructs containing chick lens connexins with swapped IL domains (Cx50*46L, Cx50*43L, Cx43*50L and Cx43*46L, each with a short FLAG tag at the C-terminus) were generated as illustrated in Fig. 1A. The exogenous expression of lens connexins and their chimeric mutants were achieved by infecting lens connexin-deficient chicken embryonic fibroblast (CEF) cells with recombinant retroviruses. Western blots showed that Cx50, Cx46, Cx43, Cx50*46L, Cx50*43L, Cx43*50L and Cx43*46L were all exogenously expressed at comparable levels when compared with the β-actin control. To verify that the IL domain of Cx50 is essential for the interaction with the CT domain of AQP0, lysates of CEF cells expressing exogenous wild-type or chimeric connexins, as well as a RCAS(A) vehicle control were subjected to pull-down assays using the CT domain of AQP0 fused to GST as an affinity matrix. A single band was observed in the precipitated fraction of the AQP0(CT) pull-down from both Cx50 and Cx43*50L, but not from lysates containing other connexins, or vehicle-expressing cells (Fig. 1B, lower panel). These results suggest that the IL domain of Cx50 is sufficient to mediate the interaction with AQP0.
Fig. 1.
The IL domain of Cx50 is sufficient in mediating the interaction with AQP0. (A) Schematic representation of chimeric connexins with swapped IL domains. Cx50*46L was generated by fusing Cx50 lacking the IL domain with the IL from Cx46. Similarly, Cx50*43L was generated by fusing Cx50 lacking the IL domain with the IL from Cx43; Cx43*50L was generated by fusing Cx43 lacking the IL domain with the IL from Cx50, and Cx43*46L was generated by fusing Cx43 lacking the IL domain with the IL from Cx46. (B) Recombinant retroviruses containing RCAS(A) vehicle (lane 1), Cx50 (lane 2), Cx46 (lane 3), Cx43 (lane 4), Cx50*46L (lane 5), Cx50*43L (lane 6), Cx43*50L (lane 7) and Cx43*46L (lane 8) were infected into CEF cells. Six days after infection, cell lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against FLAG (upper panel) or β-actin (middle panel). Glutathione agarose beads conjugated with GST-tagged AQP0(CT) were used to pull down proteins from the lysates of infected CEF cells. The corresponding eluted fractions were immunoblotted with anti-FLAG antibody (bottom panel).
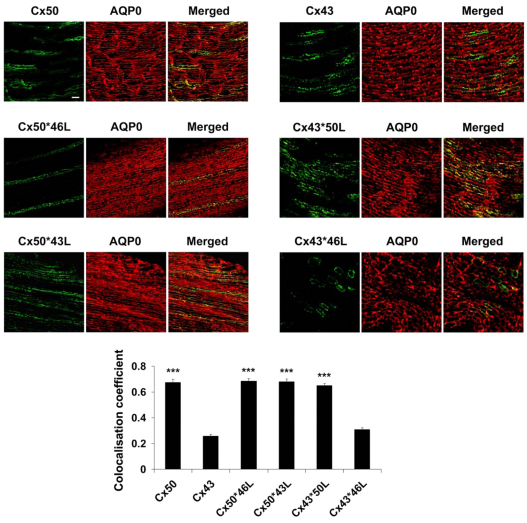
To determine whether the IL domain of Cx50 is involved in the association with AQP0 in the chick lens recombinant retroviruses containing exogenous wild-type or chimeric connexins were introduced into the lens vesicle lumen using microinjection approaches (Jiang, 2001; Jiang and Goodenough, 1998). Lens development offers a unique time frame in which the lens vesicle is formed with a large central lumen. Immunofluorescence studies showed that exogenous connexins expressed in the chick lens in vivo were primarily localized in the lens fibers with less expression in the lens epithelial cells. Both merged images and quantitative colocalization analysis indicated that Cx50, Cx50*46L, Cx50*43L and Cx43*50L all colocalized well with endogenous AQP0 in the differentiating lens fibers (Fig. 2). This colocalization can be explained by direct interactions with AQP0 through the IL domain of Cx50 (Cx50, Cx43*50L) or by indirect interactions through homomeric or heteromeric oligomerization with endogenous Cx50 (Cx50, Cx50*43L and Cx50*46L). By contrast, exogenous Cx43 and Cx43*46L did not colocalize with endogenous AQP0 and appeared to form different gap junction plaques when compared with endogenous lens fiber connexins. Without the IL domain of Cx50, as well as the capability to interact with endogenous lens fiber connexins, Cx43 and Cx43*46L have no ability to interact with endogenous AQP0 in lens fibers. Together, these results suggest that the IL domain of Cx50 is important in mediating the interaction between Cx50 and AQP0 in the differentiating lens fibers in vivo.
Fig. 2.
The IL domain of Cx50 is crucial for the interaction with AQP0 in the differentiating lens fibers. Recombinant retroviruses containing Cx50, Cx43, Cx50*46L, Cx50*43L, Cx43*50L or Cx43*46L were microinjected into chick lens vesicle at stage 18. The injected lenses were dissected out at embryonic day 18 and cryosections of the embedded chick lenses were immunolabeled with antibody against FLAG (green) or against AQP0 (red). The primary antibodies were detected by fluorescein-conjugated anti-mouse IgG for anti-FLAG antibody and Rhodamine-conjugated anti-rabbit IgG for anti-AQP0 antibody. Immunostaining was visualized by confocal fluorescence microscopy. The corresponding merged images (Merged) are shown on the right. Colocalization coefficients between exogenous connexins and endogenous AQP0 were measured on the confocal images by using NIH ImageJ software with the Intensity Correlation Analysis plugin. The data are presented below as mean ± s.e.m. (n=6). (***P<0.001 compared with Cx43).
AQP0 increases Cx50-mediated intercellular communication in CEF cells and paired Xenopus oocytes
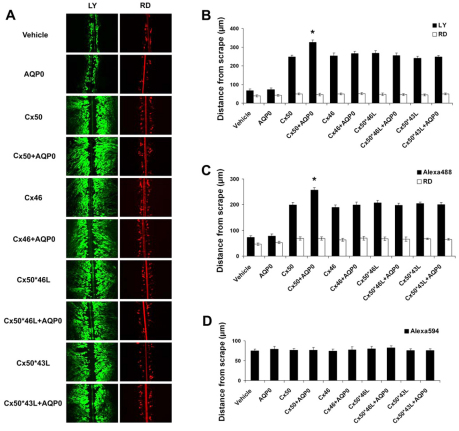
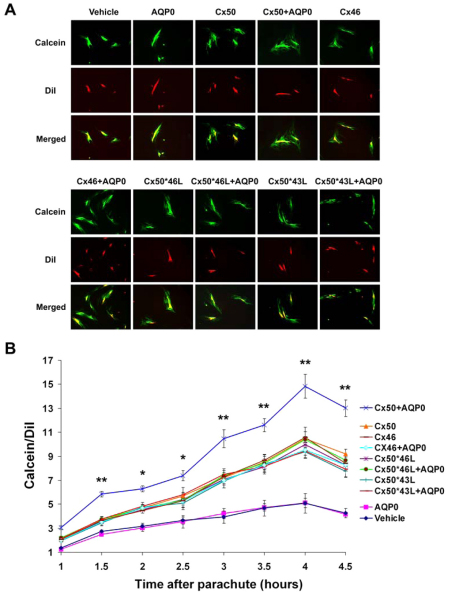
To determine whether the Cx50–AQP0 interaction has any effect on the function of Cx50 gap junctions, Cx50 and its chimeras were expressed in CEF cells in the absence or presence of AQP0. Coupling levels were assessed by scrape-loading dye transfer with three different types of tracer molecules: Lucifer yellow (LY), Alexa Fluor 488 or Alexa Fluor 594. Rhodamine dextran (RD) served as a non-transferring control. The extent of dye transfer was quantified by measuring the distance of dye travel from the scrape line. As shown in Fig. 3A,B, CEF cells expressing exogenous Cx50 plus AQP0 showed an approximately 25% increase in LY transfer over cells expressing Cx50 alone, or other chimeras in the absence or presence of AQP0, all of which showed similar coupling levels. A similar result was also obtained when using Alexa Fluor 488/RD (Fig. 3C). Gap junctions formed by Cx50 are reported to be impermeable to Alexa Fluor 594 (Mr, 759 Da), which is a larger dye with similar charge and molecular structure as Alexa Fluor 488 (Dong et al., 2006). Consistent with previous reports that Cx50 channels are much less permeable to the larger Alexa Fluor 594 (Dong et al., 2006), no Alexa Fluor 594 dye transfer was detected in CEF cells expressing Cx50 or other chimeric mutants in the absence or presence of AQP0 (Fig. 3D). A non-invasive parachute dye transfer approach was further used to complement the scrape-loading studies. This method measures dye transfer through newly formed gap junctions between pre-loaded donor cells and unlabeled recipient cells (Goldberg et al., 1995). Immunofluorescence microscopy with merged images showed the dye transfer emanating from pre-loaded cells through gap junctions (Fig. 4A). CEF cells expressing Cx50 showed a larger increase in calcein transfer in the presence of AQP0 even after a short period of incubation, compared with those expressing Cx50 in the absence of AQP0 or other chimeric mutants in the presence or absence of AQP0 (Fig. 4B). Cells infected with retroviruses containing RCAS(A) vehicle or AQP0 were used as negative controls because they can not form gap junctions.
Fig. 3.
The Cx50–AQP0 interaction enhances Cx50 intercellular gap junction coupling by using scrape-loading dye transfer assay. CEF cells were infected with recombinant retroviruses containing RCAS(A), AQP0, Cx50, Cx50 plus AQP0, Cx46, Cx46 plus AQP0, Cx50*46L, Cx50*46L plus AQP0, Cx50*43L or Cx50*43L plus AQP0. Six days after infection, the scrape-loading dye transfer assay was performed using RD as a tracer dye (A,B,C) and LY (A,B), Alexa Fluor 488 (C) or Alexa Fluor 594 (D) as a transferring dye. The extent of dye transfer was quantified by measuring the distance from scrape lines to the dye front for LY (B), Alexa Fluor 488 (C), Alexa Fluor 594 (D), and RD (B and C). The data are presented as mean ± s.e.m. (n=3). (*P<0.05, compared with Cx50).
Fig. 4.
The Cx50–AQP0 interaction enhances Cx50 intercellular gap junction coupling using parachute dye transfer assay. Six days after infection with recombinant retroviruses containing RCAS(A), AQP0, Cx50, Cx50 plus AQP0, Cx46, Cx46 plus AQP0, Cx50*46L, Cx50*46L plus AQP0, Cx50*43L or Cx50*43L plus AQP0, a time-course analysis of parachute dye transfer assay was performed at different time points after parachute using Dil as a tracer dye and calcein as a transferring dye. (A) Immunofluorescence images show the calcein transfer emanating from pre-loaded cells through gap junctions into neighboring cells. (B) The extent of dye transfer was quantified by measuring the area of calcein-fluorescence-stained cells and comparing this with the area of DiI-stained cells. The data are presented as mean ± s.e.m. (n=3). (*P<0.05 and **P<0.01, compared with Cx50).
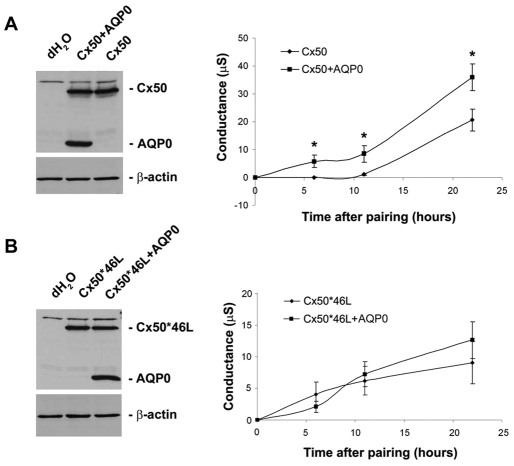
The enhancement of Cx50 gap junction coupling by AQP0 was further confirmed in paired Xenopus oocytes. Paired oocytes were analyzed for junctional conductance by the dual voltage-clamp method. Oocyte pairs expressing Cx50 plus AQP0 exhibited a higher total conductance compared with those expressing Cx50 alone (Fig. 5A). In addition, the initial establishment of coupling was significantly accelerated (evident at 5 hours opposed to >10 hours without AQP0), as was the average rate of junction formation. No significant difference was observed for oocyte pairs expressing Cx50*46L in the absence or presence of AQP0 (Fig. 5B). Differences in the rate of channel formation and conductance levels of Cx50 and Cx50*43L in the absence of AQP0 probably reflect functional consequences of the chimeric construct, because all experiments were conducted in the same oocyte batches to control for variability between batches. Together, these results suggest that the interaction between Cx50 and AQP0 increases Cx50-mediated intercellular coupling, either through assisting the assembly of Cx50 gap junctions, inhibition of degradation or regulation of Cx50 channel gating.
Fig. 5.
The channel assembly rate and total conductance of Cx50 are increased when coexpressed with AQP0 in paired Xenopus oocytes. Oocytes were pretreated with Cx38 antisense oligonucleotide. (A) Oocytes were microinjected with dH2O, Cx50 cRNA or Cx50 plus AQP0 cRNAs, respectively. Gap junction conductance was measured in paired oocytes by dual voltage clamp (right panel). Oocytes lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against Cx50, AQP0 or β-actin (left panel). The data are presented as mean ± s.e.m. (n=3). (*P<0.05, compared with Cx50). (B) Oocytes were microinjected with dH2O, Cx50*46L cRNA or Cx50*46L plus AQP0 cRNAs, respectively. Gap junction conductance was measured in paired oocytes by dual voltage clamp (right panel). Oocytes lysates were analyzed by SDS-PAGE and immunoblotted with antibodies against Cx50, AQP0 or β-actin (left panel). The data are presented as mean ± s.e.m. (n=3).
AQP0 has little effect on Cx50 hemichannel function
Cx50 forms functional hemichannels in CEF cells, which can be induced to open by mechanical stimulation (Banks et al., 2009). A dye-uptake approach was used here to determine the effect of the Cx50–AQP0 interaction on Cx50 hemichannel function. Mechanical stimulation by fluid dropping triggered a significant increase in hemichannel opening in CEF cells expressing Cx50, Cx46, Cx50 plus Cx46, or the chimeric mutant Cx50*46L, compared with untreated and vehicle controls (Fig. 6). However, hemichannel activities were not significantly affected when any of these connexins were co-expressed with AQP0. As a water channel, AQP0 cannot contribute to ion conductance by itself and does not appear to enhance hemichannel function.
Fig. 6.
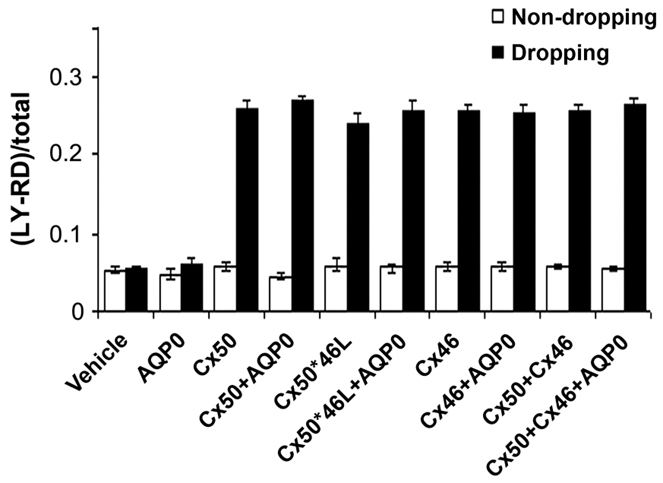
The activity of hemichannels formed by Cx50, Cx46 or chimeric mutants is not affected by AQP0. CEF cells infected with recombinant retroviruses containing RCAS(A), AQP0, Cx50, Cx46, Cx50*46L or various combinations were cultured at low cell density with minimal physical contact. After applying mechanical stimulation through fluid dropping, the numbers of cells with LY or RD dye uptake were counted under a fluorescence microscope with non-dropping groups as a negative control. The extent of dye uptake was quantified as a percentage of fluorescent cells divided by the total number of cells. The data are presented as mean ± s.e.m. (n=3).
Inhibition of the cell adhesion function of AQP0 attenuates the stimulating effect of AQP0 on Cx50 intercellular coupling
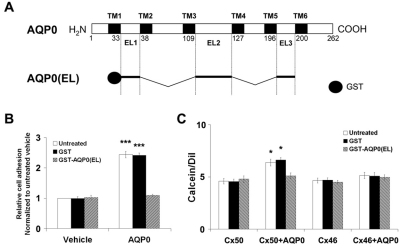
Previous studies have shown that AQP0 not only serves as a water channel but also has an important role in the cell adhesion of lens fibers (Buzhynskyy et al., 2007; Engel et al., 2008). Most recently, the cell-to-cell adhesion function of intact AQP0 was clearly demonstrated in mouse fibroblast L-cells expressing exogenous AQP0 using both a novel assay and traditional adhesion assays (Kumari and Varadaraj, 2009). To independently assess the role of cell-to-cell adhesion function of AQP0, a GST-tagged fusion protein containing three EL domains of AQP0 was generated (Fig. 7A). The cell adhesion assay results indicated that this fusion protein GST–AQP0(EL) could efficiently reduce the cell adhesiveness when AQP0 was expressed (Fig. 7B). Using the parachute dye transfer assay, we demonstrated that the enhancement of Cx50-mediated intercellular coupling initiated by AQP0 was completely blocked by pre-treatment with GST–AQP0(EL) (Fig. 7C). This strongly implicates the involvement of cell adhesion function of AQP0 in the enhancement of Cx50-mediated coupling, and is consistent with its lack of effect on Cx50 hemichannels.
Fig. 7.
A GST-tagged fusion protein containing three EL domains of AQP0 attenuates the increase of Cx50 gap junction coupling induced by AQP0. (A) Schematic illustration of AQP0 and a GST-tagged fusion protein containing all three EL domains of AQP0. GST–AQP0(EL) was generated by fusing all three EL domains of AQP0 with a GST tag at the N-terminus. (B) GST-AQP0(EL) efficiently blocks the cell adhesion of AQP0 on the cell surface. Cell adhesion assay was performed 6 days after CEF cells were infected with recombinant retroviruses containing vehicle or AQP0. Donor cells pre-loaded with DiI were mixed with the GST-tagged fusion proteins and parachuted over the unlabeled recipient cells. Plates were washed with PBS to remove non-adherent cells after 1.5-hour incubation. The extent of cell adhesion was quantified by measuring the area of DiI-stained cells, which represent adherent cells. The data are presented as mean ± s.e.m. (n=3). (***P<0.001, compared with vehicle). (C) GST–AQP0(EL) offsets the stimulatory effect of AQP0 on Cx50 gap junction coupling. Six days after infection with recombinant retroviruses containing Cx50, Cx50 plus AQP0, Cx46, or Cx46 plus AQP0, donor cells pre-loaded with calcein were mixed with the GST fusion proteins and parachuted over the unlabeled recipient cells. After incubation for 1.5 hours, the extent of dye transfer was quantified by measuring the area of calcein-stained cells and comparing this with the area of DiI-stained cells. The data are presented as mean ± s.e.m. (n=3) (*P<0.05, compared with Cx50).
Discussion
In this study, we observed that the IL domain of Cx50 has an important role in mediating the interaction with AQP0 in the lens in vivo. We also demonstrated that the Cx50–AQP0 interaction enhances the capability of Cx50 in forming functional lens fiber gap junction channels. However, this interaction has no effect on Cx50 hemichannels and does not alter the frequency of channel opening (as determined from dye-uptake studies). Indeed, increased coupling appears to be mediated through the cell-to-cell adhesion function of AQP0 and its specific interaction with Cx50. By stabilizing closer apposition of cells, specifically in domains where Cx50 connexons are concentrated, docking frequency of connexons could be enhanced, leading to increased coupling.
Lens development provides a unique time frame in which exogenous connexins can be introduced in the lens in situ through retroviral microinjection (Jiang, 2001). Immunostaining results showed that endogenous AQP0, the most abundant membrane protein in the lens fibers, is ubiquitously localized on the plasma membranes of lens fiber cells in both bow and core regions of the embryonic lens. Exogenous Cx50 colocalizes well with endogenous AQP0 in the differentiating lens fibers, whereas exogenous Cx43, which is a lens epithelial connexin, does not colocalize with endogenous AQP0. In accordance with the implication of the Cx50 CL domain in interactions with AQP0, the chimeric mutant Cx43*50L consistently colocalized with endogenous AQP0 in the differentiating lens fibers, whereas the Cx43*46L chimera showed minimal overlap with AQP0. These results confirm the role of the IL domain of Cx50 in the interaction with AQP0 in the differentiating lens fibers in situ. Other chimeras that do not contain the CL domain of Cx50, or wild-type Cx46, also colocalize with AQP0 in situ, but this is probably due to oligomerization with endogenous Cx50, which would mediate the association with AQP0.
We examined the effect of AQP0 on Cx50-mediated intercellular communication using three different approaches: invasive scrape-loading dye transfer, non-invasive parachute dye transfer and electrophysiological measurements in the Xenopus oocyte expression system. Scrape-loading dye transfer confirmed the selective transfer of LY and Alexa Fluor 488, but not Alexa Fluor 594 through Cx50 channels, as reported previously (Dong et al., 2006). These differences in dye transfer are probably due to the selectivity properties of gap junction channels, which can distinguish small molecules based on their size, shape and charge state (Nicholson et al., 2000; Weber et al., 2004). Importantly, this selectivity was not affected by association with AQP0. Scrape-loading results were also confirmed by the less invasive parachute dye-transfer assay. The differences between cells expressing both Cx50 and AQP0 and those expressing only Cx50 were even more dramatic in this assay. This might have resulted from the mode of measurement (area of dye spread as opposed to linear spread distance), but might also be due to the higher dependence of this assay on gap junction formation. This is consistent with the electrophysiological measurements in oocytes that also showed higher rates of gap junction formation in the presence of AQP0.
Besides being a component of gap junctions, connexin molecules can form functional hemichannels in nonjunctional membranes, which have a role that is distinct from that of gap junctions by allowing exchanges with the extracellular space (Goodenough and Paul, 2003). Cx50 has been reported to form functional hemichannels in Xenopus oocytes and in transfected HeLa cells (Beahm and Hall, 2002; Srinivas et al., 2005; Valiunas and Weingart, 2000), which are sensitive to both voltage and mechanical stimulation (Bao et al., 2004). We showed that hemichannels formed by two lens fiber connexins and the corresponding chimera were indeed sensitive to mechanical stimulation, demonstrated by the dye-uptake assay using fluid dropping (Banks et al., 2009). However, in contrast to its effect on gap junctions, AQP0 does not enhance the ability of Cx50 to form functional hemichannels.
Because the Cx50–AQP0 interaction enhances gap junction coupling, but not hemichannel activities, we hypothesize that the cell-to-cell adhesion function of AQP0 (Buzhynskyy et al., 2007; Chepelinsky, 2009) serves to increase the proximity of adjacent fiber cells, thus enhancing the probability of gap junction formation. Given the association between AQP0 and Cx50, this would occur selectively at sites where Cx50 connexons are concentrated. This hypothesis was tested using GST–AQP0(EL), a GST-tagged fusion protein that contains all three EL domains of AQP0. The interaction between the ELs of two apposing AQP0 molecules and/or the interaction between the ELs of AQP0 and the negatively charged lipid molecules of the adjacent cell membrane are proposed to be crucial in the formation of 11–13 nm thin junctions with extremely narrow intervening spaces between lens fibers (Kumari and Varadaraj, 2009). As expected, GST–AQP0(EL) effectively inhibited the cell adhesion of AQP0, and also inhibited the AQP0-induced enhancement of Cx50 coupling. These results suggest that the AQP0-induced enhancement of Cx50 gap junction coupling is likely to be mediated through the cell-to-cell adhesion function of AQP0.
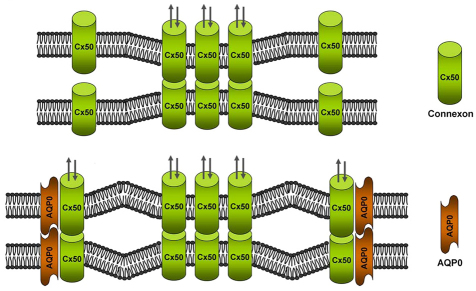
As shown in the model (Fig. 8), our data suggest that in the actively differentiating lens fibers, Cx50 forms functional gap junctions as well as hemichannels on the cell surface. These channels could be either homomeric (Cx50 alone) or heteromeric (Cx50 and Cx46). In the presence of AQP0, the C-terminus of AQP0 directly interacts with the intracellular loop of Cx50. Through the cell-to-cell adhesion function of AQP0, which pulls the plasma membranes of apposing cells closer, and its specific interaction with Cx50, more Cx50 connexons on the cell surface are able to dock with their apposing counterparts from neighboring cells to form functional gap junctions. Thus AQP0 provides a mechanism to selectively enhance the gap junction function of Cx50, while not affecting its role as a hemichannel. Cx50 and AQP0 interaction is primarily in the differentiating lens fibers around the lens equator region, where fluid and metabolic wastes are mainly disposed out based on the lens microcirculation mechanism (Mathias et al., 2007). Previous microscopy studies have shown that the density of gap junctions in the equator region is much higher than the central region of the lens, which would accommodate the high metabolic needs of the lens around this region (Jacobs et al., 2001). AQP0 mainly associates in the periphery of the large gap junctional plaques (Dunia et al., 1998). In the lens of AQP0-deficient mice, the percentage of membrane areas specialized as gap junction plaques is reduced by ~50% (Al-Ghoul et al., 2003). This in vivo evidence implies a functional role of AQP0 in gap junction formation and function. Interestingly, Cx50+/− mice have no obvious lens phenotype despite having 50% of the Cx50 protein and ~30% of the gap junctional coupling seen in the lenses of wild-type animals (Mathias et al., 2010). We have observed that the interaction between Cx50 and AQP0 occurs throughout early embryonic stages, whereas at late embryonic and early postnatal stages it mainly occurs close to the lens bow region where lens fiber cells differentiate. In the adult lens, this interaction only occurs in the narrow region close to bow region (Yu and Jiang, 2004). The gap junction coupling comparisons of WT and heterozygote animals, mentioned above, might not necessarily represent the regions of the lens where AQP and Cx50 interact. In addition, the level of gap junction coupling might not directly correlate with the expression level of Cx50, especially in the presence of AQP0. It is possible that the lack of the obvious lens phenotypes in heterozygotes could be explained by the enhancement of gap junction coupling around this region as a result of the interaction between Cx50 and AQP0. Therefore, even a ~20–30% increase of gap junction function could be physiologically important in the region containing metabolically active differentiating lens fibers that ultimately specify lens size. The physiological role of Cx50 and AQP0 interaction in the lens warrants further investigation and will be a direction of future research.
Fig. 8.
Schematic model illustrating the enhancement of Cx50-mediated intercellular communication by AQP0 in the lens fibers. In the differentiating fiber cells of lens, Cx50 forms functional gap junction channels and hemichannels on the cell surface (top). When AQP0 is also present (bottom), it pulls the plasma membranes of apposing cells much closer by functioning as an adhesion molecule. Meanwhile, the C-terminal domain of AQP0 directly interacts with the intracellular loop domain of Cx50. As a result of this, more Cx50 connexons on the cell surface are able to dock with their apposing counterparts from neighboring cells to form functional gap junction channels.
Materials and Methods
Materials
Fertilized, unincubated white leghorn chicken eggs were obtained from Ideal Poultry (Cameron, TX) and incubated in a humidified 37°C incubator. Rabbit anti-chick AQP0 polyclonal antibody was generated from rabbit against chicken AQP0 C-terminus (residues 223–262) and affinity purified as previously described (Jiang et al., 1994). Anti-FLAG monoclonal antibody (F1804) was from Sigma (St Louis, MO). mMESSAGE mMACHINE SP6 kit for in vitro transcription was from Ambion (Austin, TX). Bicinchoninic acid (BCA) microprotein assay kit was purchased from Pierce Chemical (Rockford, IL). Fluorescein-conjugated goat anti-mouse IgG and Rhodamine-conjugated goat anti-rabbit IgG were from Jackson ImmunoResearch (West Grove, PA). Paraformaldehyde (PFA, 16% stock solution) was from Electron Microscopy Science (Fort Washington, PA). Trypsin, tissue culture reagents, Alexa Fluor 488, Alexa Fluor 594, LY, Rhodamine dextran (RD), calcein acetoxymethyl ester and dialkylcarbocyanine dye (DiI) were all obtained from Invitrogen (Carlsbad, CA). PVDF membrane was from Bio-Rad (Hercules, CA). Fetal bovine serum (FBS) was from Hyclone Laboratories (Logan, UT). Vectashield® fluorescence mounting medium was from Vector Laboratories (Burlingame, CA). All other chemicals were obtained from either Sigma or Fisher Scientific (Pittsburgh, PA).
Preparation of recombinant retroviral constructs containing Cx50, Cx46, Cx43 and chimeric connexins, and generation of high-titer retroviruses
Retroviral constructs and high-titer retroviruses were prepared based on our protocol described previously (Jiang, 2001). In brief, the cDNA fragments containing Cx50, Cx46 and Cx43 were made by PCR and were constructed into the retroviral vector RCAS(A) as described previously (Jiang and Goodenough, 1998; Yin et al., 2000). For the construction of chimeric retroviral constructs Cx50*43L and Cx50*46L, a two-step PCR procedure was carried out. First, by using a Cla12NCO-Cx50 construct as the template, we obtained a fragment containing IL-deleted Cx50 (residues 1–97 and 149–400) as well as the Cla12NCO vector with the following pair of primers: sense, CTCCTGAGAACCTACATCCT; antisense, CACCGCATGCCCAAAGTACAC. Second, by using Cla12NCO-Cx43 and Cla12NCO-Cx46 constructs as the templates, PCR fragments containing the ILs of Cx43 (residues 98–150) and Cx46 (residues 98–166) were also obtained with the following pairs of primers: sense, TACGTGATGAGGAAAGAAGAG; antisense, TCCTCCACGCATCTTTACCTTG; sense, CACATTGTACGCATGGAAGAG; antisense, AGCACCTCCCATACGGATTC, respectively. Third, the two blunt-ended fragments were ligated together to generate Cx50*43L and Cx50*46L constructs. Finally, connexin fragments were isolated after digestion with ClaI and subcloned into a ClaI-linearized RCAS(A) retroviral vector. The constructs with correct insert were selected by ClaI and SalI restriction enzyme digestion. Similar procedures were used for making chimeric retroviral constructs Cx43*50L and Cx43*46L. The first fragment contained the IL-deleted Cx43 (residues 1–97 and 151–381) as well as the Cla12NCO vector was cloned by using a Cla12NCO-Cx43 construct as the template with the following pair of primers: sense, CTGCTTCGTACTTACATCATC; antisense, GAACACGTGCGCCAGGTAC. The second fragments contained the ILs of Cx50 (residues 98–148) or Cx46 (residues 98–166) were cloned by using Cla12NCO-Cx50 and Cla12NCO-Cx46 constructs as the templates with the following pair of primers: sense, CACCATGTCCGCATGGAGGAGA; antisense, GGTCCCCTCCAGGCGAAAC; sense, CACATTGTACGCATGGAAGAG; antisense, AGCACCTCCCATACGGATTC, respectively. The two fragments were fused together to generate Cx43*50L and Cx43*46L constructs. The C-termini of both wild-type and chimeric connexins were epitope-tagged with FLAG sequences to distinguish the exogenous connexins from the endogenous ones. PCR primers were synthesized at the University of Texas Health Science Center (UTHSCSA) DNA Core Facility. All the constructs generated were also sequenced at the UTHSCSA DNA Core Facility to ensure the correctness of the sequences. High-titer recombinant retroviruses were generated through transfection with these constructs into CEF cells (1–5×108 c.f.u. per ml).
Chick lens microinjection
High-titer recombinant retroviruses containing wild-type and chimeric connexins were prepared as described above. Microinjection of retroviruses into the lens of chicken embryos was based on our previously published procedure (Jiang and Goodenough, 1998). Briefly, chicken eggs were opened under sterile condition at the developmental stage 18 (approximately 65–68 hours of incubation). The amnionic membrane was removed to provide unimpeded access to the developing eye. About 10–40 nl concentrated viral stock (titers, 1-5×108 c.f.u. per ml) were directly microinjected into the lumen of the right lens vesicle using a glass pipette with a tip opening diameter of approximately 10 μm. Viral stocks were colored with Fast Green to visualize the filling of lens vesicle lumen. The left lens was kept intact to serve as a counterlateral control. After injection, the eggshell opening was sealed with tape and the embryo was returned to 39°C for further incubation. The injected lenses were dissected on embryonic day 18 for immunofluorescence studies.
Immunofluorescence and confocal laser microscopy
The dissected embryo lenses were fixed in 2% PFA for 30 minutes at room temperature, and then immersed in 1 M sucrose at 4°C overnight. After being immersed in Tissue-Tek compound for 5 minutes, the dissected lenses were quickly frozen in liquid nitrogen. Sagittal and coronal sections (10–20 mm) were collected and prepared at −20°C as described (Paul et al., 1991). For dual immunolabeling of exogenous connexins and endogenous AQP0, lens sections were first incubated in phosphate buffered saline (PBS) for 5 minutes, then in blocking solution containing 2% normal goat serum, 2% fish skin gelatin, 0.25% Triton X-100 and 1% bovine serum albumin (BSA) in PBS for 30 minutes, and finally with monoclonal anti-FLAG antibody (1:1000 dilution) mixed with affinity-purified anti-AQP0 antibody (1:400 dilution) in blocking solution overnight at 4°C. Sections were washed four times for 5 minutes each in PBS and then incubated with fluorescein-conjugated goat anti-mouse IgG against anti-FLAG (1:500 dilutions in blocking solution) plus Rhodamine-conjugated goat anti-rabbit IgG against anti-AQP0 (1:500 dilution in blocking solution) for 2 hours at room temperature. After four washes in PBS for 5 minutes each, a drop of mounting medium was added before being covered by a glass coverslip. Control experiments were included: labeling with each primary antibody individually to ensure that binding of one antibody was not sterically hindered by the other; labeling with mixed secondary antibodies only to detect any nonspecific cross-reactivity between secondary antibodies; and labeling with pre-immune antibody to determine any background bindings. The specimens were analyzed using an Olympus FV-500 confocal laser scanning microscope (Olympus Optical, Tokyo, Japan). Acquisition conditions were kept constant for each sample. FITC was excited at 488 nm by an argon laser with a corresponding excitation wavelength range 505–525 nm using a barrier filter. Rhodamine was excited at 543 nm with a HeNe-G laser with a corresponding excitation wavelength of 560 nm using a long-pass barrier filter. Quantitative colocalization analysis of exogenous connexins and endogenous AQP0 signals was performed by using NIH ImageJ software with the Intensity Correlation Analysis plugin. Colocalization coefficients measured here are Mander's Colocalization coefficients for channel 1 (green) because there are more red pixels (endogenous AQP0 signal) than green pixels (exogenous connexin signal).
Scrape-loading dye transfer assay
CEF cells expressing exogenous Cx50, Cx46, chimeric mutants, AQP0 or various combinations by retroviral infection were grown to confluence to maximize the cell–cell contact. The morphology of CEF cells makes it difficult to visualize dye transfer through individual cells. Therefore, a total distance of dye transfer from scrape line was measured. Scrape-loading dye transfer was performed based on a modified procedure (El-Fouly et al., 1987). Briefly, cells were scratched in the presence of two fluorescent dyes: LY (Mr, 457 Da) or Alexa Fluor 488 (Mr, 570 Da), which can pass through gap junctions, plus RD (Mr, 10 kDa) which is too large to pass through gap junctions. Therefore, LY and Alexa Fluor 488 staining indicates the degree of gap junction coupling and RD serves as a tracer dye for cells originally receiving the dyes. Cells were washed three times with Hanks Balanced Salt Solution (HBSS) plus 1% BSA for 5 minutes each, and then plates were scraped lightly with a 26.5 gauge needle in a dye mixture containing 1% LY and 1% RD or 1 mM Alexa Fluor 488 and 1% RD in PBS. After incubation for 15 minutes, cells were washed with HBSS three times, twice with PBS and then fixed in fresh 2% PFA for 30 minutes. Dye transfer was examined using a Zeiss Axiovert 35 fluorescence microscope (Carl Zeiss International, Oberkochen, Germany) in which LY, Alexa Fluor 488 and RD could be detected by using fluorescein and RD filters, respectively. For scrape loading with Alexa Fluor 594 (Mr, 759 Da), CEF cells were just scraped in 1 mM Alexa Fluor 594 in PBS and detected by using RD filter. Acquisition conditions were kept consistent for all measurements and no threshold adjustments were used. The extent of dye transfer was quantified by measuring the distance from the scrape lines to the travel front for LY, Alexa Fluor 488, Alexa Fluor 594 or RD-stained cells. At least five images per condition tested with six measurements per image were used to assess the extent of dye transfer.
Cell parachute dye-transfer assay
This assay was conducted based on a published protocol (Goldberg et al., 1995) with some modifications. Briefly, CEF cells expressing exogenous Cx50, Cx46, chimeric mutants, AQP0 or various combinations by retroviral infection were grown to confluence in 60 mm and 100 mm plates, respectively. RCAS(A) vehicle was used as a control. The donor cells cultured in 60 mm plates were incubated with calcein AM and DiI for 20 minutes at 37°C. Gap junction intercellular communication can be followed by simultaneously labeling cells with calcein AM as a cytosolic tracer dye along with the membrane label, DiI (Goldberg et al., 1995). Once inside the cell, calcein AM is cleaved by non-specific esterases into calcein (Mr, 622.54 Da), which is spectrally similar to LY and Alexa Fluor 488. DiI fluoresces with similar wavelength spectra as RD and indicates the cell boundaries of preloaded cells. Preloaded cells from 60 mm plates were layered (‘parachuted’) over the top of the unlabeled recipient cells cultured in 100 mm plates at a 1:150 donor to receiver ratio. Cells were allowed to attach for various periods (1, 1.5, 2, 2.5, 3, 3.5 and 4 hours), and then fixed in fresh 2% PFA and examined with a fluorescence microscope. Competitive blocking with GST fusion proteins were done based on a smaller scale of parachute dye transfer to minimize the usage of fusion proteins prepared. Donor cells from 35 mm plates preloaded with calcein AM and DiI were trypsinized and premixed with GST or GST-AQP0(EL) at 100 ng/ml before parachuted over the unlabeled recipient cells in 35 mm plates. Cells were examined under fluorescence microscope after a 1.5 hour incubation. For calcein dye transfer, the threshold was adjusted to clearly distinguish the dye-transfer boundaries for each image used for measurement. Images were converted to a binary black–white scale, with black representing the original calcein fluorescence. Finally, the black pixels were converted to a percentage of the total black–white field and reported as a calcein-positive area. Five representative images for each condition tested were used to assess calcein dye transfer per measurement. The extent of dye transfer was quantified by measuring the ratios of the area of calcein-stained cells to the area of DiI-stained cells.
Dye-uptake assay
The dye-uptake assay was performed as previously described (Cherian et al., 2005) with some modifications. Briefly, CEF cells expressing exogenous Cx50, Cx46, Cx50*46L, AQP0 or various combinations by retroviral infection were grown at low cell density to ensure the majority of cells were not physically in contact. LY was used as a tracer for hemichannel activity with RD as a negative control. CEF cells were mechanically stimulated by dropping Ca2+-free MEM from pipette at a fixed distance, which led to the opening of hemichannels on the cell surface. Dye uptake was conducted in the presence of 0.4% LY and 0.4% RD for 5 minutes, and the cells were washed with medium containing 1.8 mM Ca2+ and then fixed with 1% PFA. Similar fields were observed under the fluorescence microscope. Approximately eight images for each condition tested were collected. Dye uptake was presented as the number of fluorescent cells divided by the total number of cells.
In vitro transcription, Xenopus oocyte microinjection, electrophysiological measurement and preparation of oocyte samples for western blots
cDNAs encoding full-length Cx50, AQP0 and the chimeric mutant Cx50*46L were subcloned into a pSP64T transcription vector. The constructs were confirmed by sequencing, linearized with EcoRI, and transcribed using the mMESSAGE mMACHINE in vitro transcription kit in accordance with the manufacturer's instructions. RNA concentrations were estimated by non-denaturing gel electrophoresis with ethidium bromide staining. Diluted working stock solutions were prepared with RNase-free water and stored at −80°C. Oocytes taken from ovarian lobe tissue of Xenopus laevis were defolliculated and microinjected with a 5 ng connexin cRNA and/or 5 ng AQP0 cRNA along with 6 ng Cx38 antisense RNA for endogenous Cx38 suppression (Barrio et al., 1991). Oocytes were constantly incubated in half-strength L15 medium with 2 mM Ca2+ (Sigma) during the whole process.
Two days after cRNA injection, oocytes were stripped of their vitelline membrane and paired in agar wells. Gap junctional currents were examined within 16–24 hours of pairing, using dual electrode voltage clamps by using GeneClamp 500B voltage clamps (Axon Instruments, Foster City, CA). A 1× L-head stage was used for voltage recording and a 10× MG head stage was used for passing current. Bath potential was actively clamped to 0 mV using a 100× VG head stage. Resistances for electrodes were between 0.5 and 3 MΩ with 150 mM KCl solution. The amplifiers were interfaced to a computer through a Digidata 1200 A/D converter. Data were acquired and analyzed using Pclamp 8 software (Axon Instruments). Oocytes were clamped at −30 mV and transjunctional conductances were measured using a 10 mV pulse.
After measurements, oocytes were collected in ice-cold lysis buffer (5 mM Tris, 5 mM EDTA, 5 mM EGTA, pH 8.0) plus 2 mM phenylmethylsulfonyl fluoride, 10 mM N-ethylmaleimide, 2 mM sodium vanadate and 100 μM leupeptin, and lysed with a 20 gauge needle. Extracts were centrifuged at 8000 g at 4°C for 10 minutes to remove debris. The oocytes lysates were then subjected to SDS-PAGE and immunoblotting with specific antibodies.
Preparation of a GST-tagged AQP0(CT) fusion protein and analysis of protein pull-down
A GST-tagged fusion protein containing the CT domain of AQP0 was prepared as follows. Briefly, a cDNA fragment encoding the CT domain of AQP0 (residues 223–262) was generated by PCR using chick AQP0 cDNA clone as a template with the following pair of primers: sense, GGAATTCCATATGCTGTGTCCGCGGGCG; antisense, CCGCTCGAGCAGCCCCTGCGTC TTC and was subcloned into the expression vector pGEX-2T. The recombinant fusion protein was expressed in E. coli, induced with 1 mM IPTG, and then isolated and purified with glutathione–agarose beads. For pull-down experiments, crude membranes obtained from CEF cells were first preincubated with glutathione–agarose beads at 4°C for 2 hours to eliminate any nonspecific binding to the beads. The supernatant fraction of the mixture was saved and incubated with the corresponding GST-tagged AQP0(CT) fusion protein and glutathione-agarose beads overnight at 4°C. The beads were then washed four times with ice-cold GST lysis buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA, 0.5% NP-40, pH 8.0) plus protease inhibitors. The pull-down samples were isolated from the beads by boiling in SDS sample loading buffer for 5 minutes and subjected to SDS-PAGE and immunoblotting.
Culture of CEF cells, retroviral expression, and preparation of cell membranes
CEF cells were plated at 2.5×105 cells in 60 mm culture plates with Dulbecco's modified Eagle's medium plus 10% fetal calf serum, 2% chick serum, 1% pyruvate and 1% penicillin/streptomycin. CEF cells were infected on the second day with high-titer retroviruses. After reaching confluence, CEF cells were digested with 0.05% trypsin and passaged into 60 mm or 100 mm culture plates. At confluence, cells were collected in ice-cold lysis buffer and lysed with a 26.5 gauge needle. Cell lysates were centrifuged for 5 minutes at 1000 g to remove cell debris. Crude membranes were further pelleted at 100,000 g for 30 minutes (TLA-100.3 rotor, Beckman) at 4°C.
SDS gel electrophoresis, fluorography and western blot
Briefly, crude membrane samples resuspended in lysis buffer and boiled in 0.6% SDS were analyzed by 10% SDS-PAGE. Western blots were performed by probing with anti-FLAG (1:1000 dilution), affinity-purified anti-AQP0 (1:400 dilution) or anti-β-actin (1:5000 dilution) antibody. Primary antibodies were detected with horseradish-peroxidase-conjugated goat anti-rabbit IgG (1:5000 dilution) or anti-mouse IgG (1:5000 dilution) using chemiluminescence reagent kit (ECL). The intensity of the bands on western blots was quantified by densitometry (ImageJ Software, NIH).
Preparation of a GST-tagged AQP0(EL) fusion protein and cell adhesion assay
GST–AQP0(EL), a GST-tagged fusion protein containing all three EL domains of AQP0, was prepared as follows. Briefly, a cDNA fragment encoding all three EL domains (residues 33–38, 109–127 and 196–200) of AQP0 was generated by PCR using chick AQP0 cDNA clone as a template with the following pair of primers: sense, GCATGGATCCCCGCTGGGCCCCGGGCCCCCCGGCCGCCGTGCGCGGC; antisense, ACATGAATTCGTTGGTGAAGTTGCGCGGACCCACGCTGGGGTGCAG and was subcloned into the expression vector pGEX-2T. The recombinant fusion protein was expressed in E. coli, induced with 1 mM IPTG, and purified with glutathione–agarose beads. Cell adhesion assay was performed based on a published protocol (Kumari and Varadaraj, 2009) with some modifications. Briefly, CEF cells infected with retroviruses containing RCAS(A) or AQP0 were grown to confluence in 35 mm plates. RCAS(A) was used as a vehicle control. Equal numbers of donor cells preloaded with DiI were premixed with GST or GST–AQP0(EL) at 100 ng/ml and parachuted over the unlabeled recipient cells at a 1:50 donor to receiver ratio. After incubation for 1.5 hours at 37°C, plates were washed with PBS to remove non-adherent cells and examined under fluorescence microscope for cell adhesion. At least five representative images for each condition tested were used to assess cell adhesion per measurement.
Statistical analysis
Data were analyzed with one-way ANOVA and Newman–Keuls multiple comparison test along with GraphPad Prism (GraphPad Software, La Jolla, CA). Data are presented as the mean ± s.e.m. of multiple measurements. Asterisks represent the degree of significance in comparison with controls (*P<0.05; **P<0.01; ***P<0.001). Some of the data presented were normalized to controls for easy comparison, and the variables were analyzed as described above.
Supplementary Material
Acknowledgments
The work was supported by grants from the National Institute of Health (EY12085) (J.X.J.), (GM55437) (B.J.N.) and the Welch Foundation (AQ-1507) (J.X.J.). Confocal images were generated in the Core Optical Imaging Facility, which is supported by UTHSCSA, NIH-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center) and (NIH-NIA P01AG19316), and we thank the staff for their assistance. Deposited in PMC for release after 12 months.
References
- Al-Ghoul K. J., Kirk Y., Kuszak A. J., Zoltoski R. K., Shiels A., Kuszak J. R. (2003). Lens structure in MIP-deficient mice. Anat. Rec. 273, 714-730 [DOI] [PubMed] [Google Scholar]
- Banks E. A., Toloue M. M., Shi Q., Zhou Z. J., Liu J., Nicholson B. J., Jiang J. X. (2009). Connexin mutation that causes dominant congenital cataracts inhibits gap junctions, but not hemichannels, in a dominant negative manner. J. Cell Sci. 122, 378-388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Sachs F., Dahl G. (2004). Connexins are mechanosensitive. Am. J. Physiol. 287, C1389-C1395 [DOI] [PubMed] [Google Scholar]
- Barrio L. C., Suchyna T., Bargiello T., Xu L. X., Roginski R. S., Bennett M. V. L., Nicholson B. J. (1991). Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by voltage. Proc. Natl. Acad. Sci. USA 88, 8410-8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm D. L., Hall J. E. (2002). Hemichannel and junctional properties of connexin 50. Biophys. J. 82, 2016-2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhynskyy N., Hite R. K., Walz T., Scheuring S. (2007). The supramolecular architecture of junctional microdomains in native lens membranes. EMBO Rep. 8, 51-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepelinsky A. B. (2003). The ocular lens fiber membrane specific protein MIP/aquaporin 0. J. Exp. Zool. 300A, 41-46 [DOI] [PubMed] [Google Scholar]
- Chepelinsky A. B. (2009). Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital iinherited cataracts. Handb. Exp. Pharmacol. 190, 265-297 [DOI] [PubMed] [Google Scholar]
- Cherian P. P., Siller-Jackson A. J., Gu S., Wang X., Bonewald L. F., Sprague E., Jiang J. X. (2005). Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol. Biol. Cell 16, 3100-3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Liu X., Li H., Vertel B. M., Ebihara L. (2006). Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J. Physiol. 576, 787-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunia I., Recouvreur M., Nicolas P., Kumar N., Bloemendahl H., Benedetti E. L. (1998). Assembly of connexins and MP26 in lens fiber plasma membranes studied by SDS-fracture immunolabeling. J. Cell Sci. 111, 2109-2120 [DOI] [PubMed] [Google Scholar]
- El-Fouly M. H., Trosko J. E., Chang C. C. (1987). Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168, 422-430 [DOI] [PubMed] [Google Scholar]
- Engel A., Fujiyoshi Y., Gonen T., Walz T. (2008). Junction-forming aquaporins. Curr. Opin. Struct. Biol. 18, 229-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Donovan A. K., Ledee D. R., Zelenka P. S., Fariss R. N., Chepelinsky A. B. (2004). GammaE-crystallin recruitment to the plasma membrane by specific interaction between lens MIP/aquaporin-O and gammaE-crystallin. Invest. Opthalmol. Vis. Sci. 45, 863-871 [DOI] [PubMed] [Google Scholar]
- Fan J., Fariss R. N., Purkiss A. G., Slingsby C., Sandilands A., Quinlan R., Wistow G., Chepelinsky A. B. (2005). Specific interation between lens MIP/aquaporin-0 and two members of the γ-crystallin family. Mol. Vis. 11, 76-87 [PubMed] [Google Scholar]
- Fotiadis D., Hasler L., Müller D. L., Stahlberg H., Kistler J., Engel A. (2000). Surface tongue-groove contours on lens MIP facilitate cell-to-cell adherence. J. Mol. Biol. 300, 779-789 [DOI] [PubMed] [Google Scholar]
- Gerido D. A., White T. W. (2004). Connexin disorders of the ear, skin, and lens. Biochim. Biophys. Acta 1662, 159-170 [DOI] [PubMed] [Google Scholar]
- Girsch S. J., Peracchia C. (1991). Calmodulin interacts with a C-terminus peptide from the lens membrane protein MIP26. Curr. Eye Res. 10, 839-849 [DOI] [PubMed] [Google Scholar]
- Goldberg G. S., Bechberger J. F., Naus C. C. G. (1995). A pre-loading method of evaluating gap junctional communication by fluorescent dye transfer. Biotechniques 18, 490-497 [PubMed] [Google Scholar]
- Gong X., Cheng C., Xia C. (2007). Connexins in lens development and cataractogenesis. J. Membr. Biol. 218, 9-12 [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Paul D. L. (2003). Beyond the gap: Functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 4, 285-294 [DOI] [PubMed] [Google Scholar]
- Jacobs M. D., Soeller C., Cannell M. B., Donaldson P. J. (2001). Quantifying changes in gap junction structure as a function of lens fiber cell differentiation. Cell Commun. Adhes. 8, 349-353 [DOI] [PubMed] [Google Scholar]
- Jiang J. X. (2001). Use of retroviruses to express connexins. Methods Mol. Biol. 154, 159-174 [DOI] [PubMed] [Google Scholar]
- Jiang J. X., Goodenough D. A. (1996). Heteromeric connexons in lens gap junction channels. Proc. Natl. Acad. Sci. USA 93, 1287-1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. X., Goodenough D. A. (1998). Retroviral expression of connexins in embryonic chick lens. Invest. Ophthalmol. Vis. Sci. 39, 537-543 [PubMed] [Google Scholar]
- Jiang J. X., White T. W., Goodenough D. A., Paul D. L. (1994). Molecular cloning and functional characterization of chick lens fiber connexin45.6. Mol. Biol. Cell 5, 363-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S., Varadaraj K. (2009). Intact AQP0 performs cell-to-cell adhesion. Biochem. Biophys. Res. Commun. 390, 1034-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey Rose K. M., Gourdie R. G., Prescott A. R., Quinlan R. A., Crouch R. K., Schey K. L. (2006). The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest. Opthalmol. Vis. Sci. 47, 1562-1570 [DOI] [PubMed] [Google Scholar]
- Mathias R. T., Kistler J., Donaldson P. (2007). The lens circulation. J. Membr. Biol. 216, 1-16 [DOI] [PubMed] [Google Scholar]
- Mathias R. T., White T. W., Gong X. (2010). Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 90, 179-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mese G., Richard G., White T. W. (2007). Gap junctions: Basic structure and function. J. Invest. Dermatol. 127, 2516-2524 [DOI] [PubMed] [Google Scholar]
- Nicholson B. J., Weber P. A., Cao F., Chang H., Lampe P., Goldberg G. (2000). The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz. J. Med. Biol. Res. 33, 369-378 [DOI] [PubMed] [Google Scholar]
- Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. (1991). Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 115, 1077-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M., Kronengold J., Bukauskas F. F., Bargiello T. A., Versellis V. K. (2005). Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys. J. 88, 1725-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V., Weingart R. (2000). Electrical properties of gap junction hemichannels identified in transfected cells. Pflugers Arch. 440, 366-379 [DOI] [PubMed] [Google Scholar]
- Weber P. A., Chang H. C., Spaeth K. E., Nitsche J. M., Nicholson B. J. (2004). The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 87, 958-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Jedrzejewski P. T., Jiang J. X. (2000). Casein kinase II phosphorylates lens connexin 45.6 and is involved in its degradation. J. Biol. Chem. 275, 6850-6856 [DOI] [PubMed] [Google Scholar]
- Yu X. S., Jiang J. X. (2004). Interaction of major intrinsic protein (aquaporin-0) with fiber connexins in lens development. J. Cell Sci. 117, 871-880 [DOI] [PubMed] [Google Scholar]
- Yu X. S., Yin X., Lafer E. M., Jiang J. X. (2005). Developmental regulation of the direct interaction between the intracellular loop of connexin 45.6 and the C-terminus of major intrinsic protein (aquaporin-0). J. Biol. Chem. 280, 22081-22090 [DOI] [PubMed] [Google Scholar]
- Zampighi G. A., Eskandari S., Hall J. E., Zampighi L., Kreman M. (2002). Micro-domains of AQP0 in lens equatorial fibers. Exp. Eye Res. 75, 505-519 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.