Abstract
In a genome-wide association study (GWAS) of late-onset Alzheimer's disease (AD), we found an association between common haplotypes of the GAB2 gene and AD risk in carriers of the apolipoprotein E (APOE) ε4 allele, the major late-onset AD susceptibility gene. We previously proposed the use of fluorodeoxyglucose positron emission tomography (FDG-PET) measurements as a quantitative presymptomatic endophenotype, more closely related to disease risk than the clinical syndrome itself, to help evaluate putative genetic and non-genetic modifiers of AD risk. In this study, we examined the relationship between the presence or absence of the relatively protective GAB2 haplotype and PET measurements of regional-to-whole brain FDG uptake in several AD-affected brain regions in 158 cognitively normal late-middle-aged APOEε4 homozygotes, heterozygotes, and non-carriers. GAB2 haplotypes were characterized using Affymetrix Genome-Wide Human SNP 6.0 Array data from each of these subjects. As predicted, the possibly protective GAB2 haplotype was associated with higher regional-to-whole brain FDG uptake in AD-affected brain regions in APOEε4 carriers. While additional studies are needed, this study supports the association between the possibly protective GAB2 haplotype and the risk of late-onset AD in APOEε4 carriers. It also supports the use of brain-imaging endophenotypes to help assess possible modifiers of AD risk.
Keywords: Alzheimer's disease, fluorodeoxyglucose positron emission tomography
INTRODUCTION
Alzheimer's disease (AD) is the most common cause of disabling cognitive impairment in older adults. Past research has led to the identification of well-established associations between four genes and AD risk. While more than 200 mutations of the presenilin 1 (PS1), presenilin 2 (PS2), and amyloid precursor protein (APP) genes account for the majority of early-onset AD cases with autosomal dominant inheritance, the apolipoprotein E (APOE) ε4 allele accounts for the majority of late-onset AD (LOAD) cases with dementia onset after age 60 (Corder et al., 1993; Farrer et al., 1997; Papassotiropoulos et al., 2006). The APOE gene has three variants, ε2, ε3, and ε4. The ε2 allele is associated with a lower LOAD risk, while each copy of the ε4 allele in a person's genotype is associated with higher LOAD risk and a younger median age at dementia onset (Corder et al., 1993; Farrer et al., 1997). Twin studies suggest that 80% of LOAD cases are attributable to heritable factors (Gatz et al., 2006). While the identification of LOAD susceptibility genes besides APOE has been elusive (Bertram et al., 2007), the need for doing so remains a pressing matter as the discovery of new susceptibility genes will provide new targets for developing improved AD therapeutics. Sufficiently powered genome wide association studies (GWAS) and next generation sequencing technologies now in development may help characterize the genetic variants that contribute to LOAD risk.
In the first reported GWAS of LOAD, we found an association between common variants of the GAB2 (Grb2-associated binding factor 2) gene on chromosome 11 and the risk of AD in APOEε4 carriers (Reiman et al., 2007). The study implicated five single nucleotide polymorphisms (SNPs) surviving Bonferroni correction for more than 300,000 independent comparisons in the ε4 carrier group (rs901104, rs1385600, rs4945261, rs7115850, rs2373115), as well as a 189 kb-pair haplotype block encompassing the entire GAB2 gene and ranging from rs901104 to rs2373115. With these findings, we proposed the existence of a common risk GAB2 haplotype, a less common protective GAB2 haplotype, and a rare neutral GAB2 haplotype. Based on the HapMap CEU (Caucasian European) population, consisting of Utah residents with Northern and Western European ancestry from the CEPH (Centre d'Etude du Polymorphisme Humain) collection (http://hapmap.ncbi.nlm.nih.gov/index.html.en), the population frequency for the risk haplotype is 0.836, 0.112 for the protective haplotype, and 0.036 for the neutral haplotype. Several independent groups have confirmed the association between these GAB2 variants in APOEε4 carriers ((Feulner et al., 2009; Ikram et al., 2009; Nacmias et al., 2009; Schjeide et al., 2009; Sleegers et al., 2009), though with lower odds ratios than in the original report, raising the possibility of a common phenomenon in genetic associations studies known as “winners curse” (Nakaoka and Inoue, 2009). Other studies have failed to confirm the association (but have limited power to refute an association with adequate statistical power) (Harold et al., 2009; Li et al., 2008; Lin et al., 2009; Miyashita et al., 2009; Ramirez-Lorca et al., 2009). Based on meta-analyses of existing data, GAB2 is currently listed as the 18th most replicated LOAD susceptibility gene on the AlzGene website (http://www.alzgene.org). Based on genetic association, neuronal transcriptomic, and siRNA (short interfering RNA) data, as well as the known pathways associated with GAB2, we postulated that the Gab2 protein is involved with reducing tau phosphorylation and neurofibrillary tangle formation and that a loss-of-function isoform might contribute to an increase in the vulnerability to phosphorylated tau pathology in individuals already at risk for AD. Additional studies are needed to determine the extent to which GAB2 variants modify AD risk in APOEε4 carriers, the extent to which they may do so in ε4 noncarriers, and the biological basis for any confirmed association.
AD is associated with characteristic and progressive reductions in regional fluorodeoxyglucose positron emission tomography (FDG-PET) measurements of the cerebral metabolic rate for glucose (CMRgl), some of which are apparent years before the onset of symptoms (Alexander et al., 2002; Langbaum et al., 2009; Minoshima et al., 1997; Thal et al., 2006). Using data from an ongoing longitudinal study of initially cognitively normal, late-middle-aged people with two copies, one copies and no copies of the APOEε4 allele, we previously demonstrated an association between APOEε4 gene dose, reflecting three levels of genetic risk for late-onset AD, and lower CMRgl bilaterally in each of the brain regions preferentially affected in symptomatic patients with AD. Based on these findings, we proposed using FDG-PET as a quantitative presymptomatic endophenotype—a biological measurement more closely related to disease susceptibility than the clinical syndrome itself, to help evaluate the individual and aggregate effects of putative genetic and non-genetic modifiers of AD. We subsequently used the data from our subjects to evaluate several suggested modifiers of AD risk, testing the hypothesis that risk factors would be associated with lower regional-to-whole brain FDG uptake in AD affected brain regions.
In the present study, we initially tested the hypothesis that the possibly protective GAB2 haplotype is associated with higher regional-to-whole brain FDG uptake (i.e. that noncarriers of the protective GAB2 haplotype have lower regional-to-whole brain FDG uptake than protective GAB2 haplotype carriers) in APOEε4 carriers. We then explored the generalizability of this difference in the APOEε4 non-carrier and overall subject groups.
MATERIALS & METHODS
Subjects
Newspaper advertisements were previously used to recruit cognitively normal volunteers 47-68 years of age who reported a first-degree family history of probable AD, understood they would not receive any information about their APOE genotype, provided their informed consent, and were studied under guidelines approved by the human subjects committees at Banner Good Samaritan Medical Center and the Mayo Clinic (Reiman et al., 1996). Venous blood samples were drawn and APOE genotypes characterized with analysis involving restriction fragment length polymorphisms (Crook et al., 1994; Hixson and Vernier, 1990; Reiman et al., 1996). Originally, one APOEε4 heterozygote (with the ε3ε4 genotype) and two ε4 non-carriers were matched to a different ε4 homozygote for their gender, age (within 3 years), and educational level (within 2 years). They denied having an impairment in memory or other cognitive skills, had scores of at least 28 on the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) and less than 10 on the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960), did not satisfy criteria for a current psychiatric disorder using a structured psychiatric interview, and had a normal neurological exam. Study subjects have been assessed every two years using a medical examination, clinical ratings, neuropsychological tests, volumetric MRI and FDG-PET as previously described (Reiman et al., 2001; Reiman et al., 1996). The subjects include 57 carriers of the protective GAB2 haplotype and 101 non-carriers of the protective GAB2 haplotype, including 33 and 48, respectively in the APOEε4 carrier group and 24 and 53, respectively in the APOEε4 non-carrier group.
For the PET analyses described below, we performed a comparison of the APOEε4 carriers with the protective GAB2 haplotype versus the APOEε4 carriers without the protective GAB2 haplotype to test the hypothesis that the lower-risk GAB2 haplotype group would have higher metabolism than the comparator group in the brain regions preferentially affected by AD. We then compared the protective GAB2 haplotype carrier and non-carrier groups in the overall subject group to explore the extent to which findings might be generalized to individuals irrespective of their APOEε4 carrier or non-carrier status. We additionally performed this comparison in only APOEε4 non-carriers to explore the generalizability of our hypothesis in this subgroup.
DNA Isolation
DNA was isolated from collected whole blood and was resuspended in reduced EDTA TE buffer (10mM Tris HCl, 0.1mM EDTA, pH 8.0) to an approximate concentration of 50 ng/uL. Isolated DNA for all samples was assessed for quality by running samples on a 1% TAE (Tris-acetate-EDTA) gel. If replacement samples were available for noticeably degraded samples, these were re-assessed and used for genotyping if samples passed quality control measures. Degraded samples were not genotyped. The concentration of double-stranded DNA for each sample was measured using Invitrogen's Quant-iT Picogreen dsDNA Assay Kit (Carlsbad, CA).
SNP genotyping & haplotype analysis
Approximately 500 ng (50 ng/uL) samples were analyzed on the Affymetrix Genome-Wide Human SNP Array 6.0 (Santa Clara, CA), which probes for over 906,600 SNPs, according to the manufacturer's protocol. In summary, samples were separately digested by Sty or Nsp restriction enzymes, adaptor ligated, PCR (polymerase chain reaction) amplified and purified using polystyrene beads, fragmented, labeled, and hybridized to arrays. Arrays were washed and stained on Affymetrix GeneChip Fluidics Stations 450.
Following array scanning using Affymetrix's GeneChip Scanner 3000 7G, Birdsuite (Broad Institute; Cambridge, MA) was used to call SNP genotypes based off of CEL files (McCarroll et al., 2008). 1 sample that demonstrated a low call rate (71%) was dropped from further analysis.
Using Haploview 4.1 (Broad Institute, Cambridge, MA), 16 SNPs distributed across the GAB2 gene were analyzed. Using this method, three haplotypes were found across a single block spanning the GAB2 gene. As previously noted, these haplotypes were described in our previously reported GWAS (Reiman et al., 2007). These haplotypes include a common risk haplotype, a less common protective haplotype, and a rare neutral haplotype. Based on the SNP calls for each subject, GAB2 haplotypes were determined for each individual. Identification of protective haplotype carrier and non-carrier groups permitted us to perform PET comparisons.
Image Acquisition & Analysis
PET was performed as previously described (Reiman et al., 1996; Reiman et al., 2005) using the HR+ scanner (Siemens, Knoxville, TN) in the 3D mode, a transmission scan, the intravenous injection of 5-8 mCi of [18F] FDG and a 60-minute dynamic sequence of emission scans as the participants, who had fasted for at least 4 hours, lay quietly with eyes closed in a darkened room. The reconstructed images consisted of 63 horizontal slices with a center-to-center slice separation of 2.46 mm, an axial field of view of 15.5 cm, an in-plane resolution of 4.2-5.1 mm full width at half-maximum (FWHM), and an axial resolution of 4.6-6.0 mm FWHM. Voxel-based analyses were performed using the PET images (counts relative to the whole brain uptake) acquired during the last 30 minutes.
The automated brain mapping algorithm SPM5 (Wellcome Department of Cognitive Neurology, London, U.K.) was used to linearly and non-linearly deform each person's PET image into the coordinates of a standard brain atlas, normalize data for the variation in absolute whole brain measurements using proportionate scaling, and smooth the images using a three-dimensional Gaussian filter to a spatial resolution of 12mm. General linear model (GLM) based voxel-wise statistical ANOVA (analysis of variance) was initially used to generate statistical parametric maps of significant regional-to-whole brain CMRgl differences (P<0.005, uncorrected for multiple comparisons) in the protective carrier versus protective haplotype non-carrier groups and in APOEε4 carriers to test the hypothesis that the lower-risk GAB2 haplotype group would have higher measurements than the comparator group in the brain regions preferentially affected by AD. It was then used to compare the GAB2 haplotype carrier and non-carrier groups in the APOEε4 non-carrier and overall subject groups to explore the extent to which findings might be generalized to individuals irrespective of their APOEε4 carrier or non-carrier status. In order to test our hypotheses, significance levels were then adjusted for the number of resolution elements in the AD-affected posterior cingulate, precuneus, parietotemporal, and frontal brain regions postulated to be preferentially affected in cognitively normal persons at genetic risk for AD using the small-volume correction procedure in SPM. To examine the differential effects of APOE on the impact of GAB2 on regional-to-whole brain FDG uptake, a two factor ANOVA (analysis of variance) was performed, and to examine the presence of an epistatic interaction between GAB2 and APOE, a GAB2 x APOE interaction term was included in a separate SPM analysis.
Following Birdsuite analysis of the genetic data, a total of 158 subjects were included for PET analysis: One subject was not included in the PET analysis due to low call rates (71%) and 3 other subjects were not included because no calls were made for at least one GAB2 tag SNP that is needed to determine the haplotype. Based on Haploview analysis, three possible GAB2 haplotypes across one haplotype block in our cohort of 158 healthy subjects were identified (Supplementary Figure 1). As previously described (Reiman et al., 2007), these haplotypes include a common risk haplotype (population frequency (pf) of this cohort = 0.731), a less common protective haplotype (pf = 0.183), and a rare neutral haplotype (pf = 0.044). Table 2 lists the SNPs that characterize the three GAB2 haplotypes within the haplotype block.
Table 2.
GAB2 haplotype block
| Haplotype | PF | rs901104 | rs1385600 | rs1007837 | rs2450130 | rs2510054 | rs2510038 | rs2511170 | rs4945261a | rs7101429 | rs10793294a | rs4291702 | rs11602622 | rs10899467 | rs2458640a | rs10793302a | rs2373115 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk | 0.731 | C | T | A | A | G | C | A | G | A | A | C | A | G | A | C | G |
| Protective | 0.183 | T | C | G | C | A | T | G | A | G | C | T | G | T | C | T | T |
| Neutral | 0.044 | C | T | A | A | G | C | A | G | A | C | C | A | G | C | C | G |
PF=population frequency in 158 analyzed samples
GAB2 haplotype tag SNP
RESULTS
Characteristics of protective GAB2 haplotype carriers and non-carriers with and without the APOEε4 allele are shown in Table 1. The four sub-groups did not differ significantly in their age, gender, clinical ratings, or neuropsychological test scores with the following exceptions: APOEε4 carriers with the protective GAB2 haplotype had a higher educational level than those without the GAB2 protective haplotype (P=0.004, uncorrected for multiple comparisons) and demonstrated better performance on the WAIS-R Digit Span sub-test (P=0.02, uncorrected for multiple comparisons).
Table 1.
Protective GAB2 haplotype carrier and non-carrier characteristics, clinical ratings, and neuropsychological scoresa
| protective haplotype carrier | protective haplotype non-carrier | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | APOEε4 carrier (n=33) |
APOEε4 non-carrier (n=24) |
Overall (n=57) |
APOEε4 carrier (n=48) |
APOEε4 non-carrier (n=53) |
Overall (n=101) |
APOEε4 carrier P-value |
APOEε4 non-carrier P-value |
Overall P-value |
| Age | 59.5±6.8 | 61.7±7.8 | 60.4±7.2 | 60.5±4.9 | 60.5±5.4 | 60.5±5.2 | 0.26 | 0.26 | 0.47 |
| Gender (F/M) | 23/10 | 16/8 | 39/18 | 33/15 | 33/20 | 66/35 | 0.81 | 0.49 | 0.69 |
| Yrs of education | 16.8±2.9 | 15.9±2.0 | 16.4±2.6 | 15.2±2.2 | 15.8±2.3 | 15.5±2.3 | 0.004 | 0.43 | 0.01 |
| MMSE | 29.7±0.7 | 29.7±0.6 | 29.7±0.6 | 29.8±0.6 | 29.6±0.7 | 29.7±0.7 | 0.21 | 0.32 | 0.42 |
| HAM-D | 1.6±1.9 | 2.4±2.1 | 1.9±2.0 | 1.5±1.7 | 2.3±2.7 | 1.9±2.3 | 0.36 | 0.40 | 0.35 |
| AVLT | |||||||||
| Total learning | 50.2±10.3 | 47.2±9.4 | 49.0±9.9 | 48.4±9.1 | 48.3±8.8 | 48.4±8.9 | 0.21 | 0.32 | 0.35 |
| STM | 9.8±3.3 | 9.4±3.3 | 9.7±3.3 | 9.5±3.3 | 9.4±3.0 | 9.4±3.1 | 0.33 | 0.46 | 0.33 |
| LTM | 9.5±3.9 | 8.7±3.5 | 9.2±3.7 | 9.1 ±3.4 | 9.0±3.1 | 9.0±3.2 | 0.31 | 0.37 | 0.41 |
| Complex figure test | |||||||||
| Copy | 34.8±1.7 | 34.4±3.2 | 34.7±2.4 | 34.7±2.2 | 34.2±2.0 | 34.5±2.1 | 0.38 | 0.42 | 0.30 |
| Recall | 20.0±7.8 | 17.7±7.2 | 19.0±7.5 | 19.1 ±7.5 | 17.9±7.5 | 18.4±7.5 | 0.30 | 0.46 | 0.32 |
| BNT | 56.2±4.2 | 56.7±3.5 | 56.4±3.9 | 57.5±2.8 | 56.1 ±4.2 | 56.8±3.6 | 0.07 | 0.28 | 0.28 |
| WAIS-R | |||||||||
| Information | 12.3±2.2 | 12.4±2.1 | 12.4±2.2 | 11.8±2.0 | 12.2±2.4 | 12.0±2.2 | 0.15 | 0.37 | 0.18 |
| Digit span | 12.4±2.6 | 11.2±3.1 | 11.9±2.9 | 11.1±2.8 | 10.9±3.0 | 11.0±2.9 | 0.02 | 0.36 | 0.03 |
| Block design | 12.7±2.9 | 12.7±3.0 | 12.7±2.9 | 12.3±2.9 | 12.3±2.4 | 12.3±2.6 | 0.27 | 0.29 | 0.20 |
| Arithmetic | 12.3±2.8 | 11.7±3.0 | 12.1 ±2.9 | 11.7±3.1 | 12.2±2.3 | 12.0±2.7 | 0.17 | 0.24 | 0.41 |
| Similarities | 12.9±2.4 | 12.5±2.4 | 12.7±2.4 | 12.4±1.8 | 12.5±2.1 | 12.5±1.9 | 0.19 | 0.49 | 0.25 |
| COWAT | 47.3±11.4 | 48.1±10.1 | 47.6±10.8 | 46.8±11.4 | 44.2±9.8 | 45.4±10.6 | 0.41 | 0.06 | 0.11 |
| WMS-R orientation | 13.9±0.3 | 13.9±0.6 | 13.9±0.5 | 13.9±0.3 | 13.9±0.3 | 13.9±0.3 | 0.46 | 0.34 | 0.34 |
For 2 subjects, only age and gender data is available.
P-values listed in the far right column were calculated between protective GAB2 haplotype carriers versus non-carriers in the APOEε4 carrier group, the APOEε4 non-carrier group, and in all samples, respectively. P-values were calculated using an unpaired Student's t-test (uncorrected for multiple comparisons). The significance of gender ratios was calculated using a chi-square test.
Abbreviations: STM=short term memory, LTM=long term memory, BNT=Boston Naming Test, MMSE=Mini Mental State Examination, HAM-D=Hamilton Depression Rating Scale, AVLT=Auditory verbal learning test, WAIS-R=Wechsler Adult Intelligence Scale-Revised, COWAT=Controlled Oral Word Association Test, WMS-R=Wechsler Memory Scale-Revised
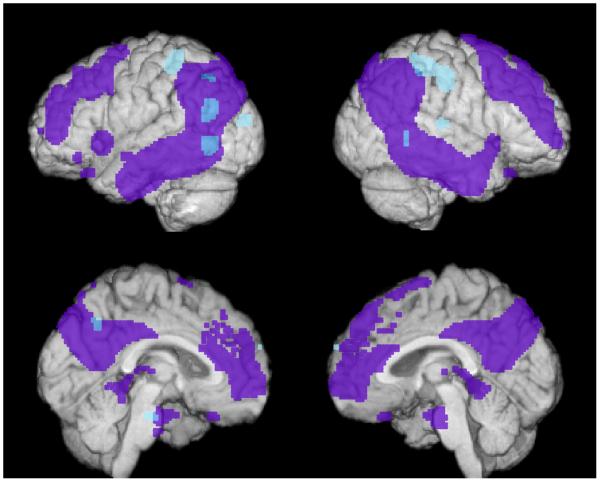
To test our hypothesis that the GAB2 protective haplotype may be associated with higher regional-to-whole brain FDG uptake in APOEε4 carriers, we analyzed PET images from 158 cognitively normal late-middle-aged subjects. As predicted, APOEε4 carriers with the protective GAB2 haplotype had higher regional-to-whole brain FDG uptake than APOEε4 carriers without the protective GAB2 haplotype in bilateral temporal, bilateral parietal and left occipital regions, areas that are in the vicinity of and overlap with regions previously found to show preferential CMRgI decreases in patients with probable AD (P<0.005, uncorrected for multiple comparisons, Figure 1, Table 3). The increases in FDK uptake remained significant after covarying the data for each subject's educational level and the WAIS-R Digit Span test (P<0.005, uncorrected for multiple comparisons). This 5.44% increase in regional-to-whole brain FDG uptake (with means of 1.6806 for GAB2 protective haplotype carriers and 1.5939 for GAB2 protective haplotype non-carriers; p=0.000314) in the left temporal cortex remained significant after correction for multiple regional comparisons. Additional increases (P<0.005, uncorrected for multiple comparisons) outside of characteristic AD increases were observed in bilateral parietal areas, the right temporal cortex, and the left occipital cortex (in a post hoc analysis of decreases in regional-to-whole brain FDG uptake in the APOEε4 carrier group, we failed to detect any associations between the protective GAB2 haplotype and lower regional-to-whole brain FDG uptake in AD affected brain regions at P<0.005, uncorrected for multiple comparisons).
Figure 1. APOEε4 carriers with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without the protective haplotype in brain regions preferentially affected by AD.
Brain regions with higher regional-to-whole brain FDG uptake (5.44%) in APOEε4 carriers with the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. The APOEε4 carriers with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without the protective haplotype in regions in vicinity of and overlapping with regions preferentially affected in patients with probably AD. These regions include bilateral temporal, parietal, and left occipital regions.
Table 3.
Location and magnitude of maximally significant regional-to-whole brain FDG uptake increases in AD-affected brain regions in APOEε4 carriers with the protective GAB2 haplotype than those without this haplotype
| Brain Region |
Atlas coordinates (mm)a (X,Y,Z) |
Z-score | P-valueb |
|---|---|---|---|
| Temporal (right) | (52, −48, −2) | 2.65 | 0.004 |
| Temporal (left) | (−40,−62, 20) | 3.60 | 0.0001c |
| Parietal 1 (right) | (56, −18, 44) | 3.29 | 0.001 |
| Parietal 1 (left) | (56, −42,− 32) | --d | 0.002 |
| Parietal 2 (left) | (−26,−60, 46) | 2.82 | 0.002 |
| Parietal 2 (right) | (50, −32, 53) | -- d | 0.0009 |
| Occipital (left) | (−38,−64, 22) | 3.49 | 0.0002 |
The coordinates were obtained from (Tournoux, 1988). X is the distance to the right (+) or left (−) of the midline, Y is the distance anterior (+) or posterior (−) to the anterior commissure, and Z is the distance superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures.
The reported significance level is one-tailed and uncorrected for multiple comparisons.
Remains significant after correction for multiple comparisons.
No voxel survived small-volume correction.
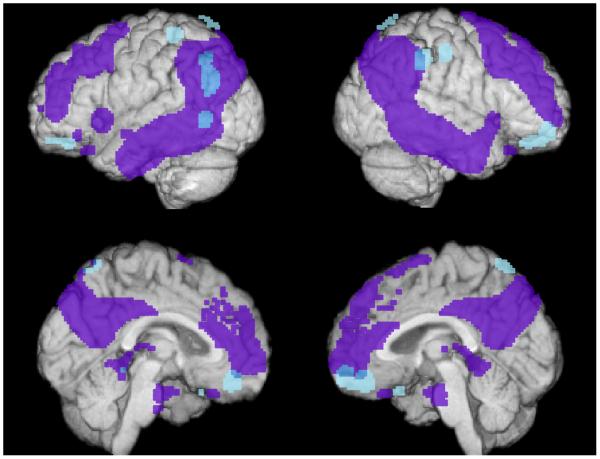
We further compared the association of GAB2 protective haplotype carriers in APOEε4 carriers versus APOEε4 non-carriers. As expected, APOEε4 carriers with the GAB2 protective haplotype had higher regional-to-whole brain FDG uptake in regions in the vicinity of and overlapping with AD regions (Figure 2 and Table 4; P<0.005, uncorrected for multiple comparisons). These regions include left temporal, right frontal, bilateral parietal regions, and the precuneus. Additionally, we evaluated whether an interaction between the GAB2 and APOE genes affects regional-to-whole brain FDG uptake. We found GAB2*APOE interaction effects in regions overlapping with and outside of AD affected brain regions. These areas include the precuneus, parietal, temporal, postcentral regions, as well as other areas (uncorrected P<0.005; Supplementary Figure 5).
Figure 2. APOEε4 carriers with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than APOEε4 non-carriers with the protective GAB2 haplotype in brain regions preferentially affected by AD.
Brain regions with higher regional-to-whole brain FDG uptake in APOEε4 carriers with the protective GAB2 haplotype than in APOEε4 non-carriers with the GAB2 protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower CMRgI in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. APOEε4 carriers with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than APOEε4 non-carriers with the protective haplotype in regions outside of and overlapping with regions preferentially affected in patients with probably AD. These regions include the precuneus, and frontal, temporal, and parietal areas.
Table 4.
Location and magnitude of maximally significant regional-to-whole brain FDG uptake increases in AD-affected brain regions in APOEε4 carriers with the protective GAB2 haplotype compared to APOEε4 non-carriers with the protective GAB2 haplotype
| Brain Region |
Atlas coordinates (mm)a (X,Y,Z) |
Z-score | P-valueb |
|---|---|---|---|
| Temporal (left) | (−42,−62,22) | 3.24 | 0.001 |
| Frontal (right) | (16,58,−14) | 3.03 | 0.001 |
| Parietal (left) | (−44,−36,62) | 2.80 | 0.003 |
| Parietal (right) | (56,−18,46) | 2.89 | 0.002 |
| Precuneus (right) | (4,−62,70) | 3.04 | 0.001 |
The coordinates were obtained from (Tournoux, 1988). X is the distance to the right (+) or left (−) of the midline, Y is the distance anterior (+) or posterior (−) to the anterior commissure, and Z is the distance superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures.
The reported significance level is one-tailed and uncorrected for multiple comparisons.
In additional exploratory analyses, we compared GAB2 protective haplotype carriers versus non-carriers in the overall subject and APOEε4 non-carrier groups. As predicted, in the overall subject group, carriers of the protective GAB2 haplotype had higher regional-to-whole brain FDG uptake compared to non-carriers in right temporal regions previously found to demonstrate characteristic decreases in regional-to-whole brain FDG uptake in patients with probable AD (P<0.005, uncorrected for multiple comparisons; Supplementary Figure 2 and Supplementary Table 1). The increase in regional-to-whole brain FDG uptake in the right temporal cortex remained significant after correction for multiple regional comparisons. Additional increases (P<0.005, uncorrected for multiple comparisons) outside of characteristic AD regions were observed in bilateral occipital and parietal areas. We additionally treated APOE genotype as a covariable in order to evaluate GAB2 haplotype effects on regional-to-whole brain FDG uptake that are distinct from APOE genotype. In occipital, and bilateral temporal and parietal regions, we identified regions of increased regional-to-whole brain FDG uptake in carriers of the protective GAB2 haplotype compared to non-carriers in areas in the vicinity of and overlapping with AD regions (P<0.005, uncorrected for multiple comparisons; Supplementary Figure 3 and Supplementary Table 2).
In the analysis of only APOEε4 non-carriers, carriers of the protective GAB2 haplotype also had higher regional-to-whole brain FDG uptake compared to non-carriers of the protective GAB2 haplotype in left temporal regions (P<0.005, uncorrected for multiple comparisons; Supplementary Figure 4 and Supplementary Table 3) which have been found to show preferential decreases in regional-to-whole brain FDG uptake in patients with probable AD. This increase in regional-to-whole brain FDG uptake in the left temporal cortex did not remain significant after correction for multiple regional comparisons. Additional increases (P<0.005, uncorrected for multiple comparisons) were observed in bilateral occipital and frontal regions.
DISCUSSION
In a previous GWAS survey of more than 300,000 SNPs, we found an association between GAB2 haplotypes and risk of late-onset AD in APOEε4 carriers (Reiman et al., 2007). In this study, we used FDG-PET as a quantitative presymptomatic endophenotype to further evaluate this haplotype. As predicted, APOEε4 carriers with the possibly protective GAB2 haplotype had higher regional-to-whole brain FDG uptake than APOEε4 carriers without this GAB2 haplotype in brain regions known to be preferentially hypometabolic in patients with probable AD. Our findings provide further support for the possibility that common GAB2 haplotypes modify AD risk in APOEε4 carriers and, to a smaller extent, non-carriers, and provide further support for the role of brain imaging in the presymptomatic assessment of putative modifiers of AD risk.
As we have previously noted, reductions in regional-to-whole brain FDG uptake in AD brains may reflect a decrease in the density or activity of terminal neuronal fields or peri-synaptic cells (Magistretti and Pellerin, 1996; Schwartz et al., 1979), metabolic dysfunctions (Mark et al., 1997; Piert et al., 1996), or both. Based on our previous GWAS, neuronal gene expression, histopathological, and siRNA findings, we postulated that the Gab2 protein may be involved in a pathway that reduces tau phosphorylation and subsequent neurofibrillary tangle formation so that a loss-of-function isoform may increase susceptibility to tangle formation in APOEε4 carriers, who are already at risk for LOAD (Reiman et al, 2007). Consistent with that possibility, carriers of the protective GAB2 haplotype and the fully functional Gab2 gene may have less tau phosphorylation than non-carriers of this haplotype in individuals who are already at risk for AD due to their APOEε4 carrier status or for other reasons. The assessment of biomarkers such as cerebrospinal fluid total-tau and phospho-tau levels could address our hypothesis further, while longitudinal follow-up studies comparing rates of memory decline and clinical conversion to mild cognitive impairment and AD are needed to confirm the posited GAB2 haplotype effect.
Among this study's limitations, our relatively small subject samples may have prevented us from detecting even more significant regional-to-whole brain FDG uptake differences between APOEε4 carriers with and without the protective GAB2 haplotype. Similarly our relatively small sample size of GAB2 protective haplotype carriers prevents us from evaluating regional-to-whole brain FDG uptake differences between APOEε4 carriers and non-carriers within that group with adequate statistical power. In conclusion, this study finds that APOEε4 carriers, and to a smaller extent, APOEε4 non-carriers, with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without this haplotype in AD-affected brain regions, and that an epistatic interaction between GAB2 and APOE may influence regional-to-whole brain FDG uptake. It provides further support for the possibility that the protective GAB2 haplotype reduces AD risk in APOEε4 carriers, and it supports the use of quantitative presymptomatic brain-imaging endophenotypes to help assess possible modifiers of AD risk. We also previously proposed that FDG-PET could be used in cognitively normal late-middle-aged subjects to help evaluate the individual and aggregate effects of putative genetic and non-genetic modifiers of AD risk (Reiman et al., 2005). In this way, it could complement observational studies of older AD cases and controls, helping to address potentially confounding effects of differential survival as related to the risk factor in question, inaccurate or biased recall of non-genetic risk factors, and easier access to accurate measurements of the risk factors in question (such as mid-life cholesterol levels (Reiman et al., 2009)). It could also complement prospective longitudinal cohort studies by providing information about the risk factor without having to study many more subjects or wait many years to determine whether or when they go on to develop symptoms.
Supplementary Material
Haploview analysis of 158 samples identified a haplotype block that encompasses the GAB2 gene. SNPs 1-2, 4-6, and 8-18 are listed consecutively in Table 2.D' values are shown in separate squares; empty boxes indicate a D' value of 1.0.
Brain regions with higher regional-to-whole brain FDG uptake in carriers of the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. Carriers of the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without the protective haplotype in the right temporal region which is preferentially affected in patients with probable AD.
After covarying out APOE genotype, brain regions with higher regional-to-whole brain FDG uptake in carriers of the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. Carriers of the protective GAB2 haplotype have higher FDG uptake than those without the protective haplotype in temporal, parietal, and occipital regions.
Brain regions with higher regional-to-whole brain FDG uptake in APOEε4 non-carriers with the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower regional-to-whole brain CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. APOEε4 non-carriers with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without the protective haplotype in the left temporal region, which is preferentially affected in patients with probable AD.
Brain regions affected by GAB2*APOE interaction (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower regional-to-whole brain CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. GAB2*APOE interaction effects were primarily identified in the precuneus, parietal, temporal, and postcentral regions.
ACKNOWLEDGMENTS
We would like to dedicate this work to our colleague Christopher Heward who passed away while this study was being completed. We thank Patti Aguilar, Sandra Yee-Benedetto, David Branch, Sandra Goodwin, Debbie Intorcia, Jennifer Keppler, Barbara Knight, Les Mullen, Anita Prouty, Stephanie Reeder, Desiree Van Egmond, Justin Venditti, and Denise Brown for their assistance. We also thank Bruce Henslin, the study coordinator, Dr. Rosa Rademakers for performing APOE testing, and Mrs. Xiaofen Liu and Dr. Napatkamon Ayutyanont from the Banner PET center. This study was supported by Kronos Science Laboratory (to EMR), the National Institute of Mental Health (R01MH57899 to EMR), the National Institute on Aging (R01AG031581 and P30AG19610 to EMR), the Arizona Alzheimer's Disease Center (AG023193 to EMR), the National Institute of Neurological Disorders and Stroke (R01 NS059873 to MJH), and the state of Arizona (to EMR, MJH, and RJC). We thank our research volunteers for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer's Disease Treatment Studies. Am J Psychiatry. 2002;159:738–745. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods. 1994;53:125–127. doi: 10.1016/0165-0270(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Feulner TM, Laws SM, Friedrich P, Wagenpfeil S, Wurst SH, Riehle C, Kuhn KA, Krawczak M, Schreiber S, Nikolaus S, Forstl H, Kurz A, Riemenschneider M. Examination of the current top candidate genes for AD in a genome-wide association study. Mol Psychiatry. 2009;6:6. doi: 10.1038/mp.2008.141. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Ikram MA, Liu F, Oostra BA, Hofman A, van Duijn CM, Breteler MM. The GAB2 gene and the risk of Alzheimer's disease: replication and meta-analysis. Biol Psychiatry. 2009;65:995–999. doi: 10.1016/j.biopsych.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, Jagust WJ, Reiman EM. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45:1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wetten S, Li L, St Jean PL, Upmanyu R, Surh L, Hosford D, Barnes MR, Briley JD, Borrie M, Coletta N, Delisle R, Dhalla D, Ehm MG, Feldman HH, Fornazzari L, Gauthier S, Goodgame N, Guzman D, Hammond S, Hollingworth P, Hsiung GY, Johnson J, Kelly DD, Keren R, Kertesz A, King KS, Lovestone S, Loy-English I, Matthews PM, Owen MJ, Plumpton M, Pryse-Phillips W, Prinjha RK, Richardson JC, Saunders A, Slater AJ, St George-Hyslop PH, Stinnett SW, Swartz JE, Taylor RL, Wherrett J, Williams J, Yarnall DP, Gibson RA, Irizarry MC, Middleton LT, Roses AD. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- Lin K, Tang M, Han H, Guo Y, Lin Y, Ma C. GAB2 is not associated with late-onset Alzheimer's disease in Chinese Han. Neurol Sci. 2009;19:19. doi: 10.1007/s10072-009-0178-8. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular bases of brain energy metabolism and their relevance to functional brain imaging: evidence for a prominent role of astrocytes. Cereb Cortex. 1996;6:50–61. doi: 10.1093/cercor/6.1.50. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, Wysoker A, Shapero MH, de Bakker PI, Maller JB, Kirby A, Elliott AL, Parkin M, Hubbell E, Webster T, Mei R, Veitch J, Collins PJ, Handsaker R, Lincoln S, Nizzari M, Blume J, Jones KW, Rava R, Daly MJ, Gabriel SB, Altshuler D. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008;40:1166–1174. doi: 10.1038/ng.238. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Miyashita A, Arai H, Asada T, Imagawa M, Shoji M, Higuchi S, Urakami K, Toyabe S, Akazawa K, Kanazawa I, Ihara Y, Kuwano R. GAB2 is not associated with late-onset Alzheimer's disease in Japanese. Eur J Hum Genet. 2009;17:682–686. doi: 10.1038/ejhg.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacmias B, Tedde A, Bagnoli S, Cellini E, Guarnieri BM, Piacentini S, Sorbi S. Implication of GAB2 gene polymorphism in Italian patients with Alzheimer's disease. J Alzheimers Dis. 2009;16:513–515. doi: 10.3233/JAD-2009-1005. [DOI] [PubMed] [Google Scholar]
- Nakaoka H, Inoue I. Meta-analysis of genetic association studies: methodologies, between-study heterogeneity and winner's curse. J Hum Genet. 2009;54:615–623. doi: 10.1038/jhg.2009.95. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Fountoulakis M, Dunckley T, Stephan DA, Reiman EM. Genetics, transcriptomics, and proteomics of Alzheimer's disease. J Clin Psychiatry. 2006;67:652–670. doi: 10.4088/jcp.v67n0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer's disease determined by dynamic FDG-PET. J Nucl Med. 1996;37:201–208. [PubMed] [Google Scholar]
- Ramirez-Lorca R, Boada M, Saez ME, Hernandez I, Mauleon A, Rosende-Roca M, Martinez-Lage P, Gutierrez M, Real LM, Lopez-Arrieta J, Gayan J, Antunez C, Gonzalez-Perez A, Tarraga L, Ruiz A. GAB2 gene does not modify the risk of Alzheimer's disease in Spanish APOE 4 carriers. J Nutr Health Aging. 2009;13:214–219. doi: 10.1007/s12603-009-0061-6. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci U S A. 2005;102:8299–8302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Langbaum JB, Lee W, Reschke C, Bandy D, Alexander GE, Caselli RJ. Higher serum total cholesterol levels in late middle age are associated with glucose hypometabolism in brain regions affected by Alzheimer's disease and normal aging. Neuroimage. 2009;49:169–176. doi: 10.1016/j.neuroimage.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Webster JA, Myers AJ, Hardy J, Dunckley T, Zismann VL, Joshipura KD, Pearson JV, Hu-Lince D, Huentelman MJ, Craig DW, Coon KD, Liang WS, Herbert RH, Beach T, Rohrer KC, Zhao AS, Leung D, Bryden L, Marlowe L, Kaleem M, Mastroeni D, Grover A, Heward CB, Ravid R, Rogers J, Hutton ML, Melquist S, Petersen RC, Alexander GE, Caselli RJ, Kukull W, Papassotiropoulos A, Stephan DA. GAB2 alleles modify Alzheimer's risk in APOE epsilon4 carriers. Neuron. 2007;54:713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schjeide BM, Hooli B, Parkinson M, Hogan MF, DiVito J, Mullin K, Blacker D, Tanzi RE, Bertram L. GAB2 as an Alzheimer disease susceptibility gene: follow-up of genomewide association results. Arch Neurol. 2009;66:250–254. doi: 10.1001/archneurol.2008.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz WJ, Smith CB, Davidsen L, Savaki H, Sokoloff L, Mata M, Fink DJ, Gainer H. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science. 1979;205:723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Bettens K, Brouwers N, Engelborghs S, van Miegroet H, De Deyn PP, Van Broeckhoven C. Common variation in GRB-associated Binding Protein 2 (GAB2) and increased risk for Alzheimer dementia. Hum Mutat. 2009;30:E338–344. doi: 10.1002/humu.20909. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Kantarci K, Reiman EM, Klunk WE, Weiner MW, Zetterberg H, Galasko D, Pratico D, Griffin S, Schenk D, Siemers E. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:6–15. doi: 10.1097/01.wad.0000191420.61260.a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournoux T. Co-Planar Stereotactic Atlas of the Human Brain. Georg Thieme Verlag/Thieme Medical Publishers; Stuttgart, New York: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Haploview analysis of 158 samples identified a haplotype block that encompasses the GAB2 gene. SNPs 1-2, 4-6, and 8-18 are listed consecutively in Table 2.D' values are shown in separate squares; empty boxes indicate a D' value of 1.0.
Brain regions with higher regional-to-whole brain FDG uptake in carriers of the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. Carriers of the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without the protective haplotype in the right temporal region which is preferentially affected in patients with probable AD.
After covarying out APOE genotype, brain regions with higher regional-to-whole brain FDG uptake in carriers of the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. Carriers of the protective GAB2 haplotype have higher FDG uptake than those without the protective haplotype in temporal, parietal, and occipital regions.
Brain regions with higher regional-to-whole brain FDG uptake in APOEε4 non-carriers with the protective GAB2 haplotype than in those without the protective haplotype (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower regional-to-whole brain CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. APOEε4 non-carriers with the protective GAB2 haplotype have higher regional-to-whole brain FDG uptake than those without the protective haplotype in the left temporal region, which is preferentially affected in patients with probable AD.
Brain regions affected by GAB2*APOE interaction (P<0.005, uncorrected for multiple comparisons) are shown in blue. Brain regions with lower regional-to-whole brain CMRgl in previously studied probable AD patients versus controls (Alexander et al., 2002) are shown in purple. GAB2*APOE interaction effects were primarily identified in the precuneus, parietal, temporal, and postcentral regions.