Abstract
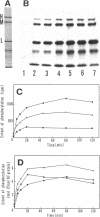
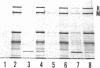
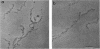
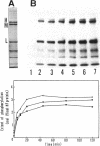
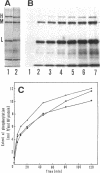
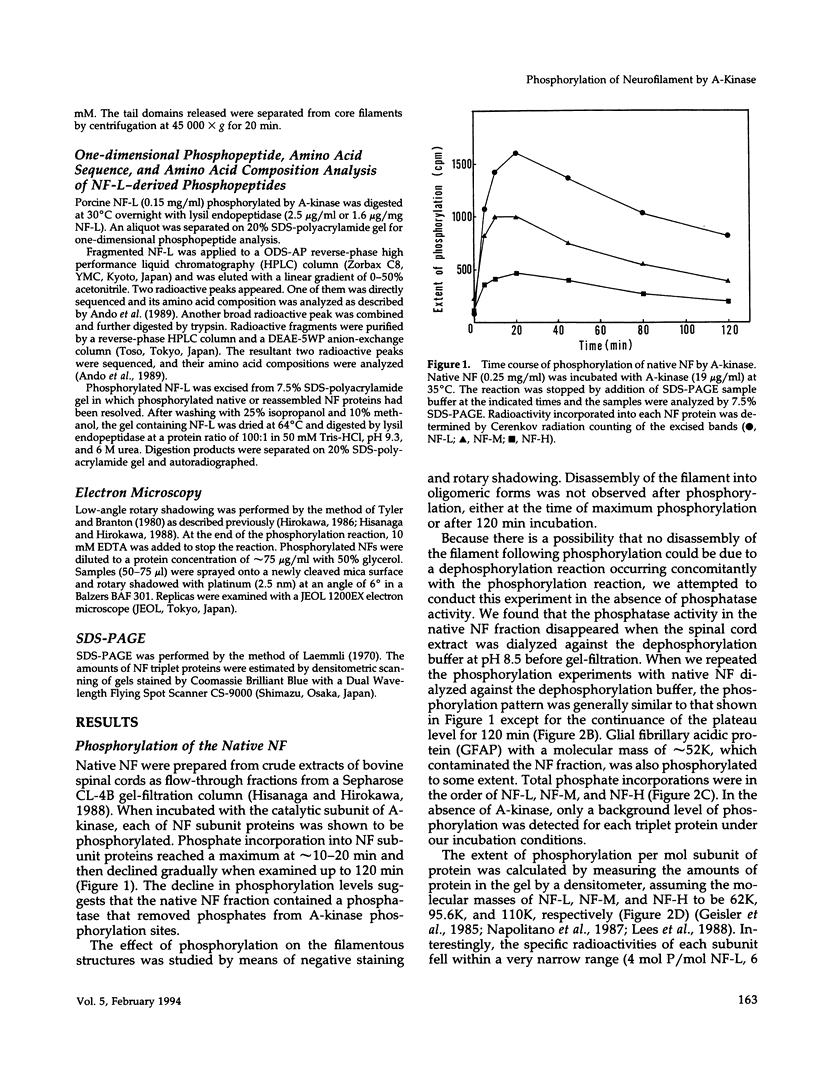
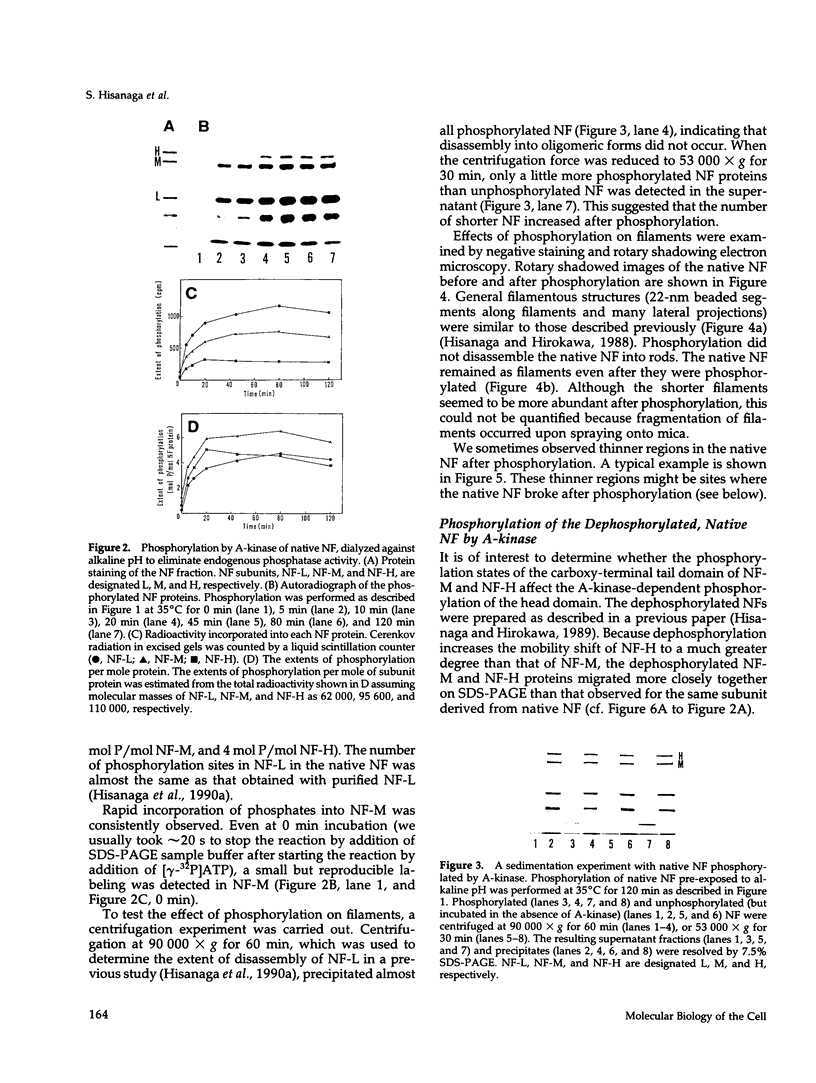
Phosphorylation of neurofilament-L protein (NF-L) by the catalytic subunit of cAMP-dependent protein kinase (A-kinase) inhibits the reassembly of NF-L and disassembles filamentous NF-L. The effects of phosphorylation by A-kinase on native neurofilaments (NF) composed of three distinct subunits: NF-L, NF-M, and NF-H, however, have not yet been described. In this paper, we examined the effects of phosphorylation of NF proteins by A-kinase on both native and reassembled filaments containing all three NF subunits. In the native NF, A-kinase phosphorylated each NF subunit with stoichiometries of 4 mol/mol for NF-L, 6 mol/mol for NF-M, and 4 mol/mol for NF-H. The extent of NF-L phosphorylation in the native NF was nearly the same as that of purified NF-L. However, phosphorylation did not cause the native NFs to disassemble into oligomers, as was the case for purified NF-L. Instead, partial fragmentation was detected in sedimentation experiments and by electron microscopic observations. This is probably not due to the presence of the three NF subunits in NF or to differences in phosphorylation sites because reassembled NF containing all three NF subunits were disassembled into oligomeric forms by phosphorylation with A-kinase and the phosphorylation by A-kinase occurred at the head domain of NF-L whether NF were native or reassembled. Disassembling intermediates of reassembled NF containing all three NF subunits were somewhat different from disassembling intermediates of NF-L. Thinning and loosening of filaments was frequently observed preceding complete disassembly. From the fact that the thinning was also observed in the native filaments phosphorylated by A-kinase, it is reasonable to propose the native NF is fragmented through a process of thinning that is stimulated by phosphorylation in the head domain of the NF subunits.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Tanabe K., Gonda Y., Sato C., Inagaki M. Domain- and sequence-specific phosphorylation of vimentin induces disassembly of the filament structure. Biochemistry. 1989 Apr 4;28(7):2974–2979. doi: 10.1021/bi00433a035. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Smith K. E., Takeda M. Assembly and exchange of intermediate filament proteins of neurons: neurofilaments are dynamic structures. J Cell Biol. 1989 Apr;108(4):1495–1506. doi: 10.1083/jcb.108.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin B. J., Lee V. M. Individual neurofilament subunits reassembled in vitro exhibit unique biochemical, morphological and immunological properties. Brain Res. 1991 Aug 16;556(2):196–208. doi: 10.1016/0006-8993(91)90307-h. [DOI] [PubMed] [Google Scholar]
- Ching G. Y., Liem R. K. Assembly of type IV neuronal intermediate filaments in nonneuronal cells in the absence of preexisting cytoplasmic intermediate filaments. J Cell Biol. 1993 Sep;122(6):1323–1335. doi: 10.1083/jcb.122.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. H., Rosevear E., Goldman R. D. Phosphorylation and disassembly of intermediate filaments in mitotic cells. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1885–1889. doi: 10.1073/pnas.86.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci A., Pant H. C. Association of cyclic-AMP-dependent protein kinase with neurofilaments. Biochem J. 1992 Mar 1;282(Pt 2):477–481. doi: 10.1042/bj2820477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner R., Kahn M. Differential extraction of keratin subunits and filaments from normal human epidermis. J Cell Biol. 1990 Apr;110(4):1149–1168. doi: 10.1083/jcb.110.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. Cyclic AMP-dependent protein kinase-induced vimentin filament disassembly involves modification of the N-terminal domain of intermediate filament subunits. FEBS Lett. 1988 Jul 4;234(1):73–78. doi: 10.1016/0014-5793(88)81306-1. [DOI] [PubMed] [Google Scholar]
- Evans R. M. Phosphorylation of vimentin in mitotically selected cells. In vitro cyclic AMP-independent kinase and calcium-stimulated phosphatase activities. J Cell Biol. 1989 Jan;108(1):67–78. doi: 10.1083/jcb.108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer J., Leterrier J. F. Influence of the phosphorylation state of neurofilament proteins on the interactions between purified filaments in vitro. Biochem J. 1988 Jun 15;252(3):655–660. doi: 10.1042/bj2520655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Plessmann U., Weber K. The complete amino acid sequence of the major mammalian neurofilament protein (NF-L). FEBS Lett. 1985 Mar 25;182(2):475–478. doi: 10.1016/0014-5793(85)80357-4. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Phosphorylation of desmin in vitro inhibits formation of intermediate filaments; identification of three kinase A sites in the aminoterminal head domain. EMBO J. 1988 Jan;7(1):15–20. doi: 10.1002/j.1460-2075.1988.tb02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Self-assembly in Vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol. 1981 Sep 25;151(3):565–571. doi: 10.1016/0022-2836(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Georges E., Lefebvre S., Mushynski W. E. Dephosphorylation of neurofilaments by exogenous phosphatases has no effect on reassembly of subunits. J Neurochem. 1986 Aug;47(2):477–483. doi: 10.1111/j.1471-4159.1986.tb04526.x. [DOI] [PubMed] [Google Scholar]
- Gonda Y., Nishizawa K., Ando S., Kitamura S., Minoura Y., Nishi Y., Inagaki M. Involvement of protein kinase C in the regulation of assembly-disassembly of neurofilaments in vitro. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1316–1325. doi: 10.1016/0006-291x(90)90667-c. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. 270K microtubule-associated protein cross-reacting with anti-MAP2 IgG in the crayfish peripheral nerve axon. J Cell Biol. 1986 Jul;103(1):33–39. doi: 10.1083/jcb.103.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. Cross-linker system between neurofilaments, microtubules, and membranous organelles in frog axons revealed by the quick-freeze, deep-etching method. J Cell Biol. 1982 Jul;94(1):129–142. doi: 10.1083/jcb.94.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Glicksman M. A., Willard M. B. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984 Apr;98(4):1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S., Gonda Y., Inagaki M., Ikai A., Hirokawa N. Effects of phosphorylation of the neurofilament L protein on filamentous structures. Cell Regul. 1990 Jan;1(2):237–248. doi: 10.1091/mbc.1.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S., Hirokawa N. Dephosphorylation-induced interactions of neurofilaments with microtubules. J Biol Chem. 1990 Dec 15;265(35):21852–21858. [PubMed] [Google Scholar]
- Hisanaga S., Hirokawa N. Molecular architecture of the neurofilament. II. Reassembly process of neurofilament L protein in vitro. J Mol Biol. 1990 Feb 20;211(4):871–882. doi: 10.1016/0022-2836(90)90080-6. [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Hirokawa N. Structure of the peripheral domains of neurofilaments revealed by low angle rotary shadowing. J Mol Biol. 1988 Jul 20;202(2):297–305. doi: 10.1016/0022-2836(88)90459-7. [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Hirokawa N. The effects of dephosphorylation on the structure of the projections of neurofilament. J Neurosci. 1989 Mar;9(3):959–966. doi: 10.1523/JNEUROSCI.09-03-00959.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S., Ikai A., Hirokawa N. Molecular architecture of the neurofilament. I. Subunit arrangement of neurofilament L protein in the intermediate-sized filament. J Mol Biol. 1990 Feb 20;211(4):857–869. doi: 10.1016/0022-2836(90)90079-2. [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Ishiguro K., Uchida T., Okumura E., Okano T., Kishimoto T. Tau protein kinase II has a similar characteristic to cdc2 kinase for phosphorylating neurofilament proteins. J Biol Chem. 1993 Jul 15;268(20):15056–15060. [PubMed] [Google Scholar]
- Hisanaga S., Kusubata M., Okumura E., Kishimoto T. Phosphorylation of neurofilament H subunit at the tail domain by CDC2 kinase dissociates the association to microtubules. J Biol Chem. 1991 Nov 15;266(32):21798–21803. [PubMed] [Google Scholar]
- Inagaki M., Gonda Y., Matsuyama M., Nishizawa K., Nishi Y., Sato C. Intermediate filament reconstitution in vitro. The role of phosphorylation on the assembly-disassembly of desmin. J Biol Chem. 1988 Apr 25;263(12):5970–5978. [PubMed] [Google Scholar]
- Inagaki M., Nishi Y., Nishizawa K., Matsuyama M., Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987 Aug 13;328(6131):649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. K., Xu Z., Wong P. C., Cleveland D. W. Neurofilaments are obligate heteropolymers in vivo. J Cell Biol. 1993 Sep;122(6):1337–1350. doi: 10.1083/jcb.122.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. A. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988 Jul;7(7):1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Preferential phosphorylation of the 150,000 molecular weight component of neurofilaments by a cyclic AMP-dependent, microtubule-associated protein kinase. J Cell Biol. 1981 Sep;90(3):755–760. doi: 10.1083/jcb.90.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J., Winkfein R. J., Paudel H. K., Wang J. H. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J Biol Chem. 1992 Dec 25;267(36):25922–25926. [PubMed] [Google Scholar]
- Liem R. K., Hutchison S. B. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982 Jun 22;21(13):3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- Minami Y., Sakai H. Dephosphorylation suppresses the activity of neurofilament to promote tubulin polymerization. FEBS Lett. 1985 Jun 17;185(2):239–242. doi: 10.1016/0014-5793(85)80914-5. [DOI] [PubMed] [Google Scholar]
- Miyasaka H., Okabe S., Ishiguro K., Uchida T., Hirokawa N. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J Biol Chem. 1993 Oct 25;268(30):22695–22702. [PubMed] [Google Scholar]
- Nakamura Y., Takeda M., Angelides K. J., Tanaka T., Tada K., Nishimura T. Effect of phosphorylation on 68 KDa neurofilament subunit protein assembly by the cyclic AMP dependent protein kinase in vitro. Biochem Biophys Res Commun. 1990 Jun 15;169(2):744–750. doi: 10.1016/0006-291x(90)90394-3. [DOI] [PubMed] [Google Scholar]
- Napolitano E. W., Chin S. S., Colman D. R., Liem R. K. Complete amino acid sequence and in vitro expression of rat NF-M, the middle molecular weight neurofilament protein. J Neurosci. 1987 Aug;7(8):2590–2599. [PMC free article] [PubMed] [Google Scholar]
- Nixon R. A., Shea T. B. Dynamics of neuronal intermediate filaments: a developmental perspective. Cell Motil Cytoskeleton. 1992;22(2):81–91. doi: 10.1002/cm.970220202. [DOI] [PubMed] [Google Scholar]
- Okabe S., Miyasaka H., Hirokawa N. Dynamics of the neuronal intermediate filaments. J Cell Biol. 1993 Apr;121(2):375–386. doi: 10.1083/jcb.121.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder H. M., Eden P. A., Ingram V. M. Brain protein kinase PK40erk converts TAU into a PHF-like form as found in Alzheimer's disease. Biochem Biophys Res Commun. 1993 Jun 15;193(2):639–647. doi: 10.1006/bbrc.1993.1672. [DOI] [PubMed] [Google Scholar]
- Roder H. M., Ingram V. M. Two novel kinases phosphorylate tau and the KSP site of heavy neurofilament subunits in high stoichiometric ratios. J Neurosci. 1991 Nov;11(11):3325–3343. doi: 10.1523/JNEUROSCI.11-11-03325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty K. T., Link W. T., Pant H. C. cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6844–6848. doi: 10.1073/pnas.90.14.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag R. K., Nixon R. A. Identification of Ser-55 as a major protein kinase A phosphorylation site on the 70-kDa subunit of neurofilaments. Early turnover during axonal transport. J Biol Chem. 1991 Oct 5;266(28):18861–18867. [PubMed] [Google Scholar]
- Sihag R. K., Nixon R. A. Phosphorylation of the amino-terminal head domain of the middle molecular mass 145-kDa subunit of neurofilaments. Evidence for regulation by second messenger-dependent protein kinases. J Biol Chem. 1990 Mar 5;265(7):4166–4171. [PubMed] [Google Scholar]
- Skalli O., Chou Y. H., Goldman R. D. Intermediate filaments: not so tough after all. Trends Cell Biol. 1992 Oct;2(10):308–312. doi: 10.1016/0962-8924(92)90121-3. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Wong J., Hutchison S. B., Liem R. K. An isoelectric variant of the 150,000-dalton neurofilament polypeptide. Evidence that phosphorylation state affects its association with the filament. J Biol Chem. 1984 Sep 10;259(17):10867–10874. [PubMed] [Google Scholar]
- Zackroff R. V., Idler W. W., Steinert P. M., Goldman R. D. In vitro reconstitution of intermediate filaments form mammalian neurofilament triplet polypeptides. Proc Natl Acad Sci U S A. 1982 Feb;79(3):754–757. doi: 10.1073/pnas.79.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]